Abstract
Analysis of the only complete early cercopithecoid (Old World monkey) endocast currently known, that of 15-million-year (Myr)-old Victoriapithecus, reveals an unexpectedly small endocranial volume (ECV) relative to body size and a large olfactory bulb volume relative to ECV, similar to extant lemurs and Oligocene anthropoids. However, the Victoriapithecus brain has principal and arcuate sulci of the frontal lobe not seen in the stem catarrhine Aegyptopithecus, as well as a distinctive cercopithecoid pattern of gyrification, indicating that cerebral complexity preceded encephalization in cercopithecoids. Since larger ECVs, expanded frontal lobes, and reduced olfactory bulbs are already present in the 17- to 18-Myr-old ape Proconsul these features evolved independently in hominoids (apes) and cercopithecoids and much earlier in the former. Moreover, the order of encephalization and brain reorganization was apparently different in hominoids and cercopithecoids, showing that brain size and cerebral organization evolve independently.
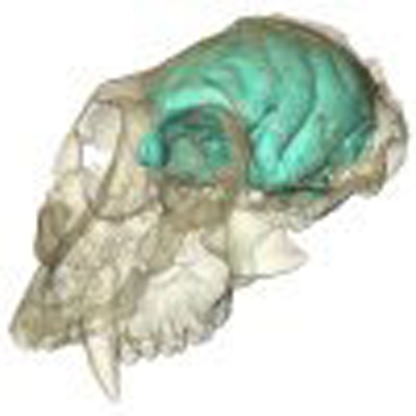 The evolution of the brain in Old World monkeys (cercopithecoids) is poorly understood. Here the authors describe a complete endocast of Victoriapithecus, a 15 Myr old cercopithecoid, which shows that the brain size was much smaller and the olfactory bulbs much larger than in any extant catarrhine primate.
The evolution of the brain in Old World monkeys (cercopithecoids) is poorly understood. Here the authors describe a complete endocast of Victoriapithecus, a 15 Myr old cercopithecoid, which shows that the brain size was much smaller and the olfactory bulbs much larger than in any extant catarrhine primate.
The relationship between the external morphology, cytoarchitecture and function of the brain is better understood for macaques than for other non-human primates because of their extensive use in neuroscience research1,2,3,4. However, it is not known when and in what order cercopithecoids evolved their distinctive pattern of cerebral sulci, brain size, relative size of major brain structures such as the olfactory bulbs, and inferred sensory and behavioural adaptations. Until now, the absence of complete hominoid and cercopithecoid cranial fossils from between 32 and 7 Myr ago necessitated a reliance on phylogenetic comparative studies of living primates, fossils outside this time period or incomplete fossils to reconstruct such events5,6. Such evidence indicated that the last common ancestor of cercopithecoids and hominoids had a small olfactory bulb and enhanced visual system, reflecting a change from reliance on olfactory to visual reproductive signalling7,8,9. In the absence of fossil evidence, disagreements persist as to whether increased brain size precedes, follows or evolves independently from increased gyrification and brain reorganization10,11,12,13,14.
The well-preserved 15-Myr-old adult male cranium KNM-MB 29100 of Victoriapithecus from Maboko Island, Kenya currently includes the only intact neurocranium of a Miocene catarrhine before 6 Myr15. Victoriapithecus represents a cercopithecoid clade that postdates the earliest fossil hominoid (Rukwapithecus) and cercopithecoid (Nsungwepithecus) by 10 million years (Myr); however, retention of a crista obliqua on the upper molars indicates that it is more primitive than the last common ancestor of extant Colobinae and Cercopithecinae16,17. Using high-resolution computed tomography (CT) we digitally extracted and reconstructed the endocast of KNM-MB 29100 to assess its bearing on the evolutionary relationship between brain size and complexity in the cercopithecoid lineage, and catarrhines in general.
Results
Endocranial volume
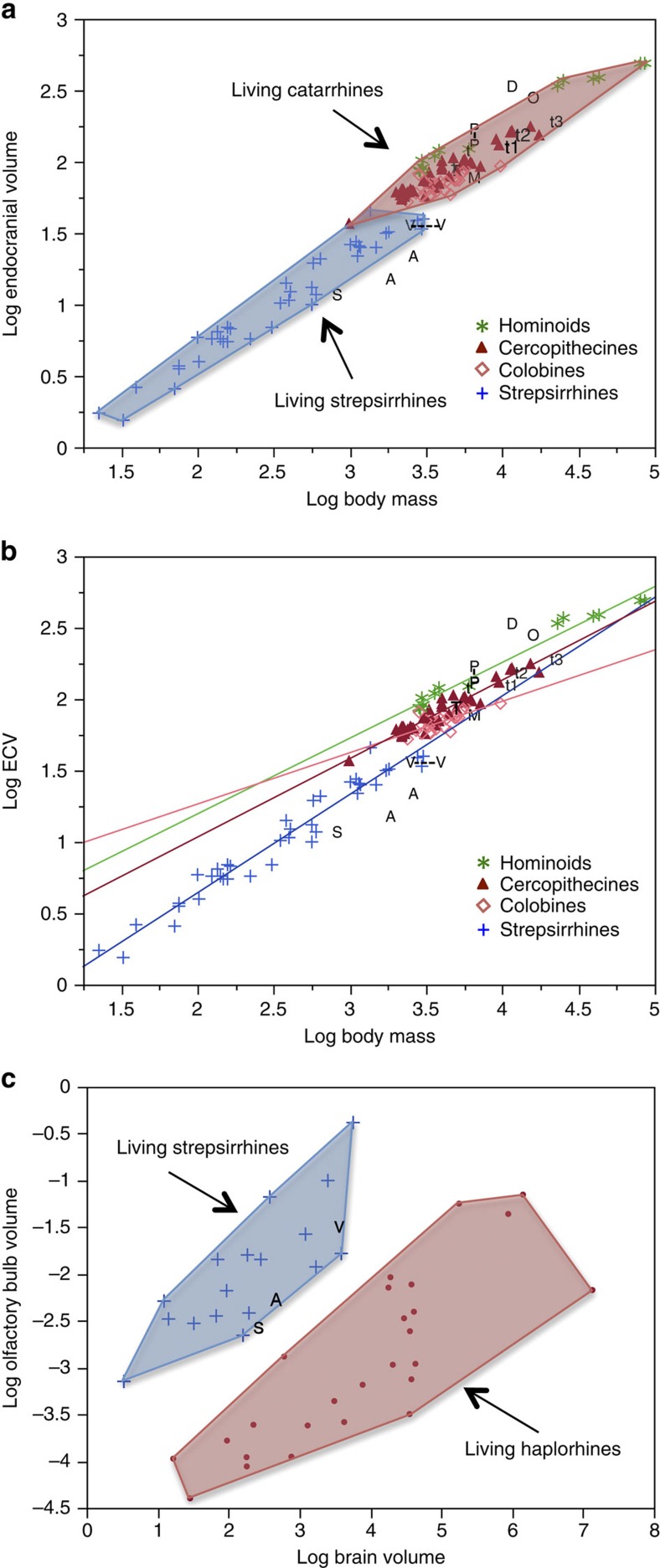
The endocast of KNM-MB 29100 is well preserved and shows remarkably clear impressions of the cerebral sulci and gyri (Fig. 1; Supplementary Movie 1). After correcting for some distortions, an endocranial volume (ECV) of 35.6 cm3 was obtained (Supplementary Fig. 1), substantially less than the 54 cm3 previously inferred15. Body mass estimates on the basis of the cranial dimensions of KNM-MB 29100 converge between 6 and 7 kg (ref. 18), although its upper molar dimensions are among the largest sampled for the species and indicate the individual was closer to 10.5 kg (ref. 18). In comparison, the largest postcranial estimates of body mass for Victoriapithecus do not extend higher than 5.0–5.5 kg (refs 19, 20). In this study we use a conservative body mass range of 5–7 kg for KNM-MB 29100 as a compromise between these estimates. Relative to this body mass range, the newly measured Victoriapithecus ECV places the large male below the range of all known extant and fossil crown catarrhines (Supplementary Table 1, Fig. 2a)21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38. Victoriapithecus falls just below the best-fit regression line for extant strepsirrhines when assuming a postcrania-based body mass of 5 kg (Fig. 2b), and further below that line (overlapping Indri) when using a body mass of 6–7 kg for KNM-MB 29100 (ref. 21). ECVs for the Oligocene stem catarrhine Aegyptopithecus and stem anthropoid Simonsius (=Parapithecus) fall somewhat further below the strepsirrhine regression than Victoriapithecus, indicating that 15 Myr ago the latter had only a slightly larger relative brain size than 32-Myr-old Oligocene stem catarrhines22,23.
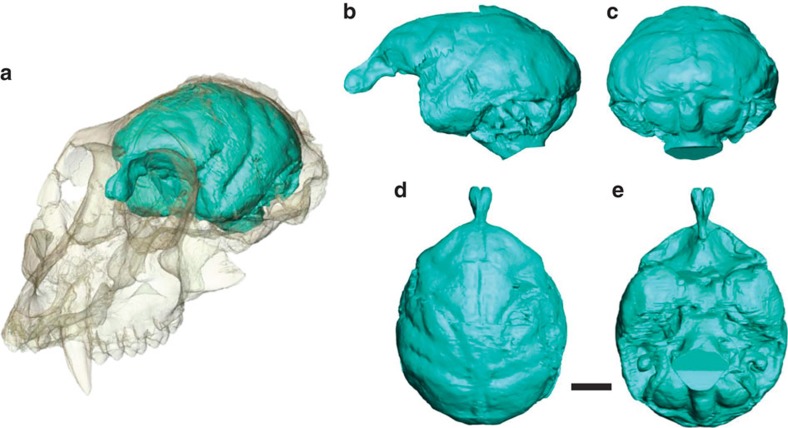
Figure 1. Endocast of V. macinessi (KNM-MB 29100).
(a) Three-quarter view, shown inside the cranium rendered transparent; (b) lateral; (c) posterior; (d) superior and (e) inferior (basal) views. Scale bar, 1 cm.
Figure 2. Analysis of the brain and olfactory bulb size.
(a) Bivariate double logarthmic plot of ECV in cm3 against body mass in g for extant strepsirrhines and catarrhines from Isler21, with data superimposed for Victoriapithecus (V), Oligocene Simonsius (S)22 and Aegyptopithecus (A)23; Miocene hominoids Proconsul (P)24,25,26,27, Turkanapithecus (T)25,26, Dryopithecus (D)25,26,28, Oreopithecus (O)25,26,29,30,31,32,33; the Miocene colobine Mesopithecus (M)5,18; Plio-Pleistocene cercopithecines T. darti (t1)18,35, T. brumpti (t3)35,36; and T. oswaldi (t2)18,35,37,38; and extant T. gelada18,35. Maximum convex polygons are fit to species means. (b) Ordinary least squares regressions for strepsirrhines, colobines, cercopithecines and hominoids using the data in a. (c) Double logarithmic plot of olfactory bulb volume against brain volume for extant primates43,45 and Victoriapithecus. Brain volume data for V, S22 and A23 are represented by ECV in cm3. Olfactory bulb volume for fossil specimens is represented by olfactory fossa volume in cm3 with maximum convex polygons fit to species means.
Among cercopithecoids Miopithecus talapoin has an ECV similar to Victoriapithecus, but an average body mass of 1.5–1.9 kg (ref. 21). Cercopithecoids with slightly lower body masses than predicted for KNM-MB 29100, between 4 and 5 kg, have ECVs roughly twice that of Victoriapithecus ranging from 51 to 82 cm3 (average 65.6, n=6 species) if female and 53–71 cm3 (average 63, n=11 species) if male21. Of these species, colobine monkeys have the smallest ECVs relative to body mass, a phenomenon related to their folivorous diet21,37. Since dental morphology and microwear indicate that Victoriapithecus was clearly frugivorous, diet did not contribute to its extremely small brain size16.
Compared with Victoriapithecus, the estimated ECV for the 8- to 9-Myr-old colobine Mesopithecus is substantially larger, falling within the extant catarrhine cluster but just below the modern colobine regression line relative to its inferred body mass5,18 (Fig. 2a,b). Directly measured ECVs for four species of the Plio-Pleistocene cercopithecine Theropithecus are similarly larger relative to body mass than Victoriapithecus but are all smaller than living T. gelada, and plot towards the edge of the catarrhine cluster where species with the smallest ECVs per body mass are found35,37(Fig. 2a). The limited fossil cercopithecoid data indicate that ECV had increased in late-Miocene and Plio-Pleistocene Old World monkeys relative to the very small volume found in middle Miocene Victoriapithecus. While ECV increases evolved independently in colobine and cercopithecine subfamilies, neither lineage had reached modern ECV level until the Holocene.
Existing evidence indicates that Miocene apes (excluding Afropithecus) were substantially more encephalized than contemporary cercopithecoids, although assessing exactly how different their ECVs were will require an improved fossil record. ECV estimates for incomplete skulls of 17- to 18-Myr-old Proconsul24,25,26,27,10-Myr-old Dryopithecus25,26,28 and 8-Myr-old Oreopithecus25,26,29,30,31,32,33, obtained from regressions of extant anthropoid cranial dimensions against ECV, all fall either within the range of great apes, hylobatids or cercopithecoids (lower end) when considered relative to estimated body mass5,22,23,24 (Fig. 2a). However, since similar methods15 overestimated the ECV of Victoriapithecus by 34% compared with the direct measurements obtained here, these apparently large hominoid ECV estimates could be an artefact of methodology. The earliest conclusive evidence that hominoids reached ECV levels of extant apes comes from the late-Miocene Sahelanthropus cranium, for which ECV falls within the range of chimpanzees relative to its estimated body mass39,40,41.
Olfaction
Victoriapithecus differs from modern anthropoids in having much larger olfactory bulbs that project anteriorly as in strepsirrhines and Aegyptopithecus23,42 (Supplementary Fig. 2). Relative to ECV, the olfactory fossa volume is large (0.22 cm3), falling within the lower range of strepsirrhines and upper-most range of anthropoids, similar to Aegyptopithecus and Simonsius (Fig. 2c)22,23,43. Therefore, olfactory bulb reduction must have occurred in cercopithecoids after 15 Myr, although it is already reduced in the 17- to 18-Myr-old hominoid Proconsul44. Measurement of a large olfactory bulb relative to the brain size in Aegyptopithecus had previously demonstrated that olfactory bulb reduction occurred independently in platyrrhine and catarrhine primates23,43,45,46. The Victoriapithecus evidence further reveals that olfactory reduction was not present in the last common ancestor of hominoids and cercopithecoids, but instead evolved independently in these two clades and at least 2 Myr later in cercopithecoids than hominoids.
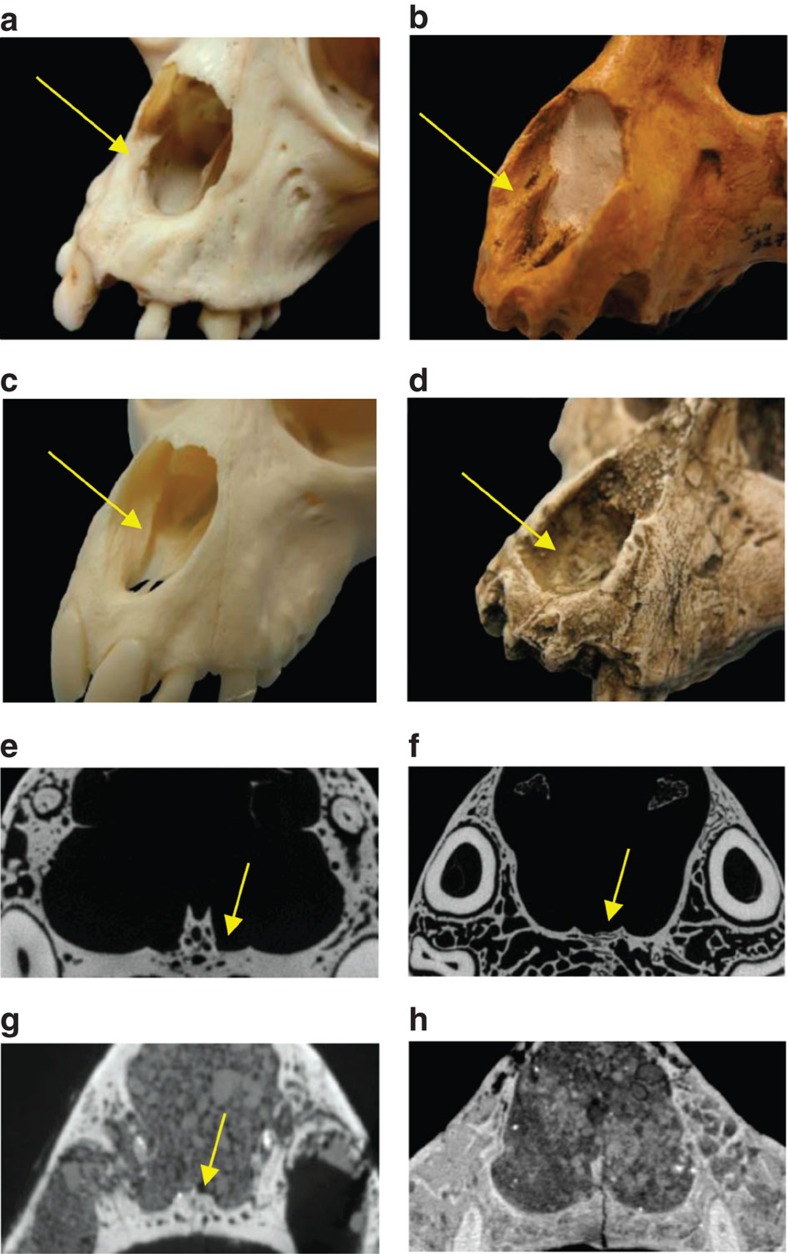
Although the olfactory bulbs of Victoriapithecus are larger compared with those of crown catarrhines, and more similar in size to Aegyptopithecus, its olfactory system may have differed significantly from the latter. Mammalian olfaction consists of two distinct parts, the main olfactory bulb, which is typically used to detect volatile odorant molecules, and the vomeronasal organ (VNO) used to detect odorant molecules of high molecular weight such as water-soluble pheromones47. Two bony structures associated with a functioning VNO in extant strepsirrhines, tarsiers and platyrrhines, an atrioturbinal ridge in the nasal complex and a vomeronasal groove along the maxillary palate, are present in Aegyptopithecus but absent in Victoriapithecus and living crown catarrhines47,48,49,50,51 (Fig. 3a–d). Miocene hominoids Afropithecus and Proconsul similarly lack VNO-related structures; however, the presence of an atrioturbinal ridge in two small-bodied early Miocene non-cercopithecoid catarrhines Limnopithecus and Kalepithecus indicates that some catarrhine lineages retained VNO function during the Miocene48. In contrast to Aegyptopithecus and these small-bodied catarrhines Victoriapithecus, Afropithecus and Proconsul would have relied only on their main olfactory bulbs rather than VNO for the detection of socially relevant olfactory stimuli, possibly including pheromones (Fig. 3e–h)52,53,54,55.
Figure 3. Interior nasal anatomy and vomeronasal complex in primates.
Atrioturbinal ridges extend outwards on the premaxilla in (a) Ateles and (b) Aegpyptopithecus (arrows) but are absent in (c) Macaca and (d) Victoriapithecus, whose nasal ridges descend inferiorly and terminate within the nasal cavity. The vomeronasal groove (VNG) is a U- or J-shaped depression (arrows) along the bony maxillary in palate47 in (e) Potto, (f) Alouatta and (g) the stem catarrhine Aegyptopithecus but is absent in (h) Victoriapithecus.
Cerebral organization
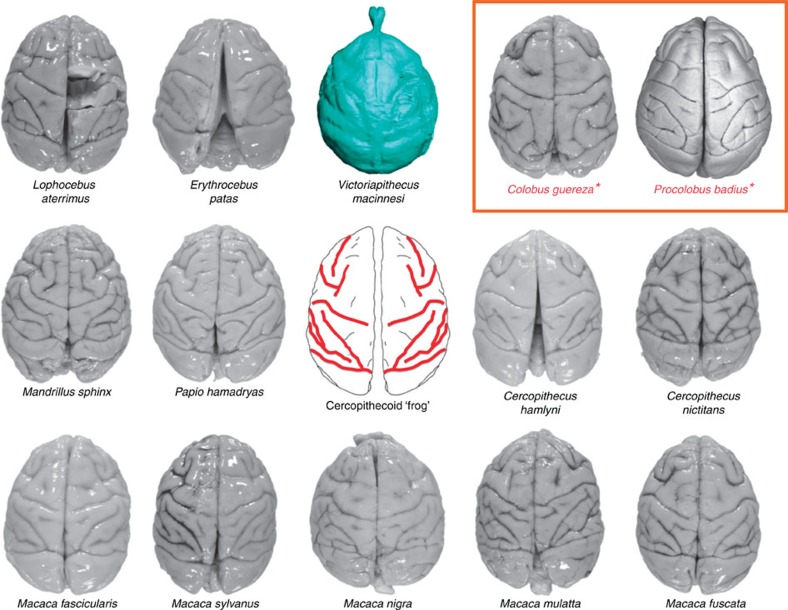
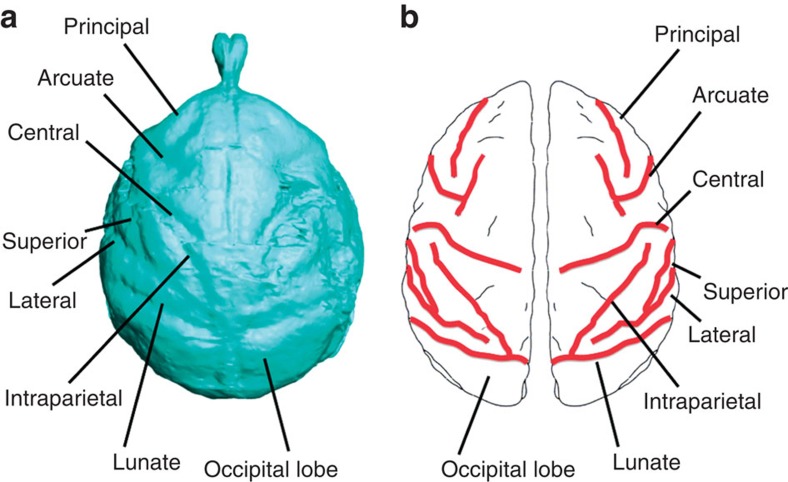
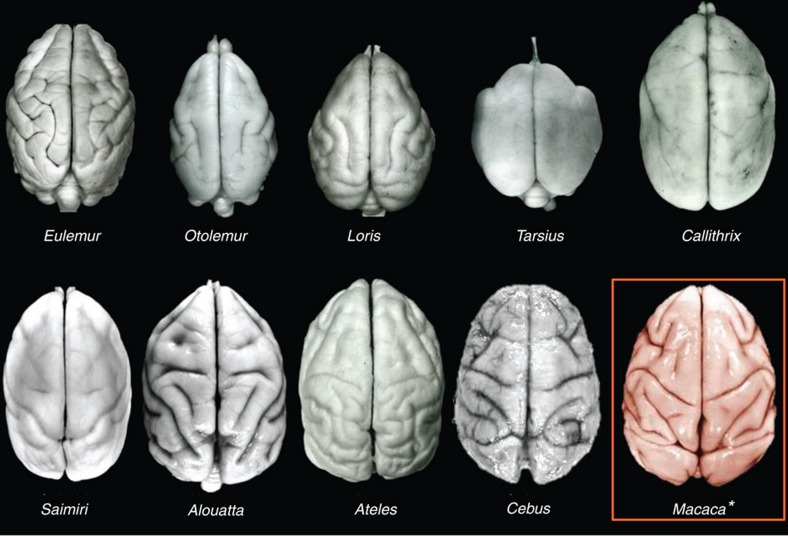
Notwithstanding its small ECV and large olfactory bulbs, the cerebral cortex of Victoriapithecus is reorganized relative to Oligocene anthropoids and exhibits the modern cercopithecoid pattern of sulci and gyri. In superior view, the sulci of all cercopithecoids, including Victoriapithecus, are arranged in a highly distinctive frog-shaped pattern (Fig. 4). The arms of the frog are formed by the principal and arcuate sulci that demarcate the prefrontal cortex; the central sulcus (primarily an anthropoid trait) borders the frontal cortex posteriorly forming the top of the frog's thigh; the intraparietal sulcus separates the back of the frog's thigh from the calf; the superior temporal sulcus forms the frog's shin; and the lunate sulcus forms the bottom of its foot and borders the occipital lobe anteriorly (Fig. 5). No other primate has this cercopithecoid sulcal pattern with the exception of the platyrrhine Cebus in which it convergently evolved5,6,56,57 (Fig. 6). Unlike variation seen in platyrrhine sulcal patterns, the frog-shaped pattern is highly conserved across cercopithecoids, although some differences exist between the two subfamilies56,57.
Figure 4. Superior views of extant cercopithecoid brains compared with Victoriapithecus endocast.
All display the distinctive pattern we describe as ‘frog-like' (centre image). Orange box marks the colobine species. Brains not scaled to actual size. Brain images from the Primate Brain Bank, Netherlands Institute for Neuroscience, the Netherlands, except Procolobus badius which was provided by K. Zilles.
Figure 5. Sulci on the superior aspect of the Victoriapithecus endocast.
(a) CT-based reconstruction and (b) line drawing highlighting the basic sulcal configuration representative of all cercopithecoids.
Figure 6. Comparison of sulcal patterns in primates.
Superior views of strepsirrhines (Eulemur, Otolemur and Loris), Tarsius, and platyrrhines (Callithrix, Saimiri, Alouatta, Ateles and Cebus) compared with the cercopithecoid Macaca. Among extant primates, only Cebus converges on the cercopithecoid sulcal pattern. Brain images provided by K. Zilles except for Cebus and Macaca which are from the Primate Brain Bank, Netherlands Institute for Neuroscience, the Netherlands.
In contrast to Victoriapithecus, Aegyptopithecus has smooth and featureless frontal lobes lacking both principal (=rectus) and arcuate sulci5,23,42 as in strepsirrhines and platyrrhines excluding Cebus. Aegyptopithecus also has smooth occipital and temporal lobes lacking inferior occipital sulci, dimpling of the temporal lobes and anterior and posterior middle temporal sulci, all of which occur in Victoriapithecus. Principal sulci are shared by all crown catarrhines and must have been retained from an ancestral condition more recent than Aegyptopithecus. The presence of arcuate sulci in Victoriapithecus, all other cercopithecoids, the 10-Myr-old hominoid Dryopithecus25, extant great apes and humans indicates they may have been present in the eucatarrhine common ancestor. The unique and complex pattern of sulci occurring in the prefrontal region of hylobatids and the early Miocene Proconsul5,58 could therefore be interpreted as derived, although their lack of an arcuate sulcus has previously been interpreted as primitive5,25,56,58. Alternatively, the arcuate sulcus might have evolved independently and convergently in hominoids and cercopithecoids, as it did in Cebus. In macaques, areas around the arcuate sulcus are involved in visual working memory, hand–eye coordination and mirror neurons activated by observing the movements of others59. Neurons in the macaque inferior temporal lobe are involved in visual pattern recognition including the processing of colours as well as place, face and object recognition59,60. In particular, the posterior middle temporal sulci, found in Victoriapithecus but not in Aegyptopithecus, concerns an area where sharply tuned colour-selective neurons are concentrated in macaque brains60. Therefore, the presence of additional temporal lobe sulci in Victoriapithecus compared with Oligocene anthropoids suggests that it had already evolved a more complex visual system than Aegyptopithecus in spite of their similarly small ECVs.
Differences between Victoriapithecus and extant cercopithecoid cerebral cortices are indicated by the more anterior position of various sulcal landmarks relative to endocast length and height in the former (Supplementary Table 2). Victoriapithecus shares with Aegyptopithecus the lack of an obvious precentral superior sulcus, and frontal lobes that are more V-shaped anteriorly, shorter anteroposteriorly and lower relative to length than in extant cercopithecoids. Among the Miocene apes, the frontal lobes of Proconsul (17–18 Myr), Turkanapithecus (17 Myr) and Dryopithecus (10 Myr) are substantially broader and less constricted anteriorly than both Aegyptopithecus and Victoriapithecus. Only Afropithecus (17 Myr) has a small V-shaped frontal cortex among Miocene hominoids.
The Victoriapithecus motor cortex (the frog's thigh) appears to have been narrower and the superior temporal gyrus shorter than in extant cercopithecoids as indicated by the more anterior position of the confluence of lateral and superior temporal sulci. In addition, the inferior temporal lobe is large and uniquely has a large posterior inferior temporal region that is continuous with the occipital lobe, creating a distinct thickening in the occipital lobe between the short lunate and upwardly curving inferior occipital sulcus that is much less anteriorly positioned than in any extant cercopithecoid we observed.
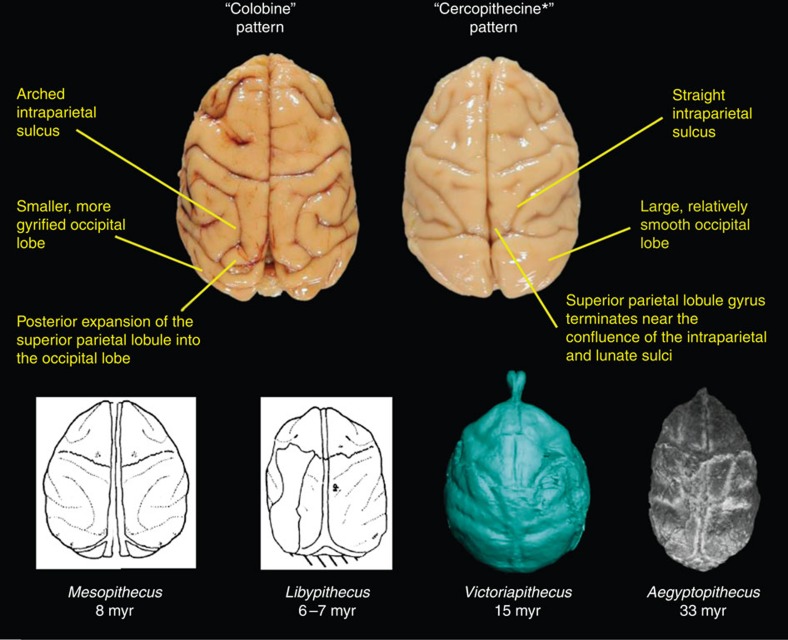
Of the two extant cercopithecoid subfamilies, the Victoriapithecus cerebral cortex is more similar to those of cercopithecines than colobines. Only extant colobines and the late-Miocene colobine Mesopithecus have intraparietal sulci that diverge laterally at their posterior ends as the superior parietal lobule (SPL) expands and impinges on a shorter but more gyrified occipital lobe56,57. Victoriapithecus shares with extant cercopithecines and the late-Miocene colobine Libypithecus intraparietal sulci that are straight and converge posteriorly as they approach the lunate sulcus, posterior ends of the superior parietal gyrus that are V-shaped and a more anteriorly positioned lunate sulcus5 (Fig. 7). Because Aegyptopithecus and Victoriapithecus share relatively large occipital lobes and unexpanded SPLs with cercopithecines (Supplementary Table 2), it is likely that the colobine condition is derived56. Lateral and superior temporal sulci converge in Victoriapithecus as in most cercopithecines, and some colobines including Libypithecus and Semnopithecus, but remain separate in most colobines, and some fossil cercopithecines including Paradolichopithecus arvernensis5 and T. oswaldi56,57,61. The only trait Victoriapithecus shares uniquely with colobines is asymmetry of the prefrontal cortex resulting from a superiorly directed extension from the principal sulcus occurring only on the right side in the Miocene monkey56.
Figure 7. Differences between extant colobine and cercopithecine superior parietal lobes.
Superior views of the brains of Colobus guereza (left) and Macaca fascicularis (right) show that owing to posterior expansion of the SPL in colobines, the intraparietal sulcal appears to be arched in lateral view, whereas in the cercopithecine the intraparietal sulcus is straight. Aegyptopithecus42 and the fossil colobine Libypithecus5 appear to have straight intraparietal sulci, whereas Mesopithecus5 has an arched intraparietal sulcas and some expansion of SPL. Both Mesopithecus and Libypithecus have anteroposteriorly short occipital lobes, unlike Victoriapithecus and Aegyptopithecus. Extant cercopithecoid brain images were provided by the Primate Brain Bank, Netherlands Institute for Neuroscience, the Netherlands.
Discussion
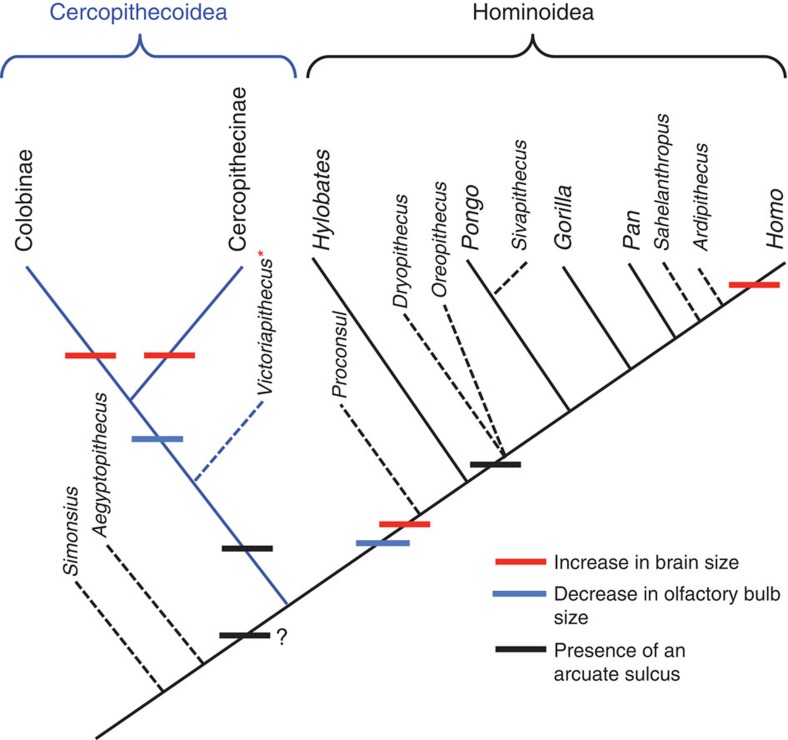
The combination in Victoriapithecus of modern cercopithecoid cerebral complexity and gyrification with a strepsirrhine-like small ECV and large olfactory bulbs is unexpected in an Old World monkey that postdates the hominoid/cercopithecoid divergence by 10–15 Myr (Fig. 8). This is especially true because encephalization has been linked with increased gyrification in anthropoid evolution, and in particular in the genus Homo10,11,62. However, recent evidence that brain size and gyrification are controlled by different genes in catarrhine primates suggests that encephalization and cerebral complexity could evolve independently14, and that either one could precede the other. The discovery that complex gyrification evolved before increased brain size in cercopithecoids underlines the finding that the notably small but highly gyrified brain of the tool-making hominin H. floresiensis63 is perhaps not as remarkable as it may seem. Diversity in the patterning of encephalization and gyrification is also seen in the evolutionary history of terrestrial and aquatic cetartiodactyls with encephalization preceding gyrification in cetaceans, but gyrification preceding encephalization in terrestrial artiodactyls64,65.
Figure 8. Cladogram of fossil and extant taxa catarrhines discussed in this paper.
The appearance of major brain changes discussed in this paper are indicated.
Following the evolution of the distinctive frog-like pattern of cercopithecoid sulci in Victoriapithecus by the middle Miocene, it was retained in both colobine and cercopithecine subfamilies resulting in far less intergeneric sulcal variation in extant Old World monkeys than is seen in hominoids, platyrrhines and strepsirrhines56. We are uncertain why this pattern was so successful that it remained static for the past 15 Myr, but it convergently evolved in Cebus monkeys that are among the most intelligent of platyrrhines66. Sulcal differences between colobines and cercopithecines are restricted to greater asymmetry of prefrontal sulci and SPL expansion with related changes in the intraparietal sulcus in colobines. We hypothesize that SPL expansion in colobines may be an adaptation for folivory since in macaques V6 and PE regions in that area appear to be devoted to proprioception and the reaching and grasping of objects such as occurs for prolonged periods during the harvesting of leaves67,68. Similar expansion of the SPL is seen in extant hylobatids, which are known to include large amounts of leaves in their diets55,69, but does not occur in highly frugivorous Aegyptopithecus, Victoriapithecus or extant cercopithecine monkeys42,56.
Convergent evolution appears to have been a hallmark of catarrhine brain evolution, with reduction in olfactory bulb volume, widening and expansion of the frontal lobe and increased ECV having evolved independently in hominoids and cercopithecoids as well as in colobines and cercopithecines. In addition, the absence of the arcuate sulcus in Proconsul and hylobatids indicates that this sulcus may not have been present in the last common ancestor of hominoids and cercopithecoids, in which case it convergently evolved in cercopithecoids and hominids. Fossil evidence has already shown that the prefrontal cruciate sulcus evolved independently in five major carnivore clades, indicating that convergent evolution of prefrontal sulci such as the arcuate sulcus is possible70. Alternatively, absence of the sulcus in Proconsul and hylobatids may represent a convergent loss or a shared derived condition.
The timing of olfactory bulb reduction and increased ECV appears to have been very different in cercopithecoids than hominoids. Existing evidence indicates that reduction of the olfactory bulb and evolution of modern catarrhine ECV levels had evolved by 17–18 Myr ago in Proconsul24,25,26,27,44. In contrast, olfactory bulb size and ECV in 15-million-year-old cercopithecoids had changed only slightly relative to 32-million-year-old Oligocene anthropoids. Late-Miocene and Plio-Pleistocene monkeys approach modern cercopithecoid ECV levels; however, Old World monkey brains did not fully reach their extant size until the Holocene5,21,35,37, with increased ECV evolving independently in colobines and cercopithecines. A pattern of ECV increase similar to that of cercopithecoids occurred in terrestrial artiodactyls, for which ECV relative to body size changed little between the Oligocene and Miocene, but increased dramatically during the Holocene and did so independently in several different lineages65.
In conclusion, differences in the brain and cognitive evolution between hominoids and cercopithecoids can now be traced back to the early Miocene (17–18 Myr). With cercopithecoids showing cerebral and visual system complexity preceding encephalization and olfactory bulb reduction, and hominoids exhibiting frontal lobe expansion and encephalization before gyrification, it appears that some morphological and functional similarities between extant macaque and hominoid brains may have evolved convergently. The combination of a modern cercopithecoid sulcal pattern with strepsirrhine ECV and olfactory bulb size in Victoriapithecus refutes hypotheses that increased brain size is the major factor causing the development of cerebral complexity in anthropoids. Instead, evidence from the brain of Victoriapithecus shows that cerebral complexity and brain size, and changes in visual and olfactory systems, are influenced by different sets of selective pressures and therefore evolve independently.
Methods
CT scan information and visualization
KNM-MB 29100 was CT scanned with the BIR ACTIS 225/300 of the Max Planck Institute for Evolutionary Anthropology, Leipzig, at the time installed at the National Museums of Kenya in Nairobi. The isotropic voxel size is 0.044 mm. Avizo 7.1 and 8.0 (Visualization Sciences Group) and Geomagic Studio 2013 (Geomagic Inc.) were used for visualization, segmentation, reconstruction and quantification.
Correction for cranial distortion
To calculate the ECV of KNM-MB 29100, the preserved endocast was corrected for distortion in three areas (Supplementary Fig. 1). The endocranial surface associated with the inferiorly depressed frontal squama was realigned with that of the parietals, filling smaller areas bilaterally by surface interpolation. The central part of both orbital roofs is fragmented and pushed superiorly. The associated surfaces were removed from the endocast and filled by interpolation based on the surrounding, well-preserved areas. Lastly, a small distorted area of the endocast associated with the left temporal lobe was interpolated. The ECV of the reconstructed endocast is 35.6 cm3. Since parts of the right frontoparietal area are not as well preserved as the left side, we also calculated ECV values for two endocast reconstructions based on the left half combined with its mirror image. One version uses an overall best-fit midsagittal plane, whereas the other applies the additional constraint that the original foramen magnum size is maintained. The associated ECVs, 35.5 and 36.2 cm3, respectively, bracket the value obtained for the full endocast.
Additional information
How to cite this article: Gonzales, L.A. et al. Cerebral complexity preceded enlarged brain size and reduced olfactory bulbs in Old World monkeys. Nat. Commun. 6:7580 doi: 10.1038/ncomms8580 (2015).
Supplementary Material
Supplementary Figures 1-2, Supplementary Tables 1-2 and Supplementary References
The KNM-MB 29100 endocast rotating around a supero-inferior axis. The surface reconstruction, based on high-resolution CT images, is of the endocast as preserved in the fossil, without corrections for distortion.
Acknowledgments
We thank the National Museums of Kenya for access to specimens in their care, and the Primate Brain Bank, Netherlands Institute for Neuroscience for permission to use photographs of extant cercopithecoid and Cebus brains; Karl Zilles and the Institute of Neuroscience and Medicine, Research Centre Juelich, Germany for providing photographs of extant platyrrhine, strepsirrhine and Procolobus badius; D. Boyer and T. Ryan for giving us access to CT images of Aegyptopithecus through the online data archive Morphosource. We are grateful to K. Allen, R. David, P. Gunz, A. Guthrie, J.-J. Hublin, H. Temming and A. Tzo for support at various stages of the research. I. Arney provided new Victoriapithecus body mass estimates based on the talus. Victoriapithecus cranium KNM-MB 29100 was excavated with the support of NSF Grant 9505778 to B.R.B. and M.L.M. CT scanning and analysis of the cranium was funded by the Max Planck Society.
Footnotes
Author contributions L.A.G. and F.S. analysed CT scans and reconstructed the KNM-MB 29100 endocast. B.R.B. and L.A.G. were primarily responsible for writing the text with substantial input from F.S. and M.L.M. Comparative figures and tables were primarily compiled by L.A.G., F.S. and B.R.B.
References
- Logothetis N. K., Guggenberger H., Peled S. & Pauls J. Functional imaging of the monkey brain. Nat. Neurosci. 2, 555–562 (1999). [DOI] [PubMed] [Google Scholar]
- Van Essen D. C. & Dierker D. L. Surface based and probabilistic atlases of primate cerebral cortex. Neuron 56, 209–225 (2007). [DOI] [PubMed] [Google Scholar]
- McLaren D. et al. A population-average MRI-based atlas collection of the rhesus macaque. NeuroImage 45, 52–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D. et al. Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nat. Methods 9, 277–282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radinsky L. The fossil evidence of anthropoid brain evolution. Am. J. Phys. Anthropol. 41, 15–28 (1974). [Google Scholar]
- Barton R. A. Primate brain evolution: integrating comparative, neurophysiological and ethological data. Evol. Anthropol. 15, 224–236 (2006). [Google Scholar]
- Liman E. R. & Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc. Natl Acad. Sci. USA 100, 3328–3332 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. & Webb D. M. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc. Natl Acad. Sci. USA 100, 8337–8341 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y., Wiebe V., Przeworski M., Lancet D. & Pääbo S. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2, E5 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerison H. J. In Primate Brain Evolution eds Armstrong E., Falk D. 77–84Springer (1982). [Google Scholar]
- Finlay B. L. & Darlington R. B. Linked regularities in the development and evolution of mammalian brains. Science 268, 1578–1584 (1995). [DOI] [PubMed] [Google Scholar]
- Barton R. A. & Harvey P. H. Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058 (2000). [DOI] [PubMed] [Google Scholar]
- Smaers J. B. & Soligo C. Brain reorganization not relative brain size, primarily characterizes anthropoid brain evolution. Proc. R Soc. Biol. Sci. 280, 20130269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. et al. On the genetic architecture of cortical folding and brain volume in primates. NeuroImage 53, 1103–1108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benefit B. R. & McCrossin M. L. Earliest known Old World monkey skull. Nature 388, 368–371 (1997). [DOI] [PubMed] [Google Scholar]
- Benefit B. R. Victoriapithecus: the key to Old World monkey and catarrhine origins. Evol. Anthropol. 7, 155–174 (1999). [Google Scholar]
- Stevens N. J. et al. Palaeontological evidence for an Oligocene divergence between Old World monkeys and apes. Nature 497, 611–614 (2013). [DOI] [PubMed] [Google Scholar]
- Delson E. et al. Body mass in Cercopithecidae (Primates, Mammalia): estimation and scaling in extinct and extant taxa. Anthropol. Pap. Am. Mus. 83, 1–159 (2000). [Google Scholar]
- Arney I. D., Benefit B. R. & McCrossin M. L. The mass of Victoriapithecus macinessi revised using foot remains. Am. J. Phys. Anthropol. 60, 71–72 (2015). [Google Scholar]
- Harrison T. New postcranial remains of Victoriapithecus from the middle Miocene of Kenya. J. Hum. Evol. 18, 3–54 (1989). [Google Scholar]
- Isler K. et al. Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J. Hum. Evol. 55, 967–978 (2008). [DOI] [PubMed] [Google Scholar]
- Bush E. C., Simons E. L. & Allman J. M. High-resolution computed tomography study of the cranium of a fossil anthropoid primate, Parapithecus grangeri: new insights into the evolutionary history of primate sensory systems. Anat. Rec. 281, 1083–1087 (2004). [DOI] [PubMed] [Google Scholar]
- Simons E. L., Seiffert E. R., Ryan T. M. & Attia Y. A remarkable female cranium of the early Oligocene anthropoid Aegyptopithecus zeuxis. Proc. Natl Acad. Sci. USA 104, 8731–8736 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. C., Falk D., Smith R. & Pickford M. The skull of Proconsul africanus: reconstruction and cranial capacity. Nature 305, 525–527 (1983). [Google Scholar]
- Begun D. R. & Kordos L. In The Evolution of Thought. Evolutionary Origins of Great Ape Intelligence eds Russon A. E., Begun D. R. 260–279Cambridge University Press (2004). [Google Scholar]
- Alba D. M. Cognitive inferences in fossil apes (Primates, Hominoidea): does encephalization reflect intelligence? J. Anthropol. Sci. 88, 11–48 (2010). [PubMed] [Google Scholar]
- Manser J. & Harrison T. Estimates of cranial capacity and encephalization in Proconsul and Turkanapithecus. Am. J. Phys. Anthropol. 28, 189 (1999). [Google Scholar]
- Kordos L. & Begun D. R. A new cranium of Dryopithecus from Rudabanya, Hungary. J. Hum. Evol. 41, 689–700 (2001). [DOI] [PubMed] [Google Scholar]
- Straus W. L. & Schon M. A. Cranial capacity of Oreopithecus bambolii. Science 132, 670–672 (1960). [DOI] [PubMed] [Google Scholar]
- Schultz A. H. Einige Beobachtungen und Masse am Skelett von Oreopithecus im Vergleich mit anderen catarrhinen Primaten. Z. Morph. Anthropol. 50, 136 (1960). [Google Scholar]
- Szalay F. & Berzi A. Cranial anatomy of Oreopithecus. Science 180, 183–185 (1973). [DOI] [PubMed] [Google Scholar]
- Jungers W. L. Body size and morphometric affinities of the appendicular skeleton of Oreopithecus bambolii (IGF 11778). J. Hum. Evol. 16, 445–456 (1987). [Google Scholar]
- Harrison T. New estimates of cranial capacity, body size and encephalization in Oreopithecus bambolii. Am. J. Phys. Anthropol. 78, 237 (1989). [Google Scholar]
- Conroy G. C. Problems of body-weight estimation in fossil primates. Int. J. Primatol. 8, 115–137 (1987). [Google Scholar]
- Elton S., Bishop L. C. & Wood B. Comparative context of Plio-Pleistocene hominin brain evolution. J. Hum. Evol. 41, 1–27 (2001). [DOI] [PubMed] [Google Scholar]
- Krentz H. in Theropithecus: The Rise and Fall of a Primate Genus ed. Jablonski N. G. 383–442Cambridge University Press (1993). [Google Scholar]
- Martin R. D. in Theropithecus: the Rise and Fall of a Primate Genus ed. Jablonski N. G. 273–298Cambridge University Press (1993). [Google Scholar]
- Jolly C. J. The classification and natural history of Theropithecus (Simopithecus) (Andrews, 1916), baboons of the African Plio-Pleistocene. Bull. Br. Mus. Nat. Hist. Geol. 22, 1–123 (1972). [Google Scholar]
- Brunet M. et al. A new hominid from the upper Miocene of Chad, central Africa. Nature 418, 145–151 (2002). [DOI] [PubMed] [Google Scholar]
- Zollikofer C. P. E. et al. Virtual cranial reconstruction of Sahelanthropus tchadensis. Nature 434, 755–759 (2005). [DOI] [PubMed] [Google Scholar]
- Bienvenu T. et al. The endocast of Sahelanthropus tchadensis, the earliest known hominid (7 Ma, Chad). Am J. Phys. Anthropol. 15, 80–81 (2013). [Google Scholar]
- Simons E. L. New endocasts of Aegyptopithecus, oldest well preserved record of the brain in Anthropoidea. Am. J. Sci. 293-A, 383–390 (1993). [Google Scholar]
- Baron G., Frahm H. D., Bhatnagar K. P. & Stephan H. Comparison of brain structure volumes in Insectivora and Primates. III. Main olfactory bulb (MOB). J. Hirnforsch. 24, 551–568 (1983). [PubMed] [Google Scholar]
- Fleagle J. G. & Kay R. F. in New Interpretations of Ape and Human Ancestry eds Ciochon R. L., Corruccini R. S. 181–210Plenum Press (1983). [Google Scholar]
- Barton R. A., Purvis A. & Harvey P. H. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Phil. Trans. Biol. Sci. 348, 381–392 (1995). [DOI] [PubMed] [Google Scholar]
- Heritage S. Modeling olfactory bulb evolution through primate phylogeny. PLoS ONE 9, e113904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. D. et al. The vomeronasal organ of New World monkeys (Platyrrhini). Anat. Rec. 294, 2158–2178 (2011). [DOI] [PubMed] [Google Scholar]
- Rossie J. B. Anatomy of the nasal cavity and paranasal sinuses in Aegyptopithecus and early Miocene African catarrhines. Am. J. Phys. Anthropol. 126, 250–267 (2005). [DOI] [PubMed] [Google Scholar]
- Smith T. D. et al. The vomeronasal organ of Lemur catta. Am. J. Primatol. 77, 229–238 (2015). [DOI] [PubMed] [Google Scholar]
- Smith T. D., Siegel M. I. & Bhatnagar K. P. Observations on the vomeronasal organ of prenatal Tarsius bancanus borneanus with implications for ancestral morphology. J. Anat. 203, 473–481 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. C. et al. The vomeronasal complex of nocturnal strepsirhines and implications for the ancestral condition in primates. Anat. Rec. 296, 1881–1894 (2013). [DOI] [PubMed] [Google Scholar]
- Setchell J. M. et al. Chemical composition of scent-gland secretions in an Old World monkey (Mandrillus sphinx): influence of sex, male status, and individual identity. Chem. Senses 35, 205–220 (2010). [DOI] [PubMed] [Google Scholar]
- Fortes-Marco L., Lazuna E. & Martinez-Garcia F. Of pheromones and kairomones: what receptors mediate innate emotional response. Anat. Rec. 296, 1346–1363 (2013). [DOI] [PubMed] [Google Scholar]
- Charpentier M. J. E., Mboumba S., Ditsoga C. & Drea C. M. Nasopalatine ducts and flehmen behavior in the mandrill: reevaluating olfactory communication in Old World primates. Am. J. Primatol. 75, 703–713 (2013). [DOI] [PubMed] [Google Scholar]
- Drea C. M. D'scent of man: a comparative survey of primate chemosignaling in relation to sex. Horm. Behav. 68, 117–133 (2015). [DOI] [PubMed] [Google Scholar]
- Connolly C. J. External Morphology of the Primate Brain CC Thomas, Springfield (1950). [Google Scholar]
- Falk D. External Neuroanatomy of Old World Monkeys (Cercopithecoidea). Contrib. Primatol. 15, 1–95 (1978). [PubMed] [Google Scholar]
- Falk D. in New Interpretations of Ape and Human Ancestry eds Ciochon R. L., Corruccini R. S. 239–248Springer (1983). [Google Scholar]
- Gerbella M., Belmalih A., Borra E., Rozzi S. & Luppino G. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb. Cortex 20, 141–168 (2010). [DOI] [PubMed] [Google Scholar]
- Yasuda M., Banno T. & Komatsu H. Color selectivity of neurons in the posterior inferior temporal cortex of the macaque monkey. Cereb. Cortex 20, 1630–1646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D. Sulcal patterns of fossil Theropithecus baboons: phylogenetic and functional implications. Int. J. Primatol. 2, 57–69 (1981). [Google Scholar]
- Armstrong E., Schleicher A., Omram H., Curtis M. & Zilles K. The ontogeny of human gyrification. Cereb. Cortex 5, 56–63 (1995). [DOI] [PubMed] [Google Scholar]
- Falk D. et al. The brain of LB1, Homo floresiensis. Science 308, 242–245 (2005). [DOI] [PubMed] [Google Scholar]
- Marino L. Cetacean brain evolution: multiplication generates complexity. Int. J. Comp. Psychol. 17, 1–16 (2004). [Google Scholar]
- Orliac M. J. & Gilissen E. Virtual endocranial cast of earliest Eocene Diacodexis (Artiodactyla, Mammalia) and morphological diversity of early artiodactyl brains. Proc. R Soc. Lond. B Biol. 279, 3670–3677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Skolnikoff S. Spontaneous tool use and sensorimotor intelligence in Cebus compared with other monkeys and apes. Behav. Brain Sci. 12, 561–627 (1989). [Google Scholar]
- Bakola S., Gamerini M., Passarelli L., Fattori P. & Galetti C. Cortical connection of parietal field PEc in the macaque: linking sensation for the control of limb action. Cereb. Cortex 20, 2592–2604 (2010). [DOI] [PubMed] [Google Scholar]
- Hadjidimitrakis K., Breveglieri R., Bosco A. & Fattori P. Three-dimensional eye position signals shape both in peripersonal space and arm movement activity in the medial posterior parietal cortex. Front. Integr. Neurosci. 6, 37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder A. in The Gibbons: New Perspectives On Small Ape Socioecology and Population Biology eds Lappan S., Whittaker D. 133–159Springer (2009). [Google Scholar]
- Radinsky L. An example of parallelism in carnivore brain evolution. Evolution 25, 518–522 (1971). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-2, Supplementary Tables 1-2 and Supplementary References
The KNM-MB 29100 endocast rotating around a supero-inferior axis. The surface reconstruction, based on high-resolution CT images, is of the endocast as preserved in the fossil, without corrections for distortion.