Abstract
Purpose
Five-year breast cancer survivors, diagnosed after 65 years of age, may develop more incident comorbidities than similar populations free of cancer. We investigated if older breast cancer survivors have a similar comorbidity burden 6–15 years after cancer diagnosis to matched women free of breast cancer at start of follow-up and if incident comorbidities are associated with all-cause mortality.
Methods
In this prospective cohort study, 1,361 older five-year early stage breast cancer survivors diagnosed between 1990 and 1994 and 1,361 age- and health system-matched women were followed for ten years. Adjudicated medical record review captured prevalent and incident comorbidities during follow-up or until death as collected from the National Death Index.
Results
Older five-year breast cancer survivors did not acquire incident comorbidities more often than matched women free of breast cancer in the subsequent 10 years (HR=1.0, 95%CI: 0.93,1.1). Adjusted for cohort membership, women with incident comorbidities had a higher mortality rate than those without incident comorbidities (HR=4.8, 95%CI: 4.1,5.6). A breast cancer history continued to be a hazard for mortality 6–15 years after diagnosis (HR=1.3, 95%CI: 1.1,1.4).
Conclusions
We found that older breast cancer survivors who developed comorbidities had an increased all-cause mortality rate even after adjusting for age and prevalent comorbidity burden. Additionally, survivors acquire comorbidities at a rate similar to older women free of breast cancer. These results highlight the association between comorbidity burden and long-term mortality risk among older breast cancer survivors and their need for appropriate oncology and primary care follow-up.
Keywords: breast cancer, survivorship, comorbidity, geriatrics, managed care, mortality
INTRODUCTION
Breast cancer predominantly affects older women [1,2], yet modest advances have been made in their survival relative to cohorts of younger breast cancer patients [3,4]. An association between age and mortality has been found in older five-year breast cancer survivors [4] and the development of incident comorbidities, such as congestive heart failure, is independently associated with aging and cancer therapy [5,6]. It is unclear, however, whether older women who have undergone cancer therapy are more likely to develop comorbidities than older women without a history of cancer [7–9]. Furthermore, it is unknown if incident comorbidities acquired in older breast cancer survivors are more often fatal than in comparable reference populations.
The unknown impact of incident comorbid disease following breast cancer and its treatment is an important barrier to improving survival among older women [6,10]. Previous investigations suggest that older breast cancer survivors have increased comorbidity burden three years after diagnosis compared to women without breast cancer matched for age, prevalent comorbidities at baseline, and health system [11]. In a cohort of older breast cancer survivors followed for a median of 85 months after primary breast cancer, survivors experienced a 40% higher all-cause mortality hazard for each unit increase in the Charlson Comorbidity Index (CCI) after accounting for prevalent comorbidities at baseline and incident comorbidities during follow-up [12]. Limitations of these studies include differential loss to follow-up, selection bias, and misclassification associated with self-report of comorbid disease. Furthermore, no investigation has examined the impact of incident comorbidities following breast cancer treatment on ten-year all-cause mortality among older five-year breast cancer survivors.
Therefore, we investigated whether incident comorbidities accumulate more frequently and rapidly among a cohort of older five-year breast cancer survivors (aged ≥65 years at diagnosis) than among a comparison cohort matched for age and geography. We also assessed whether incident comorbidities impact all-cause mortality differently among survivors than matched comparators.
METHODS
Setting, Design, and Subjects
This analysis was conducted in the longitudinal Breast Cancer Treatment Effectiveness in Older Women (BOW) cohort study, as previously described [13]. Eligible women were enrolled at one of six integrated health care systems within the Cancer Research Network [14]. This study was reviewed and approved by the Institutional Review Boards of each participating institution and at the Boston University Medical Center. The requirement for informed consent was waived.
BOW initially identified a cohort of older women (aged ≥65 years) diagnosed with early stage invasive breast cancer (American Joint Committee on Cancer [AJCC] Stage I, IIA, or IIB) between 01/01/1990 and 12/31/1994 [15]. For this study, we restricted the cohort to five-year survivors of primary breast cancer diagnosis (n=1,361) [16]. For each survivor we selected a comparison woman of the same age from the same health system, who was free of breast cancer at the matched survivor’s date of breast cancer diagnosis (index date) and survived for five years after index date. Comparator women remained enrolled if they received a breast cancer diagnosis between the index date and start of observational follow-up.
Data Collection and Analytic Variables
We collected data elements from electronic sources and through standardized medical record reviews conducted at each site by medical record abstractors trained via web-based technology who entered data directly into a computer-based, menu-driven data collection system [17,18]. The medical record abstractor inter-rater reliability across sites was a weighted average of 95% agreement (range 89%–98%) [18]. A study of medical record reviews conducted during the five years after breast cancer diagnosis showed good inter-rater reliability for the CCI [19] and little bias from comorbidity misclassification [20]. Women were followed from five years after their cancer diagnosis until death, health system disenrollment, or through 15 years after index date, whichever occurred first.
All-Cause Mortality
We obtained date of death using the National Death Index through December 31, 2009.
Comorbidity
We collected from medical records information on incident comorbid conditions included in the Charlson Comorbidity Index (CCI) [19]. We estimated the CCI, updated continually, for years 6–15 after index date. Prevalent breast cancer was not included in the CCI, however, incident primary breast cancers were scored during follow-up for both cohorts. The CCI only captures some of the comorbidities identified as potential late effects of breast cancer treatment. We therefore collected incident occurrences of pulmonary emboli and osteoporotic fractures of the hip, wrist, and vertebra during follow-up, excluding fractures diagnosed as secondary to metastatic breast cancer. We calculated a modified Charlson Comorbidity Index (mCCI) score using the CCI with the addition of pulmonary emboli and osteoporotic fractures (assigned index weight of one point).
Demographic data
Age was categorized as 70–74, 75–79, and 80+ years at the beginning of follow-up. We gathered information on race and ethnicity using the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) coding instructions [21]. Women of Hispanic ethnicity were identified and grouped into SEER race categories. For those with unknown ethnicity, the patient’s last name was identified via a validated computer program search to assign ethnicity [13].
Statistical Analyses
We tabulated the distribution of demographic factors, breast cancer treatment characteristics (survivor cohort only), prevalent comorbidities at beginning of follow-up (five years after index date), and incident comorbidities acquired during years 6–15 after index date.
To compare the incidence of comorbidities among survivors with the comparison cohort during follow-up, we fit Cox proportional hazards regression models to estimate hazard ratios (HR) and 95% confidence interval (95% CI) bounds for each comorbidity separately as well as for a dichotomous composite (presence/absence) of any incident comorbidity we ascertained. HRs were adjusted for age category, health system, and existence of any prevalent comorbidity.
We tabulated the distribution of all-cause mortality by age group and cohort type. We fit an Anderson-Gill Cox proportional hazard model, adjusting for age group, cohort, and presence of any comorbidity at beginning of follow-up to examine the effect of acquiring incident comorbidities on all-cause mortality among survivors. Additional Anderson-Gill Cox proportional hazard models were fit to each individual cohort, adjusting for age group and presence of any comorbidity at beginning of follow-up, to determine the adjusted hazard ratios of covariates within each cohort. Incident comorbidity (presence/absence) was included in the models as a time-varying covariate to account for accumulation of time between incident comorbidity and the censoring event. All statistical analyses were performed in SAS version 9 (SAS Institute, Cary, NC).
RESULTS
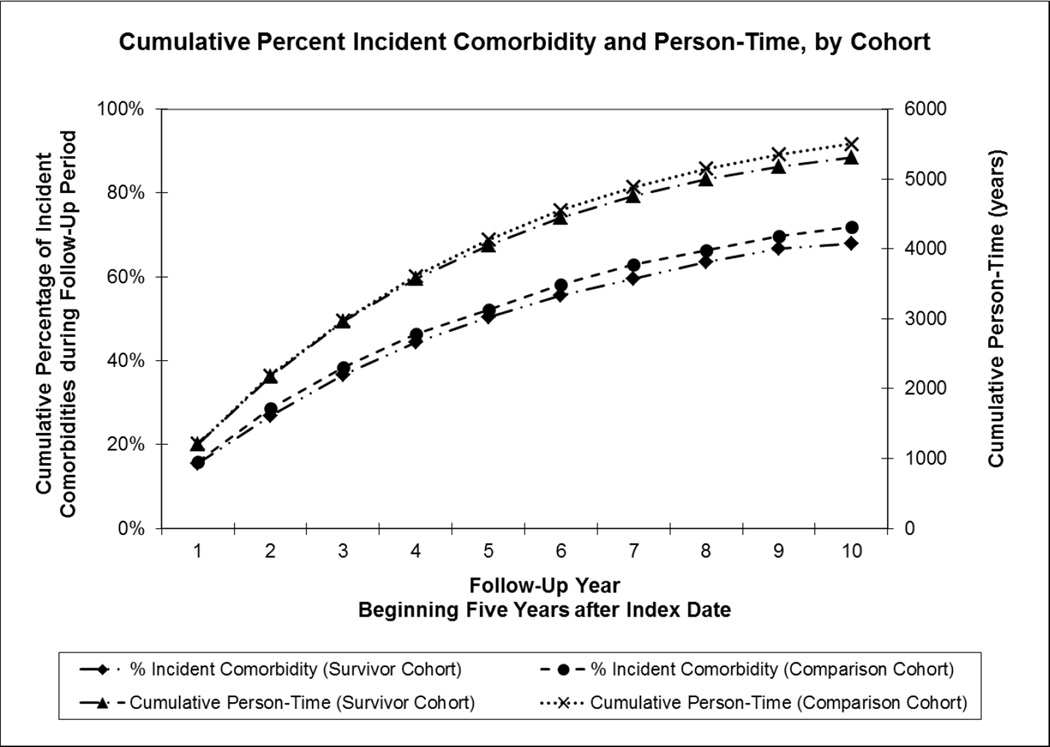
We identified 1,361 breast cancer survivors who met the eligibility criteria (five-year survivors diagnosed with early stage breast cancer at age 65 years and older) and were followed beginning five years after diagnosis for a median of 3.3 years (total person-years= 5,679). In the comparison cohort, 1,361 women were matched to the breast cancer cohort and were followed for a median of 3.7 years after five-year survival (total person-years = 6,062). The accumulation of person-time during follow-up is shown in Figure 1 (right axis) for each cohort. Age was evenly distributed across age categories and women were predominately Caucasian in both the survivor (82%) and comparison cohorts (84%, Table 1). Follow-up was censored in 319 women for disenrollment from the health care system and in 278 women for death.
Fig 1. Accumulation of Person-Time and Incident Comorbidities in Older Five-Year Early Breast Cancer Survivors and Matched Comparison Cohort.
Cumulative percentage of incident comorbidities for each year during the 10 year follow-up period beginning five years after Index Date is plotted on left y-axis for survivor cohort (dash-dot-dot line with diamond marker) and comparison cohort (dashed line with circle marker). Cumulative person-time in years for the same period is plotted on the right y-axis for survivor cohort (dash-dot line with triangle marker) and comparison cohort (dotted line with x marker).
Table 1.
Characteristics of Older Five-Year Survivors of Early Breast Cancer and Matched Comparison Cohort
| Breast Cancer Survivor Cohorta (n = 1,361) |
Comparison Cohortb (n = 1,361) |
||||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sociodemographic | |||||
| Age Category at Beginning of Follow-up (years) | |||||
| 70–74 | 502 | 37 | 502 | 37 | |
| 75–79 | 417 | 31 | 417 | 31 | |
| 80+ | 442 | 32 | 442 | 32 | |
| Race/ethnicity | |||||
| Caucasian, Non-Hispanic | 1115 | 82 | 1147 | 84 | |
| African-American, Non-Hispanic | 137 | 10 | 125 | 9.2 | |
| Hispanic | 72 | 5.3 | 62 | 4.6 | |
| Asian/Pacific Islander | 37 | 2.7 | 27 | 2.0 | |
| Native American | 0 | 0 | 0 | 0 | |
| Comorbidity Burden at Beginning of Follow-up | |||||
| Prevalent Comorbidities included in mCCIc (index weight) | |||||
| Myocardial infarction | (1) | 80 | 5.9 | 80 | 5.9 |
| Congestive heart failure | (1) | 140 | 10 | 117 | 8.6 |
| Peripheral vascular disease | (1) | 60 | 4.4 | 67 | 4.9 |
| Cerebrovascular disease | (1) | 96 | 7.1 | 98 | 7.2 |
| Dementia | (1) | 64 | 4.7 | 76 | 5.6 |
| Chronic pulmonary disease | (1) | 172 | 13 | 184 | 14 |
| Connective tissue disease | (1) | 36 | 2.6 | 62 | 4.6 |
| Ulcer disease | (1) | 75 | 5.5 | 73 | 5.4 |
| Diabetes | (1) | 202 | 15 | 169 | 12 |
| Mild liver disease | (1) | 7 | 0.51 | 6 | 0.44 |
| Hemiplegia | (2) | 24 | 1.8 | 25 | 1.8 |
| Moderate or severe renal disease | (2) | 17 | 1.2 | 15 | 1.1 |
| Diabetes with end organ damage | (2) | 31 | 2.3 | 31 | 2.3 |
| Leukemia, lymphoma or tumord | (2) | 112 | 8.2 | 75 | 5.5 |
| Moderate or severe liver disease | (3) | 14 | 1.0 | 2 | 0.15 |
| AIDS | (6) | 0 | 0.00 | 0 | 0.00 |
| Metastatic solid tumor | (6) | 6 | 0.44 | 4 | 0.29 |
| Modified Charlson Comorbidity Indexe | |||||
| mCCI 0 | 741 | 54 | 755 | 55 | |
| mCCI 1–2 | 502 | 37 | 487 | 36 | |
| mCCI 3+ | 118 | 8.7 | 119 | 8.7 | |
Older five-year survivors diagnosed 1990–1994 with early stage I and II breast cancer at age 65 or older followed for 10 years beginning five years after index date (date of breast cancer diagnosis).
Comparison cohort matched for health system and age, who were free of breast cancer at matched index date and followed for 10 years beginning five years after index date.
Women may be counted in multiple comorbidities.
Prevalent breast cancer at index date excluded.
Prevalent breast cancer at index date excluded from modified Charlson Comorbidity Index (mCCI), which is calculated from the sum of comorbidity weights listed in the table (Charlson et al 1987).
Breast Cancer
Within the survivor cohort, 60% of women were diagnosed with AJCC Stage I breast cancer (n=812) and the remaining 40% were diagnosed with Stage IIA or IIB breast cancer (n=549). About half of the women received breast conserving surgery (n=648, n=523 of whom also received radiation therapy) while the other half underwent mastectomy (n=699). Less than 10% of the survivor cohort received chemotherapy (n=121) and two-thirds received adjuvant tamoxifen (n=905).
Prevalent Comorbidities at Initiation of Follow-up Period
At the beginning of follow-up, the prevalence of comorbidities was similar in the cohorts (Table 1). The five most common prevalent conditions in the survivor and comparison cohorts were diabetes (15% vs. 12%), chronic pulmonary disease (13% vs. 14%), congestive heart failure (10% vs. 8.6%), cerebrovascular disease (7.1% vs. 7.2%), and history of malignancy other than breast cancer (8.2% vs. 5.5%). Other comorbidities frequencies were similar in the cohorts. For prevalent comorbidity score, the modified Charlson Comorbidity Index (mCCI) score was calculated using the traditional CCI comorbidity index weights (Table 2) with the addition of pulmonary emboli and osteoporotic fractures (each given an index weight of one point). At the beginning of follow-up, 54% of survivors and 55% of comparators were free of prevalent comorbidities (mCCI=0). Only 8.7% of women in each cohort had a mCCI≥3 (Table 1).
Table 2.
Incident Comorbidities after 10 Years of Follow-up in Older Five-Year Early Breast Cancer Survivors and Matched Comparison Cohort
| Breast Cancer Survivor Cohorta (n = 1,361) |
Comparison Cohortb (n = 1,361) |
Adjusted Hazard Ratio |
|||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Adj HRc | (95 % CI) | ||
| Any incident comorbidity acquired in 10 year observation period | 870 | 64 | 911 | 67 | 1.0 | (0.93,1.1) | |
| Frequency of Incident Comorbid Conditions and Adjusted Hazard Ratios from Individual Modelsd (index weight) | |||||||
| Myocardial infarction | (1) | 91 | 6.7 | 123 | 9.0 | 0.75 | (0.57,0.99) |
| Congestive heart failure | (1) | 197 | 14 | 219 | 16 | 0.92 | (0.76,1.1) |
| Peripheral vascular disease | (1) | 76 | 5.6 | 93 | 6.8 | 0.84 | (0.62,1.1) |
| Cerebrovascular disease | (1) | 145 | 11 | 176 | 13 | 0.85 | (0.68,1.1) |
| Dementia | (1) | 248 | 18 | 257 | 19 | 1.0 | (0.85,1.2) |
| Chronic pulmonary disease | (1) | 112 | 8.2 | 127 | 9.3 | 0.92 | (0.72,1.2) |
| Connective tissue disease | (1) | 29 | 2.1 | 24 | 1.8 | 1.3 | (0.74,2.2) |
| Ulcer disease | (1) | 46 | 3.4 | 53 | 3.9 | 0.92 | (0.62,1.4) |
| Diabetes | (1) | 149 | 11 | 117 | 8.6 | 1.4 | (1.1,1.8) |
| Mild liver disease | (1) | 8 | 0.59 | 10 | 0.73 | 0.85 | (0.33,2.1) |
| Hemiplegia | (2) | 35 | 2.6 | 50 | 3.7 | 0.72 | (0.47,1.1) |
| Moderate or severe renal disease | (2) | 79 | 5.8 | 83 | 6.1 | 1.0 | (0.74,1.4) |
| Diabetes with end organ damage | (2) | 76 | 5.6 | 66 | 4.9 | 1.2 | (0.88,1.7) |
| Leukemia, lymphoma or tumore | (2) | 123 | 9.0 | 115 | 8.7 | 1.1 | (0.87,1.5) |
| Moderate or severe liver disease | (3) | 4 | 0.29 | 5 | 0.37 | 0.82 | (0.22,3.1) |
| AIDS | (6) | 0 | 0 | 0 | 0 | – | – |
| Metastatic solid tumor | (6) | 37 | 2.7 | 31 | 2.3 | 1.2 | (0.77,2.0) |
| Pulmonary embolismf | (1) | 11 | 0.81 | 19 | 1.4 | 0.61 | (0.29,1.3) |
| Osteoporotic fracturef | (1) | 231 | 17 | 238 | 17 | 1.0 | (0.83,1.2) |
Older five-year survivors diagnosed 1990–1994 with early stage I and II breast cancer at age 65 or older followed for 10 years beginning five years after index date (date of breast cancer diagnosis).
Comparison cohort matched for health system and age, who were free of breast cancer at matched index date and followed for 10 years beginning five years after index date.
Hazard ratio (HR) adjusted for study site location, age, and presence of prevalent comorbidity. CI = Confidence Interval.
Women may be counted in multiple comorbidities.
Prevalent breast cancer at index date excluded in modified Charlson Comorbidity Index (CCI).
Comorbidities added to original CCI to create modified CCI (Charlson et al 1987).
Incident Comorbidities during Follow-up Period
In the survivor cohort, 355 women acquired one comorbidity while 570 women acquired more than one comorbidity. A total of 383 women in the comparison cohort acquired one comorbidity and 595 women acquired more than one comorbidity. The most common incident comorbidities in the survivor and comparison cohorts were dementia (18% vs. 19%), osteoporotic fracture (17% vs. 17%), congestive heart failure (14% vs. 16%), cerebrovascular disease (11% vs. 13%), and diabetes (11% vs. 8.6%, Table 2).
The cohorts did not accumulate incident comorbidities at different rates during the follow-up period (HR=1.0 adjusted for age and prevalent comorbidities, 95% CI: 0.93,1.1; Figure 1, left axis). Compared to the comparison cohort, survivors had no notable difference in individual incident comorbidities during the ten year follow-up period except for diabetes (HR=1.4, 95% CI: 1.1,1.8) and myocardial infarction (HR=0.75, 95% CI: 0.57,0.99, Table 2).
Association between Incident Comorbidity and Mortality
Among women who acquired incident comorbidities, 21% died within the next two years and 57% survived through end of follow-up. The time-varying model inclusive of survivors and comparators found that a breast cancer diagnosis was associated with increased mortality in years 6–15 after diagnosis, compared with matched women (HR=1.3, 95% CI: 1.1,1.4, Table 3). Regardless of cohort, mortality was also associated with having any prevalent comorbidity at the beginning of follow-up (HR=1.8, 95% CI: 1.6, 2.1) and with the development of incident comorbidities during follow-up (HR=4.8, 95% CI: 4.1,5.6). As expected, older age was also associated with mortality hazard, even after accounting for breast cancer history, prevalent comorbidities, and incident comorbidities (Table 3). In the models fit to each cohort separately (Table 4), the adjusted mortality hazard ratio after an incident comorbidity was nearly two-fold greater in the comparison cohort (HR = 7.2, 95% CI: 5.6,9.3) than the survivor cohort (HR=3.7, 95% CI: 3.0,4.5). All other covariate estimates were similar between cohort models.
Table 3.
All-Cause Mortality after 10 Years of Follow-up in Older Five-Year Early Breast Cancer Survivors and Matched Comparison Cohort
| Total Deaths, n | Adjusted HRc | (95% CI) | |
|---|---|---|---|
| Age Category at Index Date (years) | |||
| 70–74 | 263 | 1 (ref) | – |
| 75–79 | 289 | 1.3 | (1.1,1.6) |
| 80+ | 508 | 2.8 | (2.4,3.3) |
| Cohort | |||
| Breast Cancer Survivorsa | 593 | 1.3 | (1.1,1.4) |
| Comparisonb | 467 | 1 (ref) | – |
| Any Prevalent Comorbidity (at 5 Years after Index Date) | 614 | 1.8 | (1.6,2.1) |
| Incident Comorbidity (6–15 Years after Index Date) | 773 | 4.8 | (4.1,5.6) |
Older five-year survivors (n=1,361) diagnosed 1990–1994 with early stage I and II breast cancer at age 65 or older followed for 10 years beginning five years after index date (date of breast cancer diagnosis).
Comparison cohort (n=1,361) matched for health system and age, who were free of breast cancer at matched index date and followed for 10 years beginning five years after index date.
Hazard ratio (HR) adjusted for study site location, age, and presence of prevalent comorbidity. CI = Confidence Interval.
Table 4.
All-Cause Mortality Stratified by Cohort (Older Five-Year Early Breast Cancer Survivors and Matched Comparison Cohort).
| Breast Cancer Survivorsa | Comparison Cohortb | |||
|---|---|---|---|---|
| Adjusted HRc | (95% CI) | Adjusted HRc | (95% CI) | |
| Age Category at Index Date (years) | ||||
| 70–74 | 1 (ref) | – | 1 (ref) | – |
| 75–79 | 1.2 | (0.92,1.4) | 1.6 | (1.2,2.0) |
| 80+ | 2.6 | (2.1,3.1) | 3.4 | (2.7,4.3) |
| Any Prevalent Comorbidity (at 5 Years after Index Date) | 1.7 | (1.4,2.0) | 2.0 | (1.7,2.4) |
| Incident Comorbidity (Years 6–15 after Index Date) | 3.7 | (3.0,4.5) | 7.2 | (5.6,9.3) |
Older five-year survivors (n=1,361) diagnosed 1990–1994 with early stage I and II breast cancer at age 65 or older followed for 10 years beginning five years after index date (date of breast cancer diagnosis).
Comparison cohort (n=1,361) matched for health system and age, who were free of breast cancer at matched index date and followed for 10 years beginning five years after index date.
Hazard ratio (HR) adjusted for health system, age, and presence of prevalent comorbidity. CI = Confidence Interval.
DISCUSSION
In this study of five-year breast cancer survivors diagnosed at age 65 or older, older survivors did not develop more comorbidities 6–15 years after diagnosis and treatment than age-matched women free of breast cancer. However, despite comparable incident comorbidity development, survivors were slightly more likely to die in the 10 year follow-up period beginning five years after cancer diagnosis and treatment independent of age and prevalent comorbidity burden. In both cohorts, newly acquired comorbidities were strongly associated with all-cause mortality.
The results of our study are concordant with prior literature investigating comorbidities in older breast cancer survivors and their association with mortality. Previous studies concluded that despite an increased comorbidity frequency within one year of diagnosis [22,11], survivors have the same comorbidity burden five years after diagnosis as matched women free of breast cancer [11]. We observed an equivalent prevalent comorbidity burden among both cohorts at start of follow-up. Additionally, the rate of acquired comorbidities in our survivor cohort was also similar to another survivor cohort [23]. Our work extends this earlier survivor comorbidity burden research by investigating comorbidity burden in a population of older breast cancer survivors matched with a comparison cohort from the same source population over a longer survival period than previously studied. We have previously observed within this cohort that starting at diagnosis, women who receive left-sided radiotherapy have increased rates of cardiovascular disease [24] which may account for increased rates of cardiovascular comorbidities in our population. Similarly, anthracycline chemotherapy is associated with cardiovascular toxicity [25], however, we did not have specific regimen information to further investigate this within our population. A study investigating the impact of comorbidities among older five-year breast cancer survivors found a low to moderate comorbidity burden was associated with a two-fold mortality risk, whereas a severe comorbidity burden was associated with a three-fold risk mortality compared to survivors without comorbidities [26]. Other studies have suggested that increased comorbidity burden is associated with increased all-cause mortality risk in older breast cancer survivors [27,28,12]; however, none of these prior studies included a matched comparison group. In our study we found that acquiring any comorbidity in years 6–15 after index date was associated with a 4.8-fold increase in mortality hazard after adjusting for cohort membership.
Our work addresses a gap in the literature by determining the effect of incident comorbidities on mortality in a population of older five-year breast cancer survivors 15 years after diagnosis in a prospective matched cohort study. Importantly, we found that older five-year survivors continue to have a slightly increased hazard of mortality, even after adjusting for covariates of age, race/ethnicity, prevalent comorbidity, and incident comorbidity during years 6–15 after diagnosis and treatment. This finding emphasizes the importance of all women receiving proper treatment regardless of age, comorbid conditions, or breast cancer history. Although follow-up was censored for health care system disenrollment, survivors generally remain with the insurer who covered their diagnosis and treatment. Thus, disenrollment is likely due to factors beyond womens’ control and loss to follow-up is not likely to be differential.
No earlier studies have compared a cohort of older breast cancer survivors with a population-based matched comparison cohort. As noted previously, the adjusted HR for incident comorbidity in the comparison cohort was nearly two-fold greater than that of the survivor cohort (Table 4). We endeavored to explain this result through a series of analyses, none of which were illuminating, and are therefore not presented here. Possible explanations of the observed interaction include selection bias associated with surviving breast cancer and its treatment and/or surveillance bias associated with more in-depth investigation and management of incident conditions. Deaths from causes other than breast cancer may be more affected by age and comorbidity; the mortality hazard in the comparator group may therefore be more influenced by age comorbidity than the survivor group in which the effects of age and comorbidity are diluted by breast cancer deaths. Conversely, as the first matched comparison cohort study to investigate these outcome measures, it is potentially a spurious finding that may not be confirmed in future studies.
Our measure of comorbidity burden, the CCI, was originally designed to include fatal comorbid conditions [19]. The included diseases were selected based on mortality data from decades ago and may not represent the most important comorbidities in this population [29]. Quan et al. re-weighted the CCI with more recent administrative data and found no large change in the association between CCI and mortality [30]. We captured additional comorbidity data for pulmonary emboli and osteoporotic fractures which have been determined to occur in older cancer survivors [31,32]. There is no gold standard measure of comorbidity in cancer populations [29,6]; as such, we calculated the mCCI given our access to data and methods of data collection. Importantly, older survivors have an incremental increase in mortality driven primarily by new comorbidity burden acquired 6–15 years after diagnosis and treatment. These findings highlight the importance of care coordination between oncology and primary care providers and appropriate long-term follow-up in older breast cancer survivors [33,34].
The strengths of our study include a well-matched comparison cohort with high quality data obtained through adjudicated medical record collection with good inter-rater reliability [17,20]. We repeatedly collected comorbidity data that allowed us to implement time-varying analyses while accounting for censored events, which is critical in longitudinal studies. Our work utilized an index of comorbidity burden previously associated with all-cause mortality in breast cancer patients [19]. Additionally, our analyses extended the follow-up period observing older breast cancer survivors to determine the association of incident comorbidities and mortality.
This study is not without limitations, however. The possibility of a more complete medical history obtained in the survivor cohort versus the comparison cohort may have led to information bias. Prior studies have shown that comorbidities are similar among women with and without breast cancer at diagnosis [35] and five years after diagnosis [22,11], and our measures of comorbidity burden are therefore unlikely to have substantially different information quality. Additional unmeasured parameters, such as body mass index, may leave residual confounding in the findings of the association between comorbidity burden and mortality [22]. While this survivor cohort had breast cancer diagnoses between 1990 and 1994, the standards of care and primary treatment for early stage invasive breast cancer in older women have only changed modestly since this cohort was identified. Epidemiologic studies of the rate of late effects of more current treatments will need to be conducted in a new survivorship cohort after an appropriate person-time has accumulated. While our study investigated early stage survivors with low prevalence of receipt of radiation therapy and chemotherapy, the inclusion of advanced stage breast cancer survivors in future studies may provide more insight into the late effects of radiation therapy and chemotherapy. We sought to enroll a diverse population of survivors and purposefully undersampled eligible non-hispanic white women at the largest health system site [13], however, our resultant survivor population consisted of a higher proportion of non-hispanic white women than in the general population which may limit conclusions drawn for other races and ethnicities.
The results of this study demonstrate that comorbidity burden in older breast cancer survivors is similar to age-matched women free of breast cancer in an extended observational period of 6–15 years after cancer diagnosis and treatment. Furthermore, older survivors retain a marginal increase in mortality rate 6–15 years after diagnosis, even after adjusting for age and comorbidity burden. It is important, therefore, that patients receive optimal breast cancer therapy regardless of age and comorbidity. Additionally, as the rates of five-year and 10-year breast cancer survival increase [2] and the population ages, these findings underscore the importance of preventing and treating the comorbidities in older survivors of breast cancer as well as in older women in general.
ACKNOWLEDGEMENTS
Sponsor’s Role: This study was funded by Public Health Service grant R01CA093772 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (PI: RA Silliman). The funding sponsors played no part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Sponsors had no access to the data and did not perform any of the study analysis.
ABBREVIATIONS
- AJCC
American Joint Committee on Cancer
- BOW
Breast cancer treatment effectiveness in Older Women cohort study
- CCI
Charlson comorbidity index
- CI
Confidence interval
- HR
Hazard ratio
- mCCI
modified Charlson comorbidity index
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
This article was prepared while Dr. Geiger was employed at Wake Forest School of Medicine. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Ethical Standards: This study complies with the current laws of the United States of America. This study was reviewed and approved by the Institutional Review Boards of each participating institution and at the Boston University Medical Center.
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Yancik R, Ries LAG. Cancer and aging in America - Demographic and epidemiologic perspectives. Hematology-Oncology Clinics of North America. 2000;14(1):17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures 2011–2012. Atlanta: American Cancer Society, Inc.; 2011. [Google Scholar]
- 3.Smith BD, Jiang J, McLaughlin SS, Hurria A, Smith GL, Giordano SH, Buchholz TA. Improvement in breast cancer outcomes over time: Are older women missing out? J Clin Oncol. 2011;29(35):4647–4653. doi: 10.1200/JCO.2011.35.8408. [DOI] [PubMed] [Google Scholar]
- 4.van de Water W, Markopoulos C, van de Velde CJH, Seynaeve C, Hasenburg A, Rea D, Putter H, Nortier JWR, de Craen AJM, Hille ETM, Bastiaannet E, Hadji P, Westendorp RGJ, Liefers GJ, Jones SE. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. J Am Med Assoc. 2012;307(6):590–597. doi: 10.1001/jama.2012.84. [DOI] [PubMed] [Google Scholar]
- 5.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28(26):4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 6.Geraci JM, Escalante CP, Freeman JL, Goodwin JS. Comorbid disease and cancer: The need for more relevant conceptual models in health services research. J Clin Oncol. 2005;23(30):7399–7404. doi: 10.1200/JCO.2004.00.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancik R, Havlik RJ, Wesley MN, Ries L, Long S, Rossi WK, Edwards BK. Cancer and comorbidity in older patients: A descriptive profile. Ann Epidemiol. 1996;6(5):399–412. doi: 10.1016/s1047-2797(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM. Assessing the impact of comorbidity in the older population. Ann Epidemiol. 1996;6(5):376–380. doi: 10.1016/s1047-2797(96)00060-9. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106(7):1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: Challenge and opportunity. Semin Radiat Oncol. 2003;13(3):248–266. doi: 10.1016/S1053-4296(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 11.Hanchate AD, Clough-Gorr KM, Ash AS, Thwin SS, Silliman RA. Longitudinal patterns in survival, comorbidity, healthcare utilization and quality of care among older women following breast cancer diagnosis. J Gen Intern Med. 2010;25(10):1045–1050. doi: 10.1007/s11606-010-1407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahern TP, Lash TL, Thwin SS, Silliman RA. Impact of acquired comorbidities on all-cause mortality rates among older breast cancer survivors. Med Care. 2009;47(1):73–79. doi: 10.1097/MLR.0b013e318180913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enger SM, Thwin SS, Buist DS, Field T, Frost F, Geiger AM, Lash TL, Prout M, Yood MU, Wei F, Silliman RA. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24(27):4377–4383. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner EH, Greene SM, Hart G, Field TS, Fletcher S, Geiger AM, Herrinton LJ, Hornbrook MC, Johnson CC, Mouchawar J, Rolnick SJ, Stevens VJ, Taplin SH, Tolsma D, Vogt TM. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005;(35):3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 15.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edn. New York: Springer; 2010. [Google Scholar]
- 16.Bosco JLF, Lash TL, Prout MN, Buist DSM, Geiger AM, Haque R, Wei FF, Silliman RA Investigators B. Breast cancer recurrence in older women five to ten years after diagnosis. Cancer Epidem Biomar. 2009;18(11):2979–2983. doi: 10.1158/1055-9965.EPI-09-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thwin SS, Clough-Gorr KM, McCarty MC, Lash TL, Alford SH, Buist DS, Enger SM, Field TS, Frost F, Wei F, Silliman RA. Automated inter-rater reliability assessment and electronic data collection in a multi-center breast cancer study. BMC Med Res Methodol. 2007;7:23. doi: 10.1186/1471-2288-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avila CC, Quinn VP, Geiger AM, Kerby TJ, St Charles M, Clough-Gorr KM. Webinar Training: An acceptable, feasible and effective approach for multi-site medical record abstraction: the BOWII experience. BMC Res Notes. 2011;4:430. doi: 10.1186/1756-0500-4-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Lash TL, Fox MP, Thwin SS, Geiger AM, Buist DS, Wei F, Field TS, Yood MU, Frost FJ, Quinn VP, Prout MN, Silliman RA. Using probabilistic corrections to account for abstractor agreement in medical record reviews. Am J Epidemiol. 2007;165(12):1454–1461. doi: 10.1093/aje/kwm034. [DOI] [PubMed] [Google Scholar]
- 21.Adamo MBJC, Ruhl JL, Dickie LA, editors. SEER Program Coding and Staging Manual. Bethesda, MD: National Cancer Institute; 2011. NIH Publication Number 11–5581. [Google Scholar]
- 22.Danese MD, O'Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756–1765. doi: 10.1093/annonc/mdr486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braithwaite D, Moore DH, Satariano WA, Kwan ML, Hiatt RA, Kroenke C, Caan BJ. Prognostic impact of comorbidity among long-term breast cancer survivors: Results from the LACE study. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haque R, Yood MU, Geiger AM, Kamineni A, Avila CC, Shi J, Silliman RA, Quinn VP. Long-term safety of radiotherapy and breast cancer laterality in older survivors. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2120–2126. doi: 10.1158/1055-9965.EPI-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. J Am Med Assoc. 1991;266(12):1672–1677. [PubMed] [Google Scholar]
- 26.Houterman S, Janssen-Heijnen MLG, Verheij CDGW, Louwman WJ, Vreugdenhil G, van der Sangen MJC, Coebergh JWW. Comorbidity has negligible impact on treatment and complications but influences survival in breast cancer patients. Brit J Cancer. 2004;90(12):2332–2337. doi: 10.1038/sj.bjc.6601844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel G, Wedding U, Rohrig B, Katenkamp D. The impact of comorbidity on the survival of postmenopausal women with breast cancer. J Cancer Res Clin Oncol. 2004;130(11):664–670. doi: 10.1007/s00432-004-0594-3. [DOI] [PubMed] [Google Scholar]
- 28.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarfati D. Review of methods used to measure comorbidity in cancer populations: No gold standard exists. J Clin Epidemiol. 2012 doi: 10.1016/j.jclinepi.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 31.Cabanillas ME, Lu H, Fang S, Du XL. Elderly patients with non-Hodgkin lymphoma who receive chemotherapy are at higher risk for osteoporosis and fractures. Leuk Lymphoma. 2007;48(8):1514–1521. doi: 10.1080/10428190701471973. [DOI] [PubMed] [Google Scholar]
- 32.Chavez-MacGregor M, Zhao H, Kroll M, Fang S, Zhang N, Hortobagyi GN, Buchholz TA, Shih YC, Giordano SH. Risk factors and incidence of thromboembolic events (TEEs) in older men and women with breast cancer. Ann Oncol. 2011;22(11):2394–2402. doi: 10.1093/annonc/mdq777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nekhlyudov L. "Doc, Should I See You or My Oncologist?" A primary care perspective on opportunities and challenges in providing comprehensive care for cancer survivors. J Clin Oncol. 2009;27(15):2424–2426. doi: 10.1200/JCO.2008.21.4023. [DOI] [PubMed] [Google Scholar]
- 34.Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489–2495. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 35.Harlan LC, Klabunde CN, Ambs AH, Gibson T, Bernstein L, McTiernan A, Meeske K, Baumgartner KB, Ballard-Barbash R. Comorbidities, therapy, and newly diagnosed conditions for women with early stage breast cancer. J Cancer Surviv. 2009;3(2):89–98. doi: 10.1007/s11764-009-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]