Abstract
Background:
Tongue squamous cell carcinoma (TSCC) is increasing in incidence, especially among young patients and preferably females. Infection with human papilloma virus (HPV) has been suggested as a cause of SCC in the head and neck, and the proportion of oropharyngeal cancers caused by HPV has steadily increased.
Methods:
Samples from 109 patients with primary TSCC were analysed for the presence of HPV16 by in situ hybridisation and for expression of its surrogate marker p16 and the HPV receptor syndecan-1 by immunhistochemistry.
Results:
No evidence of HPV16 DNA was observed in the tumours, although one-third showed p16 staining. There was no difference in the expression of the primary HPV receptor, syndecan-1, between TSCC and a group of tonsil SCC.
Conclusion:
Whereas p16 is expressed in some TSCCs, HPV16 is undetectable, therefore, p16 cannot be used as a surrogate marker for high-risk HPV-infection in this tumour. Despite presence of the HPV-receptor syndecan-1 in TSCC, HPV prefers the tonsillar environment. Lack of p16 associates with worse prognosis primarily in patients aged ⩽40 years with tongue SCC. The improved prognosis seen in p16-positive TSCC can be due to induction of a senescent phenotype or an inherent radiosensitivity due to the ability of p16 to inhibit homologous recombination repair.
Keywords: squamous cell carcinoma, tongue, HPV, p16, syndecan-1
Squamous cell carcinoma of the head and neck (SCCHN) is a collective term for tumours of several different locations within the head and neck area showing widely varying histology dependent on location. Even within the limited area of the oral cavity, there are differences in the expression of proteins and miRNAs between sites that are seen also between tumour-free samples (Boldrup et al, 2011; Boldrup et al, 2012). The most commonly tumour affected site within the oral cavity is the tongue, and tongue squamous cell carcinoma (TSCC) is an increasing group of tumours especially among young patients (Hilly et al, 2013; Troeltzsch et al, 2014) and preferably females (Patel et al, 2011). The reason for this increase is so far not known, even if it is clear that this age group, defined as being ⩽40 years, has not been exposed to the known risk factors for this disease, smoking and alcohol, for the same duration and extent as many of the older patients.
Recently, infection with human papilloma virus (HPV) has been suggested as a cause of SCCHN, especially among young patients, and the proportion of oropharyngeal cancers caused by HPV has steadily increased in recent years (Marur et al, 2010; Chaturvedi et al, 2011). Thus an increase in HPV infection incidence could possibly relate also to the increased incidence of TSCC in young patients. In order to infect an epithelial cell, HPV first has to bind to and then enter the cell (Rautava and Syrjänen, 2012). In this binding process, a heparin sulphate proteoglycan, syndecan-1, has been pinpointed as the primary receptor for HPV in keratinocytes (Shafti-Keramat et al, 2003). Infection seems to be restricted to tumour only, as HPV-positive oropharyngeal tumours show complete lack of active HPV around the tumour and also lack field cancerisation (Rietbergen, et al, 2014). These observations emphasise that HPV-positive and -negative tumours are two distinct groups, at least in the oropharyngeal area (Pannone et al, 2011), where HPV-associated tumours often present at higher clinical stage with advanced nodal disease, despite being of smaller size. Despite this advanced clinical stage, the prognosis and overall disease-free survival for patients suffering from these tumours are superior to that of patients with non-HPV-associated tumours (Shah et al, 2009).
There are several ways of analysing HPV infection available (Schlecht et al, 2011), including PCR and in situ hybridisation for specific HPV types, most commonly the high-risk-type HPV16. It has also been proposed that expression of p16 correlates with HPV infection. The tumour-suppressor p16 (also called Cdkn2a) is a cdk (cyclin-dependent kinase) inhibitor, which inhibits binding of cdks 4 and 6 to cyclin D1. This in turn inhibits phosphorylation of Rb, which is needed for release of E2F to enable entry into the cell cycle (Witkiewicz et al, 2011). Apart from being a tumour suppressor, p16 is also a surrogate marker for high-risk HPV infection and has been found to be upregulated in HPV-positive oropharyngeal cancers. HPV infection could lead to accumulation of p16 protein via targeting of Rb (Witkiewicz et al, 2011). p16 is expressed in a wide variety of SCCs, other than those originating from cervix, and seems to be a reliable surrogate marker for high-risk HPV also in oropharynx. However, p16 is not a specific marker of HPV status in non-oropharyngeal SCC (Doxtader and Katzenstein, 2012). In a small group of 25 young (<40 years) patients with TSCC, p16 positivity correlated with improved relapse-free survival (Harris et al, 2011). This finding is in accordance with another study of oral SCC where patients with cancer with lower p16 expression were more likely to develop a recurrence (Shah et al, 2009).
In this study, we clarified the clinical and prognostic importance of HPV 16 and p16 in a large group of SCC tumours in the mobile tongue. By comparing the expression of the HPV receptor syndecan-1 in a subgroup of these TSCC with a group of tonsillar cancers, we also wanted to clarify whether there is any difference in the expression of this receptor between these sites, which could explain the differences in incidence of HPV infection between them.
Materials and Methods
Materials
Samples from 96 patients with primary TSCC and formalin-fixed, paraffin-embedded biopsies available at Clinical Pathology, Umeå University Hospital, Sweden, and 13 patients available at the Second University of Naples, Multidisciplinary Department of Medical, Surgical and Dental Specialties, Naples, Italy were included in the project. Both patient groups had been treated during a period of 15 years. Of the 109 patients, 54 were men and 55 were women with a mean age of 63.5 years, ranging from 19 to 93 years. Patients were grouped into three groups based on age at diagnosis: ⩽40, 41–65, and >65 years. For clinical data, see Table 1. The majority of patients (66%) had received preoperative radiotherapy followed by surgery and 31% were primarily treated with surgery.
Table 1. Patient data, including age at diagnosis, gender and TNM stage.
| Age at diagnosis, years | Number | Male/female ratio | T1 | T2 | T3 | T4 | N0 | N+ | M0 | M1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ⩽40 | 16 14.7% | 7/9 1 : 1.3 | 2 | 10 | 2 | 2 | 12 | 4 | 16 | 0 | 16 |
| 41–65 | 38 34.9% | 26/12 2.2 : 1 | 14 | 11 | 9 | 4 | 27 | 11 | 37 | 1 | 38 |
| >65 | 55 50.4% | 21/34 1 : 1.6 | 15 | 19 | 8 | 13 | 41 | 14 | 54 | 1 | 55 |
| 109 | 31 | 40 | 19 | 19 | 80 | 29 | 107 | 2 | 109 |
Abbreviations: M=distant metastasis; N=nodal metastasis; T=tumour size.
The mean follow-up time was 45.5 months (ranging from 1 to 179 months). At the end of the study, survival was measured as: alive disease free, alive with disease, dead of disease, dead of other disease or dead with disease but not with oral cancer as first cause of death. Data on survival and cause of death were obtained from the clinical files or the Swedish Death Registry.
A group of 65 patients with tonsillar carcinoma (17 women and 48 men) were included in the analysis of syndecan-1 expression. The mean age within this group was 59.9 years (range 45–87). This group of tumours had been analysed previously for HPV presence and p16 expression (Loizou et al, 2015). The project was approved by the local Ethical Committee (dnr 03–201 and dnr 08-003M).
Immunohistochemistry
For detection of p16, the antibody (Santa Cruz Biotechnology, Dallas, TX, USA) was diluted 1 : 200. Slides were pretreated in Tris-EDTA pH 8.0, and staining was performed in a Ventana staining machine (Ventana Medical Sytems Inc, Roche, Tuscon, AZ, USA) according to the supplier's recommendations. Eighty-nine of the tongue tumours and 65 cases of tonsillar cancer were also stained with an antibody detecting syndecan-1 (Abcam, Cambridge, UK) diluted 1 : 100, after pretreatment in citrate buffer pH 6.0. Staining was performed in a Ventana staining machine.
Scoring
Samples were scored for proportion of tumour cells expressing p16 and syndecan-1 and for intensity of staining. Proportion of tumour cells expressing the proteins was divided into six stages, where 1=0–4%, 2=5–19%, 3=20–39%, 4=40–59%, 5=60–79% and 6=80–100%, and intensity in four stages, with 0=negative, 1=weak, 2=intermediate and 3=strong staining. By multiplying the percentage of tumour cells expressing the protein with intensity, a quick score (QS) ranging from 0 to 18 was obtained (Detre et al, 1995). The p16-stained slides were evaluated independently by three of the authors NS, KS and KN. Results were then compared, and cases of disagreement were discussed in a joint session. The syndecan-stained slides were evaluated by KN only.
HPV16 in situ hybridisation
In situ hybridisation was used to investigate the presence of HPV16 DNA in 71 of the samples (all 36 p16-positive and 35 p16-negative tumours). HPV16 plasmid DNA was obtained from ATCC (LGC Standards, Middlesex, UK), amplified and purified using HiPure Plasmid Maxi-prep Kit (Invitrogen, Paisley, UK). Plasmid DNA was labelled by nick-translation (Invitrogen) for 90 min at 14 °C in the presence of digoxigenin-16-dUTP (Roche, West Sussex, UK) and purified by repeated ethanol precipitation in the presence of 100 × excess of salmon sperm and Cot-1 DNA (Invitrogen). Sections were dewaxed, endogenous peroxidase activity was blocked in H2O2 in methanol and tissue digested with varying concentrations of proteinase K (Sigma) in 50 mM Tris pH 7.5 and 1 mM EDTA pH 8.0. Probe (1 ng μl−1) in hybridisation buffer was applied, and sections were coverslipped before rapid high temperature micowave-mediated denaturation, as previously described (Coates et al, 1987; Coates et al, 1991). After overnight hybridisation at 42 °C, sections were washed twice in 2 × SCC at room temperature, twice in 0.1 × SCC at 45 °C and once in 2 × SCC at room temperature, each for 5 min. For immunoshistochemical detection, sections were incubated with mouse anti-digoxin (1/5000; Sigma) followed by biotinylated anti-mouse and avidin–biotin peroxidase complex (Elite ABC Kit, Vector Laboratories (Cambridgeshire, UK), used according to the manufacturer's instructions) and detection with an intensified DAB/imidazole reaction. Nuclei were lightly stained with haematoxylin, dehydrated, cleared and mounted in resin for light microscopy. A positive control section (cervix) was performed with each batch of tumours analysed.
Statistical analysis
SPSS version 22 (IBM Corporation, New York, NY, USA) was used for statistical analysis. QSs were correlated to clinical data. For calculation of P-values, Chi2-test was used, and in survival analysis 2- and 5-year survival was used. A P-value <0.05 was considered statistically significant.
Results
Clinical data
The majority of tumours were localised on the lateral border of the mobile tongue (67%), 20% on the ventral side and 2% on the dorsal side. In 11%, lesions were so widespread that it was not possible to state the prime localisation of the lesion on the mobile tongue. There was a statistically significant correlation between patients suffering from an extended lesion showing both lower survival rate and disease-free status (P=0.009 for 2-year survival, 0.027 for 5-year survival and 0.022 for status). Patients in the young age group (⩽40 years) showed lower survival and disease-free rate compared with the older age patients (>65 years). Gender did not affect survival. A statistically significant correlation was seen between T and staging and survival rate and disease-free condition, with decreased survival with increased T (P=0.000 for 2-year survival, 0.002 for 5-year survival and 0.001 for status) and higher stage (P=0.000 for 2-year survival, 0.010 for 5-year survival and 0.003 for status). There was also a statistically significant correlation between node positive, N+, tumours and poor 2-year survival rate (P=0.040; Table 2).
Table 2. Patient outcome at end of the study, with female:male ratio given in parenthesis.
|
Status (female:male) |
2-year survival |
5-year survival |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis, years | ADF/DAD | AWD/DOD/DWD | Yes | No | Not passed | Yes | No | Not passed | Relapse | Never free of tumour | |
| ⩽40 | 5 (2 : 3) | 11 (7 : 4) | 8 | 8 | 0 | 5 | 11 | 0 | 11 | 0 | 16 |
| 41–65 | 25 (5 : 20) | 13 (7 : 6) | 26 | 12 | 0 | 19 | 13 | 6 | 8 | 6 | 38 |
| >65 | 23 (13 : 10) | 32 (21 : 11) | 25 | 26 | 4 | 13 | 28 | 14 | 10 | 19 | 55 |
| 53 | 56 | 59 | 46 | 4 | 37 | 52 | 20 | 29 | 25 | 109 | |
Abbreviations: ADF=alive, disease-free, AWD=alive with disease, DAD=dead of other disease; DOD=dead of disease, DWD=dead with disease but not with oral cancer as the first cause of death.
Immunohistochemistry
p16
Of the 109 tumour samples, 73 (67%) were negative for the presence of p16 independent of site of lesion. Weak expression (defined as a QS of 1–5) was seen in 19%, and a QS of 6–18 in 14% (Table 3 and Figure 1). Comparing p16 expression between age groups, 75% of tumours in patients aged ⩽40 years were p16 negative, compared with 66% within the other two age groups (Table 3). At the 5-year follow-up, 4 of the 12 p16-negative young patients (33%) were alive, compared with 62% of the patients aged 41–65 years having passed 5-year follow-up.
Table 3. p16 status in relation to patient age, N status and 2- and 5-year survival, respectively.
| p16 QS | Patients aged ⩽40 years | Patients aged 41–65 years | Patients aged >65 years | N0 | N+ | 2-year survival | 5-year survival | |
|---|---|---|---|---|---|---|---|---|
| 0 | 12 (75%) | 25 (66%) | 36 (66%) | 58 (72%) | 15 (52%) | 44 (75%) | 28 (76%) | 73 (67%) |
| 1–5 | 2 (12.5%) | 9 (24%) | 10 (18%) | 15 (19%) | 6 (21%) | 9 (15%) | 5 (13%) | 21 (19%) |
| 6–18 | 2 (12.5%) | 4 (10%) | 9 (16%) | 7 (9%) | 8 (27%) | 6 (10%) | 4 (11%) | 15 (14%) |
| Total | 16 | 38 | 55 | 80 | 29 | 59/105 | 37/89 | 109 |
Abbreviation: N=nodal metastasis; QS=quick score. Concerning 2-year survival, 4 patients had not been followed that long, and in the analysis of 5-year survival, 20 patients had too short follow-up.
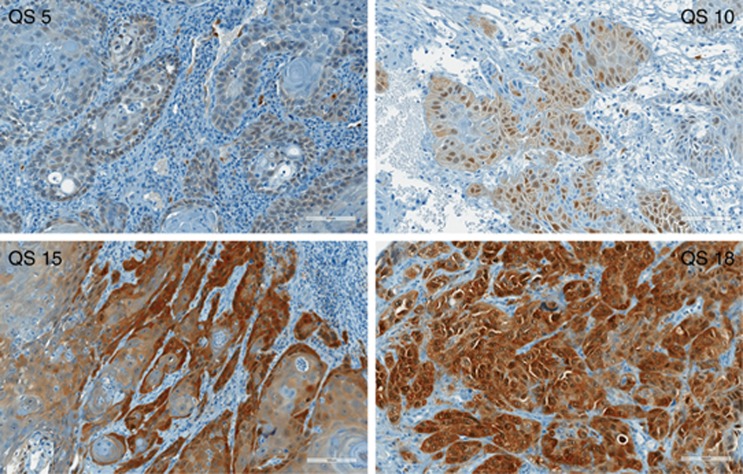
Figure 1.
p16 expressing tongue SCC with a QS of 5, 10, 15 and 18 respectively.
Of the 29 node positive, N+, tumours, 52% were p16 negative. No correlation was seen between p16 and localisation of the lesion, age, gender, grading or relapse.
Syndecan
All 89 tongue SCCs analysed for syndecan expression were positive, with 82% having a QS of 6–18. There was no statistically significant correlation between expression of p16 and syndecan-1. Most patients with a QS of 6–18, 65%, were N0. Similar results were seen for patients with tonsillar carcinoma, with all tumours expressing the receptor, and the majority, 74%, having a QS of 6–18 (Table 4 and Figure 2).
Table 4. Syndecan status in relation to age of patients with tongue and tonsillar SCC, respectively.
|
Tongue SCC |
Tonsil SCC |
|||||
|---|---|---|---|---|---|---|
| Syndecan QS | ⩽40 years | 41–65 years | >65 years | ⩽40 years | 41–65 years | >65 years |
| 0 | 0 | 0 | 0 | — | 0 | 0 |
| 1–5 | 3 | 5 | 8 | — | 14 | 3 |
| 6–18 | 11 | 29 | 33 | — | 36 | 12 |
| Total | 14 | 34 | 41 | — | 50 | 15 |
Abbreviation: QS=quick score; SCC, squamous cell carcinoma.
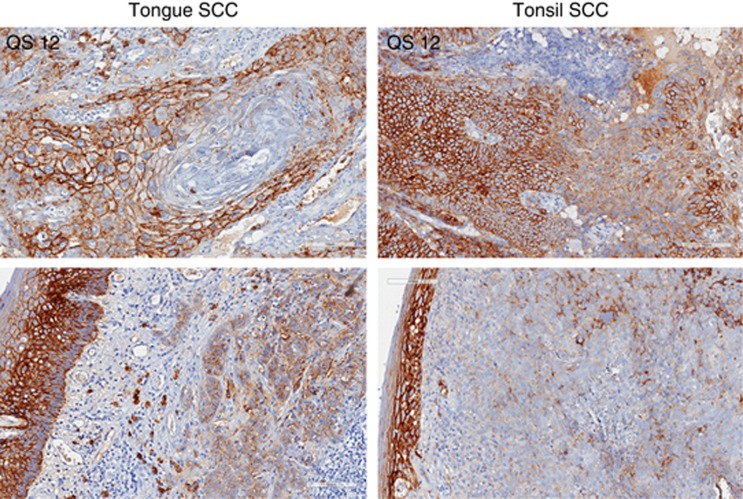
Figure 2.
Expression of syndecan in tongue SCC, left panel, and tonsillar SCC, right panel. The two photos at the bottom show intense syndecan staining in surface epithelium in contrast to considerably weaker expression in tumour cells in the connective tissue.
HPV16 in situ hybridisation
In the 71 samples analysed, including all 36 p16-positive samples, no evidence of HPV16 DNA was observed in the tumour tissue. The technique used is able to detect low copy number viral DNA (approximately two copies of HPV DNA per cell; Coates et al, 1991; Herrington et al, 1991), and a positive control of human cervical epithelium with histological evidence of productive infection showed the presence of a hybridisation signal throughout the epithelium, including basal cells that contain only a few viral DNA copies per cell.
Discussion
Squamous cell carcinoma of the head and neck remains a significant problem and is the eighth most common cause of cancer death worldwide. The aetiology of epithelial cancers of the head and neck is considered to be a multifactorial, sequential process. Several factors are involved in oral carcinogenesis, such as age, gender, ethnicity, lifestyle, genetic background, status of health and exposure to one or more oncogenic factors. The two major lifestyle risk factors in SCCHN are tobacco use and alcohol; however, 15–20% of patients do not have any known tobacco or alcohol exposure (Patel et al, 2011). The disease incidence has also been seen increasing among younger patients who often lack these traditional risk factors, and one unique subgroup of patients identified is young patients with TSCC. The tongue is the most common site for oral cancer development, and TSCC is more aggressive than other SCCs of the oral cavity, with properties of rapid local invasion and high regional relapse rate. TSCCs also show a more split invasive growth pattern and a more intense inflammatory response at the tumour interface compared with the whole group of SCCHN tumours (Lundqvist et al, 2012).
TSCCs may not genomically differ when comparing young and older patients (Pickering et al, 2014), and it has been hypothesised that the increasing incidence of SCCHN in young patients is related to infection with high-risk subtypes of the HPV. In normal oral mucosa, the incidence of HPV infection is very low (Migaldi et al, 2012), whereas a recent meta-analysis showed a strong association between HPV and oral potentially malignant lesions and oral carcinoma (Syrjanen et al, 2011). HPV-positive tumours are generally found in the oropharynx and have been associated with younger patients who are less likely to be smokers or drinkers and show improved response to therapy and overall survival (Pannone et al, 2011; Sand and Jalouli, 2014). The proportion of SCCHN that are potentially HPV related (cancers of the tongue base and the Waldeyer's ring) increased in the past 30 years, perhaps as a result of changing sexual behaviours, and nowadays about 18% of oropharyngeal cancers and >90% of tonsillar cancers worldwide are HPV associated (Pannone et al, 2011; Loizou et al, 2015).
We have previously not been able to detect HPV in TSCCs using PCR and Luminex, whereas 91% of tonsillar carcinomas were HPV positive using the same PCR method (Loizou et al, 2015). Based on the clinical impact of mapping HPV status in oropharyngeal SCC (Pannone et al, 2011), we were encouraged to go further in this analysis and used here in situ hybridisation for detection of HPV16. However, no virus could be detected in TSCCs using this highly sensitive method, capable of detecting very low viral copy numbers in both experimental situations and clinical samples (Coates et al, 1991; Herrington et al, 1991). These data therefore suggest that HPV16 is either not present or is present at extremely low levels in the majority of p16-positive TSCCs, including those arising in young patients. However, due to the variable fixation and processing of these clinical samples, which influences the sensitivity of in situ hybridisation, we cannot completely exclude the presence of HPV16, and it is also possible that other high-risk HPV types are present, although other studies consistently demonstrate that HPV16 is the most prevalent type found in the oral cavity (Chaturvedi et al, 2011; Schlecht et al, 2011).
Molecular profiling of HPV-positive tumours has shown them to be commonly associated with p16 overexpression, whereas tumours not associated with HPV are seldom p16 positive. The lack of p16 expression defines a subgroup of oropharyngeal cancer patients with increased risk of local recurrence and worse clinical outcome (Shah et al, 2009). p16 protein overexpression has thus been proposed as a surrogate marker of HPV infection even if not all studies confirm its prognostic significance in SCCHN (Gröbe et al, 2013). In our group of tongue tumours, 67% were p16 negative. Considering age, 75% of young patients were p16 negative compared with the other age groups where 66% of the tumours were p16 negative. Looking at follow-up for the p16-negative young patients, the majority (67%) was either still suffering from tumour or dead of or with disease. Even if differences are not statistically significant (the group of young patients is limited), results are in accordance with the previously shown worse outcome for p16-negative tumours (Shah et al, 2009) and also in line with the study of Harris et al (2011) showing the importance of p16 as a prognostic marker. Our results are further in concordance with the worse prognosis shown for young patients with TSCC (Lundqvist et al, 2012). The cases showing p16 expression in the absence of detectable HPV could in turn be explained by infection with other HPV types (Jordan et al, 2012), other unidentified infectious agents (Harris et al, 2011), or molecular alterations in the p16 pathway independent of infection with high-risk HPV, which may include transcriptional upregulation by oncogenic transcription factors such as Ets and Myc, alterations of Ras-MAPK pathways or loss of Rb (reviewed in Li et al, 2011; Romagosa et al, 2011; Witkiewicz et al, 2011). Indeed, high-level expression of p16 in the absence of HPV is well recognised outside of oropharyngeal and cervical cancer (Doxtader and Katzenstein, 2012; Hoffmann et al, 2012; Bussu et al, 2013). Thus, although unrelated to HPV, p16 expression in tongue SCC reflects an oncogenic process that is different from p16-negative cancers and provides an improved prognosis.
Another interesting finding was that expression of the primary receptor for HPV, syndecan-1, did not differ between these tongue SCC and a group of tonsillar SCC with a high percentage, 91%, of HPV infection (Loizou et al, 2015). This indicates that conditions for entering the tissue are fairly similar between tongue and tonsil, at least considering receptor availability, yet the virus seems to prefer the tonsillar environment. Recently, there has been a discussion on the impact of co-infection with various viruses, for example, HSV and EBV, where the latter preferably infects cells in a lymphocytic environment. Results are, however, not conclusive (Sand and Jalouli, 2014), still it is tempting to speculate that an explanation for the absence of HPV infection seen in tongue SCC could be lack of viral collaboration.
Taken together, we have shown that HPV16 is undetectable in tongue SCC. It can also be concluded that p16 cannot be used as a surrogate marker for HPV infection in tongue SCC, at least not when using the methods currently at hand. Looking at the prime receptor for HPV, syndecan-1, conditions for enabling entrance in the tissue are the same in the tongue as in the tonsil that gives room for speculation on the potential value of co-infection with other viruses or factors more common in the tonsillar area.
In concert with HPV-positive OSCC showing overall better outcome than HPV-negative oral cancers (Pannone et al, 2011), we suggest that lack of p16 expression in TSCC is an indicator of worse prognosis primarily in young patients suffering from this devastating disease. Although the mechanism(s) for improved prognosis in p16-positive TSCC is unclear, it may relate to either the induction of a senescent phenotype and thereby slow tumour growth (Ramagosa et al, 2011; Witkiewicz et al, 2011) or to an inherent radiosensitivity due to impaired DNA double-strand break repair capacity (Rieckmann et al, 2013), which in turn may relate to the ability of p16 to directly inhibit homologous recombination repair in HPV-positive SCCHN (Dok et al, 2014).
Acknowledgments
This study was supported by grants from Lion's Cancer Research Foundation, LP 13–1997 and LP 13–1998, Umeå University, the Swedish Cancer Society Contract number 14 0752, Västerbotten County Council and RECAMO; CZ 1.05/2.1.00/03.0101 grant LO1413.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Boldrup L, Coates PJ, Laurell G, Nylander K. Differences in p63 expression in SCCHN tumours of different sub-sites within the oral cavity. Oral Oncol. 2011;47:861–865. doi: 10.1016/j.oraloncology.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Boldrup L, Coates PJ, Laurell G, Nylander K. p63 transcriptionally regulates BNC1, a Pol I and Pol II transcription factor that regulates ribosomal biogenesis and epithelial differentiation. Eur J Cancer. 2012;48 (9:1401–1406. doi: 10.1016/j.ejca.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Bussu F, Sali M, Vellone VG, Zannoni GF, Autorino R, Dinapoli N, Santangelo R, Martucci R, Graziani C, Miccickè F, Almadori G, Galli J, Delogu G, Sanguinetti M, Rindi G, Valentini V, Paludetti G. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker. Br J Cancer. 2013;108:1157–1162. doi: 10.1038/bjc.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29 (32:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PJ, Hall PA, Butler MG, d'Ardenne AJ. Rapid technique of DNA-DNA in situ hybridisation on formalin fixed tissue sections using microwave irradiation. J Clin Pathol. 1987;40 (8:865–869. doi: 10.1136/jcp.40.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PJ, Slavin G, D'Ardenne AJ. Persistence of Epstein-Barr virus in Reed-Sternberg cells throughout the course of Hodgkin's disease. J Pathol. 1991;164 (4:291–297. doi: 10.1002/path.1711640404. [DOI] [PubMed] [Google Scholar]
- Detre S, Saccani Jotti G, Dowsett M. A ‘quickscore' method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dok R, Kalev P, van Limbergen EJ, Asbagh LA, Vázquez I, Hauben E, Sablina A, Nuyts S. p16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Res. 2014;74:1739–1751. doi: 10.1158/0008-5472.CAN-13-2479. [DOI] [PubMed] [Google Scholar]
- Doxtader EE, Katzenstein ALA. The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: a study of 137 cases. Hum Pathol. 2012;43:327–332. doi: 10.1016/j.humpath.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Gröbe A, Hanken H, Kluwe L, Schöllchen M, Tribius S, Pohlenz P, Clauditz T, Grob T, Simon R, Sauter G, Heiland M, Blessmann M. Immunohistochemical analysis of p16 expression, HPV infection and its prognostic utility in oral squamous cell carcinoma. J Oral Pathol Med. 2013;42 (9:676–681. doi: 10.1111/jop.12086. [DOI] [PubMed] [Google Scholar]
- Harris SL, Thorne LB, Seaman WT, Hayes DN, Couch ME, Kimple RJ. Association of p16INK4a overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head and Neck. 2011;33:1622–1627. doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]
- Herrington CS, Graham AK, McGee JO. Interphase cytogenetics using biotin and digoxigenin labelled probes: III. Increased sensitivity and flexibility for detecting HPV in cervical biopsy specimens and cell lines. J Clin Pathol. 1991;44 (1:33–38. doi: 10.1136/jcp.44.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilly O, Shkedy Y, Hod R, Soudry E, Mizrachi A, Hamzany Y, Bachar G, Shpitzer T. Carcinoma of the oral tongue in patients younger than 30 years: comparison with patients older than 60 years. Oral Oncol. 2013;49 (10:987–990. doi: 10.1016/j.oraloncology.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Tribius S, Quabius ES, Henry H, Pfannenschmidt S, Burkhardt C, Görögh T, Halec G, Hoffmann AS, Kahn T, Röcken C, Haag J, Waterboer T, Schmitt M. HPV DNA, E6*I-mRNA expression and p16INK4A immunohistochemistry in head and neck cancer—how valid is p16INK4A as surrogate marker. Cancer Lett. 2012;323:88–96. doi: 10.1016/j.canlet.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, Jiang B, Wakely P, Xiao W, Gillison ML. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36 (7:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16INK4A and their relevance to cancer. Biochemistry. 2011;50:5566–5582. doi: 10.1021/bi200642e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou C, Laurell G, Lindquist D, Öfverman C, Stefansson K, Nylander K, Olofsson K.2015Incidence of tonsillar cancer in northern Sweden: impact of human papilloma virus Oncol Lett http://dx.doi.org/10.3109/00016489.2015.1048378 . [DOI] [PMC free article] [PubMed]
- Lundqvist L, Stenlund H, Laurell G, Nylander K. The importance of stromal inflammation in squamous cell carcinoma of the tongue. J Oral Pathol Med. 2012;41 (5:379–383. doi: 10.1111/j.1600-0714.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- Marur S, D́Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaldi M, Pecorari M, Forbicini G, Nanni N, Grottola A, Grandi T, Delle Donne G, Leocata P, Trovato D, Sgambato A. Low prevalence of human papillomavirus infection in the healthy oral mucosa of a northern Italian population. J Oral Path Med. 2012;41:16–20. doi: 10.1111/j.1600-0714.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- Pannone G, Santoro A, Carinci F, Bufo P, Papagerakis SM, Rubini C, Campisi G, Giovanelli L, Contaldo M, Serpico R, Mazzotta M, Lo Muzio L. Double demonstration of oncogenic high risk human papilloma virus DNA and HPV-E7 protein in oral cancers. Int J Immunpathol Pharmacol. 2011;24 (2S:95–101. doi: 10.1177/03946320110240S217. [DOI] [PubMed] [Google Scholar]
- Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, Hayes DN, Shores C, Chera BS. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29:1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- Pickering CR, Zhang J, Neskey DM, Zhao M, Jasser SA, Wang J, Ward A, Tsai CJ, Ortega Alves MV, Zhou JH, Drummond J, El-Naggar AK, Gibbs R, Weinstein JN, Wheeler DA, Wang J, Frederick MJ, Myers JN. Squamous cell carcinoma of the oral tongue in young non-smokers is genomically similar to tumors in older smokers. Clin Cancer Res. 2014;20 (14:3842–3848. doi: 10.1158/1078-0432.CCR-14-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautava J, Syrjänen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol. 2012;6:S3–S15. doi: 10.1007/s12105-012-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann T, Tribius S, Grob TJ, Meyer F, Busch CJ, Petersen C, Dikomey E, Kriegs M. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242–246. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Rietbergen MM, Braakhuis BJM, Moukhtari N, Bloemena E, Bring A, Sie D, Ylstra B, Baatenburg de Jong RJ, Snijders PJF, Brakenhoff RH, Leemans CR. No evidence for active human papillomavirus (HPV) in fields surrounding HPV-positive oropharyngeal tumours. J Oral Pathol Med. 2014;43:137–142. doi: 10.1111/jop.12123. [DOI] [PubMed] [Google Scholar]
- Romagosa C, Simonetti S, Lòpez-Vicente L, Mazo A, Lleonart ME, Castellvi J, Cajal SR. p16INK4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30:2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- Sand L, Jalouli J. Viruses and oral cancer. Is there a link. Microbes Infect. 2014;16:371–378. doi: 10.1016/j.micinf.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, Smith RV, Burk RD, Prystowsky MB. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Modern Pathol. 2011;24 (10:1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NG, Trivedi TI, Tankshali RA, Goswami JV, Jetly DH, Shukla SN, Shah PM, Verma RJ. Prognostic significance of molecular markers in oral squamous cell carcinoma: a multivariate analysis. Head Neck. 2009;31:1544–1556. doi: 10.1002/hed.21126. [DOI] [PubMed] [Google Scholar]
- Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparin sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77 (24:13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjanen S, Lodi G, von Bultzingslowen I, Aliko A, Arduino A, Campisi G, Challacombe S, Ficarra G, Flaitz C, Zhou HM, Maeda H, Miller C, Jontell M. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17 (Suppl. 1:58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- Troeltzsch M, Knösel T, Eichinger C, Probst F, Troeltzsch M, Woodlock T, Mast G, Ehrenfeld M, Otto S. Clinicopathologic features of oral squamous cell carcinoma: do they vary in different age groups. J Oral Maxillofac Surg. 2014;72 (7:1291–1300. doi: 10.1016/j.joms.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Witkiewicz AK, Knudsen KE, Picker AP, Knudsen ES. The meaning of p16ink4a expression in tumor. Functional significance, clinical associations and future developments. Cell Cycle. 2011;10 (15:2497–2503. doi: 10.4161/cc.10.15.16776. [DOI] [PMC free article] [PubMed] [Google Scholar]