Abstract
Background:
Analysis of a microRNA (miRNA) expression signature of bladder cancer (BC) by deep-sequencing revealed that clustered miRNAs microRNA (miR)-451a, miR-144-3p, and miR-144-5p were significantly downregulated in BC tissues. We hypothesised that these miRNAs function as tumour suppressors in BC. The aim of this study was to investigate the functional roles of these miRNAs and their modulation of cancer networks in BC cells.
Methods:
The functional studies of BC cells were performed using transfection of mature miRNAs. Genome-wide gene expression analysis, in silico analysis, and dual-luciferase reporter assays were applied to identify miRNA targets. The association between miR-144-5p levels and expression of the target genes was determined, and overall patient survival as a function of target gene expression was estimated by the Kaplan–Meier method.
Results:
Gain-of-function studies showed that miR-144-5p significantly inhibited cell proliferation by BC cells. Four cell cycle-related genes (CCNE1, CCNE2, CDC25A, and PKMYT1) were identified as direct targets of miR-144-5p. The patients with high CCNE1 or CCNE2 expression had lower overall survival probabilities than those with low expression (P=0.025 and P=0.032).
Conclusion:
miR-144-5p functions as tumour suppressor in BC cells. CCNE1 and CCNE2 were directly regulated by miR-144-5p and might be good prognostic markers for survival of BC patients.
Keywords: microRNA, miR-144-5p, CCNE1, CCNE2, bladder cancer, prognostic marker
Bladder cancer (BC) is the fifth most commonly diagnosed cancer and the eighth most common cause of death in cancer patients in the 40 countries of the European Union (Ferlay et al, 2013). BC can be classified into two categories: non-muscle-invasive BC (NMIBC), and muscle-invasive BC (MIBC). Most BC patients (70–80%) are diagnosed with NMIBC, and recurrence rates are high (50–70%) in this group. Moreover, 15% of recurrent bladder tumours progress to MIBC. The 5-year survival rate for patients with NMIBC is close to 90%, whereas that for patients with MIBC is only approximately 60%. Furthermore, nearly 80% of patients with lymph node metastases die in the first 5 years after diagnosis (Meeks et al, 2012). The molecular mechanisms of recurrence and muscle invasion are not well understood. Patients with advanced bladder cancer (with or without metastases) are generally treated with combination chemotherapy consisting of gemcitabine and cisplatin, but progression-free survival is of limited duration.
A substantial amount of evidence has suggested that microRNAs (miRNAs) are aberrantly expressed in many human cancers and have significant roles in human oncogenesis and metastasis (Di Leva and Croce, 2010). Our recent study of BC miRNA signatures by deep-sequencing revealed that 60 miRNAs were significantly downregulated in BC tissues (Itesako et al, 2014), suggesting that these miRNAs were potential candidates for tumour-suppressive miRNAs. Using our previous PCR-based BC signatures and present deep-sequence signature, we have focussed on downregulated clustered miRNAs and sequentially investigated their functional significance. Thus far, we have shown that microRNA (miR)-1/133a, miR-195/497, and miR-23b/27b clustered miRNAs function as tumour suppressors through their targeting of several oncogenic genes in BC cells (Yoshino et al, 2011; Itesako et al, 2014; Chiyomaru et al, 2015).
Deep-sequencing-based BC signatures revealed that miR-451a, miR-144-3p, and miR-144-5p clustered miRNAs were downregulated in BC tissues. The aim of the present study was to investigate the functional significance of miR-451a/144-3p/144-5p and to identify the molecular targets regulated by these miRNAs in BC cells. Our data demonstrated that restoration of miR-144-5p significantly inhibited cancer cell proliferation through their targeting of oncogenic genes (CCNE1, CCNE2, CDC25A, and PKMYT1) that promoted progress through the cell cycle. The discovery of molecular targets mediated by tumour-suppressive miRNAs provides important insights into the potential mechanisms of BC oncogenesis and suggests novel therapeutic strategies and tumour markers for the treatment of BC.
Materials and Methods
Clinical specimens and cell culture
The tissue specimens for quantitative real-time reverse transcription PCRs (qRT–PCRs) were collected from BC patients (n=60) who had received cystectomy (n=10) or transurethral resection of their bladder tumours (n=50) at Kagoshima University Hospital between 2003 and 2013. Normal bladder epithelia (n=22) were derived from patients with noncancerous disease. The specimens were staged according to the American Joint Committee on Cancer-Union Internationale Contre le Cancer tumour–node–metastasis classification and histologically graded (LH et al, 2009). Our study was approved by the Bioethics Committee of Kagoshima University; written prior informed consent and approval were obtained from all patients. Patient details and clinicopathological characteristics are listed in Supplementary Table S1.
We used two human BC cell lines: T24, which was invasive and obtained from the American Type Culture Collection (Manassas, VA, USA); and BOY, which was established in our laboratory from an Asian male patient, aged 66 years, who was diagnosed with stage III BC with lung metastasis (Takemoto et al, 1997). These cell lines were maintained in minimum essential medium supplemented with 10% fetal bovine serum in a humidified atmosphere of 5% CO2 and 95% air at 37 °C (Inoguchi et al, 2014; Itesako et al, 2014).
Tissue collection and RNA extraction
Tissues were immersed in RNAlater (Ambion, Austin, TX, USA) and stored at −20 °C until RNA extraction was conducted. Total RNA, including miRNA, was extracted using the mirVana miRNA Isolation Kit (Ambion) following the manufacturer's protocol. The integrity of the RNA was checked with an RNA 6000 Nano Assay Kit and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) following the manufacturer's protocol.
Quantitative real-time reverse transcription PCR
Stem-loop RT–PCR (TaqMan MicroRNA Assays; P/N: 001141 for miR-451a, P/N: 002676 for miR-144-3p, and P/N: 002148 for miR-144-5p; Applied Biosystems, Foster City, CA, USA) was used to quantify miRNAs according to previously published conditions (Ichimi et al, 2009). TaqMan probes and primers for cyclin E1: CCNE1 (P/N: Hs 01026536_m1; Applied Biosystems), cyclin E2: CCNE2 (P/N: Hs00180319_m1), cell division cycle 25A: CDC25A (P/N: Hs00947994_m1), and protein kinase, membrane-associated tyrosine/threonine 1: PKMYT1 (P/N: Hs00993620_m1) were assay-on-demand gene expression products. We used human GUSB (P/N: Hs99999908_m1) and RNU48 (P/N: 001006), respectively, as internal controls, and the ΔΔCt method was employed to calculate the fold changes.
Transfection with mature miRNA
As described elsewhere (Chiyomaru et al, 2010), T24 and BOY cells were transfected with Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) and Opti-MEM (Invitrogen) with 10 nM mature miRNA. Mature miRNAs and negative-control miRNA (Applied Biosystems) were used in gain-of-function experiments.
Cell proliferation and flow cytometry assays
Cell proliferation was determined with an XTT assay (Roche Applied Sciences, Tokyo, Japan) that was performed as described previously (Chiyomaru et al, 2010; Itesako et al, 2014).
BC cell lines were transiently transfected with transfection reagent only (mock), miR-control, or miR-144-5p in six-well tissue culture plates, as described earlier (Inoguchi et al, 2014). Cells were harvested by trypsinisation 72 h after transfection and washed in cold phosphate-buffered saline. For the cell cycle analysis, cells were stained with PI using the Cycletest PLUS DNA Reagent Kit (BD Biosciences, Bedford, MA, USA) following the protocol and analysed by CyAn ADP analyser (Beckman Coulter, Brea, CA, USA). The percentages of the cells in the G0/G1, S, and G2/M phases were determined and compared. Experiments were performed in triplicate.
Genome-wide gene expression and in silico analysis for the identification of genes regulated by miR-144-5p
To identify target genes of miR-144-5p, we used genome-wide gene expression analysis and in silico analysis. We attempted to identify miR-144-5p target genes using miR-144-5p-transfected BC cell line T24. A SurePrint G3 Human GE 8 × 60 K Microarray (Agilent) was used for expression profiling of miR-144-5p transfectants. The current microarray data were deposited by the Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) and were assigned GEO accession number GSE66498. Gene expression data were adapted to Kyoto Encyclopaedia of Genes and Genomes pathway categories by the GENECODIS program (http://genecodis.dacya.ucm.es). Expression data from BC specimens used publicly available gene expression data sets in the GEO database (accession number: GSE11783 + GSE31684). The data were normalized and analysed with the GeneSpring software (Agilent) as described previously (Chiyomaru et al, 2015). We merged these data sets and selected putative miR-144-5p target genes using microRNA.org (August, 2010 release, http://www.microrna.org). The strategy for investigation of the target genes is shown in Supplementary Figure S1.
Plasmid construction and dual-luciferase reporter assays
Partial wild-type sequences of the 3′-untranslated regions (UTR) of CCNE1, CCNE2, CDC25A, and PKMYT1 or those with a deleted miR-144-5p target site (positions 91–96 of CCNE1 3′-UTR, 843–848 of CCNE2 3′-UTR, 775–780 of CDC25A 3′-UTR, and 14–19 of PKMYT1 3′-UTR) were inserted between the XhoI and PmeI restriction sites in the 3′-UTR of the hRluc gene in the psiCHECK-2 vector (C8021; Promega, Madison, WI, USA). The procedure for dual-luciferase reporter assay was described previously (Inoguchi et al, 2014; Itesako et al, 2014).
Statistical analysis
Relationships between two or three variables and numerical values were analysed using the Mann–Whitney U-test or Bonferroni-adjusted Mann–Whitney U-test. Spearman's rank test was used to evaluate the correlation between the expression levels of miR-144-5p and its target genes. We estimated overall survival in 60 BC patients by using the Kaplan–Meier method. Patients were divided into two groups according to the median value of CCNE1 and CCNE2 expression, and the differences between the two groups were evaluated by the log-rank tests. We used the Expert Stat View software, version 4 (Cary, NC, USA), for these analyses.
Results
The expression levels of miR-451a, miR-144-3p, and miR-144-5p
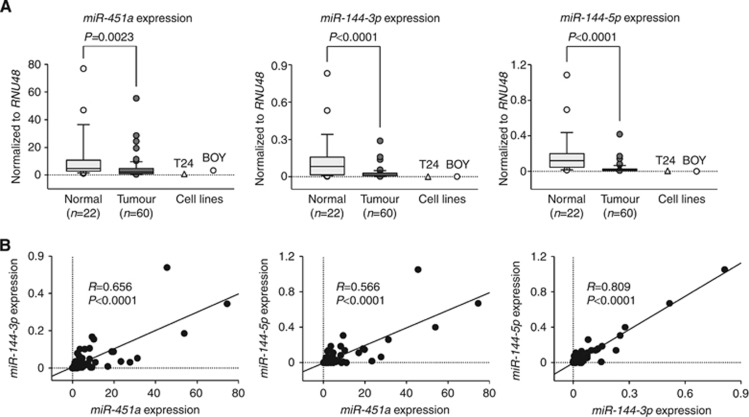
We evaluated the expression levels of miR-451a, miR-144-3p, and miR-144-5p in BC tissues (n=60), normal bladder epithelia (n=22), and BC cell lines (T24 and BOY). The expression levels of miR-451a, miR-144-3p, and miR-144-5p were significantly reduced in tumour tissues and BC cell lines compared with normal bladder epithelia (Figure 1A). The expression levels of miR-451a, miR-144-3p, and miR-144-5p were analysed for their correlation with one another. Correlation coefficients of 0.656, 0.566, and 0.809 with P<0.0001 indicated that their expression levels were highly correlated with each other (Figure 1B). To avoid incidental results caused by outliers, we have excluded over ±1.0 s.d. samples and found that there still remained significant differences in the expression levels of the miRNAs between tumour tissues and normal bladder epithelia (Supplementary Figure S2A). Similarly, there still remained significant positive correlations between the expression levels of the miRNAs (Supplementary Figure S2B). On the other hand, there were no significant relationships between any of the clinicopathological parameters (i.e., tumour stage, grade, infiltration, or survival rate) and the expression levels of miR-451a, miR-144-3p, and miR-144-5p (data not shown).
Figure 1.
The expression levels of miR-451a, miR-144-3p, and miR-144-5p. (A) qRT–PCR showed that the expression levels of miR-451a, miR-144-3p, and miR-144-5p were significantly lower in BC tissues and BC cell lines (T24 and BOY) than in non-BC tissues. (B) The correlated expression of miR-451a, miR-144-3p, and miR-144-5p. The correlation coefficients 0.656, 0.566, and 0.809 with P<0.0001 indicated that miR-451a, miR-144-3p, and miR-144-5p expression levels were highly correlated with each other.
Effect of miR-144-5p transfection on cell growth and cell cycle
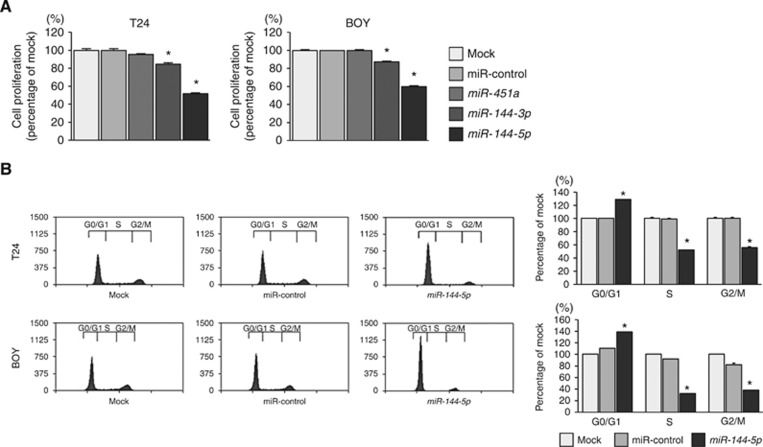
We performed gain-of-function studies using the transfectants of those miRNAs to investigate the functional roles of miR-451a, miR-144-3p, and miR-144-5p. The XTT assay showed that miR-144-3p and miR-144-5p transfectants significantly inhibited cancer cell proliferation comparison with mock or miR-control transfectants (P<0.0001, Figure 2A).
Figure 2.
Effects of miR-451a, miR-144-3p, and miR-144-5p transfection on the functionality of BC cell lines. (A) The XTT assay showed significant inhibition of cell proliferation in miR-144-3p and miR-144-5p transfectants in comparison with mock or miR-control transfectants. *P<0.0001. (B) Flow cytometric analysis of cell cycle phase distribution in mock, miR-control, or miR-144-5p transfectants. The bar charts represent the percentage of mock in G0/G1, S, and G2/M phases. In miR-144-5p transfectants, the fraction of cells in the G0/G1 phase was significantly larger compared with mock or miR-control transfectants. *P<0.0001.
Restoration of miR-144-5p significantly reduced cell proliferation in T24 and BOY cells, therefore we hypothesised that miR-144-5p induced cell cycle arrest in BC. With regard to the cell cycle distribution, the fraction of cells in the G0/G1 phase was significantly larger in miR-144-5p transfectants in comparison with mock or miR-control transfectants (P<0.0001, Figure 2B). These results suggested that miR-144-5p restoration induced G0/G1 arrest in BC cells.
Identification of molecular pathways modulated by miR-144-5p and putative target genes in BC cells
To gain further insight into molecular mechanisms and pathways regulated by tumour-suppressive miR-144-5p in BC cells, we performed genome-wide gene expression analysis using miR-144-5p-transfected cells, miR-144-5p-T24. In our microarray, a total of 1196 genes were downregulated <−1.0 (log2 ratio) in miR-144-5p-T24 transfectants, compared with negative control cells. The GENECODIS analysis categorised 75 significantly enriched signalling pathways (Supplementary Table S2). We focussed on the ‘cell cycle' pathway and the 23 genes listed within this pathway. Of those 23 genes, 15 genes were upregulated in the GEO database, and of those 15 genes, four genes (CCNE2, PKMYT1, CDC25A, and CCNE1) contained putative binding sites for miR-144-5p in their 3′-UTR according to the microRNA.org database (Table 1). Our strategy for this selection of miR-144-5p target genes is shown in Supplementary Figure S1. The details of the top five enriched pathways excluding ‘cell cycle' such as ‘DNA replication', ‘p53 signalling pathway', ‘pathways in cancer', and ‘peroxisome' are shown in Supplementary Table S3.
Table 1. Upregulated genes involved in ‘cell cycle'.
|
Gene expression omnibus |
|||||
|---|---|---|---|---|---|
| Entrez gene ID | Gene symbol | Description | Fold change (log2 ratio) | P-value | Target site |
| 991 | CDC20 | Cell division cycle 20 | 4.06 | 1.05E-03 | − |
| 9134 | CCNE2 | Cyclin E2 | 3.46 | 1.05E-03 | + |
| 9133 | CCNB2 | Cyclin B2 | 3.40 | 1.05E-03 | − |
| 9088 | PKMYT1 | Protein kinase, membrane-associated tyrosine/threonine 1 | 3.35 | 1.05E-03 | + |
| 891 | CCNB1 | Cyclin B1 | 3.12 | 1.05E-03 | − |
| 993 | CDC25A | Cell division cycle 25A | 3.09 | 1.05E-03 | + |
| 890 | CCNA2 | Cyclin A2 | 3.07 | 1.05E-03 | − |
| 898 | CCNE1 | Cyclin E1 | 2.73 | 1.05E-03 | + |
| 1869 | E2F1 | E2F transcription factor 1 | 2.33 | 1.05E-03 | − |
| 1870 | E2F2 | E2F transcription factor 2 | 1.89 | 1.05E-03 | − |
| 4173 | MCM4 | Minichromosome maintenance complex component 4 | 1.84 | 1.05E-03 | − |
| 4998 | ORC1 | Origin recognition complex, subunit 1 | 1.74 | 1.27E-02 | − |
| 2810 | SFN | Stratifin | 1.37 | 5.49E-03 | − |
| 4176 | MCM7 | Minichromosome maintenance complex component 7 | 1.28 | 1.05E-03 | − |
| 4175 | MCM6 | Minichromosome maintenance complex component 6 | 1.23 | 2.94E-03 | − |
CCNE1, CCNE2, CDC25A, and PKMYT1 were directly regulated by miR-144-5p
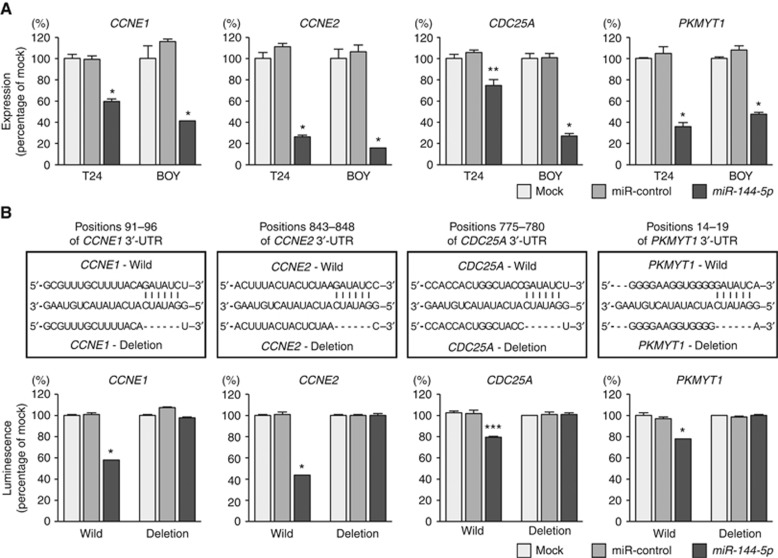
We performed qRT–PCR to confirm that restoration of miR-144-5p resulted in the downregulated expression of CCNE1, CCNE2, CDC25A, and PKMYT1 in T24 and BOY cells. The mRNA levels of CCNE1, CCNE2, CDC25A, and PKMYT1 were significantly reduced in miR-144-5p transfectants in comparison with mock or miR-control transfectants (P<0.005, Figure 3A).
Figure 3.
Direct regulation of CCNE1, CCNE2, CDC25A, and PKMYT1 by miR-144-5p. (A) The expression of CCNE1, CCNE2, CDC25A, and PKMYT1 were significantly repressed in miR-144-5p transfectants in comparison with mock or miR-control transfectants. GUSB was used as an internal control. *P<0.0001; **P=0.0022. (B) miR-144-5p binding sites in the 3′-UTRs of CCNE1, CCNE2, CDC25A, and PKMYT1 mRNAs. Dual-luciferase reporter assays using vectors encoding putative miR-144-5p target sites for wild-type or deleted regions. Normalised data were calculated as ratios of Renilla/firefly-luciferase activities. The luminescence intensity was significantly reduced by co-transfection with miR-144-5p and the vector carrying the wild-type, whereas transfection with the deletion vector blocked the decrease in luminescence. These data suggested that miR-144-5p bound directly to specific site in the 3′-UTRs of CCNE1, CCNE2, CDC25A, and PKMYT1 mRNAs. *P<0.0001; ***P=0.0002.
We performed dual-luciferase reporter assays in T24 cells to determine whether the four genes were directly regulated by miR-144-5p. The microRNA.org database predicted that there was one binding site for miR-144-5p in the 3′-UTRs of CCNE1 (positions 91–96), CCNE2 (positions 843–848), CDC25A (positions 775–780), and PKMYT1 (positions 14–19). We used vectors encoding the partial wild-type sequence of the 3′-UTR of the mRNA, including the predicted miR-144-5p target sites. We found that the luminescence intensity was significantly reduced by co-transfection with miR-144-5p and the vector carrying the wild-type 3′-UTR, whereas transfection with the deletion vector (binding site had been removed) blocked the decrease in luminescence (P<0.0005, Figure 3B). These data suggested that miR-144-5p bound directly to specific sites in the 3′-UTR of CCNE1, CCNE2, CDC25A, and PKMYT1 mRNAs.
Expression of CCNE1, CCNE2, CDC25A, and PKMYT1 in BC clinical specimens
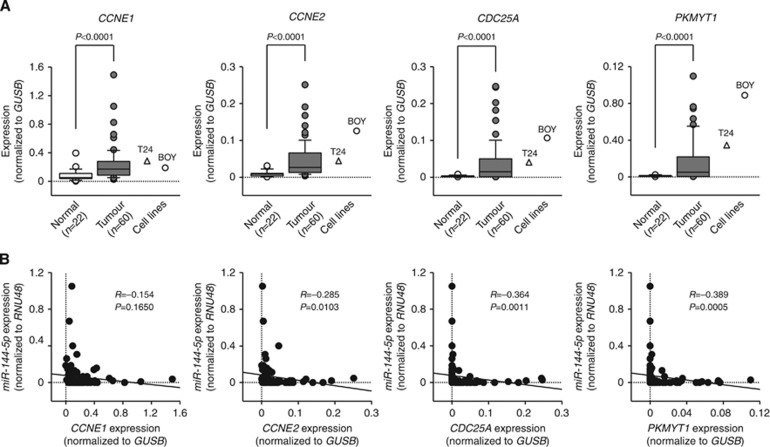
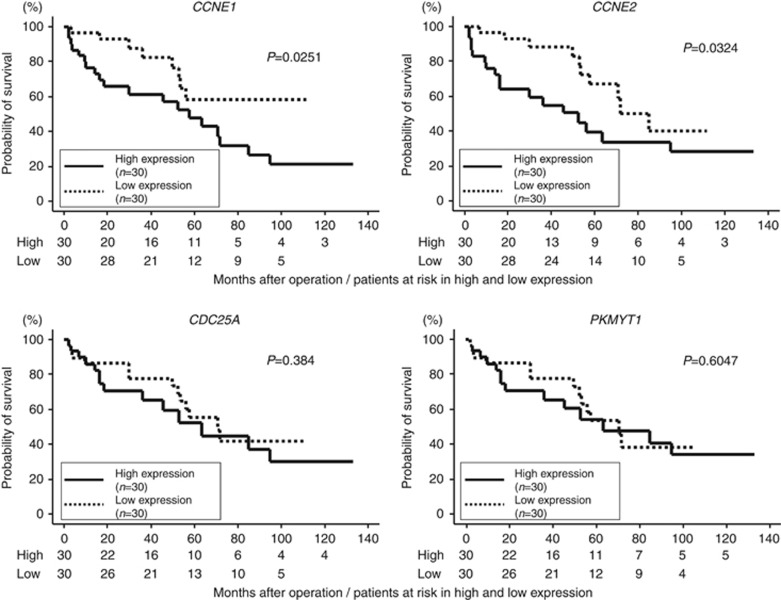
The qRT–PCR analysis showed that the expression levels of CCNE1, CCNE2, CDC25A, and PKMYT1 were significantly upregulated in 60 BC specimens and BC cell lines compared with 22 normal specimens (P<0.0001, Figure 4A). Spearman's rank test showed negative correlations between the expression of miR-144-5p and that of CCNE2, CDC25A, and PKMYT1 (R=−0.285, −0.364, and −0.389, respectively, P<0.05, Figure 4B). To determine whether the levels of CCNE1, CCNE2, CDC25A, and PKMYT1 mRNA in tumour tissues correlated with clinicopathological factors, we analysed those gene expression levels in human tumour samples. Only CCNE1 expression increased from the NMIBC to MIBC (P=0.0498, Supplementary Figure S3). Kaplan–Meier analysis showed that the high CCNE1 and CCNE2 expression groups had significantly lower overall survival probabilities compared with the low CCNE1 and CCNE2 expression groups (P=0.0251 and P=0.0324, respectively, Figure 5).
Figure 4.
Expression levels of four miR-144-5p target genes. (A) The expression levels of CCNE1, CCNE2, CDC25A, and PKMYT1 were significantly upregulated in BC tissues and BC cell lines in comparison with the normal bladder tissues. (B) The correlated expression levels between miR-144-5p and CCNE1, CCNE2, CDC25A, and PKMYT1. Expression levels of miR-144-5p and CCNE2 or CDC25A or PKMYT1 were significantly negatively correlated with each other.
Figure 5.
Kaplan–Meier survival plots for high and low expression groups for CCNE1, CCNE2, CDC25A, and PKMYT1 determined for 60 patients. Overall survival was significantly prolonged in patients with low CCNE1 and CCNE2 expression vs patients with high expression (P=0.0251 and P=0.0324, respectively).
Discussion
Aberrantly expressed miRNAs are frequently annotated in BC expression signatures. We suggest that they might be driver molecules for BC development, progression, and metastasis. Several expression signatures showed that miR-1, miR-26a, miR-29a/c, miR-133a/b, miR-143, miR-145, and miR-195 were downregulated in BC tissues, suggesting that these miRNAs function as tumour suppressors (Yoshino et al, 2013c). Interestingly, aberrantly expressed miR-1/133a, miR-29a/b, miR-143/145, and miR-195/497 are located close together in the human genome, constituting a cluster of miRNAs. Our previous studies demonstrated that miR-1/133a, miR-143/145, and miR-195/497 clustered miRNAs functioned as tumour suppressors via their targeting of several oncogenic pathways in BC cells and other types of cancers (Nohata et al, 2011, 2012; Yoshino et al, 2011, 2013a; Kawakami et al, 2012; Kojima et al, 2012, 2014; Yamasaki et al, 2012; Itesako et al, 2014). Identification of tumour-suppressive miRNAs and their mediated molecular pathways adds significantly to our understanding of BC oncogenesis.
Deep-sequencing analysis seems to be superior to array-based or PCR-based methods that can only determine a limited number of known miRNAs. In the future, deep-sequencing analysis is likely to become the gold standard for comprehensive miRNA analysis in cancer genomics. Our deep-sequence-based signature showed that miR-451a, miR-144-3p, and miR-144-5p clustered miRNAs were downregulated in BC tissues. In the present study, downregulation of the cluster was validated in BC clinical specimens and suggested that these miRNAs function as tumour suppressors in BC cells.
In this study, we focussed on miR-144-5p because it showed the strongest antitumour effects in BC cells. At present, there are few reports about miR-144-5p in human cancers. To better understand BC oncogenesis, we identified target genes and pathways of miR-144-5p using in silico analysis. Recent miRNA studies in our laboratory have utilised this strategy to identify novel molecular targets and pathways regulated by tumour-suppressive miRNAs in several cancers, including BC (Yoshino et al, 2013b; Fukumoto et al, 2014; Goto et al, 2014; Inoguchi et al, 2014; Nishikawa et al, 2014; Chiyomaru et al, 2015). Molecular target searches revealed that four cell cycle-promoting genes, CCNE1, CCNE2, CDC25A, and PKMYT1, were directly targeted by miR-144-5p in BC cells. Overexpression of these genes was confirmed in BC clinical specimens. Furthermore, Kaplan–Meier analysis showed that high CCNE1 and CCNE2 expression groups had significantly lower overall survival. These results suggest that these genes might have the potential to be good prognostic markers and therapeutic targets in BC.
Cyclin E proteins have critical roles in the G1 phase and in the G1–S phase transition with cyclin-dependent kinase 2 (CDK2). Overexpression of CCNE1 and CCNE2 has been reported in many types of human cancers, including BC. It is well established that these genes act as oncogenes (Donnellan and Chetty, 1999). Our present data clearly showed that restoration of miR-144-5p function in BC cells inhibited the expression of both CCNE1 and CCNE2 and significantly induced G1 arrest in BC cells. In the in vitro studies, CCNE/CDK2 activation has important role in tumourigenesis (Hwang and Clurman, 2005). Fu et al (2014) showed that overexpression of CCNE1 was associated with aggressiveness in clinical BC specimens. In addition, CCNE1/CDK2 inhibition significantly reduced tumour growth in trastuzumab-resistant HER2± breast cancer cell (Scaltriti et al, 2011). These results lead to an idea that CCNE-targeted therapy by miR-144-5p might be a new strategy in human cancers.
Our present data also showed that the CDC25A gene was regulated by miR-144-5p in BC cells. Overexpression of CDC25A has been reported in various cancers and contributes to cancer cell progression (Brunetto et al, 2013). Thus CDC25A is considered an oncogene, as it can cooperate with CCNE1/2 in promoting G1/S cell cycle transition in BC cells. Direct regulation of cell cycle regulators CCNE1/2 and CDC25A by tumour-suppressive miR-144-5p is important in developing new treatment of BC.
In previous studies of miRNA regulation of CCNE1, CCNE2, and CDC25A in cancers, it was shown that CCNE1 was regulated by miR-15 in glioma cells (Xia et al, 2009), miR-7 in HCC (Zhang et al, 2014), miR-132 in osteosarcoma cells (Wang et al, 2014), miR-195-5p in breast cancer (Luo et al, 2014), and miR-497 in cervical carcinoma cells (Han et al, 2014). In contrast, CCNE2 was regulated by miR-26a in pancreatic cancer (Deng et al, 2013), miR-1699 in ovarian cancer cells (Lee et al, 2012), miR-200 in MALT lymphoma (Cai et al, 2012), and miR-449 in gastric cancer (Bou Kheir et al, 2011). CDC25A is regulated by several miRNAs, such as the miR-195/497 cluster in HCC (Furuta et al, 2013), let-7 in lung cancer cells (Johnson et al, 2007), and miR-125b in glioma cells (Shi et al, 2010). We have recently reported that miR-195 and miR-497 act as tumour suppressors in BC cells and that they target BIRC5, a member of the inhibitor of apoptosis family (Itesako et al, 2014). Interestingly, our unpublished data show that the tumour-suppressive miR-195/497 cluster targets CCNE1, CCNE2, and CDC25A in BC cells (data not shown). Our tumour-suppressive miRNAs studies suggest that activation of the cell cycle and inhibition of cancer cell apoptosis might have roles in the recurrence of BC cancer. These findings suggest that molecules promoting G1/S cell cycle transition in BC cells would be critically important targets for BC therapeutic strategies.
Conclusions
Downregulation of the miR-451a, miR-144-3p, and miR-144-5p cluster was frequently observed in BC cells, and miR-144-5p significantly inhibited cancer cell proliferation by inducing cell cycle arrest. Cell cycle regulator genes, CCNE1, CCNE2, and CDC25A, were directly modulated by miR-144-5p. Furthermore, the expression levels of CCNE1 and CCNE2 might be good disease prognostic markers in BC.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Numbers 26293354, 25462490, and 26462416.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License
Supplementary Material
References
- Bou Kheir T, Futoma-Kazmierczak E, Jacobsen A, Krogh A, Bardram L, Hother C, Gronbaek K, Federspiel B, Lund AH, Friis-Hansen L. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol Cancer. 2011;10:29. doi: 10.1186/1476-4598-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetto E, Ferrara AM, Rampoldi F, Talarico A, Cin ED, Grassini G, Spagnuolo L, Sassi I, Ferro A, Cuorvo LV, Barbareschi M, Piccinin S, Maestro R, Pecciarini L, Doglioni C, Cangi MG. CDC25A protein stability represents a previously unrecognized target of HER2 signaling in human breast cancer: implication for a potential clinical relevance in trastuzumab treatment. Neoplasia. 2013;15:579–590. doi: 10.1593/neo.122054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Liu X, Cheng J, Li Y, Huang X, Li Y, Ma X, Yu H, Liu H, Wei R. MicroRNA-200 is commonly repressed in conjunctival MALT lymphoma, and targets cyclin E2. Graefes Arch Clin Exp Ophthalmol. 2012;250:523–531. doi: 10.1007/s00417-011-1885-4. [DOI] [PubMed] [Google Scholar]
- Chiyomaru T, Enokida H, Tatarano S, Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N, Nakagawa M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102:883–891. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyomaru T, Seki N, Inoguchi S, Ishihara T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako T, Nakagawa M, Enokida H. Dual regulation of receptor tyrosine kinase genes EGFR and c-Met by the tumor-suppressive microRNA-23b/27b cluster in bladder cancer. Int J Oncol. 2015;46:487–496. doi: 10.3892/ijo.2014.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, He M, Chen L, Chen C, Zheng J, Cai Z. The loss of miR-26a-mediated post-transcriptional regulation of cyclin E2 in pancreatic cancer cell proliferation and decreased patient survival. PLoS One. 2013;8:e76450. doi: 10.1371/journal.pone.0076450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G, Croce CM. Roles of small RNAs in tumor formation. Trends Mol Med. 2010;16:257–267. doi: 10.1016/j.molmed.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan R, Chetty R. Cyclin E in human cancers. FASEB J. 1999;13:773–780. doi: 10.1096/fasebj.13.8.773. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Fu YP, Kohaar I, Moore LE, Lenz P, Figueroa JD, Tang W, Porter-Gill P, Chatterjee N, Scott-Johnson A, Garcia-Closas M, Muchmore B, Baris D, Paquin A, Ylaya K, Schwenn M, Apolo AB, Karagas MR, Tarway M, Johnson A, Mumy A, Schned A, Guedez L, Jones MA, Kida M, Hosain GM, Malats N, Kogevinas M, Tardon A, Serra C, Carrato A, Garcia-Closas R, Lloreta J, Wu X, Purdue M, Andriole GL, Jr, Grubb RL, 3rd, Black A, Landi MT, Caporaso NE, Vineis P, Siddiq A, Bueno-de-Mesquita HB, Trichopoulos D, Ljungberg B, Severi G, Weiderpass E, Krogh V, Dorronsoro M, Travis RC, Tjonneland A, Brennan P, Chang-Claude J, Riboli E, Prescott J, Chen C, De Vivo I, Govannucci E, Hunter D, Kraft P, Lindstrom S, Gapstur SM, Jacobs EJ, Diver WR, Albanes D, Weinstein SJ, Virtamo J, Kooperberg C, Hohensee C, Rodabough RJ, Cortessis VK, Conti DV, Gago-Dominguez M, Stern MC, Pike MC, Van Den Berg D, Yuan JM, Haiman CA, Cussenot O, Cancel-Tassin G, Roupret M, Comperat E, Porru S, Carta A, Pavanello S, Arici C, Mastrangelo G, Grossman HB, Wang Z, Deng X, Chung CC, Hutchinson A, Burdette L, Wheeler W, Fraumeni J, Jr, Chanock SJ, Hewitt SM, Silverman DT, Rothman N, Prokunina-Olsson L. The 19q12 bladder cancer GWAS signal: association with cyclin E function and aggressive disease. Cancer Res. 2014;74 (20:5808–5818. doi: 10.1158/0008-5472.CAN-14-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto I, Kinoshita T, Hanazawa T, Kikkawa N, Chiyomaru T, Enokida H, Yamamoto N, Goto Y, Nishikawa R, Nakagawa M, Okamoto Y, Seki N. Identification of tumour suppressive microRNA-451a in hypopharyngeal squamous cell carcinoma based on microRNA expression signature. Br J Cancer. 2014;111:386–394. doi: 10.1038/bjc.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Kozaki K, Tanimoto K, Tanaka S, Arii S, Shimamura T, Niida A, Miyano S, Inazawa J. The tumor-suppressive miR-497-195 cluster targets multiple cell-cycle regulators in hepatocellular carcinoma. PLoS One. 2013;8:e60155. doi: 10.1371/journal.pone.0060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Nishikawa R, Kojima S, Chiyomaru T, Enokida H, Inoguchi S, Kinoshita T, Fuse M, Sakamoto S, Nakagawa M, Naya Y, Ichikawa T, Seki N. Tumour-suppressive microRNA-224 inhibits cancer cell migration and invasion via targeting oncogenic TPD52 in prostate cancer. FEBS Lett. 2014;588:1973–1982. doi: 10.1016/j.febslet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Han J, Huo M, Mu M, Liu J, Zhang J. [miR-497 suppresses proliferation of human cervical carcinoma HeLa cells by targeting cyclin E1] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30:597–600. [PubMed] [Google Scholar]
- Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24 (17:2776–2786. doi: 10.1038/sj.onc.1208613. [DOI] [PubMed] [Google Scholar]
- Ichimi T, Enokida H, Okuno Y, Kunimoto R, Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama K, Tsujimoto G, Nakagawa M, Seki N. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer. 2009;125:345–352. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- Inoguchi S, Seki N, Chiyomaru T, Ishihara T, Matsushita R, Mataki H, Itesako T, Tatarano S, Yoshino H, Goto Y, Nishikawa R, Nakagawa M, Enokida H. Tumour-suppressive microRNA-24-1 inhibits cancer cell proliferation through targeting FOXM1 in bladder cancer. FEBS Lett. 2014;588:3170–3179. doi: 10.1016/j.febslet.2014.06.058. [DOI] [PubMed] [Google Scholar]
- Itesako T, Seki N, Yoshino H, Chiyomaru T, Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa M, Enokida H. The microRNA expression signature of bladder cancer by deep sequencing: the functional significance of the miR-195/497 cluster. PLoS One. 2014;9:e84311. doi: 10.1371/journal.pone.0084311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Enokida H, Chiyomaru T, Tatarano S, Yoshino H, Kagara I, Gotanda T, Tachiwada T, Nishiyama K, Nohata N, Seki N, Nakagawa M. The functional significance of miR-1 and miR-133a in renal cell carcinoma. Eur J Cancer. 2012;48:827–836. doi: 10.1016/j.ejca.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Kojima S, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M, Seki N. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J Cancer. 2012;106:405–413. doi: 10.1038/bjc.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Enokida H, Yoshino H, Itesako T, Chiyomaru T, Kinoshita T, Fuse M, Nishikawa R, Goto Y, Naya Y, Nakagawa M, Seki N. The tumor-suppressive microRNA-143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J Hum Genet. 2014;59:78–87. doi: 10.1038/jhg.2013.121. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jeong W, Kim JH, Kim J, Bazer FW, Han JY, Song G. Distinct expression pattern and post-transcriptional regulation of cell cycle genes in the glandular epithelia of avian ovarian carcinomas. PLoS One. 2012;7:e51592. doi: 10.1371/journal.pone.0051592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LH S, MK G, C W.2009TNM Classification of Malignant Tumour, International Union Against cancer (UICC)7th edn,262–265.Wiley-Liss: New York, NY, USA [Google Scholar]
- Luo Q, Wei C, Li X, Li J, Chen L, Huang Y, Song H, Li D, Fang L. MicroRNA-195-5p is a potential diagnostic and therapeutic target for breast cancer. Oncol Rep. 2014;31:1096–1102. doi: 10.3892/or.2014.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T, Sternberg CN, Sonpavde G. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–533. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Nishikawa R, Goto Y, Sakamoto S, Chiyomaru T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya Y, Ichikawa T, Seki N. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci. 2014;105:802–811. doi: 10.1111/cas.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohata N, Hanazawa T, Enokida H, Seki N. microRNA-1/133a and microRNA-206/133b clusters: dysregulation and functional roles in human cancers. Oncotarget. 2012;3:9–21. doi: 10.18632/oncotarget.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohata N, Hanazawa T, Kikkawa N, Sakurai D, Sasaki K, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nakagawa M, Okamoto Y, Seki N. Identification of novel molecular targets regulated by tumor suppressive miR-1/miR-133a in maxillary sinus squamous cell carcinoma. Int J Oncol. 2011;39:1099–1107. doi: 10.3892/ijo.2011.1096. [DOI] [PubMed] [Google Scholar]
- Scaltriti M, Eichhorn PJ, Cortes J, Prudkin L, Aura C, Jimenez J, Chandarlapaty S, Serra V, Prat A, Ibrahim YH, Guzman M, Gili M, Rodriguez O, Rodriguez S, Perez J, Green SR, Mai S, Rosen N, Hudis C, Baselga J. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci USA. 2011;108 (9:3761–3766. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Zhang J, Pan T, Zhou J, Gong W, Liu N, Fu Z, You Y. MiR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Res. 2010;1312:120–126. doi: 10.1016/j.brainres.2009.11.056. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Shirahama T, Miyauchi T, Matsusako T, Kaneda N, Muramatsu H, Ozawa M, Ohi Y, Muramatsu T. Metanestin, a glycoprotein with metastasis-associated expression in transitional cell carcinoma of the urinary bladder. Int J Cancer. 1997;74 (1:7–14. doi: 10.1002/(sici)1097-0215(19970220)74:1<7::aid-ijc2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Shen F, Kang Y. miR-132 targeting cyclin E1 suppresses cell proliferation in osteosarcoma cells. Tumour Biol. 2014;35:4859–4865. doi: 10.1007/s13277-014-1637-2. [DOI] [PubMed] [Google Scholar]
- Xia H, Qi Y, Ng SS, Chen X, Chen S, Fang M, Li D, Zhao Y, Ge R, Li G, Chen Y, He ML, Kung HF, Lai L, Lin MC. MicroRNA-15b regulates cell cycle progression by targeting cyclins in glioma cells. Biochem Biophys Res Commun. 2009;380:205–210. doi: 10.1016/j.bbrc.2008.12.169. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Yoshino H, Enokida H, Hidaka H, Chiyomaru T, Nohata N, Kinoshita T, Fuse M, Seki N, Nakagawa M. Novel molecular targets regulated by tumor suppressors microRNA-1 and microRNA-133a in bladder cancer. Int J Oncol. 2012;40:1821–1830. doi: 10.3892/ijo.2012.1391. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N, Nakagawa M. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer. 2011;104:808–818. doi: 10.1038/bjc.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Enokida H, Itesako T, Kojima S, Kinoshita T, Tatarano S, Chiyomaru T, Nakagawa M, Seki N. Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in renal cell carcinoma. Cancer Sci. 2013;104:1567–1574. doi: 10.1111/cas.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Enokida H, Itesako T, Tatarano S, Kinoshita T, Fuse M, Kojima S, Nakagawa M, Seki N. Epithelial-mesenchymal transition-related microRNA-200s regulate molecular targets and pathways in renal cell carcinoma. J Hum Genet. 2013;58:508–516. doi: 10.1038/jhg.2013.31. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013;10:396–404. doi: 10.1038/nrurol.2013.113. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hu S, Zhang X, Wang L, Zhang X, Yan B, Zhao J, Yang A, Zhang R. MicroRNA-7 arrests cell cycle in G1 phase by directly targeting CCNE1 in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2014;443:1078–1084. doi: 10.1016/j.bbrc.2013.12.095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.