Abstract
Purpose
To compare the impact of sustained supplementation using different macular carotenoid formulations on macular pigment (MP) and visual function in early age-related macular degeneration (AMD).
Patients and methods
Sixty-seven subjects with early AMD were randomly assigned to: Group 1 (20 mg per day lutein (L), 0.86 mg per day zeaxanthin (Z); Ultra Lutein), Group 2 (10 mg per day meso-zeaxanthin (MZ), 10 mg per day L, 2 mg per day Z; Macushield; Macuhealth), Group 3 (17 mg per day MZ, 3 mg per day L, 2 mg per day Z). MP was measured using customised heterochromatic flicker photometry and visual function was assessed by measuring contrast sensitivity (CS) and best-corrected visual acuity (BCVA). AMD was graded using the Wisconsin Age-Related Maculopathy Grading System (AREDS 11-step severity scale).
Results
At 3 years, a significant increase in MP from baseline was observed in all groups at each eccentricity (P<0.05), except at 1.75° in Group 1 (P=0.160). Between 24 and 36 months, significant increases in MP at each eccentricity were seen in Group 3 (P<0.05 for all), and at 0.50° in Group 2 (P<0.05), whereas no significant increases were seen in Group 1 (P>0.05 for all). At 36 months, compared with baseline, the following significant improvements (P<0.05) in CS were observed: Group 2—1.2, 6, and 9.6 cycles per degree (c.p.d.); Group 1—15.15 c.p.d.; and Group 3—6, 9.6, and 15.15 c.p.d. No significant changes in BCVA, or progression to advanced AMD, were observed.
Conclusion
In early AMD, MP can be augmented with a variety of supplements, although the inclusion of MZ may confer benefits in terms of panprofile augmentation and in terms of CS enhancement.
Introduction
Age-related macular degeneration (AMD) is characterised by a spectrum of degenerative changes at the macula, which include drusen and/or hyper-/hypopigmentary changes (known as early AMD), atrophic changes (geographic atrophy, GA, a form of advanced AMD), and choroidal neovascularisation (neovascular or ‘wet AMD', another form of advanced AMD).1
Macular pigment (MP) is a yellow pigment located in the macular region of the human retina, and is composed of lutein (L), zeaxanthin (Z), and meso-zeaxanthin (MZ).2 MP filters short-wavelength blue light (and therefore limits photooxidative damage passively) and its constituent carotenoids act as antioxidants by neutralizing free radicals.3, 4
In the current study, known as the Meso-zeaxanthin Ocular Supplementation Trial (MOST) AMD study, we compared the effect of sustained supplementation with some or all of MP's constituent carotenoids on visual function, and evaluated the impact of such supplementation on vision and disease progression. Observations that MZ, the dominant carotenoid in the epicentre of the MP's spatial profile, may offer advantages in terms of MP augmentation across its spatial profile5 and in terms of enhancement of visual function6 prompted this investigation. The 8-week7 and 12-month8 reports of the MOST AMD study have been published. In the current study, we present new data on a 3-year follow-up of subjects in the MOST AMD study. Of note, this is the first study to monitor MP, visual function, and AMD status in response to supplementation with all three macular carotenoids in patients with early AMD, over a 36-month period.
Materials and methods
The design and methodology of the MOST AMD study has been reported previously.8 In brief, MOST AMD is a single-blind, randomised controlled clinical trial. Clinical assessments were carried out at the Institute of Eye Surgery (http://www.ioes.ie/), Waterford, Ireland. Before study enrolment, an eligibility screening visit was conducted by an ophthalmologist with a special interest in retinal disease (SB). The eligibility criteria included early AMD (one to eight on AREDS 11-step severity scale9 in at least one eye (the study eye), confirmed by the Ocular Epidemilogy Reading Center at the University of Wisconsin, Madison, WI, USA); best-corrected visual acuity (BCVA) ≥6/12 in the study eye; and no other ocular pathology.
Subjects were randomly assigned to one of three parallel groups: Group 1—20 mg L, 0.86 mg Z (Ultra Lutein supplied by Natural Organics, Inc., Melville, NY, USA); Group 2—10 mg MZ, 10 mg L, 2 mg Z (Macushield (Macuvision Europe Limited, Solihull, UK)/Macuhealth LMZ3 (MacuHealth LLC, Birmingham, MI, USA)); Group 3—17 mg MZ, 3 mg L, 2 mg Z (supplied by Industrial Organica, Monterrey, Mexico (not commercially available)). The above treatment groups (formulations) were selected to be comparable total concentrations of macular carotenoids (ie 22 mg). Of note, however, discrepancies between label claim and measured values of the supplements used in this trial have been reported previously, and in particular, the finding that the Group 1 supplement contained small amounts of MZ (0.30 mg).10, 11 This has implications for the findings presented below.
The supplements were prepared in a soft gel capsule. Subjects were instructed to take one capsule daily with a meal. All study supplements were indistinguishable in terms of external appearance, and were packaged in identical containers. Study visits were conducted at baseline, 12 months, 24 months, and 36 months.
Ethics
Ethics approval was granted by the Waterford Regional Hospital Ethics Committee. Written and informed consent was obtained from each subject before study enrolment. The tenets of the Declaration of Helsinki were adhered to in all study procedures.
Outcome measures
The primary outcome measure was change in MP as measured by customized heterochromatic flicker photometry (cHFP) at 36 months. Secondary outcome measures included BCVA, letter contrast sensitivity (CS), serum concentrations of macular carotenoids, and grade of AMD.
Study procedures
MP measurement
MP was measured using the Macular Densitometer (Macular Metrics, Corp., Providence, RI, USA) at 0.25°, 0.5°, 1.0°, and 1.75° retinal eccentricity, with a reference point at 7°.12
Serum L, Z, and MZ analysis
Serum L, Z, and MZ were quantified by high-performance liquid chromatography using methodology described previously.7, 13
Visual acuity
BCVA was measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) logarithm of the minimum angle of resolution (LogMAR) chart (Test Chart 2000 PRO; Thomson Software Solutions, Hatfield, Hertfordshire, UK) viewed at 4 m.
Letter CS
Letter CS was assessed using the LogMAR ETDRS (Test Chart 2000 PRO; Thomson Software Solutions) chart at five different spatial frequencies (1.2, 2.4, 6.0, 9.6, and 15.15 c.p.d., respectively) viewed at 4 m.
Retinal photography and AMD grading
Following prior pupillary dilation (0.5% proxymetacaine hydrochloride, 2.5% phenylephrine hydrochloride, and 1% tropicamide), 45° stereoscopic color fundus photographs were taken in three retinal photographic fields (optic disc, macula, temporal to macula) using a Zeiss Visucam 200 (Carl Zeiss Meditec AG, Jena, Germany). Photographs were transferred to the Ocular Epidemiology Reading Center at the University of Wisconsin via an encrypted system. Photographs were graded in a masked manner using a modified version of the Wisconsin Age-Related Maculopathy Grading System14, 15 and adhered to the AREDS 11-step severity scale.9
Statistical analysis
One eye (the study eye) of each subject comprised the unit of analysis. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 21.0 (IBM, Armonk, NY, USA). To compare the effects of the three supplements (on each outcome measure, over time), we used repeated-measures analysis of variance, and contingency table analysis, as appropriate. Cognisant that this exploratory study would likely have insufficient power for such analyses, however, we did some additional analyses. In fact, and beyond the previously reported 12-month data,8 we decided upon two strands of analysis: (a) between supplement group analysis over time: despite the small sample sizes, supplement groups were compared with each other, for changes in each outcome variable over the 3 years of the study. For interval outcome variables (MP, serum carotenoids, BCVA, CS), the method of analysis was repeated-measures analysis of variance, with time as a within-subjects factor and supplement as a between-subjects factor; we used the Greenhouse–Geisser correction for lack of sphericity. Post hoc analysis, with Bonferroni adjustment for multiple testing, was used where appropriate. For categorical outcome variables (AMD grade), we used contingency table analysis to compare supplements; (b) within-supplement group changes in each outcome variable, over the 3 years of the study. We used paired t-tests analysis here.
Tests of significance, for all t-test analyses, were two-tailed, and the 5% level of significance was used throughout. With the exception of post hoc analyses for the repeated-measures analysis of variance, we did not correct for multiple tests.
Results
Sixty-seven subjects were enrolled at baseline, with 47 subjects completing the final study visit at 36 months. Only those subjects who completed each study visit were included in analysis. Therefore, if a subject attended his/her 12- or 24-month visit, but did not complete the 36-month visit, he/she was not included in the analysis. Where a subject did complete a study visit, but where a variable was not measured or recorded, that subject was also excluded from all analyses relating to that variable. Exclusions occurred only in the MP and CS analysis because data were not available at all study visits (MP analysis: 5 subjects; CS analysis: 6 subjects). We have also included the sample size in all tables for clarity.
Baseline characteristics (eg age, gender, smoking status, education) of participants in intervention groups have been described previously, and the intervention groups were statistically comparable in terms of these variables.8
MP and its constituent carotenoids in serum
Macular pigment
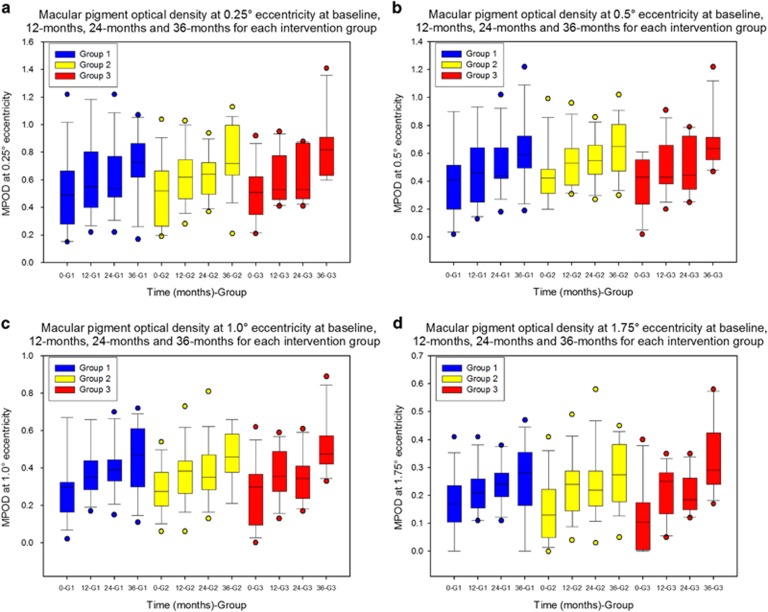
(a) Comparing supplement groups In the repeated-measures analysis of change in MP (at 0.25°, 0.5°, 1.0°, and 1.75°), the within-subjects Time × Supplement interaction effect was not significant (P=0.759, 0.726, 0.703, 0.110, respectively, using the Greenhouse–Geisser adjustment for lack of sphericity). Thus, the effect (on MP levels) over time, at any eccentricity, does not differ significantly between supplement groups. The boxplots in Figure 1 graphically illustrate these findings.
Figure 1.
Macular pigment response at different retinal eccentricities over the course of the MOST AMD study. Boxplots representing macular pigment optical density at four time points (baseline, 12 months, 24 months, and 36 months) for each intervention group: Group 1—20 mg L and 0.86 mg Z; Group 2—10 mg MZ, 10 mg L, and 2 mg Z; Group 3—17 mg MZ, 3 mg L, and 2 mg Z Macular pigment was measured at 0.25° (a), 0.5° (b), 1.0° (c), and 1.75° (d) eccentricity using cHFP. 0-G1, Baseline Group 1; 12-G1, 12 months Group 1; 24-G1, 24 months Group 1; 36-G1, 36 months Group 1; 0-G2, Baseline Group 2; 12-G2, 12 months Group 2; 24-G2, 24 months Group 2; 36-G2, 36 months Group 2; 0-G3, Baseline Group 3; 12-G3, 12 months Group 3; 24-G3, 24 months Group 3; 36-G3, 36 months Group 3. MPOD, macular pigment optical density.
(b) Within-supplement group analyses of MP are given in Table 1.
Table 1. Within-supplement group analysis of macular pigment by intervention groups.
| Intervention | N | Baseline, mean±SD | 36 Months, mean±SD | %Δ | Sig. | Baseline, mean±SD | 12 Months, mean±SD | %Δ | Sig. | 12 Months, mean±SD | 24 Months, mean±SD | %Δ | Sig. | 24 Months, mean±SD | 36 Months, mean±SD | % Δ | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MP at 0.25 | |||||||||||||||||
| Group 1 | 13 | 0.51±0.29 | 0.72±0.24 | 41 | 0.004 | 0.51±0.29 | 0.61±0.30 | 20 | 0.039 | 0.61±0.30 | 0.61±0.25 | 0 | 0.896 | 0.61±0.25 | 0.72±0.24 | 18 | 0.134 |
| Group 2 | 16 | 0.50±0.24 | 0.76±0.23 | 52 | 0.001 | 0.50±0.24 | 0.63±0.21 | 26 | 0.001 | 0.63±0.21 | 0.64±0.17 | 2 | 0.802 | 0.64±0.17 | 0.76±0.23 | 19 | 0.095 |
| Group 3 | 12 | 0.51±0.20 | 0.85±0.25 | 67 | 0.000 | 0.51±0.20 | 0.62±0.19 | 22 | 0.021 | 0.62±0.19 | 0.62±0.19 | 0 | 0.924 | 0.62±0.19 | 0.85±0.25 | 37 | 0.003 |
| MP at 0.5 | |||||||||||||||||
| Group 1 | 13 | 0.41±0.28 | 0.62±0.26 | 51 | 0.000 | 0.41±0.28 | 0.47±0.27 | 15 | 0.194 | 0.47±0.26 | 0.53±0.21 | 13 | 0.092 | 0.53±0.21 | 0.62±0.26 | 16 | 0.087 |
| Group 2 | 16 | 0.45±0.21 | 0.64±0.20 | 42 | 0.000 | 0.45±0.21 | 0.54±0.18 | 20 | 0.011 | 0.54±0.18 | 0.55±0.16 | 2 | 0.343 | 0.55±0.16 | 0.64±0.20 | 16 | 0.034 |
| Group 3 | 12 | 0.39±0.19 | 0.68±0.20 | 74 | 0.000 | 0.39±0.19 | 0.50±0.20 | 22 | 0.016 | 0.50±0.20 | 0.50±0.20 | 0 | 0.879 | 0.50±0.20 | 0.68±0.20 | 36 | 0.011 |
| MP at 1.0 | |||||||||||||||||
| Group 1 | 13 | 0.30±0.19 | 0.45±0.19 | 50 | 0.006 | 0.30±0.19 | 0.38±0.15 | 27 | 0.053 | 0.38±0.15 | 0.40±0.14 | 5 | 0.339 | 0.40±0.14 | 0.45±0.18 | 13 | 0.298 |
| Group 2 | 16 | 0.29±0.13 | 0.46±0.15 | 59 | 0.000 | 0.29±0.13 | 0.37±0.16 | 28 | 0.010 | 0.37±0.16 | 0.38±0.16 | 3 | 0.730 | 0.38±0.16 | 0.46±0.15 | 21 | 0.071 |
| Group 3 | 12 | 0.26±0.17 | 0.52±0.16 | 100 | 0.000 | 0.26±0.17 | 0.37±0.14 | 42 | 0.010 | 0.37±0.14 | 0.35±0.13 | −6 | 0.473 | 0.35±0.13 | 0.52±0.16 | 49 | 0.011 |
| MP at 1.75 | |||||||||||||||||
| Group 1 | 13 | 0.17±0.11 | 0.23±0.19 | 35 | 0.160 | 0.17±0.11 | 0.22±0.09 | 29 | 0.055 | 0.22±0.09 | 0.24±0.08 | 9 | 0.256 | 0.24±0.08 | 0.23±0.19 | −4 | 0.870 |
| Group 2 | 16 | 0.15±0.12 | 0.28±0.11 | 87 | 0.000 | 0.15±0.12 | 0.24±0.11 | 60 | 0.007 | 0.24±0.11 | 0.24±0.13 | 0 | 0.793 | 0.24±0.13 | 0.28±0.11 | 17 | 0.383 |
| Group 3 | 12 | 0.12±0.13 | 0.34±0.14 | 183 | 0.000 | 0.12±0.13 | 0.21±0.09 | 75 | 0.006 | 0.21±0.09 | 0.21±0.07 | 0 | 0.899 | 0.21±0.07 | 0.34±0.14 | 62 | 0.003 |
Abbreviations: MP, macular pigment; N, number of subjects; SD, standard deviation; Sig., significance; %Δ, percentage change.
Macular pigment was measured at 0.25°, 0.5°, 1.0°, and 1.75° eccentricity using customized heterochromatic flicker photometry. Statistical significance was tested using paired t-test. Level of significance set at P <0.05. The calculated percentage change from baseline to 36 months, calculated as the 36-month value minus baseline value divided by baseline value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from baseline to 12 months, calculated as the 12-month value minus baseline value divided by baseline value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from 12 to 24 months, calculated as the 24-month value minus the 12-month value divided by the 12-month value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from 24 to 36 months, calculated as the 36-month value minus the 24-month value divided by the 24-month value, multiplied by 100 (−, negative change; +, positive change).
Group 1, 20 mg lutein and 0.86 mg zeaxanthin; Group 2, 10 mg meso-zeaxanthin, 10 mg lutein, and 2 mg zeaxanthin; Group 3, 17 mg meso-zeaxanthin, 3 mg lutein, and 2 mg zeaxanthin.
Serum concentrations of lutein
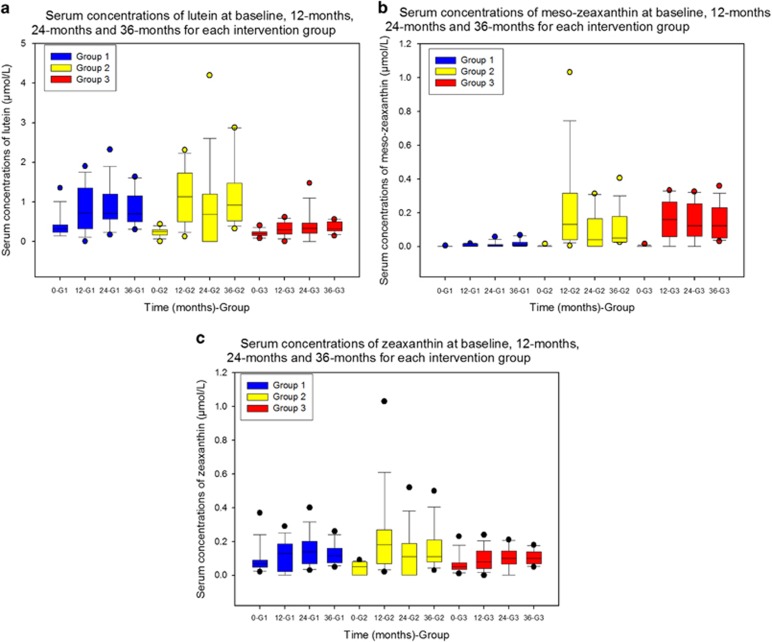
(a) Comparing supplement groups In the repeated-measures analysis of change in serum L, the within-subjects Time × Supplement interaction effect was significant (P=0.029, using the Greenhouse–Geisser adjustment for lack of sphericity). Thus, the effect (on serum L levels) over time differs significantly between the supplements used. Post hoc analysis indicates that increases in serum L over time in groups 1 and 2 are comparable (P=1, after Bonferroni adjustment for multiple testing), and each of these groups exhibit significantly greater increases than group 3 (P=0.029 and P=0.004, respectively, after Bonferroni adjustment for multiple testing). The boxplots in Figure 2a graphically illustrate these findings.
Figure 2.
Serum response of L, MZ, and Z over the course of the MOST AMD study. Boxplots representing serum concentrations of L (a), MZ (b), and zeaxanthin (c) at four time points (baseline, 12 months, 24 months, and 36 months) for each intervention group: Group 1—20 mg L and 0.86 mg Z; Group 2—10 mg MZ, 10 mg L, and 2 mg Z; Group 3—17 mg MZ, 3 mg L, and 2 mg Z. Serum macular carotenoids were analysed by HPLC and expressed as μmol/L; 0-G1, Baseline Group 1; 12-G1, 12 months Group 1; 24-G1, 24 months Group 1; 36-G1, 36 months Group 1; 0-G2, Baseline Group 2; 12-G2, 12 months Group 2; 24-G2, 24 months Group 2; 36-G2, 36 months Group 2; 0-G3, Baseline Group 3; 12-G3, 12 months Group 3; 24-G3, 24 months Group 3; 36-G3, 36 months Group 3.
(b) Within-supplement group analyses of serum L are given in Table 2.
Table 2. Within-supplement group analysis of serum macular carotenoids by intervention groups.
| Intervention | N | Baseline, mean±SD | 36 Months, mean±SD | %Δ | Sig. | Baseline, mean±SD | 12 Months, mean±SD | %Δ | Sig. | 12 Months, mean±SD | 24 Months, mean±SD | %Δ | Sig. | 24 Months, mean±SD | 36 Months, mean±SD | % Δ | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lutein | |||||||||||||||||
| Group 1 | 14 | 0.39±0.31 | 0.81±0.44 | 108 | 0.006 | 0.39±0.31 | 0.81±0.58 | 108 | 0.014 | 0.81±0.58 | 0.90±0.57 | 11 | 0.616 | 0.90±0.57 | 0.81±0.44 | −10 | 0.412 |
| Group 2 | 15 | 0.24±0.11 | 1.14±0.83 | 375 | 0.001 | 0.24±0.11 | 1.11±0.67 | 363 | 0.000 | 1.11±0.67 | 0.85±1.05 | −23 | 0.336 | 0.85±1.05 | 1.14±0.83 | 34 | 0.250 |
| Group 3 | 13 | 0.20±0.08 | 0.36±0.13 | 80 | 0.001 | 0.20±0.08 | 0.31±0.17 | 55 | 0.021 | 0.31±0.17 | 0.39±0.36 | 26 | 0.367 | 0.39±0.36 | 0.36±0.13 | −8 | 0.694 |
| Zeaxanthin | |||||||||||||||||
| Group 1 | 14 | 0.09±0.09 | 0.13±0.06 | 44 | 0.124 | 0.09±0.09 | 0.12±0.09 | 33 | 0.314 | 0.12±0.09 | 0.15±0.09 | 25 | 0.251 | 0.15±0.09 | 0.13±0.06 | −13 | 0.202 |
| Group 2 | 15 | 0.04±0.03 | 0.16±0.12 | 300 | 0.001 | 0.04±0.03 | 0.22±0.24 | 450 | 0.012 | 0.22±0.24 | 0.13±0.14 | −41 | 0.221 | 0.13±0.14 | 0.16±0.12 | 23 | 0.298 |
| Group 3 | 13 | 0.06±0.05 | 0.11±0.04 | 83 | 0.005 | 0.06±0.05 | 0.09±0.06 | 50 | 0.031 | 0.09±0.06 | 0.10±0.07 | 11 | 0.653 | 0.10±0.07 | 0.11±0.04 | 10 | 0.799 |
| Meso-zeaxanthin | |||||||||||||||||
| Group 1 | 14 | 0.00±0.00 | 0.02±0.02 | 0.010 | 0.00±0.00 | 0.01±0.01 | 0.008 | 0.01±0.01 | 0.01±0.02 | 0 | 0.393 | 0.01±0.02 | 0.02±0.02 | 100 | 0.371 | ||
| Group 2 | 15 | 0.00±0.00 | 0.11±0.11 | 0.001 | 0.00±0.00 | 0.22±0.27 | 0.007 | 0.22±0.27 | 0.09±0.11 | −59 | 0.083 | 0.09±0.11 | 0.11±0.11 | 22 | 0.314 | ||
| Group 3 | 13 | 0.00±0.00 | 0.14±0.10 | 0.000 | 0.00±0.00 | 0.16±0.11 | 0.000 | 0.16±0.11 | 0.15±0.11 | −6 | 0.911 | 0.15±0.11 | 0.14±0.10 | −7 | 0.743 | ||
Abbreviations: N, number of subjects; SD, standard deviation; Sig., significance; %Δ, percentage change.
Serum Lutein, Zeaxanthin and Meso-zeaxanthin were analysed by HPLC and expressed as μmol/L. Statistical significance tested using paired t-test. Level of significance set at P<0.05. The calculated percentage change from baseline to 36 months, calculated as the 36-month value minus baseline value divided by baseline value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from baseline to 12 months, calculated as the 12-month value minus baseline value divided by baseline value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from 12 to 24 months, calculated as the 24-month value minus the 12-month value divided by the 12-month value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from 24 to 36 months, calculated as the 36-month value minus the 24-month value divided by the 24-month value, multiplied by 100 (−, negative change; +, positive change).
Group 1, 20 mg lutein and 0.86 mg zeaxanthin; Group 2, 10 mg meso-zeaxanthin, 10 mg lutein, and 2 mg zeaxanthin; Group 3, 17 mg meso-zeaxanthin, 3 mg lutein, and 2 mg zeaxanthin.
Serum concentrations of MZ
(a) Comparing supplement groups In the repeated-measures analysis of change in serum MZ, the within-subjects Time × Supplement interaction effect was significant (P=0.011, using the Greenhouse–Geisser adjustment for lack of sphericity). Thus, the effect over time (on serum levels of MZ) differs significantly between the supplement groups. Post hoc analysis indicates that increases in MZ over time in Groups 2 and 3 are comparable (P=1, after Bonferroni adjustment for multiple testing), and each of these groups exhibits significantly greater increases than Group 1 (P=0.001 for both, after Bonferroni adjustment for multiple testing). The boxplots in Figure 2b graphically illustrate these findings.
(b) Within-supplement group analyses of serum MZ are given in Table 2.
Serum concentrations of zeaxanthin
(a) Comparing supplement groups In the repeated-measures analysis of change in serum Z, the within-subjects Time × Supplement interaction effect was not significant (P=0.081, using the Greenhouse–Geisser adjustment for lack of sphericity). Thus, the effect over time does not differ significantly between the supplements. The boxplots in Figure 2c graphically illustrate these findings.
(b) Within-supplement group analyses of serum Z are given in Table 2.
Changes in visual function
(a) Comparing supplement groups There were no significant Time × Supplement interaction effects for any vision-related outcome measures (BCVA, letter CS at any spatial frequency), indicating that the observed effects over time in terms of these variables (see below) did not differ between intervention groups.
Best-corrected visual acuity
Within-supplement group analysis There were no significant within-supplement changes in BCVA (P>0.05, for all), with the exception of a statistically significant improvement in Group 3 between 12 and 24 months.
Contrast sensitivity
Within-supplement group analysis of CS are given in Table 3. At 36 months, compared with baseline, the following significant improvements (P<0.05) in CS were observed: Group 2—1.2, 6, and 9.6 c.p.d.; Group 1—15.15 c.p.d.; Group 3—6, 9.6, and 15.15 c.p.d.
Table 3. Within-supplement group analysis of letter contrast sensitivity by intervention groups.
| Intervention | N | Baseline, mean±SD | 36 Months, mean±SD | % Δ | Sig. | Baseline, mean±SD | 12 Months, mean±SD | % Δ | Sig. | 12 Months, mean±SD | 24 Months, mean±SD | %Δ | Sig. | 24 Months, mean±SD | 36 Months, mean±SD | % Δ | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Letter CS 1.2cpd | |||||||||||||||||
| Group 1 | 12 | 1.87±0.25 | 1.89±0.16 | 1 | 0.817 | 1.87±0.25 | 1.96±0.23 | 5 | 0.222 | 1.96±0.23 | 1.76±0.22 | −10 | 0.000 | 1.76±0.22 | 1.89±0.16 | 7 | 0.059 |
| Group 2 | 15 | 1.71±0.24 | 1.86±0.18 | 9 | 0.012 | 1.71±0.24 | 1.93±0.29 | 13 | 0.004 | 1.93±0.29 | 1.85±0.25 | −4 | 0.207 | 1.85±0.25 | 1.86±0.18 | 1 | 0.861 |
| Group 3 | 13 | 1.75±0.31 | 1.82±0.20 | 4 | 0.432 | 1.75±0.31 | 1.89±0.27 | 8 | 0.069 | 1.89±0.27 | 1.86±0.24 | −2 | 0.602 | 1.86±0.24 | 1.82±0.20 | −2 | 0.494 |
| Letter CS 2.4cpd | |||||||||||||||||
| Group 1 | 12 | 1.76±0.30 | 1.87±0.17 | 6 | 0.227 | 1.76±0.30 | 1.89±0.33 | 7 | 0.077 | 1.89±0.33 | 1.70±0.25 | −10 | 0.011 | 1.70±0.25 | 1.87±0.17 | 10 | 0.065 |
| Group 2 | 15 | 1.68±0.31 | 1.81±0.21 | 8 | 0.087 | 1.68±0.31 | 1.86±0.31 | 11 | 0.000 | 1.86±0.31 | 1.78±0.26 | −4 | 0.221 | 1.78±0.26 | 1.81±0.21 | 2 | 0.657 |
| Group 3 | 13 | 1.63±0.31 | 1.78±0.21 | 9 | 0.083 | 1.63±0.31 | 1.85±0.29 | 13 | 0.005 | 1.85±0.29 | 1.77±0.22 | −4 | 0.225 | 1.77±0.22 | 1.78±0.21 | 1 | 0.947 |
| Letter CS 6cpd | |||||||||||||||||
| Group 1 | 12 | 1.42±0.30 | 1.60±0.15 | 13 | 0.112 | 1.42±0.30 | 1.49±0.41 | 5 | 0.224 | 1.49±0.41 | 1.39±0.26 | −7 | 0.200 | 1.39±0.26 | 1.60±0.15 | 15 | 0.037 |
| Group 2 | 15 | 1.37±0.24 | 1.52±0.25 | 11 | 0.040 | 1.37±0.24 | 1.44±0.30 | 5 | 0.079 | 1.44±0.30 | 1.39±0.34 | −3 | 0.357 | 1.39±0.34 | 1.52±0.25 | 9 | 0.164 |
| Group 3 | 13 | 1.23±0.44 | 1.52±0.27 | 24 | 0.034 | 1.23±0.44 | 1.55±0.29 | 26 | 0.005 | 1.55±0.29 | 1.48±0.20 | −5 | 0.357 | 1.48±0.20 | 1.52±0.27 | 3 | 0.547 |
| Letter CS 9.6cpd | |||||||||||||||||
| Group 1 | 12 | 1.14±0.31 | 1.35±0.16 | 18 | 0.043 | 1.14±0.31 | 1.14±0.32 | 0 | 0.959 | 1.14±0.32 | 1.14±0.28 | 0 | 1.000 | 1.14±0.28 | 1.35±0.16 | 18 | 0.031 |
| Group 2 | 15 | 1.06±0.27 | 1.27±0.34 | 20 | 0.024 | 1.06±0.27 | 1.17±0.39 | 10 | 0.072 | 1.17±0.39 | 1.06±0.37 | −9 | 0.115 | 1.06±0.37 | 1.27±0.34 | 20 | 0.025 |
| Group 3 | 13 | 0.94±0.48 | 1.30±0.22 | 38 | 0.020 | 0.94±0.48 | 1.17±0.44 | 24 | 0.031 | 1.17±0.44 | 1.23±0.27 | 5 | 0.503 | 1.23±0.27 | 1.30±0.22 | 6 | 0.201 |
| Letter CS 15.15cpd | |||||||||||||||||
| Group 1 | 12 | 0.75±0.32 | 1.02±0.23 | 36 | 0.033 | 0.75±0.32 | 0.83±0.31 | 11 | 0.055 | 0.83±0.31 | 0.79±0.29 | −5 | 0.509 | 0.79±0.29 | 1.02±0.23 | 29 | 0.011 |
| Group 2 | 15 | 0.70±0.37 | 0.91±0.38 | 30 | 0.083 | 0.70±0.37 | 0.78±0.44 | 11 | 0.278 | 0.78±0.44 | 0.60±0.47 | −23 | 0.013 | 0.60±0.47 | 0.91±0.38 | 52 | 0.029 |
| Group 3 | 13 | 0.61±0.48 | 0.97±0.25 | 59 | 0.019 | 0.61±0.48 | 0.81±0.38 | 33 | 0.028 | 0.81±0.38 | 0.93±0.35 | 15 | 0.169 | 0.93±0.35 | 0.97±0.25 | 4 | 0.555 |
Abbreviations: CS, contrast sensitivity; cpd, cycles per degree; N, number of subjects; SD, standard deviation; Sig., level of significance set at P<0.05; %Δ, percentage change.
Letter contrast sensitivity was assessed using Thompson Test Chart PRO and recorded in the logarithm of contrast sensitivity (Log CS) units. Statistical significance was tested using paired t-test. The calculated percentage change from baseline to 36 months, calculated as the 36-month value minus baseline value divided by baseline value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from baseline to 12 months, calculated as the 12-month value minus baseline value divided by baseline value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from 12 to 24 months, calculated as the 24-month value minus the 12-month value divided by the 12-month value, multiplied by 100 (−, negative change; +, positive change); the calculated percentage change from 24 to 36 months, calculated as the 36-month value minus the 24-month value divided by the 24-month value, multiplied by 100 (−, negative change; +, positive change).
Group 1: 20 mg lutein and 0.86 mg zeaxanthin; Group 2: 10 mg meso-zeaxanthin, 10 mg lutein, and 2 mg zeaxanthin; Group 3: 17 mg meso-zeaxanthin, 3 mg lutein, and 2 mg zeaxanthin.
Changes in grade of AMD
Because of the limited number of subjects in this study, we collapsed adjacent grades of AMD, as follows: AREDS grades 1–3 (representing eyes at low risk of progression to advanced AMD), and AREDS grades 4–8 (representing eyes at high risk of progression to advanced AMD). In terms of this collapsed and simplified classification, intervention groups were statistically similar in terms of baseline findings (P=0.44, χ2 test). Using this simplified and modified system, no study eye in any intervention group progressed from low risk to high risk of progression to advanced AMD over the course of the study period, and no study eye regressed from high risk to low risk of progression to advanced AMD in any intervention group, and finally, no subject progressed to advanced AMD (AREDS grades 9–11) over the study period. Given that findings were identical for all three intervention groups, there was no need for statistical investigation of differences between intervention groups in terms of changes in risk for progression to advanced AMD.
We also investigated clinically meaningful change in AMD grade along the AREDS 11-step scale, defined as a change of at least two steps along this scale. Thus, an increase of two steps between baseline and final visit at 36 months was considered clinically meaningful disease progression and a decrease of two steps was considered a clinically meaningful disease regression. On this basis, there was no clinically meaningful change in AMD grade in 43 (93%) study eyes, whereas 3 (7%) study eyes (one subject in Group 1 and two subjects in Group 3) exhibited a clinically meaningful progression along the AREDS 11-step scale, and these observed changes were not statistically different between intervention groups (P=0.29, Fisher's exact test).
Discussion
The present study reports on the impact of sustained supplementation with different carotenoid formulations on serum concentrations of MP's constituent carotenoids, MP, visual function (BCVA and letter CS), and disease progression in subjects with early AMD.
The strengths of this study include: (1) it is a randomized clinical trial comparing three different formulations containing some or all of MP's constituent carotenoids, with a follow-up of 3 years; (2) MP was measured using a validated technique at regular intervals throughout the study period; (3) assessment of visual function was not restricted to BCVA, and included CS; (4) assessment of AMD morphology was performed by an accredited reading centre in a masked manner.
Serum response to supplementation reflected the carotenoid content of the supplement used. For example, serum L exhibited an increase in all three supplementation groups, but to a greater extent in Groups 1 and 2, where intake of L was at least three times the typical dietary intake of this carotenoid.16, 17 Similarly, a significant rise in serum Z was noted following supplementation, but that was comparable across supplement groups, reflecting similar concentrations of this carotenoid in each of the three formulations tested. Finally, serum MZ response is noteworthy for several reasons. First, MZ was detected in the serum of patients supplemented with a formulation with no declared MZ content. However, we have shown that MZ is indeed present in commercially available formulations containing L, including Ultra Lutein, the Group 1 supplement used in this study.10 Finally, it is also worth noting that serum L and serum Z responses were unaffected by the presence of substantial concentrations of MZ (10 mg or more) in the formulation used, thereby allaying previously expressed concerns that the inclusion of MZ in a supplement may adversely impact upon the circulating bioavailability of the other two macular carotenoids.
MP increased significantly in all groups at each eccentricity (with the exception of Group 1 at 1.75°) at 3 years. It is surprising to see that MP did not increase at 1.75° in Group 1, given that L is the dominant carotenoid at this locus, and this seemingly counterintuitive observation might be because subjects in Group 1 were bioconverting L to MZ at the macula.18, 19 Consistent with this hypothesis, only groups that received supplemental MZ exhibited significant augmentation of MP across the spatial profile of this pigment.
In terms of MP increase over the course of the study, it was observed that MP continues to increase further and significantly in the third year of supplementation (but only in groups supplemented with meaningful concentrations of MZ) following a relative plateau in the second year of supplementation. Indeed, MP did not increase significantly between 12 and 24 months in any intervention group, at any eccentricity. Although the exact mechanism of macular carotenoid uptake has not been fully elucidated, it is plausible that there are several mediators (eg binding proteins, enzymes) that influence the capture, accumulation, and stabilisation of these carotenoids at the macula,20 but further research is needed to understand these mechanisms.
There was no significant change in BCVA over the course of the present study, other than a transient improvement between 12 and 24 months in Group 3. Murray et al21 reported the impact of supplemental L on MP and visual acuity in patients with early AMD in a randomised, double-blind, placebo-controlled, multicentre 12-month trial. At the end of their study, there was no change in BCVA in the L group, whereas BCVA in the placebo group had deteriorated significantly.21 In the present study, there was a nonsignificant increase in BCVA in all intervention groups, consistent with the view that BCVA stabilised over the 3-year period of the study in this cohort of patients with early AMD. The CARMA trial, a randomised controlled trial of L, Z, and coantioxidants vs placebo, reported no significant change in BCVA at 1 year, although there was a demonstrable benefit in terms of differential BCVA between intervention and placebo groups at 3 years.22, 23 Of note, visual acuity, which is a measure of the spatial resolving power of the visual system and remains the most commonly used measure of vision in clinical practice,24 is probably not sensitive enough to detect subtle but important changes in visual function experienced when monitoring subjects with early AMD.25
CS measures the threshold between visible and invisible at a given spatial frequency, and could be loosely described as ‘faintness appreciation'26 and is a better tool than BCVA for assessing visual function in early AMD.25 In Group 2 (a supplement with a formulation containing all three of MP's constituent carotenoids), there was a statistically significant improvement in CS at the lowest spatial frequency (2.4 c.p.d.), whereas this was not observed for Groups 1 and 3. At the highest spatial frequency (15.15 c.p.d.), letter CS improved in Groups 1 and 3 at 36 months, but not in Group 2. At intermediate spatial frequencies (6 and 9.6 c.p.d.), however, only supplementation with formulations containing appreciable amounts of MZ (Groups 2 and 3) resulted in a significant improvement in letter CS. Although some, but not all, previous studies have reported improvements in CS following supplementation with macular carotenoids in subjects with early AMD, our results suggest that those studies that failed to report an improvement in CS may be explained, at least in part, by a lack of MZ in the supplement formulation used.23, 27 Finally, an important and novel finding of the current study rests on the observation that further and significant improvements in CS are experienced beyond 24 months of supplementation with MP's constituent carotenoids, suggesting that sustained supplementation is indeed necessary to exert a beneficial effect on visual function.
With respect to AMD, only three study eyes exhibited clinically meaningful disease progression (1 subject from Group 1 and 2 subjects from Group 3), and no study eye progressed to advanced AMD over the 3-year study period. This study is not adequately powered or designed to make meaningful comment on AMD progression.
The current study compared the impact of supplementation with different carotenoid formulations on visual function, and our findings suggest that a formulation containing MZ yields benefits in terms of MP augmentation and in terms of CS enhancement. Further, sustained supplementation appears necessary, for at least 3 years, if MP is to be augmented maximally and CS is to be optimised over that period of time. Of note, modest visual benefits were observed in the current study. Future clinical trials should examine the impact of supplementation with formulations containing MZ and Z at similar doses. The Central Retinal Enrichment Supplementation Trial (CREST), currently underway, will also add to our understanding of the role of the macular carotenoids, including MZ, on vision in healthy and diseased eyes.28
Limitations of the MOST AMD study include its small numbers and the fact that it is a single blind clinical trial with no placebo arm. With respect to the use of placebo in the current study, we believe that the findings arising from the secondary analysis of the AREDS2 may render the use of placebo in patients with early (including intermediate) AMD ethically questionable.29, 30 Of note, the term early AMD in this study includes patients with intermediate AMD (as defined by AREDS). However, the absence of placebo may render it difficult to demonstrate clinical efficacy of the different carotenoid formulations used in this study and our results should be interpreted with full appreciation of this limitation. We used the single-blind design because the current study was the first clinical trial to compare the impact of supplementation with three different carotenoid formulations (including MZ) on visual function in subjects with early AMD and therefore we wanted to monitor more closely the effects of the three carotenoid formulations in terms of response among these subjects. Statistically, this exploratory study was underpowered for a direct comparison of the three supplements. Differences in effects between supplements were, in general, likely to be small, meaning that impractically large numbers of subjects would have been required to obtain statistically significant results.
In conclusion, we report that the inclusion of MZ in a supplement formulation seems to confer benefits in terms of MP augmentation and in terms of enhanced CS in subjects with early AMD. An important and novel finding rests on the observation that sustained supplementation with the macular carotenoids seems necessary to maximally augment MP and to optimise CS over a 3-year period in patients with early AMD.
Acknowledgments
This study was funded by a grant from the Howard Foundation, Cambridge, UK. KOA and JMN (the principal investigator) are currently funded by the European Research Council (ERC), grant reference number: 281096. We would like to thank Industrial Orgánica and Macuvision Europe for providing the study supplements.
JMN and SB do consultancy work for nutraceutrical companies in a personal capacity and as directors of Nutrasight Consultancy Limited. ANH is a ‘honorary director' of Howard Foundation Holdings Limited and Nutriproducts Limited, which licence and supply nutraceutical ingredients. DIT is a consultant of Howard Foundation Holdings Limited. All other authors declare no conflict of interest.
References
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Akkali MC, Loughman J, Howard AN, Beatty S. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp Eye Res. 2012;101:9–15. doi: 10.1016/j.exer.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Loughman J, Nolan JM, Howard AN, Connolly E, Meagher K, Beatty S. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Invest Ophthalmol Vis Sci. 2012;53 (12:7871–7880. doi: 10.1167/iovs.12-10690. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Loskutova E, Howard AN, Moran R, Mulcahy R, Stack J, et al. Macular pigment, visual function, and macular disease among subjects with Alzheimer's disease: an exploratory study. J Alzheimers Dis. 2014;42 (4:1191–1202. doi: 10.3233/JAD-140507. [DOI] [PubMed] [Google Scholar]
- Sabour-Pickett S, Beatty S, Connolly E, Loughman J, Stack J, Howard A, et al. Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. Retina. 2014;34 (9:1757–1766. doi: 10.1097/IAE.0000000000000174. [DOI] [PubMed] [Google Scholar]
- Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, et al. The age-related eye disease study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher KA, Thurnham DI, Beatty S, Howard AN, Connolly E, Cummins W, et al. Serum response to supplemental macular carotenoids in subjects with and without age-related macular degeneration. Br J Nutr. 2013;110:289–300. doi: 10.1017/S0007114512004837. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Meagher K, Kashani S, Beatty S. What is meso-zeaxanthin, and where does it come from. Eye (Lond) 2013;27:899–905. doi: 10.1038/eye.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten BR, Hammond BR, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci. 1999;40:2481–2489. [PubMed] [Google Scholar]
- Thurnham DI, Tremel A, Howard AN. A supplementation study in human subjects with a combination of meso-zeaxanthin, (3R,3'R-zeaxanthin and (3R,3'R,6'R-lutein. Br J Nutr. 2008;100:1307–1314. doi: 10.1017/S0007114508971336. [DOI] [PubMed] [Google Scholar]
- Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- Sparrow JM, Dickinson AJ, Duke AM. The Wisconsin Age-related Macular Degeneration grading system: performance in an independent centre. Ophthalmic Epidemiol. 1997;4:49–55. doi: 10.3109/09286589709058061. [DOI] [PubMed] [Google Scholar]
- O'Neill ME, Carroll Y, Corridan B, Olmedilla B, Granado F, Blanco I, et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br J Nutr. 2001;85:499–507. doi: 10.1079/bjn2000284. [DOI] [PubMed] [Google Scholar]
- O'Connell ED, Nolan JM, Stack J, Greenberg D, Kyle J, Maddock L, et al. Diet and risk factors for age-related maculopathy. Am J Clin Nutr. 2008;87:712–722. doi: 10.1093/ajcn/87.3.712. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Zhao dY, Serban B, Bernstein PS. Identification of 3-methoxyzeaxanthin as a novel age-related carotenoid metabolite in the human macula. Invest Ophthalmol Vis Sci. 2007;48:1435–1440. doi: 10.1167/iovs.06-1046. [DOI] [PubMed] [Google Scholar]
- Loane E, Nolan JM, O'Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol. 2008;53:68–81. doi: 10.1016/j.survophthal.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Murray IJ, Makridaki M, van der Veen RL, Carden D, Parry NR, Berendschot TT. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: the CLEAR study. Invest Ophthalmol Vis Sci. 2013;54:1781–1788. doi: 10.1167/iovs.12-10715. [DOI] [PubMed] [Google Scholar]
- Beatty S, Nolan JM, Muldrew KA, Woodside J, Stevenson MR, Chakravarthy U. Visual outcome after antioxidant supplementation. Ophthalmology. 2013;120:645. doi: 10.1016/j.ophtha.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Beatty S, Chakravarthy U, Nolan JM, Muldrew KA, Woodside JV, Denny F, et al. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120:600–606. doi: 10.1016/j.ophtha.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Charalampidou S, Loughman J, Nolan J, Stack J, Cassidy L, Pesudovs K, et al. Prognostic indicators and outcome measures for surgical removal of symptomatic nonadvanced cataract. Arch Ophthalmol. 2011;129 (9:1155–1161. doi: 10.1001/archophthalmol.2011.111. [DOI] [PubMed] [Google Scholar]
- Kleiner RC, Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Arch Ophthalmol. 1988;106:55–57. doi: 10.1001/archopht.1988.01060130061028. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Bex P. Measuring contrast sensitivity. Vision Res. 2013;90:10–14. doi: 10.1016/j.visres.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Layana A, Recalde S, Alaman AS, Robredo PF. Effects of lutein and docosahexaenoic acid supplementation on macular pigment optical density in a randomized controlled trial. Nutrients. 2013;5:543–551. doi: 10.3390/nu5020543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuffo KO, Beatty S, Stack J, Dennison J, O'Regan S, Meagher KA, et al. Central Retinal Enrichment Supplementation Trials (CREST): Design and Methodology of the CREST Randomized Controlled Trials. Ophthalmic Epidemiol. 2014;21:111–123. doi: 10.3109/09286586.2014.888085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age Related Eye Disease Study 2 Research Group Lutein+zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL, III, Elman MJ, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 Report No. 3. JAMA Ophthalmol. 2014;132 (2:142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]