Abstract
Background
Antiallergic effect of 20(S)-protopanaxatriol (PPT), an intestinal metabolite of ginseng saponins, was investigated in guinea pig lung mast cells and mouse bone marrow-derived mast cells activated by a specific antigen/antibody reaction.
Methods
Increasing concentrations of PPT were pretreated 5 min prior to antigen stimulation, and various inflammatory mediator releases and their relevant cellular signaling events were measured in those cells.
Results
PPT dose-dependently reduced the release of histamine and leukotrienes in both types of mast cells. Especially, in activated bone marrow-derived mast cells, PPT inhibited the expression of Syk protein, cytokine mRNA, cyclooxygenase-1/2, and phospholipase A2 (PLA2), as well as the activities of various protein kinase C isoforms, mitogen-activated protein kinases, PLA2, and transcription factors (nuclear factor-κB and activator protein-1).
Conclusion
PPT reduces the release of inflammatory mediators via inhibiting multiple cellular signaling pathways comprising the Ca2+ influx, protein kinase C, and PLA2, which are propagated by Syk activation upon allergic stimulation of mast cells.
Keywords: 20(S)-protopanaxatriol, inflammatory mediators, mast cells, Panax ginseng
1. Introduction
It has been reported that Panax ginseng Meyer exerts a variety of pharmacological effects on the immune system [1]. In the studies with human and rodents, ginseng extracts or various ginseng components exhibited mitogenic activity on T and B lymphocytes [2,3], and immunomodulatory effects in a variety of diseases [4,5]. Ginseng saponin enhances the phagocytic activity of macrophage by increasing the intracellular Ca2+ level ([Ca2+]i)and the protein kinase C activity [6], and ginsenoside Rh1 inhibits cyclooxygenase-2 (COX-2) expression and nuclear factor (NF)-κB activation [7]. However, a person engaged in Korean ginseng wholesale was recently reported to be suffering from occupational asthma [8].
In the inflammatory and allergic responses, protopanaxadiol Rb1 inhibits histamine release and leukotrienes (LTs) in guinea pig lung mast cells (GPMCs) activated by specific antigen/antibody reaction [9]. Ginseng ameliorates chronic histopathologic changes in murine asthma [10]. Ginsan, a polysaccharide derived from P. ginseng, also has tumoricidal activities [11], antiseptic activity [12], and antiasthmatic effects in a mouse OVA (ovalbumin)-induced asthma model [13].
A variety of ginseng saponin metabolites are formed by intestinal bacteria when total ginseng extracts or total ginseng saponin is administered orally to a human or rat, respectively, and absorbed into the systemic circulation [14,15], and then their metabolites including nonsugar moieties of ginsenosides (aglycones) are detected in blood and in urine. Compound K [16] and Rh2 [17] among various ginseng metabolites have potent inhibitory activity on β-hexosaminidase release from RBL-2H3 cells.
Mast cells are well known as major effector cells for immunoglobulin (Ig) E-mediated immediate hypersensitivity and chronic allergic reactions such as asthma. Antigen-specific IgE bound to the high-affinity receptor (FcεRI) on the mast cell membrane encounters the multivalent antigen, and then the cells are activated. Activated mast cells secrete preformed mediators [histamine, tryptase, chymase, tumor necrosis factor (TNF)α, and other proteins] as well as newly synthesized proinflammatory mediators such as prostaglandin D2, LTs, cytokines, and chemokines [18–21]. These mediators have been postulated to be responsible for airway inflammation and remodeling in allergic asthma [19,22].
Although 20(S)-dihydroprotopanaxadiol and 20(S)-dihydroprotopanaxatriol have distinctive immunological responses [23,24], the effects of 20(S)-protopanaxatriol (PPT, Fig. 1A), one of the ginseng saponin metabolites formed by human intestinal bacteria, on the inflammatory mediator release during mast cell activation, which is a well-known event in allergic asthma, has not been reported yet. Therefore, we examined the effects of PPT on the release of inflammatory mediators from the guinea pig lung mast cells (GPMCs) and mouse bone marrow-derived mast cells (BMMCs) activated by a specific antigen/antibody reaction to determine the role of ginseng intestinal metabolites of orally administered ginseng extract.
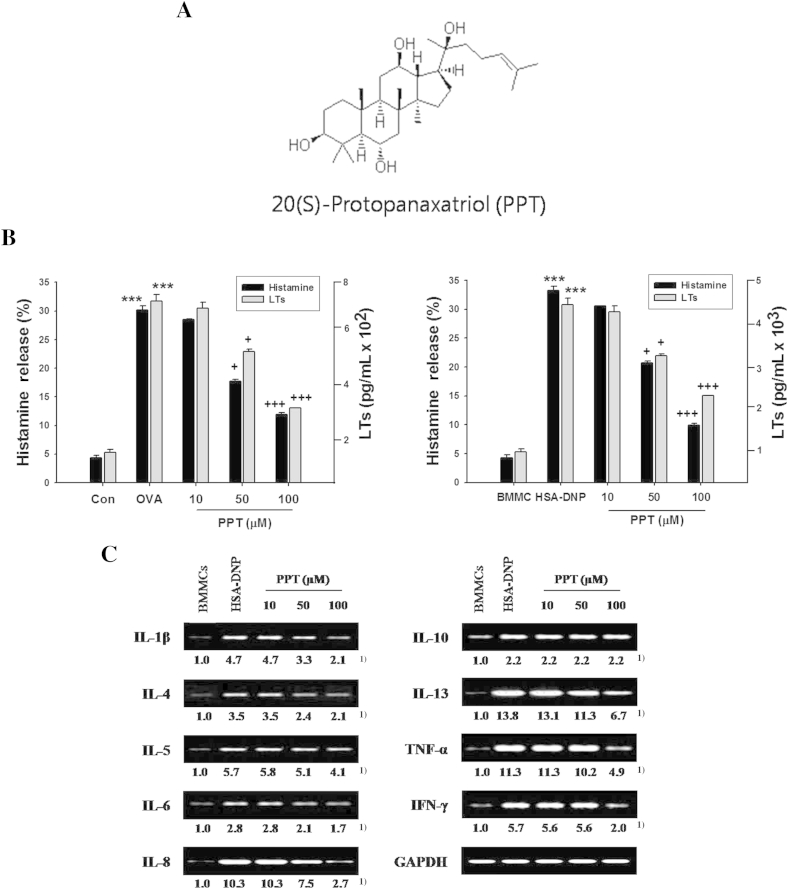
Fig. 1.
Effects of 20(S)-protopanaxatriol (PPT) on the release of mediators or mRNA expression of cytokines in activated mast cells. Guinea pig mast cells (GPMCs; 4 × 105 cells) were sensitized with anti-ovalbumin (OVA) antibody and then stimulated with OVA challenge. Mouse bone marrow-derived mast cells (BMMCs; 1 × 106 cells) were sensitized and challenged with anti-dinitrophenyl immunoglobulin E antibody and human serum albumin–dinitrophenyl (HSA-DNP), respectively, as described in Materials and methods. PPT (10μM, 50μM, or 100μM) was pretreated 5 min prior to the OVA challenge. (A) Chemical structure of PPT. (B) Amounts of histamine and leukotrienes (LTs) released in GPMCs (left panel) and BMMCs (right panel). Histamine release (%) was expressed as the percentage of the total histamine in nonstimulated cells. LTs were determined as pg/L × 106 cells. (C) Expressions of cytokine mRNA in activated BMMCs. The total number of experiments were eight (n = 8). ***, p < 0.001 versus control (Con); +p < 0.05; +++p < 0.05 versus OVA challenge. 1) Numbers below the bands indicate the ratio of band intensity of each band versus that of control and GAPDH mRNA.
2. Materials and methods
2.1. Animals
Female Hartley guinea pigs weighing 200–250 g and female BALB/c mice weighing 20 g (age 8 weeks) were purchased from Samtako BioKorea (Osan, Korea) and maintained in specific pathogen-free conditions prior to being sacrificed. All animals were housed in accordance with the guideline from the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), and all protocols were approved by the Institutional Review Board and conducted in the Laboratory Animal Research Center of Sungkyunkwan University, Suwon, Korea.
2.2. Reagents
OVA (fraction V), collagenase (type I), elastase (type I, porcine pancreatic), human serum albumin–dintrophenyl (HSA-DNP), Fluo-3 AM, and percoll were purchased from Sigma-Aldrich Chemical Co (St Louis, MO, USA); leukotriene immunoassay kit and [3H]arachidonic acid were purchased from PerkinElmer (Waltham, MA, USA); and gelatin was purchased from Difco Laboratories (Detroit, MI, USA). PPT (molecular weight, 476.7) was prepared by the chemical modification of ginseng saponin with periodic acid as described previously [25]. Its structure is shown in Fig. 1A. Other chemicals and reagents used in this experiment were of the best grade.
The PPT was dissolved in 50mM stock solution in dimethyl sulfoxide (DMSO), and diluted prior to use. The percentage (%) of DMSO in experimental solution or media was 0.01%, 0.02%, and 0.04% for 10μM, 50μM, or 100μM PPT, respectively. These percentages of DMSO did not affect release of mediators (data not shown). The concentration of PPT was chosen in preliminary experiments.
2.3. Immunization and preparation of anti-OVA antibody
Ten outbred female guinea pigs were immunized by injecting a mixture of 50 μg/200 μL OVA and complete Freund's adjuvant into a foot pad. After 1 week, 100 μg/200 μL OVA was injected intradermally at one side of the back of the animals and 200 μg/200 μL of OVA at the other side of the back of the animals. One week later, mice were sacrificed for getting sera, and the sera were kept in aliquots at −70°C until use [26]. To separate IgG1 antibody from sera, guinea pig serum was applied to anti-IgG2 affinity column and the column was washed with 0.1M citric acid (pH 2.1). IgG1 passed through and it was concentrated under pressure for the experiment. The titers of anti-OVA antibody were in the range 1,600–3,200 using passive cutaneous anaphylaxis method [26]. The sera were used for preparing the sensitized GPMCs.
2.4. GPMC preparation
GPMCs were isolated and purified as reported previously [26]. Briefly, each lung isolated from eight unsensitized guinea pigs was perfused with 50 mL of the modified Tyrode's buffer (TGCM buffer: 137mM NaCl, 0.36mM NaH2PO4, 2.6mM KCl, 1mM CaCl2, 1.5mM MgCl2, 119mM NaHCO3, 5.5mM glucose, 1 g/L gelatin, pH 7.4). After removing large airways and blood vessels, the lungs were chopped with a McIlwain tissue chopper (The Mickle Laboratory Engineering Co., Gomshall, Surrey, UK). Pooled tissue was treated three times with collagenase (125 U/g tissue) and elastase (5 U/g tissue) for 15 min, 15 min, and 25 min. The freed cells were separated by filtrating through mesh and Nytex mesh (100 μm). The cells were washed with Tyrode's buffer without CaCl2 and MgCl2 containing gelatin (TG buffer) and laid over gradients consisting of 10 mL Percoll (density, 1.045 g/mL), and then centrifuged at 800 × g for 20 min. Pelleted cells containing mast cells (∼3.5 × 108 cells/gradient) were resuspended in TG buffer and applied in discontinuous Percoll (1 mL) density gradients (density gradients: 1.06 g/mL, 1.07 g/mL, 1.08 g/mL, 1.09 g/mL, and 1.10 g/mL) for centrifugation at 800 × g for 20 min. The mast cells were collected with the highest purity and number (1–2 × 107) from the bands between the 1.09 g/mL and 1.10 g/mL densities. This gradient band was removed, washed with TGCM buffer. The partially purified mast cells were identified with Alcian blue staining, and the viability was assessed using trypan blue exclusion assay. The purity of the mast cell preparation was 80–90% and the cell viability was consistently > 98%.
2.5. Culture of BMMCs
Bone marrow cells were flushed from femurs and tibias of BALB/c mice. RBC was lysed using 0.1M NH4Cl and remaining cells were washed, resuspended, and cultured for 5 weeks in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum and 50% WEHI-3B conditioned media, which contains interleukin (IL)-3. The media were changed twice per week. BMMCs were confirmed by May-Grünwald Giemsa staining [27]. Briefly, BMMCs were collected onto object glasses by cytospin (400 × g, 3 min). Cells were fixed in methanol for 2–3 min and then stained with May-Grünwald solution for 15 min, followed by Giemsa solution for 10 min and by washing steps in H2O. The purity of BMMCs was > 99%.
2.6. Sensitization and measurements of mediator release in the activated mast cells
As described previously [26], the partially purified GPMCs (4 × 105) were sensitized with anti-OVA antibody (1 × 106 cells/mL antibody) for 45 min at 37°C in a shaking water bath. The cells were then washed, suspended in TGCM buffer, and challenged with 1.0 μg/mL OVA for 10 min or 20 min for histamine and LT release, respectively. After terminating the reaction on ice bath, supernatant was taken for measuring the amounts of the released histamine and LTs.
BMMCs (1 × 106 cells) were sensitized with anti-DNP IgE antibody (0.1 μg/mL) overnight at 37°C. The cells were washed, and then challenged with 1.0 ng/mL HSA-NP) for time periods indicated (optimal time, 6 h) at 37°C in Tyrode's buffer [27].
In all experiments, optimal time and concentration for activation of GPMCs and BMMCs were first determined in preliminary experiments, and both types of mast cells were treated with PPT (10μM, 50μM, or 100μM) 5 min prior to the antigen challenge.
2.7. Histamine assay
Amounts of histamine release in each supernatant obtained after activation of GPMCs or BMMCs were quantified by the automated fluorometric analyzer (with dialysis; Series 300 Analyzer; Astoria Clackamas, OR, USA). The detection limit of this assay is approximately 5 ng/mL of histamine. Amounts of histamine release were expressed as the percentage of the total histamine in nonstimulated cells [26].
2.8. LT immunoassay
The amounts of LTs in each supernatant obtained from GPMCs or BMMCs were determined using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions. Briefly, 50 μL samples were incubated with anti-LT antiserum (diluted 1:120) and acetylcholinesterase-linked LTs (diluted 1:120) in wells that were coated with mouse monoclonal antibodies for 18 h at room temperature. After rinsing with washing buffer, color was developed using Ellman's reagent, and the plates were read at 412 nm with a spectrophotometer. The concentrations of LTs were then calculated using standard curves generated with specific LTs standards and using analysis tools on the Cayman Chemical website (http://www.caymanchem.com/app/template/analysis%2CEIA.vm/a/z).
2.9. Reverse transcription–polymerase chain reaction
Total cellular RNA was isolated from BMMCs (1 × 106 cells) using Trizol reagent. Reverse transcription–polymerase chain reaction (RT-PCR) was performed in a final volume of 50 μL using a amfiRivert one-step RT-PCR kit (GenDEPOT, Barker, TX, USA) in an automated thermal cycler (BIOER Technology, Hangzhou, China). PCR assays were performed for 35 cycles. Each cycle consisted of the following steps: denaturation at 94°C for 30 s, annealing at 56°C for 45 s, and extension at 72°C for 1 min. PCR products were analyzed using 1.0% agarose gel containing ethidium bromide.
The primer sequences used were as follows: IL-1β sense, 5′-TGA AGG GCT GCT TCC AAA CCT TTG ACC-3′; IL-1β antisense, 5′-TGT CCA TTG AGG TGG AGA GCT TTC AGG-3′; IL-4 sense, 5′-TCG GCA TTT TGA ACG AGG TC-3′; IL-4 antisense, 5′-GAA AAG CCC GAA AGA GTC TC-3′; IL-5 sense, 5′-ATG GAG ATT CCC ATG AGC AC-3′; IL-5 antisense, 5′-GTC TCT CCT CGC CAC ACT TC-3′; IL-6 sense, 5′-TGG AGT CAC AGA AGG AGT GGC TAA G-3′; IL-6 antisense, 5′-TCT GAC CAC AGT GAG GAA TGT CCA C-3′; IL-8 sense, 5′-CAA ACC TTT CCA CCC CAA AT-3′; IL-8 antisense, 5′-ATT GCA TCT GGC AAC CCT AC-3′; IL-10 sense, 5′-CAT GGG TCT TGG GAA GAG AA-3′; IL-10 antisense, 5′-CAT TCC CAG AGG AAT TGC AT-3′; IL-13 sense, 5′-CAG CTC CCT GGT TCT CTC AC-3′; IL-13 antisense, 5′-CCA CAC TCC ATA CCA TGC TG-3′; TNFα sense, 5′-TTA TCT CTC AGC TCC ACG CC-3′; TNFα antisense, 5′-TGC GCA CTG AAA GCA TGA TC-3′; interferon-γ sense, 5′-GCT CTG AGA CAA TGA ACG CT-3; interferon-γ antisense, 5′-AAA GAG ATA ATC TGG CTG TGC-3′; GAPDH sense, 5′-GAT GCA GGG ATG ATG TTC TG-3′; and GAPDH antisense, 5′-GTG AAG GTC GGT AAC GG-3′ [28].
2.10. Measurements of the intracellular Ca2+ level in the activated mast cells
The sensitized GPMCs (4 × 105 cells) or BMMCs (4 × 105 cells) were incubated for 30 min after adding Fluo-3 AM (5μM) and placed on a glass slide treated with poly-l-lysine, and then OVA (1.0 μg/mL) for GPMCs or HSA-DNP for BMMCs was flowed out on a glass slide for stimulation. The cells were treated with PPT (10μM, 50μM, or 100μM) 5 min prior to each antigen challenge (OVA or HSA-DNP). The [Ca2+]i of both types of mast cells were monitored for 10 min after each antigen stimulation using a confocal laser scanning microscopy (Leica TCS NT Confocal Microscopy, Leica, Heidelberg, Germany) [24,26]. [Ca2+]i was estimated with LSM500 software (Mitutoyo America Corporation, Aurora, IL, USA). Relative intensity (RI) indicated the ratio of optical fluorescence density versus the control (RI = 1).
2.11. Immunoprecipitation for Syk
Immunoprecipitation of Syk protein was performed according to method provided previously [29]. Briefly, agarose conjugate (50 μL) was washed twice with washing buffer (PBS, pH 7.4), centrifuged for 10 s at 12,000 × g at room temperature and then resuspended in washing buffer. Agarose conjugate was added to 10 μl anti-Syk antibody (Upstate, Lake Placid, NY, USA), incubated for 60 min at room temperature with gentle mixing, and then centrifuged at 3,000 × g for 2 min at 4°C. Samples were washed with 1 mL washing buffer, centrifuged at 3,000 × g for 2 min at 4°C, and this step was repeated at least twice. Cell lysates (200 μg protein) were added to agarose conjugate-bound antibody, and incubated overnight at 4°C with gentle mixing. Immunoprecipitated complexes were washed with washing buffer and centrifuged at 3,000 × g for 2 min at 4°C. Pellets were washed with 1 mL washing buffer and centrifuged at 3,000 × g for 2 min at 4°C. This step was repeated at least three times. The pellet was resuspended with 25–100 μL Laemmli sample buffer [0.125M Tris HCl (pH 6.8), 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue]. Samples were heated at 95°C for 5 min, centrifuged for 30 s at 12,000 × g at room temperature, and then the supernatants collected (immunoprecipitation sample).
Immunoprecipitation samples were stored in sample buffer at −70°C until assay. Samples and molecular weight standards were run with known concentrations on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and immunoblotting performed.
2.12. Measurements of protein kinase C activity in the activated GPMCs
The sensitized GPMCs (1 × 106 cells) were preincubated in the presence of histone (0.2 mg/mL), phosphatidylserine (40 μg/mL), and [γ-32P] ATP (1μM) at 30°C for 5 min. The cells were treated with 100μM PPT 5 min prior to OVA stimulation. The changes in the protein kinase C (PKC) activity in mast cells were measured at 10 min after 1.0 g/mL OVA stimulation. The reaction was stopped by adding 1 mL of 10% TCA at 4°C for 30 min. The reaction mixtures were filtered through a glass fiber disk (GF/B; Whatman, Maidstone, Kent, UK) to remove unreacted [γ-32P] ATP, and then were washed four times with 20mM tetrasodium pyrophosphate and once with absolute ethanol. After drying the glass fiber disk, radioactivity was measured with a liquid scintillation counter [26].
2.13. Measurements of phospholipase A2 activity in the activated GPMCs
GPMCs (1 × 106 cells) were preincubated with [3H] arachidonic acid (1 μCi) at 37°C for 1 h. The labeled mast cells were washed twice and resuspended in TGCM buffer. The cells were sensitized by anti-OVA antibody (1 × 106 cells/mL antibody) at 37°C for 45 min and then stimulated with 1.0 μg/mL OVA at 37°C for 10 min, and the reaction were stopped by the addition of 1N formic acid. The cells were treated with 100μM PPT 5 min prior to the OVA challenge. The mast cells were then centrifuged at 800 × g for 10 min. Reactivities released in supernatant were counted by adding Aquasol (PerkinElmer, Boston, MA, USA) for liquid scintillation spectrometry [30].
2.14. Immunoblotting for signaling molecules in the activated mast cells
The activated GPMCs (1 × 106 cells) or BMMCs (1 × 106 cells) were homogenized in lysis buffer [10mM HEPES (pH 7.9), 10mM KCl, 0.1mM EDTA, 0.1mM EGTA, 1mM DTT, 0.5mM PMSF, 2.0 μg/mL aprotinin, 2.0 μg/mL leupeptin], and allowed to swell on ice for 10 min. Cell lysates (μg) were subjected to 8% or 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Bioscience, Amersham, Buckinghamshire, UK). Membranes were washed with PBS containing 0.1% Tween 20 (PBST), and then blocked for 1 h in PBST containing 5% skim milk. After the membranes were washed with PBST, they were incubated for 60 min at room temperature with antibodies against Syk, PKC isotypes (α, βI, βII), extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), p38, COX-1/2, secreted phospholipase A2 (PLA2), actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), as well as p-PKCs, p-ERK, p-JNK, p-p38, diluted with PBST (1:1,000). Membranes were washed with PBST and treated with horseradish peroxidase-conjugated rabbit anti-goat IgG (diluted to 1:5,000–1:10,000; Zymed Laboratory Inc., San Francisco, CA, USA) in PBST for 60 min. After washing, the protein bands were visualized using enhanced chemiluminescent solution (Amersham Biosciences) [28].
2.15. Electrophoretic mobility shift assay
To prepare nuclear extracts, BMMCs (1 × 106 cells) were washed twice with ice-cold PBS and resuspended in 1 mL ice-cold buffer A (10mM Hepes/KOH pH 7.9, 10mM KCl, 1.5mM MgCl2, 0.5mM DTT, 0.2mM PMSF, 1 μg/mL leupeptin, and 1 μg/mL aprotinin. After incubation on ice for 15 min, the cells were lysed by adding Nonidet P40 (10 μL 10% Nonidet P40, to a final concentration of 0.625%, v/v) and immediately vortexed for 10 s. Nuclei were harvested by centrifugation at 20,000 × g for 1 min and resuspended in 40 μL ice-cold buffer C (20mM Hepes/KOH pH 7.9, 0.42M NaCl, 1.5mM MgCl2, 0.2mM EDTA, 0.5mM DTT, 25% glycerol, 0.2mM PMSF, 1 μg/mL leupeptin, and 1 μg/mL aprotinin). After incubation at 4°C for 20 min on a shaking platform, the nuclei were clarified by centrifugation at 15,000 × g for 10 min. The supernatant (nuclear extract) was then transferred to a new tube and quantified using Bradford's method. Nuclear extracts were stored at −70°C until required [28].
Ten microliters of a mixture of NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′, 3′-TCA ACT CCC CTG AAA GGG TCC G-5′) or activator protein-1 (AP-1; 5′-CGC TTG ATG AGT CAG CCG GAA-3′, 3′-GCG AAC TAC TCA GTC GGC CTT-5′) oligonucleotide (1.75μM), T4 polynucleotide kinase 10 × buffer, [α-32P]ATP (10 μCi; 3,000 Ci/mmol), nuclease-free water, and T4 polynucleotide kinase (5–10 U/μL) were incubated at 37°C for 30 min. The reaction was stopped by adding 1 μL EDTA (0.5M). After adding 89 μL Tris-EDTA buffer (10mM Tris-HCl pH 8.0, 1mM EDTA), unincorporated nucleotides were separated from the DNA probe by G-25 spin column chromatography. The nuclear extract and gel shift binding 5 × buffer [20% glycerol, 5mM MgCl2, 2.5mM EDTA, 2.5mM DTT, 250mM NaCl, 50mM Tris-HCl pH 7.5, and 0.25 mg/mL poly(dI-dC)] were incubated at room temperature for 10 min, and then 20–30 fmol of 32P-labeled NF-κB or AP-1 oligonucleotide was added and incubated at room temperature for 20 min. After stopping the reaction, 1 μL of 10 × gel loading buffer was added to each reaction. Reaction mixtures were electrophoresed on 6% polyacrylamide gels, and the gels were analyzed using FLA-2000 (Fujifilm, Tokyo, Japan).
2.16. Statistical analysis
Experimental data were expressed as mean ± standard error of the mean. An analysis of variance (ANOVA) was used for statistical analysis. Significance between control and experimental groups was determined by Scheffe's posthoc test using SPSS version 21 (SPSS Inc., Chicago, IL, USA). The bands for RT-PCR, western blot and electrophoretic mobility shift assay are representative of four independent experiment (n = 4). A p value of 0.05 was regarded as statistically significant.
3. Results
3.1. Effect of PPT on the release of histamine and LTs in the activated GPMCs and BMMCs
It is well known that histamine and LTs are released from mast cells when activated by antigen/antibody reaction. Two types of mast cells, GPMCs and mouse bone marrow-derived mast cells BMMCs were used for this study since commercially available antibodies for guinea pigs have certain limitations for investigating signaling molecules related to allergic responses. In addition, it is known that mast cells are different in their function, depending on their location in the types of organs (lung, bone marrow, etc.) and species (guinea pigs, mice, etc.).
In order to determine whether PPT causes some effects on the release of inflammatory mediators (histamine and LTs) in quiescent GPMCs or BMMCs, unsensitized each types of mast cells pretreated with various concentrations of PPT (5μM, 10μM, 50μM, 100μM, or 300μM) for 5 min were challenged by OVA (1.0 μg/mL), or both types of mast cells sensitized with anti-OVA antibody were challenged with various concentrations of PPT (5μM, 10μM, 50μM, 100μM, or 300μM) for 30 min or 1 h. It was confirmed that PPT itself did not cause any effects on the mediator releases if not antigen/antibody stimulation (data not shown).
Effects of PPT on the mediator release were examined in GPMCs or BMMCs activated by each specific antigen/antibody reaction. In the activated GPMCs, PPT pretreatment (10μM, 50μM, or 100μM) dose-dependently inhibited histamine release by 5.4%, 41.4%, and 60.5%, respectively (28.6 ± 0.05% for 10μM; 17.7 ± 0.34% for 50μM; and 11.9 ± 0.25% for 100μM PPT), compared with that of OVA stimulation (30.2 ± 0.72%; Fig. 1B, left panel). In the activated BMMCs, histamine release was inhibited by PPT pretreatment by 7.9%, 37.6%, and 70.1% (30.6 ± 0.07% for 10μM; 20.7 ± 0.35% for 50μM; and 9.9 ± 0.26% for 100μM PPT), compared with that of OVA challenge (33.2 ± 0.76%; Fig. 1B, right panel).
Patterns of LT secretion in the activated GPMCs or BMMCs were similar to those of histamine release in both types of mast cells. PPT (10μM, 50μM, or 100μM) inhibited LTs release by 3.4%, 27.6% and 55.4%, respectively, in activated GPMCs (6.5 ± 0.04 × 102 pg/mL for 10μM PPT; 4.9 ± 0.39 × 102 pg/mL for 50μM PPT; 3.0 ± 0.09 × 102 pg/mL for 100μM PPT), compared with that of OVA challenge (6.8 ± 0.13 × 102 pg/mL; Fig. 1B, left panel). In activated BMMCs, PPT inhibited LTs release by 4.7%, 21.9%, and 45.7% (4.1 ± 0.06 × 103 pg/mL for 10μM PPT; 3.3 ± 0.11 × 103 pg/mL for 50μM PPT; and 2.3 ± 0.12 × 103 pg/mL for 100μM PPT), compared with that of OVA challenge (4.3 ± 0.07 × 103 pg/mL; Fig. 1B, right panel).
Histamine and LTs release in the unstimulated GPMCs or BMMCs were 1.3 ± 0.27% and 4.3 ± 0.50%, and 1.3 ± 0.09 × 102 pg/mL and 0.2 ± 0.00 × 103 pg/mL, respectively. Total amounts of histamine in the unstimulated GPMCs or BMMCs were 2,356 ± 70.7 ng/4 × 105 cells and 758 ± 31.3 ng/L × 106 cells, respectively.
3.2. Effect of PPT on the expressions of cytokine mRNA in activated BMMCs
In addition to the release of histamine and LTs, mast cells secrete various types of cytokines, particularly inflammatory cytokines (IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, TNF-α, and interferon-γ) [20]. Thus, whether the mRNA levels of each cytokines were altered by PPT was examined in the activated BMMCs. PPT pretreatment (10μM, 50μM, or 100μM) inhibited the expressions of all cytokines' mRNA in dose-dependent manner, except IL-10 mRNA expression, which was increased in the activated BMMCs (Fig. 1C).
3.3. Effect of PPT on [Ca2+]i in activated GPMCs and BMMCs
Increments of [Ca2+]i are crucial for degranulation in activated mast cells [20,26,31,32]. In activated GPMCs, changes in the [Ca2+]i were monitored for 10 min after OVA challenge (data shown only for 5 min). [Ca2+]i reached the maximum level (approximately 1.9 folds of basal level) at 3 min and was maintained until 10 min. PPT pretreatment (100μM) strongly prevented the OVA-induced rise in [Ca2+]i and kept it at the basal level (Fig. 2A). In the activated BMMCs, [Ca2+]i increased steadily, reached the maximum at 5 min (approximately 1.9 folds of basal level), and was maintained until 10 min (data shown only for 5 min). PPT (10μM, 50μM, or 100μM) pretreatment significantly suppressed [Ca2+]i (Fig. 2B). Especially, PPT inhibited [Ca2+]i in activated GPMCs more prominently than in activated BMMCs. Effects on the changes in [Ca2+]i and the activities of PKC and PLA2 in GPMCs were tested only at the highest concentration of PPT since it was hard to purify GPMCs and thus only limited GPMCs were available.
Fig. 2.
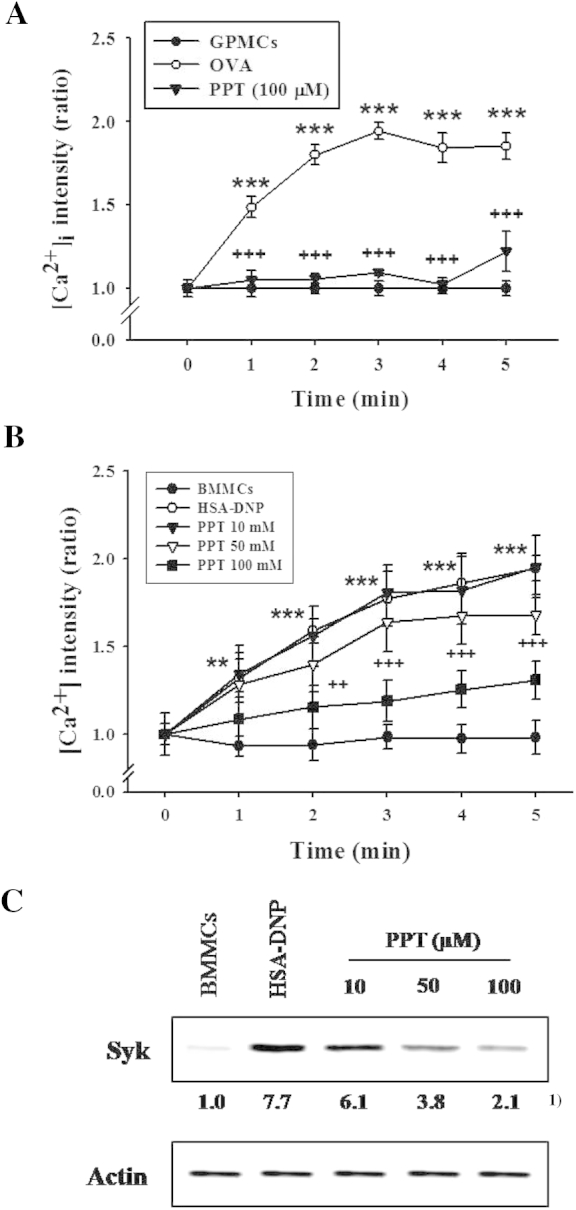
Effects of 20(S)-protopanaxatriol (PPT) on the intracellular Ca2+ level ([Ca2+]i) and expression of Syk kinase protein in activated mast cells. The sensitized guinea pig mast cells (GPMCs) or mouse bone marrow-derived mast cells (BMMCs; 4 × 105) were incubated for 30 min after adding Fluo-3 AM (5μM) and fixed on a glass slide treated with poly-l-lysine, and then ovalbumin (OVA; 1.0 μg/mL) or human serum albumin–dinitrophenyl (HSA-DNP), respectively, was flowed out on a glass slide for stimulation. The [Ca2+]i was monitored for 10 min using confocal microscopy, and Syk protein expression after immunoprecipitation was determined with western blot, as described in Materials and methods. PPT (10μM, 50μM, or 100μM) was pretreated 5 min prior to the OVA challenge. (A and B) [Ca2+]i in (A) GPMCs and (B) BMMCs. In total there were eight experiments. **p < 0.01; ***p < 0.001 versus control; ++, p < 0.01; +++, p < 0.001 versus OVA or HSA-DNP challenge. C, Syk protein. 1) Numbers below the bands indicate the ratio of band intensity of each band versus that of control and actin. The data are representative of four independent experiments.
3.4. Effect of PPT on the expression of Syk protein in the activated BMMCs
As PPT reduced the [Ca2+]i elevated in IgE-induced mast cell activation, it was examined whether Syk kinase, an upstream signaling molecule of [Ca2+]i influx was affected by PPT pretreatment. PPT (10μM, 50μM, or 100μM) significantly reduced the increased level of expression of Syk kinase in BMMCs when activated by antigen/antibody stimulation (Fig. 2C).
3.5. Effect of PPT on the PKC activity in the activated GPMCs and BMMCs
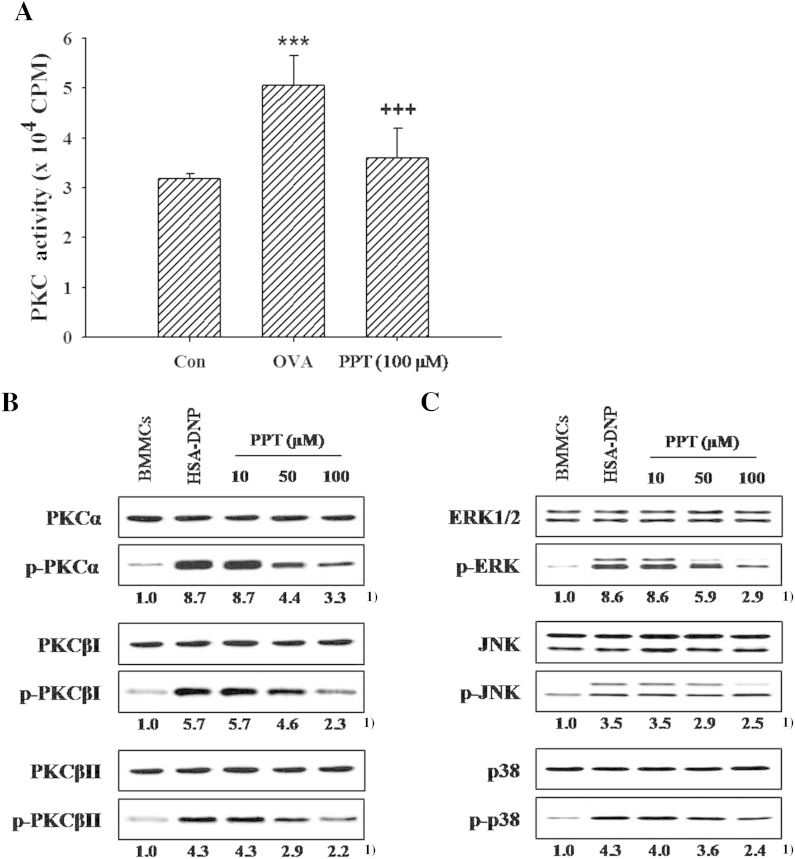
It has been reported that PKC is activated by an elevation of [Ca2+]i level in the stimulated B cells and mast cells [26,31]. The changes in the PKC activity in both types of mast cells were measured after stimulation. The activity of PKC was enhanced in OVA-challenged GPMCs by 59.3%. The OVA-induced increment in PKC activity was remarkably counteracted by 100μM PPT (Fig. 3A).
Fig. 3.
Effects of 20(S)-protopanaxatriol (PPT) on the activities of protein kinase C (PKC) or mitogen-activated protein (MAP) kinases in activated mast cells. The sensitized guinea pig mast cells (GPMCs; 1 × 106 cells) incorporated with [γ-32P]ATP (1μM) and sensitized mouse bone marrow-derived mast cells (BMMCs; 1 × 106 cells) were stimulated by each antigen/antibody reaction as described in Materials and methods. PPT (10μM, 50μM, or 100μM) was pretreated 5 min prior to the antigen challenge. The PKC activity was measured by the phosphorylated protein at 30 min after OVA challenge, and activities of PKC isoforms and MAP kinases were determined using western blot. (A) PKC activity in GPMCs. The data are expressed as mean ± standard error of the mean (n = 8). *** p < 0.001 versus control (Con). +++p < 0.001 versus OVA challenge. (B and C) The phosphorylation of PKCs and MAP kinases in BMMCs. 1) Numbers below the bands indicate the ratio of band intensity of each band versus those of control and total protein. The data are representative of four independent experiments. ERK, extracellular signal-regulated kinase; JNK, c-jun N-terminal kinase; OVA, ovalbumin; HSA-DNP, human serum albumin–dinitrophenyl.
The Ca2+-dependent PKC isoforms (PKCα, βI, and βII) are activated in mast cells, and then the activated PKC isoforms regulate mitogen-activated protein (MAP) kinases [18,33]. Fig. 3B showed that PKC isoforms (α, βI, βII) are phosphorylated by antigen/antibody reaction in BMMCs, and PPT (10μM, 50μM, or 100μM) inhibited the phosphorylation of PKC isoforms in the activated BMMCs. In addition, we observed the phosphorylations of ERK, JNK, and p38 MAP kinases in activated BMMCs. PPT (10μM, 50μM, or 100μM) reduced the augmented phosphorylation of MAP kinases in activated BMMCs (Fig. 3C).
3.6. Effect of PPT on the PLA2 activity in activated GPMCs and BMMCs
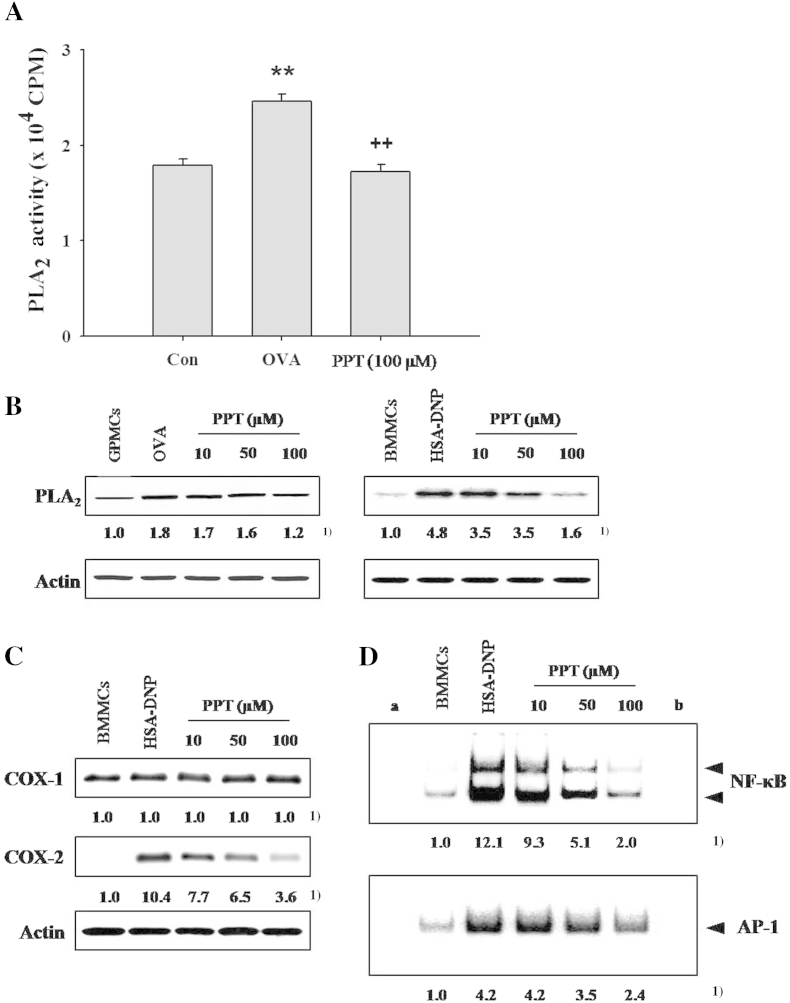
The changes in the PLA2 activity in GPMCs were assayed after OVA stimulation. OVA increased the PLA2 activity by 38% of basal activity in stimulated GPMCs. The OVA-induced increment in the enzyme activity was decreased by 30.2% when the cells were pretreated with 100μM PPT (Fig. 4A).
Fig. 4.
Effects of 20(S)-protopanaxatriol (PPT) on the phospholipase A2 (PLA2) activity or expression of cyclooxygenase (COX)-1/2, and the DNA binding activities of transcription factors, nuclear factor (NF)-κB and activator protein (AP)-1 in activated mast cells. GPMCs (1 × 106) pre-incubated with [3H] arachidonic acid (1 μCi) and BMMCs (1 × 106 cells) were sensitized and challenged by each antigen/antibody reaction. The experimental details and PPT treatment were as described in Materials and methods. (A) PLA2 activity in GPMCs. The data are expressed as mean ± standard error of the mean (n = 8). ** p < 0.01 versus control (Con). ++, p < 0.01 versus ovalbumin (OVA) challenge. (B) The expression of PLA2 protein in guinea pig mast cells (GPMCs) or mouse bone marrow-derived mast cells (BMMCs). (C) Expression of COX-1/2 in BMMCs. (D) Activities of transcription factors, NF-κB and AP-1 in BMMCs. 1) Numbers below the bands indicate the ratio of band intensity of each band versus those of control and actin protein or total protein. (a) Negative control; (b) competition. The data are representative of four independent experiments (n = 4). HSA-DNP, human serum albumin–dinitrophenyl.
In GPMCs and BMMCs, the level of protein expression of PLA2 was enhanced upon stimulation with OVA challenge (Fig. 4B). PPT pretreatment (10μM, 50μM, or 100μM) dose-dependently reduced the PLA2 expression in each type of activated mast cells.
3.7. Effect of PPT on the activity of COX-1/2 in the activated BMMCs
It is well known that prostaglandins (PGs) are produced by the actions of both constitutive enzyme COX-1 and inducible enzyme COX-2. In mast cells, certain amount of COX-1 is expressed and COX-2 is induced by the antigen/antibody stimulation [34,35]. It was also observed that expression of COX-2 was enhanced in activated BMMCs, but COX-1 was not affected (Fig. 4C). PPT decreased dose-dependently only the induced level of COX-2 expression by antigen stimulation, but not that of COX-1 expression (Fig. 4C).
3.8. Effect of PPT on the activity of transcription factors in the activated BMMCs
NF-κB and AP-1 are transcription factors controlling the expressions of inflammatory cytokines in mast cells. These transcription factors were upregulated in the activated BMMCs. PPT (10μM, 50μM, or 100μM) inhibited the DNA binding activities of transcription factors in a dose-dependent manner (Fig. 4D).
4. Discussion
Mast cells participate in the induction of innate and adaptive immune responses [20,36]. Mast cells influence both health and diseases, such as allergy, arthritis, autoimmunity, and neoplasia, by releasing and producing a variety of mediators [20,26].
Ginsenosides and ginsan, the major active saponin and polysaccharide components of ginseng, respectively, have many biological activities including inflammatory responses in mast cells as well as allergic inflammation [7,16,37]. Herein, we demonstrated that PPT, one of the ginseng saponin metabolites formed by human intestinal bacteria and of nonsugar moiety of ginsenoside (aglycone), reduced the release of mediators by [Ca2+]i level, PLA2, PKC, and NF-κB/AP-1 activities which are downstream signals regulated by Syk kinase in FcεRI-mediated mast cell activation.
The antigen-mediated activation of mast cells via engagement of the IgE bound to FcεRI receptor represents an initial event in the development of type I hypersensitivity reaction, and results in degranulation, with the release of various inflammatory mediators, such as histamine, LTs, and cytokines [20,26,32]. That is, IgE-mediated signaling pathways lead to the release of preformed mediators (histamine, tryptase, etc.) by PKC activity that is accompanied by Ca2+ influx, which is critical for the downstream propagation signals by Syk-mediated phosphorylation and the activation of PLCγ or PLD enzyme [18,20,38,39]. Therefore, we examined the effects of PPT on the well-known signaling pathways in mediator release from IgE-mediated mast cell activation. Our data (Figs. 1B, 2, 3A, 3B) suggest that PPT reduces histamine release via the inhibition of PKC activity caused by blocking of the rise in [Ca2+]i through regulating Syk in FcεRI-mediated mast cell activations. Similarly, it was reported that ginsenoside Rb1, which has a sugar moiety in its structure, inhibited mediator release in activated GPMCs [9]. However, there has been a report that ginseng saponin enhanced [Ca2+]i and PKC activity in macrophage activation [6]. This may be due to the cell types or the sort of ginsenoside components used in each separate experiment.
Rapid hydrolysis of phospholipids in mast cell membrane also occurs through the activation of cytosolic phospholipase A2 (cPLA2) in IgE-mediated signaling pathways. Hydrolyzed phospholipids were converted into arachidonic acids. Then, enzymes such as COX-1/2 or 5-lipoxigenase (LOX) convert them into PGs and LTs, respectively, which affects the early phase of allergic responses in mast cells [34,35,38,40,41]. A rise in [Ca2+]i is necessary for translocation of cPLA2 to the cell membrane in FcεRI-mediated mast cell activation [18,42]. It has been reported that ginsenoside Rh1 inhibited COX-2 [7]. Our data (Figs. 1B, 2A, 2B, 4A–C) suggest that PPT-mediated reduction in [Ca2+]i leads to the decrements in production of LTs via inhibiting the activities of cPLA2 and most likely LOX during mast cell activations. In addition, we observed that PPT causes reduction of COX-1/2 expression, which may result in PG production in combination with the inhibition of PLA2 activity and the reduction of [Ca2+]i. This indicates that PPT may concurrently inhibit the COX-2 and LOX activities, thereby reducing early phase of allergic responses.
IgE-mediated signaling pathways induce production of inflammatory cytokines via activation of MAP kinases during activation of mast cells [32,43,44]. NF-κB and AP-1 are pleiotropic transcription factors that play an important role in regulating the expression of multiple genes including Th2 cytokines [20], and allergic inflammation is associated with increased NF-κB activity in animal lung tissues and BAL cells [28,45]. A rise of [Ca2+]i level also leads to the activation of MAP kinases and NF-κB, which play important roles in the control of IgE-mediated cytokine synthesis in mast cells [46–49]. Our results (Figs. 1C, 2, 3C, 4D) suggest that PPT inhibits production of inflammatory cytokines via inhibiting an increase of cellular Ca2+ level, the activities of MAP kinases and NF-κB/AP-1 in the IgE-mediated activated mast cells. Similarly, ginsenosides have been reported to inhibit NF-κB activation as well as histamine release in certain cell lines [7,37]. However, IL-10, which is known as an anti-inflammatory cytokine, was not inhibited in the PPT pretreatment.
PPT pretreatment exerted antiallergic effects in the same manner in both types of mast cells when activated by specific antigen/antibody reaction. It can be inferred that PPT acts via regulating expression of Syk kinase, which is critical for the downstream propagation of signals in FcεRI-mediated mast cell activation, although further upstream signaling molecules of Syk and [Ca2+]i were not defined in this experiment. This can be supported by the previous reports that a rise of [Ca2+]i triggered the induction of activities of cellular signaling molecules such as PKCs, MAP kinases, PLA2, and NF-κB/AP-1 [18,20,38,39,42,46–49].
A variety of ginsenosides affect allergic and inflammatory responses with their characteristic effects. Ginsenosides Rb1, Rg1, Rg2, and Rc exert antiallergic effects by inhibiting histamine release from mast cells [7,9,16,17] and antiallergic effects in murine model [10,13]. In addition, PPT, an intestinal metabolite of ginsenosides (an aglycone moiety) inhibited the release and production of inflammatory mediators (histamine, LTs, and cytokines, respectively) in the GPMCs as well as in BMMCs, which is compatible with the observations by other laboratories that ginseng metabolites such as compound K [16] and Rh2 [17] inhibit release of mediators such as β-hexosaminidase from RBL-2H3 cells. Thus, the sugar moiety attached at their triterpenoid structures is not supposed to affect the mediator release during mast cell activation.
Ginsenoside Re, which induces allergic responses (unpublished data), exists in very low amounts in ginseng extracts. An allergic asthma observed in personnel exposed to ginseng for long periods, such as the workers engaged in Korean ginseng wholesale may be due to ginsenoside Re [8].
The blood concentration of PPT after oral intake of ginseng extract would be much lower than that of PPT used in this experiment. Therefore, it can be supposed that the cumulative blood concentration of ginseng metabolites when taken for longer periods will be much more close to the concentration of PPT used in this experiment. Thus, it is necessary to measure the blood concentrations of ginseng metabolites after long-term use of ginseng extract for more appropriately evaluating the dose-dependent effects of intestinal ginseng metabolites in vivo.
In conclusion, we suggest that PPT, one of the intestinal ginseng metabolites, inhibits the release and production of inflammatory mediators by affecting the cellular signaling pathways initiated by Syk activation in the FcεRI receptor stimulation and it may be used as an auxiliary substance for treating allergy-induced asthma.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by the grant from the Korean Society of Ginseng funded Korea Ginseng Corp (2002) for C.H.L. and from the Samsung Biomedical Research Institute, Sungkynkwan University School of Medicine for J.Y.R.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Jai Youl Ro, Email: jyro426@med.skku.ac.kr.
Chang Ho Lee, Email: changholee57@gmail.com.
References
- 1.Kang S., Min H. Ginseng, the ‘immunity boost’: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizuno M., Yamada J., Terai H., Kozukue N., Lee Y.S., Tsuchida H. Differences in immunomodulating effects between wild and cultured Panax ginseng. Biochem Biophys Res Commun. 1994;200:1672–1678. doi: 10.1006/bbrc.1994.1644. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., Wang S., Liu H., Yang L., Nan G. Stimulatory effect of saponin from Panax ginseng on immune function of lymphocytes in the elderly. Mech Ageing Dev. 1995;83:43–53. doi: 10.1016/0047-6374(95)01618-a. [DOI] [PubMed] [Google Scholar]
- 4.Scaglione F., Ferrara F., Dugnani S., Falchi M., Santoro G., Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp Clin Res. 1990;16:537–542. [PubMed] [Google Scholar]
- 5.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 6.Hu S., Concha C., Cooray R., Holmberg O. Ginseng-enhanced oxidative and phagocytic activities of polymorphonuclear leucocytes from bovine peripheral blood and stripping milk. Vet Res. 1995;26:155–161. [PubMed] [Google Scholar]
- 7.Park E.K., Choo M.K., Han M.J., Kim D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 8.Kim K.M., Kwon H.S., Jeon S.G., Park C.H., Sohn S.W., Kim D.I., Kim S.S., Chang Y.S., Kim Y.K., Cho S.H. Korean ginseng-induced occupational asthma and determination of IgE binding components. J Korean Med Sci. 2008;23:232–235. doi: 10.3346/jkms.2008.23.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ro J.Y., Ahn Y.S., Kim K.H. Inhibitory effect of ginsenoside on the mediator release in the guinea pig lung mast cells activated by specific antigen-antibody reactions. Int J Immunopharmacol. 1998;20:625–641. doi: 10.1016/s0192-0561(98)00062-9. [DOI] [PubMed] [Google Scholar]
- 10.Babayigit A., Olmez D., Karaman O., Bagriyanik H.A., Yilmaz O., Kivcak B., Erbil G., Uzuner N. Ginseng ameliorates chronic histopathologic changes in a murine model of asthma. Allergy Asthma Proc. 2008;29:493–498. doi: 10.2500/aap.2008.29.3137. [DOI] [PubMed] [Google Scholar]
- 11.Song J.Y., Han S.K., Son E.H., Pyo S.N., Yun Y.S., Yi S.Y. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2002;2:857–865. doi: 10.1016/s1567-5769(01)00211-9. [DOI] [PubMed] [Google Scholar]
- 12.Ahn J.Y., Song J.Y., Yun Y.S., Jeong G., Choi I.S. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 2006;46:187–197. doi: 10.1111/j.1574-695X.2005.00021.x. [DOI] [PubMed] [Google Scholar]
- 13.Lim Y.J., Na H.S., Yun Y.S., Choi I.S., Oh J.S., Rhee J.H., Cho B.H., Lee H.C. Suppressive effects of ginsan on the development of allergic reaction in murine asthmatic model. Int Arch Allergy Immunol. 2009;150:32–42. doi: 10.1159/000210378. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa H., Sung J.H., Matsumiya S., Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- 15.Bae E.A., Park S.Y., Kim D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 16.Choo M.K., Park E.K., Han M.J., Kim D.H. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522. doi: 10.1055/s-2003-40653. [DOI] [PubMed] [Google Scholar]
- 17.Park E.K., Choo M.K., Kim E.J., Han M.J., Kim D.H. Antiallergic activity of ginsenoside Rh2. Biol Pharm Bull. 2003;26:1581–1584. doi: 10.1248/bpb.26.1581. [DOI] [PubMed] [Google Scholar]
- 18.Chang W.C., Nelson C., Parekh A.B. Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion, and expression of c-fos through ERK-dependent and -independent pathways in mast cells. FASEB J. 2006;20:2381–2383. doi: 10.1096/fj.06-6016fje. [DOI] [PubMed] [Google Scholar]
- 19.Brown J.M., Wilson T.M., Metcalfe D.D. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- 20.Kalesnikoff J., Galli S.J. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakim-Rad K., Metz M., Maurer M. Mast cells: makers and breakers of allergic inflammation. Curr Opin Allergy Clin Immunol. 2009;9:427–430. doi: 10.1097/ACI.0b013e32832e9af1. [DOI] [PubMed] [Google Scholar]
- 22.Okayama Y., Ra C., Saito H. Role of mast cells in airway remodeling. Curr Opin Immunol. 2007;19:687–693. doi: 10.1016/j.coi.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Kim M.Y., Cho J.Y. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J Ginseng Res. 2013;37:293–299. doi: 10.5142/jgr.2013.37.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim M.Y., Cho J.Y. 20S-dihydroprotopanaxatriol modulates functional activation of monocytes and macrophages. J Ginseng Res. 2013;37:300–307. doi: 10.5142/jgr.2013.37.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka O., Nagai M., Shibata S. Chemical studies on the oriental plant drugs. XVI. The stereochemistry of protopanaxadiol, a genuine sapogenin of ginseng. Chem Pharm Bull. 1966;14:1150–1156. doi: 10.1248/cpb.14.1150. [DOI] [PubMed] [Google Scholar]
- 26.Ro J.Y., Lee B.C., Kim J.Y., Chung Y.J., Chung M.H., Lee S.K., Jo T.H., Kim K.H., Park Y.I. Inhibitory mechanism of aloe single component (alprogen) on mediator release in guinea pig lung mast cells activated with specific antigen-antibody reactions. J Pharmacol Exp Ther. 2000;292:114–121. [PubMed] [Google Scholar]
- 27.Kim J.Y., Kim D.Y., Ro J.Y. Granule formation in NGF-cultured mast cells is associated with expressions of pyruvate kinase type M2 and annexin I proteins. Int Arch Allergy Immunol. 2008;146:287–297. doi: 10.1159/000121463. [DOI] [PubMed] [Google Scholar]
- 28.Kim D.Y., Park J.W., Jeoung D., Ro J.Y. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur J Pharmacol. 2009;612:98–105. doi: 10.1016/j.ejphar.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 29.Lin X., Takemori H., Katoh Y., Doi J., Horike N., Makino A., Nonaka Y., Okamoto M. Salt-inducible kinase is involved in the ACTH/cAMP-dependent protein kinase signaling in Y1 mouse adrenocortical tumor cells. Mol Endocrinol. 2001;15:1264–1276. doi: 10.1210/mend.15.8.0675. [DOI] [PubMed] [Google Scholar]
- 30.Reddy S.T., Winstead M.V., Tischfield J.A., Herschman H.R. Analysis of the secretory phospholipase A2 that mediates prostaglandin production in mast cells. J Biol Chem. 1997;272:13591–13596. doi: 10.1074/jbc.272.21.13591. [DOI] [PubMed] [Google Scholar]
- 31.Takata M., Sabe H., Hata A., Inazu T., Homma Y., Nukada T., Yamamura H., Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbu E.A., Zhang J., Siraganian R.P. The limited contribution of Fyn and Gab2 to the high affinity IgE receptor signaling in mast cells. J Biol Chem. 2010;285:15761–15768. doi: 10.1074/jbc.M110.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehrenbach K., Lessmann E., Zorn C.N., Kuhny M., Grochowy G., Krystal G., Leitges M., Huber M. Steel factor enhances supraoptimal antigen-induced IL-6 production from mast cells via activation of protein kinase C-beta. J Immunol. 2009;182:7897–7905. doi: 10.4049/jimmunol.0801773. [DOI] [PubMed] [Google Scholar]
- 34.Boyce J.A. Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications. Chem Immunol Allergy. 2005;87:59–79. doi: 10.1159/000087571. [DOI] [PubMed] [Google Scholar]
- 35.Lin T.Y., London C.A. Characterization and modulation of canine mast cell derived eicosanoids. Vet Immunol Immunopathol. 2010;135:118–127. doi: 10.1016/j.vetimm.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall J.S., Jawdat D.M. Mast cells in innate immunity. J Allergy Clin Immunol. 2004;114:21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa H., Takada Y., Shishodia S., Jayaprakasam B., Nair M.G., Aggarwal B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006;5:1434–1445. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 38.Kimata M., Shichijo M., Miura T., Serizawa I., Inagaki N., Nagai H. Ca2+ and protein kinase C signaling for histamine and sulfidoleukotrienes released from human cultured mast cells. Biochem Biophys Res Commun. 1999;257:895–900. doi: 10.1006/bbrc.1999.0557. [DOI] [PubMed] [Google Scholar]
- 39.Turner H., Kinet J.P. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 40.Hirasawa N., Santini F., Beaven M.A. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. Indications of different pathways for release of arachidonic acid and secretory granules. J Immunol. 1995;154:5391–5402. [PubMed] [Google Scholar]
- 41.Triggiani M., Granata F., Frattini A., Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta. 2006;1761:1289–1300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Leslie C.C. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- 43.Lee Y.N., Tuckerman J., Nechushtan H., Schutz G., Razin E., Angel P. c-Fos as a regulator of degranulation and cytokine production in FcepsilonRI-activated mast cells. J Immunol. 2004;173:2571–2577. doi: 10.4049/jimmunol.173.4.2571. [DOI] [PubMed] [Google Scholar]
- 44.Kettner A., Di Matteo M., Santoni A. Insulin potentiates FcepsilonRI-mediated signaling in mouse bone marrow-derived mast cells. Mol Immunol. 2010;47:1039–1046. doi: 10.1016/j.molimm.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Bureau F., Bonizzi G., Kirschvink N., Delhalle S., Desmecht D., Merville M.P., Bours V., Lekeux P. Correlation between nuclear factor-kappaB activity in bronchial brushing samples and lung dysfunction in an animal model of asthma. Am J Respir Crit Care Med. 2000;161:1314–1321. doi: 10.1164/ajrccm.161.4.9907010. [DOI] [PubMed] [Google Scholar]
- 46.Kempuraj D., Huang M., Kandere-Grzybowska K., Basu S., Boucher W., Letourneau R., Athanassiou A., Theoharides T.C. Azelastine inhibits secretion of IL-6, TNF-alpha and IL-8 as well as NF-kappaB activation and intracellular calcium ion levels in normal human mast cells. Int Arch Allergy Immunol. 2003;132:231–239. doi: 10.1159/000074304. [DOI] [PubMed] [Google Scholar]
- 47.Macian F.N.F.A.T. proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 48.Klein M., Klein-Hessling S., Palmetshofer A., Serfling E., Tertilt C., Bopp T., Heib V., Becker M., Taube C., Schild H. Specific and redundant roles for NFAT transcription factors in the expression of mast cell-derived cytokines. J Immunol. 2006;177:6667–6674. doi: 10.4049/jimmunol.177.10.6667. [DOI] [PubMed] [Google Scholar]
- 49.Perkins N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]