Abstract
Patients with end-stage liver disease frequently need invasive cardiac procedures in preparation for liver transplantation. As a consequence of impaired hepatic function these patients often have a prolonged prothrombin time (PT) and elevated International Normalized Ratio (INR). To determine whether an abnormal PT/INR is predictive of bleeding complications from invasive cardiac procedures, we reviewed retrospectively, for bleeding complications, the databases and case records of our series of patients with advanced cirrhosis who underwent cardiac catheterization. One hundred and fifty-seven patients underwent isolated right heart catheterization (RHC), and 83 underwent left (LHC) or combined left and right sided heart catheterization. INRs ranged from 0.93-2.35. There were no major procedure-related complications. Several patients in each group required blood transfusion for gastrointestinal bleeding, but not for procedure-related bleeding. There was no significant change in hemoglobin after RHC or LHC, and no correlation between pre-procedure INR and change in post-procedure hemoglobin. When comparing patients with normal (≤1.5) and elevated (>1.5) INRs, no significant difference in hemoglobin post-procedure was found in either group. In conclusion, despite an elevated INR, patients with end-stage liver disease can safely undergo invasive cardiac procedures. INR elevation does not predict catheterization-related bleeding complications in this patient population.
Keywords: Liver disease, cardiac catheterization, bleeding
Introduction
At present there are few published data regarding the bleeding risk from cardiac procedures in patients with end-stage liver disease (ESLD). Three single center studies have been published comparing patients with ESLD to matched cohorts, and have found a similar to slightly higher procedure-related complication rate in those with liver disease1-3. Of particular interest were the findings that pre-procedure International Normalized Ratio (INR) was associated with bleeding risk3, and in a second study, that treatment of INRs >1.6 with fresh frozen plasma (FFP) might have reduced bleeding risk2. Therefore we sought to determine whether an elevated INR is predictive of complications from cardiac catheterization in ESLD patients.
Methods
After approval by the Medical University of South Carolina Institutional Review Board, we searched our liver transplant and heart catheterization databases for patients undergoing invasive cardiac procedures between 5/2003 and 8/2009. Patients were divided into those undergoing isolated right heart catheterization (RHC), and a left heart catheterization group (LHC) comprised of those undergoing left heart catheterization with or without an associated RHC.
We collected demographic, laboratory and procedural data for each patient. Model for End-Stage Liver Disease (MELD) scores and body-mass index (BMI) were calculated for each patient4.
Venous access in the isolated RHC group was predominately in an internal jugular vein (90/157, 57%) with the remainder being femoral. A 7 French sheath was most commonly used (139/157, 89%). The majority of patients with combined RHC and LHC had femoral venous access (58/66, 89%) with a 7 French sheath (65/66, 98%). Arterial access was uniformly in a femoral artery, with 4 French (40/83, 48%) and 6 French (34/83, 41%) sheaths most commonly used. Neither ultrasound guidance nor micropuncture technique was routinely used during the time period of this study.
Daily notes and radiology reports were searched for any evidence of vascular complication or significant bleeding at the catheterization sites or elsewhere. We specifically looked for the development of arteriovenous fistulas, aneurysms or pseudoaneurysms, evidence of retroperitoneal bleeding, hematomas, or intracranial bleeding. Outpatients with an uncomplicated course were discharged post procedure, and as a result follow up laboratory data was only available in 62% with isolated RHC, and 70% of patients in the LHC group.
Administration of platelets and fresh frozen plasma within 24 hours of the procedure was also recorded, as well as red blood cell infusion at any time afterwards. The decision to transfuse FFP or platelets was at the discretion of the cardiologist performing the procedure.
Statistical analysis was performed with Spearman's rank correlation coefficient to look for a relationship between INR and post-procedural changes in hemoglobin. Post-procedural differences in hemoglobin were also compared between subgroups with normal (≤1.5) and elevated (>1.5) INRs, using Student's t-test. A p-value <0.05 was considered statistically significant.
Results
One hundred and fifty-seven patients were identified as having anisolated RHC, and 83 were identified in the LHC group, a large majority (66/83) of whom underwent associated RHC. The mean INR in patients' undergoing isolated RHC was 1.5±0.3 (0.93-2.35), and in the LHC group the mean INR was 1.38±0.3 (0.94-2.15). Demographic, laboratory and procedural data are included in Table 1.
Table 1.
|
|
||||||
|---|---|---|---|---|---|---|
| Variable | RHC (n=157) | P | LHC (n=83) | P | ||
|
|
||||||
| INR≤1.5 61% | INR>1.5 39% | INR≤1.5 72% | INR>1.5 28% | |||
| Age (years) | 55±7.9 | 52±10 | 0.07 | 57±9.2 | 52±7.8 | 0.02 |
| Men | 57% | 62% | 0.84 | 73% | 61% | 0.78 |
| Cuacasian | 79% | 89% | 0.65 | 78% | 91% | 0.81 |
| Weight (kg) | 90±22 | 89±22 | 0.76 | 93±17 | 90±25 | 0.52 |
| Body Mass Index (Kg/m2) | 31±7 | 29±6 | 0.24 | 31±5 | 30±7.3 | 0.62 |
| Systolic blood pressure (mmHg) | 125±21 | 121±18 | 0.24 | 122±23 | 121±21 | 0.91 |
| Diastolic blood pressure (mmHg | 72±12 | 67±13 | 0.01 | 65±13 | 64±14 | 0.76 |
| Hemoglobin (g/dL) | 12±2.0 | 10±2.1 | <0.01 | 12±2.1 | 10±1.9 | <0.01 |
| Platelets (cm3) | 101±57 | 105±102 | 0.79 | 97±53 | 77±32 | 0.1 |
| Creatinine (mg/dL) | 1.35±1.0 | 1.57±1.7 | 0.31 | 1.49±1.2 | 1.58±1.2 | 0.76 |
| MELD | 13±5.3 | 21±7.4 | <0.01 | 14±6.4 | 22±6.4 | <0.01 |
| International Normalized Ratio | 1.3±0.14 | 1.8±0.21 | <0.01 | 1.25±0.14 | 1.74±0.18 | <0.01 |
| Concurrent RHC | 75% (45) | 91% (21) | 0.12 | |||
No major vascular complications or procedure-related bleeding events were identified in any patient. Of those with complete laboratory data, there was no significant difference between pre and post-procedure hemoglobin in either the isolated RHC (10.5g/dLvs 10.5g/dL, p=0.83) or LHC groups (11.1g/dLvs 11g/dL, p=0.83). In patients with isolated RHC, there was no significant change in hemoglobin in either the normal or elevated INR groups (9.7g/dLvs 9.7g/dL, p=0.98, and 11.1g/dLvs 10.9g/dL, p=0.1, respectively). Similarly, in the LHC group, no significant change in hemoglobin was found in either the normal or elevated INR groups (9.5g/dLvs 9.6g/dL, p=0.87, and 9.7g/dLvs 9.7g/dL, p=0.99, respectively) (Figure 1). To look for a relationship between post-procedure changes in hemoglobin and INR, corresponding values were plotted, but no correlation was seen in either the RHC (ρ = 0.1176; p=0.248) or LHC (ρ = -0.0463; p=0.820) groups (Figure 2).
Figure 1.
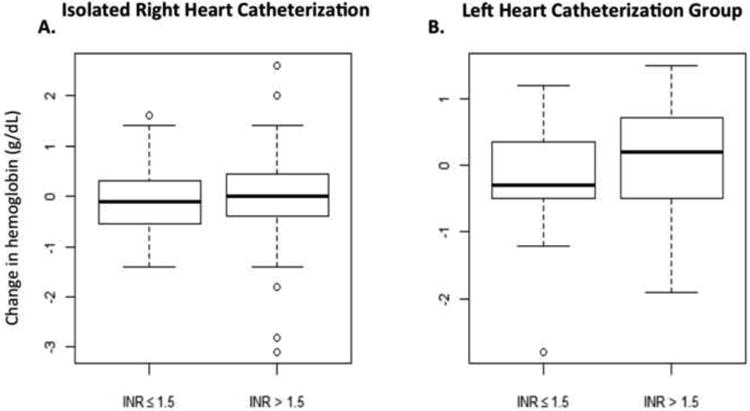
Changes in hemoglobin (g/dL) pre- to post-procedure, are plotted on the vertical axis against INR on the horizontal axis, from patients undergoing isolated right heart catheterization (A) and in the leftheart catheterization group (B). The bold middle line shows the median; the top and bottom lines of the box represent the 25th and 75th percentiles and the whiskers represent 1.5 multiplied by the interquartile range (IQR).
Figure 2.
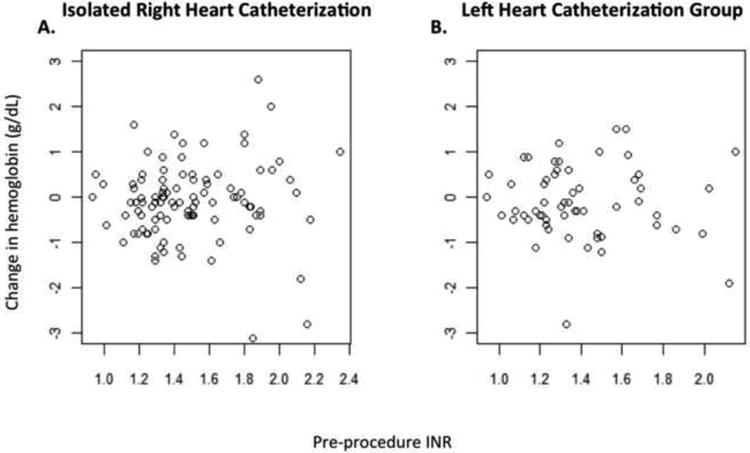
Scatter plots of change in hemoglobin pre- to post-catheterization versus pre-procedure INR. No correlation between change in hemoglobin and INR was identifiable in either the isolated right heart catheterization (A) or left heart catheterization group (B).
Of the 157 isolated RHC patients, 11 received FFP before the procedure. The mean INR in these patients was 2.4 (range 1.9-3.3). Despite transfusion of at least 2 units of FFP, only 1 patient had their INR decrease to <1.9. Two patients with platelets <50,000/mm3 received platelet transfusions. Finally, 3 patients received packed red cells. In 2 of these cases, gastrointestinal bleeding caused anemia; in the other, a fall in hemoglobin was attributed to hemodilution as there was no overt or concealed bleeding.
Among the 83 patients in the LHC group, 6 received pre-procedure FFP. The mean INR in these patients was 2.3 (range 1.9-3.3). After transfusion INR fell below 1.5 in only 1 case. Four patients with platelet counts <40,000/mm3 received platelet transfusions. Three patients received packed red blood cell infusion. In each case anemia was attributed to gastrointestinal bleeding.
Discussion
In the current study, we sought an association between INR and bleeding associated with cardiac catheterization in patients with ELSD, and found none. Our data suggest that a moderately elevated INR poses no risk for invasive procedures in ESLD despite concern frequently expressed by those who perform such procedures. Even when our patients were dichotomized into normal and elevated INR groups, no significant difference in procedural complications was identified. This is especially important as one of the highest reported reasons for blood product administration is the correction of an elevated INR in preparation for an invasive procedure5.
Historically, an elevated PT and its derived INR have been used to predict bleeding from invasive procedures. While these laboratory variables are associated with increased risk of hemorrhage in patients treated with warfarin-type anticoagulants, their utility and ability to predict bleeding in patients with cirrhosis is unclear 6,7. Patients with ESLD, by virtue of impaired hepatic synthetic function, have a characteristic coagulation profile that is now referred to as “rebalanced hemostasis” 8. Hepatic production of both pro-coagulant (Factors II, VII, IX, X) and anticoagulant (Protein C, Protein S, Anti-thrombin) factors is reduced7. This resets hemostatic balance despite an increase in PT/INR. Continued understanding of the complex interplay between those factors that promote and those that impair hemostasis has cast doubt on whether the PT/INR is predictive of bleeding risk in patients with ESLD, or merely serves as a marker of hepatic synthetic impairment 7.
Our findings are in accordance with results of several published studies9. Segal and Dzik, in a large systematic review of studies involving patients with an elevated PT or INR, did not find a significant increase in bleeding, although not all of the studies they reviewed included patients with ESLD 10. One study included in their review is worth highlighting. In this prospective investigation of patients with liver disease undergoing central venous access placement, only one major bleeding complication was observed, and this was in a patient with an INR of 1.5. In fact when comparing patients with INRs above and below 5, the only statistically different finding was for superficial hematomas, which were found in 12.4% of those with an INR >5, and in only 5.6% of those with an INR <5 11.
Not only do these published results suggest that INR may not accurately predict those at high risk of bleeding, but there is also little evidence to suggest that correction of INR by administration of FFP has any beneficial clinical effect 12,13. Consequently, the potential risk and significant cost associated with blood product transfusion may be avoided in this patient population. Bleeding complications with invasive procedures may still occur in patients with ESLD - as they can in patients without liver disease – but when they do, INR elevation is not likely to be the cause.
There are several limitations inherent in our retrospective study in which only major bleeding and vascular events are likely to have been recorded, whereas small hematomas or increased bruising might have been missed or not considered clinically significant enough to have been reported. There is undoubtedly the possibility of selection bias where in those considered most at risk for procedural complications did not undergo catheterization. It may also be that the operating physicians, aware of elevated INRs, took extra care to avoid bleeding complications.
Despite a patient population with elevated INRs, high MELD scores, renal dysfunction, and thrombocytopenia, we did not identify any serious catheter-related complications, nor could we find any association between INR and bleeding risk. Our conclusion, inferred from our current findings and results of studies in the literature, is that correction of INR with FFP, which puts patients at risk of volume overload and Transfusion-Related Acute Lung Injury14, is unnecessary to reduce a theoretical risk of catheterization-related bleeding due to INR elevation. These results should be confirmed prospectively and include ESLD patients with higher INRs than seen in our population.
Acknowledgments
This publication was supported by the South Carolina Clinical & Translational Research Institute, Medical University of South Carolina's CTSA, NIH/NCRR Grant Number UL1RR029882. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCRR.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaitkus PT, Dickens C, McGrath MK. Low bleeding risk from cardiac catheterization in patients with advanced liver disease. Cathet Cardiovasc Interv. 2005;65:510–512. doi: 10.1002/ccd.20398. [DOI] [PubMed] [Google Scholar]
- 2.Pillarisetti J, Patel P, Duthuluru S, Roberts J, Chen W, Genton R, Wiley M, Candipan R, Tadros P, Gupta K. Cardiac catheterization in patients with end-stage liver disease: safety and outcomes. Cathet Cardiovasc Interv. 2011;77:45–48. doi: 10.1002/ccd.22591. [DOI] [PubMed] [Google Scholar]
- 3.Sharma M, Yong C, Majure D, Zellner C, Roberts JP, Bass NM, Ports TA, Yeghiazarians Y, Gregoratos G, Boyle AJ. Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation. Am J Cardiol. 2009;103:742–746. doi: 10.1016/j.amjcard.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 4.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 5.Dzik W, Rao A. Why do physicians request fresh frozen plasma? Transfusion. 2004;44:1393–1394. doi: 10.1111/j.0041-1132.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107:1692–1711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 7.Roberts LN, Patel RK, Arya R. Haemostasis and thrombosis in liver disease. Br J Haematol. 2010;148:507–521. doi: 10.1111/j.1365-2141.2009.08021.x. [DOI] [PubMed] [Google Scholar]
- 8.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878–885. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 9.Malloy PC, Grassi CJ, Kundu S, Gervais DA, Miller DL, Osnis RB, Postoak DW, Rajan DK, Sacks D, Schwartzberg MS, Zuckerman DA, Cardella JF. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20:S240–249. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413–1425. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 11.Fisher NC, Mutimer DJ. Central venous cannulation in patients with liver disease and coagulopathy--a prospective audit. Intensive Care Med. 1999;25:481–485. doi: 10.1007/s001340050884. [DOI] [PubMed] [Google Scholar]
- 12.Youssef WI, Salazar F, Dasarathy S, Beddow T, Mullen KD. Role of fresh frozen plasma infusion in correction of coagulopathy of chronic liver disease: a dual phase study. Am J Gastroenterol. 2003;98:1391–1394. doi: 10.1111/j.1572-0241.2003.07467.x. [DOI] [PubMed] [Google Scholar]
- 13.Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–152. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 14.Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, Hubmayr RD, Gajic O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]


