Abstract
Background
Korean Red Ginseng has been used as a traditional oriental medicine to treat illness and to promote health for several thousand years in Eastern Asia. It is widely accepted that ginseng saponins, ginsenosides, are the major active ingredients responsible for Korean Red Ginseng’s therapeutic activity against many kinds of illness. Although the crude saponin fraction (CSF) displayed antiplatelet activity, the molecular mechanism of its action remains to be elucidated.
Methods
The platelet aggregation was induced by collagen, the ligand of integrin αIIβI and glycoprotein VI. The crude saponin’s effects on granule secretion [e.g., calcium ion mobilization and adenosine triphosphate (ATP) release] were determined. The activation of mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated protein kinase 1/2 (ERK1/2), c-Jun N-terminal kinases (JNKs), and p38 MAPK, and phosphoinositide 3-kinase (PI3K)/Akt was analyzed by immunoblotting. In addition, the activation of integrin αIIbβIII was examined by fluorocytometry.
Results
CSF strongly inhibited collagen-induced platelet aggregation and ATP release in a concentration-dependent manner. It also markedly suppressed [Ca2+]i mobilization in collagen-stimulated platelets. Immunoblotting assay revealed that CSF significantly suppressed ERK1/2, p38, JNK, PI3K, Akt, and mitogen-activated protein kinase kinase 1/2 phosphorylation. In addition, our fraction strongly inhibited the fibrinogen binding to integrin αIIbβ3.
Conclusion
Our present data suggest that CSF may have a strong antiplatelet property and it can be considered as a candidate with therapeutic potential for the treatment of cardiovascular disorders involving abnormal platelet function.
Keywords: crude saponin fraction, Korean Red Ginseng, mitogen-activated protein kinase, phosphatidylinositol-3-kinase, platelet aggregation
1. Introduction
Platelet aggregation is essential for the normal hemostatic process when blood vessels are injured. However, aberrant platelet activation causes cardiovascular diseases such as thrombosis, atherosclerosis, and myocardial infarction [1]. Several agonists [e.g., collagen, thrombin, and adenosine diphosphate (ADP)] can bind to glycoprotein receptors or G-protein coupled receptors, and lead to subsequent activation of intracellular downstream signaling molecules. The activation of these receptors can stimulate phospholipase C, which decomposes phosphatidylinositol 4,5-bisphosphate into two second messengers, namely, inositol-1,4,5-trisphosphate (IP3) and diacylglycerol [2,3]. IP3 binds to IP3 receptor of calcium storage pool (i.e., dense tubular system) and releases Ca2+ into the cytoplasm. Thus, the intracellular calcium ion ([Ca2+]i) concentration can be increased by more than 10-fold. [Ca2+]i can bind to the calcium-binding protein calmodulin and activate myosin light-chain kinase. This kinase causes the phosphorylation of the regulatory light chain of myosin, which in turn causes the activation of platelet [4,5]. As the result, platelet aggregation occurs and the activation of platelet that goes beyond this range causes cardiovascular diseases such as thrombosis, atherosclerosis, and myocardial infarction. Therefore, the suppression of platelet function by natural products represents a promising approach to the prevention of thrombosis.
To suppress or prevent the atherosclerosis and thrombosis, many antiplatelet drugs have been developed [6]. However, these antiplatelet drugs have had various side effects in some patients, such as gastrointestinal side effects, hemorrhage, and reduction of platelets [7–10]. As a result, increasing attention has been paid to dietary supplements, prevention of cardiovascular diseases for the safety of drug use, and development of oriental medicines for treatment [11].
Korean Red Ginseng has been used as a traditional oriental medicine to treat illness and promote health for several thousand years. Moreover, it is reported that Korean Red Ginseng is an effective folk medicine against stress, heart failure, hypertension, diabetes, and so on [12,13]. Korean Red Ginseng contains many active ingredients such as polysaccharides, peptides, fatty acids, mineral oils, and ginsenosides. Among these components, ginseng saponin (i.e., ginsenoside) is one of the well-known bioactive ingredients in ginseng, and thus far more than 30 ginsenosides have been isolated and characterized. Ginsenosides are classified into protopanaxadiol (PPD) and protopanaxatriol (PPT) saponins. PPD includes ginsenoside Rb1 (G-Rb1), G-Rb2, G-Rc, and G-Rd, whereas PPT contains G-Rg1, G-Re, G-Rf, and G-Rg2.
Although the antiplatelet activities of saponin fraction and several ginsenosides have been reported [14–17], the molecular mechanism of its action is not yet well understood.
In this study, we have demonstrated that the crude saponin fraction (CSF) has strong inhibitory activities on collagen-induced platelet aggregation. In addition, we determined the molecular mechanism underlying the antiplatelet activity of CSF.
2. Materials and Methods
2.1. Materials
CSF was obtained from KGC Research Institute (Daejeon, Korea). CSF was analyzed by HPLC and the composition of ginsenosides is as follows: 134 mg/g of G-Rg1, 106 mg/g of G-Re, 52 mg/g of G-Rf, 181 mg/g of G-Rb1, 165 mg/g of G-Rc, 108 mg/g of G-Rb2, 18 mg/g of Rb3, 72 mg/g of Rd, 44 mg/g of F2, 28 mg/g of Rg3. Collagen was obtained from Chrono-Log Co. (Havertown, PA, USA). Fura-2-acetoxymethyl ester (Fura-2/AM) and dimethyl sulfoxide (DMSO) were from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies to phospho-p44/42 [p-extracellular signal-regulated protein kinase 1/2 (p-ERK1/2)], p44/42 (T-ERK1/2), phospho-p38 (p-p38), p38 (T-p38), phospho-SAPK (phospho-stress-activated protein kinase)/c-Jun N-terminal kinase (p-JNK), SAPK/JNK (T-JNK), phospho-phosphoinositide 3-kinase (phospho-PI3K; p-p85), PI3K (T-p38), phospho-mitogen-activated protein kinase kinase (p-MEK1/2), MEK1/2 (T-MEK1/2), phospho-Akt (p-Akt), and Akt (T-Akt) were acquired from Cell Signaling Technology (Beverly, MA, USA). Adenosine triphosphate (ATP) assay kits were purchased from Biomedical Research Service Center (Buffalo, NY, USA). Fibrinogen Alexa Fluor 488 conjugate was obtained from Molecular Probes (Eugene, OR, USA). All other chemicals were of reagent grade.
2.2. Experimental animals
Male Sprague Dawley rats (300–350 g) were obtained from Orient Co. (Seoul, Korea). The animals were acclimated for 1 wk before the experiments, and housed in an air-conditioned animal room with a 12/12-h light/dark cycle at a temperature of 22 ± 1°C and humidity of 50% ± 10%. All animals were provided with a laboratory diet and water ad libitum. All experimental protocols involving the use of animals were conducted in accordance with the National Institutes of Health guidelines and approved by the Committee on Animal Care at the Kyungpook National University.
2.3. Washed rat platelet preparation
Blood was withdrawn from the abdominal vein of rats and collected directly into an anticoagulant citrate dextrose solution that contained 0.8% citric acid, 2.2% trisodium citrate, and 2% dextrose (w/v). Washed platelets were prepared as previously described. In brief, platelet-rich plasma (PRP) was obtained by centrifuging rat blood samples at 230g for 10 min. Platelets were precipitated by centrifugation of the PRP at 800g for 15 min and washed with HEPES buffer (137mM NaCl, 2.7mM KCl, 1mM MgCl2, 5.6mM glucose, and 3.8mM HEPES; pH 6.5) containing 0.35% bovine serum albumin and 0.4mM ethylene glycol tetraacetic acid (EGTA). The washed platelets were resuspended in HEPES buffer (pH 7.4) and the cell dilution was adjusted to 4 × 108 cells/mL.
2.4. Platelet aggregation
Platelet aggregation was evaluated as previously described [18]. Aggregation was monitored by measuring light transmission with an aggregometer (Chrono-Log Co.) at constant stirring speed (1,200 rpm). The washed platelets (3 × 108/mL) were preincubated at 37°C for 2 min with either CSF or vehicle and then stimulated with 2.5 mg/mL collagen. The mixture was further incubated for 5 min with stirring at 170g. The vehicle concentration was less than 0.1% to minimize the effect of this reagent.
2.5. [Ca2+]i measurement
The intracellular calcium ion concentration ([Ca2+]i) was measured with Fura-2/AM as previously described [19]. In brief, the platelets were incubated with 5μM of Fura-2/AM for 30 min at 37°C and washed. The Fura-2-loaded platelets (3 × 108/mL) were then preincubated with MAE for 2 min at 37°C in the presence of 1mM CaCl2, and subsequently stimulated with collagen for 5 min. Fluorescent signals were recorded using a Hitachi F-2500 fluorescence spectrofluorometer (Hitachi, Japan). Light emission was measured at 510 nm, with simultaneous excitation at 340 and 380 nm that changed every 0.5 seconds. Fura-2 fluorescence in the cytosol measured with the spectrofluorometer was calculated as previously described by Schaeffer and Blaustein [20] using the following formula: [Ca2+]i 224nM × (F – Fmin)/(Fmax – F), where 224nM is the dissociation constant of the Fura-2–Ca2+ complex, and Fmin and Fmax represent the fluorescence intensity levels at very low and very high Ca2+ concentrations, respectively. In our experiment, Fmax was the fluorescence intensity of the Fura-2–Ca2+ complex at 510 nm after the platelet suspension containing 1mM of CaCl2 had been solubilized with Triton X-100 (0.1%). Fmin was the fluorescence intensity of the Fura-2–Ca2+ complex at 510 nm after the platelet suspension containing 20mM Tris/3mM of EGTA had been solubilized with Triton X-100 (0.1%). F represented the fluorescence intensity of the Fura-2 complex at 510 nm after the platelet suspension was stimulated with collagen with or without crude saponin fraction in the presence of 1mM CaCl2.
2.6. ATP release assay
Washed platelets (3 × 108/mL) were preincubated for 2 min at 37°C with various concentrations of CSF and then stimulated with 2.5 μg/mL collagen. After the reaction was terminated, the cells were centrifuged and the supernatants were used for the assay. ATP release was measured with a luminometer (GloMax 20/20; Promega, Madison, WI, USA) using an ATP assay kit (Biomedical Research Service Center, Buffalo, NY, USA) according to manufacturer’s instructions.
2.7. Immunoblotting
Platelet suspensions (3 × 108/mL) were preincubated with CSF or vehicle [0.1% (v/v) DMSO] at 37°C for 2 min. Platelet activation was induced by the addition of 2.5 μg/mL collagen and the reaction was allowed to proceed for 5 min. After terminating the reaction, lysates were then prepared by solubilizing and centrifuging the platelets in sample buffer [0.125M Tris–HCl, pH 6.8; 2% sodium dodecyl sulfate, 2% β-mercaptoethanol, 20% glycerol, 0.02% bromophenol blue, 1 μg/mL phenylmethyl sulfonyl fluoride, 2 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 μg/mL pepstatin A]. Protein concentration was determined using a bicinchoninic acid assay (Pro-Measure; iNtRON Biotechnology, Seoul, Korea). Total cell proteins (30 μg) from the platelet lysate were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes in transfer buffer (25mM Tris, pH 8.5; 0.2M glycine, and 20% methanol). The membranes were blocked in Tris-buffered saline and Tween-20 containing 5% nonfat dry milk and incubated with primary antibody diluted in a blocking solution. The blots were then incubated with horseradish peroxidase-conjugated secondary antibody. Antibody binding was visualized using enhanced chemiluminescence (iNtRON Biotechnology).
2.8. Assessment of fibrinogen binding to integrin αIIbβ3
Binding of fibrinogen Alexa Fluor 488 conjugate to washed platelets was quantified by flow cytometry. In brief, washed platelets (3 × 108/mL) were preincubated for 2 min with various concentrations of CSF at room temperature in the presence of 0.1mM CaCl2. The platelets were then stimulated with collagen for 5 min, immediately incubated thereafter with fibrinogen Alexa Fluor 488 (20 μg/mL) for 5 min, and finally fixed with 0.5% paraformaldehyde at 4°C for 30 min. The platelets were pelleted by centrifugation at 2,000g at 4°C and resuspended in 500 μL phosphate-buffered saline. Because the activation of integrin αIIbβ3 is largely dependent on the generation of Ca2+, nonspecific binding of fibrinogen to integrin αIIbβ3 was measured by assessing fibrinogen binding in the presence of the calcium chelator EGTA (1mM). The fluorescence of each platelet sample was analyzed using a FACS Calibur cytometer (BD Biosciences, San Jose, CA, USA), and data were analyzed using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA).
2.9. Statistics
Data were analyzed with a one-way analysis of variance followed by a post hoc Dunnett test to measure statistical significance of the differences observed (SAS Institute Inc., Cary, NC, USA). All data are presented as the mean ± standard deviation. A p value of 0.05 or less was considered to be statistically significant.
3. Results
3.1. Inhibitory effect of CSF on collagen-induced platelet aggregation
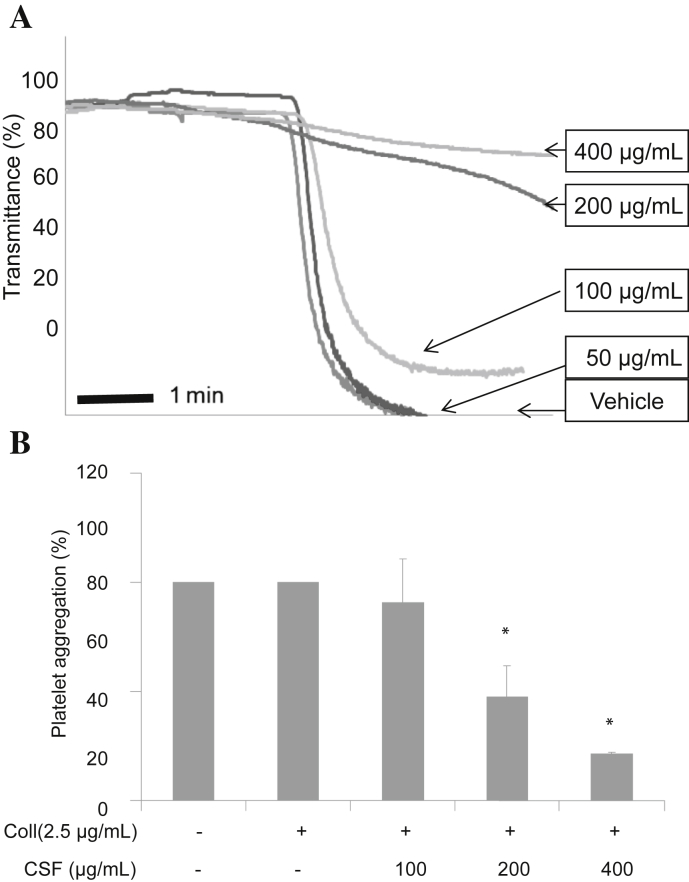
The concentration of collagen used (i.e., 2.5 μg/mL) could induce full activation and aggregation of rat platelet. Therefore, 2.5 μg/mL of collagen was used as the ligand to induce platelet aggregation for further experiments. Although washed platelets were strongly activated by 2.5 μg/mL of collagen in the presence of 1mM CaCl2, CSF significantly inhibited collagen-induced platelet aggregation in a concentration-dependent manner (Fig. 1).
Fig. 1.
The inhibitory effect of crude saponin fraction (CSF) on platelet aggregation induced by collagen. Platelets (3 × 108/mL) were preincubated with or without CSF (50–400 μg/mL) in the presence of 1mM CaCl2 for 2 min at 37°C. The platelet aggregation was then induced by 2.5 μg/mL of collagen, and the extent of aggregation was measured with a Chrono-Log aggregometer (A). The aggregation reaction was terminated after 5 min, and the percentage aggregation rate was calculated. Each graph shows the mean ± standard deviation of at least four independent experiments (B). * p < 0.001 compared with the agonist control. Coll, collagen.
3.2. Inhibitory effect of CSF on the granule release
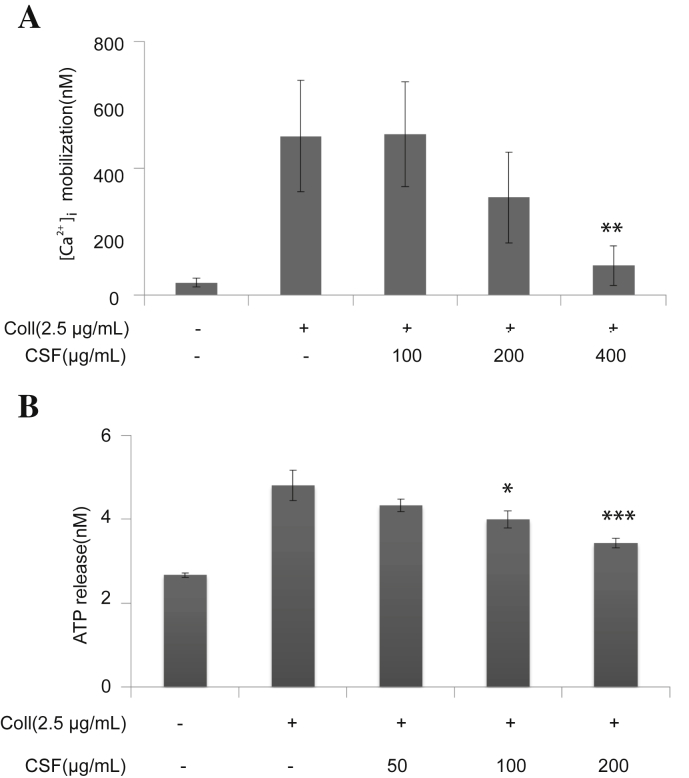
It is well-studied that intracellular calcium ion [Ca2+]i plays a critical role in the agonist-induced platelet aggregation. That is, [Ca2+]i activates downstream signaling molecules, and thus, it is the prerequisite for full activation of platelet. Therefore, we have analyzed the inhibitory effect of CSF on the Ca2+ mobilization in rat platelets. When the platelets were stimulated by collagen, [Ca2+]i was significantly increased. However, this was markedly diminished by the pretreatment of CSF in a concentration-dependent manner (Fig. 2A).
Fig. 2.
(A) The inhibitory effect of crude saponin fraction (CSF) on collagen-induced [Ca2+]i. Washed platelets (3 × 108/mL) were incubated with a calcium fluorophore (5 μM; Fura-2-acetoxymethyl ester) and stimulated with collagen (2.5 μg/mL). [Ca2+]i was then measured as described in the “Materials and Methods” section. (B) Effects of CSF on granule secretion from the collagen-stimulated platelets. Washed platelets (3 × 108/mL) were preincubated with CSF at the indicated concentrations and stirred in an aggregometer for 2 min before stimulation with 2.5 μg/mL collagen for 5 min. The reaction was terminated, and adenosine triphosphate (ATP) release was determined by ATP assay. Assay was performed as described in the “Materials and Methods” section. Bar graphs show the mean ± standard deviation of at least four independent experiments. * p < 0.05, ** p < 0.005, *** p < 0.001 compared with the agonist control. Coll, collagen.
In the following study, we have analyzed whether CSF modulated the ATP granule secretion. Collagen induced ATP release from dense granules. It was revealed that the pretreatment of platelets with CSF showed a significant decrease in a concentration-dependent manner (Fig. 2B).
3.3. Inhibitory effect by CSF on the integrin αIIbβ3 activation
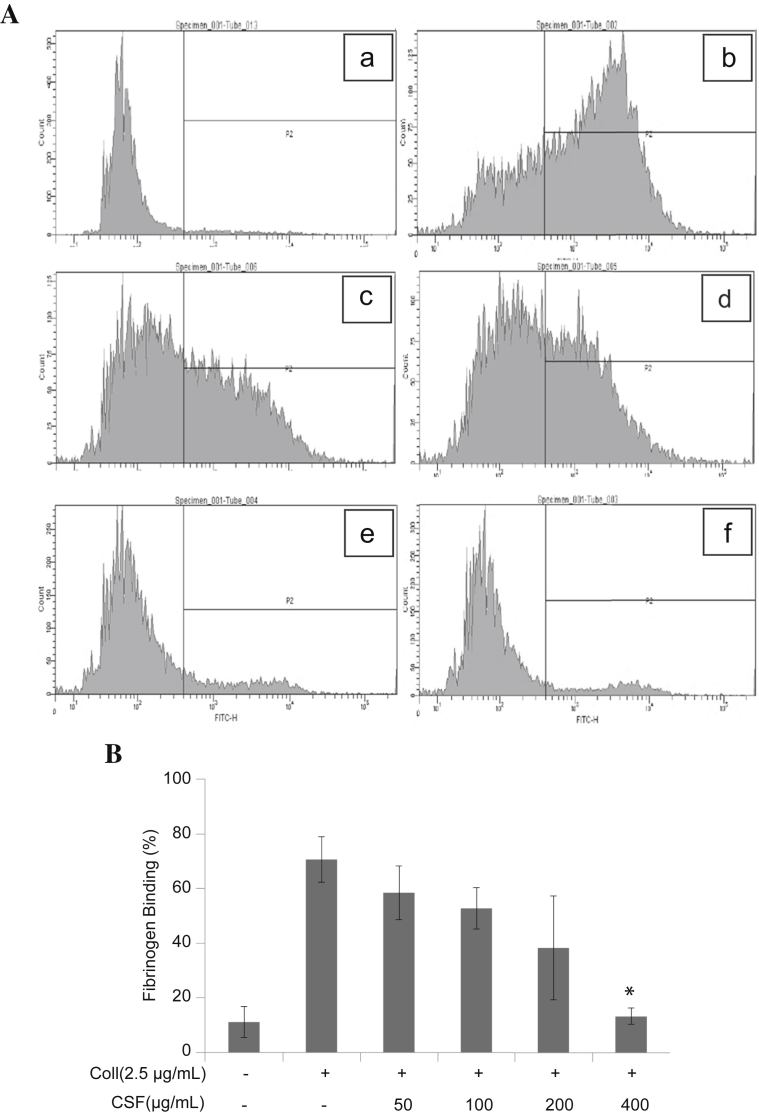
To prompt intravascular platelet aggregation in a stable manner, binding of fibrinogen to platelet integrin αIIbβ3 should occur. Therefore, we analyzed the influence of CSF on integrin αIIbβ3 activation using fluorocytometry assay. As shown in Fig. 3, whereas resting platelet did not activate the integrin αIIbβ3, collagen (2.5 μg/mL) markedly increased the fibrinogen binding to active integrin αIIbβ3. CSF dose-dependently and significantly reduced collagen (2.5 μg/mL)-stimulated fibrinogen binding to integrin αIIbβ3.
Fig. 3.
Effects of crude saponin fraction (CSF) on fibrinogen binding to integrin αIIbβ3 in collagen-activated platelets. (A) The inhibitory effects of CSF on fibrinogen binding to integrin αIIbβ3 in collagen-activated platelets were measured by flow cytometry. Washed platelets (3 × 108/mL) were pretreated with vehicle (dimethyl sulfoxide) or CSF at concentrations ranging from 50 to 400 μg/mL. Collagen was then incubated with human fibrinogen labeled with Alexa Fluor 488 (20 μg/mL) for 5 minutes. The cells were subsequently fixed with 0.5% paraformaldehyde at 4°C for 30 min. (B) Graphs showing fluorescent intensity present the data from one experiment but are representative of four independent trials. Data are expressed as the mean fluorescence intensity of fibrinogen-positive platelets. Each graph presents the results expressed as percentage of gated cells. * p < 0.001 compared with the agonist control. Coll, collagen.
3.4. Inhibitory effect of CSF on the mitogen-activated protein kinase phosphorylation
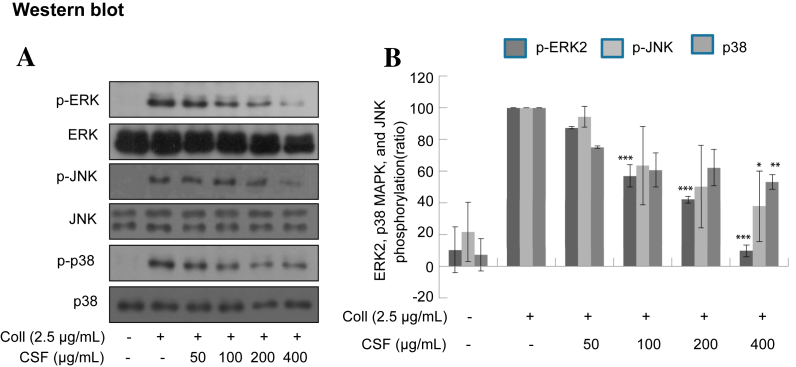
It has been well established that mitogen-activated protein kinases (MAPKs), p38, JNK, and ERK, are acutely, but transiently, activated by collagen in washed platelets. The activation of MAPK plays an important role in the secretion of platelet granules. Here, we determined whether collagen-induced MAPK phosphorylation could be regulated by CSF. As shown in Fig. 4, CSF suppressed MAPK phosphorylation in a concentration-dependent manner. Especially, ERK1/2 phosphorylation was more eminently reduced than others.
Fig. 4.
(A) Effects of crude saponin fraction on collagen-induced phosphorylation of mitogen-activated protein kinases. Washed platelets (3 × 108/mL) were preincubated for 2 min with vehicle or crude saponin fraction at the indicated concentration. The platelets were then stimulated with 2.5 μg/mL collagen for 5 min at 37°C. After terminating the reactions, total cell proteins were extracted. The proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were then probed with antibodies against phospho-p44/42, p44/42, phospho-p38, p38, phospho-SAPK/JNK, and SAPK/JNK (JNK). Antibody binding was visualized by chemiluminescence. All immunoblots are representative of three or four independent experiments. (B) The bar graph shows that the density of specific band was measured and the data are presented as the mean +/− standard deviation of three or four independent trials. * p < 0.05, ** p < 0.005, *** p < 0.001 versus vehicle control. Coll, collagen; ERK, extracellular signal-regulated protein kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase.
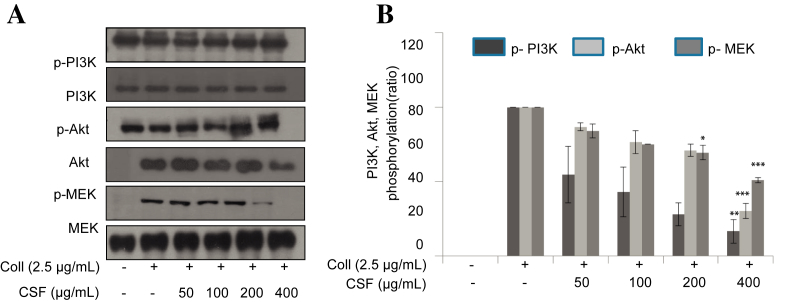
3.5. Inhibitory effect of CSF in the phosphorylation of protein involving PI3K, Akt, and MEK1/2
It is well-known that PI3K and Akt are other important mediators of platelet aggregation. We, therefore, investigated whether CSF modulated PI3K and Akt activation. As shown in Fig. 5, CSF strongly attenuated PI3K and Akt phosphorylation in a concentration-dependent manner. In addition, MEK1/2 is an upstream signaling molecule of MAPK pathway, which is especially required for ERK1/2 activation. CSF significantly reduced collagen-induced ERK phosphorylation. We then analyzed whether CSF inhibits MEK1/2 phosphorylation. As shown in Fig. 5, CSF abolished MEK1/2 phosphorylation in a concentration-dependent manner.
Fig. 5.
Effects of crude saponin fraction on collagen-induced phosphorylation of PI3K, Akt, and MEK1/2. Washed platelets (3 × 108/mL) were preincubated for 2 min with vehicle or crude saponin fraction at the indicated concentration. (A) The platelets were then stimulated with 2.5 μg/mL collagen for 5 min at 37°C. After terminating the reactions, total cell proteins were extracted. The proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were then probed with antibodies against phospho-PI3K, phospho-Akt, and phospho-MEK1/2. Antibody binding was visualized by chemiluminescence. (B) The bar graph shows that the density of specific band was measured and the data are presented as the mean +/− standard deviation of three or four independent trials. * p < 0.05, *** p < 0.001 versus vehicle control. Coll, collagen; MEK, mitogen-activated protein kinase kinase; PI3K, phosphoinositide 3-kinase.
4. Discussion
Although platelet aggregation is important in the process of normal hemostasis in the case of damage to blood vessels, aberrant activation of platelets can cause cardiovascular diseases such as thrombosis, atherosclerosis, and myocardial infarction [21,22]. Therefore, it is worthwhile to develop antiplatelet drugs isolated from natural products. Among them, Korean Red Ginseng is a good candidate for developing antiplatelet agents, because it has been used in traditional oriental medicine and has various efficacies such as anti-inflammatory, antioxidant, antidiabetic, and liver-protection functions. Several ginsenosides isolated from Korean Red Ginseng have been reported to have the function to suppress platelet activation individually [23,24]. In addition, ginsenoside from Korean Red Ginseng was also reported to have preventive effects on cardiovascular diseases. Thus, this study investigated whether CSF in Korean Red Ginseng suppresses the process of platelet aggregation and the underlying molecular mechanism of its antiplatelet action.
Because collagen is a potent agonist for platelet and a component of subendothelial matrix in blood vessel, it was used to induce platelet aggregation. As expected, CSF significantly and dose-dependently impaired collagen-induced platelet aggregation. To elucidate the mechanism of its antiplatelet action, we further analyzed downstream signaling using intracellular calcium concentration, dense granule secretions, protein phosphorylations (e.g., MAPKs and PI3K, Akt), and integrin signaling. A platelet contains approximately two to seven dense granules, each of which enclose various substances and coagulation factors, such as ATP, ADP, and Ca2+ [25,26]. These materials play a crucial role in constricting damaged blood vessels and aggregating platelets. We found that CSF significantly blocked calcium-ion mobilization and ATP secretion to a lesser extent in collagen-activated platelets (Fig. 2). This indicated that CSF impaired dense granule secretion, which is an earlier phase of platelet activation process. By contrast, the activation process of integrin αIIbβ3 proteins called inside-out signaling is the late and final stage of platelet activation and aggregation. Moreover, CSF dose-dependently and significantly inhibited the fibrinogen binding to active integrin αIIbβ3 proteins. The principal consequence of adhesion and activation in platelets is a change in the ligand-binding function of integrin αIIbβ3, which leads to aggregation. This is mediated by adhesive substrates bound to the membranes of activated platelets. Therefore, our results indicated that CSF impaired both the earlier and later phases of platelet activation process.
By contrast, the phosphorylation of signaling molecules, including MAPKs (i.e., ERK1/2, p38 MAPK, and JNK) and PI3K/Akt are important steps for outside-in signaling and inside-out signaling [27,28]. We investigated collagen-induced MAPK phosphorylation and found that ERK, p38, and JNK are inhibited by CSF concentration-dependent platelet aggregation. Although the physiological roles of ERK, p38, and JNK are not yet clear, results of many studies have confirmed that these are associated with the pathway of integrin αIIbβ3 and PLA2/TXA2 [29,30]. In addition, CSF blocked collagen-induced PI3K/Akt phosphorylation. The PI3K/Akt signaling pathway is significant in platelet activation and aggregation processes. The activation of platelet membrane receptor induces the phosphorylation of PI3K and Akt. Furthermore, it was reported that PI3K signaling is associated with the temporary activation of integrin αIIbβ3. Our results show that collagen-induced PI3K/Akt phosphorylation was blocked by CSF. In addition, CSF inhibited PI3K activity by direct enzyme assay, suggesting that the inhibitory acting point of CSF could be PI3K in the platelet activation and aggregation.
In conclusion, CSF inhibited collagen-induced platelet aggregation in a concentration-dependent manner. By analyzing downstream signaling pathway, we found that CSF blocked granule secretion and fibrinogen binding to active integrin αIIbβ3. In addition, we found that CSF impaired PI3K/Akt phosphorylation and directly blocked PI3K enzyme activity, which is the putative acting point of antiplatelet characterization. Finally, CSF has a potent antiplatelet activity, suggesting it as a potent natural drug without side effects in the therapeutic treatment of aberrant platelet activation-related disorders, including thrombosis, atherosclerosis, and stroke.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
This research was supported by a grant (2013) from the Korean Society of Ginseng Funded by the Korean Ginseng Corporation, and by the National Research Foundation of Korea grant funded by the Korean government (MSIP; Grant No. 2011-0018829).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Schwartz S.M., Heimark R.L., Majesky M.W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70:1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J., Irvine R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 3.Jennings L.K. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103:4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Pasqui A.L., Capecchi P.L., Ceccatelli L., Mazza S., Gistri A. Laghi Pasini F, Di Perri T. Nitroprusside in vitro inhibits platelet aggregation and intracellular calcium translocation. Effect of haemoglobin. Thromb Res. 1991;61:113–122. doi: 10.1016/0049-3848(91)90238-r. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980;287:863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- 6.Patrono C., Coller B., FitzGerald G.A., Hirsh J., Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt D.L., Topol E.J. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov. 2003;2:15–28. doi: 10.1038/nrd985. [DOI] [PubMed] [Google Scholar]
- 8.Gum P.A., Kottke-Marchant K., Welsh P.A., White J., Topol E.J. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 9.Eisert W.G. How to get from antiplatelet to antithrombotic treatment. Am J Ther. 2001;8:443–449. doi: 10.1097/00045391-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Rezkalla S.H., Benz M. Antiplatelet therapy from clinical trials to clinical practice. Clin Med Res. 2003;1:101–104. doi: 10.3121/cmr.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu F.B. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr. 2003;78:544S–551S. doi: 10.1093/ajcn/78.3.544S. [DOI] [PubMed] [Google Scholar]
- 12.Jia L., Zhao Y., Liang X.J. Current evaluation of the millennium phytomedicine-ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.K., Park J.H. Trends in ginseng research in 2010. J Ginseng Res. 2011;35:389–398. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura Y., Okuda H., Arichi S. Effects of various ginseng saponins on 5-hydroxytryptamine release and aggregation in human platelets. J Pharm Pharmacol. 1988;40:838–843. doi: 10.1111/j.2042-7158.1988.tb06285.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuo S.C., Teng C.M., Lee J.C., Ko F.N., Chen S.C., Wu T.S. Antiplatelet components in Panax ginseng. Planta Med. 1990;56:164–167. doi: 10.1055/s-2006-960916. [DOI] [PubMed] [Google Scholar]
- 16.Lee W.M., Kim S.D., Park M.H., Cho J.Y., Park H.J., Seo G.S., Rhee M.H. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: critical roles of ERK2 and cAMP. J Pharm Pharmacol. 2008;60:1531–1536. doi: 10.1211/jpp/60.11.0015. [DOI] [PubMed] [Google Scholar]
- 17.Lee D.H., Cho H.J., Kang H.Y., Rhee M.H., Park H.J. Total saponin from Korean Red Ginseng inhibits thromboxane A2 production associated microsomal enzyme activity in platelets. J Ginseng Res. 2012;36:40–46. doi: 10.5142/jgr.2012.36.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D.H., Cho H.J., Kim H.H., Rhee M.H., Ryu J.H., Park H.J. Inhibitory effects of total saponin from Korean Red Ginseng via vasodilator-stimulated phosphoprotein-Ser(157) phosphorylation on thrombin-induced platelet aggregation. J Ginseng Res. 2013;37:176–186. doi: 10.5142/jgr.2013.37.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh W.J., Endale M., Park S.C., Cho J.Y., Rhee M.H. Dual roles of quercetin in platelets: phosphoinositide-3-kinase and MAP kinases inhibition, and cAMP-dependent vasodilator-stimulated phosphoprotein stimulation. Evid Based Complement Alternat Med. 2012;2012:485262. doi: 10.1155/2012/485262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaeffer J., Blaustein M.P. Platelet free calcium concentrations measured with fura-2 are influenced by the transmembrane sodium gradient. Cell Calcium. 1989;10:101–113. doi: 10.1016/0143-4160(89)90050-x. [DOI] [PubMed] [Google Scholar]
- 21.Park H.J., Lee J.H., Song Y.B., Park K.H. Effects of dietary supplementation of lipophilic fraction from Panax ginseng on cGMP and cAMP in rat platelets and on blood coagulation. Biol Pharm Bull. 1996;19:1434–1439. doi: 10.1248/bpb.19.1434. [DOI] [PubMed] [Google Scholar]
- 22.Jung K.Y., Kim D.S., Oh S.R., Lee I.S., Lee J.J., Park J.D., Kim S.I., Lee H.K. Platelet activating factor antagonist activity of ginsenosides. Biol Pharm Bull. 1998;21:79–80. doi: 10.1248/bpb.21.79. [DOI] [PubMed] [Google Scholar]
- 23.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 24.Radad K., Gille G., Liu L., Rausch W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 25.Israels S.J., Gerrard J.M., Jacques Y.V., McNicol A., Cham B., Nishibori M., Bainton D.F. Platelet dense granule membranes contain both granulophysin and P-selectin (GMP-140) Blood. 1992;80:143–152. [PubMed] [Google Scholar]
- 26.Unsworth A.J., Smith H., Gissen P., Watson S.P., Pears C.J. Submaximal inhibition of protein kinase C restores ADP-induced dense granule secretion in platelets in the presence of Ca2+ J Biol Chem. 2011;286:21073–21082. doi: 10.1074/jbc.M110.187138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam F., Kauskot A., Rosa J.P., Bryckaert M. Mitogen-activated protein kinases in hemostasis and thrombosis. J Thromb Haemost. 2008;6:2007–2016. doi: 10.1111/j.1538-7836.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 28.Adam F., Kauskot A., Nurden P., Sulpice E., Hoylaerts M.F., Davis R.J., Rosa J.P., Bryckaert M. Platelet JNK1 is involved in secretion and thrombus formation. Blood. 2010;115:4083–4092. doi: 10.1182/blood-2009-07-233932. [DOI] [PubMed] [Google Scholar]
- 29.Calderwood D.A. Integrin activation. J Cell Sci. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 30.Ruggeri Z.M. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]