Abstract
Background
Ginseng total saponin (GTS) contains various ginsenosides. These ginsenosides are widely used for treating cardiovascular diseases in Asian communities. The aim of this study was to study the effects of GTS on cardiac injury after global ischemia and reperfusion (I/R) in isolated guinea pig hearts.
Methods
Animals were subjected to normothermic ischemia for 60 minutes, followed by 120 minutes of reperfusion. GTS significantly increased aortic flow, coronary flow, and cardiac output. Moreover, GTS significantly increased left ventricular systolic pressure and the maximal rate of contraction (+dP/dtmax) and relaxation (−dP/dtmax). In addition, GTS has been shown to ameliorate electrocardiographic changes such as the QRS complex, QT interval, and RR interval.
Results
GTS significantly suppressed the biochemical parameters (i.e., lactate dehydrogenase, creatine kinase-MB fraction, and cardiac troponin I levels) and normalized the oxidative stress markers (i.e., malondialdehyde, glutathione, and nitrite). In addition, GTS also markedly inhibits the expression of interleukin-1β (IL-1β), IL-6, and nuclear factor-κB, and improves the expression of IL-10 in cardiac tissue.
Conclusion
These data indicate that GTS mitigates myocardial damage by modulating the biochemical and oxidative stress related to cardiac I/R injury.
Keywords: antioxidative enzymes, cardioprotection, hemodynamics, myocardial infarction, Panax ginseng
1. Introduction
According to the World Health Organization, myocardial infarction (MI) is the leading cause of mortality worldwide [1]. The primary manifestations of myocardial ischemia and reperfusion (I/R) are myocyte death and contractile dysfunction [2,3]. Numerous studies have suggested that treatment with cardioprotective drugs can significantly reduce MI [4,5]. Recently, traditional herbs have been suggested to improve heart disease [6,7].
Panax ginseng Meyer belongs to the Araliaceae family and is a perennial herbal medicine [8]. Ginseng has been used as a folk medicine for several thousand years: P. ginseng is extensively used for preventing cardiovascular diseases in Asian countries [9,10]. We had previously reported that ginseng total saponin (GTS) protects against I/R-induced injury in isolated rat hearts [11]. However, to our knowledge, there is no study that evaluated the effects of GTS in guinea pigs. Therefore, this study was designed to examine the effects of GTS on I/R injury in isolated guinea pig hearts. The primary aim of this study was to evaluate hemodynamic functions such as aortic flow, coronary flow, and cardiac output and, in particular, the left ventricular systolic pressure (LVSP) and the maximal rate of contraction (+dP/dtmax) and relaxation (−dP/dtmax). We also aimed to determine whether GTS reduces abnormal electrocardiographic (ECG) changes such as QRS complex, QT interval, and RR interval, and whether GTS inhibits the levels of biochemical markers such as lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB), cardiac troponin I (cTnI), and malondialdehyde (MDA). In addition, we established whether GTS increases antioxidant parameters, such as glutathione (GSH) and nitrite, and protects cardiomyocytes through the regulation of inflammatory cytokines and nuclear factor-kappa B (NF-κB) activity.
2. Materials and methods
2.1. Animals
Forty-five male Duncan-Hartley guinea pigs weighing 300–350 g were purchased from Samtako (Seoul, Korea) and used in this study. All animals were housed in colony cages at an ambient temperature of 22 ± 2°C and humidity of 45 ± 10°C with alternating 12-hour light and dark cycle. All animals had access to standard food and water ad libitum for 1 week prior to the experiment. All animal experiments were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
2.2. Test drugs
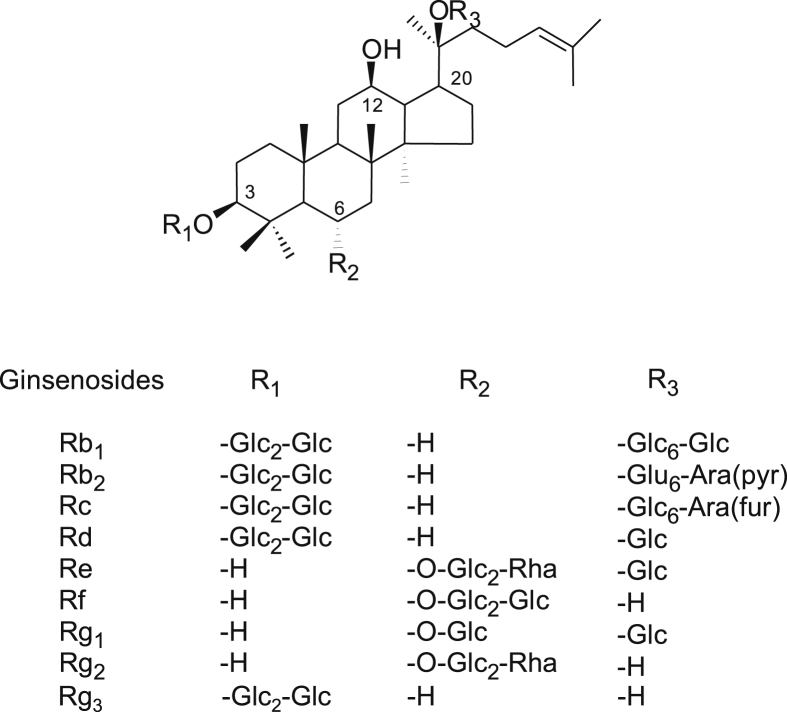
GTS was obtained from Korea Ginseng Corporation (Daejeon, Korea; Fig. 1). Ginsenosides Rg1, Re, Rf, Rh1, Rb1, Rc, Rb2, Rd, Rg3s, and Rg3r were purchased from Chromadex Co. (Irvine, CA, USA) and ginsenosides Rg2s and Rg2r were obtained from Embo Laboratory (Seoul, Korea; Fig. 2). Modified Krebs–Henseleit bicarbonate (KH) solution consisted of NaCl 120.0mM, NaHCO3 25mM, KCl 4.8mM, KH2PO4 1.2mM, CaCl2 1.25mM, MgSO4 1.2mM, and glucose 11.0mM. All reagents were guaranteed grade, and high-performance liquid chromatography-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany).
Fig. 1.
Structures of the nine representative ginsenosides. They differ at three side chains attached to the common steroid ring. Superscript numbers indicate the carbon in the glucose ring that links the two carbohydrates. Ara (pyr), arabinopyranoside; Glc, glucopyranoside; Rha, rhamnopyranoside.
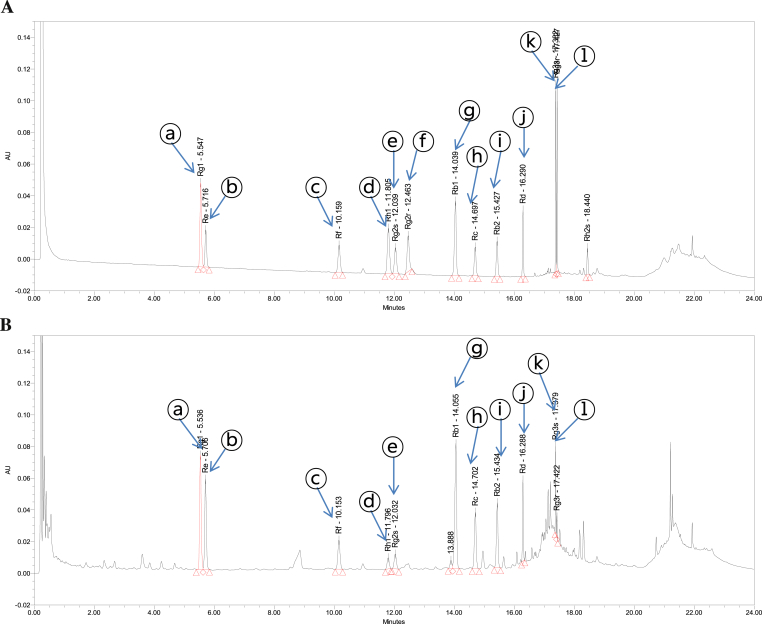
Fig. 2.
Ultraperformance liquid chromatography chromatogram of the (A) ginsenosides and (B) ginseng total saponin. Peak identification: ⓐ, Rg1; ⓑ, Re; ⓒ, Rf; ⓓ, Rh1; ⓔ, Rg2s; ⓕ, Rg2r; ⓖ, Rb1; ⓗ, Rc; ⓘ, Rb2; ⓙ, Rd; ⓚ, Rg3s; and ⓛ, Rg3r.
2.3. Preparation of GTS and liquid chromatography
The ultraperformance liquid chromatography was performed using a Waters ACQUITY system equipped with a binary solvent delivery pump, an auto sampler, and a photodiode array detector. The mobile phase consisted of acetonitrile and water at a flow rate of 0.6 mL/min. Gradient elution was as follows: isocratic elution with 15% for 0.5 minutes, followed by a 14-minute gradient to 30%, 2 2-minute gradients to 40%, 3.5-minute gradient minutes to 90%, then isocratic elution with 90% in for 2 minutes, and then finally returned to 15% in 4 minutes.
2.4. Experimental protocols
The animals were divided into the following five groups (n = 9 in each group): Normal control (N/C), guinea pigs orally received 0.2% (vol/vol) starch in tap water once daily for 14 days and their hearts were not subjected to I/R; GTS control, animals were fed with 200 mg/kg GTS orally once per day for 14 days and their hearts were not subjected to I/R; I/R control, animals received 0.2% starch for 14 days and then I/R was induced for 60 minutes and 120 minutes, respectively; 100GTS + I/R, animals were treated with 100 mg/kg GTS for 14 days and then I/R was induced for 60 minutes and 120 minutes, respectively; 200GTS + I/R, animals received 200 mg/kg GTS and then I/R was induced for 60 minutes and 120 minutes, respectively. At the end of reperfusion, the coronary effluents and left ventricle tissues were quickly frozen and fixed in 10% formalin (−80°C) for biochemical examination, respectively.
2.5. Preparation of isolated heart
After GTS administration for 14 days, animals were anesthetized with pentobarbital (30 mg/kg) intraperitoneally. The heart was excised as described previously [12]. In brief, standard perfusion was carried out at 37°C with a modified KH solution. The veins entering the right atrium were ligated, so that coronary sinus effluent passed into the right ventricle and was ejected through the pulmonary artery. Coronary flow was continuously recorded with a flow meter. The left atrium was cannulated through an opening by uniting the pulmonary orifices. This allowed for the natural filling and contraction of the left atrial appendage [13].
2.6. Measurement of hemodynamic parameters
To evaluate the effects of GTS, hemodynamic data after reperfusion were compared for changes in aortic flow, coronary flow, cardiac output, and LVSP. Aortic flow was measured by the flow volume ejected from the cannula located 100 cm above the heart. Coronary flow was measured by the collection of perfusate from the pulmonary trunk (mL/min). Cardiac output was evaluated by summing the aortic and coronary effluents. LVSP was evaluated by a pressure transducer connected to the aortic cannula. In this study, the maximal rate of contraction (+dP/dtmax) and relaxation (−dP/dtmax) are considered indices of ventricular contractility [14]. The +dP/dtmax and −dP/dtmax values were analyzed at 30-minute intervals for reperfusion.
2.7. Preparation of ECG recording
For ECG recording, electrodes were placed on the epicardial surface and signals from electrodes were amplified by an electric amplifier (AB-621G; Nihon Kohden, Tokyo, Japan), evaluated on a computer (PC-9801VX; NEC, Tokyo, Japan) through an A/D converter (Analog-Pro Jr., Canopus Electric, Kobe, Japan), and analyzed with WAVE MASTER II and WM Read (Canopus Electric) as described previously [15,16]. If rhythm is irregular during the equilibration, the heart was discarded.
2.8. Biochemical, oxidative stress, and anti-inflammatory assays
The coronary effluent was collected after reperfusion. Myocardial injury was assessed using the LDH level [17], CK-MB activity [18], and cTnI level [19]. Evidence of oxidative stress was evaluated from left ventricle tissue using MDA and GSH analyses. In brief, tissues were homogenized in 0.1M phosphate buffer (pH 7.4) with ULTRA TURRAX homogenizer (IKA T18 basic; Wilmington, NC, USA). The homogenates were centrifuged at 2,000 × g for 10 minutes, and the supernatants were removed and assayed for MDA, GSH, and inflammatory cytokines [i.e., interleukin-1β (IL-1β), IL-6, and IL-10] and NF-κB. The levels of MDA [20], GSH [21], and inflammatory cytokines [22] were measured using the methods referenced. The tissue nitric oxide (NO) levels were correlated with the nitrite levels determined from myocardial homogenates using the diazotization method [23].
2.9. Statistical analysis
All statistics were calculated using SigmaPlot for Windows version 12.0 (SYSTAT Software, Inc., IL, USA). Data were subjected to one-way analysis of variance (ANOVA) and one-way repeated measures ANOVA. If a statistical significance was established, the values from the control group and those from the remaining groups were compared using the Bonferroni t test. For all studies, statistical significance was considered at p < 0.05.
3. Results
3.1. Ultraperformance liquid chromatography analysis for ginsenosides contents
The contents of ginsenosides in GTS were as follows: Rg1, 46.17 mg/g; Re, 38.05 mg/g; Rf, 14.04 mg/g; Rh1, 4.57 mg/g; Rg2s, 7.36 mg/g; Rb1, 83.92 mg/g; Rc, 33.74 mg/g; Rb2, 35.31 mg/g; Rd, 18.67 mg/g; Rg3s, 9.09 mg/g; Rg3r, 2.35 mg/g; in addition, other minor ginsenosides and components were also present (Fig. 2).
3.2. Effect of GTS on the hemodynamics
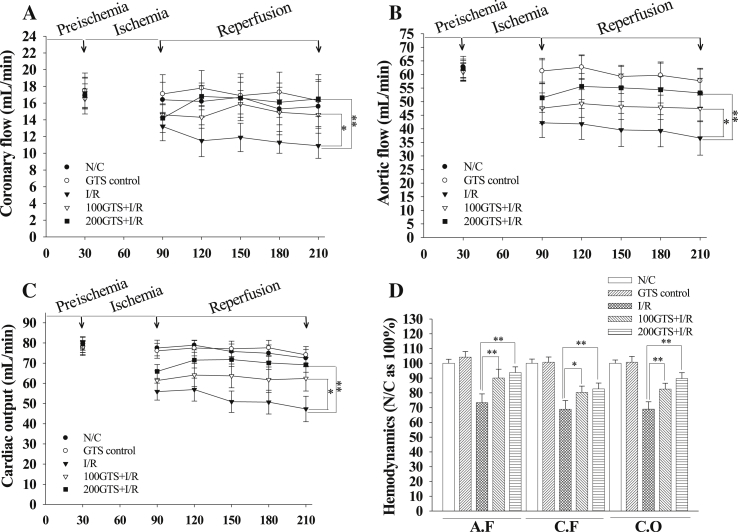
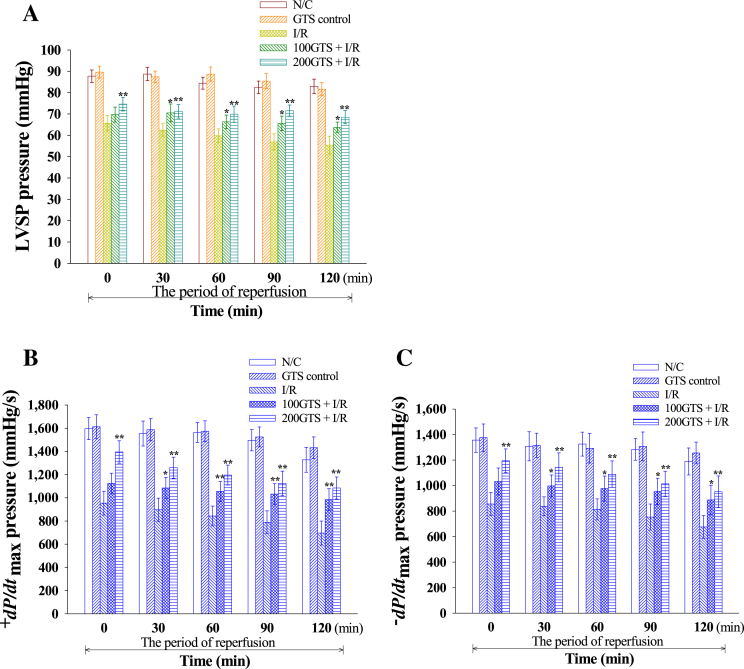
Aortic flow, coronary flow, and cardiac output were significantly decreased by reperfusion for 120 minutes to an average of 68.91 ± 3.81%, 73.75 ± 2.95%, and 68.99 ± 4.97%, respectively. However, administration of GTS increased aortic flow, coronary flow, and cardiac output to an average of 82.54 ± 4.23%, 89.94 ± 3.97%, and 82.56 ± 3.95% in 100GTS + I/R, and to an average of 82.69 ± 3.97%, 93.76 ± 3.84%, and 82.69 ± 3.77% in 200GTS + I/R, respectively (Fig. 3). Furthermore, I/R induction significantly decreased average LVSP values to 60.1 ± 3.5 mmHg as compared with N/C (84.6 ± 3.0 mmHg). However, administration of GTS increased LVSP to an average of 67.6 ± 3.3 mmHg in 100GTS + I/R and to an average of 71.1 ± 3.1 mmHg in 200GTS + I/R, respectively (Fig. 4A). Likewise, compared with an average +dP/dtmax value of 1,507.6 ± 97.4 mmHg in the N/C group, I/R induction resulted in a significant decrease in average +dP/dtmax values to 836.1 ± 97.9 mmHg, but administration of GTS significantly increased the +dP/dtmax values to 1,056.5 ± 89.2 mmHg in 100GTS + I/R and to 1,212.0 ± 94.9 mmHg in 200GTS + I/R, respectively (Fig. 4B). Under the same conditions, the −dP/dtmax values were 1,292.7 ± 98.8 mmHg and 787.3 ± 87.5 mmHg in the N/C and I/R control, respectively. Whereas administration of GTS increased –dP/dtmax to an average of 969.6 ± 101.5 mmHg and 1,078.5 ± 106.8 mmHg in 100GTS + I/R and 200GTS + I/R, respectively (Fig. 4C). As shown in Fig. 5, there was no difference between hemodynamic parameters such as LVSP and ±dP/dtmax between the N/C and GTS control. These results suggest that GTS itself did not influence heart function.
Fig. 3.
Effects of 100 mg/kg and 200 mg/kg ginseng total saponin (GTS) between (A) coronary flow, (B) aortic flow, (C) cardiac output, and (D) average percent for 120-minute reperfusion on these hemodynamic parameters. Each histogram represents the mean ± standard deviation (n = 9). *p < 0.05, **p < 0.01 compared with the ischemia and reperfusion (I/R) group, respectively. N/C, normal control.
Fig. 4.
Effects of 100 mg/kg and 200 mg/kg ginseng total saponin (GTS) on (A) left ventricular systolic pressure (LVSP), (B) +dP/dtmax, and (C) −dP/dtmax. These hemodynamic parameters were estimated at 30-minute intervals throughout the 120-minute reperfusion period. Results were representative of nine independent experiments. Values are expressed as mean ± standard deviation. *p < 0.05, **p < 0.01 compared with ischemia and reperfusion (I/R). N/C, normal control.
Fig. 5.
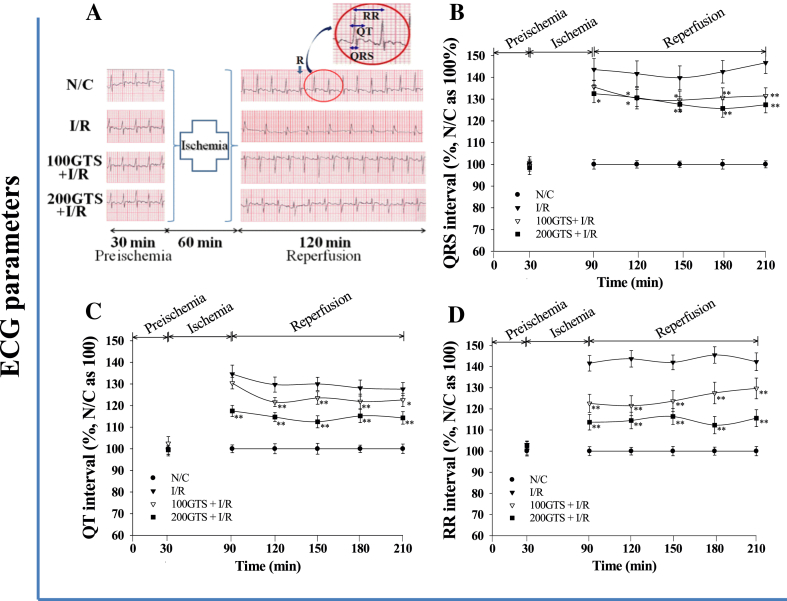
Effects of 100 mg/kg and 200 mg/kg ginseng total saponin (GTS) on representative electrocardiogram tracings. (A) Enlarged electrocardiographic (ECG) patterns such as QRS complex, QT interval, and RR interval are shown (circled area on ECG of the N/C lane). These pictures were representative of ECG patterns in each group. Effects of 100 mg/kg and 200 mg/kg GTS on (B) QRS, (C) QT interval, and (D) RR interval. Values are expressed as mean ± standard deviation for eight independent experiments in each group. *p < 0.05, **p < 0.01 compared with I/R. I/R, ischemia and reperfusion; N/C, normal control.
3.3. Effects of GTS on QRS, QT, and RR intervals
The N/C group represents a normal ECG pattern. The GTS control also did not show any changes in the ECG pattern compared with N/C (Fig. 5A). On ECG patterns in I/R control, the QRS interval, representative of the atrioventricular conduction time, tends to be significantly prolonged compared with N/C (Fig. 5B). In I/R control, the average QRS values were 142.88 ± 5.32% during reperfusion. However, the QRS interval was decreased in 100GTS + I/R and 200GTS + I/R. The average values of the QRS interval were 131.54 ± 4.08% in 100GTS + I/R and 128.76 ± 3.84% in 200GTS + I/R compared with 142.88 ± 5.32% in I/R control. In addition, the average QT interval in I/R control was 130.0 ± 3.47%. Whereas the average values of QT interval were 124.1 ± 2.85% in 100GTS + I/R and 114.84 ± 2.9% in 200GTS + I/R compared with I/R control (i.e., 130.0 ± 3.47%; Fig. 5C). In addition, the average RR interval in the I/R group was 143.02 ± 3.85% compared with 100% in the N/C group. The RR interval in the 100GTS + I/R and 200GTS + I/R groups was shorter than that in the I/R control group (Fig. 5D). Average values of RR interval were 125.0 ± 4.77% and 114.5 ± 3.8% in 100GTS + I/R and 200GTS + I/R, respectively.
3.4. Effects on biochemical, oxidative stress, and inflammatory indicators
In the 100 mg/kg GTS group, the LDH, CK-MB, and cTnI levels were significantly lower than I/R control. The MDA levels in the I/R group were higher than in the N/C group, whereas administration of GTS decreases the MDA level. In addition, the GSH and nitrite levels were significantly increased in 100GTS + I/R and 200GTS + I/R than in I/R control. However, the nitrite levels in 200GTS + I/R were not statistically significantly different from 100GTS + I/R (Table 1). Meanwhile, the expressions of IL-1β, IL-6, and NF-κB were significantly lower in the 100GTS + I/R and 200GTS + I/R groups than in the I/R control group. Moreover, the expression of IL-10 in 100GTS + I/R and 200GTS + I/R was significantly increased compared with I/R control, respectively (Table 2).
Table 1.
Effect of GTS on cardiac markers and oxidative stress, and nitrite levels after I/R-induced myocardial injury
| Periods | Group | LDH (IU/L) | CK-MB (IU/L) | cTnI (mg/L) | MDA (nmol/g protein) | GSH (nmol/g protein) | Nitrite (mmol/g protein) |
|---|---|---|---|---|---|---|---|
| After reperfusion | N/C | 54.5 ± 11.3 | 41.3 ± 7.2 | 1.12 ± 0.13 | 18.7 ± 2.2 | 37.35 ± 1.98 | 264.68 ± 29.2 |
| I/R | 313.8 ± 26.41) | 153.5 ± 13.61) | 4.35 ± 0.241) | 143.8 ± 12.41) | 9.34 ± 2.221) | 119.73 ± 39.81) | |
| 100GTS + I/R | 254.2 ± 32.73) | 114.7 ± 15.83) | 3.56 ± 0.253) | 106.5 ± 19.62) | 14.32 ± 3.872) | 176.3 ± 42.72) | |
| 200GTS + I/R | 186.3 ± 29.83) | 91.5 ± 12.93) | 2.91 ± 0.183) | 95.7 ± 17.43) | 19.65 ± 1.963) | 247.6 ± 32.73) |
Results are expressed as the mean ± standard deviation in each group.
1) Significantly different (p < 0.01) from N/C.
2) Significantly different (p < 0.05) from I/R control.
3) Significantly different (p < 0.01) from I/R control.
CK-MB, creatine kinase-MB; cTnI, cardiac troponin I; GSH, glutathione; GTS, ginseng total saponin; I/R, ischemia and reperfusion; LDH, lactate dehydrogenase; MDA, malondialdehyde; N/C, normal control.
Table 2.
Effect of GTS on inflammation and apoptosis in left ventricle tissues of guinea pig
| Periods | Group | IL-1β (pg/mL) | IL-6 (pg/mL) | IL-10 (pg/mL) | NF-κB (IOD) | Β-actin (IOD) | IOD ratio |
|---|---|---|---|---|---|---|---|
| After reperfusion | N/C | 13.86 ± 3.79 | 5.42 ± 1.97 | 514.86 ± 87.7 | 34,764.52 ± 2,965.7 | 10,263.71 ± 379.65 | 3.17 ± 0.32 |
| I/R | 35.96 ± 6.841) | 16.56 ± 3.751) | 628.63 ± 92.11) | 52,569.91 ± 3,267.11) | 10,735.91 ± 543.721) | 4.97 ± 0.431) | |
| 100GTS + I/R | 26.19 ± 4.522) | 11.07 ± 2.432) | 714.32 ± 106.52) | 48,219.53 ± 3,154.92) | 10,549.17 ± 515.262) | 4.23 ± 0.392) | |
| 200GTS + I/R | 22.62 ± 4.173) | 9.64 ± 2.073) | 784.37 ± 78.23) | 43,854.27 ± 3,269.93) | 10,583.92 ± 437.653) | 3.88 ± 0.353) |
Results are expressed as the mean ± standard deviation in each group.
1) Significantly different (p < 0.01) from N/C.
2) Significantly different (p < 0.05) from I/R control.
3) Significantly different (p < 0.01) from I/R control.
GTS, ginseng total saponin; I/R, ischemia and reperfusion; IL, interleukin; IOD, integrated optical density; N/C, normal control; NF-κB, nuclear factor-kappa B.
4. Discussion
We examined the influence of GTS on cardiac I/R injury on isolated guinea pig hearts and evaluated its cardioprotective effects. A previous report demonstrated that GTS provides cardioprotection against I/R injury in rats [11], but to the best of our knowledge, there is no study that evaluated the effects of GTS in guinea pigs. The results of this study can be divided into three points. First, GTS increases coronary flow, aortic flow, and LVSP in a dose-dependent manner. However, there was no significant difference in cardiac output between 100 mg/kg GTS and 200 mg/kg GTS. The reason for no difference in cardiac output may be attributed to the fact that cardiac output is calculated by the summation of coronary flow and aortic flow. The increase in coronary flow may be due to the “preconditioning-like role” of GTS. Second, GTS inhibited ventricular dysfunction as proved by the improvements in −dP/dtmax, which indicates diastolic function, and +dP/dtmax, which indicates systolic function. In addition, we examined I/R-induced changes in the QRS complex, QT interval, and RR interval. These differences may be due to cardiac injury induced by I/R, because the QT interval indicates the ventricular depolarization and repolarization times [24]. Therefore, these results suggest that I/R injury can change the repolarization time. These differences between QT and RR intervals may have important meaning from a clinical point of view [24]. Administration of GTS decreased the production of pathological ECG patterns, suggesting the cardioprotective action of GTS. Third, the occurrence of I/R injury as a result of various processes leads to the formation of reactive oxygen species (ROS), which causes severe tissue damage [25], eventually leading to cell death [26]. It is well-known that ROS are eliminated by antioxidant mechanisms [25]. In our study, MDA, which is an index of lipid damage by ROS, was found to be decreased in the GTS groups. In addition, GSH provides a defense mechanism against ROS, and in the GTS groups a decrease in ROS production was noted. Decreased levels of LDH, CK-MB, and cTnI in the GTS groups suggested that there is lower cardiac I/R injury. In this study, GTS also increased the nitrite level, which is an index of NO production [27]. The increased nitrite level might induce cardioprotective effects by a preconditioning role related to NO production [9]. Furthermore, administration of GTS elevated the expression of IL-10, anti-inflammatory cytokine, and inhibited the activation of some proinflammatory cytokines including IL-1β and IL-6. NF-κB serves as an upstream signaling factor to modulate the expression of apoptotic genes [22]. Yang et al [28] found that the NF-κB mediated apoptosis, which was a crucial parameter for cardiac injury. In addition, it was reported that inhibition of NF-κB increases the myocardial function [22]. In this regard, we found that GTS decreased the expression of NF-κB against I/R injury. This result was consistent with those reported previously [5,29]. Taken together, it can be suggested that the beneficial influences of inflammatory cytokines and NF-κB play a role in myocardial protection. In conclusion, the results of this study suggest a cardioprotective effect for GTS.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012R1A1A4A01011658) and, this work was supported by the 2012 grant from the Korean Society of Ginseng.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Ostadal B. The past, the present and the future of experimental research on myocardial ischemia and protection. Pharmacol Rep. 2009;61:3–12. doi: 10.1016/s1734-1140(09)70002-7. [DOI] [PubMed] [Google Scholar]
- 2.Gross G.J., Kersten J.R., Warltier D.C. Mechanisms of postischemic contractile dysfunction. Ann Thorac Surg. 1999;68:1898–1904. doi: 10.1016/s0003-4975(99)01035-8. [DOI] [PubMed] [Google Scholar]
- 3.Verma S., Fedak P.W., Weisel R.D., Butany J., Rao V., Maitland A., Li R.K., Dhillon B., Yau T.M. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–2336. doi: 10.1161/01.cir.0000016602.96363.36. [DOI] [PubMed] [Google Scholar]
- 4.Buja L.M. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim J.H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhar M.L., Dhar M.M., Dhawan B.N., Mehrotra B.N., Ray C. Screening of Indian plants for biological activity. Indian J Exp Biol. 1968;6:232–247. [PubMed] [Google Scholar]
- 7.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart diseases. The Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 8.Park Y.H., Kim Y.C., Park S.U., Lim H.S., Kim J.B., Cho B.K., Bae H.H. Age-dependent distribution of fungal endophytes in Panax ginseng roots cultivated in Korea. J Ginseng Res. 2012;36:327–333. doi: 10.5142/jgr.2012.36.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H., Hou S.Z., Luo P., Zeng B., Wang J.R., Wong Y.F., Jiang Z.H., Liu L. Ginseng protects rodent hearts from acute myocardial ischemia-reperfusion injury through GR/ER-activated RISK pathway in an endothelial NOS-dependent mechanism. J Ethnopharmacol. 2011;17;135:287–298. doi: 10.1016/j.jep.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Kim T.H., Lee S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48:1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Kim J.H. Protective roles of ginseng saponin in cardiac ischemia and reperfusion injury. J Ginseng Res. 2009;33:283–293. [Google Scholar]
- 12.Massoudy P., Becker B.F., Gerlach E. Bradykinin accounts for improved postischemic function and decreased glutathione release of guinea pig heart treated with the angiotensin-converting enzyme inhibitor ramiprilat. J Cardiovasc Pharmacol. 1994;23:632–639. doi: 10.1097/00005344-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Massoudy P., Beblo S., Raschke P., Zahler S., Becker B.F. Influence of intact left atrial appendage on hemodynamic parameters of isolated guinea pig heart. Eur J Med Res. 1998;3:470–474. [PubMed] [Google Scholar]
- 14.Guo L., Dong Z., Guthrie H. Validation of a guinea pig Langendorff heart model for assessing potential cardiovascular liability of drug candidates. J Pharmacol Toxicol Methods. 2009;60:130–151. doi: 10.1016/j.vascn.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Minematsu T., Ohtani H., Yamada Y., Sawada Y., Sato H., Iga T. Quantitative relationship between myocardial concentration of tacrolimus and QT prolongation in guinea pigs: pharmacokinetic/pharmacodynamic model incorporating a site of adverse effect. J Pharmacokinet Pharmacodyn. 2001;28:533–554. doi: 10.1023/a:1014460404352. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani H., Hanada E., Yamamoto K., Sawada Y., Iga T. Pharmacokinetic pharmacodynamic analysis of the electrocardiographic effects of terfenadine and quinidine in rats. Biol Pharm Bull. 1996;19:1189–1196. doi: 10.1248/bpb.19.1189. [DOI] [PubMed] [Google Scholar]
- 17.Asha S., Radha E. Effect of age and myocardial infarction on serum and heart lactic dehydrogenase. Exp Gerontol. 1985;20:67–70. doi: 10.1016/0531-5565(85)90010-5. [DOI] [PubMed] [Google Scholar]
- 18.Gerhardt W., Ljungdahl L., Herbert A.K. Troponin T and CK-MB (mass) in early diagnosis of ischemic myocardial injury. Clin Biochem. 1993;26:231–240. doi: 10.1016/0009-9120(93)90122-m. [DOI] [PubMed] [Google Scholar]
- 19.Bertinchant J.P., Larue C., Pernel I., Ledermann B., Fabbro-Peray P., Beck L., Calzolari C., Trinquier S., Nigond J., Pau B. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin Biochem. 1996;29:587–594. doi: 10.1016/s0009-9120(96)00105-1. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Beutler E. The glutathione instability of drug sensitive red cells: a new method for the in vitro detection of drug sensitivity. J Lab Clin Med. 1957;49:84–95. [PubMed] [Google Scholar]
- 22.Chen Z., Wu Z., Huang C., Zhao Y., Zhou Y., Zhou X., Lu X., Mao L., Li S. Effect of lipoxin A4 on myocardial ischemia reperfusion injury following cardiac arrest in a rabbit model. Inflammation. 2013;36:468–475. doi: 10.1007/s10753-012-9567-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang G.F., Satake M., Horita K. Spectrophotometric determination of nitrate and nitrite in water and some fruit samples using column preconcentration. Talanta. 1998;46:671–678. doi: 10.1016/s0039-9140(97)00325-1. [DOI] [PubMed] [Google Scholar]
- 24.Yi P., Zhongwei S. Characterization of QT and RR interval series during acute myocardial ischemia by means of recurrence quantification analysis. Med Biol Eng Comput. 2011;49:25–31. doi: 10.1007/s11517-010-0671-5. [DOI] [PubMed] [Google Scholar]
- 25.Kaul N., Siveski-Iliskovic N., Hill M., Slezak J., Singal P.K. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993;30:55–67. doi: 10.1016/1056-8719(93)90008-3. [DOI] [PubMed] [Google Scholar]
- 26.Robicsek F., Schaper J. Reperfusion injury: fact or myth? J Card Surg. 1997;12:133–137. doi: 10.1111/j.1540-8191.1997.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 27.Ikizler M., Erkasap N., Dernek S., Kural T., Kaygisiz Z. Dietary polyphenol quercetin protects rat hearts during reperfusion: enhanced antioxidant capacity with chronic treatment. Anadolu Kardiyol Derg. 2007;7:404–410. [PubMed] [Google Scholar]
- 28.Yang J., Jiang H., Yang J., Ding J.W., Chen L.H., Li S., Zhang X.D. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-κB signaling pathway. Mol Cell Biochem. 2009;330:39–46. doi: 10.1007/s11010-009-0098-1. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem Int. 2011;58:119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]