Abstract
Background
Panax ginseng has been used to prolong longevity and is believed to be useful for improving skin complexion. Ginsenosides are the most active components isolated from ginseng, and ginsenoside Rg3 (G-Rg3) in particular has been demonstrated to possess antioxidative, antitumorigenic, and anti-inflammatory properties. The aim of this study was to examine the ability of G-Rg3 to inhibit melanogenesis.
Methods
The effects of G-Rg3 on melanin contents and the protein levels of tyrosinase, microphthalmia-associated transcription factor (MITF), and tyrosinase-related protein 1 (TRP1) were evaluated. Melanogenesis-regulating signaling molecules such as Akt and extracellular signal-regulated kinase (ERK) were also examined to explore G-Rg3-induced antimelanogenic mechanisms.
Results
G-Rg3 was found to significantly inhibit the synthesis of melanin in normal human epidermal melanocytes and B16F10 cells in a dose-dependent manner. The activity of cellular tyrosinase and the expression of MITF, tyrosinase, and TRP1 were all reduced, whereas ERK was strongly activated. PD98059 (a specific inhibitor of ERK) attenuated the G-Rg3-induced inhibition of melanin synthesis and tyrosinase activity.
Conclusion
Taken together, these results showed that G-Rg3 induces the activation of ERK, which accounts for its antimelanogenic effects. G-Rg3 may be a promising safe skin-whitening agent, adding to the long list of uses of P. ginseng for the enhancement of skin beauty.
Keywords: antimelanogenic effect, ginsenoside Rg3, Panax ginseng
1. Introduction
Melanin is the pigment responsible for skin and hair color that is synthesized in melanosomes by melanocytes. Although melanin plays an important protective role against UV light, melanin overproduction and accumulation can cause serious cutaneous pigmentary disorders such as freckles, melasma, and age spots [1,2]. Thus, the inhibition of melanogenesis has been the focus of medicinal and cosmetic treatments for skin pigmentary diseases [1,2]. Although most of previous antimelanogenesis experiments were carried out in B16 melanoma cells, reduction of melanin activity in human normal epidermal melanocytes should be demonstrated to confirm the effect of possible whitening agents in human skin [3–5]. Melanogenesis is regulated by the key enzyme, tyrosinase, and additional enzymatic proteins such as tyrosinase-related proteins TRP1 and TRP2 [1–4]. Microphthalmia-associated transcriptional factor (MITF) plays a critical role in the regulation of melanin synthesis and transcription of the key enzyme tyrosinase [3–5].
Panax ginseng has been commonly used as an herbal medicine in Asia for more than 2,000 years and currently occupies an important place among the tonic remedies used in Oriental medicine. In North America, ginseng species such as Panax quinquefolius represent an important industry for both the domestic and export markets [6,7]. Currently, over 40 ginsenosides have been explored and classified into several types in accordance with their specific chemical structures, such as protopanaxadiols, protopanaxatriols, and oleanolic acids [6–8]. Recently, many investigational studies have shown that ginsenosides are biologically active components in antioxidant, antineoplastic, anti-inflammatory, and biomodulatory processes [7,9–13]. Ginsenoside Rg3 (G-Rg3), a tetracyclic triterpenoid saponin monomer, is the primary bioactive component of ginseng extract and has been reported to have various biological effects, including antioxidant effects that may influence melanogenesis [7,8,14,15]. However, the inhibitory effect of G-Rg3 on melanogenesis has not been reported to date. In this study, we have evaluated the inhibitory effect of G-Rg3 on melanin biosynthesis in B16F10 cells and normal human melanocytes. In addition, the molecular mechanisms underlying the antimelanogenic action of G-Rg3 were further evaluated.
2. Materials and methods
2.1. Chemicals and antibodies
Arbutin, alpha-melanocyte-stimulating hormone (α-MSH), l-3,4-dihydroxyphenylalanine (l-DOPA), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and PD98059 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies recognizing phospho-extracellular signal-regulated kinase (p-ERK, No. 9101) and phospho-AKT (p-AKT, No. 9271) were obtained from Cell Signaling Technology (Danvers, MA, USA). Antityrosinase (H-109), TRP1 (H-90), MITF (H-50), and actin (H-300) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Cell culture and cell viability assay
B16F10 mouse melanoma cells (CRL 6475) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Normal human melanocytes (neonatal/moderately pigmented) were cultured in medium 254 supplemented with human melanocyte growth supplement (Cascade Biologics, Invitrogen, Carlsbad, CA, USA). Melanocytes at between three and seven passages were used for analysis. The melanocyte culture was fed two times weekly and incubated in a humidified atmosphere at 37°C and 5% CO2.
After incubating the cells with 20, 40, 60, 80, or 100μM of G-Rg3 for 48 h at 37°C in an atmosphere containing 5% CO2, MTT was added to each well at one tenth of the volume of media. The cells were incubated at 37°C for 3 h, and dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a spectrophotometer.
2.3. Measurement of melanin content
Normal human melanocytes and B16F10 cells were pretreated with various concentrations (20, 40, and 60μM) of G-Rg3 or with 50 μg/mL arbutin control for 72 h. Cell pellets were then dissolved in a 200 μL aliquot of 1N NaOH in 10% DMSO at 100°C for 30 min and centrifuged at 13,000 rpm for 10 min. The relative melanin content was measured using a microplate reader at 415 nm. The value of each measurement is expressed as a percentage change from the control.
2.4. Tyrosinase activity assay
Tyrosinase activity was estimated by measuring the rate of dopachrome formation from l-DOPA. Cells grown in six-well plates were treated with 200nM α-MSH in the presence of 20, 40, or 60μM G-Rg3 or 50 μg/mL arbutin (control) in DMEM for 72 h. The cells were then washed in ice-cold phosphate-buffered saline and lysed in 150 μL of sodium phosphate buffer (0.1M, pH 6.8) containing 0.5% Triton X-100 and 0.1mM phenylmethanesulfonyl fluoride. The cellular extract was centrifuged at 13,000 rpm for 20 min at 4°C. The tyrosinase substrate, 10 μL of 10mM l-DOPA, was added to 90 μL of the supernatant sample. Dopachrome formation was assayed by measuring the absorbance at 492 nm.
2.5. Western blot analysis
B16F10 cells were cultured in 60-mm diameter dishes with or without 200nM α-MSH and 20, 40, or 60μM G-Rg3. Cell pellets were harvested and lysed using radioimmunoprecipitation assay lysis buffer (EMD Millipore, Billerica, MA, USA). The samples were resolved by 4–20% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and then exposed to the appropriate primary antibodies such as MITF, tyrosinase, TRP1, p-ERK, p-AKT, and β-actin. The proteins were visualized by an enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA) using horseradish peroxidase-conjugated antirabbit secondary antibodies.
2.6. Statistical analysis
The data in this report are representative of three or more experiments under same conditions and are expressed as the mean ± standard error of the mean. Differences between means were tested for significance by one-way analysis of variance test using a statistical software package (SPSS version 18.0; SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Effects of G-Rg3 on cell viability
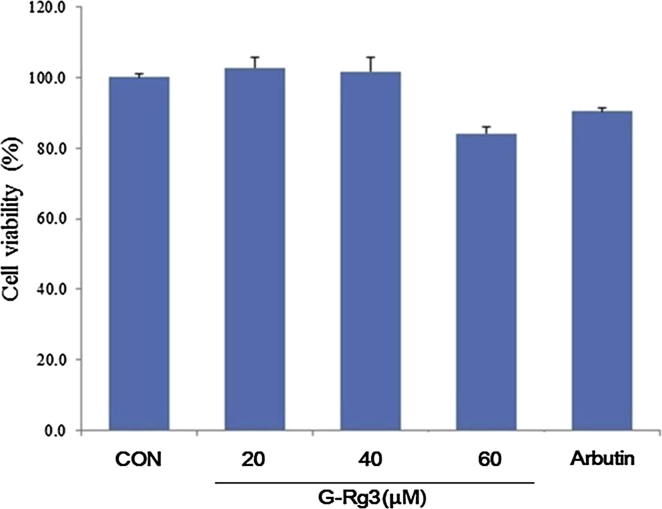
To exclude the possibility that the inhibitory effects of G-Rg3 on melanogenesis are caused by suppression of cell growth, a cell viability test was performed. Treatment with G-Rg3 at concentrations ranging from 20 to 60μM had no cytotoxic effect in B16F10 cells. Based on these results, G-Rg3 concentrations in the range of 20–60μM were used for the remainder of the experiments. In normal human melanocyte, no significant changes in cell viability were found at concentrations as high as 40μM G-Rg3, and more than 80% of cells were alive at 60μM G-Rg3 concentration (Fig. 1).
Fig. 1.
Cell viability assay after ginsenoside Rg3 (G-Rg3) treatment in normal human melanocytes. After incubating cells with 20, 40, or 60μM of G-Rg3 for 48 h at 37°C in an atmosphere containing 5% CO2, 3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide was added to each well at one tenth of the volume of media. Data are expressed as the mean ± standard error of the mean of three independent experiments carried out in triplicate. CON, control cells.
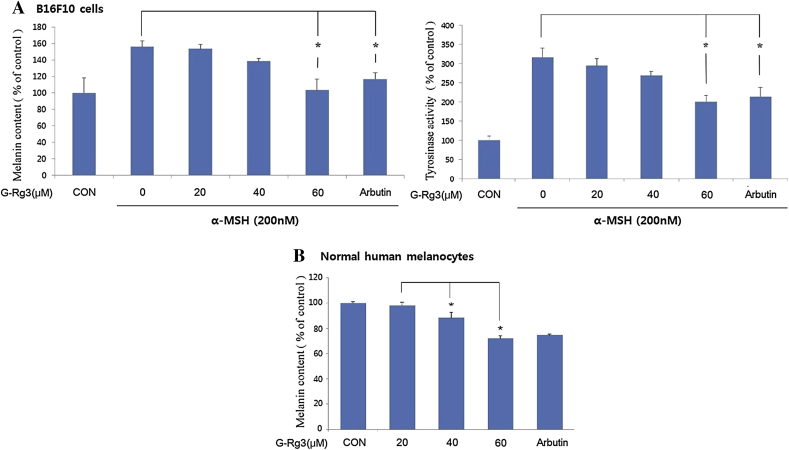
3.2. Inhibition of melanin biosynthesis and tyrosinase activity
To investigate the antimelanogenic mechanism of G-Rg3, cells were exposed to tyrosinase inhibitors α-MSH (200nM) or arbutin (50 μg/mL) in the presence of G-Rg3 (20–60μM), and subsequently, the melanin content and cellular tyrosinase activity of cells were measured. The melanin content was found to be remarkably reduced by the G-Rg3 treatment in a dose-dependent manner in both B16F10 cells (Fig. 2A) and normal human melanocytes (Fig. 2B). At concentration ≥ 40μM, G-Rg3 caused a significant reduction in melanin content in normal human melanocytes (Fig. 2B). Tyrosinase activity also showed a consistent and dose-dependent decrease in response to the G-Rg3 treatment (Fig. 2A). The melanin content and tyrosinase activity in the 60μM G-Rg3-treated and arbutin-treated cells were significantly lower than those in α-MSH-treated cells (p < 0.05). Moreover, the melanin content and tyrosinase activity in 60μM G-Rg3-treated cells were remarkably lower than the levels seen in arbutin-treated cells. These results indicate that G-Rg3 inhibits tyrosinase activity and melanin production without affecting the cellular viability. Moreover, the inhibitory activity of G-Rg3 on melanogenesis is due to inhibition of the melanogenesis pathway involving the key melanogenesis enzyme, tyrosinase.
Fig. 2.
(A) Inhibitory effect of ginsenoside Rg3 (G-Rg3) on melanin synthesis and cellular tyrosinase activity in B16F10 cells. For the cellular tyrosinase activity, cells were exposed to 200nM alpha-melanocyte-stimulating hormone (α-MSH) in the presence of G-Rg3 (20, 40, or 60μM) or arbutin (50 μg/mL) as a positive control for tyrosinase inhibition for 72 h. (B) In normal human melanocytes, G-Rg3 inhibited melanin contents in a dose-dependent manner. Each value for the treated cells is reported as a percentage relative to the control cells (CON; i.e., normal human melanocytes without α-MSH). Data are expressed as the mean ± standard error of the mean of three independent experiments carried out in triplicate. * p < 0.05 compared with α-MSH-treated cells.
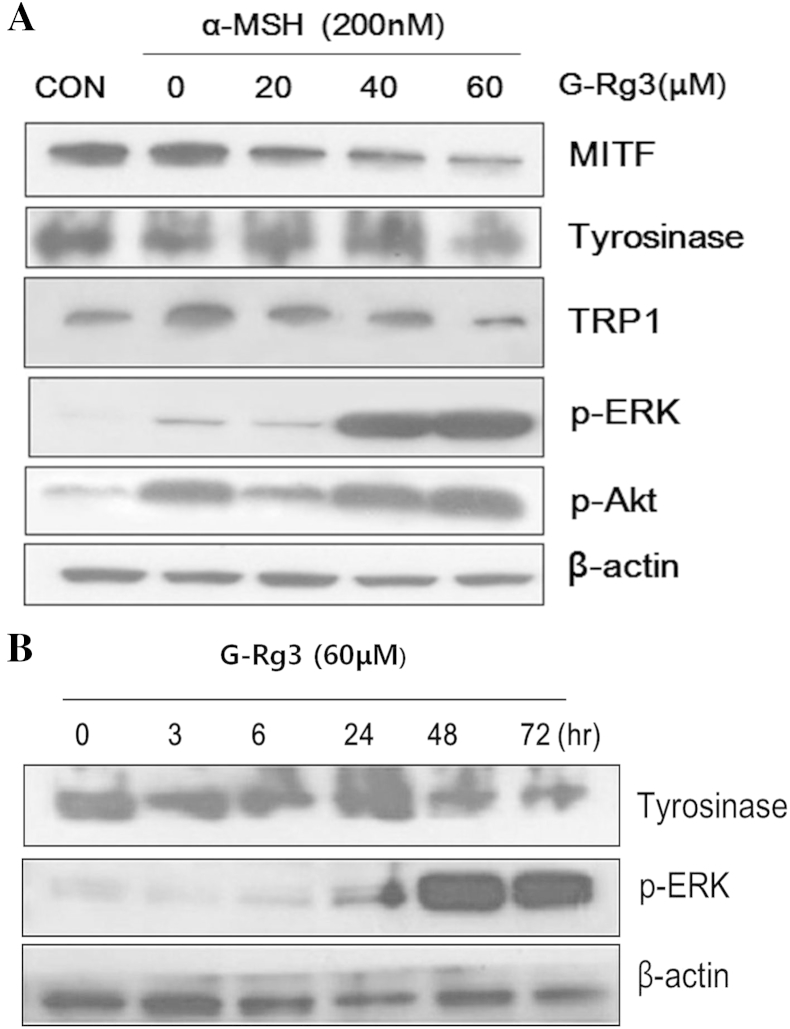
3.3. Effects of G-Rg3 on melanogenic protein expression and intracellular signaling involved in melanogenesis
To determine whether the inhibitory activity of G-Rg3 is related to the melanogenesis pathways involving MITF, tyrosinase, and TRP1, B16F10 cells were treated with G-Rg3, followed by stimulation with α-MSH for 48 h. The cells were then analyzed by Western blot analysis. The protein levels of MITF, tyrosinase, and TRP1 in α-MSH-stimulated cells decreased following treatment with G-Rg3 (Fig. 3A). The decreased expression levels of MITF, tyrosinase, and TRP1 were most prominent following treatment with 60μM G-Rg3, consistent with the melanin content and tyrosinase activity measurements. These results indicate that G-Rg3 suppresses the expression of tyrosinase protein by downregulating the expression of MITF, a master transcription factor of the tyrosinase gene.
Fig. 3.
(A) Effect of ginsenoside Rg3 (G-Rg3) on the expression of melanogenesis-related proteins and intracellular signaling proteins in B16F10 cells. Cells were exposed to 200nM alpha-melanocyte-stimulating hormone (α-MSH) at different doses of G-Rg3 (20, 40, and 60μM) for 48 h. The control cells (CON) were without α-MSH. The protein expression levels of tyrosinase, tyrosinase-related protein 1 (TRP1), microphthalmia-associated transcription factor, phospho-extracellular signal-regulated kinase (p-ERK), and p-AKT were then examined by Western blot analysis. Normalization for loading differences in densitometry values was carried out using β-actin. (B) Time-dependent effect of G-Rg3 on the expression of tyrosinase and p-ERK during the incubation period. Cells were exposed to 60μM G-Rg3 for the indicated times with 200nM α-MSH stimulation, and the protein expression levels of tyrosinase and p-ERK were examined by Western blot analysis. Normalization for loading differences in densitometry values was carried out using β-actin. Note the distinct changes in expression levels of the tyrosinase and p-ERK proteins at 48 and 72 h.
As for the intracellular signaling, ERK and/or Akt activation is well-known to be involved in the regulation of melanogenesis. We then investigated the effects of G-Rg3 on the ERK and AKT signaling pathways. Akt signaling was activated inconsistently. The activation of p-ERK was induced by the G-Rg3 treatment, most notably at concentrations of 40 and 60μM (Fig. 3A). Moreover, serial time-dependent blotting of ERK activation revealed that p-ERK levels were markedly increased 48 h after the α-MSH treatment (Fig. 3B). These results suggest that the suppressive mechanism of G-Rg3 in melanogenesis is related to the activation of ERK signaling.
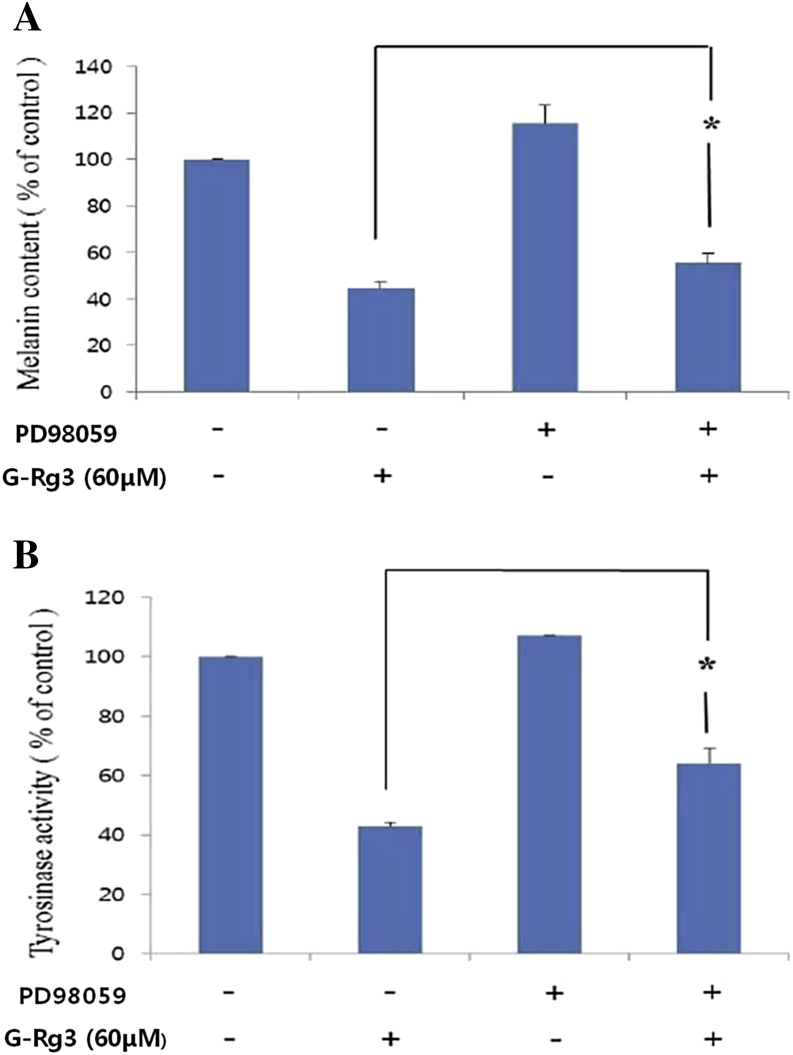
3.4. Role of the ERK pathway in the inhibition of melanin synthesis
We cultured B16F10 cells with or without PD98059 (a specific inhibitor of the ERK pathway) for 72 h to determine whether G-Rg3 inhibits melanogenesis through the ERK pathway. Treatment with PD98059 inhibited the G-Rg3-induced antimelanogenic effect in α-MSH-stimulated cells and led to the recovery of tyrosinase activity and melanin content. The melanin content and tyrosinase activities of cells co-treated with G-Rg3 and α-MSH were significantly increased following treatment with PD98059 (p < 0.05, Fig. 4). These results suggest that G-Rg3-mediated ERK activation contributes to reduced melanin synthesis through MITF and its downstream expression of tyrosinase and TRP1.
Fig. 4.
Effects of PD98059 on melanin synthesis (A) and cellular tyrosinase activity (B) in ginsenoside Rg3 (G-Rg3)-treated B16F10 cells. Cells were exposed to 200nM alpha-melanocyte-stimulating hormone (α-MSH) after pretreatment with or without G-Rg3 (60μM) and PD98059 (20μM). Each treated cell is reported as a percentage relative to that in α-MSH-treated control cells. Data are reported as the mean ± standard error of the mean of three independent experiments carried out in triplicate. * p < 0.05 compared with α-MSH and G-Rg3 co-treated cells.
4. Discussion
The abnormal production and accumulation of melanin on the exposed area such as the face and neck are characteristic of several common hyperpigmentation disorders such as melasma, postinflammatory hyperpigmentation, freckles, uneven skin tone, and solar lentigo. These skin disorders can pose a serious esthetic problem for many people, especially for those in Asian countries. Although many pharmacological skin-whitening agents such as hydroquinone, arbutin, kojic acid, vitamin C, retinol, and azelaic acid, and numerous botanical compounds are available, there is a great interest in identifying other natural skin-whitening agents for safe application [6,16–18]. Although hydroquinone has been the mainstay treatment for hyperpigmentation, its clinical potential has been complicated by a number of adverse reactions including contact dermatitis, irritation, transient erythema, burning or prickling sensations, leukoderma, chestnut spots on the nails, hypochromia, and ochronosis [1,19–22]. As a result, many studies have been conducted in attempts to develop new and safe skin-whitening agents.
Today, natural extracts are a valuable resource for the development of new drugs. Ginseng, which refers to the root of P. ginseng and its related species, has been used for thousands of years in Asian countries and exhibits a wide range of medicinal effects [10–14,23]. The pharmacological effects of ginseng have been demonstrated in the central nervous system as well as in the cardiovascular, endocrine, and immune systems [15,24–28]. In addition, ginseng and its constituents have been ascribed to have antineoplastic, antistress, and antioxidant activities [27–30]. The herb ginseng has many active components, and numerous studies have suggested that ginseng has a variety of beneficial effects. The active constituents found in most ginseng species are ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids. However, most of the pharmacological actions of ginseng are attributed to ginsenosides [7,15,24,27–30]. Approximately 40 ginsenoside compounds have been identified to date, each having different pharmacological effects and underlying mechanisms due to their different chemical structures. Based on their chemical structures, the ginsenosides are generally classified into two groups, namely, the protopanaxadiols and protopanaxatriol groups. The sugar moieties in the protopanaxadiol group attach to position 3 of dammarane-type triterpene such as in Rb1, Rb2, Rc, Rd, Rg3, Rh2, and Rh3, whereas the sugar moieties in the protopanaxatriol group attach to position 6 of dammarane-type triterpene such as in Re, Rf, Rg1, Rg2, and Rh1. Among these compounds, the most commonly studied ginsenosides are Rb1, Rg1, Rg3, Re, Rd, and Rh1 [25–27,30].
G-Rg3, a tetracyclic triterpenoid saponin monomer, is one of the most active ingredients found in ginseng extracts and is reported to possess various biological effects such as antineoplastic, anti-inflammatory, antistress, and antioxidant activities [7,8,14,15,24]. G-Rg3 exerts protective effects attributed to its antioxidant ability by increasing cellular antioxidant enzyme levels and acting as a free-radical scavenger. Moreover, G-Rg3 has been shown to have the strongest antioxidant activity among all ginsenosides [25,26,30]. Oxidative stress caused by excess reactive oxygen species and ultraviolet light is causally linked to skin disorders. Inhibition of reactive species and scavenging of reactive species are thought to reduce hyperpigmentation [28,29]. Antioxidants, such as ascorbic acid derivatives and α-tocopherol, may prevent or delay pigmentation [29].
Because of these potential roles, we hypothesized that G-Rg3 may possess an antimelanogenic effect. To date, there have been no reported scientific studies addressing this possibility. Therefore, we investigated the inhibitory effects of G-Rg3 on α-MSH-induced melanogenesis and the possible underlying molecular mechanisms.
Melanogenesis is regulated by various intracellular signaling mechanisms by the action of protein kinases such as cyclic adenosine monophosphate-dependent protein kinase A, protein kinase C-α, protein kinase C-β, mitogen-activated protein kinase, and phosphatidylinositol 3-kinase (PI3K). Among these, ERK was shown to regulate the expression and function of melanogenic enzymes and MITF [5,31]. Moreover, inhibition of the ERK pathway in B16F10 melanoma cells leads to hyperpigmentation by the upregulation of tyrosinase activity and cellular differentiation. In addition, the PI3K/Akt signaling pathway downregulates the expression of MITF and causes antimelanogenic effect [1,18–21].
Previous studies have also shown that tyrosinase, TRP1, and TRP2 are regulated by MITF, which is downregulated by both ERK and AKT activation [1,19–21]. Our present results show that G-Rg3 downregulates the expression of MITF, tyrosinase, and TRP1, leading to a reduction in the activity of cellular tyrosinase and melanin content. Furthermore, G-Rg3 induces the phosphorylation of ERK; however, the addition of PD98059, a specific inhibitor of the ERK pathway, attenuates the G-Rg3-induced inhibition of melanin synthesis and tyrosinase activity. Taken together, G-Rg3 inhibits melanogenesis by activating the ERK pathway-mediated suppression of MITF and downstream signaling molecules such as tyrosinase and TRP1. To our knowledge, this is the first report on the inhibitory effects of G-Rg3 on melanin production. These results indicate that G-Rg3 is a potential new and safe skin-whitening agent for enhancing skin beauty and for the treatment of hyperpigmentation disorders.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by a 2010 Samsung Biomedical Research Institute grant (Grant No. C-B0-313-1).
Contributor Information
Sung Eun Chang, Email: csesnumd@gmail.com.
Ga-Young Lee, Email: gyleemd@gmail.com.
References
- 1.Briganti S., Camera E., Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16:101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 2.Böhm M., Luger T.A. Alpha-melanocyte-stimulating hormone. Its current significance for dermatology. Hautarzt. 2004;55:436–445. doi: 10.1007/s00105-004-0729-0. [Article in German] [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi Y., Hearing V.J. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35:193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.S., Hwang E.S., Lee J.E., Kim S.Y., Kwon S.B., Park K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J Cell Sci. 2003;116:1699–1706. doi: 10.1242/jcs.00366. [DOI] [PubMed] [Google Scholar]
- 6.Weber D.A., Wheat J.M., Currie G.M. Cancer stem cells and the impact of Chinese herbs, isolates and other complementary medical botanicals: a review. Zhong Xi Yi Jie He Xue Bao. 2012;10:493–503. doi: 10.3736/jcim20120503. [DOI] [PubMed] [Google Scholar]
- 7.Wei X., Chen J., Su F., Su X., Hu T., Hu S. Stereospecificity of ginsenoside Rg3 in promotion of the immune response to ovalbumin in mice. Int Immunol. 2012;24:465–471. doi: 10.1093/intimm/dxs043. [DOI] [PubMed] [Google Scholar]
- 8.Joo S.S., Yoo Y.M., Ahn B.W., Nam S.Y., Kim Y.B., Hwang K.W. Lee do I. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol Pharm Bull. 2008;31:1392–1396. doi: 10.1248/bpb.31.1392. [DOI] [PubMed] [Google Scholar]
- 9.Rivera E., Daggfeldt A., Hu S. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Vet Immunol Immunopathol. 2003;91:19–27. doi: 10.1016/s0165-2427(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 10.Rivera E., Hu S., Concha C. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine. 2003;21:1149–1157. doi: 10.1016/s0264-410x(02)00518-2. [DOI] [PubMed] [Google Scholar]
- 11.Hu S., Concha C., Lin F., Persson Waller K. Adjuvant effect of ginseng extracts on the immune responses to immunization against Staphylococcus aureus in dairy cattle. Vet Immunol Immunopathol. 2003;91:29–37. doi: 10.1016/s0165-2427(02)00264-7. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y.L., Kong X.F., Rui R., Wang D., Li X. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int Immunopharmacol. 2004;4:975–982. doi: 10.1016/j.intimp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Rivera E., Ekholm Pettersson F., Inganäs M., Paulie S., Grönvik K.O. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine. 2005;23:5411–5419. doi: 10.1016/j.vaccine.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.So M.W., Lee E.J., Lee H.S., Koo B.S., Kim Y.G., Lee C.K., Yoo B. Protective effects of ginsenoside Rg3 on human osteoarthritic chondrocytes. Mod Rheumatol. 2013;23:104–111. doi: 10.1007/s10165-012-0635-8. [DOI] [PubMed] [Google Scholar]
- 15.Wei X., Su F., Su X., Hu T., Hu S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia. 2012;83:636–642. doi: 10.1016/j.fitote.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Tu C.X., Lin M., Lu S.S., Qi X.Y., Zhang R.X., Zhang Y.Y. Curcumin inhibits melanogenesis in human melanocytes. Phytother Res. 2012;26:174–179. doi: 10.1002/ptr.3517. [DOI] [PubMed] [Google Scholar]
- 17.Jang J.Y., Lee J.H., Kang B.W., Chung K.T., Choi Y.H., Choi B.T. Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells. Exp Dermatol. 2009;18:232–237. doi: 10.1111/j.1600-0625.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu L.C., Lin Y.Y., Yang S.Y., Weng Y.T., Tsai Y.T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signaling pathways. J Biomed Sci. 2011;18:74. doi: 10.1186/1423-0127-18-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang J.Y., Lee J.H., Jeong S.Y., Chung K.T., Choi Y.H., Choi B.T. Partially purified Curcuma longa inhibits alpha-melanocyte-stimulating hormone-stimulated melanogenesis through extracellular signal-regulated kinase or Akt activation-mediated signalling in B16F10 cells. Exp Dermatol. 2009;18:689–694. doi: 10.1111/j.1600-0625.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim D.S., Lee H.K., Park S.H., Chae C.H., Park K.C. AVS-1357 inhibits melanogenesis via prolonged ERK activation. Pharmazie. 2009;64:532–537. [PubMed] [Google Scholar]
- 21.Olivares C., Solano F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009;22:750–760. doi: 10.1111/j.1755-148X.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- 22.Engasser P.E., Maibach H.I. Cosmetics and dermatology: bleaching creams. J Am Acad Dermatol. 1981;5:143–147. doi: 10.1016/s0190-9622(81)70082-3. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.S., Kim D.S., Kim S.I. Ginsenoside Rh2 and Rh3 induce differentiation of HL-60 cells I into granulocytes: modulation of protein kinase C isoforms during differentiation by ginsenoside Rh2. Int J Biochem Cell Biol. 1998;30:327–338. doi: 10.1016/s1357-2725(97)00141-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z.Q. Chemical insights into ginseng as a resource for natural antioxidants. Chem Rev. 2012;112:3329–3355. doi: 10.1021/cr100174k. [DOI] [PubMed] [Google Scholar]
- 25.Lee T.F., Shiao Y.J., Chen C.F., Wang L.C. Effect of ginseng saponins on beta-amyloid-suppressed acetylcholine release from rat hippocampal slices. Planta Med. 2001;67:634–637. doi: 10.1055/s-2001-17366. [DOI] [PubMed] [Google Scholar]
- 26.Lü J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou T.H., Ding H.Y., Hung W.J., Liang C.H. Antioxidative characteristics and inhibition of alpha-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp Dermatol. 2010;19:742–750. doi: 10.1111/j.1600-0625.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 28.Kang K.S., Yokozawa T., Yamabe N., Kim H.Y., Park J.H. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C A Meyer. Biol Pharm Bull. 2007;30:917–921. doi: 10.1248/bpb.30.917. [DOI] [PubMed] [Google Scholar]
- 29.Kang K.S., Kim H.Y., Baek S.H., Yoo H.H., Park J.H., Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 30.Kang K.S., Yamabe N., Kim H.Y., Okamoto T., Sei Y., Yokozawa T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol. 2007;113:225–232. doi: 10.1016/j.jep.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 31.Kim D.S., Kim S.Y., Chung J.H., Kim K.H., Eun H.C., Park K.C. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell Signal. 2002;14:779–785. doi: 10.1016/s0898-6568(02)00024-4. [DOI] [PubMed] [Google Scholar]