Abstract
Inflammation within the brainstem microvasculature has been associated with chronic cardiovascular diseases. We found that the expression of several enzymes involved in arachidonic acid (AA) - leukotriene B4 (LTB4) production was altered in NTS of SHR. LTB4 produced from AA by 5-lipoxygenase (5LOX) is a potent chemoattractant of leukocytes. Leukotriene B4-12-hydroxydehydrogenase (LTB4-12-HD), which degrades leukotriene B4 (LTB4), was down-regulated compared to Wistar-Kyoto rats (WKY). Quantitative RT-PCR revealed that LTB4-12-HD was reduced by 63 and 58% in the NTS of adult SHR and pre-hypertensive (PH) SHR respectively, compared to age-matched WKY rats (n=6). 5LOX gene expression was up-regulated in the NTS of SHR (~50%; n=6). LTB4 levels were increased in the NTS of the SHR (17%; n=10, p<0.05). LTB4 receptors BLT1 (but not BLT2), were expressed on astroglia in the NTS but not neurons or vessels. Microinjection of LTB4 into the NTS of WKY rats increased both leukocyte adherence and arterial pressure for over 4 days (peak: +15 mmHg; P<0.01). In contrast, blockade of NTS BLT1 receptors lowered blood pressure in the SHR (peak: -13 mmHg; P<0.05) but not WKY rats. Thus, excessive amounts of LTB4 in NTS of SHR possibly as a result of up-regulation of 5LOX and down regulation of LTB412-HD, can induce inflammation. Since blockade of NTS BLT1 receptors lowered arterial pressure in the SHR their endogenous activity may contribute to the hypertensive state of this rodent model. Thus, inflammatory reactions in the brainstem are causally associated with neurogenic hypertension.
Keywords: hypertension, inflammation, sympathetic nervous system, brainstem
Introduction
High blood pressure (hypertension) is a major contributor to stroke, heart attacks and kidney disease. It has escalated to pandemic proportions (0.9 billion currently) and expected to rise further to 1.4 billion by 20251. The most common form of human hypertension is neurogenic hypertension characterized by excessive sympathetic activity that not only increases vascular resistance and cardiac output, which raises blood pressure, but damages end organs causing stiffening of arteries and the chambers of the heart2. The finding that this pathological rise in sympathetic activity precedes the onset of hypertension in humans3 suggests it is an early event in the disease process therefore making it an important therapeutic target. However, the remarkable statistic that ~23% of hypertensive patients who take multiple anti-hypertensive (polypill) medication are resistant to therapy4 emphasizes the need to discover new ways to control excessive sympathetic nerve activity to bring blood pressure under control.
It is likely that immune cells communicate with the brain across the blood brain barrier and vagal afferents and that the autonomic nervous system modulates the immune system2-7. Such cross-talk occurs during systemic infection, stress and cardiovascular disease and results in alterations in animal behavior, autonomic vasomotor tone and ventilation, for example. Evidence is accumulating for immune to brain signaling in a number of pathophysiological conditions including hypertension (reviewed in2,5). In this regard, leukocyte counts in SHR are 50-100% higher than controls and leukocyte-endothelial interactions are abundant in the SHR8. Vascular inflammation in the SHR was associated with elevated expression of interleukin-1β (IL-1β), IL-6, and TNF-α9-12. Angiotensin II promotes leukocyte-endothelial interactions contributing to vascular inflammation13 whereas candesartan decreases inflammatory cytokines13,14. T cells play an important role in vascular inflammation in hypertension15, angiotensin II infusion- and DOCA salt- induced hypertension whereas the T cell modulating agent, mycophenolate mofetil, can prevent hypertension16,17 Immune-to-brain-signaling involves the release of pro-inflammatory cytokines from intra-luminar and extravasated leukocytes, and from microglial cells activated by leukocytes2. It is speculated that inflammatory molecules may also diffuse across the blood brain barrier to effect neuronal activity and synaptic function. At the level of the nucleus tractus solitarii (NTS), a major region that governs both the sensitivity of the baroreceptor reflex and the set point of arterial pressure, and the structure studied in the present study, we described a unique pattern of expression of cytokines and chemokines within the NTS of the SHR relative to the normotensive rat18. Subsequently, we have found functional roles for the chemokine (C-C motif) ligand 5 (Ccl5 or RANTES -Regulated upon Activation, Normal T-cell Expressed and Secreted) and inter-leukin 6 (Il6) in the NTS in modulating arterial pressure and baroreceptor reflex function respectively19,20. Further, we reported an increase in a chemoattractant protein in the NTS of the spontaneously hypertensive rat (SHR) called junctional adhesion molecule-A (JAM-A)21. When over expressed in normotensive rats, JAM-A induced leukocyte adhesion in the brainstem microvasculature and induced mild hypertension in a normotensive animal21.
Given these previous findings and the importance and power of immune-to-brain signaling, the present study has sought to determine whether other molecules are involved in mediating leukocyte adhesion in the brainstem of hypertensive human and SHR. Here, we employed microarray screening of the NTS from SHR and WKY rats and identified a major difference in arachidonic acid metabolism in the SHR including a down regulation of leukotriene B4 12-hydroxydehydrogenase (LTB4-12-HD), the enzyme that degrades leukotriene B4 - a powerful chemoattractant of leukocytes22. We further demonstrate that the NTS of the SHR has elevated levels of LTB4 and that this has functional consequences for its hypertensive state.
Methods
Procedures were carried out according to the UK Home Office guidelines on animals (Scientific Procedures) Act 1986. They were also approved by the University of Bristol’s Animal Ethic Committee. All animals were housed individually, allowed normal rat chow and drinking water ad libitum, and kept on a 12 h light/12 h dark cycle. Human brain tissue studies were approved by Frenchay Hospital (Bristol) ethics committee.
NTS transcriptomic analysis and data handling
Affymetrix 230 2.0 Gene chips were used. Tissue from NTS was micro-dissected from brain slices from 11-13 week old age-matched adult male inbred Wistar Kyoto (WKY) rats and spontaneously hypertensive rats (SHR). Five replicates were made for each rat strain. For further details, see http://hyper.ahajournals.org.
Quantitative RT-PCR of whole NTS and primer sequences
Isolation of microvasculature from the medulla oblongata and quantitative RT-PCR
Quantitative RT-PCR of human brainstem
Fresh frozen brainstem tissue was thawed and transected coronally at the level of the NTS, which was identified as a distinct translucent structure in the dorsomedial medulla. At the level of the area postrema a 2-3 mm diameter (1-2 mm thick) piece of NTS was cut out using a scapel under a binocular microscope. Subjects were male and either had a medical history of uncomplicated essential hypertension (>140/90 mmHg; n=3) or were normotensive (n=4). For RNA extraction and primer sequences used, see http://hyper.ahajournals.org.
LTB4 content in medulla oblongata of WKY and SHR
NTS microinjection and immunohistochemistry for leukocytes
BLT1 receptor immuncytochemistry in SHR
Blood pressure responses to NTS microinjections in (i)Anaesthetized and (ii) Conscious rats
Procedures were as we have described previously21,23 and given in full in http://hyper.ahajournals.org.
Data analysis
Group data were expressed as mean ± SEM. To evaluate time-dependent changes of cardiovascular variables by injecting LTB4 or the BLT1 receptor antagonist into the NTS, we used repeated-measures ANOVA and the Bonferroni’s post hoc test. An unpaired t-test was also used for comparisons between two groups (e.g. comparison of gene expression levels). Differences were considered significant if p≤0.05.
Results
Catalogue of gene expression in the NTS
We present here lists of genes that, with a high degree of statistical confidence, represent comprehensive descriptions of the RNA populations expressed in the NTS of WKY (15,402 probesets, S1) and SHR (13,618 probesets, S2) – see http://www.vasopressin.org/#/data-bank/3755442 for full details. Our genetic data were also submitted to the NCBI gene expression and hybridization array data repository (GEO); the GEO accession number is: Series GSE8796. Combination of these lists provides a basis from which statistical testing was conducted. Of 15,870 probesets that were considered to be present in all the independent microarrays from both SHR and WKY NTS, 85 were significantly regulated differentially by greater than 1.5-fold (54-down regulated, 31-up regulated). We identified a clear down-regulation of LTB4-12-DH (or prostaglandin reductase 1) in the NTS of SHR. This drove the hypothesis that this pathway may contribute to the hypertensive phenotype of SHR. Hence, we first validated the array result using RT-PCR, second investigated the expression of other components of this pathway and third, proceeded to establish its functional significance to blood pressure control.
RT-PCR analysis of expression of the components of the arachidonic acid signaling cascade in SHR & WKY rats
(i) NTS
We confirmed the array data by showing that LTB4-12-HD gene expression level was significantly lower in both young and adult SHR than that of age-matched WKY (adult WKY & SHR: 1.13 ± 0.25 vs 0.42 ± 0.04 respectively, n=6 for each strain, p<0.05; young WKY & SHR, 1.07 ± 0.20 vs 0.45 ± 0.10 respectively, n=6 for each strain, p<0.05, Table 1, Fig 1A). The level of 5LOX gene was significantly higher in the NTS of both young and adult SHR compared to age-matched WKY rats (adult WKY & SHR: 1.02 ± 0.08 vs 1.52 ± 0.23 respectively, n=6 for each strain, p<0.05; young WKY & SHR, 1.02 ± 0.10 vs 1.47 ± 0.09 respectively, n=6 for each strain, p<0.05, Fig 1A). The level of LTA4H gene expression was not different between SHR and WKY (adult WKY & SHR: 1.01 ± 0.07 vs 1.09 ± 0.05 respectively, n=6 for each strain, p<0.05; young WKY & SHR, 1.05 ± 0.14 vs 0.98 ± 0.15 respectively, n=6 for each strain, Fig 1A). Note that both the rostral ventrolateral medulla (RVLM) and hypothalamic paraventricular nucleus also exhibited lower levels of LTB4-12-HD in SHR versus WKY rats (Table 1).
Table 1. Gene expression in rat RVLM, PVN and brainstem blood vessels.
| Enzyme | RVLM (15 weeks old) | PVN (15 weeks old) | Rat brainstem blood vessels (3 weeks old) | |||
|---|---|---|---|---|---|---|
| WKY (n=6) |
SHR (n=6) |
WKY (n=5) |
SHR (n=5) |
WKY (n=4) |
SHR (n=4) |
|
| 5ALOX | 1.01±0.1 | 1.39±0.09* | - | - | - | - |
| LTA4H | 1.01±0.1 | 0.98±0.09 | - | - | - | - |
| LTB4-12HD | 1.04±0.1 | 0.17±0.03† | 1.03±0.1 | 0.73±0.11* | 1.01±0.1 | 0.49±0.08* |
Expression of target genes relative to ß-actin gene in each sample were derived using the comparative (2-ΔΔCT) method16. Fold differences were calculated against the average of age matched WKY rats.
P<0.05,
P<0.01
Fig. 1. Altered arachidonic acid metabolism in the NTS of SHR and WKY rats.
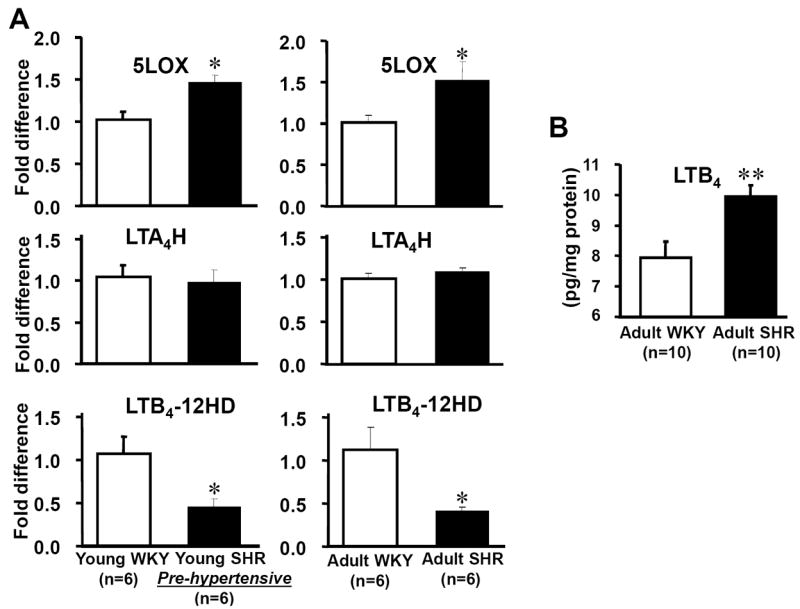
In A, 5LOX mRNA was up-regulated in both adult and pre-hypertensive SHR compared to age-matched WKY. In comparison, LTB4-12-HD mRNA expression level was reduced in both adult and pre-hypertensive SHR compared to age-matched WKY. In contrast, the level of LTA4H mRNA expression was not different between SHR and WKY. The reduced expression levels of LTB4-12-HD mRNA were associated with an increase in LTB4 content within the brainstem of SHR (B). *p<0.05, **p<0.01.
(ii) Brainstem blood vessels
Consistent with brainstem tissue, there was also a reduction of LTB4-12-HD gene in isolated microvessels extracted from the brainstem of 3 week old rats strains (SHR, 0.49 ± 0.08 vs WKY 1.01 ± 0.05; n=4, P<0.05; Table 1).
(iii) Human hypertensive NTS tissue
As with the SHR:WKY rat difference, a similar difference was found in human NTS where hypertensive subjects had lowered expression levels of LTB4-12-HD compared to controls (hypertensives: 0.42 ± 0.05 vs. control: 1.02 ± 0.10, n=4; p<0.01).
Levels of LTB4 in the medulla oblongata of SHR and WKY rats
When the LTB4 quantity was normalized to the total protein concentration, the concentration of LTB4 in was higher in the SHR versus WKY rats (WKY: 7.95 pg LTB4/mg protein, SHR: 9.94 pg LTB4/mg protein, n=10, p<0.01, Fig. 1B and see http://hyper.ahajournals.org). Thus, either the production of LTB4 is increased and/or its degradation decreased in the NTS of SHR relative to WKY rats.
BLT1 and BLT2 receptor expression in the NTS
mRNA expression of LTB4 receptors indicated the presence of BLT1 receptors in the NTS of both SHR and WKY rats (Fig. 2). BLT2 receptor expression was hardly detectable (Fig 2A). At the protein level, BLT1 receptors were detected immunocytochemically in the NTS of SHR and found to be co-localised with cells that were immunopositive for GFAP but not NeuN or RECA (Fig 2B). Co-localisation with GFAP was relatively dense and supports substantial numbers of BLT1 receptors on astrocytes.
Fig. 2. BLT1 receptors in the NTS.
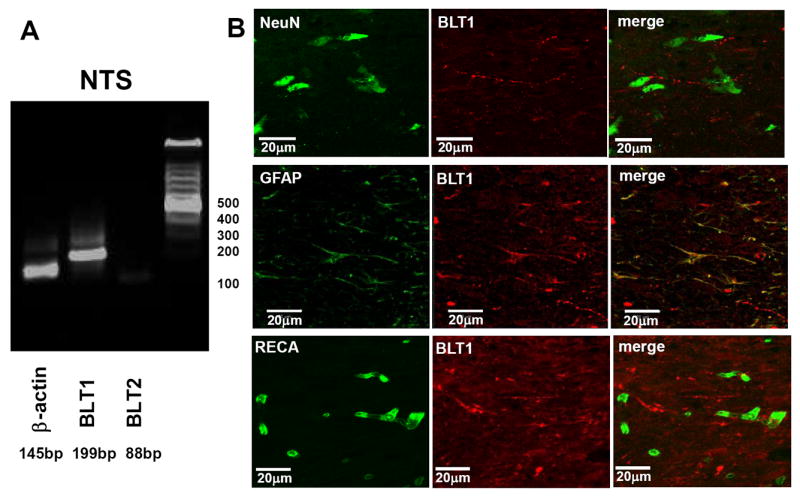
Gene expression of BLT1, but not BLT2 receptors, was identified in WKY and SHR (A, data from WKY rat). B: Immunofluorescence labeling indicated that BLT1 receptor expression was apparent on glial but not neuronal or endothelial cell types within the NTS of SHR. Glial cells were identified using antibodies against glial fibrillary acidic protein (GFAP), NeuN for neurones and RECA for endothelial cells.
LTB4 in the NTS of WKY rats: acute and chronic effects on cardiovascular control
Acute
In normotensive anesthetized WKY rats, a depressor site was located in the NTS as determined by glutamate microinjection (range -30 to -60 mmHg drop in arterial pressure). Within a one hour observation period neither arterial pressure nor HR changed following LTB4 microinjection into this NTS depressor site (SBP, before: 105±7 after: 107±8 mmHg; HR, before: 323±23 after: 322±25 bpm; n=6). After one hour glutamate still produced a quatitatively similar fall in arterial pressure suggesting that the structure had maintained its viability.
Chronic
In conscious telemetered WKY rats, baseline levels of SBP, HR and sBRG were 113±3 mmHg, 365±6 bpm and 0.92±0.08 msec/mmHg, respectively. After NTS microinjection of LTB4 the maximum increment of SBP was +15±3 mmHg relative to pre-injection levels; this occurred two days after the injection (P<0.001, Fig. 3A) and remained above baseline levels for post-injection days 3 (P<0.05) and 4 (P<0.05). The peak mean arterial pressure response increased from 93±3 to 107±4 mmHg (P<0.05). In contrast, HR and sBRG did not change (P>0.1). Three days post LTB4 injection the LF + VLF power of SBP variability increased from 7.40±0.34 to 10.69±1.29 mmHg2 (P<0.05) and the change in the LTB4 injection group was higher than that in the control group (3.30±0.99 vs 0.22±0.74 mmHg2, p<0.05; Fig 3B) indirectly suggesting raised sympathetic vasomotor activity. Consistently, LF SBP increased from 0.20±0.3 to 0.87±0.3 mmHg2 (P<0.05). Both the changes in LF:HF and HF power of HR variability were not different between LTB4 injection and control groups (P>0.1). Post-hoc analysis at the conclusion of the experiment (ie day 6 post-injection) failed to find leukocyte adhesion (ie CD4 immunoreactivity) in the NTS suggesting that any inflammatory response was either undetectable or transient and resolved by the time arterial pressure had returned to control levels.
Fig. 3. LTB4 and BLT1 receptor manipulation in NTS evokes arterial pressure responses.
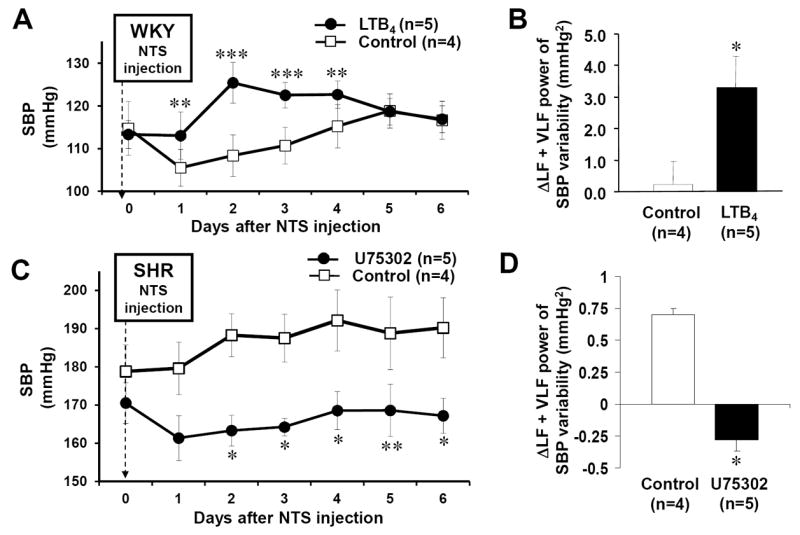
A, 2-4 days after NTS injection of LTB4, SBP was elevated significantly in WKY rats. B, 3 days after LTB4 injection, LF+VLF power of SBP variability was increased, suggesting activation of vasomotor sympathetic activity. C, U75302 (to block BLT1 receptors) in NTS of SHR lowered both arterial pressure and the LF+VLF power of SBP variability (D). Differences are relative to control injections. *p<0.05, **p<0.01 and ***p<0.001.
Chronic blockade of BLT1R in the NTS of WKY and SHR on cardiovascular control
In conscious rats fitted with radio transmitters, resting SBP was: 171±8 (SHR, U75 group), 179±5 (SHR, acsf) and 121±4 mmHg (Wistar, U75302). SBP decreased maximally by -13±5 mmHg (n=5; P=0.05) following NTS microinjection of U75302 in SHR. This reduction in SBP persisted for up to 6 days relative to baseline and SHR treated with acsf (Fig 3C; P<0.05). Mean arterial pressure fell from 134±5 to 115±3 mmHg (P<0.05). In SHR treated with BLT1 receptor blocker, LF+VLF power of SBP decreased significantly relative to baseline (from: 4±0.2 to 3.3±0.2 mmHg2 P<0.05; Fig 3D). This reduction remained significant for up to 6 days thereby accompanying the pressure fall. LF SBP also fell (4.0±0.2 to 3.3±0.2 mmHg2; P<0.05). No such changes were observed in the SHR acsf and Wistar rat groups (Fig 3D). None of the three rat groups showed any significant change in heart rate, heart rate spectra or sBRG. In control SHR microinjected with acsf a rise of 12±1 mmHg was noted (n=4; P<0.05) whereas no change was observed in Wistar rats receiving U75302.
BLT1 receptor modulation of inflammation in the NTS
We found that LTB4 microinjection into the NTS of WKY rats produced inflammation as detected from elevated CD4 immunoreactivity after 2 days (Fig 4A; n=4). We confirmed our original finding of high endogenous CD4 immunoreactivity in the NTS of the SHR21, which was minimal in WKY rats (Fig 4B). However, blocking BLT1 receptors in the SHR failed to reduce endogenous CD4 immunoreactivity in the NTS of the SHR (Fig 4C; n=4) suggesting that maintaining inflammation is not dependent upon continuous BLT1 receptor activation. Thus, these data indicate a potential role for LTB4 in evoking inflammation in the NTS of WKY rats but that blockade of endogenous BLT1 receptor activity is insufficient to reduce CD4 immunoreactivity in the SHR.
Fig. 4. BLT1 receptor modulation of inflammation in the NTS.
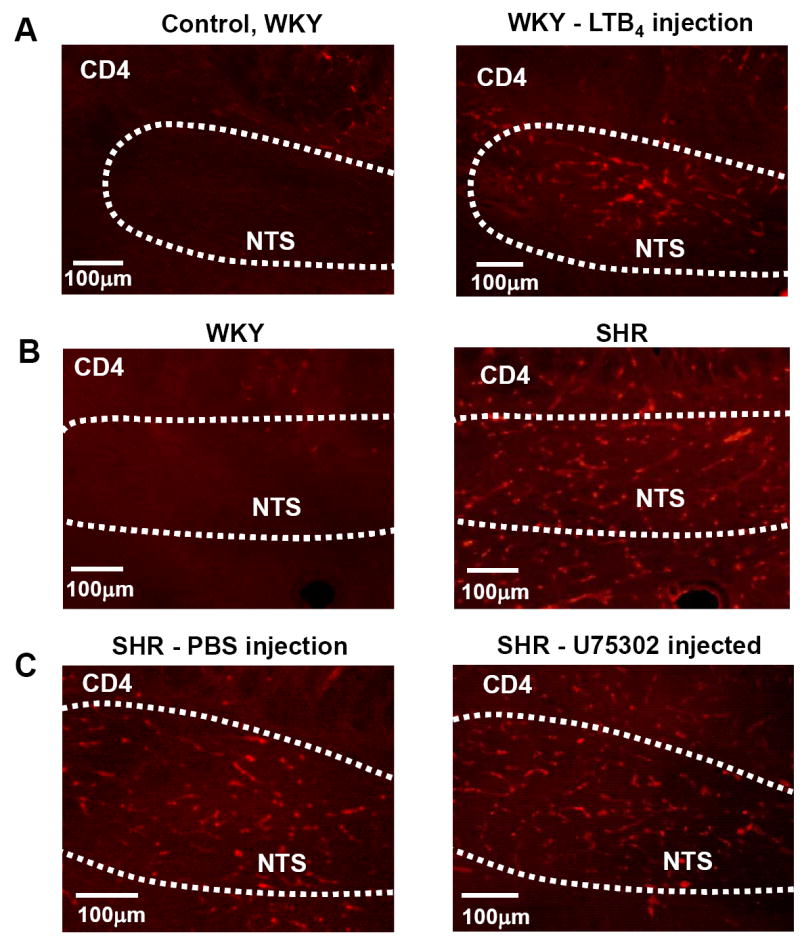
A. LTB4 microinjection into the NTS of a WKY rat induced CD4 immunoreactivity relative to rats injected with vehicle control. B. The NTS of the SHR is inflamed relative to WKY rat as indicated by the greater CD4 immunoreactivity. C. Blockade of BLT1 receptors in the NTS of the SHR did not affect the endogenous leukocyte accumulation.
Discussion
The novel findings of this study are that in both adult and young pre-hypertensive SHR LTB4-12-HD gene was down-regulated in the NTS while 5LOX gene was up-regulated compared to age-matched WKY rats. Based on this, we predicted excessive amounts of the arachidonic acid metabolite LTB4 in the NTS of the SHR, which we have confirmed. We also described the presence of BLT1 receptors on glial cells in the NTS. We found that a single injection of LTB4 in NTS produced hypertension in normotensive rats while BLT1 receptor antagonism lowered arterial pressure in the SHR but not normotensive rats. Although BLT1 receptor blockade was unable to reduce CD4 immunoreactivity in the NTS of SHR, LTB4 could induce inflammation in this brainstem region of normotensive rats. Our findings indicated that a high level of LTB4 in the NTS may have roles in both the development and maintenance of the hypertension in the SHR, and that this is likely mediated via a BLT1 receptor signaling process involving astrocytes.
Altered arachidonic acid - LTB4 metabolic system in the NTS of SHR
5-lipoxygenase is a lipoxygenase present in the central nervous system24. Its up regulation in the NTS of the SHR together with the down regulation of LTB4-12-HD, the enzyme that causes dehydrogenation of LTB4 to 12-oxo-LTB4, could both cause the elevated levels of LTB4 that we found. Since this imbalance was seen in PHSHR these changes are not secondary to the hypertension but occur early in the pathology and could therefore be involved in the establishment of hypertension. Although our sample number was low, the observation that LTB4-12-HD was lower in the NTS of human hypertensives lends both credence to the relevance of our rat transcriptomic data to human essential hypertension and the applicability of the animal model for understanding human neurogenic hypertension. These changes are unlikely to be unique to the NTS since LTB4-12-HD was also down regulated in the rostral ventrolateral medulla and hypothalamic paraventricular nucleus (Table). Thus, the magnitude of the blood pressure responses reported herein may well be amplified if the LTB4 signaling pathways are modulated in this additional brainstem site simultaneously.
Potential sources of LTB4 in the NTS of the SHR
Generally, LTB4 synthesis is increased by inflammatory mediators including endotoxin, complement fragments, tumor necrosis factor and interleukins25. In the brain, astrocytes26 and oligodendrocytes27 are both potential source of LTB4. However, since our data indicate reduced levels of LTB4-12-HD in isolated brainstem vessels LTB4 may also be released from the endothelium and/or vascular muscle cells in the SHR. This is consistent with the evidence that both these cell types have been shown to synthesize this leukotriene28,29. If of endothelial origin, LTB4 could be released into the blood stream to attract leukocytes (as well as into the brain to activate glial cells). As confirmed in the present study, and found previously21 there is leukocyte accumulation in the NTS capillaries of SHR but not WKY rats. This may, in part, be due to the greater adhesiveness of leukocytes in the SHR than in normotensive rats30. It may also be accentuated by the high level of endothelial JAM-A expression in the NTS21, which has a binding site for leukocytes31-33 but also due to the high level of LTB4 as detected in the present study. The types of leukocytes will need to be identified in future studies. Since leukocytes can synthesize LTB422, we cannot rule out their contribution to the raised levels of this LTB4 in the medulla oblongata of the SHR described herein. Given these multiple mechanisms for attracting leukocytes to the NTS in the SHR, and that LTB4 production may be a product of an inflammatory processes, it is perhaps unsurprising that blocking BLT1 receptors was ineffective in reducing CD4 immunoreactivity.
Potential actions of LTB4 in the NTS of the SHR
LTB4 is one of the most potent chemoattractants and activators of leukocytes and has a primary role in inflammatory diseases22,34. We found that microinjection of the LTB4 into the NTS or normotensive rats induced leukocyte adherence. These data are consistent with the role of BLT1receptor activation as a major stimulus driving leukocyte accumulation22,34-36. Since BLT1 receptor antagonism blocks neutrophil activation37 it may reduce the inflammatory response in the NTS. This is relevant since LTB4 stimulates the production of a number of pro-inflammatory cytokines that augment and prolong tissue inflammation. For example, it stimulates the release of mono-chemoattractant protein-138 (MCP-1) via the NF-κB pathway in human monocytes. Interestingly, we found that MCP-1 was higher in the NTS of the SHR18, but it is unclear whether this relates causally to the high LTB4 levels in the NTS of the SHR. LTB4 can increase levels of IL-639 but this chemokine was down regulated in the NTS of the SHR18and its expression not altered after NTS injection of LTB440. The latter support the notion of a specific type of inflammatory condition in the NTS of the SHR as proposed previously18. In contrast, LTB4 decreased the expression of Ccl5 in the NTS of normotensive rats40. Because endogenous Ccl5 was down regulated in the NTS of the SHR it is tempting to speculate that this is triggered by the elevated levels of LTB4 in this nucleus, but this awaits confirmation. Ccl5 receptors have been associated with enhancing glutamate transmission and were previously found on NTS neurons40. Their activation resulted in a lowering of arterial pressure that was significantly more pronounced in the SHR than the normotensive rat40. As LTB4 lowers Ccl5 expression it is suggested that Ccl5’s restraining effect on arterial pressure may be depressed in the SHR, which could contribute to its hypertensive state. Ccl5 also contributes directly to monocyte-leukocyte-activation and could further support the aforementioned actions of LTB4 and JAM-A in white cell aggregation in the SHR. As Ccl5 stimulates the production and release of specific pro-inflammatory arachidonic acid products, including LTB4 from monocytes41, we suggest that leukocyte aggregation in the NTS could lead to further LTB4 production through a positive feedback/wind-up mechanism.
BLT1 receptors and potential downstream inter-cellular signaling in the NTS
In the present study, gene expression of BLT1 receptors predominated over BLT2 receptors in the NTS. BLT1 receptor is a G-protein-coupled receptor 22,42 and previously described on leukocytes43. Our immunofluorescence labeling suggested that BLT1 receptors were located on glial cells in the NTS. It is hypothesized that leukocyte accumulation in the NTS induced, in part, by LTB4 acting of BLT1 receptors, release both cytokines and reactive oxygen species (ROS) such as super oxide; these are established products from such cells44,45 and known to activate central neurones including brainstem cardiovascular neurones46,47. ROS and some types of cytokines can cross the blood brain barrier48. Whether leukocytes extravasate into the NTS was not confirmed21 and a role for LTB4 in diapedesis is controversial35,38,49. However, it is known that astrocytes are a potential source for cytokines and ROS production50-52. On physical contact with leukocytes, astrocytes release MCP-153. MCP-1 receptors are present on central neurones54, however its functional role has yet to be identified in the NTS. Taken together, we propose that leukocyte accumulation, even if intra-lumenally, may well influence central neuronal circuits via cytokines, chemokines and ROS activation that originates from adhered white cells or LTB activation of glial cells via BLT1 receptors. This may dramatically alter neuronal function leading to neurogenic hypertension. Since we have found that gene expression of LTB4-12-HD was down regulated in the RVLM and hypothalamic paraventricular nucleus, it remains to be established whether LTB4 also affects theses other regions of the SHR resulting in hypertension.
Perspectives
Our findings raise the intriguing and novel possibility that there is a link between high levels of leukocyte adhesion in the cardiovascular control regions of the brain with excessive levels of sympathetic nerve activity. We surmise that by antagonising either leukocyte adhesion and or reducing inflammation in the brainstem of the SHR one might predict a reduction in the pro-inflammatory status thereby alleviating the symptoms of hypertension. With the demonstration that BLT1 receptor activation can itself induce leukocyte adherence in the brainstem and trigger hypertension in normotensive rats, anti-inflammatory therapy may prove an effective anti-hypertensive strategy. Whilst anti-inflammatory drugs are generally ineffective anti-hypertensive agents, we argue that the inflammatory status of the brainstem is relatively specific18. Anti-inflammatory agents tested to date may be inappropriate or not cross the blood brain barrier. However, minocycline given centrally restricts the pressor response induced by angiotensin II infusion and decreases the numbers of activated microglia and mRNAs for interleukin (IL) 1beta, IL-6, and tumor necrosis factor-alpha, but increase mRNA for IL-10 (anti-inflammatory) in the hypothalamus55. Since we found that LTB4-12-HD gene is also down-regulated in the NTS of humans with a history of essential hypertension, we suggest that LTB4-12-HD itself might be an effective target for therapeutic intervention as proposed previously56. Moreover, it may also be an early diagnostic indicator to predict whether a subject is likely to develop hypertension before it has manifested itself especially since this gene was down regulated in the pre-hypertensive SHR. Such early diagnosis might stimulate anti-inflammatory approaches as an effective preventative approach to hypertension.
Novelty and Significance.
1) What is new?
Leukotriene B4 (LTB4), a major chemoattractant for immune cells, is elevated in NTS of SHR and human hypertensives
LTB4 receptor stimulation in NTS is pro-hypertensive whereas their blockade is anti-hypertensive
LTB4 receptors exist on astrocytes in NTS
2) What is relevant?
The brainstem microvasculature of the SHR is inflamed; this occurs before the onset of hypertension and may involve LTB4
Appropriate anti-inflammatory treatment may provide an anti-hypertensive strategy
3) Summary
Arachidonic acid metabolism is altered in SHR brainstem; this is associated with inflammatory reactions that appear causally related with neurogenic hypertension
Acknowledgments
Sources of funding
The study was financially supported by the British Heart Foundation (BS/93003), the Japan Society for the Promotion Science (19-07458), the Biotechnology and Biological Science Research Council and the NIH. JFRP was in receipt of a Royal Society Wolfson Research Merit Award. We are acknowledge the generous donation of from Pfizer (USA).
Footnotes
Disclosures
None
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Zubcevic J, Waki H, Raizada MK. Paton JFR Autonomic-Immune-Vascular Interaction: An Emerging Concept for Neurogenic Hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–222. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Plump A. Accelerating the pulse of cardiovascular R&D. Nat Rev Drug Discov. 2010;9:823–824. doi: 10.1038/nrd3315. [DOI] [PubMed] [Google Scholar]
- 5.Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin Pharmacol. 2011;11:156–161. doi: 10.1016/j.coph.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrats J, Schiltz JC, García-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid-Schönbein GW, Seiffge D, DeLano FA, Shen K, Zweifach BW. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–330. doi: 10.1161/01.hyp.17.3.323. [DOI] [PubMed] [Google Scholar]
- 9.Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT(1) receptor blockade ameliorates brain inflammation. Neuropsychopharm. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benicky J, Sánchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol. 2009;29:781–792. doi: 10.1007/s10571-009-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnevale D, Mascio G, Ajmone-Cat MA, D’Andrea I, Cifelli G, Madonna M, Cocozza G, Frati A, Carullo P, Carnevale L, Alleva E, Branchi I, Lembo G, Minghetti L. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging. 2010;33:205, e19–29. doi: 10.1016/j.neurobiolaging.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neurosci. 2010;171:852–858. doi: 10.1016/j.neuroscience.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Sanz-Rosa D, Oubiña MP, Cediel E, de Las Heras N, Vegazo O, Jiménez J, Lahera V, Cachofeiro V. Effect of AT1 receptor antagonism of vascular and circulating inflammatory mediators in SHR: role of NF-kappaB/IkappaB system. Am J Physiol Heart Circ Physiol. 2005;288:H111–H115. doi: 10.1152/ajpheart.01061.2003. [DOI] [PubMed] [Google Scholar]
- 15.Viel EC, Lemarié CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol. 2010;298:H938–H944. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 16.Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 17.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waki H, Gouraud SS, Maeda M, Paton JF. Specific inflammatory condition in nucleus tractus solitarii of the SHR: novel insight for neurogenic hypertension? Auton Neurosci. 2008;142:25–31. doi: 10.1016/j.autneu.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Gouraud SS, Waki H, Bhuiyan MER, Takagishi M, Cui HE, Kohsaka A, Paton JFR, Maeda M. Downregulation of chemokine Ccl5 gene expression in the NTS of SHR may be prohypertensive. J Hypertens. 2011;29:732–740. doi: 10.1097/HJH.0b013e328344224d. [DOI] [PubMed] [Google Scholar]
- 20.Takagishi M, Waki H, Bhuiyan MER, Gouraud SS, Kohsaka A, Cui H, Yamazaki T, Paton JFR, Maeda M. IL-6 microinjected into the nucleus tractus solitarii attenuates cardiac baroreceptor reflex function in rats. Am J Physiol. 2010;298:R183–R190. doi: 10.1152/ajpregu.00176.2009. [DOI] [PubMed] [Google Scholar]
- 21.Waki H, Li B-H, Kasparov S, Murphy D, Paton JFR. Over expression of junctional adhesion molecule -1 in nucleus tractus solitarii is pro-hypertensive in the rat. Hypertension. 2007;49:1321–1327. doi: 10.1161/HYPERTENSIONAHA.106.085589. [DOI] [PubMed] [Google Scholar]
- 22.Yokomizo T, Izumi T, Shimizu T. Leukotriene B4: Metabolism and signal transduction. Arch Biochem Biophysics. 2001;385:231–241. doi: 10.1006/abbi.2000.2168. [DOI] [PubMed] [Google Scholar]
- 23.Waki H, Katahira K, Kasparov S, Murphy D, Paton JFR. Automating the analysis of cardiovascular autonomic function from chronic measurements of arterial pressure in conscious rats. Exp Physiol. 2006;91:201–213. doi: 10.1113/expphysiol.2005.031716. [DOI] [PubMed] [Google Scholar]
- 24.Manev H, Uz T, Sugaya K, Qu T. Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J. 2000;14:1464–1469. doi: 10.1096/fj.14.10.1464. [DOI] [PubMed] [Google Scholar]
- 25.Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- 26.Hartung HP, Heininger K, Toyka KV. Primary rat astroglial cultures can generate leukotriene B4. J Neuroimmunol. 1988;19:237–243. doi: 10.1016/0165-5728(88)90005-7. [DOI] [PubMed] [Google Scholar]
- 27.Shirazi Y, Imagawa DK, Shin ML. Release of leukotriene B4 from sublethally injured oligodendrocytes by terminal complement complexes. J Neurochem. 1987;48:271–278. doi: 10.1111/j.1471-4159.1987.tb13158.x. [DOI] [PubMed] [Google Scholar]
- 28.Cole OF, Fan TP, Lewis GP. Endothelial (aortic) cells in culture synthesise LTB4. Cell Biol Int Rep. 1986;10:407–413. doi: 10.1016/0309-1651(86)90035-4. [DOI] [PubMed] [Google Scholar]
- 29.Ibe BO, Raj JU. Leukotriene metabolism by intrapulmonary vessels of newborn lambs: effect of platelet-activating factor. Exp Lung Res. 2001;27:331–348. doi: 10.1080/019021401750193601. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda S, Yasu T, Kobayashi N, Ikeda N, Schmid-Schönbein GW. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ Res. 2004;95:100–108. doi: 10.1161/01.RES.0000133677.77465.38. [DOI] [PubMed] [Google Scholar]
- 31.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 32.Fraemohs L, Koenen RR, Ostermann G, Heinemann B, Weber C. The functional interaction of the beta 2 integrin lymphocyte function associated antigen-1 with junctional adhesion molecule-A is mediated by the I domain. J Immunol. 2004;173:6259–6264. doi: 10.4049/jimmunol.173.10.6259. [DOI] [PubMed] [Google Scholar]
- 33.Naik UP, Ehrlich YH, Kornecki E. Mechanisms of platelet activation by a stimulatory antibody: cross-linking of a novel platelet receptor for monoclonal antibody F11 with the Fc gamma RII receptor. Biochem J. 1995;310:155–162. doi: 10.1042/bj3100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claesson HE, Odlander B, Jakobsson PJ. Leukotriene B4 in the immune system. Int J Immunopharmacol. 1992;14:441–449. doi: 10.1016/0192-0561(92)90174-j. [DOI] [PubMed] [Google Scholar]
- 35.Nohgawa M, Sasada M, Maeda A, Asagoe K, Harakawa N, Takano K, Yamamoto K, Okuma M. Leukotriene B4-activated human endothelial cells promote transendothelial neutrophil migration. J Leukoc Biol. 1997;62:203–209. doi: 10.1002/jlb.62.2.203. [DOI] [PubMed] [Google Scholar]
- 36.Lindbom L, Hedqvist P, Dahlén SE, Lindgren JA, Arfors KE. Leukotriene B4 induces extravasation and migration of polymorphonuclear leukocytes in vivo. Acta Physiol Scand. 1982;116:105–108. doi: 10.1111/j.1748-1716.1982.tb10607.x. [DOI] [PubMed] [Google Scholar]
- 37.Souza DG, Coutinho SF, Silveira MR, Cara DC, Teixeira MM. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur J Pharmacol. 2000;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Zhao A, Wong F, Ayala JM, Struthers M, Ujjainwalla F, Wright SD, Springer MS, Evans J, Cui J. Leukotriene B4 strongly increases monocyte chemoattractant protein-1 in human monocytes. Arterioscler Thromb Vasc Biol. 2004;24:1783–1788. doi: 10.1161/01.ATV.0000140063.06341.09. [DOI] [PubMed] [Google Scholar]
- 39.Brach MA, de Vos S, Arnold C, Gruss HJ, Mertelsmann R, Herrmann F. Leukotriene B4 transcriptionally activates interleukin-6 expression involving NK-chi B and NF-IL6. Eur J Immunol. 1992;22:2705–2711. doi: 10.1002/eji.1830221034. [DOI] [PubMed] [Google Scholar]
- 40.Gouraud SS, Waki H, Bhuiyan MER, Takagishi M, Cui HE, Kohsaka A, Paton JFR, Maeda M. Downregulation of chemokine Ccl5 gene expression in the NTS of SHR may be prohypertensive. J Hypertens. 2011;29:732–740. doi: 10.1097/HJH.0b013e328344224d. [DOI] [PubMed] [Google Scholar]
- 41.Shanmugham LN, Petrarca C, Castellani ML, Frydas S, Vecchiet J, Conti P, Tete S. Rantes potentiates human macrophage aggregation and activation responses to calcium ionophore (A23187) and activates arachidonic acid pathways. J Biol Regul Homeost Agents. 2006;20:15–23. [PubMed] [Google Scholar]
- 42.Toda A, Yokomizo T, Shimizu T. Leukotriene B4 receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:575–585. doi: 10.1016/s0090-6980(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 43.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 44.Crockett-Torabi E, Ward PA. The role of leukocytes in tissue injury. Eur J Anaesthesiol. 1996;13:235–246. doi: 10.1046/j.1365-2346.1996.00982.x. [DOI] [PubMed] [Google Scholar]
- 45.Krotz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 46.Mayorov DN, Head GA, De Matteo R. Tempol attenuates excitatory actions of angiotensin II in the rostral ventrolateral medulla during emotional stress. Hypertension. 2004;44:101–106. doi: 10.1161/01.HYP.0000131290.12255.04. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–206. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 48.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11:973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 49.Thureson-Klein A, Hedqvist P, Lindbom L. Leukocyte diapedesis and plasma extravasation after leukotriene B4: lack of structural injury to the endothelium. Tissue Cell. 1986;18:1–12. doi: 10.1016/0040-8166(86)90002-9. [DOI] [PubMed] [Google Scholar]
- 50.Safieh-Garabedian B, Haddad JJ, Saade NE. Cytokines in the central nervous system: targets for therapeutic intervention. Curr Drug Targets CNS Neurol Disord. 2004;3:271–280. doi: 10.2174/1568007043337300. [DOI] [PubMed] [Google Scholar]
- 51.Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93:182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 52.Andjelkovic AV, Kerkovich D, Pachter JS. Monocyte:astrocyte interactions regulate MCP-1 expression in both cell types. J Leukoc Biol. 2000;68:545–552. [PubMed] [Google Scholar]
- 53.Andjelkovic AV, Kerkovich D, Pachter JS. Monocyte:astrocyte interactions regulate MCP-1 expression in both cell types. J Leukoc Biol. 2000;68:545–552. [PubMed] [Google Scholar]
- 54.Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: Functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- 55.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bäck M. Leukotrienes: potential therapeutic targets in cardiovascular diseases. Bull Acad Natl Med. 2006;190:1511–1518. [PubMed] [Google Scholar]


