Abstract
Background:
The melanocortin-1-receptor (MC1R) gene regulates human pigmentation and is highly polymorphic in populations of European origins. The aims of this study were to evaluate the association between MC1R variants and the risk of non-melanoma skin cancer (NMSC), and to investigate whether risk estimates differed by phenotypic characteristics.
Methods:
Data on 3527 NMSC cases and 9391 controls were gathered through the M-SKIP Project, an international pooled-analysis on MC1R, skin cancer and phenotypic characteristics. We calculated summary odds ratios (SOR) with random-effect models, and performed stratified analyses.
Results:
Subjects carrying at least one MC1R variant had an increased risk of NMSC overall, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC): SOR (95%CI) were 1.48 (1.24–1.76), 1.39 (1.15–1.69) and 1.61 (1.35–1.91), respectively. All of the investigated variants showed positive associations with NMSC, with consistent significant results obtained for V60L, D84E, V92M, R151C, R160W, R163Q and D294H: SOR (95%CI) ranged from 1.42 (1.19–1.70) for V60L to 2.66 (1.06–6.65) for D84E variant. In stratified analysis, there was no consistent pattern of association between MC1R and NMSC by skin type, but we consistently observed higher SORs for subjects without red hair.
Conclusions:
Our pooled-analysis highlighted a role of MC1R variants in NMSC development and suggested an effect modification by red hair colour phenotype.
Keywords: pooled-analysis, pigmentation genes, genetic epidemiology, basal cell carcinoma, squamous cell carcinoma
Non-melanoma skin cancers (NMSC) are the most common malignancies in fair-skinned populations with a continuing increase in incidence during recent decades (Levi et al, 2001; Bath-Hextall et al, 2007; Flohil et al, 2011; Lomas et al, 2012). According to the estimates of the American Cancer Society, more than two million NMSC are diagnosed annually in the US (Housman et al, 2003; Rogers et al, 2010; American Cancer Society, 2012). In 1992, among US Medicare beneficiaries, NMSC ranked among the top five most costly cancers to treat (Housman et al, 2003). Moreover, from 1992 to 2006 in the same population, there was a 77% increase in the total number of skin-cancer-related procedures (∼94% NMSC (Rogers et al, 2010)). The vast majority of NMSC are basal cell carcinomas (BCC) and squamous cell carcinomas (SCC) with a BCC/SCC incidence ratio in immunocompetent patients of 4 : 1. BCC is the most common cancer in populations of European origin and accounts for 29% of all cancers (DePinho, 2000), although it is less likely to be lethal and rarely metastasises. Previous studies identified solar UV irradiation, fair skin, red hair and freckles as the most relevant risk factors for NMSC development (Rosso et al, 1996; Zanetti et al, 1996; IARC, 2012).
The melanocortin-1-receptor (MC1R) gene is involved in the genetics of human pigmentation. Binding of α-melanocyte-stimulating hormone (α-MSH) to MC1R stimulates the synthesis of melanin-activating adenylate cyclase enzyme, thereby elevating intracellular cyclic adenosine monophosphate (cAMP). Pigmentation is determined by regulation of the melanin proportion of photoprotective eumelanin and phaeomelanin, the latter being potentially mutagenic because it generates free radicals following UV exposure (Garcia-Borron et al, 2005).
MC1R is a highly polymorphic gene: more than 100 non-synonymous variants have been described to date (Garcia-Borron et al, 2005; Gerstenblith et al, 2007; Perez Oliva et al, 2009). Functional analysis of some of these variants revealed partial loss of the receptor's ability to stimulate cAMP pathway, leading to a quantitative shift of melanin synthesis from eumelanin to phaeomelanin (Duffy et al, 2004). Phaeomelanin is associated with the ‘red hair colour' (RHC) phenotype, characterised by fair skin, red hair, freckles and sun sensitivity (solar lentigines and low tanning response) (Box et al, 1997). Variant alleles of the following six single nucleotide polymorphisms rs1805006 (D84E), rs11547464 (R142H), rs1805007 (R151C), rs1110400 (I155T), rs1805008 (R160W) and rs1805009 (D294H) were defined as ‘R' alleles for their association with the RHC phenotype in population or familial association studies. The rs1805005 (V60L), rs2228479 (V92M) and rs885479 (R163Q) variants seem to have a lower association with RHC phenotype and have been designated as ‘r' alleles (Garcia-Borron et al, 2005).
Previous studies reported that the risk of NMSC is higher among carriers of MC1R variants (Smith et al, 1998; Bastiaens et al, 2001; Kennedy et al, 2001; Han et al, 2006; Scherer and Kumar, 2010). However, it is not well known which variants are mostly associated with NMSC and whether the association completely depends on pigmentation characteristics.
The first aim of this study was to evaluate the association between specific and combined MC1R variants and the risk of NMSC through a large multicenter pooled-analysis of individual data from the melanocortin-1-receptor gene, skin cancer and phenotypic characteristics (M-SKIP) project. The second aim was to evaluate whether risk estimates differed by phenotypic characteristics.
Materials and Methods
Data for the present analyses were gathered through the M-SKIP project, which was previously described (Raimondi et al, 2012). Briefly, we collected data from epidemiological studies on MC1R variants, sporadic cutaneous melanoma (CM), NMSC and phenotypic characteristics associated with skin cancer from 33 investigators who agreed to participate in the M-SKIP project. Participant investigators sent their data along with a signed statement declaring that their original study was approved by an Ethics Committee and/or that study subjects provided a written consent to participate in the original study. We created a pooled database, including data on 8301 CM cases, 3542 NMSC cases and 15 589 controls.
For the present study, we identified in the M-SKIP database 8 independent case–control studies on NMSC (Kennedy et al, 2001; Dwyer et al, 2004; Scherer et al, 2008; Brudnik et al, 2009; Liu et al, 2009; Nan et al, 2009; Ferrucci et al, 2012; Andresen et al, 2013) that overall included data on 2587 BCC cases, 788 SCC cases, 152 cases with both BCC and SCC, and 9391 controls.
Statistical analysis
First, we compared population characteristics reported in publications of non-participant authors with those of studies included in our analysis, to assess the representativeness of our study population. Categorical and continuous variables were compared by the χ2-test and by the Wilcoxon two-sample test, respectively. Small-study effects was graphically represented by funnel plots and formally assessed by Egger's test. We verified the departure of frequencies of each MC1R variant from expectation under Hardy–Weinberg (HW) equilibrium by the χ2-test in controls for each included study.
We first pooled BCC and SCC together to evaluate the association between MC1R variants and NMSC risk overall, and then performed separate analyses to test the association of MC1R variants with BCC and SCC risk. For the first step, we considered all 3527 cases (2587 BCC, 788 SCC and 152 with both), while for the second step we first considered 2739 cases for BCC (2587 BCC and 152 with both) and then 940 cases for SCC (788 SCC and 152 with both). For all the analyses, controls are subjects free of any skin cancer.
We previously tested different inheritance models and found that the dominant model was the one with the lowest Akaike's Information Criterion for almost all the studies and variants, therefore we assumed this model of inheritance in the pooled analyses (Pasquali et al, 2015). For each study, we calculated the odds ratio (OR) with 95% confidence interval (CI) of MC1R variants by applying logistic regression to the data. Beyond MC1R, each model included, if available, the following covariates: age, sex, intermittent and chronic sun exposure, lifetime and childhood sunburns, and smoking status. Coding and standardisation of the variables in the M-SKIP database has been described elsewhere (Raimondi et al, 2012). For each study, we imputed missing data with multiple imputation models for variables with <20% of missing data, by using the iterative Markov chain Monte Carlo method, as previously described (Schafer, 1997). We choose this method because several data sets presented non-monotone missing data patterns and because it is robust to minor departures from the assumptions of multivariate normality (Schafer, 1997). For each imputation procedure, five data sets were generated, which was considered an adequate number for multiple imputation (Rubin, 1996). The results from the five logistic regression models applied to the imputed data sets were then combined for the inference with proc mianalyze (SAS software, Cary, NC, USA).
We performed the analysis using two different criteria to define the reference category for MC1R: the first one was applied to the four studies where MC1R was sequenced, and it used the wild-type (WT) subjects as a reference category for each variant; the second one was applied to the four studies where MC1R gene was not sequenced, and it used, for each study, subjects without any of the tested MC1R variants as a reference category for each variant. For this latter analysis, it should be noted that reference category includes both WT and carriers of any MC1R variant, which was not specifically assessed in each original study (Table 1).
Table 1. Description of the 8 case–control studies included in the pooled-analysis of BCC and SCC.
|
Mean age (s.d.) |
Males (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| First author, publication year | Country | MC1R genotyping variables | Controls type | N cases/N controls | Cases | Controls | Cases | Controls | Available confoundersa |
|
BCC | |||||||||
| Kennedy et al, 2001 | The Netherlands | All | Hospital | 341/378 | 62 (10) | 58 (11) | 54 | 42 | Continuous and intermittent sun exposure, sunburns, smoking status |
| Dwyer et al, 2004 | Australia | V60L D84E R151C R160W D294H | Population | 157/290 | 44 (9) | 44 (10) | 48 | 46 | Continuous and intermittent sun exposure, sunburns |
| Scherer et al, 2008 | Hungary, Romania, Slovakia | All | Hospital | 529/532 | 65 (10) | 60 (12) | 45 | 51 | Intermittent sun exposure |
| Brudnik et al, 2009 | Poland | V60L D84E V92M R142H R151C I155T R160W R163Q D294H | Hospital | 110/489 | 68 (12) | 43 (19) | 43 | 40 | — |
| Nan et al, 2009 | USA | V60L V92M R151C I155T R160W R163Q D294H | Population | 299/323 | 64 (7) | 59 (7) | 0 | 0 | Sunburns |
| Rotterdam Study (Liu et al, 2009) | The Netherlands | V60L R142H R151C R160W R163Q | Population | 927/6559 | 73 (8) | 72 (9) | 48 | 41 | — |
| Ferrucci et al, 2012 | USA | All | Hospital | 376/383 | 35 (5) | 35 (6) | 32 | 30 | Continuous and intermittent sun exposure, sunburns, smoking status |
| Total | 2739/8954 | 62 (15) | 66 (14) | 40 | 40 | ||||
|
SCC | |||||||||
| Kennedy et al, 2001 | The Netherlands | All | Hospital | 151/378 | 66 (8) | 58 (11) | 66 | 42 | Continuous and intermittent sun exposure, sunburns, smoking status |
| Dwyer et al, 2004 | Australia | V60L D84E R151C R160W D294H | Population | 144/290 | 50 (6) | 44 (10) | 54 | 46 | Continuous and intermittent sun exposure, sunburns |
| Nan et al, 2009 | USA | V60L V92M R151C I155T R160W R163Q D294H | Population | 286/307 | 65 (7) | 60 (7) | 0 | 0 | Sunburns |
| Rotterdam Study (Liu et al, 2009) | The Netherlands | V60L R142H R151C R160W R163Q | Population | 272/6559 | 74 (8) | 72 (9) | 53 | 41 | — |
| Andresen et al, 2013 | Norway | All | Hospitalb | 87/130 | 56 (11) | 63 (10) | 63 | 62 | — |
| Total | 940/7664 | 65 (11) | 69 (11) | 40 | 40 | ||||
Abbreviations: BCC=basal cell carcinoma; MC1R=melanocortin-1-receptor; SCC=squamous cell carcinoma; s.d.=standard deviation.
Beyond age and gender, which were available in all the studies.
Controls are subjects with functional renal grafts at time of invitation.
In all analyses, we took into account all the identified variants and calculated the OR for: (1) carrying at least one MC1R variant; (2) carrying just one MC1R variant; and (3) carrying two or more MC1R variants. Finally, a MC1R score was calculated, by summing across the MC1R alleles, giving a value of 1 to ‘r' and 2 to ‘R' variants. To calculate this score, we considered both common and rare variants and classified them as previously suggested (Davies et al, 2012).
Following the two-stage analysis approach, we pooled study-specific ORs using a random-effects model, implemented by the DerSimonian–Laird method. When there were more than one OR calculated in a single study (i.e., analysis by MC1R score), we took into account the correlation between the ORs by using the multivariate approach of van Houwelingen et al (2002). We evaluated homogeneity among study-specific estimates by the Q-statistic and I2, which represents the percentage of total variation across studies that is attributable to heterogeneity rather than to chance. We considered that statistically significant heterogeneity existed when the P-value was ⩽0.10. When significant heterogeneity was revealed, we performed sensitivity analysis and meta-regression by year of publication of the study, geographic area where the study was carried out, MC1R genotyping methodology, deviation from HW equilibrium, type of controls and DNA source. To evaluate the robustness of the results, we also compared the pooled-OR obtained using the M-SKIP data set with the meta-OR calculated by pooling risk estimates reported in studies from not-participating investigators also using DerSimonian–Laird random-effects models.
We computed the attributable risk (AR) in the population for the presence of at least one MC1R variant and for the MC1R variants found to be statistically significantly associated with NMSC by using the Miettinen's formula: (OR−1/OR) × proportion of cases exposed, with the corresponding 95%CI.
Finally, we performed a stratified analysis, to investigate whether the observed association between MC1R variants and NMSC varied according with different phenotypic characteristics. Phenotypic characteristics were taken into account only in stratified analysis and were not considered as confounders in previous analyses because they are likely in the pathway between MC1R and NMSC. Study-specific ORs were adjusted by age, sex, intermittent and chronic sun exposure, lifetime and childhood sunburns, and smoking status, where available. The hypothesis of homogeneity of ORs among strata was tested by meta-regression models with random-effects and restricted maximum likelihood estimates, after the calculation of strata-specific OR in each study. The correlation between the ORs calculated in the same studies was taken into account by using the approach of van Houwelingen et al (2002).
The analysis was carried out using SAS (version 9.2, Cary, NC, USA) and STATA (version 11.2, Lakeway, TX, USA ).
Results
Studies included in our pooled-analysis did not differ from studies from not-participating investigators according to publication period, study area, phenotype assessment, source of controls, genotyping methodology, mean age of cases and controls, sex distribution of cases and controls.
Table 1 summarises the eight case–control studies included in the pooled analyses, four of which provided information on both BCC and SCC, three on BCC only and one on SCC only. The studies were published between 2001 and 2013 and the majority of them were carried out in Europe (N=4 out of 7 (57%) and N=3 out of 5 (60%), for BCC and SCC subgroups, respectively). For the BCC analysis, hospital controls were recruited in four studies (57%) and population controls in three studies (43%), while for the SCC analysis population controls were included in three studies (60%) and hospital controls in the remaining two studies (40%). All the studies included patients with histological confirmed diagnosis, except the Nurses Health Study (Nan et al, 2009) in which histological and self-reported diagnosis were collected. For this latter study, however, the validity of self-reported diagnosis was reported to be 90% (Nan et al, 2009). A complete sequencing analysis of the MC1R coding region was performed in three studies (43%) in BCC subgroup and in two studies (40%) in SCC subgroup. In general, cases were of a similar age, or slightly older than controls, and except for a study which only included women the sex distribution was similar between cases and controls. Individual information on age and sex were available for each study, but information on other potential confounders varied between studies. Among the eight included studies, no deviation from HW equilibrium was observed for the following MC1R variants: V60L, D84E, V92M, I155T and R163Q. Deviation from HW equilibrium was observed in one study for R142H (Liu et al, 2009) and R151C (Dwyer et al, 2004), and in two studies for R160W (Brudnik et al, 2009; Liu et al, 2009) and D294H (Brudnik et al, 2009; Andresen et al, 2013). Further information on cases identification is presented in Supplementary Table S1.
Association between combined MC1R variants and NMSC
We found that subjects carrying any MC1R variant had a significantly increased risk of NMSC (Table 2) compared with subjects without any MC1R assessed variant. In more detail, carrying at least one MC1R variant increased the risk of NMSC overall, BCC and SCC: summary OR (SORs) (95%CI) were 1.48 (1.24–1.76), 1.39 (1.15–1.69) and 1.61 (1.35–1.91), respectively.
Table 2. Summary odds ratios for the association between combined MC1R variants and non-melanoma skin cancer and heterogeneity estimates.
|
All studies |
Sequenced studies only |
|||||
|---|---|---|---|---|---|---|
| Variant | N studies (N cases/N controls) | SOR (95%CI) | Q-test P-value | I2 (%) | N studies (N cases/N controls) | SOR (95%CI) |
|
All | ||||||
| Wild typea | 8 (1162/4419) | Reference | — | — | 4 (360/539) | Reference |
| Any variant | 8 (2365/4972) | 1.48 (1.24–1.76) | 0.01 | 60.5 | 4 (1074/884) | 1.78 (1.50–2.11) |
| 1 variant | 9 (1628/3942) | 1.40 (1.19–1.65) | 0.24 | 22.7 | 4 (670/650) | 1.54 (1.29–1.85) |
| 2+ variants | 8 (737/1030) | 1.80 (1.49–2.17) | 0.001 | 70.5 | 4 (404/234) | 2.49 (1.99–3.12) |
| Scoreb 1 | 8 (776/2043) | 1.24 (1.02–1.51) | 0.27 | 19.2 | 4 (350/371) | 1.41 (1.15–1.73) |
| Scoreb 2 | 8 (1041/2183) | 1.61 (1.33–1.96) | 0.31 | 15.3 | 4 (422/354) | 1.81 (1.47–2.22) |
| Scoreb 3 | 8 (351/439) | 1.93 (1.52–2.46) | 0.001 | 69.5 | 4 (199/111) | 2.68 (2.01–3.57) |
| Scoreb ⩾4 | 8 (197/307) | 1.80 (1.37–2.38) | 0.02 | 57.5 | 4 (103/48) | 2.68 (1.81–3.96) |
|
BCC | ||||||
| Wild typea | 7 (937/4288) | Reference | — | — | 3 (322/506) | Reference |
| Any variant | 7 (1802/4666) | 1.39 (1.15–1.69) | 0.01 | 63.6 | 3 (924/787) | 1.75 (1.46–2.09) |
| 1 variant | 7 (1244/3720) | 1.31 (1.08–1.60) | 0.21 | 28.6 | 3 (581/588) | 1.52 (1.26–1.83) |
| 2+ variants | 7 (558/946) | 1.70 (1.36–2.12) | 0.002 | 70.8 | 3 (343/199) | 2.48 (1.96–3.15) |
| Scoreb 1 | 7 (610/1925) | 1.17 (0.94–1.46) | 0.26 | 22.8 | 3 (308/344) | 1.39 (1.12–1.72) |
| Scoreb 2 | 7 (786/2046) | 1.51 (1.21–1.87) | 0.27 | 20.5 | 3 (359/307) | 1.78 (1.43–2.21) |
| Scoreb 3 | 7 (264/399) | 1.80 (1.37–2.36) | 0.001 | 72.1 | 3 (176/91) | 2.85 (2.10–3.86) |
| Scoreb ⩾4 | 7 (142/296) | 1.62 (1.19–2.20) | 0.07 | 48.7 | 3 (81/45) | 2.36 (1.56–3.56) |
|
SCC | ||||||
| Wild typea | 5 (272/3783) | Reference | — | — | 2 (45/175) | Reference |
| Any variant | 5 (668/3881) | 1.61 (1.35–1.91) | 0.42 | 0 | 2 (192/333) | 2.17 (1.44–3.28) |
| 1 variant | 5 (452/3124) | 1.55 (1.24–1.94) | 0.70 | 0 | 2 (113/237) | 1.89 (1.22–2.92) |
| 2+ variants | 5 (216/757) | 2.10 (1.60–2.76) | 0.19 | 35.3 | 2 (79/96) | 2.80 (1.71–4.57) |
| Scoreb 1 | 5 (190/1561) | 1.23 (0.92–1.64) | 0.59 | 0 | 2 (51/120) | 1.51 (0.91–2.52) |
| Scoreb 2 | 5 (308/1755) | 1.94 (1.48–2.53) | 0.79 | 0 | 2 (80/145) | 2.28 (1.42–3.67) |
| Scoreb 3 | 5 (105/318) | 2.28 (1.59–3.28) | 0.11 | 46.5 | 2 (34/49) | 2.49 (1.37–4.54) |
| Scoreb ⩾4 | 5 (65/247) | 2.33 (1.50–3.61) | 0.10 | 48.2 | 2 (27/19) | 4.93 (2.28–10.64) |
Abbreviations: CI=confidence interval; MC1R=melanocortin-1-receptor; SOR=summary odds ratio.
Note: significant ORs and P-values are in bold.
For studies that did not sequence MC1R gene it includes both wild-type and carriers of any MC1R variant not specifically assessed in the original study.
Score calculated as detailed in Davies et al (2012).
Carriers of two or more MC1R variants always presented higher SORs compared with subjects carrying one MC1R variant: SORs (95%CI) were 1.80 (1.49–2.17) for NMSC overall, 1.70 (1.36–2.12) for BCC and 2.10 (1.60–2.76) for SCC (Table 2).
We observed a significant linear trend for one point increase in MC1R score for NMSC overall, BCC and SCC: per-point SOR (95%CI) were 1.25 (1.14–1.36), P<0.0001; 1.22 (1.11–1.34), P<0.0001 and 1.28 (1.17–1.41), P<0.0001, respectively (results not shown).
We then restricted the analysis only to the studies that sequenced the MC1R gene. ORs for these studies were always higher than ORs obtained both on the whole group of studies and on the studies with MC1R not sequenced (P<0.0001, results not shown).
Association between single MC1R variants and NMSC
The nine most prevalent MC1R variants in the M-SKIP database were: V60L, D84E, V92M, R142H, R151C, I155T, R160W, R163Q and D294H. Table 3 and Supplementary Figure S1 present SORs for the association of each variant with NMSC in the whole group of eight studies, using as reference group for each variant the subjects without any MC1R assessed variant. Table 3 also presents results obtained by restricting the analysis only to the studies that sequenced the MC1R gene. We found positive associations between all the investigated MC1R variants and NMSC, with SORs always higher than 1.00 for both the whole group of studies and the sequenced studies. Particularly, in the whole group of studies, a statistically significant association with NMSC overall was found for all variants except R142H and I155T. Furthermore, we observed a significant association with BCC for six MC1R variants: V60L, D84E, V92M, R151C, R160W and D294H, and a significant association with SCC for six variants: V60L, V92M, R151C, I155T, R160W and D294H. Significant heterogeneity was found for 5 variants in the NMSC pooled analyses (D84E, R151C, I155T, R160W and R163Q) and for five variants in BCC (V60L, R151C, I155T, R160W and R163Q).
Table 3. Allele frequency, summary odds ratios for the association between single MC1R variants and non-melanoma skin cancer and heterogeneity estimates.
|
All studies |
Sequenced studies only |
|||||||
|---|---|---|---|---|---|---|---|---|
| MC1R variant | NMSC | Allele frequency in controls (%) | N studies (N cases/N controls) | SOR (95%CI)a | Q-test P-value | I2 (%) | N studies (N cases/N controls) | SOR (95%CI)a |
| V60L | All | 9.7% | 8 (3403/9129) | 1.42 (1.19–1.70) | 0.13 | 36.5 | 4 (1434/1423) | 1.73 (1.39–2.16) |
| BCC | 7 (2664/8695) | 1.39 (1.12–1.72) | 0.07 | 48.6 | 3 (1246/1293) | 1.75 (1.39–2.20) | ||
| SCC | 5 (886/7406) | 1.53 (1.22–1.93) | 0.67 | 0 | 2 (237/508) | 1.98 (1.16–3.38) | ||
| D84E | All | 0.5% | 5 (1720/1703) | 2.66 (1.06–6.65) | 0.07 | 53.1 | 4 (1434/1423) | 3.16 (1.06–9.42) |
| BCC | 4 (1396/1573) | 3.52 (1.49–8.31) | 0.16 | 42.2 | 3 (1246/1293) | 4.55 (1.75–11.82) | ||
| SCC | 3 (375/788) | 1.96 (0.45–8.55) | 0.13 | 50.5 | 2 (237/508) | 2.01 (0.18–22.76) | ||
| V92M | All | 8.8% | 6 (2125/2541) | 1.56 (1.23–1.97) | 0.20 | 30.4 | 4 (1434/1423) | 1.74 (1.38–2.20) |
| BCC | 5 (1651/2105) | 1.46 (1.09–1.96) | 0.13 | 44.3 | 3 (1246/1293) | 1.74 (1.37–2.22) | ||
| SCC | 3 (523/814) | 1.94 (1.32–2.85) | 0.85 | 0 | 2 (237/508) | 1.81 (1.05–3.10) | ||
| R142H | All | 0.6% | 4 (2389/7469) | 1.20 (0.74–1.94) | 0.40 | 0 | 3 (1347/1293) | 1.37 (0.66–2.84) |
| BCC | 4 (2123/7469) | 1.12 (0.68–1.87) | 0.41 | 0 | 3 (1246/1293) | 1.29 (0.63–2.64) | ||
| SCC | 2 (412/6554) | 1.59 (0.59–4.24) | 0.83 | 0 | 1 (150/378) | 1.87 (0.31–11.22) | ||
| R151C | All | 6.0% | 8 (3465/9229) | 1.99 (1.50–2.65) | 0.002 | 67.7 | 4 (1434/1423) | 2.57 (1.90–3.48) |
| BCC | 7 (2698/8796) | 1.86 (1.35–2.56) | 0.004 | 69.1 | 3 (1246/1293) | 2.52 (1.68–3.77) | ||
| SCC | 5 (915/7509) | 2.10 (1.53–2.87) | 0.21 | 31.3 | 2 (237/508) | 3.16 (1.82–5.50) | ||
| I155T | All | 1.0% | 5 (2040/2408) | 1.80 (0.87–3.72) | 0.06 | 52.0 | 3 (1347/1293) | 2.38 (1.25–4.53) |
| BCC | 5 (1655/2105) | 1.54 (0.69–3.45) | 0.07 | 53.6 | 3 (1246/1293) | 2.33 (1.23–4.44) | ||
| SCC | 2 (434/681) | 4.60 (1.44–14.75) | 0.42 | 0 | 1 (150/378) | 11.46 (0.93–140.38) | ||
| R160W | All | 8.5% | 8 (3475/9238) | 1.67 (1.37–2.05) | 0.08 | 43.4 | 4 (1434/1423) | 1.92 (1.52–2.43) |
| BCC | 7 (2702/8805) | 1.61 (1.26–2.06) | 0.03 | 55.9 | 3 (1246/1293) | 1.89 (1.47–2.42) | ||
| SCC | 5 (920/7515) | 1.97 (1.57–2.47) | 0.72 | 0 | 2 (237/508) | 2.66 (1.60–4.43) | ||
| R163Q | All | 4.9% | 7 (3217/9097) | 1.50 (1.11–2.02) | 0.05 | 51.0 | 4 (1434/1423) | 1.93 (1.22–3.06) |
| BCC | 6 (2574/8660) | 1.39 (0.99–1.94) | 0.05 | 54.3 | 3 (1246/1293) | 1.69 (0.96–2.99) | ||
| SCC | 4 (792/7374) | 1.37 (0.90–2.08) | 0.24 | 28.6 | 2 (237/508) | 1.85 (0.60–5.73) | ||
| D294H | All | 1.2% | 7 (2393/2816) | 2.06 (1.45–2.93) | 0.86 | 0 | 4 (1434/1423) | 2.37 (1.39–4.05) |
| BCC | 6 (1788/2382) | 1.77 (1.17–2.68) | 0.97 | 0 | 3 (1246/1293) | 2.09 (1.17–3.72) | ||
| SCC | 4 (656/1095) | 2.90 (1.70–4.96) | 0.39 | 0.6 | 2 (237/508) | 5.07 (1.79–14.36) | ||
Abbreviations: BCC=basal cell carcinoma; CI=confidence interval; MC1R=melanocortin-1-receptor; NMSC=non-melanoma skin cancer; SCC=squamous cell carcinoma; SOR=summary odds ratio.
Note: significant ORs and P-values are in bold.
For studies that did not sequence MC1R gene reference category includes both wild-type and carriers of any MC1R variant not specifically assessed in the original study. For studies that sequenced MC1R gene it includes only wild type.
By meta-regression, we found that genotype methodology significantly affected the risk estimates for some analysed variants, probably due to different reference categories. Restricting the analysis only to the studies that sequenced the MC1R gene, ORs were almost always higher than ORs obtained both on the whole group of studies and on the studies with MC1R not sequenced (P<0.0001, results not shown). ORs significance was consistent for all MC1R variants with the only exception of the I155T that reached statistical significance for NMSC overall and BCC, while lost statistical power to confirm the association with SCC. Other possible sources of between-study heterogeneity, as publication year, study area, deviation from HW equilibrium, source of controls and source of DNA, seemed not to play a role to the observed heterogeneity. Otherwise, sensitivity analysis indicated that the heterogeneity may be attributable to single studies: when we excluded the studies that were outliers in the corresponding funnel plot, we obtained similar pooled-ORs than the original analysis, but with no more evidence of heterogeneity among study-specific estimates (results not shown).
Funnel plots for each MC1R variant are presented in Supplementary Figure S2 for the whole set of eight studies. We found suggestion of small-study effects for R151C and R160W variants, with P-values of 0.021 and 0.006, respectively.
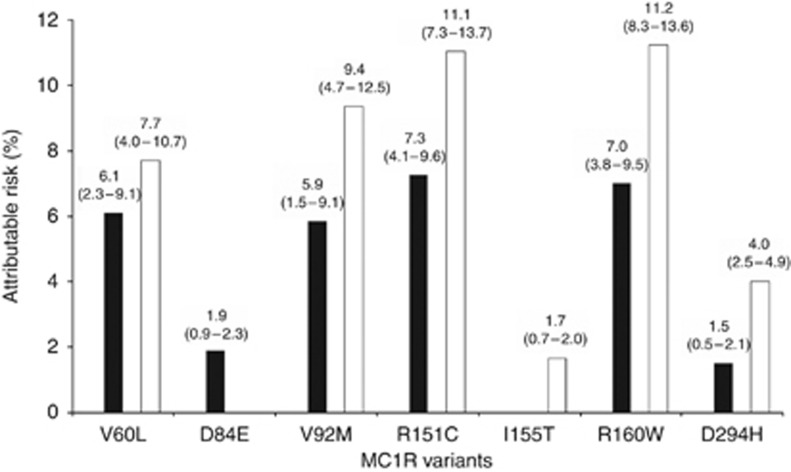
Figure 1 presents AR for MC1R variants significantly associated with NMSC in the previous analysis. The highest AR for both BCC and SCC was observed for R151C (7.3% and 11.1%, respectively), followed by R160W (7.0% and 11.2%, respectively).
Figure 1.
Attributable risksa with 95% confidence intervals in the population for non-melanoma skin cancer according to different MC1R variantsb (percentages). Black bars represent BCC, white bars represent SCC. aMiettinen's formula (OR−1/OR × proportion cases exposed). bOnly variants significantly associated with BCC and/or SCC are represented.
Meta-ORs calculated for studies not included in the M-SKIP project were similar to those obtained from our pooled-analysis for all variants (results not shown).
Analysis stratified by phenotypic characteristics
Table 4 presents SORs for the association between NMSC and any MC1R variant stratified by skin type, hair colour and freckles. For this analysis, subjects without any MC1R assessed variant were the reference group for each variant. We consistently observed higher SORs for the association between MC1R and NMSC for subjects without red hair and without freckles. For hair colour, the difference between SOR of red-haired and not red-haired subjects was statistically significant for SCC and borderline for NMSC overall (P=0.01 and 0.06, respectively), indicating that MC1R is more important for subjects with darker hair colour than with red hair. Similarly, although not significant, subjects without freckles have greater MC1R-associated risk than subjects with freckles. Stratified analyses on the four studies that sequenced the MC1R gene were not feasible due to the limited sample size in each strata-specific analysis.
Table 4. Stratified analysis for any MC1R variants and non-melanoma skin cancer association, according with skin type, hair colour and freckles.
| Phenotypic characteristic | Strata | MC1R variant | N studies (N cases/N controls) | SOR (95%CI) | P-valuea |
|---|---|---|---|---|---|
|
All | |||||
| Skin type | I/II | Wild typeb | 7 (224/279) | 1.00 (reference) | 0.29 |
| Any variant | 7 (861/738) | 1.42 (1.12–1.80) | |||
| III/IV | Wild typeb | 7 (382/736) | 1.00 (reference) | ||
| Any variant | 7 (872/972) | 1.66 (1.38–1.99) | |||
| Hair colour | Red | Wild typeb | 4 (17/31) | 1.00 (reference) | 0.06 |
| Any variant | 4 (105/211) | 0.67 (0.32–1.44) | |||
| Other | Wild typeb | 7 (971/4145) | 1.00 (reference) | ||
| Any variant | 7 (1796/4394) | 1.40 (1.18–1.66) | |||
| Freckles | Yes | Wild typeb | 3 (171/217) | 1.00 (reference) | 0.51 |
| Any variant | 3 (545/428) | 1.52 (1.08–2.15) | |||
| No | Wild typeb | 3 (122/190) | 1.00 (reference) | ||
| Any variant | 3 (275/214) | 1.79 (1.18–2.71) | |||
|
BCC | |||||
| Skin type | I/II | Wild typeb | 6 (176/258) | 1.00 (reference) | 0.13 |
| Any variant | 6 (642/654) | 1.28 (0.97–1.69) | |||
| III/IV | Wild typeb | 6 (289/633) | 1.00 (reference) | ||
| Any variant | 6 (638/766) | 1.63 (1.31–2.05) | |||
| Hair colour | Red | Wild typeb | 4 (13/31) | 1.00 (reference) | 0.23 |
| Any variant | 4 (90/211) | 0.78 (0.33–1.83) | |||
| Other | Wild typeb | 6 (753/4020) | 1.00 (reference) | ||
| Any variant | 6 (1292/4118) | 1.31 (1.08–1.57) | |||
| Freckles | Yes | Wild type^ | 3 (116/217) | 1.00 (reference) | 0.66 |
| Any variant | 3 (431/428) | 1.52 (1.04–2.23) | |||
| No | Wild type^ | 3 (103/190) | 1.00 (reference) | ||
| Any variant | 3 (222/214) | 1.71 (1.09–2.67) | |||
|
SCC | |||||
| Skin type | I/II | Wild typeb | 4 (54/129) | 1.00 (reference) | 0.41 |
| Any variant | 4 (251/297) | 2.02 (1.34–3.06) | |||
| III/IV | Wild typeb | 4 (96/271) | 1.00 (reference) | ||
| Any variant | 4 (245/385) | 1.62 (1.18–2.24) | |||
| Hair colour | Red | Wild typeb | 2 (6/27) | 1.00 (reference) | 0.01 |
| Any variant | 2 (21/170) | 0.34 (0.11–1.07) | |||
| Other | Wild typeb | 5 (263/3749) | 1.00 (reference) | ||
| Any variant | 5 (593/3662) | 1.59 (1.33–1.90) | |||
| Freckles | Yes | Wild typeb | 2 (58/137) | 1.00 (reference) | 0.11 |
| Any variant | 2 (127/216) | 1.31 (0.86–2.02) | |||
| No | Wild typeb | 1 (25/125) | 1.00 (reference) | ||
| Any variant | 1 (82/167) | 2.32 (1.33–4.03) | |||
Abbreviations: BCC=basal cell carcinoma; CI=confidence interval; MC1R=melanocortin-1-receptor; SCC=squamous cell carcinoma; SOR=summary odds ratio.
Note: significant ORs and P-values are in bold.
Overall P-value for any significant difference among strata-specific ORs.
Reference category for SORs is subjects without any of the assessed MC1R variants.
Discussion
We found a statistically increased risk of NMSC for carriers of at least one MC1R variant, with slightly higher SOR observed for SCC than for BCC. Although these two kinds of tumours share many similarities, they present rather different incidence rates and aetiological factors. It has been suggested that neoplastic transformation of epithelial cells requires significantly less UV for BCC than for SCC (Rosso et al, 1996). Cumulative exposure to sunlight was indeed found to be the main risk factor for SCC, while intermittent sun exposure plays a major role in BCC development (IARC, 2012).
We consistently observed significant MC1R-associated NMSC risk only for subjects without red hair, while in subjects with red hair MC1R seemed not to have an effect in addition to phenotype. Previous studies (Bastiaens et al, 2001; Liboutet et al, 2006; Scherer et al, 2008; Ferrucci et al, 2012; Andresen et al, 2013) also suggested that MC1R variants had an independent role in NMSC by phenotypic characteristics, and a similar finding has been observed for melanoma development (Pasquali et al, 2015). MC1R may therefore contribute to skin carcinogenesis through other mechanisms than pigmentation. MC1R signalling has been implicated in a number of key biological pathways involved in cell cycle control (April and Barsh, 2007), apoptosis (Hauser et al, 2006), and activation of DNA repair mechanisms and antioxidant defenses (Bohm et al, 2005; Kadekaro et al, 2010; Maresca et al, 2010; Kadekaro et al, 2012). In addition, stimulation of MC1R also activates the MAPK pathway and regulates target genes involved in inflammation through the NF-κb pathway (Wikberg et al, 2000). Finally, α-MSH affects proliferation and differentiation of both melanocytes and keratinocytes (Slominski et al, 1991).
Concerning individual variants, all of them showed a positive association with NMSC, with a consistent statistically significant association with either NMSC overall, BCC and SCC observed for V60L, V92M, R151C, R160W and D294H. Functional studies revealed that these variants resulted in inefficient or even absent activation of the cAMP pathway downstream. Specifically, V60L, R151C and R160W variants reduced cell-surface expression with a corresponding impairment in cAMP activation, while the loss-of-function phenotype of the D294H variant is probably due to inability to properly undergo the agonist-induced transition to the active state and/or to impaired coupling to the Gs protein (Schioth et al, 1999; Beaumont et al, 2007; Herraiz et al, 2012). Only a marginal effect of the V92M substitution on cell-surface expression or ability to activate the cAMP and ERK cascades has been reported (Beaumont et al, 2007; Herraiz et al, 2012).
The R163Q variant reached statistical significance only for the analysis of NMSC overall, while the D84E variant was associated with both NMSC overall and BCC. Furthermore, the I155T variant was associated only with SCC, although after restricting analysis to sequenced studies there was a suggestive association of I155T with BCC and NMSC overall. For D84E and I155T, receptor impairment in cAMP coupling is largely accounted by reduced cell-surface expression (Beaumont et al, 2007; Sanchez-Laorden et al, 2007, 2009). It is not clear whether our results are attributable to a specific role of the above-mentioned variants in the pathogenesis of each tumour type. This would need to be further investigated in functional studies focused on the carcinogenic mechanisms leading to BCC and SCC, respectively.
Finally, the R142 variant did not show a statistically significant association with NMSC, probably due to its low allele frequency (<1%) and, consequently, limited statistical power.
In previous studies, contradictory results were reported for the associations of the most common MC1R variants and NMSC (Smith et al, 1998; Jones et al, 1999; Bastiaens et al, 2001; Dwyer et al, 2004; Han et al, 2006; Liboutet et al, 2006; Scherer et al, 2008; Andresen et al, 2013), probably due to small sample size of single studies, especially for variants with relatively low allele frequencies.
To the best of our knowledge, our study has for the first time put together and meta-analysed results from different studies on MC1R and NMSC, thus providing powerful estimates of the association between single and combined MC1R variants and NMSC risk in populations living in different geographical areas. Moreover, the availability of individual data from each study allowed a stratified analysis by phenotypic characteristics to be performed, and thus the independent contribution of MC1R variants on NMSC risk to be assessed. We were also able to provide both separate risk estimates for BCC and SCC and a combined risk estimate for NMSC overall. A further strength is that we took into account in our centralised statistical analysis all the available confounders, with a homogeneous plan of analysis and homogeneous definition of co-variables. Our results might have an important impact on public health since MC1R variants may be considered, along with other epidemiological risk factors, in both primary and secondary prevention strategies for NMSC, the most common neoplasm in populations of European origin. The improved identification of at risk subjects might enable public-health messages and early diagnostic procedures to be targeted to the population at risk.
One limitation of our study is that MC1R gene sequencing was completed in only four out of the eight studies included in our pooled-analysis, and thus we were able to compare carriers of MC1R variants with WT subjects only in a small subset of studies. As was previously pointed out (Williams et al, 2011; Pasquali et al, 2015), the inclusion of some MC1R variants in the reference category for the analyses would lead to underestimate the true risk of disease, because MC1R variants are very common among populations of European origins (66–67% of our study population had at least one variant). We sought to overcome this problem in our analysis by excluding from the reference category all the MC1R variants that were specifically assessed in each study. Since the nine most common variants were examined in the majority of studies, the reference category would mainly include WT and rare variants that were observed in ∼4% of the study subjects in the M-SKIP data set. We performed separate stratified analyses on subjects with and without red hair, skin type I/II and freckles, but unfortunately we could not compare subjects with and without any of these at risk phenotypic characteristics because they were collected jointly only in two studies (Kennedy et al, 2001; Ferrucci et al, 2012). Lack of availability of information on other genes in most studies prevented the analysis of possible gene–gene interactions. Other genes have been indeed involved in NMSC development and include pigmentation genes like ASIP, TYR, TYRP1 OCA2, SLC, POMC and IRF4 (Nan et al, 2009; Scherer and Kumar, 2010), and, for BCC, the inactivating mutations in the PTCH gene (Liboutet et al, 2006). Since we carried out a retrospective pooled-analysis, we did not perform centralised sequencing. However, previous studies (Harland et al, 2008; Davies et al, 2012) reported excellent concordance in sequencing data from different centres. Finally, differences in the assessment of sun exposure did not allow us to use this variable in stratified analysis, although it was possible to take it into account the adjustment for confounders.
In conclusion, our pooled-analysis provided evidence for a role of all the most common variants in NMSC development, with consistent significant association with NMSC overall found for the MC1R variants V60L, D84E, V92M, R151C, R160W, R163Q and D294H. Since the contribution of MC1R variants in addition to phenotype in NMSC risk was mainly observed in subjects with no red-hair or no freckles, prevention strategies involving avoidance of indoor and outdoor ultraviolet radiation should not only be recommended for fair skin phenotypes, and MC1R assessment may be tailored to darker-pigmented subjects.
Acknowledgments
This work was supported by the Italian Association for Cancer Research (grant number: MFAG 11831). The M-SKIP study group consists of the following members: Principal Investigator: Sara Raimondi (the European Institute of Oncology, Milan, Italy); Advisory Committee members: Philippe Autier (the International Prevention Research Institute, Lyon, France), Maria Concetta Fargnoli (the University of L'Aquila, Italy), José C García-Borrón (the University of Murcia, Spain), Jiali Han (Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA), Peter A Kanetsky (Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA), Maria Teresa Landi (the National Cancer Institute, NIH, Bethesda, MD, USA), Julian Little (the University of Ottawa, Canada), Julia Newton-Bishop (the University of Leeds, UK), Francesco Sera (the UCL Institute of Child Health, London, UK); Consultants: Saverio Caini (ISPO, Florence, Italy), Sara Gandini and Patrick Maisonneuve (the European Institute of Oncology, Milan, Italy); Participant Investigators: Albert Hofman, Manfred Kayser, Fan Liu, Tamar Nijsten and Andre G. Uitterlinden (the Erasmus MC University Medical Center, Rotterdam, The Netherlands), Rajiv Kumar and Dominique Scherer (the German Cancer Research Center, Heidelberg, Germany), Eduardo Nagore (the Instituto Valenciano de Oncologia, Valencia, Spain), Johan Hansson and Veronica Hoiom (Karolinska Institutet, Stockholm, Sweden), Paola Ghiorzo and Lorenza Pastorino (the University of Genoa, Italy), Nelleke A. Gruis and Jan Nico Bouwes Bavinck (the Leiden University Medical Center, The Netherlands), Paula Aguilera, Celia Badenas, Cristina Carrera, Josep Malvehy, Miriam Potrony Mateu, Susana Puig, Joan Anton Puig-Butille, Gemma Tell (the Hospital Clinic, IDIBAPS and CIBERER, Barcelona, Spain), Terence Dwyer (the Murdoch Childrens Research Institute, Victoria, Australia), Leigh Blizzard and Jennifer Cochrane (the Menzies Research Institute Tasmania, Hobart, Australia), Ricardo Fernandez-de-Misa (the Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain), Wojciech Branicki (the Institute of Forensic Research, Krakow, Poland), Tadeusz Debniak (the Pomeranian Medical University, Polabska, Poland), Niels Morling and Peter Johansen (the University of Copenhagen, Denmark), Susan Mayne, Allen Bale, Brenda Cartmel and Leah Ferrucci (the Yale School of Public Health and Medicine, New Haven, CT, USA), Ruth Pfeiffer (the National Cancer Institute, NIH, Bethesda, MD, USA), Giuseppe Palmieri (the Istituto di Chimica Biomolecolare, CNR, Sassari, Italy), Gloria Ribas (the Fundación Investigación Clínico de Valencia Instituto de Investigación Sanitaria- INCLIVA, Spain), Alexander Stratigos and Katerina Kypreou (the University of Athens, Andreas Sygros Hospital, Athens, Greece), Anne Bowcock, Lynn Cornelius and M. Laurin Council (the Washington University School of Medicine, St Louis, MO, USA), Tomonori Motokawa (POLA Chemical Industries, Yokohama, Japan), Sumiko Anno (the Shibaura Institute of Technology, Tokyo, Japan), Per Helsing and Per Arne Andresen (the Oslo University Hospital, Norway), Terence H. Wong (the University of Edinburgh, UK) and the GEM Study Group. Participants in the GEM Study Group are as follows: Coordinating Center, Memorial Sloan-Kettering Cancer Center, New York, NY, USA: Marianne Berwick (PI, currently at the University of New Mexico), Colin Begg (Co-PI), Irene Orlow (Co-Investigator), Urvi Mujumdar (Project Coordinator), Amanda Hummer (Biostatistician), Klaus Busam (Dermatopathologist), Pampa Roy (Laboratory Technician), Rebecca Canchola (Laboratory Technician), Brian Clas (Laboratory Technician), Javiar Cotignola (Laboratory Technician), Yvette Monroe (Interviewer). Study Centers: The University of Sydney and The Cancer Council New South Wales, Sydney (Australia): Bruce Armstrong (PI), Anne Kricker (co-PI), Melisa Litchfield (Study Coordinator). Menzies Centre for Population Health Research, the University of Tasmania, Hobart (Australia): Terence Dwyer (PI), Paul Tucker (Dermatopathologist), Nicola Stephens (Study Coordinator). The British Columbia Cancer Agency, Vancouver (Canada): Richard Gallagher (PI), Teresa Switzer (Coordinator). The Cancer Care Ontario, Toronto (Canada): Loraine Marrett (PI), Beth Theis (Co-Investigator), Lynn From (Dermatopathologist), Noori Chowdhury (Coordinator), Louise Vanasse (Coordinator), Mark Purdue (Research Officer). David Northrup (Manager for CATI). Centro per la Prevenzione Oncologia Torino, Piemonte (Italy): Roberto Zanetti (PI), Stefano Rosso (Data Manager), Carlotta Sacerdote (Coordinator). The University of California, Irvine (USA): Hoda Anton-Culver (PI), Nancy Leighton (Coordinator), Maureen Gildea (Data Manager). The University of Michigan, Ann Arbor (USA): Stephen Gruber (PI), Joe Bonner (Data Manager), Joanne Jeter (Coordinator). The New Jersey Department of Health and Senior Services, Trenton (USA): Judith Klotz (PI), Homer Wilcox (Co-PI), Helen Weiss (Coordinator). The University of North Carolina, Chapel Hill (USA): Robert Millikan (PI), Nancy Thomas (Co-Investigator), Dianne Mattingly (Coordinator), Jon Player (Laboratory Technician), Chiu-Kit Tse (Data Analyst). The University of Pennsylvania, Philadelphia, PA (USA): Timothy Rebbeck (PI), Peter Kanetsky (Co-Investigator), Amy Walker (Laboratory Technician), Saarene Panossian (Laboratory Technician). Consultants: Harvey Mohrenweiser, The University of California, Irvine, Irvine, CA (USA); Richard Setlow, The Brookhaven National Laboratory, Upton, NY (USA).
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License
Supplementary Material
References
- American Cancer Society 2012. Skin cancer: Basal and Squamous CellAmerican Cancer Society; Available from http://www.cancer.org/cancer/skincancer-basalandsquamouscell/detailedguide/skin-cancer-basal-and-squamous-cell-what-is-basal-and-squamous-cell . [Google Scholar]
- Andresen PA, Nymoen DA, Kjaerheim K, Leivestad T, Helsing P. Susceptibility to cutaneous squamous cell carcinoma in renal transplant recipients associates with genes regulating melanogenesis independent of their role in pigmentation. Biomark Cancer. 2013;5:41–47. doi: 10.4137/BIC.S12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April CS, Barsh GS. Distinct pigmentary and melanocortin 1 receptor-dependent components of cutaneous defense against ultraviolet radiation. PLoS Genet. 2007;3:e9. doi: 10.1371/journal.pgen.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens MT, ter Huurne JA, Kielich C, Gruis NA, Westendorp RG, Vermeer BJ, Bavinck JN, Leiden Skin Cancer Study Team Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. Am J Hum Genet. 2001;68:884–894. doi: 10.1086/319500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath-Hextall F, Leonardi-Bee J, Smith C, Meal A, Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer. 2007;121:2105–2108. doi: 10.1002/ijc.22952. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SN, Newton RA, James MR, Stow JL, Duffy DL, Sturm RA. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum Mol Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280:5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6:1891–1897. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- Brudnik U, Branicki W, Wojas-Pelc A, Kanas P. The contribution of melanocortin 1 receptor gene polymorphisms and the agouti signalling protein gene 8818A>G polymorphism to cutaneous melanoma and basal cell carcinoma in a Polish population. Exp Dermatol. 2009;18:167–174. doi: 10.1111/j.1600-0625.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Davies JR, Randerson-Moor J, Kukalizch K, Harland M, Kumar R, Madhusudan S, Nagore E, Hansson J, Hoiom V, Ghiorzo P, Gruis NA, Kanetsky PA, Wendt J, Pjanova D, Puig S, Saiag P, Schadendorf D, Soufir N, Okamoto I, Affleck P, Garcia-Casado Z, Ogbah Z, Ozola A, Queirolo P, Sucker A, Barrett JH, van Doorn R, Bishop DT, Newton-Bishop J. Inherited variants in the MC1R gene and survival from cutaneous melanoma: a BioGenoMEL study. Pigment Cell Melanoma Res. 2012;25:384–394. doi: 10.1111/j.1755-148X.2012.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, Hayward NK, Martin NG, Sturm RA. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13:447–461. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- Dwyer T, Stankovich JM, Blizzard L, FitzGerald LM, Dickinson JL, Reilly A, Williamson J, Ashbolt R, Berwick M, Sale MM. Does the addition of information on genotype improve prediction of the risk of melanoma and nonmelanoma skin cancer beyond that obtained from skin phenotype. Am J Epidemiol. 2004;159:826–833. doi: 10.1093/aje/kwh120. [DOI] [PubMed] [Google Scholar]
- Ferrucci LM, Cartmel B, Molinaro AM, Gordon PB, Leffell DJ, Bale AE, Mayne ST. Host phenotype characteristics and MC1R in relation to early-onset basal cell carcinoma. J Invest Dermatol. 2012;132:1272–1279. doi: 10.1038/jid.2011.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohil SC, de Vries E, Neumann HA, Coebergh JW, Nijsten T. Incidence, prevalence and future trends of primary basal cell carcinoma in the Netherlands. Acta Derm Venereol. 2011;91:24–30. doi: 10.2340/00015555-1009. [DOI] [PubMed] [Google Scholar]
- Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum Mutat. 2007;28:495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- Han J, Kraft P, Colditz GA, Wong J, Hunter DJ. Melanocortin 1 receptor variants and skin cancer risk. Int J Cancer. 2006;119:1976–1984. doi: 10.1002/ijc.22074. [DOI] [PubMed] [Google Scholar]
- Harland M, Goldstein AM, Kukalizch K, Taylor C, Hogg D, Puig S, Badenas C, Gruis N, ter Huurne J, Bergman W, Hayward NK, Stark M, Tsao H, Tucker MA, Landi MT, Scarra GB, Ghiorzo P, Kanetsky PA, Elder D, Mann GJ, Holland EA, Bishop DT, Bishop JN, GenoMEL, the Melanoma Genetics Consortium A comparison of CDKN2A mutation detection within the Melanoma Genetics Consortium (GenoMEL) Eur J Cancer. 2008;44:1269–1274. doi: 10.1016/j.ejca.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser JE, Kadekaro AL, Kavanagh RJ, Wakamatsu K, Terzieva S, Schwemberger S, Babcock G, Rao MB, Ito S, Abdel-Malek ZA. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19:303–314. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- Herraiz C, Journe F, Ghanem G, Jimenez-Cervantes C, Garcia-Borron JC. Functional status and relationships of melanocortin 1 receptor signaling to the cAMP and extracellular signal-regulated protein kinases 1 and 2 pathways in human melanoma cells. Int J Biochem Cell Biol. 2012;44:2244–2252. doi: 10.1016/j.biocel.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, Chen GJ. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- IARC Radiation. IARC Monogr Eval Carcinog Risk Chem Hum. 2012;100D:35–101. [Google Scholar]
- Jones FI, Ramachandran S, Lear J, Smith A, Bowers B, Ollier WE, Jones P, Fryer AA, Strange RC. The melanocyte stimulating hormone receptor polymorphism: association of the V92M and A294H alleles with basal cell carcinoma. Clin Chim Acta. 1999;282:125–134. doi: 10.1016/s0009-8981(99)00017-0. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, Cheng T, Kadakia M, Abdel-Malek Z. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res. 2012;10:778–786. doi: 10.1158/1541-7786.MCR-11-0436. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Leachman S, Kavanagh RJ, Swope V, Cassidy P, Supp D, Sartor M, Schwemberger S, Babcock G, Wakamatsu K, Ito S, Koshoffer A, Boissy RE, Manga P, Sturm RA, Abdel-Malek ZA. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J. 2010;24:3850–3860. doi: 10.1096/fj.10-158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- Levi F, Te VC, Randimbison L, Erler G, La Vecchia C. Trends in skin cancer incidence in Vaud: an update, 1976-1998. Eur J Cancer Prev. 2001;10:371–373. doi: 10.1097/00008469-200108000-00011. [DOI] [PubMed] [Google Scholar]
- Liboutet M, Portela M, Delestaing G, Vilmer C, Dupin N, Gorin I, Saiag P, Lebbe C, Kerob D, Dubertret L, Grandchamp B, Basset-Seguin N, Soufir N. MC1R and PTCH gene polymorphism in French patients with basal cell carcinomas. J Invest Dermatol. 2006;126:1510–1517. doi: 10.1038/sj.jid.5700263. [DOI] [PubMed] [Google Scholar]
- Liu F, van Duijn K, Vingerling JR, Hofman A, Uitterlinden AG, Janssens AC, Kayser M. Eye color and the prediction of complex phenotypes from genotypes. Curr Biol. 2009;19:R192–R193. doi: 10.1016/j.cub.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- Maresca V, Flori E, Bellei B, Aspite N, Kovacs D, Picardo M. MC1R stimulation by alpha-MSH induces catalase and promotes its re-distribution to the cell periphery and dendrites. Pigment Cell Melanoma Res. 2010;23:263–275. doi: 10.1111/j.1755-148X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer. 2009;125:909–917. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali E, Garcia-Borron JC, Fargnoli MC, Gandini S, Maisonneuve P, Bagnardi V, Specchia C, Liu F, Kayser M, Nijsten T, Nagore E, Kumar R, Hansson J, Kanetsky PA, Ghiorzo P, Debniak T, Branicki W, Gruis NA, Han J, Dwyer T, Blizzard L, Landi MT, Palmieri G, Ribas G, Stratigos A, Council ML, Autier P, Little J, Newton-Bishop J, Sera F, Raimondi S, M-SKIP Study Group MC1R variants increased the risk of sporadic cutaneous melanoma in darker-pigmented Caucasians: a pooled-analysis from the M-SKIP project. Int J Cancer. 2015;136:618–631. doi: 10.1002/ijc.29018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Oliva AB, Fernendez LP, Detorre C, Herraiz C, Martinez-Escribano JA, Benitez J, Lozano Teruel JA, Garcia-Borron JC, Jimenez-Cervantes C, Ribas G. Identification and functional analysis of novel variants of the human melanocortin 1 receptor found in melanoma patients. Hum Mutat. 2009;30:811–822. doi: 10.1002/humu.20971. [DOI] [PubMed] [Google Scholar]
- Raimondi S, Gandini S, Fargnoli MC, Bagnardi V, Maisonneuve P, Specchia C, Kumar R, Nagore E, Han J, Hansson J, Kanetsky PA, Ghiorzo P, Gruis NA, Dwyer T, Blizzard L, Fernandez-de-Misa R, Branicki W, Debniak T, Morling N, Landi MT, Palmieri G, Ribas G, Stratigos A, Cornelius L, Motokawa T, Anno S, Helsing P, Wong TH, Autier P, Garcia-Borron JC, Little J, Newton-Bishop J, Sera F, Liu F, Kayser M, Nijsten T, GEM Study Group, M-SKIP Study Group Melanocortin-1 receptor, skin cancer and phenotypic characteristics (M-SKIP) project: study design and methods for pooling results of genetic epidemiological studies. BMC Med Res Methodol. 2012;12:116–2288-12-116. doi: 10.1186/1471-2288-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- Rosso S, Zanetti R, Martinez C, Tormo MJ, Schraub S, Sancho-Garnier H, Franceschi S, Gafa L, Perea E, Navarro C, Laurent R, Schrameck C, Talamini R, Tumino R, Wechsler J. The multicentre south European study ‘Helios'. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73:1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation after 18+ year. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- Sanchez-Laorden BL, Herraiz C, Valencia JC, Hearing VJ, Jimenez-Cervantes C, Garcia-Borron JC. Aberrant trafficking of human melanocortin 1 receptor variants associated with red hair and skin cancer: Steady-state retention of mutant forms in the proximal golgi. J Cell Physiol. 2009;220:640–654. doi: 10.1002/jcp.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Jimenez-Cervantes C, Garcia-Borron JC. Regulation of human melanocortin 1 receptor signaling and trafficking by Thr-308 and Ser-316 and its alteration in variant alleles associated with red hair and skin cancer. J Biol Chem. 2007;282:3241–3251. doi: 10.1074/jbc.M606865200. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. Chapman and Hall: New York; 1997. [Google Scholar]
- Scherer D, Bermejo JL, Rudnai P, Gurzau E, Koppova K, Hemminki K, Kumar R. MC1R variants associated susceptibility to basal cell carcinoma of skin: interaction with host factors and XRCC3 polymorphism. Int J Cancer. 2008;122:1787–1793. doi: 10.1002/ijc.23257. [DOI] [PubMed] [Google Scholar]
- Scherer D, Kumar R. Genetics of pigmentation in skin cancer—a review. Mutat Res. 2010;705:141–153. doi: 10.1016/j.mrrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Phillips SR, Rudzish R, Birch-Machin MA, Wikberg JE, Rees JL. Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem Biophys Res Commun. 1999;260:488–491. doi: 10.1006/bbrc.1999.0935. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Wortsman J. Can some melanotropins modulate keratinocyte proliferation. J Invest Dermatol. 1991;97:747. doi: 10.1111/1523-1747.ep12484829. [DOI] [PubMed] [Google Scholar]
- Smith R, Healy E, Siddiqui S, Flanagan N, Steijlen PM, Rosdahl I, Jacques JP, Rogers S, Turner R, Jackson IJ, Birch-Machin MA, Rees JL. Melanocortin 1 receptor variants in an Irish population. J Invest Dermatol. 1998;111:119–122. doi: 10.1046/j.1523-1747.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- Wikberg JE, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, Skottner A. New aspects on the melanocortins and their receptors. Pharmacol Res. 2000;42:393–420. doi: 10.1006/phrs.2000.0725. [DOI] [PubMed] [Google Scholar]
- Williams PF, Olsen CM, Hayward NK, Whiteman DC. Melanocortin 1 receptor and risk of cutaneous melanoma: a meta-analysis and estimates of population burden. Int J Cancer. 2011;129:1730–1740. doi: 10.1002/ijc.25804. [DOI] [PubMed] [Google Scholar]
- Zanetti R, Rosso S, Martinez C, Navarro C, Schraub S, Sancho-Garnier H, Franceschi S, Gafa L, Perea E, Tormo MJ, Laurent R, Schrameck C, Cristofolini M, Tumino R, Wechsler J. The multicentre south European study ‘Helios'. I: Skin characteristics and sunburns in basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73:1440–1446. doi: 10.1038/bjc.1996.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.