Abstract
Background
Ginsenoside Rb1 (G-Rb1), the major active constituent of ginseng, improves insulin sensitivity and exerts antidiabetic effects. We tested whether the insulin-sensitizing and antidiabetic effects of G-Rb1 results from a reduction in ectopic fat accumulation, mediated by inhibition of lipolysis in adipocytes.
Methods
Obese and diabetic db/db mice were treated with daily doses of 20 mg/kg G-Rb1 for 14 days. Hepatic fat accumulation was evaluated by measuring liver weight and triglyceride content. Levels of blood glucose and serum insulin were used to evaluate insulin sensitivity in db/db mice. Lipolysis in adipocytes was evaluated by measuring plasma-free fatty acids and glycerol release from 3T3-L1 adipocytes treated with G-Rb1. The expression of relevant genes was analyzed by western blotting, quantitative real-time polymerase chain reaction, and enzyme-linked immunosorbent assay kit.
Results
G-Rb1 increased insulin sensitivity and alleviated hepatic fat accumulation in obese diabetic db/db mice, and these effects were accompanied by reduced liver weight and hepatic triglyceride content. Furthermore, G-Rb1 lowered the levels of free fatty acids in obese mice, which may contribute to a decline in hepatic lipid accumulation. Corresponding to these results, G-Rb1 significantly suppressed lipolysis in 3T3-L1 adipocytes and upregulated the perilipin expression in both 3T3-L1 adipocytes and mouse epididymal fat pads. Moreover, G-Rb1 increased the level of adiponectin and reduced that of tumor necrosis factor-α in obese mice, and these effects were confirmed in 3T3-L1 adipocytes.
Conclusion
G-Rb1 may improve insulin sensitivity in obese and diabetic db/db mice by reducing hepatic fat accumulation and suppressing adipocyte lipolysis; these effects may be mediated via the upregulation of perilipin expression in adipocytes.
Keywords: adipocyte, ginsenoside Rb1, insulin resistance, liver triglycerides, perilipin
1. Introduction
Panax ginseng has been used for the treatment and prevention of various diseases for several millennia in oriental medicine [1]. In previous clinical and pharmacological studies, ginseng and its active components have been reported to be effective in the treatment of diabetes [2]. Ginsenosides are the major active constituents responsible for the pharmacological properties of ginseng, and G-Rb1 is the most abundant among > 40 ginsenosides [3,4]. Our previous studies showed that G-Rb1 stimulates glucose uptake through the insulin-like signaling pathway in 3T3-L1 adipocytes [5], and facilitates adipogenesis of 3T3-L1 preadipocytes by enhancing the expression of peroxisome proliferator-activated receptor γ2 (PPARγ2) and CCAAT/enhancer-binding protein α [6]. In addition, we found that ginsenoside Rb1 can bind to PPARγ as a ligand and inhibited lipolysis in 3T3-L1 adipocytes [7]. Another study reported that ginsenoside Rb1 has antiobesity and antihyperglycemic effects in diet-induced obese rats [8]. Furthermore, recent studies demonstrate that G-Rb1 can reduce liver fat accumulation in high fat diet (HFD)-induced obese rats and mice [9,10].
In the pathogenesis of insulin resistance and diabetes, ectopic fat deposition, which is defined as the storage of triglycerides within cells of nonadipose tissue, is postulated to play an important role in the development of obesity-mediated insulin resistance [11]. In the obese and diabetic state, the level of circulating free fatty acids (FFAs) is often elevated, and most FFAs have spilled over from adipose tissue [11]. Circulating FFAs contribute to the incidence of insulin resistance in two ways. One is that FFAs interfere with the insulin-signaling pathway, and another is that they lead to excessive accumulation of intracellular lipid products in the liver and muscle [11,12]. Intrahepatic lipid content, and not visceral fat mass, is primarily related to hepatic insulin resistance [13]. Several interventional studies have demonstrated that a reduction in liver fat in patients with type 2 diabetes mellitus (T2DM) can improve insulin sensitivity and glucose metabolism [14,15]. Thus, reducing the ectopic fat content represents an effective strategy for the treatment of insulin resistance and T2DM.
Several factors contribute to lipid accumulation in the liver, including increased FFA release from visceral fat depots, increased lipogenesis, and reduced fatty acid oxidation in the liver. Failure to suppress FFA release from adipose tissue is known to be more important for fat accumulation in the liver [16]. Lipolysis in adipocytes is regulated by a complex signaling cascade, among which perilipin plays a central role in the regulation of lipolysis [17]. Perilipin double-regulates triacylglycerol (TG) metabolism by blocking lipase from approaching droplets to reduce the rate of basal lipolysis and facilitate hormonally stimulated lipolysis [18,19]. In 3T3-L1 adipocytes, the stimulation of lipolysis by tumor necrosis factor-α (TNFα) is in part mediated by promoting the rapid degradation of perilipin. Moreover, overexpression of perilipin in adipocytes inhibited TNF-α induced lipolysis [20]. In perilipin-null mice, basal adipocyte lipolysis was increased, and the development of glucose intolerance and insulin resistance was also promoted, probably due to the elevated levels of FFA [21].
Based on these observations, we hypothesized that G-Rb1 exerts insulin-sensitizing and antidiabetic effects in part by inhibiting lipolysis in adipocytes to reduce ectopic lipid accumulation in the liver. To test this hypothesis, we investigated the effect of G-Rb1 on hepatic lipid accumulation in obese diabetic db/db mice as well as the effect of G-Rb1 on the expression of perilipin in adipocytes.
2. Materials and methods
2.1. Materials
G-Rb1, rosiglitazone, and dimethyl sulfoxide were obtained from Sigma–Aldrich (St Louis, MO, USA). Recombinant human insulin was purchased from Lily (Fegersheim, France) and recombinant mouse TNFα from Gibco (Grand Island, NY, USA). Perilipin A goat polyclonal antibody, adipose triglyceride lipase (ATGL) rabbit monoclonal antibody, and abhydrolase domain-containing 5 (ABHD5) goat polyclonal antibody from Abcam (Cambridge, MA, USA). Hormone sensitive lipase (HSL) rabbit polyclonal antibody and phospho-HSL (Ser563) rabbit polyclonal antibody were purchased from Cell Signaling Technology (Beverly, MA, USA). β-actin mouse monoclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The BCA protein assay kit and West Pico chemiluminescent substrate were purchased from Pierce (Rockford, IL, USA). Unless otherwise specified, all other reagents were of analytic grade.
2.2. Animals and treatment
Male obese and diabetic db/db mice and their nondiabetic counterparts, male C57BLKS/J mice, were obtained from MTE Ltd. (Jiangsu, China). Mice were maintained on a 12/12 h light/dark cycle at 25°C, and provided free access to standard rodent chow and tap water. Diabetic db/db mice were randomly divided into two groups. In the G-Rb1 treated group, G-Rb1 was administered by intraperitoneal injection at a dose of 20 mg/kg body weight to nondiabetic mice, and control animals received vehicle alone. Body weight and food intake were recorded daily. After 14 d of treatment, the mice were fasted for 10 h, and blood was collected from the orbital vein of mice after they were deeply anesthetized using sodium pentobarbital (30 mg/kg, intraperitoneal). Epididymal fat and liver were surgically removed and stored at −80°C until the assays were performed. All experiments were approved by the Institutional Animal Care and Use Committee of Nanjing University of Chinese Medicine, Nanjing, China.
2.3. Measurement of plasma samples
Plasma glucose levels were measured using a glucometer. Insulin was measured using a commercially available enzyme-linked immunosorbent assay kit (Mercodia, Uppsala, Sweden). The homeostasis model assessment of basal insulin resistance was used to calculate an index from the product of the fasting concentrations of plasma glucose (mM) and plasma insulin (μU/L) divided by 22.5 [22]. FFA content was measured using the LabAssay NEFA kit (Wako, Osaka, Japan). Plasma concentrations of cholesterol, triglycerides (TG), and high- and low-density lipoprotein cholesterol were assessed with an autobiochemistry instrument using biochemical methods. Serum TNFα and adiponectin were measured using commercially available enzyme-linked immunosorbent assay kits (Millipore, St Charles, MO, USA).
2.4. Liver TG assay
The liver TG assay was performed as previously described [23]. In brief, approximately 100 mg of liver tissue was digested overnight with ethanolic KOH at 55°C, and the digest was extracted twice with 50% ethanol. After neutralization with MgCl2, the supernatant was used for triglyceride measurement using a TG kit (Sigma–Aldrich).
2.5. Histological examination of the liver
Tissue sections were cut from frozen liver samples using a microtome (Leica, Wetzlar, Germany) and mounted on gelatin-coated slides. Oil red O staining was performed as previously described [24]. In brief, the sections were fixed in 4% formaldehyde in phosphate-buffered saline for 1 h and then stained with 0.6% (w/v) Oil Red O solution (60% isopropanol, 40% water) for 1 h at room temperature. Sections were then washed three times with 60% isopropanol to remove unbound dye and photographed at 100× magnification.
2.6. Measurement of lipolysis in 3T3-L1 adipocytes
Mouse 3T3-L1 fibroblasts were obtained from the American Type Culture Collection (Manassas, VA, USA). 3T3-L1 fibroblasts were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and differentiated into adipocytes as previously described [25]. On Day 10 of differentiation, 3T3-L1 adipocytes were incubated with or without TNFα (10 ng/mL) in Dulbecco's modified Eagle's medium containing 0.2% bovine serum albumin for 12 h. Subsequently, the medium was changed. Adipocytes were treated with G-Rb1 or rosiglitazone at a concentration of 10μM in the presence of 10 ng/mL TNFα for 24 h. After treatment, the medium was removed and cells were incubated with phosphate-buffered saline for 1 h. The glycerol content in the supernatant was measured using free glycerol reagent (Sigma–Aldrich).
2.7. Western blotting analysis
Total cellular proteins of liver samples and 3T3-L1 adipocytes were extracted with homogenization buffer. Equal quantities of protein (50 μg) were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane (Amersham International, Cardiff, UK) using a Bio-Rad wet transfer unit. After blocking with 5% (w/v) nonfat dried milk in Tris-buffered saline with Tween-20 solution [25mM Tris, pH 7.5, 150mM NaCl, 0.05% (v/v) Tween-20] for 1 h at room temperature, the membranes were incubated with a goat anti-perilin A antibody (1:5000 dilution), rabbit anti-ATGL antibody (1:3000 dilution), rabbit anti-HSL antibody (1:1000 dilution), rabbit anti-phospho-HSL (Ser563) antibody (1:1000 dilution), or goat anti-ABHD5 antibody (1:3000) for 1 h at room temperature, followed by horseradish peroxidase-conjugated second antibody for 1 h at room temperature. Targeted proteins were detected with ECL kit (Amersham Biosciences). β-actin was used to normalize the amount of loaded protein.
2.8. Quantitative real-time polymerase chain reaction
Total RNA was extracted from 3T3-L1 cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed with SuperScript III Reverse Transcriptase and Oligo(dT) primer (Invitrogen). Quantitative real-time polymerase chain reaction was performed using the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The following primer sequences were used: TNFα, 5′-ACG GCA TGG ATC TCA AAG AC-3′ (forward) and 5′-CGG CAG AGA GGA GGT TGA CT-3′ (reverse); perilipin, 5′-AAC CCT GCT GGA TGG AGA-3′ (forward) and 5′-TTT GGT GCT GTT GTA GGT CTT-3′ (reverse); and β-actin, 5′-CGT TGA CAT CCG TAA AGA CC-3′ (forward) and 5′-AAC GT CCG CCT AGA AGC AC T-3′ (reverse). Reaction specificity was controlled by post-amplification melting curve analyses and gel electrophoresis of products. Quantitation of expression was achieved by normalization to β-actin expression, and the results were analyzed using the 2ΔΔCt relative quantitative method.
2.9. Statistical analysis
All values are expressed as mean ± standard deviation values. All in vitro data were obtained from at least three independent experiments. Differences between two groups were determined using Student t test. Differences among multiple groups were determined by one-way analysis of variance followed by Fisher's least square difference post hoc test using the SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). All p values < 0.05 were considered statistically significant.
3. Results
3.1. G-Rb1 improves insulin sensitivity in db/db obese diabetic mice
In G-Rb1-treated db/db mice, fasting insulin levels and homeostasis model assessment of basal insulin resistance were significantly lower than those in vehicle-treated db/db mice after 14 days of treatment (Table 1, p < 0.05), whereas fasting glucose levels showed a decreasing trend compared with those in vehicle-treated db/db mice (p > 0.05). G-Rb1 treatment had no significant effect on the amount of food intake. Body weight gain and epididymal fat weight were slightly reduced in G-Rb1-treated db/db mice in comparison with these measurements for vehicle-treated db/db mice (p > 0.05). These results suggest that the insulin sensitising effect of G-Rb1 in db/db mice may not be dependent on the inhibition of food uptake and reduction of adiposity.
Table 1.
Effects of Rb1 on fasting metabolic parameters in obese diabetic db/db mice
| Food intake (g/day) | Body weight increment (g) | Epididymal fat weight (%) | FBG (mM) | FSI (μg/L) | HOMA-IR | |
|---|---|---|---|---|---|---|
| BKS | 16.79 ± 1.59 | 1.24 ± 0.66 | 1.32 ± 0.30 | 6.38 ± 2.28 | 0.93 ± 0.98 | 0.24 ± 0.13 |
| db/db | 24.71 ± 1.21 | 4.38 ± 1.04 | 6.16 ± 0.91 | 17.42 ± 2.74 | 9.29 ± 3.03 | 7.15 ± 1.29 |
| G-Rb1 | 24.72 ± 3.15 | 2.86 ± 2.23 | 5.68 ± 0.26 | 15.7 ± 6.90 | 7.31 ± 1.69* | 4.42 ± 1.61* |
*p < 0.05 (db/db vs. G-Rb1 group).
FBG, fasting blood glucose; FSI, fasting serum insulin; HOMA-IR, homeostasis model assessment of insulin resistance.
3.2. G-Rb1 reduces liver fat accumulation in db/db obese diabetic mice
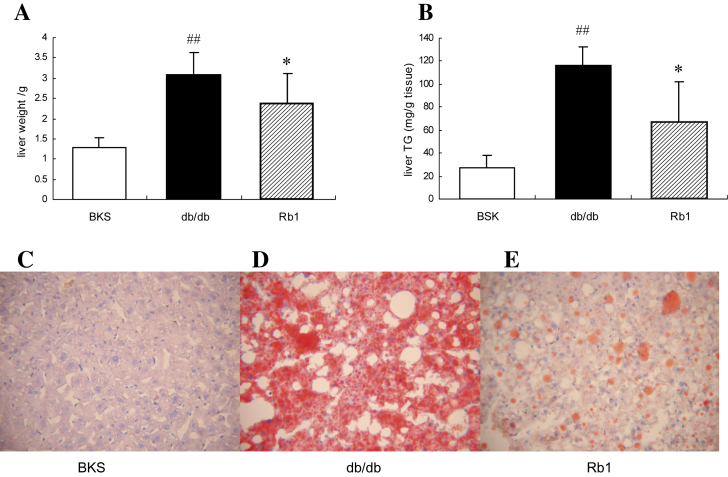
In the present study, we investigated whether G-Rb1 exerts insulin-sensitizing and antidiabetic effects in part by reducing ectopic TG accumulation in the body. The weight of the liver and hepatic TG content were significantly lower in the G-Rb1-treated db/db mice than in the vehicle-treated controls (Fig. 1A, B). Oil Red O staining, which indicates lipid droplet accumulation in the cytoplasm, revealed numerous large lipid droplets in vehicle-treated db/db mice, whereas only scattered and smaller lipid droplets in G-Rb1-treated db/db mice, and no droplets in pair-fed normal BKS mice (Fig. 1C–E).
Fig. 1.
Ginsenoside-Rb1 significantly reduced hepatic fat accumulation in obese and diabetic db/db mice. (A) Mouse liver weight. (B) Mouse liver TG content. Liver sections stained with Oil Red O from pair-fed BKS mice (C), vehicle-treated db/db mice (D), and Rb1-treated db/db mice (E), captured via light microscopy with a magnification of 100×. Data are means ± standard deviation (n = 5), ##p < 0.05 vs. BKS, * p < 0.05 vs. db/db.
3.3. G-Rb1 decreases circulating FFA levels in diabetic mice and lipolysis in adipocytes
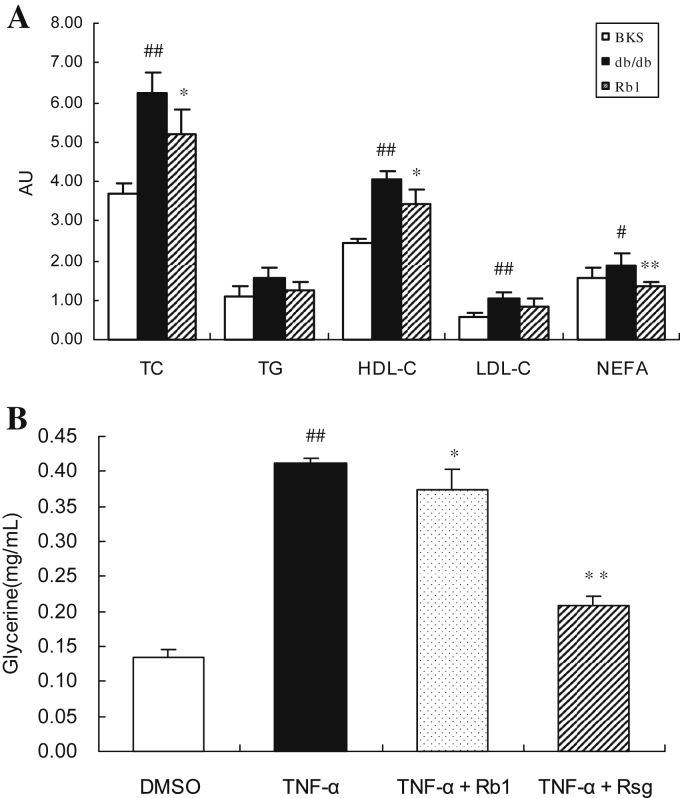
To determine the potential mechanisms by which G-Rb1 reduces hepatic fat accumulation in obese and diabetic db/db mice, circulating levels of FFAs and total cholesterol, TG, and high- and low-density lipoprotein cholesterol were tested. As shown in Fig. 2A, plasma FFA levels were significantly lower in G-Rb1-treated db/db mice than in vehicle-treated mice (p < 0.05; Fig. 2A). In addition, the plasma TG level appeared to be lower in G-Rb1-treated db/db mice than in vehicle-treated mice; however, this difference was not significant. Because low levels of circulating FFA could result from decreased lipolysis in adipocytes, we further investigated the effect of G-Rb1 on lipolysis in 3T3-L1 adipocytes. Glycerol released from TNFα-stimulated adipocytes clearly increased. The glycerol concentration in the supernatant was reduced by approximately 9% in G-Rb1-treated 3T3-L1 adipocytes (p < 0.05; Fig. 2B). These results suggest that G-Rb1 could lower circulating FFA levels by inhibiting lipolysis.
Fig. 2.
Effects of ginsenoside-Rb1 on mice blood lipid profiles and FFA release from adipocytes. (A) Plasma total cholesterol, triglycerides, and low-and high-density lipoprotein cholesterol were tested using biochemical methods and expressed as mM. Serum FFAs were measured use a nonesterified fatty acids (NEFA) test kit and expressed as mEq/L serum. Data are means ± standard deviation (n = 5), #p < 0.05 vs. BKS, ##p < 0.01 vs. BKS, * p < 0.05 vs. db/db, and ** p < 0.01 vs. db/db. (B) 3T3-L1 adipocytes were treated with TNFα only or with Rb1 simultaneously for 24 h, and then cells were incubated with phosphate-buffered saline for 1 h. The glycerol content in the supernatant was measured. Data are means ± standard deviation (n = 4), #p < 0.01 vs. dimethyl sulfoxide, * p < 0.05 vs. TNFα, and ** p < 0.01 vs. TNFα. Rsg, rosiglitazone; TNFα, tumor necrosis factor-α.
3.4. G-Rb1 upregulates perilipin expression in adipocytes
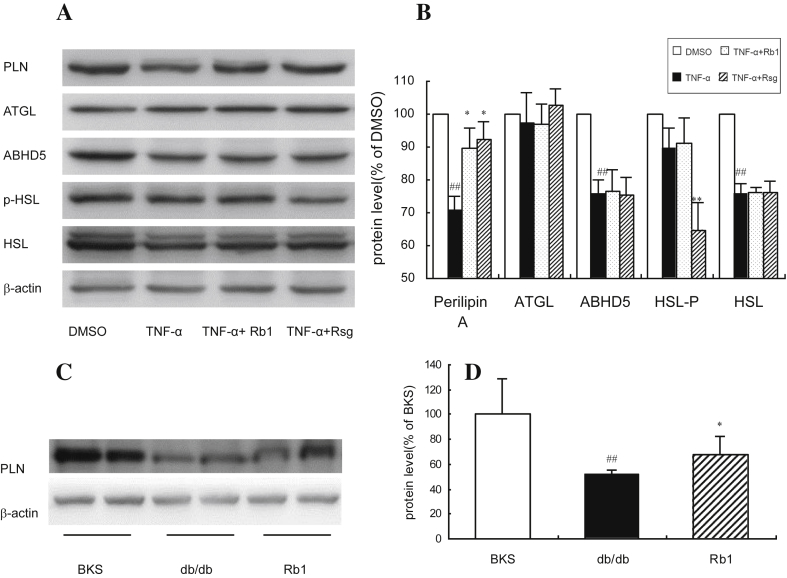
To elucidate the mechanism responsible for the effects of G-Rb1, several proteins that directly participate in lipolysis were measured. As shown in Fig. 3B, after pretreatment with TNFα, the expression of HSL, ABHD5, and perilipin A was significantly reduced (Fig. 3A, B, p < 0.01), and the expression of ATGL and HSL phosphorylation showed no significant change. The expression of perilipin A recovered when TNFα-pretreated 3T3-L1 adipocytes were incubated with G-Rb1 for 24 h. These observations were further confirmed in the epididymal fat pads of G-Rb1 or treated mice (Fig. 3C). The results suggest that the inhibiting effect of G-Rb1 on lipolysis adipocytes may result from the recovered expression of downregulated perilipin A.
Fig. 3.
Ginsenoside-Rb1 increased perilipin A expression in adipocytes. (A) and (B) Proteins were isolated from the 3T3-L1 cell lysates and analyzed by western blot analysis. The experiments were repeated three times. Data are means ± standard deviation (n = 3), #p < 0.01 vs. dimethyl sulfoxide, * p < 0.01 vs. TNFα, and ** p < 0.01 vs. TNFα (C) and (D) proteins were isolated from the epididymal fat pad lysates and analyzed by western blot analysis. The experiments were repeated three times. Data are means ± standard deviation (n = 3), ##p < 0.01 vs. BKS, and * p < 0.05 vs. db/db. β-actin was used for normalization of the amount of loaded protein. TNFα, tumor necrosis factor-α.
3.5. G-Rb1 decreases TNFα and increases adiponectin in db/db mice and adipocytes
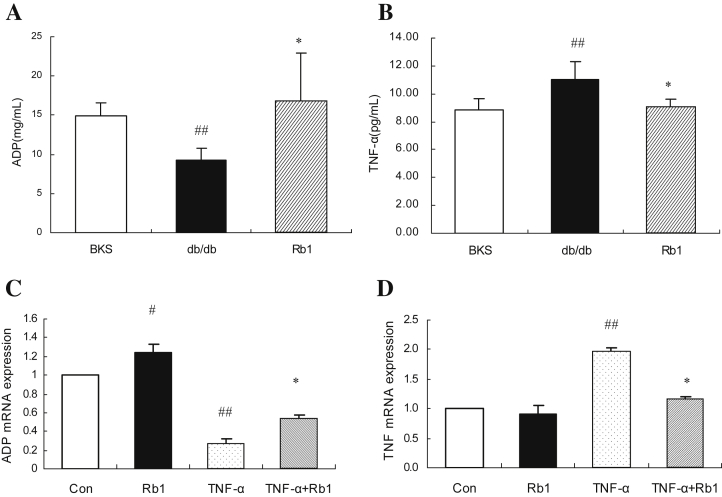
Compared with pair-fed normal BKS mice, db/db mice have lower levels of adiponectin and higher levels of TNFα. After 14-days of treatment with G-Rb1, TNFα decreased and adiponectin significantly increased in G-Rb1-treated mice compared with those in vehicle-treated db/db mice (Fig. 4A, B, p < 0.05). In 3T3-L1 adipocytes, G-Rb1 upregulated the mRNA expression of adiponectin and partially recovered the suppressed expression of adiponectin induced by TNFα (Fig. 4C, p < 0.05). By contrast, G-Rb1 reduced the overexpression of TNFα stimulated by pretreatment with TNFα (Fig. 4D, p < 0.05).
Fig. 4.
The effects of ginsenoside-Rb1 on the mRNA and concentration of adiponection and tumor necrosis factor-α (TNFα). (A) and (B) serum adiponectin and TNFα were analyzed by commercially available enzyme-linked immunosorbent assay kits. Data are means ± standard deviation (n = 5), ##p < 0.05 vs. BKS, * p < 0.05 vs. db/db (C) and (D) 3T3-L1 adipocytes were treated with TNF-α only or with Rb1 simultaneously for 24 h. Total RNA was extracted from 3T3-L1 cells and analyzed by quantitative real-time polymerase chain reaction for adiponectin and TNFα. Data are means ± standard deviation (n = 3), #p < 0.01 vs. control, and *p < 0.01 vs. TNFα.
4. Discussion
In the past decade, numerous in vitro and in vivo studies have demonstrated that ginseng and ginsenosides exert significant antidiabetic effects, such as lowering blood glucose, increasing insulin sensitivity, and regulating lipid metabolism and inflammatory pathways in both insulin-dependent and -independent manners [2]. G-Rb1, the most abundant and most important active component in ginseng, has been reported to exert antiobesity and antihyperglycemic effects in HFD-induced obese rats [8]. Our previous studies showed that G-Rb1 can promote glucose uptake and inhibit lipolysis in 3T3-L1 adipocytes [5–7]. In the present study, we found that G-Rb1 treatment improved insulin sensitivity and reduced liver fat content in obese diabetic db/db mice. Meanwhile, G-Rb1 treatment upregulated the expression of perilipin in adipose tissue and reduced the circulating FFA concentration. Because ectopic fat accumulation induced by overload of FFA is an important cause of insulin resistance [11,16], these results imply that G-Rb1 can upregulate the expression of perilipin in adipose tissue to ameliorate liver fat accumulation, which offers an improved understanding of the mechanisms of the insulin-sensitizing and antidiabetic effects of G-Rb1.
Fat accumulation within the liver occurs as a consequence of continuous oversupply of FFA together with reduced FFA oxidation in the mitochondria [26]. Shen et al [9] recently reported that, after chronic intraperitoneal administration, G-Rb1 significantly ameliorated hepatic fat accumulation in HFD-induced obese rats, and this effect was associated with elevated activation of hepatic AMP-activated protein kinase, which ultimately stimulated fatty acid oxidative gene expression and suppressed the expression of genes encoding enzymes or proteins that function in lipogenesis. Enhanced FFA release from adipose tissue in obesity is more important in the progress of fat accumulation in the liver [16]. In the present study, we found that G-Rb1 upregulated the expression of perilipin to inhibit FFA release from adipocytes and in obese diabetic db/db mice, which implies another mechanism by which G-Rb1 regulates hepatic fat accumulation.
Adipose tissue is an important site of FFA liberation into the plasma. In the fasting state, plasma FFA is derived almost exclusively from hydrolysis of TG within adipocytes [27]. The increase plasma FFAs observed in obese patients is believed to result from dysregulated lipolysis of TG in adipose cells [27]. Thus, we postulated that the decreased plasma FFA concentrations in obese and diabetic db/db mice treated with G-Rb1 may be caused by enhanced inhibition of intracellular lipolysis in adipose tissue. We found that G-Rb1 significantly prevented lipolysis in 3T3-L1 cells pretreated with TNFα in this study. Although lipolysis in adipocytes is highly regulated through modulation of the activity of multiple enzymes and conformation of the proteins that coat the adipocyte lipid droplet, perilipin has been shown to play a key role of in lipolytic activation of adipocytes [18]. Perilipin is the most abundant protein on the surface of lipid droplets, and also the major substrate of protein kinase A in hormonally stimulated lipolysis. Under basal conditions, HSL is located in the cytoplasm, while nonphosphorylated perilipin coats the lipid droplets and binds to ABHD5, which is a key activator of ATGL [18,19]. This restricts the actions of lipases on stored neutral lipids and promotes TG storage. During energy deficit, protein kinase A is activated by cAMP, which phosphorylates both perilipin and HSL. This induces a conformational change of perilipin, which facilitates the HSL docking, by which phosphorylated HSL gains access to substrates at the surface of lipid droplets. In addition, phosphorylated perilipin releases ABHD5, which then binds to ATGL and activates this enzyme [19,28]. Taysey et al [21] reported that perilipin ablation results in lean mice with reduced adipose tissue mass, high levels of basal lipolysis, and peripheral insulin resistance. The expression of perilipin A is reduced in poorly controlled patients with T2DM [29]. In our study, perilipin expression in the adipose tissue of db/db obese mice was lower than that in normal lean mice, and circulating FFA levels were elevated in obese mice. G-Rb1 partly recovered the downregulated expression of perilipin in obese mice, and reduced the level of circulated FFA, without affecting the expression of ATGL or HSL, which was confirmed in 3T3-L1 adipocytes treated with TNFα. These results indicate that the increase in perilipin induced by G-Rb1 may represent the underlying mechanism for the inhibition of lipolysis and reduction in plasma FFA concentrations.
The precise mechanism underlying the regulation of perilipin by G-Rb1 has not been identified in this study. It has been demonstrated that the expression of perilipin is regulated by PPARγ in adipocytes [30,31]. The insulin-sensitizing effect of multiple PPAR agonists is mediated via increased expression of perilipin to reduce plasma FFA concentrations and ectopic fat accumulation [32,33]. In our previous study, we found that G-Rb1 can not only promote the expression of PPARγ2 but also directly bind to PPARγ2 as a ligand to increase its activity [6,7]. Although we did not detect PPARγ expression, the upregulating effect of G-Rb1 on perilipin probably results from its activation of PPARγ in adipocytes. By contrast, TNFα production is increased in obese patients with T2DM and may lead to enhanced basal lipolysis in obesity through the downregulation of perilipin mRNA and protein expression in adipose tissue [34,35]. Our results showed that G-Rb1 could reduce TNFα concentration in obese mice and suppress the expression of TNFα in adipocytes, which may contribute to the effect of G-Rb1 on perilipin and lipolysis. In addition, we found that G-Rb1 upregulated the expression of adiponectin in adipocytes and increased the level of adiponectin in obese mice. There has been no report on the relationship between adiponectin and perilipin, but adiponectin, as an adipocytokine, that improves insulin sensitivity induced by obesity [36], also inhibits lipolysis in adipocytes [37]. Thus, the elevated adiponectin expression induced by G-Rb1 may be helpful for inhibiting the effects of G-Rb1 on lipolysis and insulin resistance.
In conclusion, this study revealed that G-Rb1, the most abundant ginsenoside in ginseng root, improves insulin sensitivity in obese and diabetic db/db mice by reducing hepatic fat accumulation and suppressing adipocyte lipolysis, which may be mediated by the upregulation of perilipin expression in adipocytes. The findings promote an improved understanding of the mechanism of the insulin-sensitizing and antidiabetic effects of G-Rb1.
Conflicts of interests
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (83073110) and a grant from the Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Material Medica (P09014).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 2.Yuan H.D., Kim J.T., Kim S.H., Chung S.H. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012;36:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim W., Mudge K.W., Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium) J Agric Food Chem. 2005;53:8498–8505. doi: 10.1021/jf051070y. [DOI] [PubMed] [Google Scholar]
- 4.Washida D., Kitanaka S. Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem Pharm Bull (Tokyo) 2003;51:1314–1317. doi: 10.1248/cpb.51.1314. [DOI] [PubMed] [Google Scholar]
- 5.Shang W., Yang Y., Zhou L., Jiang B., Jin H., Chen M. Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J Endocrinol. 2008;198:561–569. doi: 10.1677/JOE-08-0104. [DOI] [PubMed] [Google Scholar]
- 6.Shang W., Yang Y., Jiang B., Jin H., Zhou L., Liu S., Chen M. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARgamma2 and C/EBPalpha gene expression. Life Sci. 2007;80:618–625. doi: 10.1016/j.lfs.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Shang W., Yang Y., Zhou L., Jiang B., Jin H., Chen M. Ginsenoside Rb1 facilitates adipocyte differentiation and inhibits lipolysis in 3T3-L1 adipocytes. Chin J Endocrinol Metab. 2007;23:6–9. [Google Scholar]
- 8.Xiong Y., Shen L., Liu K.J., Tso P., Wang G., Woods S.C., Liu M. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 2010;59:2505–2512. doi: 10.2337/db10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen L., Xiong Y., Wang D.Q., Howles P., Basford J.E., Wang J., Xiong Y.Q., Hui D.Y., Woods S.C., Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430–1438. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang W., Yu X., Wang G., Zhao J. Effect of ginsenoside Rb1 in ameliorating insulin resistance and ectopic fat deposition in obese mice induced by high fat diet. Zhongguo Zhong Yao Za Zhi. 2013;38:4119–4123. [Article in Chinese] [PubMed] [Google Scholar]
- 11.Johnson A.M., Olefsky J.M. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 12.Ebbert J.O., Jensen M.D. Fat depots, free fatty acids, and dyslipidemia. Nutrients. 2013;5:498–508. doi: 10.3390/nu5020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W., Okunade A., Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato F., Tamura Y., Watada H., Kumashiro N., Igarashi Y., Uchino H., Maehara T., Kyogoku S., Sunayama S., Sato H. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab. 2007;92:3326–3329. doi: 10.1210/jc.2006-2384. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj M., Suraamornkul S., Pratipanawatr T., Hardies L.J., Pratipanawatr W., Glass L., Cersosimo E., Miyazaki Y., DeFronzo R.A. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 16.Yki-Järvinen H. Ectopic fat accumulation: an important cause of insulin resistance in humans. J R Soc Med. 2002;95(Suppl. 42):39–45. [PMC free article] [PubMed] [Google Scholar]
- 17.Brasaemle D.L. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Sztalryd C., Xu G., Dorward H., Tansey J.T., Contreras J.A., Kimmel A.R., Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granneman J.G., Moore H.P., Granneman R.L., Greenberg A.S., Obin M.S., Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 20.Souza S.C., Yamamoto M.T., Franciosa M.D., Lien P., Greenberg A.S. BRL 49653 blocks the lipolytic actions of tumor necrosis factor-alpha: a potential new insulin-sensitizing mechanism for thiazolidinediones. Diabetes. 1998;47:691–695. doi: 10.2337/diabetes.47.4.691. [DOI] [PubMed] [Google Scholar]
- 21.Tansey J.T., Sztalryd C., Gruia-Gray J., Roush D.L., Zee J.V., Gavrilova O., Reitman M.L., Deng C.X., Li C., Kimmel A.R. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Salmon D.M., Flatt J.P. Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int J Obes. 1985;9:443–449. [PubMed] [Google Scholar]
- 24.Ramírez-Zacarias J.L., Castro-Muñozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 25.Perrini S., Natalicchio A., Laviola L., Belsanti G., Montrone C., Cignarelli A., Minielli V., Grano M., De Pergola G., Giorgino R. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53:41–52. doi: 10.2337/diabetes.53.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Samuel V.T., Shulman G.I. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mook S., Cj Cj Halkes, Bilecen S., Cabezas M.C. In vivo regulation of plasma free fatty acids in insulin resistance. Metabolism. 2004;53:1197–1201. doi: 10.1016/j.metabol.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi T., Omatsu N., Morimoto E., Nakashima H., Ueno K., Tanaka T., Satouchi K., Hirose F., Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen T.S., Kampmann U., Nielsen R.R., Jessen N., Orskov L., Pedersen S.B., Jorgensen J.O., Lund S., Moller N. Reduced mRNA and protein expression of perilipin A and G0/G1 switch gene 2 (G0S2) in human adipose tissue in poorly controlled type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E1348–E1352. doi: 10.1210/jc.2012-1159. [DOI] [PubMed] [Google Scholar]
- 30.Arimura N., Horiba T., Imagawa M., Shimizu M., Sato R. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem. 2004;279:10070–10076. doi: 10.1074/jbc.M308522200. [DOI] [PubMed] [Google Scholar]
- 31.Nagai S., Shimizu C., Umetsu M., Taniguchi S., Endo M., Miyoshi H., Yoshioka N., Kubo M., Koike T. Identification of a functional peroxisome proliferator-activated receptor responsive element within the murine perilipin gene. Endocrinology. 2004;145:2346–2356. doi: 10.1210/en.2003-1180. [DOI] [PubMed] [Google Scholar]
- 32.Kim H.J., Jung T.W., Kang E.S., Kim D.J., Ahn C.W., Lee K.W., Lee H.C., Cha B.S. Depot-specific regulation of perilipin by rosiglitazone in a diabetic animal model. Metabolism. 2007;56:676–685. doi: 10.1016/j.metabol.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Bays H., Mandarino L., DeFronzo R.A. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 34.Ryden M., Arvidsson E., Blomqvist L., Perbeck L., Dicker A., Arner P. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem Biophys Res Commun. 2004;318:168–175. doi: 10.1016/j.bbrc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Duncan R.E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H.S. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao L., Kinney B., Schaack J., Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 2011;60:1519–1527. doi: 10.2337/db10-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]