Abstract
BACKGROUND
The relative effectiveness of 3 approaches to blood pressure control—(i) an intensive lifestyle intervention (ILI) focused on weight loss, (ii) frequent goal-based monitoring of blood pressure with pharmacological management, and (iii) education and support—has not been established among overweight and obese adults with type 2 diabetes who are appropriate for each intervention.
METHODS
Participants from the Action for Health in Diabetes (Look AHEAD) and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) cohorts who met criteria for both clinical trials were identified. The proportions of these individuals with systolic blood pressure (SBP) <140mm Hg from annual standardized assessments over time were compared with generalized estimating equations.
RESULTS
Across 4 years among 480 Look AHEAD and 1,129 ACCORD participants with baseline SBPs between 130 and 159mm Hg, ILI (OR = 1.46; 95% CI = [1.18–1.81]) and frequent goal-based monitoring with pharmacotherapy (OR = 1.51; 95% CI = [1.16–1.97]) yielded higher rates of blood pressure control compared to education and support. The intensive behavioral-based intervention may have been more effective among individuals with body mass index >30kg/m2, while frequent goal-based monitoring with medication management may be more effective among individuals with lower body mass index (interaction P = 0.047).
CONCLUSIONS
Among overweight and obese adults with type 2 diabetes, both ILI and frequent goal-based monitoring with pharmacological management can be successful strategies for blood pressure control.
CLINICAL TRIALS REGISTRY
clinicaltrials.gov identifiers NCT00017953 (Look AHEAD) and NCT00000620 (ACCORD).
Keywords: blood pressure, blood pressure control, blood pressure monitoring, comparative effectiveness, diabetes, hypertension, lifestyle intervention, obesity.
Adults who have type 2 diabetes and are overweight are increasing in number and frequently have hypertension.1,2 Weight loss and pharmacological management are 2 recommended strategies for controlling their hypertension.3 Two major clinical trials have reported results from 4 years of interventions on blood pressure in cohorts that contained many individuals with these co-morbidities. The Action for Health in Diabetes (Look AHEAD) contrasted an intensive behavioral-based lifestyle intervention (ILI) targeting ≥10% weight loss with diabetes support and education (DSE); both interventions were combined with community-care blood pressure management.4,5 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial included an intervention of conventional pharmacologic blood pressure (CBP) control (its “standard” blood pressure control arm) that was managed by the study group. It targeted systolic blood pressure (SBP) <140mm Hg, which included frequent clinic-based assessment.6,7
We compare experience with these 3 treatment approaches through 4 years among the subset of individuals from both trials who were appropriate candidates for any of the three approaches: for whom ILI and CBP were judged to be safe and good adherence was expected. We hypothesized that, compared to DSE, the ILI, and the CBP interventions would both provide better long-term blood pressure control in this subset of treatment-eligible individuals.
METHODS
Enrollment
The Look AHEAD trial enrolled 5,145 overweight or obese (body mass index (BMI) ≥25kg/m2, or ≥27kg/m2 if on insulin) volunteers with type 2 diabetes who were aged 45–76 years and had blood pressures <160/100mm Hg with or without treatment. The ACCORD Blood Pressure Trial enrolled 4,733 volunteers with type 2 diabetes (BMI ≤ 45kg/m2). They may have had antihypertensive medications adjusted prior to randomization so that their SBP rose or fell to criteria: between 130 and 160mm Hg on 0–3 medications, between 161 and 170mm Hg on 0–2 medications, or between 171 and 180mm Hg on 0 or 1 medications. Additional details appear elsewhere: Look AHEAD8–10 and ACCORD.6,11 Our analyses are limited to those eligible for either trial based on SBP ≥130mm Hg (i.e., appropriate for ACCORD), <160mm Hg (i.e., appropriate for Look AHEAD), and other criteria as described later.
Look AHEAD ILI
The Look AHEAD ILI was designed to induce and maintain ≥10% weight loss.4 During the first 6 months, participants were seen weekly with 3 group meetings and 1 individual session per month. During months 7–12, participants were seen at least twice per month: group sessions every other week and a monthly individual session. During months 13–48, participants had a monthly individual on-site session followed approximately 2 weeks later by a second individual contact (phone or email), with optional monthly group sessions. Calorie goals were 1,200–1,500 for individuals weighing ≤250 lbs (114kg) at baseline and 1,500–1,800 for individuals weighing >250 lbs. The physical activity component relied heavily on home-based exercise, with gradual progression toward a goal of 175 minutes of moderate intensity physical activity per week. Standard community-care management of blood pressure was left to participants and their medical providers, who were provided results of annual study blood pressure measurements and JNC VI recommendations for blood pressure <130/80mm Hg. If measured blood pressures exceeded 160mm Hg systolic or 100mm Hg diastolic, participants were cautioned and their physicians were notified.
Look AHEAD DSE intervention
The Look AHEAD DSE intervention was designed to promote good health practices and retention. Its initial session covered key aspects of diabetes self-care and safety.5 Subsequent 60–90 minute small group sessions, offered three times annually, provided basic information on nutrition and physical activity promotion, diabetes and stress management, and social support. Community-care of blood pressure was left to participants and the medical management offered by their health care providers. The same measurement schedule, feedback of blood pressure values, and standard recommendations were followed in DSE as in ILI.
ACCORD CBP management intervention
The ACCORD CBP intervention was designed to reach the SBP goal of <140mm Hg. Drugs were available from all the major antihypertensive drug classes to achieve this goal.6,11 Antihypertensive medication down-titration was encouraged if SBP was <135mm Hg on 2 successive visits or <130mm Hg at any single visit. Participants were scheduled to attend blood pressure assessment visits at 1 and 4 months, and every 4 months thereafter, with additional visits as needed. Specific drug regimens were not mandated. Although the ACCORD CBP intervention was primarily based on pharmacological control, participants were also provided with dietary and lifestyle recommendations to optimize their glucose control, which included teaching on dietary principles and advice to engage in regular aerobic exercise (if medically fit to do so). The main focus of nutrition intervention was on monitoring carbohydrates, fat, sodium, and alcohol intake to achieve a healthy balanced diet. Participants were encouraged to accumulate ≥30 minutes of moderate-intensity aerobic activity for ≥5 days a week, which could be in 8–10 minute increments over 24-hour periods. The ACCORD trial also had an arm targeting SBP control <120mm Hg: we do not consider this arm in our manuscript because there was not a comparable group in Look AHEAD.
Summary of the 3 interventions
In summary, the Look AHEAD DSE intervention combined annual monitoring, participant education, and community care. The Look AHEAD ILI intervention featured these 3 approaches added to ILI. The ACCORD CBP management intervention featured monitoring every 4 months, behavioral advice, and goal-based pharmacological blood pressure management.
Data collection protocols
In both trials, blood pressures were measured by certified staff after participants sat quietly for 5 minutes. Cuff sizes were determined by arm circumference. In Look AHEAD, 2 blood pressure measurements were taken 30 seconds apart with the Dinamap Monitor Pro 100 automated device. In ACCORD, 3 measurements were taken automatically at 1-minute intervals with the Omron HEM-907 device, which calculated and displayed their mean. We base our analyses on the averages of these replicate measures.
In both studies, weight and height were measured using a digital scale and stadiometer. Demographic and risk factor measures were based on self-report. Look AHEAD participants brought current prescription medications to annual clinic visits for recording and classification, however, doses were not recorded. ACCORD recorded antihypertensive medication use at each visit. In our analyses, medications are classified as ACE-inhibitors, alpha-blockers, aldosterone receptor blockers, angiotensin receptor blockers, beta-blockers, calcium channel blockers, central alpha-2 agonists, reserpine, combined alpha-beta blockers, direct vasodilators, diuretics (thiazide), diuretics (loop), or diuretics (K-sparing).
Statistical analysis
Variables collected by the 2 trials were previously mapped onto a common set of definitions and formats as part of the Look AHEAD/ACCORD pooling project, which identified subsets of participants who were eligible for both the Look AHEAD and ACCORD trials.12 It found that 21% of Look AHEAD participants were suitable candidates for the overall ACCORD trial and 72% of ACCORD participants were suitable candidates for Look AHEAD. Major reasons that Look AHEAD participants were not eligible for ACCORD were primarily due to better glycemic control and no history of early cardiovascular disease (CVD). Major reasons that ACCORD participants were not eligible for Look AHEAD were often linked to poorer health and BMI <25kg/m2. For our analyses, we applied the additional blood pressure criteria necessary for ACCORD participants to be enrolled in its hypertension control trial to the Look AHEAD cohort, which reduced the percentage of suitable Look AHEAD participants to 10% (N = 248 ILI; N = 232 DSE) to compare with 1,129 ACCORD CBP participants.
We compared characteristics at trial enrollment of these subsets of Look AHEAD and ACCORD participants using chi-square tests and analyses of variance. We define our primary measure of SBP control as <140mm Hg, as all 3 intervention groups targeted meeting or exceeding this goal. We used generalized estimating equations models to compare the rates of SBP control over time among intervention groups. We compared the consistency of differences among intervention groups across subgroups of participants, using tests of interaction to assess whether some may differentially benefit from one of the interventions.
RESULTS
Within Look AHEAD, reasonable balance between the ILI and DSE groups was achieved for the subsets of participants we describe (Table 1). Many differences reached statistical significance among the 3 groups, however, primarily due to differences between the Look AHEAD and ACCORD cohorts. Compared to ACCORD participants, the Look AHEAD cohort contained greater numbers of women, college graduates, and Hispanics and fewer nondrinkers, current smokers, insulin users, and individuals with history of CVD. Their mean BMI, SBP, and HbA1c, were higher; their mean years of diabetes duration was lower. The 2 trial cohorts had markedly different profiles of hypertension treatment, with ACCORD participants receiving more antihypertensive therapy than Look AHEAD participants at baseline.
Table 1.
Characteristics at baseline of participants grouped by study and intervention assignment
| Baseline characteristic | Look AHEAD, N (%) or Mean (SD) | ACCORD, N (%) or Mean (SD) | Chi-squared or analysis of variance, P value | |
|---|---|---|---|---|
| DSE, N = 248 | ILI, N = 232 | CBP, N = 1129 | ||
| Females | 136 (54.8%) | 127 (54.7%) | 533 (47.2%) | 0.02 |
| Education | ||||
| Less than high school | 19 (7.8%) | 19 (8.3%) | 162 (14.6%) | <0.001 |
| High school or GED | 48 (19.8%) | 33 (14.5%) | 326 (28.9%) | |
| Post high school | 84 (34.7%) | 77 (33.8%) | 375 (33.2%) | |
| College graduate | 91 (37.6%) | 99 (43.2%) | 265 (23.5%) | |
| Race/ethnicity | ||||
| African-American | 46 (18.6%) | 38 (16.4%) | 275 (24.8%) | <0.001 |
| Hispanic | 21 (8.5%) | 30 (12.9%) | 67 (6.0%) | |
| Non-Hispanic white | 174 (70.2%) | 148 (63.8%) | 690 (62.3%) | |
| Other/multiple | 7 (2.8%) | 16 (6.9%) | 75 (6.7%) | |
| Alcohol use | ||||
| None | 168 (67.7%) | 161 (69.7%) | 869 (77.0%) | <0.001 |
| 1–7/week | 69 (27.8%) | 55 (23.8%) | 231 (20.5%) | |
| >7/week | 11 (4.4%) | 15 (6.5%) | 28 (2.5%) | |
| Smoking | ||||
| Never | 127 (51.4%) | 99 (42.7%) | 511 (45.4%) | <0.001 |
| Past | 111 (44.9%) | 118 (50.9%) | 489 (43.4%) | |
| Current | 9 (3.6%) | 15 (6.5%) | 128 (11.4%) | |
| Insulin users | 62 (25.0%) | 73 (31.5%) | 416 (36.8%) | 0.001 |
| History of CVD | 51 (20.6%) | 47 (20.3%) | 356 (32.3%) | <0.001 |
| Antihypertensive medication classes | ||||
| 0 | 45 (18.2) | 60 (25.9) | 94 (8.3) | <0.001 |
| ≥1 | 91 (36.7) | 68 (29.3) | 284 (25.2) | |
| ≥2 | 112 (45.2) | 104 (44.8) | 751 (66.5) | |
| Age, years | 61.4 (5.2) | 61.4 (4.8) | 62.2 (6.9) | 0.09 |
| BMI, kg/m2 | 35.1 (4.3) | 35.0 (4.6) | 33.1 (4.9) | <0.001 |
| Systolic blood pressure | ||||
| 130–139mm Hg | 111 (44.8) | 96 (41.4) | 523 (46.3) | 0.62 |
| 140–149mm Hg | 89 (35.9) | 86 (37.1) | 372 (33.0) | |
| 150–159mm Hg | 48 (19.4) | 50 (21.6) | 234 (20.7) | |
| Diabetes duration, years | 9.2 (7.2) | 9.0 (6.7) | 10.9 (8.2) | <0.001 |
| HbA1c, % | 8.40 (0.79) | 8.36 (0.81) | 8.21 (0.98) | 0.006 |
Abbreviation: GED, Completion of the General Educational Development test.
Follow-up of Look AHEAD participants was similar between intervention groups: 97.2% and 94.8% at year 1, 94.0% and 90.0% at year 2, 92.0% and 91.1% at year 3, and 91.2% and 89.3% at year 4 for the ILI and DSE groups, respectively. Follow-up of ACCORD CBP participants was 91.9% at year 1, 89.9% at year 2, 87.4% at year 3, and 71.2% at year 4.
Figure 1A portrays the mean number of antihypertensive medication classes used over time among the 3 intervention groups. During follow-up, the ILI cohort used slightly fewer classes than the DSE participants (P = 0.07) and substantially fewer classes than CBP (P < 0.001). There was little difference between the DSE and CBP cohorts (P = 0.22). Covariate-adjustment did not materially affect these findings.
Figure 1.
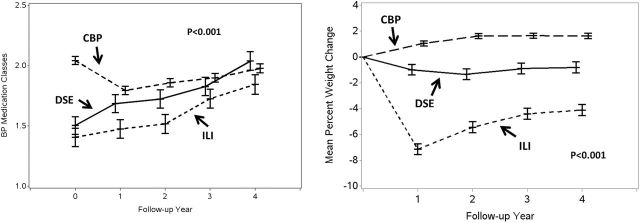
(a) Mean number of antihypertensive medication classes over time by intervention group. Classes are ACE-inhibitors, alpha-blockers/agonists, aldosterone receptor blockers, angiotensin II receptor blockers, beta-blockers, calcium channel blockers (non-DH), calcium channel blockers (DH), central alpha-2 agonists, combined A-B blockers, direct vasodilators, diuretics (thiazide), diuretics (loop), and diuretics (K-sparing). (b) Mean percent changes in weight over time by intervention group.
Figure 1B portrays weight changes from baseline in the 3 cohorts. The ILI cohort had greater weight losses over time than the DSE (P < 0.001) and CBP (P < 0.001) cohorts; the DSE cohort had modest weight losses, compared to some gain among CPB participants (P < 0.001). These were not altered with extensive covariate adjustment.
Figure 2 portrays the percentage of participants with SBP <140mm Hg over time for the 3 intervention groups. Overall, these were stable across 4 years (P = 0.77). Both the ILI (P = 0.004) and CBP (P = 0.003) yielded higher rates of blood pressure control compared to the DSE. With adjustment for baseline SBP and number of antihypertensive medicines, the odds ratios for control relative to DSE were OR = 1.48 [95% CI = 1.14–1.93] for ILI and OR = 1.40 [1.12–1.72] for CBP. Covariate adjustment for all factors in Table 1 that differed among groups did not materially alter these relationships: the adjusted odds ratios for control relative to DSE were OR = 1.46 [1.18–1.81] (P < 0.001) for ILI and OR = 1.51 [1.16–1.97] (P = 0.002) for CBP.
Figure 2.
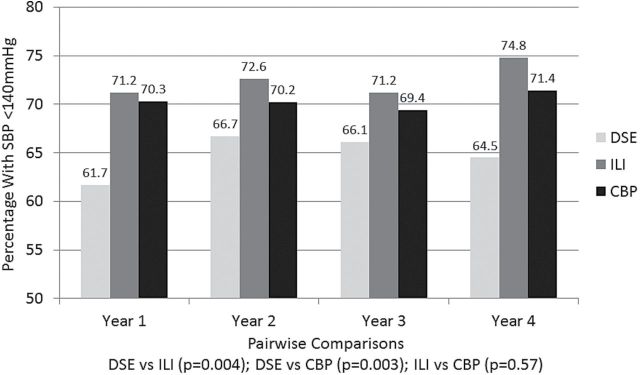
Percentage of participants with SBP <140mm Hg over time by intervention group. Inference is based on generalized estimating equations with adjustment for baseline systolic blood pressure and number of medication classes.
Table 2 describes the overall success rates of participants grouped by baseline factors in Table 1 in achieving blood pressure control. Results from tests of interactions are reported to identify subgroups that may differentially benefit from one of the interventions. Overall, better success in controlling blood pressure occurred among women, non-Hispanic whites compared to African-Americans, those using fewer antihypertensive medications at baseline, younger participants, those with lower baseline SBP, and those with shorter histories of diabetes. For only 2 subgroups was there some evidence of differential response to the interventions (P < 0.10). The strongest evidence was for BMI (P = 0.047). The success rates of CBP did not vary greatly by BMI, but were slightly higher among participants with baseline BMI <30kg/m2. The Look AHEAD interventions had the least success in this BMI group: the odds of successful control for the ILI participants were 1.64 [0.99–2.72] and 1.69 [0.87–3.27] times higher among those with baseline BMI of 30–39kg/m2 and >40kg/m2, respectively, compared to those with BMI 25–29kg/m2. There was also a more modest trend (P = 0.07) for individuals without a history of CVD to benefit differentially across the interventions. Among CBP participants there was slightly worse control among those without a history of CVD: OR = 0.85 [0.71–1.02]; for ILI and DSE participants there was slightly better control among those without a history of CVD: OR = 1.36 [0.86–2.17] and OR = 1.31 [0.82–2.10] respectively. We note, however, that all 95% confidence intervals for these subgroup comparisons include 1.
Table 2.
Relative odds for systolic blood pressure <140mm Hg by baseline characteristics and results of tests for the consistency of effects across subgroups and an interaction between subgroups and the three intervention groups
| Baseline characteristic | Odds ratio for SBP control | 95% CI | Consistency between subgroups, P value | Subgroup by intervention group interaction, P value |
|---|---|---|---|---|
| Gender | ||||
| Female | 1.21 | [1.06, 1.40] | 0.006 | 0.20 |
| Male (ref) | 1.00 | |||
| Education | ||||
| Less than high school | 0.81 | [0.64, 1.04] | ||
| High school or GED | 0.92 | [0.76, 1.12] | 0.13 | 0.95 |
| Post high school | 0.82 | [0.69, 0.98] | ||
| College graduate (ref) | 1.00 | |||
| Race/ethnicity | ||||
| African-American | 0.66 | [0.48, 0.90] | ||
| Hispanic | 0.95 | [0.62, 1.46] | 0.01 | 0.49 |
| Other/multiple | 0.81 | [0.60, 1.10] | ||
| Non-Hispanic white (ref) | 1.00 | |||
| Alcohol use | ||||
| None | 1.00 | [0.69, 1.45] | 0.71 | 0.40 |
| 1–7/week | 1.08 | [0.73, 1.59] | ||
| >7/week (ref) | 1.00 | |||
| Smoking | ||||
| Never | 0.89 | [0.71, 1.12] | 0.23 | 0.44 |
| Past | 1.01 | [0.80, 1.27] | ||
| Current (ref) | 1.00 | |||
| Insulin user | ||||
| No | 0.87 | [0.75, 1.01] | 0.06 | 0.14 |
| Yes (ref) | 1.00 | |||
| History of CVD | ||||
| No | 1.05 | [0.89, 1.23] | 0.56 | 0.07a |
| Yes (ref) | 1.00 | |||
| Antihypertensive medication classes | ||||
| 0 | 1.53 | [1.22, 1.92] | <0.001 | 0.27 |
| 1 | 1.41 | [1.20, 1.67] | ||
| ≥2 (ref) | 1.00 | |||
| Age, years | ||||
| Ten years older | 0.77 | [0.69, 0.86] | <0.001 | 0.30 |
| BMI | ||||
| 5kg/m2 greater | 1.00 | [0.93, 1.08] | 0.91 | 0.047b |
| Systolic blood pressure | ||||
| 130–139mm Hg | 1.99 | [1.66, 2.39] | <0.001 | 0.58 |
| 140–149mm Hg | 1.28 | [1.06, 1.55] | ||
| 150–159mm Hg (ref) | 1.00 | |||
| Diabetes duration | ||||
| 5 years longer | 0.91 | [0.87, 0.95] | <0.001 | 0.10 |
| HbA1c, % | ||||
| One unit higher | 0.96 | [0.89, 1.04] | 0.28 | 0.93 |
Abbreviation: GED, Completion of the General Educational Development test.
Baseline systolic blood pressure and number of medication classes are included as covariates.
aAmong CBP participants there was slightly worse control among those without a history of CVD: OR = 0.85 [95% CI: 0.71, 1.02]; Among ILI and DSE participants there was slightly better control among those without a history of CVD: OR = 1.36 [0.86, 2.17] and OR = 1.31 [0.82, 2.10], respectively.
bCompared to participants with BMI > 40kg/m2, the odds of blood pressure control among CBP participants was 1.13 [0.84, 1.52] among those with BMI 25–29kg/m2 and 1.01 [0.77, 1.32] among those with BMI 30–39kg/m2. Compared to participants with BMI 25–29kg/m2, the odds of blood pressure control among DSE participants were 0.98 [0.54, 1.78] for BMI 30–29kg/m2 and 1.30 [0.62, 2.72] for BMI ≥ 40kg/m2. Among ILI participants, these were 1.64 [0.99, 2.72] for BMI 30–39kg/m2 and 1.69 [0.88, 3.27] for BMI ≥ 40kg/m2.
Percent change in BMI, as a time-varying covariate, was related to SBP control in each intervention group in a fairly uniform manner (interaction P = 0.09). The odds of SBP control associated with a 5% loss in BMI were increased by a factor of 1.08 [1.02–1.13] among CPB participants, 1.23 [1.07–1.42] among DSE participants, and 1.19 [1.04–1.34] among ILI participants.
DISCUSSION
The Look AHEAD ILI and the ACCORD pharmacologic intervention including frequent goal-based monitoring both provided better overall success in meeting blood pressure goals than the Look AHEAD DSE intervention. The Look AHEAD ILI accomplished this with weight loss even with less use of antihypertensive medications. The ACCORD CBP did this with comparable levels of antihypertensive medication, even with a slight gain in weight. Overall, the relative effects on blood pressure control provided by the ILI and CBP interventions were fairly consistent across subgroups of participants formed by a number of risk factors for hypertension. While not statistically significant, there was some evidence that the CBP outperformed the ILI intervention for individuals with BMI <30kg/m2 and those with a history of CVD while the ILI intervention may have outperformed the CBP for heavier individuals and those free from CVD. While the groups of participants assigned to these interventions differed across a number of factors that were related to successful blood pressure control, our comparative effectiveness analysis is bolstered by the ability to confirm that all individuals included in our analyses were appropriate candidates for any of the three interventions. Nevertheless, we cannot rule out whether the relative benefits we observed for CBP compared to DSE could be attributable to unmeasured differences between the Look AHEAD and ACCORD study groups. The CBP cohort included the greatest proportion of African-Americans, a group for which the overall rate of blood pressure control was lower: including race/ethnicity subgroups as a covariate might be expected to enhance the difference between CBP and DSE.
Look AHEAD has examined the relative safety of ILI compared to DSE for serious adverse events potentially related to weight loss. Among events reported, there was a slight increased rate of fractures among the ILI group: 2.5 vs. 2.2 per 100 pyrs.11 ACCORD has reported that CBP compared to intensive blood pressure lowering (goal < 120mm Hg) has lower rates of several serious adverse events potentially related to antihypertensive treatments (e.g., hypokalemia, elevations of serum creatinine).11 Within the relatively few Look AHEAD participants included in these analyses, these events were rare and differences between protocols make cross-trial comparisons difficult.
Weight loss
Weight loss is recommended for overweight and obese individuals with type 2 diabetes.13 As expected, the Look AHEAD ILI group achieved the greatest weight losses: these individuals were provided behavioral tools and extensive training in strategies to effect lifestyle changes. These weight losses conferred greater blood pressure control than DSE, even with less use of antihypertensive medications and lower medication costs.15
Individuals with greater weight loss in all 3 interventions experienced greater blood pressure control, as might be expected,16,17 thus it appears that it may be additive to the benefit of pharmacologic approaches.
Of interest, the DSE approach of offering periodic educational and support sessions may have also been successful in promoting weight maintenance, and some overall mean weight loss, compared to the CBP intervention, which did not produce weight loss. This level was insufficient to match the blood pressure control provided by the CBP. However, individuals enrolled in Look AHEAD may have had more interest in weight loss than those in ACCORD and some weight gain among ACCORD participants may be attributable to its intensive glycemic control intervention.15
Frequent blood pressure monitoring
At enrollment, ACCORD CBP participants had been prescribed more antihypertensive medications than their Look AHEAD counterparts. Their prescription use was tapered during the first year of the CBP intervention and became comparable to that among DSE participants over time. Despite this, they were able to achieve better levels of blood pressure control. This suggests that the ACCORD frequent clinic-based monitoring, despite modest average increases in weight, was superior to participant education and community care (i.e., DSE). In addition, blood pressure medications were provided to ACCORD study participants as part of the study protocol. This may have improved compliance with prescribed therapy. While most treatment guidelines for individuals with diabetes recommend blood pressure monitoring 4 times annually and aggressive pharmacological control,14,18 the rates of blood pressure control provided by community care are often less than optimal.19,20
Strengths and limitations
Strengths of this study include well-characterized cohorts, standardized assessments, and good retention. Analyses are limited by not having data on medication doses in Look AHEAD. While individuals included in analyses simultaneously met criteria to be good candidates for all 3 of interventions, differences between the Look AHEAD and ACCORD cohorts existed and may have influenced findings. Participants were volunteers to the respective treatment approaches (e.g., pharmacotherapy vs. lifestyle change) and individual’s preferences may have influenced findings. Cohorts were limited to those with baseline SBPs of 130–159mm Hg. The clinical trials made considerable efforts to select participants and promote adherence to interventions among the volunteers: the intervention effects we describe may not be fully achieved in other settings. We have not examined the relative costs of delivering these interventions or patient treatment preferences.
Summary
Frequent goal-based blood pressure monitoring combined with pharmacological therapy and intensive lifestyle weight loss intervention combined with community care may provide comparable levels of blood pressure control among overweight and obese individuals with type 2 diabetes. We did not find strong evidence that either is preferentially effective across many subgroups of individuals based on risk factors, however there is some evidence that weight loss may be more effective among morbidly obese participants while the frequent monitoring in combination with pharmacotherapy approach may be more effective among overweight only participants. Both approaches may be superior to DSE combined with community care, however we cannot rule out that differences between the Look AHEAD and ACCORD groups may influence our findings. Future studies combining the ILI and frequent goal-based pharmacotherapy may be warranted.
DISCLOSURES
M.A.E. has received compensation for serving on advisory panels for Takeda Pharmaceuticals, Boehringer Ingelheim, Terumo Medical Corporation, and Zinfandel Pharmaceuticals and research support from AMGEN Pharmaceuticals. J.B.G. has received compensation from Merck for work as a consultant, from Bioscientifica for editorial activities, and from The Endocrine Society for the development of educational materials. Grant support has been paid to her institution for the performance of research by Merck and Bristol-Myers Squibb/Astra Zeneca, which in turn partly support her salary. J.B.G. has also received medical writing support funded by Boehringer Ingelheim for the development of a different manuscript. W.C.C. has received research funding from Merck and Lilly and compensation from Omron, Daiichi-Sankyo, Takeda, Novartis, and Janssen. No other authors report disclosures.
FUNDING
This work was supported by National Heart Lung and Blood Institute contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, and IAA #Y1-HC-9035 and IAA#Y1-HC-1010. Other components of the National Institutes of Health, including the National Institute of, Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute, contributed funding. The Center for Disease Control funded, substudies within ACCORD on cost-effectiveness and health-related quality of life. General Clinical Research Centers provide support at many sites.
ACKNOWLEDGMENTS
Look AHEAD Research Group at Year 4
Look AHEAD staff is listed alphabetically by site.
Clinical Sites
The Johns Hopkins Medical Institutions
Frederick L. Brancati, MD, MHS 1 ; Lee Swartz 2 ; Lawrence Cheskin, MD 3 ; Jeanne M. Clark, MD, MPH 3 ; Kerry Stewart, EdD 3 ; Richard Rubin, PhD 3 ; Jean Arceci, RN; Suzanne Ball; Jeanne Charleston, RN; Danielle Diggins; Mia Johnson; Joyce Lambert; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun
Pennington Biomedical Research Center
George A. Bray, MD 1 ; Kristi Rau 2 ; Allison Strate, RN 2 ; Frank L. Greenway, MD 3 ; Donna H. Ryan, MD 3 ; Donald Williamson, PhD 3 ; Brandi Armand, LPN; Jennifer Arceneaux; Amy Bachand, MA; Michelle Begnaud, LDN, RD, CDE; Betsy Berhard; Elizabeth Caderette; Barbara Cerniauskas, LDN, RD, CDE; David Creel, MA; Diane Crow; Crystal Duncan; Helen Guay, LDN, LPC, RD; Carolyn Johnson, Lisa Jones; Nancy Kora; Kelly LaFleur; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Mandy Shipp, RD; Marisa Smith; Elizabeth Tucker
The University of Alabama at Birmingham
Cora E. Lewis, MD, MSPH 1 ; Sheikilya Thomas, MPH 2 ; Monika Safford, MD 3 ; Vicki DiLillo, PhD; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Stacey Gilbert, MPH; Stephen Glasser, MD 3 ; Sara Hannum, MA; Anne Hubbell, MS; Jennifer Jones, MA; DeLavallade Lee; Ruth Luketic, MA, MBA, MPH; L. Christie Oden; Janet Raines, MS; Cathy Roche, RN, BSN; Janet Truman; Nita Webb, MA; Casey Azuero, MPH; Jane King, MLT; Andre Morgan
Harvard Center
Massachusetts General Hospital
David M. Nathan, MD 1 ; Enrico Cagliero, MD 3 ; Kathryn Hayward, MD 3 ; Heather Turgeon, RN, BS, CDE 2 ; Linda Delahanty, MS, RD 3 ; Ellen Anderson, MS, RD 3 ; Laurie Bissett, MS, RD; Valerie Goldman, MS, RD; Virginia Harlan, MSW; Theresa Michel, DPT, DSc, CCS; Mary Larkin, RN; Christine Stevens, RN; Kylee Miller, BA; Jimmy Chen, BA; Karen Blumenthal, BA; Gail Winning, BA; Rita Tsay, RD; Helen Cyr, RD; Maria Pinto
Joslin Diabetes Center
Edward S. Horton, MD 1 ; Sharon D. Jackson, MS, RD, CDE 2 ; Osama Hamdy, MD, PhD 3 ; A. Enrique Caballero, MD 3 ; Sarah Bain, BS; Elizabeth Bovaird, BSN, RN; Barbara Fargnoli, MS, RD; Jeanne Spellman, BS, RD; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center
George Blackburn, MD, PhD 1 ; Christos Mantzoros, MD, DSc 3 ; Ann McNamara, RN; Kristina Spellman, RD
University of Colorado Health Sciences Center
James O. Hill, PhD 1 ; Marsha Miller, MS, RD 2 ; Brent Van Dorsten, PhD 3 ; Judith Regensteiner, PhD 3 ; Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Lindsey Munkwitz, BS; Loretta Rome, TRS; Terra Worley, BA; Kirstie Craul, RD, CDE; Sheila Smith, BS
Baylor College of Medicine
John P. Foreyt, PhD 1 ; Rebecca S. Reeves, DrPH, RD 2 ; Henry Pownall, PhD 3 ; Ashok Balasubramanyam, MBBS 3 ; Peter Jones, MD 3 ; Michele Burrington, RD, RN; Chu-Huang Chen, MD, PhD 3 ; Allyson Clark Gardner, MS, RD; Molly Gee, MEd, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Jayne Joseph, RD; Julieta Palencia, RN; Jennifer Schmidt; Carolyn White
The University of Tennessee Health Science Center
University of Tennessee East
Karen C. Johnson, MD, MPH 1 ; Carolyn Gresham, RN 2 ; Stephanie Connelly, MD, MPH 3 ; Amy Brewer, RD, MS; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; Shirley Vosburg, RD, MPH; and J. Lee Taylor, MEd, MBA
University of Tennessee Downtown
Abbas E. Kitabchi, PhD, MD 1 ; Ebenezer Nyenwe, MD 3 ; Helen Lambeth, RN, BSN 2 ; Amy Brewer, MS, RD, LDN; Debra Clark, LPN; Andrea Crisler, MT; Debra Force, MS, RD, LDN; Donna Green, RN; Robert Kores, PhD
University of Minnesota
Robert W. Jeffery, PhD 1 ; Carolyn Thorson, CCRP 2 ; John P. Bantle, MD 3 ; J. Bruce Redmon, MD 3 ; Richard S. Crow, MD 3 ; Scott Crow, MD 3 ; Susan K Raatz, PhD, RD 3 ; Kerrin Brelje, MPH, RD; Carolyne Campbell; Jeanne Carls, MEd; Tara Carmean-Mihm, BA; Julia Devonish, MS; Emily Finch, MA; Anna Fox, MA; Elizabeth Hoelscher, MPH, RD, CHES; La Donna James; Vicki A. Maddy, BS, RD; Therese Ockenden, RN; Birgitta I. Rice, MS, RPh, CHES; Tricia Skarphol, BS; Ann D. Tucker, BA; Mary Susan Voeller, BA; Cara Walcheck, BS, RD
St. Luke’s Roosevelt Hospital Center
Xavier Pi-Sunyer, MD 1 ; Jennifer Patricio, MS 2 ; Stanley Heshka, PhD 3 ; Carmen Pal, MD 3 ; Lynn Allen, MD; Lolline Chong, BS, RD; Marci Gluck, PhD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD; Nancy Rau, MS, RD, CDE; Dori Brill Steinberg, BS
University of Pennsylvania
Thomas A. Wadden, PhD 1 ; Barbara J Maschak-Carey, MSN, CDE 2 ; Robert I. Berkowitz, MD 3 ; Seth Braunstein, MD, PhD 3 ; Gary Foster, PhD 3 ; Henry Glick, PhD 3 ; Shiriki Kumanyika, PhD, RD, MPH 3 ; Stanley S. Schwartz, MD 3 ; Michael Allen, RN; Yuliis Bell; Johanna Brock; Susan Brozena, MD; Ray Carvajal, MA; Helen Chomentowski; Canice Crerand, PhD; Renee Davenport; Andrea Diamond, MS, RD; Anthony Fabricatore, PhD; Lee Goldberg, MD; Louise Hesson, MSN, CRNP; Thomas Hudak, MS; Nayyar Iqbal, MD; LaShanda Jones-Corneille, PhD; Andrew Kao, MD; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, RD, MPH
University of Pittsburgh
John M. Jakicic, PhD 1 , David E. Kelley, MD 1 ; Jacqueline Wesche-Thobaben, RN, BSN, CDE 2 ; Lewis H. Kuller, MD, DrPH 3 ; Andrea Kriska, PhD 3 ; Amy D. Otto, PhD, RD, LDN 3 , Lin Ewing, PhD, RN 3 , Mary Korytkowski, MD 3 , Daniel Edmundowicz, MD 3 ; Monica E. Yamamoto, DrPH, RD, FADA 3 ; Rebecca Danchenko, BS; Barbara Elnyczky; David O. Garcia, MS; George A. Grove, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Nicole L. Helbling, MS, RN; Diane Ives, MPH; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, PhD, RD, LDN; Tracey Y. Murray, BS; Joan R. Ritchea; Susan Urda, BS, CTR; Donna L. Wolf, PhD
The Miriam Hospital/Brown Medical School
Rena R. Wing, PhD 1 ; Renee Bright, MS 2 ; Vincent Pera, MD 3 ; John Jakicic, PhD 3 ; Deborah Tate, PhD 3 ; Amy Gorin, PhD 3 ; Kara Gallagher, PhD 3 ; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
The University of Texas Health Science Center at San Antonio
Steven M. Haffner, MD 1 ; Helen P. Hazuda, PhD 1 ; Maria G. Montez, RN, MSHP, CDE 2 ; Carlos Lorenzo, MD 3 ; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN; Ronda Saenz, MS, RD
VA Puget Sound Health Care System/University of Washington
Steven Kahn MB, ChB 1 ; Brenda Montgomery, RN, MS, CDE 2 ; Robert Knopp, MD 3 ; Edward Lipkin, MD 3 ; Dace Trence, MD 3 ; Terry Barrett, BS; Joli Bartell, BA; Diane Greenberg, PhD; Anne Murillo, BS; Betty Ann Richmond, MEd; Jolanta Socha, BS; April Thomas, MPH, RD; Alan Wesley, BA
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico
William C. Knowler, MD, DrPH 1 ; Paula Bolin, RN, MC 2 ; Tina Killean, BS 2 ; Cathy Manus, LPN 3 ; Jonathan Krakoff, MD 3 ; Jeffrey M. Curtis, MD, MPH 3 ; Justin Glass, MD 3 ; Sara Michaels, MD 3 ; Peter H. Bennett, MB, FRCP 3 ; Tina Morgan 3 ; Shandiin Begay, MPH; Paul Bloomquist, MD; Teddy Costa, BS; Bernadita Fallis RN, RHIT, CCS; Jeanette Hermes, MS, RD; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Julie Nelson, RD; Carol Percy, RN; Patricia Poorthunder; Sandra Sangster; Nancy Scurlock, MSN, ANP-C, CDE; Leigh A. Shovestull, RD, CDE; Janelia Smiley; Katie Toledo, MS, LPC; Christina Tomchee, BA; Darryl Tonemah, PhD
University of Southern California
Anne Peters, MD 1 ; Valerie Ruelas, MSW, LCSW 2 ; Siran Ghazarian Sengardi, MD 2 ; Kathryn (Mandy) Graves Hillstrom, EdD, RD, CDE; Kati Konersman, MA, RD, CDE; Sara Serafin-Dokhan
Coordinating Center
Wake Forest University
Mark A. Espeland, PhD 1 ; Judy L. Bahnson, BA, CCRP 3 ; Lynne E. Wagenknecht, DrPH 3 ; David Reboussin, PhD 3 ; W. Jack Rejeski, PhD 3 ; Alain G. Bertoni, MD, MPH 3 ; Wei Lang, PhD 3 ; Michael S. Lawlor, PhD 3 ; David Lefkowitz, MD 3 ; Gary D. Miller, PhD 3 ; Patrick S. Reynolds, MD 3 ; Paul M. Ribisl, PhD 3 ; Mara Vitolins, DrPH 3 ; Haiying Chen, PhD 3 ; Delia S. West, PhD 3 ; Lawrence M. Friedman, MD 3 ; Brenda L. Craven, MS, CCRP 2 ; Kathy M. Dotson, BA 2 ; Amelia Hodges, BS, CCRP 2 ; Carrie C. Williams, MA, CCRP 2 ; Andrea Anderson, MS; Jerry M. Barnes, MA; Mary Barr; Daniel P. Beavers, PhD; Tara Beckner; Cralen Davis, MS; Thania Del Valle-Fagan, MD; Patricia A. Feeney, MS; Candace Goode; Jason Griffin, BS; Lea Harvin, BS; Patricia Hogan, MS; Sarah A. Gaussoin, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Michael P. Walkup, MS; Karen Wall, AAS; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco
Michael Nevitt, PhD 1 ; Ann Schwartz, PhD 2 ; John Shepherd, PhD 3 ; Michaela Rahorst; Lisa Palermo, MS, MA; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories
Santica M. Marcovina, PhD, ScD 1 ; Jessica Chmielewski 2 ; Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS 1 ; Ronald J. Prineas, MD, PhD 1 ; Charles Campbell 2 ; Zhu-Ming Zhang, MD 3 ; Teresa Alexander; Lisa Keasler; Susan Hensley; Yabing Li, MD
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities
Robert Moran, PhD 1
Hall-Foushee Communications, Inc.
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases
Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD; Robert Kuczmarski, PhD
National Heart, Lung, and Blood Institute
Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR
Centers for Disease Control and Prevention
Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD
Funding
This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche, Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene for providing vital statistics data.
ACCORD RESEARCH GROUP
Steering Committee: W.T. Friedewald (Chair), J.B. Buse (Vice Chair), J.T. Bigger, R.P. Byington, W.C. Cushman, F. Ismail-Beigi, H.C. Gerstein, H.N. Ginsberg, D.C. Goff, Jr, R.H. Grimm, Jr, J.L. Probstfield, D.G. Simons-Morton
Clinical center networks (CCNs) and clinical sites
Canadian CCN: Population Health Research Institute, Hamilton General Hospital, Canadian Diabetes Outcome Researchers (CANDOR Network), Hamilton, Ontario, Canada: H.C. Gerstein, S. Yusuf, Z. Punthakee, R. Russo, S. Anand, K. Chrysler*, B. Cracknell, T. Cukierman-Yaffe, A. Gafni, G. Guyatt*, S. Hall, J. Kaszyca, E. Lonn*, P. Mackie*, V. Reiding, N. Shehadeh*, B. Tadeson*, K. Thompson*, M. Vallis, V. Vasudeva, I. Wilderman*, D. Wright
Canadian clinical sites
McMaster Medical Centre, Hamilton, Ontario, Canada: Z. Punthakee, A. Smith, I. Stanton, S. Capes*, P. Manjoo*, T. Valla, S. Danby*, W. Harper*, P. Harvey, D. Hunt, Audrey Moroso*, Rose Otto, Ally Prebtani
Six Nations Health Services, Ohsweken, Ontario, Canada: Z. Punthakee, A. Davis, S. Capes*, K.L. Hill, V. McCarthy
Diabetes, Hypertension and Cholesterol Centre, University of Calgary, Calgary, Alberta, Canada: A.L. Edwards, D.J. Mitchell, M.A. Clearwaters, C. Dielissen*, M. Gillam, B. Hammond*, H. Jensen*, A. Kherani*, D. Lau, V. Pringle, D. Rabi, R. Sigal, C. Smith*, M. Walker*, G. Williams*
Memorial University of Newfoundland, St. John’s, Newfoundland, Canada: C. Joyce, M. Parsons, B. Rowe, J. Burton*, V. Chandurkar, S. CoadyMcDonald*, D. Gibbons*, C. Kovacs, B. Murphy*, R. Smart*, S. Varghese
University of Alberta, Edmonton, Alberta, Canada: L. Mereu, E. Ryan, P. Senior, P. Kirkland, J. Abe*, K. Dalton, W. Gendall, J. Germsheid*, D. Hartmann*, A. Jeffrys*, U. Kumar*, C. MacDonald, N. Makhani, S. Mawani*, F. Morales, B. Paty*, M. Pick*, B. Schwanke, A. Stark, M. Tennant, S. Varma*, D. Weiss-Aburto, P. WerbiskiWood, B. Woloschuk, W. Zimmerman*
Centre de Recherche Clinique de Laval, Laval, Quebec, Canada: A. Bélanger, S. Gauthier, G. Bahsali*, C. Barbeau*, E. Caponi*, R. Duchesne*, R. Dumas, P. Gauthier, J. Girouard*, N. Kandalaft*, M. Labbé, J. Palardy, M. Pilon, J. Raymond, A. Schiffrin
St. Joseph’s Health Care London, London, Ontario, Canada: I. Hramiak, S. Tereschyn, M. Driscoll, M. Gehring, J. Gonder, C. Lincoln, W. McBeth, C. McDonald, T. McDonald*, P. Pauli*, T.Paul, S. Powers*, N. Ronald, V. Trinh*, L. Vancer*, G. Walsh*
Ottawa Hospital, Division of Endocrinology and Metabolism, Ottawa, Ontario, Canada: H. Lochnan, T.C. Ooi, J. Maranger, L. Bradley*, R. Buhrmann, M. Cyr*, C. Gilchrist*, B. Hanlon*, M. Harley*, K. Jay*, T. Leech, J. Malcolm, M. McLean*, E. Parker*, R. Sigal*, K. Sullivan
Royal Victoria Hospital, Montreal, Quebec, Canada: J.F. Yale, S.A. Segal, G. Al Ansari, N. Renouf*, N. Allaire*, M.A.M.A. Alawadhi*, B. Belfer*, D.W. Blank, F. Bouchard*, S. BuoyPhang*, J. Carter, L. Coppin*, D. Dalpe*, I. Delpech*, P.M. Doran†, F. Emmian*, S. Fortin*, N. Garfield, M. Gosselin*, S. Horan*, M. Kalergis*, S. Koutelias*, C. Légaré*, A. Lombardo*, J.A. Morais, M. Quigley*, N. Renouf*, C. Riopel*, S. Riopel, J.A. Rivera, G. Rochon*, M. Roy, M. Salera*, M.H. Sherman, M. Shingler*, H.E. Staples*, L. Ulyatt*, Z. Yared*
St. Michael’s Hospital, Toronto, Ontario, Canada: L.A. Leiter, G. Booth, L. Sparrow*, H. Choi, D.C. Bedard, A. Berger*, L.A. Berndl, A. Cheng*, V. Evalmplev*, J. Goguen, A. Hanna, R.G. Josse, J.A. Kalas*, S. Perry, M. Pike*
Vancouver General Hospital, Vancouver, British Columbia, Canada: T. Elliott, K. Dawson, J. Kong, D. Albiani*, M. Inducil, R. Al Amoudi*, T. Broughton*, L. Hall*, B. Harrison*, N. Hirvi*, R. Lee*, A. Merkur*, E. Norman*, B. Paty, M. Potter, D. Stevenson*, A. Vafadaran
Health Sciences Centre Diabetes Research Group, Winnipeg, Manitoba, Canada: V. Woo, L. Berard, T. Anderlic, K. Austman*, A. Bernard*, D. Catte*, P. Darvill*, D. Hak, K.Hutchison*, L. Janzen*, T. Klopak, C. Mandock, M. Mathen*, S. Mawani*, A. Mawani*, L. Murphy†, G. Nyomba, B. Penner*, S. Pockett*, S. Russell*, F. Stockl, J. Studney*, R. Sukkau
Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada: C. Abbott, E. Ur*, M. Yuille, M. Archibald*, D. Clayton, A. Cruess, N. Davis, H. Fong*, S. Frizzell*, B. Hanway*, A. Hoskin-Mott, A. Imran, C. Ingraham*, G. McCarthy, H. Murdock*, T. Palmer, A.M. Patterson*, T. Ransom, D. Shu*, J. Tuttle*
Western CCN: University of Washington, Seattle, WA: J.L. Probstfield, C. Kingry, A.S. Line, M.A. Corson, R. Knopp*, E. Lipkin*, M.D. Sullivan, J. Johnson, C. Griswold*, K. Liebert*, A. Brown*, D. Juliano*, E.M. Kurashige*, S. Moberg*, J. Leader
Western clinical sites
Northridge Hospital Medical Center, Cardiovascular Center, Northridge, CA: K. Ariani, K. Karunaratne, M. Azizad*, C. Chow*, H. Gutierrez, J. Partamian*, J. Toven*, J. Toven, J. Mular*, S. Sanders*
White Memorial Medical Center, Clinical Hypertension Services, Los Angeles, CA: L.J. Haywood, V. Kamdar*, D.L. DeQuattro, L. Wang, Z. Song, L. Becerra, A. Qi Cai*, C. Pruitt*, V. DeQuattro†
University of Washington Medical Center at Roosevelt, Family Medical Center, Seattle, WA: R. Failor, A. Ellsworth*, N. Jackson, C. Miller, D. Britt*, S. Dobie*, I. Hirsch*, D. Khakpour*, R. Quaempts*, W. Stoffel*, W. Neighbor*, K. Cappocia*, V.Hawkins*, L.Tapp
Idaho State University, Department of Family Medicine, Pocatello, ID: R. Force, M. Macdonald, K. Pettingill, C. Liday*, S. Koester*, T. Pettinger, R. Solbrig, C. Waldron, * W. Woodhouse*, B. Hoover* S.Lusk, T. Wilcox*, D.Hachey*
Naval Medical Center San Diego, Cardiology Division, San Diego, CA: P.E. Linz, P.V. Pepper, J. Kozlowski, C. Chase*, C. Griffin, D. Samuelson*, P. Gutierrez*, C. Gonzales, M. Engle*, J. Coopersmith*, S. Griffin, R. Lammers*, J. Leon*, D. Zirkle*, S. Hollingsworth*
Oregon Health & Science University, Section of Diabetes, Portland, OR: M.C. Riddle, K.A. Hanavan, P.A. McDaniel, R. Swift, A.J. Ahmann*, S.C. Gammell-Matthews, D.M. Karl, V. Burden*, B. MacNeil, M. MacMurray, J. Weiss*, C. Carlson*, S.K. DesRochers*, D. Negreanu*, E.A. Stephens*, D. Gale*
Washington State University, Spokane, WA: C. Wysham, D. Weeks, L. Kuntsmann, L. Maxwell*, S. Yedinak, H. Pena*, J. Kistler*, J. White*
Kaiser Endocrine Clinic, San Diego, CA: J. Dudl, L. Lyons, B. House, M. Murray, R. Stevenson*, P. Wu*, A. Palma*, S. Briere*, T. Wilson*, D. Becker*, K. Harden*, C. Hawley*
Whittier Institute for Diabetes, Clinical Trials Department, La Jolla, CA: G. Dailey, M. Baron, A. Gianella, M. Jacobson*, E. Farro*, A. Philis-Tsimikas*, A. Banares*, A. Bravo-Medina*, J. Horne*, E. Esquer*, R. Morrissey*
Minnesota-Iowa CCN: Berman Center for Outcomes & Clinical Research, Minneapolis, MN: R.H. Grimm, Jr, B.R. Kirpach, M.M. Bartkoske, C.M. Boyce*, N. Druckman*, A.M. Gillett*, J.A. Levin, G.J. Livingston, A.M. Murray, H. Wood*, HealthPartners Research Foundation, Minneapolis, MN: K.L. Margolis
Minnesota-Iowa clinical sites
Hennepin ACCORD Clinic, Minneapolis, MN: S. Kempainen, M. Madden, M.Tariq Fareed*, K. Hall*, R. Moor*, K.Wood
International Diabetes Center, Minneapolis, MN: R. Bergenstal, R. Cuddihy, B. Davick*, J. Hokanson*, M. Johnson, M. Lausch*, S. List, A. Monk, R. Robinson*, K. Smith*, D. Whipple, G. Damberg, R. Hahn*, V. Koenig*, M. Magadan, S. Sabin- Smith*, P. Stewart*, E. Strock, D. Peremislov, K. Gunyou, R. Passi . C. Ashanti, L. Thomas, D. Stoffels
University of Minnesota, Minneapolis, MN: E.R. Seaquist, M.V. Mech, L.E. Benedict*, D.J. Demmon*, D. Kendall*, A.F. Kumar*, S.M. Martinson*, S.A. Miller*, C. Pease*, J.P. Rao*, J.B. Redmon*, J.E. Swanson†, J.K. Wimmer*, Y. Okorocha, M. Stiles*, C. Kodl*, C. Chadha*
University of Minnesota, Phalen Village Clinic, St. Paul, MN: KA. Peterson, L.A. Seaquist, C. Boese*, M. Cruciani*, E. Dodds, F. Parenteau Ek*, J.L. Feldman*, P. Fontaine*, C.J. Lange, T.J. Mendenhall*, A.M. Peterson*, A. Rudelt* T.M. Schrock*, D.P. Spielman*, S. Velasco*, J.C. Weinhand*
Riverside Health Partners Clinic, Department of Endocrinology, Minneapolis, MN: J.M. Sperl-Hillen, P.J. O’Connor, M.E. Busch, A. Chung*, B.K. Klein*, N. Krugen*, T. Bunkers-Lawson*, H.L. Ekstrom*, H.S. Gunderson*, B.M. Johnson*, J.H. MacIndoe*, D.J. Prewedo*, J.L. Rawl*, C.M. Roethke*, Mary Quinlan, C.R. Fox, B.M. Bate, Q.T. Cao*. M.M. Ohnstad, P.J. Meyers*
University of Iowa, Health Care Diabetes Clinical Research and Programs, Iowa City, IA: W.I. Sivitz, S.M. Wayson, T.A. Lower*, L. Larson, L.A. Ahrens*, M. Bayless, S.E. Beck*, J. Chahal, C. Chenard, G.C. Doelle, V.M. Guzman, U.M. Kabadi*, K.A. Ochs*, A. Rahhal, R.G. Spanheimer*, L. Snetselaar*, K. Smith*, D. Wells*
Ohio-Michigan CCN: Case Western Reserve University, Division of Clinical and Molecular Endocrinology, Cleveland, OH: S. Genuth, F. Ismail-Beigi, M. Thibonnier*, L. Vargo*, C. Kelly*, T. Bongorno*, A. Dolish*, L. Pavlik, M. Tiktin, S. Isteitieh* A. Bartlett, T. Kulow
Ohio-Michigan clinical sites
University Hospitals of Cleveland, Division of Endocrinology, and University Hospitals Westlake Medical, Cleveland, OH: F. Ismail-Beigi, A. Krikorian, L. Moore, L. Richardson, E. Coles-Herman*, K. Yee*, J. Frankino, M. Jing*, A. Sood, L. Hustak*, M. Julius*, L. Pavlik*, T. Ross*, L. Long*, W. Schwing*, M. Tiktin*, M.K. Sullivan*, L. Strauss*, K. Behm*, F. Eskandari*, C. Hall*, D. Hayes*, K. Horowitz, S. Isteitieh*, Z. Madhun*, E. Seeholzer*, J. Shina*, H. Taylor*, A. Schnall*, S. Huang, M. Heeg, J. Tang, J. Belkin*, M.S. Lee*, T. Joly*
St. Vincent Charity Hospital, Lipid Research Center, Cleveland, OH: L.S. Sadler, M. Griffith*, A. Hornsby*, K. Klyn, E. Ospelt, L. Long, M. DeSmit*, P. McCann, N.P. Schmidt*, C. Gottfried, T. Kulow, J. Zaletal*, M.S. Kapadia, L.Smith*
University Suburban Health Center, South Euclid, OH: A.M. Schnall, L. Dragmen, R. Ellert*, J. Smith, J. Leksan, T. Sussman, S. Huang, M. Heeg, J. Tang, J. Belkin*, M.S. Lee*, T. Joly*
Cleveland Veterans Affairs (VA) Medical Center (VAMC), Department of Medicine, and Ravenna Community Based Outpatient Clinic, Cleveland, OH: F. Ismail-Beigi, L. Hustak*, M. Julius*, W. Schwing, M. Tiktin*, J. Anselmo*, F. Eskandari*, S. Daymeyer*, C. Hall*, D. Hayes*, K. Horowitz*, S. Isteitieh*, C. Johnson*, E. Kern, M.A. Richmond*, L. Richardson, K. Roberts*, J. Shina*, A. Sood, P. Suhan*, H. Taylor*, S. Watts*, J. Martin, L. Moor*, B. Burtch*, S. Ober, G.J. Strauss, A. Leone, J. Belkin*, S. Huang*, K. Frank*, D. Stephens*, M.S. Lee*, T. Joly*
The Cleveland Clinic Foundation, Cleveland, OH: B.J. Hoogwerf*, J. Brakeman, M. Matzinger *, J. Newsome*, J. Becker*, S. Bizjack*, B. Clingman*, S. Curtas-Thomas, G. Depietro*, R. Ellert*, C. Horner, G. Bunae*, A. Hamrahian*, A. Hawkins*, T. Head*, S. Iannica*, L. Jones*, P. Kaiser, R. McCoy, A. Mehta, L. Olansky, A. Orasko, S. Reddy*, D. Ross*, L. Shockley*, E. Siraj*, M. Williams*, R. Zimmerman, M. Hamaty
Your Diabetes Endocrine Nutrition Group, Mentor, OH: D. Weiss, K.A. Fagan, T.M. Hanslik*, J. Farrell*, P. Brys*, M. Oligny, K. Prokop, K. Lenardic*, T. Karapanzcik*, S. Huang, M. Heeg, J. Tang, J. Belkin*, M.S. Lee*, T. Joly*
Medical University of Ohio, Department of Medicine, Ruppert Health Center, Toledo, OH: B. Akpunonu, R. Franco-Saenz†, J. Gilmore, M. Gilmore, L. Godfrey*, P. Ross*, B. Bauer, M. Chrisstie*, A. Lopez, P. Mulrow, C. Peters*, R. Pop-Busui*, J. Roman*, C. Smith*, J. Bick*, Z. Blust*, P.T. Nelsen, D. Marcus*
The Ohio State University Medical Center, Division of Endocrinology, Diabetes and Metabolism, Columbus, OH: K. Osei, E.A. Dziengelewski*, H. Breedlove, D. Boland*, C. Casey Boyer, S. Cataland, P. A. Kearns, J.E. Irwin, D.P. Schuster, J.L. Varga-Spangler*, T. Bowles, K. Weiland, K. Arnold; T. Evans*, J. Bouttamy, A. Letson, E. Craig, F. Davidorf
University of Cincinnati/VA Medical Center, Research Service, Cincinnati, OH: R.M. Cohen, K. Burton, J. Craig, B. Carter*, J. Harrer*, R. Hurd*, D. Lopez-Stickney*, C. Pritchard*, A. Pfefferman*, B.A. Ramlo-Halsted*, C. McCormick, C. Riley, M. Strominger*, A. Knittel, G. Groff*, C. Bailey, A. Howald, N. Anderson, J. Laver Bierschbach, M. Tyzinski*, B. Smith*, S. Krug, V. Hershberger*, R.K. Hutchins*, L.A. Raymond*
Henry Ford Health System–New Center One, Detroit, MI: A.Thomas, D.M. Kahkonen*, T. Cushman, M. Roman, A.M. Stys, K. White, M. Austin*, C. Chatterton, J.K. Francis*, C. Jones*, D. Kruger, A. McLellan*, F. Whitehouse, E. Higgins*, S. Levy, A. Schoenherr*, P. Edwards
Grunberger Diabetes Institute, Bloomfield Hills, MI: G. Grunberger, L.C. Aman*, A.H. Bandagi*, K.M. Russell*, C. Tucker, Y. Abidova*, A. Amirikia, M. Narduccio, B. Billingsly
Northeastern CCN: Columbia University College of Physicians and Surgeons, New York, NY: J.T. Bigger, C.R. Lopez-Jimenez, R. Bornholdt, L. Busaca, H.N. Ginsberg, P. Gonzales, D. Gosh*, P. Love†, A. Kosok*, E. Robinson*, R. Steinman, C. Watson, G. Reyes
Northeastern clinical sites
Jacobi Medical Center, Bronx, NY: U.K. Schubart, M. Mendoza, G. Goswami, A. Laufer*, J. Russo, N. Vincenty
Albert Einstein General Clinical Research Center, Bronx, NY: M.H. Alderman, L. Carroll, M.J. Sanguily*, J.U. Gorkin, A.C. Mayer, L. Ramos, V. Sessoms, A. Fritts Stewart*
Cornell Internal Medicine Associates, New York, NY: D. Brillon, J. Cordero, M.A. Richardson, E. Wei, F. Ganz, B.R. Meyer, J. Paley*, S. Anderson*, C. Charles*, A. Dwoskin*, R. Chiong*, K. Hyams
The Diabetes Care and Information Center of New York, Flushing, NY: D.L. Lorber, T. Arenstein*, P. Depree, A.A. Elmorsy*, J.M. Wendel, L.L. Zintl*, P. August, M. Beck*, M.D. Goldberg, M.J. Hofacker*, M. Marotta-Kollarus*, E.J.L. Ocampo, C.A. Resta, J.M. Tibaldi
The Cooper Health System, Cherry Hill, NJ: A. Bastien*, S. Grudzinski*, P. Niblack, L. Abreu*, T. Brobyn, K. Brown*, M. Casale*, D. Dougherty*, G. Haddad, K. Heintz, M. Kelly*, D. Linneman*, C. Olivia, M.A. Salvador*, P. Zee*, D. Hyman
Great Lakes Medical Clinic Research, Westfield, NY: D.F. Brautigam, R. Fischer, J.M. Chiarot, D.M. Scharf*, B. Nunn*, J. Carlson, C. Flanders*, M.R. Hagen, S. Newman, T.A. Gordon
Naomi Berrie Diabetes Center, New York, NY: R. Goland, C.H. Tuck†, P. Kringas, J. Hey-Hadavi*, J. Montes*, J. Vargas-Jerez, J. Salas-Spiegel
Ambulatory Care Network at Columbia University, New York, NY: A. Getaneh, J. Ramirez*, E.F. Vasquez*, G. Kranwinkel*, S.A. Durant
Irving Diabetes Research Unit, New York, NY: D.S. Donovan, G. Febres*, C. Hernandez*, M.A. Jonaitis, L. Mesa*
State University of New York Downstate Medical Center, Brooklyn, NY: M.A. Banerji, M. Norton, P. Patel*, V. Daly, S. Hirsch, C. Jazmin, R. Khillan, D. Mendonca, A. Relingado, E. Sandoval, M. Tiewala
Kings County, Brooklyn, NY: M.A. Banerji, M. Norton, P. Patel*, V. Daly, S. Hirsch, C. Jazmin, R. Khillan, D. Mendonca, A. Relingado, E. Sandoval, M. Tiewala
The Cooper Health System, Cherry Hill, NJ: A. Bastien*, S. Grudzinski*, P. Niblack, L. Abreu*, T. Brobyn, K. Brown*, M. Casale*, D. Dougherty*, G. Haddad, K. Heintz, M. Kelly*, D. Linneman*, C. Olivia, M.A. Salvador*, P. Zee*, D. Hyman
Southeastern CCN: Wake Forest University School of Medicine, Department of Public Health Sciences, Winston-Salem, NC: D.C. Goff, Jr, J.H. Summerson, L. Crago, C.S. Blackwell*, A. Bertoni*, R.L. Blaine*, J.K. Kirk, R.L. Spach, J. Williamson, J. Calles, J. Katula, D.B. Wishnietsky*.
Southeastern clinical sites
Duke University Medical Center, Durham, NC: M.N. Feinglos, J. Jones, M.B. Mason, M.A. Furst, W.J. Bean*, G. Gedon-Lipscomb, J.B. Green, T. Parham*, B.M. Satterwhite*, C.R. Thacker
Constant Care, Inc., Valdosta, GA: D. Padhiar, R. Noel*, N. Padhiar, S. West*, J. Braden, A Francis*
Wake Forest University School of Medicine, Department of Geriatrics/Gerontology, Winston-Salem, NC: H.H. Atkinson, M. Dibari*, J. Allen, J. Stanfield, T. Delvalle-Fagan, L.J. Gordineer, L. Gordon, M. Gordon*, S.L. Smith*, H. Yates*
Downtown Health Plaza, Winston-Salem, NC: C.F. Pedley, G. Zurek, M. Baird, B. Dunn*, W. Kinder*, S. Mauney
University of North Carolina, Diabetes Care Center, Chapel Hill, NC: J.B. Buse, M.D. Duclos, R.E. Kirby*, J.F. Largay, N.M. McDermott*, A. Goley, S.S. Braithwaite, J.M. Dostou, E.A. Fasy*, D.C. Kelly*, C.E. Metz*, J. Jeffries, D. Rubin*, K. Vukojicik
Holston Medical Group, Kingsport, TN: J.L. Miller, W. Besterman, S.M. Norton*, J. Weatherly*, S. Bishop*, B. Cross, K. Nuss*, M. Surgenor*, D. Alley*, A. Farmer*, J. Foard, J. White, Y. Wood*, B. Gross
Carolinas Medical Center Family Practice, Charlotte, NC: M. Dulin, J. Konen*, T. Barringer*, K. Andrews*, C. Hoffman*, C. Morris*, S. Norton, P. Tochiki*, G. Reinblatt*, P. Bruner*
Robeson Health Care Corporation, Fairmont Clinic, Fairmont, NC: R. Peace, D.O. Stuart*, J. Strickland, L. Cummings*, D. Craig*, J. Stanfield*, J. Morgan*
Robeson Health Care Corporation, Julian T. Pierce Clinic, Pembroke, NC: R. Peace, D.O. Stuart*, J. Strickland, L. Cummings*, D. Craig*, J. Stanfield*, G. Williams*
Wake Forest School of Medicine, Departments of Internal Medicine and Endocrinology, Winston-Salem, NC: J.R. Crouse, L. Menon, S. Marion*, D. Davis*, B. Cabrera*, J. Calles, T. Chandler, J. Ellis, E. Kouba, P. Riddle, E. Myers*
Tulane University Health Science Center, New Orleans, LA: V. Fonseca, J. John-Kalarickal*, R.H. McDuffie, N.O. Asafu-Adjaye, S.M. Leger, P. Reilly, G. Afner, F. Arrey*, S. Asnani, E. Borshard*, D. Boyd*, A.Cemo, S. Chennur*, P. Dupart, R. Garg*, G.P. Girindra*, B. Gouda*, W. Itoua-N’Ganongo*, I. Innocent-Ituah*, C. Johnson*, N. Kuhadiya, M. Kukreja*, I. Mangan-Mbondi*, S. Mason*, C. McLain, J. Naylyanya*, K. Nazereth*, S. Nazereth*, S. Singh, T. Thethi, K. Varnado*, R. Williams*
Kaiser Permanente, Clinic Atlanta Crescent Medical Center, Tucker, GA: J.I. Barzilay, M. Eley*, K. Bader, D. Curry-Ball, S. Goodman*, M. Stevens
VA CCN: Memphis VAMC, Memphis, TN: William C. Cushman, Therese S. Geraci, Sandra M. Walsh, Linda G. Coley, Marshall B. Elam, Diane I. Pickering*, C.Huff
VA clinical sites
Memphis VAMC, Hypertension/Lipid Research Clinic, Memphis, TN: M.B. Elam, C.W. Thompson*, L. Lichtermann, S. Peeples, J. Turner-Bates*, M. Heimberg, D. Childress, J. Turner, J. Jasper*, J. Coley
Baltimore VAMC, Baltimore, MD: B.P. Hamilton, J. Hamilton, G. Kuzbida*, W. Hatten, Jr, A. Lancaster, J. Haywood, J.Luck, D. Bannerman-Wood*
Carl T. Hayden VAMC, Phoenix, AZ: J. Felicetta, M. Bourne-Collo*, M.E. Svoboda, D. Clothier*, M. Deitz†, C. Flaugher*, P. Hayward*, T. Scheibe*, S. Velarde, S. Heritage*, J.P. Nelson, D. Heritage*, C.Martinez*
Atlanta VAMC Medical Service, Decatur, GA: M.E. Sweeney, D. Harrelson*, S. McConnell, F. Watson, R. Johnson, L. Whittington, M. Nanes, M. Salles, C Rice*, C. Cowden*
Ralph H. Johnson VAMC, Primary Care, Charleston, SC: J. Basile, D.B. Ham, B. North-Lee*, H.A. Baig, S.U. Rehman, J. Mixson, D. Nelson*
G. V. (Sonny) Montgomery VAMC, Research Department, Jackson, MS: K.A. Kirchner, L.A. Hinton, L. Mack*, C. Adair*, B. James*, A. Spencer, A. Jones
VA NY Harbor Healthcare System, New York, NY: L. Katz, E.A. Richardson, A.G. Goldberg*, A. Nieves*, J.E. Russo*, J. Adams*, S.A. Sochalski*, M. Coles*, S. Anderson*, M. Williams* D. Hoffman†
Washington VAMC, Washington, DC: V. Papademetriou, B. Gregory, R. Alignay*, E. Nylen, B. Rajendran, R. Hodges, S. Amodeo, A. Ross*, A. Notargiacomo*, M. Metcalfe*, P. Narayan*, D. Wojackovski*
St. Louis VAMC, St. Louis, MO: S. Giddings, E. Clark, A. Pittler, R. Davis*, P. Harris, K. Waidmann, L. Conwill, S. Felton, A. Chen
Central Arkansas Clinic Healthcare System, Little Rock, AR: D.L. Simmons, J.J. Cooper*, K. Dishongh, R. Bates*, K. Bhaghayath*, P. Choksi*, S. Conley*, S. Elbein*, F. Faas, Z. Hamid*, J. Johnson, P. Johnson*, A. Mayo*, M.S. Moriarty*, G. Nair*, D. Rani*, N. Rasouli*, S. Said*, N. Rassouli*, M. Rodriguez*, K. Thomas*, K. Watson*, D. Williams*, A. Makdissi
Coordinating Center: Wake Forest University School of Medicine, Winston-Salem, NC: R.P. Byington, W.T. Ambrosius, R.T. Anderson*, J. Barnes*, J. Beal, C. Bell*, D.E. Bonds*, S. Burton*, C. Collins, D. Cook*, B. Craven*, T. Craven, D. Dunbar*, G. W. Evans, P. Feeney*, C. D. Furberg, C. M. Greven, J. Griffin, L. Harvin, J. Hepler, L. Howard*, L.T. Howard-Perdue, M. Hough, W. Hwang*, A. Kimel, D. Lefkowitz, A. Lopina*, J. Lovato, L.C. Lovato, M.E. Miller, D. Reboussin*, S. Rushing, L. Sanders*, L. Sims, C. Stowe*, M. Walkup*, S. Wilmoth, K. Wilson*, N. Woolard
Drug Distribution Center: VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, NM: D. Raisch, R. Ringer, M. Sather, B. DelCurto, D. Garnand
ECG Reading Center: Wake Forest University School of Medicine, Winston-Salem, NC: R. Prineas*, C. Campbell, Z. Zhang, L. Kesler, S. Hall*, S. Hensley, Y. Li, E.Z. Soliman, D. Milne
Central Chemistry Laboratory: Northwest Lipid Research Laboratories, Seattle, WA: S. Marcovina, J. Chmielewski, K. Gadbois, V. Gaur, G. Strylewicz, M. Ramirez, S. Waddell, M. Mehan*
ACCORD-MIND MRI Reading Center: University of Pennsylvania, Philadelphia, PA: R.N. Bryan, C. Davatzkios, G. Moonis, L. Desiderio, S. D’Arcy*
Fundus Photograph Reading Center: University of Wisconsin Medical School, Madison, WI: M. Davis, R. Danis, S. Gangaputra, L. Hubbard, N. Robinson, J. Dingledine, D. Thayer, H. Wabers, M. Neider, B. Esser, T. Harding, R. Susman, C. Hurtenbach, V. Gama, M. Schiffman, S. Johnson*, T. Graham*
Project Office
National Heart, Lung, and Blood Institute (NHLBI), Bethesda, MD: D.G. Simons-Morton, L. Cooper*, M. Domanski, C. Nwachuku*, Y. Rosenberg, M. Salive*, P. Savage, J.L. Fleg, J.A. Cutler, N. Geller, D. Follmann*, M. Proschan*, C. Jennings, E. Schaeffer*, P. Mills, * J. Bittner*, R. Kirby, P. Frommer†, L. Fine, J. Chan*
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Bethesda, MD: J. Fradkin, S. Malozowski, C. Meyers, T. Hostetter*
National Institute on Aging (NIA), Bethesda, MD: L. Launer
National Eye Institute (NEI), Bethesda, MD: E.Y. Chew
Centers for Disease Control and Prevention (CDC), Atlanta, GA: A. Albright, K.M.V. Narayan, M. Engelgau*, P. Zhang
ACKNOWLEDGMENTS
Members of the ACCORD DSMB included: Antonio M. Gotto. Jr. (chair), Kent Bailey, Dorothy Gohdes, Steven Haffner, Roland Hiss, Kenneth Jamerson, Kerry Lee, David Nathan, James Sowers, LeRoy Walters. The following companies provided study medications, equipment, or supplies: Abbott Laboratories (Abbott Park, IL); Amylin Pharmaceutical (San Diego, CA); AstraZeneca Pharmaceuticals LP (Wilmington, DE); Bayer HealthCare LLC (Tarrytown, NY); Closer Healthcare Inc. (Tequesta, FL); GlaxoSmithKline Pharmaceuticals (Philadelphia, PA); King Pharmaceuticals, Inc. (Bristol, TN); Merck & Co., Inc. (Whitehouse Station, NJ); Novartis Pharmaceuticals, Inc. (East Hanover, NJ); Novo Nordisk, Inc. (Princeton, NJ); Omron Healthcare, Inc. (Schaumburg, IL); Sanofi-Aventis U.S. (Bridgewater, NJ); Schering-Plough Corporation (Kenilworth, NJ); Takeda Pharmaceuticals (Deerfield, IL).
Principal Investigator
Program Coordinator
Co-Investigator
No longer affiliated with study unit
Deceased
REFERENCES
- 1. Li C, Balluz LS, Ford ES, Okoro CA, Zhao G, Pierannunzi C. A comparison of prevalence estimates for selected health indicators and chronic disease conditions from the Behavioral Risk Factor Surveillance System, the National Health Interview Survey, and the National Health and Nutrition Examination Survey, 2007–2008. Prev Med 2012; 54:281–387. [DOI] [PubMed] [Google Scholar]
- 2. Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation 2012; 125:2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 1997; 157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 4. The Look AHEAD Research Group. The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006; 14:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Look AHEAD Research Group. The development and description of the comparison group in the Look AHEAD trial. Clin Trials 2011; 8:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cushman WC, Grimm RH, Cutler JA, Evans GW, Capes S, Corson MA, Sadler LS, Alderman MH, Peterson K, Bertoni A, Basile JN. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:44i–55i. [DOI] [PubMed] [Google Scholar]
- 7. Buse JB. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: design and methods. Am J Cardiol 2007; 99:S21–S33. [DOI] [PubMed] [Google Scholar]
- 8. The Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003; 24:610–628. [DOI] [PubMed] [Google Scholar]
- 9. The Look AHEAD Research Group. Baseline characteristics of the randomized cohort from the Look AHEAD (Action for Health in Diabetes) Research Study. Diab Vasc Dis Res 2006; 3:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med 2013; 369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Eng J Med 2010; 362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mount DL, Feeney P, Fabricatore AN, Coday M, Bahnson J, Byington R, Phelan S, Wilmoth S, Knowler WC, Hramiak I, Osei K, Sweeney ME, Espeland MA. Constructing common cohorts from trials with overlapping eligibility criteria from comparing effect sizes between trials: results from the Look AHEAD and ACCORD trials. Clin Trials 2009; 6:416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goff DC, Gerstein HC, Ginsberg HN, Cushman WC, Margolis KL, Byington RP, Buse JB, Genuth S, Probstfield JL, Simons-Morton DG. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rations for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:S4–S20. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2012; 35:S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espeland MA, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, Curtis JM, Egan C, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Hazuda HP, Hill JO, Hire D, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Killean T, Kitabchi AE, Knowler WC, Kriska A, Lewis CE, Miller M, Montez MG, Murillo A, Nathan DM, Nyenwe E, Patricio J, Peters AL, Pi-Sunyer X, Pownall H, Redmon JB, Rushing J, Ryan DH, Safford M, Tsai AG, Wadden TA, Wing RR, Yanovski SZ, Zhang P. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the Action for Health in Diabetes. Diabetes Care 2014;37:2548–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D. Long-term weight loss from lifestyle intervention benefits blood pressure? Hypertension 2009; 54:756–762. [DOI] [PubMed] [Google Scholar]
- 17. MacLaughlin HL, Sarafidis PA, Greenwood SA, Campbell KL, Hall WL, Macdougall IC. Compliance with a structured weight loss program is associated with reduced systolic blood pressure in obese patients with chronic kidney disease. Am J Hypertens 2012; 25:1024–1029. [DOI] [PubMed] [Google Scholar]
- 18. Fonseca V, McDuffie R, Calles J, Cohen RM, Feeney P, Feinglos M, Gerstein HC, Ismail-Beigi F, Morgan TM, Pop-Busui R, Riddle MC. Determinants of weight gain in the action to control cardiovascular risk in diabetes trial. Diabetes Care 2013; 36:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wermeling PR, Gorter KJ, Stellato RK, de Wit GA, Beulins WJ, Rutten GEHM. Effectiveness and cost-effectiveness of 3-monthly versus 6-monthly monitoring of well-controlled type 2 diabetes patients: a pragmatic randomized controlled patient-preference equivalence trial in primary care (EFFIMODI study). Diabetes Obes Metab 2014; PMID 24635880. [DOI] [PubMed] [Google Scholar]
- 20. Rückert IM, Schunk M, Holle R, Schipf S, Völzke H, Kluttig A, Greiser KH, Berger K, Müller G, Ellert U, Neuhauser H, Rathmann W, Tamayo T, Moebus S, Andrich S, Meisinger C. Blood pressure and lipid management fall far short in persons with type 2 diabetes: results from the DIAB-CORE Consortium including six German population-based studies. Cardiovasc Diabetol 2012; 11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Godley PJ, Maue SK, Farrelly EW, Frech F. The need for improved medical management of patients with concomitant hypertension and type 2 diabetes mellitus. Am J Manag Care 2005; 11:206–210. [PubMed] [Google Scholar]


