Abstract
Background
Birds possess the most diverse assemblage of haemosporidian parasites; including three genera, Plasmodium, Haemoproteus, and Leucocytozoon. Currently there are over 200 morphologically identified avian haemosporidian species, although true species richness is unknown due to great genetic diversity and insufficient sampling in highly diverse regions. Studies aimed at surveying haemosporidian diversity involve collecting and screening samples from hundreds to thousands of individuals. Currently, screening relies on microscopy and/or single or nested standard PCR. Although effective, these methods are time and resource consuming, and in the case of microscopy require substantial expertise. Here we report a newly developed real-time PCR protocol designed to quickly and reliably detect all three genera of avian haemosporidians in a single biochemical reaction.
Methods
Using available DNA sequences from avian haemosporidians we designed primers R330F and R480RL, which flank a 182 base pair fragment of mitochondrial conserved rDNA. These primers were initially tested using real-time PCR on samples from Malawi, Africa, previously screened for avian haemosporidians using traditional nested PCR. Our real time protocol was further tested on 94 samples from the Cerrado biome of Brazil, previously screened using a single PCR assay for haemosporidian parasites. These samples were also amplified using modified nested PCR protocols, allowing for comparisons between the three different screening methods (single PCR, nested PCR, real-time PCR).
Results
The real-time PCR protocol successfully identified all three genera of avian haemosporidians from both single and mixed infections previously detected from Malawi. There was no significant difference between the three different screening protocols used for the 94 samples from the Brazilian Cerrado (χ2 = 0.3429, df = 2, P = 0.842). After proving effective, the real-time protocol was used to screen 2113 Brazilian samples, identifying 693 positive samples.
Conclusions
Our real-time PCR assay proved as effective as two widely used molecular screening techniques, single PCR and nested PCR. However, the real-time protocol has the distinct advantage of detecting all three genera in a single reaction, which significantly increases efficiency by greatly decreasing screening time and cost. Our real-time PCR protocol is therefore a valuable tool in the quickly expanding field of avian haemosporidian research.
Keywords: Avian haemosporidians, Plasmodium, Haemoproteus, Leucocytozoon, Real-time PCR
Background
Haemosporidians are protozoan parasites that infect vertebrate blood cells and are transmitted by dipteran vectors [1–5]. Haemosporidians are one of the most widely studied groups of vertebrate parasites, because members of the genus Plasmodium have severe impacts on human health [6, 7] and their evolutionary history is generally not fully understood [8]. Birds possess the highest diversity of haemosporidian parasites, including three genera, Plasmodium, Haemoproteus, and Leucocytozoon [4]. Studies of avian haemosporidians have a long history being first described by Danilewsky [9] and later used as a model for human malaria [4, 6, 10]. With the discovery of rodent malaria [11] avian haemosporidians lost their importance as laboratory models. Consequently, they were relegated to the status of a group of limited interest, studied mainly in connection with impacts of these parasites on wild and domestic bird populations [4].
The past two decades have seen a dramatic increase in the study of these parasites as tools to test evolutionary theories of parasite-host interactions [12–18] and the cost of parasitism on host populations [19–24]. The growth in this field is directly tied to the development of a standard nested PCR protocol for amplifying a portion of the haemosporidian cytochrome b gene [25–27] and the subsequent development of the MalAvi database of avian haemosporidian lineages [28] (http://mbio-serv2.mbioekol.lu.se/Malavi/). Prior to the development of these resources, the main method to identify these parasites was microscopic examination of blood films, which requires expertise in making, staining, and examining such films. Although examination of blood films is an effective way for identifying and quantifying parasites [29], the expertise needed to screen blood films takes time to develop, and chronically infected birds with low parasitemia can be missed [27, 30]. Although morphological data remain essential to link genetic lineages with known morphospecies [29], molecular identification requires only minimal training, does not require quality blood films, and is generally accepted to be more sensitive than microscopy [30–34]. It is also much faster and allows screening of large numbers of samples in a relatively short time.
The PCR protocols initially developed by Bensch et al. [25], and modified by Hellgren et al. [26], and Waldenström et al. [27] are widely used today. They rely on using two nested PCR amplifications of a 478 bp fragment of the cytochrome b gene, one set of nested PCR for Haemoproteus/Plasmodium [25, 27] and a separate set for Leucocytozoon [26]. Although effective at both screening and amplifying haemosporidian parasite DNA, the time and amount of reagents necessary for running nested reactions can be limiting when screening large numbers of samples. Fallon et al. [35] worked around this issue by developing an initial standard PCR screening protocol that amplified a 154 bp fragment of the conserved rDNA region of the mitochondrial genome of Haemoproteus and Plasmodium, although it did not identify Leucocytozoon. Only positive samples from screening were subsequently amplified by regular PCR for cytochrome b and sequenced. This increased the speed at which large sets of samples could be screened, but still required the gel electrophoresis of hundreds or thousands of PCR products. Subsequently, researchers who used the Fallon et al. [35] protocol for initial screening moved to various nested PCR protocols, e.g.[36, 37], to improve the chances of amplifying haemosporidian DNA from hosts with low intensity of infection.
The use of real-time PCR to screen samples for presence of viral [38–40], bacterial [41–43], or parasite [44–46] DNA has become a useful and common method of determining pathogen prevalence in host populations. Although real-time PCR has been used for avian haemosporidians, it has generally been used to determine level of parasitemia [47–50] or for detecting specific lineages [22, 51–53]. The usefulness of real-time PCR as a large scale screening tool for haemosporidian DNA in avian blood samples has been only minimally explored [54] and never done for all three genera with a single reaction. Here we report the development of a real-time PCR protocol that can identify infections of any of three haemosporidian genera in a single screening reaction using a 182 bp fragment of the conserved RNA region of the mitochondrial genome.
Methods
Design of primers that could successfully amplify all three genera in a single real-time reaction required determining a gene region that is more conserved than the standard 478 bp fragment of the cytochrome b gene [26, 27]. The conserved rDNA region of the mitochondrial genome was a good target because it is quite conserved in avian haemosporidians and has been previously used to screen for Haemoproteus and Plasmodium infections [35]. Available avian haemosporidian mitochondrial sequences from GenBank (Table 1) that contained the conserved rDNA region were aligned using BioEdit v7.2.0 [55]. Although the primers described by Fallon et al. [35] did not match Leucocytozoon sequences, a region adjacent to these primers proved to be sufficiently conserved for detection of all three genera. The forward primer R330F and reverse primer R480RL were designed, flanking a 182 base pair fragment (Fig. 1, Table 2).
Table 1.
List of GenBank sequences used to design real-time PCR primers to detect haemosporidian rDNA. Accession numbers and the associated haemosporidian species/lineage are given
| Accession Number | Haemosporidian species/lineage |
|---|---|
| FJ168562 | Haemoproteus columbae |
| AY733087 | Haemoproteus sp. jb1. JA27 |
| AB302215 | Leucocytozoon caulleryi |
| FJ168564 | Leucocytozoon fringillinarum |
| FJ168563 | Leucocytozoon majoris |
| NC009336 | Leucocytozoon sabrezesi |
| AB250690 | Plasmodium gallinaceum |
| AB250415 | Plasmodium juxtanucleare |
| KC138226 | Plasmodium lutzi |
| NC012426 | Plasmodium relictum |
Fig. 1.
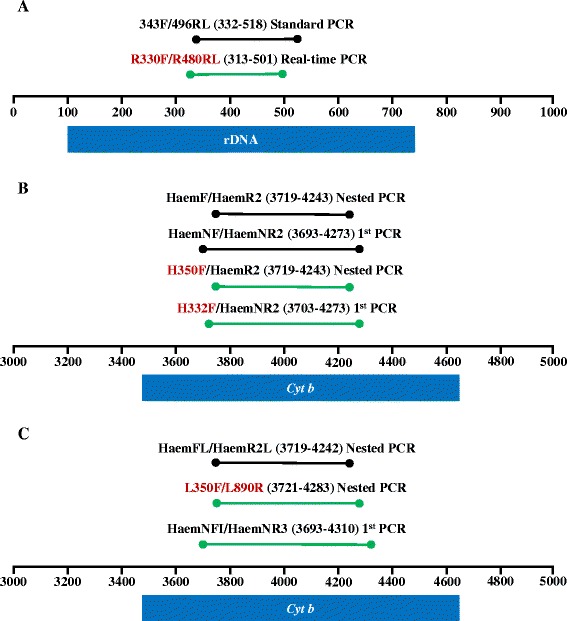
Primer positions of rDNA primers for standard and real-time PCR (a) and cytochrome b primers for nested PCR for Haemoproteus/Plasmodium (b), and Leucocytozoon (c). Blue bars denote location of the target genes on the mitochondrial genome of Plasmodium relictum (NC012426). The spans of amplified DNA fragments are indicated in parentheses behind each primer pair. Fragments in green are those that we recommend for use in avian haemosporidian detection (a) and amplification by nested PCR (b, c). Primers in red represent new primers developed for avian haemosporidians either herein or in [17]
Table 2.
Primer sequences for real-time and nested PCR protocols, along with sequence of positive control used for real time PCR reactions. Sequencing primers are also listed
| Protocol/primer | Primer sequence |
|---|---|
| Real-Time PCR – Haemoproteus, Plasmodium, Leucocytozoon | |
| R330Fa | 5′- CGTTCTTAACCCAGCTCACG - 3′ |
| R480RLa | 5′- GCCTGGAGGTWAYGTCC – 3′ |
| P. relictum – Pos. Control | 5′- GGGAACAAACTGCCTCAAGACGTTCTTAACCAGCT |
| (Accession # NC012426) | CACGCATCGCTTCTAACGGTGAACTCTCATTCCAA |
| TGGAACCTTGTTCAAGTTCAAATAGATTGGTAAGG | |
| TATAGCGTTTACTATCGAATGAAACAATGTGTTCC | |
| ACCGCTAGTGTTTGCTTCTAACATTCCATTGCTTAT | |
| AACTGTATGGACGTAACCTCCAGGCAAAGAAAAT | |
| GACCGGTC – 3′ | |
| Nested PCR – Haemoproteus and Plasmodium | |
| H332Fa | 5′ - GAGAATTATGGAGYGGATGGTG - 3′ |
| HAEMNR2b | 5′ - AGAGGTGTAGCATATCTATCTAC- 3′ |
| H350Fa | 5′ – GGTGTTTTAGATATATGCATGC - 3′ |
| HAEMR2c | 5′ - GCATTATCTGGATGTGATAATGGT - 3′ |
| Nested PCR – Leucocytozoon | |
| HAEMNFId | 5′ - CATATATTAAGAGAAITATGGAG - 3′ |
| HAEMNR3d | 5′ - ATAGAAAGATAAGAAATACCATTC - 3′ |
| L350Fe | 5′ - GGTGTTTTAGATACTTA -3′ |
| L890Re | 5′ - TACAATATGTTGAGGTGTTTG - 3′ |
| Sequencing – Haemoproteus and Plasmodium | |
| FIFIf | 5′ – GGGTCAAATGAGTTTCTGG - 3′ |
| R2f | 5′ - GCTGTATCATACCCTAAAGG - 3′ |
| Sequencing – Leucocytozoon | |
| L545Fe | 5′ - ACAAATGAGTTTCTGGGGA - 3′ |
| L825Re | 5′ - GCAATTCCAAATAAACTTTGAA - 3′ |
These primers were tested using DNA extracted from avian blood stored on Whatman FTA Classic Cards or 95 % ethanol and liver samples stored in 95 % ethanol. DNA was extracted using the Qiagen DNeasy 96 Blood and Tissue kit (Qiagen, Valencia, CA), following the Qiagen dried blood spot protocol for blood stored on Whatman FTA Classic Cards and the Qiagen tissue protocol for both blood and liver stored in 95 % ethanol. Since blood coagulates in 95 % ethanol, sterilized wooden applicators were used to transfer a small portion of the clot representing approximately 2 mm3 into each extraction tube. Both liver and coagulated blood samples required overnight incubation at 56 °C for appropriate digestion. Both the American Ornithologist’s Union (http://www.nmnh.si.edu/BIRDNET/guide) and University of North Dakota Animal Care and Use Committee guidelines (Project # 1402-1) for ethically collecting avian blood and tissue samples were strictly followed.
All reactions were carried out using iTaq universal SYBR Green Supermix on a CFX96 real-time thermocycler (Bio-Rad, Hercules, CA). The total volume of the reactions was 15 μl, with 7.5 μl of SYBR Green Supermix, 0.6 μl of each primer (10 μM concentration), 3.3 μl of molecular grade water, and 3 μl of DNA template (the volume established empirically, approximately 20 ng/μl). The following cycling conditions were used: 95 °C for 30 s, followed by 35 cycles of 95 °C for 30 s and 53 °C for 35 s (with a plate read) followed by a final melt curve analysis using instrument default settings. Positive and negative controls were included in all runs. The positive control used was a synthetic double stranded DNA product (G-Block - IDT DNA, Coralville, IA) designed from a 220 bp fragment of the conserved rDNA region of Plasmodium relictum (Accession # NC012426) (Table 1). The positive control of Plasmodium relictum produced a melt curve peak at 78.5 °C (Fig. 2).
Fig. 2.
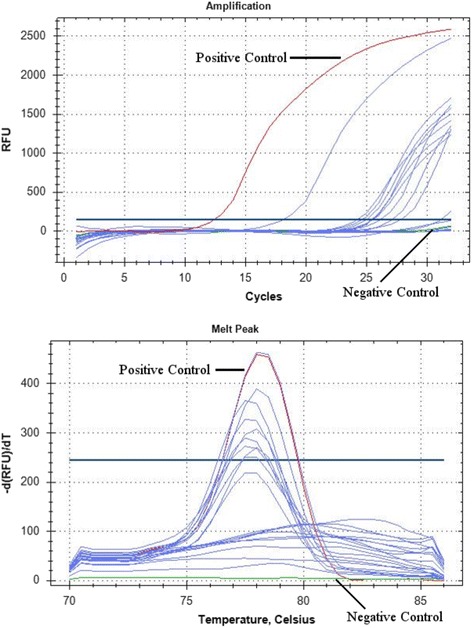
Amplification and melt peak curves from real-time PCR amplification of rDNA from avian blood samples. Positive (Plasmodium relictum) control, shown in red, and negative (water) control, shown in green, are indicated in the curves
This protocol was initially tested on samples positive for Plasmodium, Haemoproteus, or Leucocytozoon, samples with mixed infections, and known negative samples, from a previous study of haemosporidians from Malawi, Africa [17]. These samples had been previously screened by nested PCR and microscopy [17] and were from 16 host species, representing 15 genera, 13 families, and 7 orders.
To further test this protocol 94 samples were selected from 791 samples collected from the Cerrado biome of Brazil and previously screened for haemosporidian parasites [36] using the Fallon et al. protocol [35]. These samples were obtained from four host species, Myiarchus swainsoni, Neothraupis fasciata, Nystalus chacuru, and Volatinia jacarina, and were rescreened with the real-time protocol and also amplified using nested PCR protocols (described below) to amplify the cytochrome b gene. This not only allowed for testing the effectiveness of the real time protocol, but also enabled comparison between the three different screening methods (single PCR, nested PCR, real-time PCR). Results for these screening methods were analysed using a 2 × 3 chi-square contingency table using the package Rcmdr in program R [56].
Two modified nested PCR protocols were used to amplify fragments of the cytochrome b gene (Table 2). The protocol for Haemoproteus/Plasmodium was based on the standard protocol of Waldenström et al. [27] but with newly designed forward primers, H332F and H350F (Fig. 1, Table 2), which match more closely with available GenBank sequences. The protocol produces a 477 bp fragment, which is only one base pair shorter than the fragment produced by the Waldenström et al. protocol [27]. The Leucocytozoon protocol uses the initial primer sets described by Hellgren et al. [26] but with newly designed nested primers [17] (Fig. 1, Table 2). This new protocol produces a 526 bp fragment that encompasses the 478 bp fragment produced by the Hellgren protocol [17].
All nested PCRs were run using OneTaq Quick-Load 2X Master Mix with standard buffer (New England Biolabs, Ipswich, MA) in 20 μl reactions. The initial PCR amplifications included 10 μl of OneTaq Master Mix, 1 μl of each primer (10 μM concentration), 3 μl of molecular grade water, and 5 μl of template (the volume established empirically, approximately 20 ng/μl). The nested PCR amplifications differed in using 5 μl of water and 3 μl of PCR product as template. The following protocol was used for all reactions; 95 °C for 3 min, then followed by 20 cycles (first amplification)/35 cycles (nested amplification) of 95 °C for 30 s, 50 °C for 45 s, and 68 °C for one minute, followed by a final elongation at 68 °C for 5 min. Negative controls were included in all nested PCR runs. Each sample identified as positive by real-time PCR underwent two separate nested PCR amplifications, one for Haemoproteus/Plasmodium using our modified Waldenström protocol and one for Leucocytozoon [17].
PCR products were run on 1.25 % agarose gels, stained with ethidium bromide, and visualized under UV light. Positive PCR products were purified using ExoSAP-IT (Affymetrix, Santa Clara, CA) and sequenced using BigDye terminator v3.1 cycle sequencing kit (Applied Bio systems, Foster City, CA). The primers FIFI and R2 [57] were used for sequencing of Haemoproteus and Plasmodium and the primers L545F and L825R [17] were used for Leucocytozoon (Table 1). Sequencing reaction products were precipitated with ethanol, dried by vacuum centrifuge, re-suspended with 10 μl of dH2O, and run on an ABI 3100 DNA sequencer (Applied Bio systems, Foster City, CA). Forward and reverse sequences were visualized and assembled using Sequencher v.5.0.1 (Gene Codes Corp., Ann Arbor, MI). Assembled sequences were aligned using BioEdit v7.2.0 [55] and collapsed to unique haplotypes using the FaBox haplotype collapse and converter tool [58]. Sequence identities were verified with a local BLAST against the MalAvi database [28] using BioEdit v7.2.0 [55].
Results
The real-time PCR protocol successfully identified all single infections of Plasmodium, Haemoproteus, and Leucocytozoon previously detected by standard nested PCR protocol and microscopy [17] from samples collected in Malawi, Africa. For all three genera the melt peaks generally occurred between 78 to 79 °C, but variability existed, with some lineages producing peaks slightly above or below this range. The assay also detected all samples from the same collection with mixed infections of Plasmodium/Haemoproteus, Plasmodium/Leucocytozoon, Haemoproteus/Leucocytozoon, and Plasmodium/Haemoproteus/Leucocytozoon, but due to the use of a single primer set it was generally not possible to discern mixed infections with the real-time PCR assay. The intensity of infection as determined by blood films had no effect on detection by real-time PCR. It successfully detected the presence of haemosporidians in samples with only one infected red blood cell per 100 fields at 1000× magnification.
There was no significant difference between the three different screening protocols used for the 94 samples from Cerrado (χ2 = 0.3429, df = 2, P = 0.842) (Table 3). The Fallon protocol identified 49 positive samples, the real-time protocol identified 53 positive samples, and our nested PCR protocol for Haemoproteus/Plasmodium (Table 2) identified 51 positive samples (Table 3). The samples were also run using the Leucocytozoon nested PCR protocol [17] and all were negative. The real-time protocol identified 45 out of 49 samples previously identified by the Fallon protocol and 48 out of 51 samples identified by our nested PCR protocol. Two samples determined to be positive by both the Fallon et al. protocol [35] and the real-time protocol were negative by our nested PCR protocol and three samples were only found positive by the real-time protocol. Both the Fallon protocol and the real-time protocol failed to identify three samples screened as positives by our nested PCR protocol (Table 3).
Table 3.
Results of single, nested, and real-time PCR tests on 94 samples from Cerrado biome of Brazil. Only samples that were positive by at least one screening method are shown, 36 samples were negative by all three methods. Forty-two samples were positive by all three screening methods (bold text), samples with divergent results are shown individually
| Sample ID | Single PCR | Nested PCR | Real-time PCR |
|---|---|---|---|
| Various (n = 42) | Positive | Positive | Positive |
| CE0049 | Positive | Positive | |
| CE0051 | Positive | Positive | |
| CE0053 | Positive | Positive | |
| CE0058 | Positive | ||
| CE0060 | Positive | Positive | |
| CE0068 | Positive | Positive | |
| CE0071 | Positive | ||
| CE0074 | Positive | ||
| CE0076 | Positive | ||
| CE0578 | Positive | Positive | |
| CE0581 | Positive | Positive | |
| CE0592 | Positive | Positive | |
| CE0594 | Positive | Positive | |
| CE0595 | Positive | Positive | |
| CE0597 | Positive | Positive | |
| CE0598 | Positive | ||
| TOTAL | 49 | 51 | 53 |
After all the new and amended protocols were tested, the real-time protocol was used to screen 2113 samples collected from three Brazilian biomes; Amazonia, Caatinga, and Pantanal and representing 332 host species. Of these 2113 samples, 693 were identified as positive by real-time PCR. Of those 693 infected, we successfully amplified cytocrome b with nested primers in 532 samples (77 %) and confirmed their identification by sequencing. These infected individuals included single infections of Plasmodium and Haemoproteus as well as coinfections of two different haemosporidian taxa, including Haemoproteus/Haemoproteus, Haemoproteus/Plasmodium, and Plasmodium/Plasmodium. No Leucocytozoon infections have been detected in this sample which is in agreement with previous reports from the region [4, 59, 60].
Discussion
The real-time protocol presented herein is highly effective at determining the presence of haemosporidian parasites in avian blood and liver samples. It reliably identified all known positive samples from a recently published study of haemosporidians from birds sampled in Malawi [17] and matched the results of two other standard molecular screening methods. The real-time protocol also successfully detected parasites in more than 2100 samples from Brazil. The results of these three screening methods (single PCR, nested PCR, real-time PCR) were not significantly different when used to screen the same blood samples, showing that similar results were obtained regardless of the screening method employed. This is important for the comparability of results from studies where these different screening methods have been used.
Limitations exist for any screening method for haemosporidians, whether using microscopy or molecular techniques. Birds with low parasitemia during the chronic phase of infection are always difficult to detect with microscopy creating the potential for misidentification of these birds as uninfected [27, 30]. Increasing the area of the blood film screened reduces the probability of false negative results [29], but adds considerable time to the screening process, 20 to 25 min per slide [29]. Even after adding additional screening time some infections will be missed. For example, a blood film from an individual with low parasitemia rarely contains all stages of haemosporidian development that are necessary for identification and/or adequate characterization of morphological species.
With molecular techniques, including nested PCR, low intensity infections can also be missed [29]. Molecular screening techniques based on PCR and Sanger sequencing also have lower ability to distinguish and identify mixed infections [61]. This is compounded by the fact that the host DNA is much more concentrated in samples than parasite DNA which somewhat affects the ability to detect haemosporidian DNA [62] or to PCR amplify larger fragments of parasite DNA, a necessity for the nested PCR protocol. This is evident in the results from this study, where only 77 % of the 693 samples identified as positive by real-time PCR were also identified as positive by nested PCR.
The goal of any new screening method is to provide an accurate estimate of parasite prevalence and to provide advantages over already established methods. The real-time PCR protocol proved as effective as the two most widely used molecular screening methods for haemosporidian parasites in birds [27, 35]. Although all three methods likely leave a small proportion of samples undetected, there are distinct advantages of the real-time protocol. The main advantage of this protocol is its ability to reliably and quickly detect haemosporidian infections. Since real-time PCR eliminates gel electrophoresis, the result for a full 96 or 384-well PCR plate are available in one hour (or sooner if fast running protocol and corresponding PCR mix is used). With the Fallon et al. [35] or Waldenström et al. [27] protocols not only is cycling time between 2.5 to 3.5 times longer respectively, there is also the added time of gel electrophoresis before results can be determined. Thus, the real-time protocol dramatically increases throughput of sample screening.
Of the three methods, only our real-time protocol uses a single reaction to screen for Leucocytozoon in addition to Plasmodium and Haemoproteus infections. The Fallon et al. [35] protocol was not designed to target Leucocytozoon. To amplify Leucocytozoon DNA with nested PCR a separate set of nested PCR amplifications are needed, the most widely used is the protocol of Hellgren et al. [26]. Inability to screen for all three genera in one nested PCR protocol increases the time and expense of screening for Leucocytozoon infections. This has led to a strong bias towards screening for Haemoproteus and Plasmodium only and ignoring Leucocytozoon, which explains why it is understudied. This is particularly true in areas of high host diversity, where the increased cost of PCR amplifications can make screening for Leucocytozoon prohibitive. Recent studies have shown that the Leucocytozoon diversity may be high in regions with high avian diversity [17] and in specific host populations [63]. Availability of a screening method that can amplify all three genera can aid in understanding the true diversity and ecology of all three genera of avian haemosporidian parasites. Until now, the only screening methods that could detect all three genera in a single procedure were microscopy and the restriction digestion protocol of Beadell & Fleischer [64], but both take significantly more time than the real-time PCR protocol and still require the use of nested PCR to amplify DNA for sequencing.
Although real-time PCR reagents are somewhat more expensive than those for standard PCR, it is more cost effective to use real-time PCR compared to the cost of running two to three rounds of regular/nested PCRs and associated gels for all samples. The cost advantage is even more evident when time and workforce cost are taken into consideration. This is especially beneficial when screening very large sets of hundreds or thousands of samples.
Conclusions
Our real-time PCR assay proved as effective as two currently used molecular screening techniques, a single PCR screening assay [35] and nested PCR screening assays [26, 27]. However, the real-time protocol has the distinct advantage of detecting all three genera in a single reaction in at least half the time of these current methods. Therefore, throughput is significantly increased by greatly decreasing screening time and cost without loss of sensitivity. The ability to quickly and reliably screen avian blood samples is crucial for trying to understand the species richness and ecology of haemosporidian parasites, especially from highly diverse areas. The real-time protocol proposed here serves these purposes and provides a very useful tool in the expanding field of avian haemosporidian research.
Acknowledgements
JB would like to thank the Malaria Research Coordination Network for the possibility to participate in the 2014 Third Annual International Workshop on Malaria and Related Haemosporidian Parasites of Wildlife. The fruitful discussions during the workshop helped to shape this manuscript. Both the American Ornithologist’s Union [65] and University of North Dakota Animal Care and Use Committee guidelines (Project # 1402–1) for ethically collecting avian blood and tissue samples were strictly followed. This project was funded in part by the National Science Foundation as part of their biodiversity, discovery, and analysis program, project numbers DEB-1050525 to VT and DEB-1503804 to JW, and a Postdoctoral Fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (Process number 201275/2014-7) to AF.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JB and VT conceived of the idea, JB designed primers and protocols and carried out all tests, JW and AF provided samples, JB, JW, AF, and VT wrote and reviewed the manuscript. All authors approved the final version of the manuscript.
Contributor Information
Jeffrey A. Bell, Email: jeffrey.bell@my.und.edu
Jason D. Weckstein, Email: jdw342@drexel.edu
Alan Fecchio, Email: alanfecchio@yahoo.com.br.
Vasyl V. Tkach, vasyl.tkach@email.und.edu
References
- 1.Garnham PCC. Malaria parasites and other Haemosporidia. Oxford: Blackwell Scientific Publications; 1966. [Google Scholar]
- 2.Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. Washington, DC: US Government Printing Office; 1971. [Google Scholar]
- 3.Schall JJ. Malaria parasites of lizards: diversity and ecology. Adv Parasitol. 1996;37:255–333. doi: 10.1016/S0065-308X(08)60222-5. [DOI] [PubMed] [Google Scholar]
- 4.Valkiūnas G. Avian malaria parasites and other Haemosporidia. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 5.Telford SR. Haemosporidia of Reptilia: color atlas and text. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- 6.Cox FEG. History of the discovery of the malaria parasites and their vectors. Parasit Vectors. 2010;3:5. doi: 10.1186/1756-3305-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay SI, Okiro EA, Gething PW, Path AP, Tatem AJ, Guerra CA, et al. Estimate the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010; doi:10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed]
- 8.Perkins SL. Malaria’s many mates: past, present, and future of the systematics of the Order Haemosporida. J Parasitol. 2014;100:11–25. doi: 10.1645/13-362.1. [DOI] [PubMed] [Google Scholar]
- 9.Danilewsky VY. About blood parasites (Haematozoa) Russian Med. 1884;46:948–9. [Google Scholar]
- 10.Atkinson CT, van Riper C., III . Pathogenicity and epizootiology of avian hematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M, editors. Bird-parasite interactions: ecology, evolution, and behavior. New York: Oxford University Press; 1991. pp. 19–48. [Google Scholar]
- 11.Vincke W, Lips M. Un nouveau Plasmodium d’un rongeur sauvage du Congo, Plasmodium berghi n.sp. Soc Belg Med Trop. 1948;28:97–104. [PubMed] [Google Scholar]
- 12.Ricklefs RE, Fallon SM. Diversification and host switching in avian malaria parasites. Proc R Soc Lond B Biol Sci. 2002;269:885–92. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricklefs RE, Fallon SM, Bermingham E. Evolutionary relationships, cospeciations, and host switching in avian malaria parasites. Syst Biol. 2004;53:111–9. doi: 10.1080/10635150490264987. [DOI] [PubMed] [Google Scholar]
- 14.Fallon SM, Bermingham E, Ricklefs RE. Host specialization and geographic localization of avian malaria parasites: a regional analysis in the Lesser Antilles. Am Nat. 2005;165:466–80. doi: 10.1086/428430. [DOI] [PubMed] [Google Scholar]
- 15.Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol Phylogenet Evol. 2008;47:261–73. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Ricklefs RE, Outlaw DC, Svensson-Coelho MS, Medeiros MCI, Ellis VA, Latta S. Species formation by host shifting in avian malaria parasites. Proc Natl Acad Sci USA. 2014;111:14816–21. doi: 10.1073/pnas.1416356111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz HL, Hochachka WM, Engel JI, Bell JA, Tkach VV, Bates JM, et al. Parasite prevalence corresponds to host life history in a diverse assemblage of Afrotropical bids and haemosporodian parasites. PLoS One. 2015; doi:10.1371/journal.pone.0121254. [DOI] [PMC free article] [PubMed]
- 18.Olsson-Pons S, Clark NJ, Ishtiaq F, Clegg SM. Differences in host species relationships and biogeographic influences produce contrasting patterns of prevalence, community composition and genetic structure in two genera of avian malaria parasites in southern Melanesia. J Anim Ecol. 2015; doi:10.1111/1365-2656.12354. [DOI] [PubMed]
- 19.Marzal A, de Lope F, Navarro C, Møller AP. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia. 2005;142:541–5. doi: 10.1007/s00442-004-1757-2. [DOI] [PubMed] [Google Scholar]
- 20.Knowles SCL, Palinauskas V, Sheldon BC. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J Evol Biol. 2009;23:557–69. doi: 10.1111/j.1420-9101.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-de la Puente J, Merino S, Thomás G, Moreno J, Morales J, Lobato E, et al. The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett. 2010;6:663–5. doi: 10.1098/rsbl.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asghar M, Hasselquist D, Bensch S. Are chronic avian haemosporidian infections costly in wild birds? J Avian Biol. 2011;42:530–7. doi: 10.1111/j.1600-048X.2011.05281.x. [DOI] [Google Scholar]
- 23.Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon B. Fitness effects of endemic malaria infections in wild bird populations: the importance of ecological structure. J Anim Ecol. 2011;80:1196–206. doi: 10.1111/j.1365-2656.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- 24.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science. 2015;347:9–12. doi: 10.1126/science.1261121. [DOI] [PubMed] [Google Scholar]
- 25.Bensch S, Stjernman M, Hasselquist D, Östman Ö, Hansson B, Westerdahl H, et al. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc Lond B Biol Sci. 2000;267:1583–9. doi: 10.1098/rspb.2000.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucyoctyozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- 27.Waldenström J, Bensch S, Hasselquist D, Östman Ö. A new nested polymerase chain reation method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol. 2004;90:191–4. doi: 10.1645/GE-3221RN. [DOI] [PubMed] [Google Scholar]
- 28.Bensch S, Hellgren O, Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour. 2009;9:1353–8. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- 29.Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Ravinder NMS, Bensch S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol. 2008;94:1395–401. doi: 10.1645/GE-1570.1. [DOI] [PubMed] [Google Scholar]
- 30.Jarvi SI, Farias MEM, Baker H, Friefeld HB, Baker PE, Van Gelder E, et al. Detection of avian malaria (Plasmodium spp.) in native land birds of American Samoa. Conserv Genet. 2003;4:629–37. doi: 10.1023/A:1025626529806. [DOI] [Google Scholar]
- 31.Jarvi SI, Schultz JJ, Atkinson CT. PCR diagnostics underestimate the prevalence of avian malaria (Plasmodium relictum) in experimentally-infected passerines. J Parasitol. 2002;88:153–8. doi: 10.1645/0022-3395(2002)088[0153:PDUTPO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Richard FA, Sehgal RN, Jones HI, Smith TB. A comparative analysis of PCR-based detection methods of avian malaria. J Parasitol. 2002;88:819–22. doi: 10.1645/0022-3395(2002)088[0819:ACAOPB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Durrant KL, Beadell JS, Ishtiaq F, Graves GR, Olson SL, Gering E, et al. Avian haematozoa in South America: a comparison of temperate and tropical zones. Ornithol Monogr. 2006;60:98–111. doi: 10.1642/0078-6594(2006)60[98:AHISAA]2.0.CO;2. [DOI] [Google Scholar]
- 34.Fallon SM, Ricklefs RE. Parasitemia in PCR-detected Plasmodium and Haemoproteus infections in birds. J Avian Biol. 2008;39:514–22. doi: 10.1111/j.0908-8857.2008.04308.x. [DOI] [Google Scholar]
- 35.Fallon SM, Ricklefs RE, Swanson BL, Bermingham E. Detecting avian malaria: an improved polymerase chain reaction diagnostic. J Parasitol. 2003;89:1044–7. doi: 10.1645/GE-3157. [DOI] [PubMed] [Google Scholar]
- 36.Fecchio A, Lima MR, Svensson-Coelho M, Marini MA, Ricklefs RE. Structure and organization of an avian haemosporidian assemblage in a Neotropical savanna in Brazil. Parasitology. 2013;140:181–92. doi: 10.1017/S0031182012001412. [DOI] [PubMed] [Google Scholar]
- 37.Svensson-Coelho M, Blake JG, Loiselle BA, Penrose AS, Parker PG, Ricklefs RE. Diversity, prevalence, and host specificity of avian Plasmodium and Haemoproteus in a Western Amazon assemblage. Ornithol Monogr. 2013;76:1–47. doi: 10.1525/om.2013.76.1.1. [DOI] [Google Scholar]
- 38.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–71. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Liu Y, Zhang S, Wang Y, Zhao J, Miao F, et al. A SYBR-green I quantitative real-time reverse transcription-PCR assay for rabies viruses with different virulence. Virol Sin. 2014;29:131–2. doi: 10.1007/s12250-014-3378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan W, Zheng Y, Sun M, Zhang X, Qi Y, Sun J. Development of a TaqMan-based real-time reverse transcription polymerase chain reaction assay for the detection of encephalomyocarditis virus. J Virol Methods. 2014;201:60–5. doi: 10.1016/j.jviromet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Ferdin J, Cerar T, Strle F, Ruzić-Sabljić E. Evaluation of real-time PCR targeting hbb gene for Borrelia species identification. J Microbiol Methods. 2010;82:115–9. doi: 10.1016/j.mimet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Birdsell DN, Vogler AJ, Buchagen J, Clare A, Kaufman E, Naumann A, et al. TaqMan real-time PCR assays for single-nucleotide polymorphisms which identify Francisella tularensis and its subspecies and subpopulations. PLoS One. 2014; doi:10.1371/journal.pone.0107964. [DOI] [PMC free article] [PubMed]
- 43.Greiman SE, Tkach VV, Pulis E, Fayton TJ, Curran SS. Large scale screening of digeneans for Neorickettsia endosymbionts using real-time PCR reveals new Neorickesttsia genotypes, host associations and geographic records. PLoS One. 2014; doi:10.1371/journal.pone.0098543. [DOI] [PMC free article] [PubMed]
- 44.Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol. 2012;50:903–8. doi: 10.1128/JCM.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albers A, Sartono E, Wahyuni S, Yazdanbakhsh M, Maizels RM, Klarmann-Schulz U, et al. Real-time PCR identification of the Hhal tandem DNA repeat in pre – and post-patent Brugia malayi infections: a study in Indonesian transmigrants. Parasit Vectors. 2014;7:146. doi: 10.1186/1756-3305-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu W, Morris U, Aydin-Schmidt B, Msellem MI, Shakely D, Petzold M, et al. SYBR green real-time PCR-RFLP assay targeting the Plasmodium cytochrome b gene – a highly sensitive molecular tool for malaria parasite detection and species determination. PLoS One. 2015; doi:10.1371/journal.pone.0120210. [DOI] [PMC free article] [PubMed]
- 47.Bentz S, Rigaud T, Barroca M, Martin-Laurent F, Bru D, Moreau J, et al. Sensitive measure of prevalence and parasitaemia of haemosporidia from European blackbird (Turdus merula) populations: value of PCR-RFLP and quantitative PCR. Parasitology. 2006;133:685–92. doi: 10.1017/S0031182006001090. [DOI] [PubMed] [Google Scholar]
- 48.Zehtindjiev P, Ilieva M, Westerdahl H, Hansson B, Valkiūnas G, Bensch S. Dynamics of parasitemia of malaria parasites in a naturally and experimentally infected migratory songbird, the great reed warbler Acrocephalus arundinaceus. Exp Parasitol. 2008;119:99–110. doi: 10.1016/j.exppara.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Knowles SCL, Wood MJ, Alves R, Wilken TA, Bensch S, Sheldon BC. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol Ecol. 2011;20:1062–76. doi: 10.1111/j.1365-294X.2010.04909.x. [DOI] [PubMed] [Google Scholar]
- 50.van Rooyen J, Lalubin F, Glaizot O, Christe P. Avian haemosporidian persistence and co-infection in great tits at the individual level. Malar J. 2013;12:40. doi: 10.1186/1475-2875-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cellier-Holzem E, Esparza-Salas R, Garnier S, Sorci G. Effect of repeated exposure of Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int J Parasitol. 2010;40:1447–53. doi: 10.1016/j.ijpara.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Larcombe S, Bichet C, Cornet S, Faivre B, Sorci G. Food availability and competition do not modulate the costs of Plasmodium infection in dominant male canaries. Exp Parasitol. 2013;135:708–14. doi: 10.1016/j.exppara.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Biedrzycka A, Migalska M, Bielański W. A quantitative PCR protocol for detecting specific Haemoproteus lineages: molecular characterization of blood parasites in a sedge warbler population from southern Poland. J Ornithol. 2014;156:201–8. doi: 10.1007/s10336-014-1116-y. [DOI] [Google Scholar]
- 54.Friedl TWP, Groscurth E. A real-time PCR protocol for simple and fast quantification of blood parasite infections in evolutionary and ecological studies and some data on intensities of blood parasite infections in a subtropical weaverbird. J Ornithol. 2012;153:239–47. doi: 10.1007/s10336-011-0735-9. [DOI] [Google Scholar]
- 55.Hall TA. BIOEDIT: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8. [Google Scholar]
- 56.Fox J. The R commander: A basic statistics graphical user interface to R. J Stat Softw. 2005;14:1–42. [Google Scholar]
- 57.Ishtiaq F, Gering E, Rappole JH, Rahmani AR, Jhala YV, Dove CJ, et al. Prevalence and diversity of avian hamatozoan parasites in Asia: a regional survey. J Wildl Dis. 2007;43:382–98. doi: 10.7589/0090-3558-43.3.382. [DOI] [PubMed] [Google Scholar]
- 58.Villesen P. FaBox: an online toolbox for fasta sequences. Mol Ecol Notes. 2007;7:965–8. doi: 10.1111/j.1471-8286.2007.01821.x. [DOI] [Google Scholar]
- 59.White E, Greiner EE, Bennet GF, Herman CM. Distribution of the hematozoa of Neotropical birds. Rev Biol Trop. 1978;26:43–102. [PubMed] [Google Scholar]
- 60.Forrester D, Greiner E. Leucocytozoonosis. In: Atkinson CT, Thomas NB, editors. Parasitic diseases of wild birds. Ames, IA: Wiley-Blackwell; 2008. pp. 55–107. [Google Scholar]
- 61.Valkiūnas G, Bensch S, Iezhova TA, Križanauskienė A, Hellgren O, Bolshakov CV. Nested cytochrome b polymerase chain reaction diagnostics underestimate missed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol. 2006;92:418–22. doi: 10.1645/GE-3547RN.1. [DOI] [PubMed] [Google Scholar]
- 62.Freed LA, Cann RL. DNA quality and accuracy of avian malaria PCR diagnostics: a review. Condor. 2006;108:459–73. doi: 10.1650/0010-5422(2006)108[459:DQAAOA]2.0.CO;2. [DOI] [Google Scholar]
- 63.Reeves AB, Smith MM, Meixell BW, Fleskes JP, Ramey AM. Genetic diversity and host specificity varies across three genera of blood parasites in ducks of the Pacific Americas flyway. PLoS One. 2015; doi:10.1371/journal.pone.0116661. [DOI] [PMC free article] [PubMed]
- 64.Beadell JS, Fleischer RC. A restriction enzyme-based assay to distinguish between a avian haemosporidians. J Parasitol. 2005;91:683–5. doi: 10.1645/GE-3412RN. [DOI] [PubMed] [Google Scholar]
- 65.Fair JE, Jones J. Guidelines for the use of wild birds in research. In: BIRDNET, presented by the ornithological council. 2010. http://www.nmnh.si.edu/BIRDNET/guide. Accessed 30 May 2015.


