Abstract
MicroRNA (miRNA) directs post-transcriptional regulation of a network of genes by targeting mRNA. Although relatively recent in development, many miRNAs direct differentiation of various stem cells including induced pluripotent stem cells (iPSCs), a major player in regenerative medicine. An effective and safe delivery of miRNA holds the key to translating miRNA technologies. Both viral and nonviral delivery systems have seen success in miRNA delivery, and each approach possesses advantages and disadvantages. A number of studies have demonstrated success in augmenting osteogenesis, improving cardiogenesis, and reducing fibrosis among many other tissue engineering applications. A scaffold-based approach with the possibility of local and sustained delivery of miRNA is particularly attractive since the physical cues provided by the scaffold may synergize with the biochemical cues induced by miRNA therapy. Herein, we first briefly cover the application of miRNA to direct stem cell fate via replacement and inhibition therapies, followed by the discussion of the promising viral and nonviral delivery systems. Next we present the unique advantages of a scaffold-based delivery in achieving lineage-specific differentiation and tissue development.
Keywords: MicroRNA, Non-viral vector, Regenerative medicine, Scaffold, Stem cells, Tissue engineering, Viral vector
Graphical Abstract
1. Introduction
Regenerative medicine aims to restore normal functions of damaged cells, organs, or tissues [1]. It holds promise in treating trauma, burns, degenerative diseases, and other maladies that cause tissue or organ failures. Cytokines and growth factors are widely used to augment the cell-mediated tissue regeneration. However, protein delivery suffers from the inherent disadvantages of limited stability, high cost, and short half-life [2]. Gene therapy offers an alternative [3–5]. Instead of using plasmid DNA to produce the growth factors, new development of microRNA (miRNA) has attracted much interest because it can influence a wide range of cell functions including the control of proliferation, differentiation, apoptosis and other metabolic processes by down-regulating or up-regulating the expression of their target genes [6–9]. There are over 1000 miRNAs in humans and they regulate over 30% of genomic RNAs [10, 11].
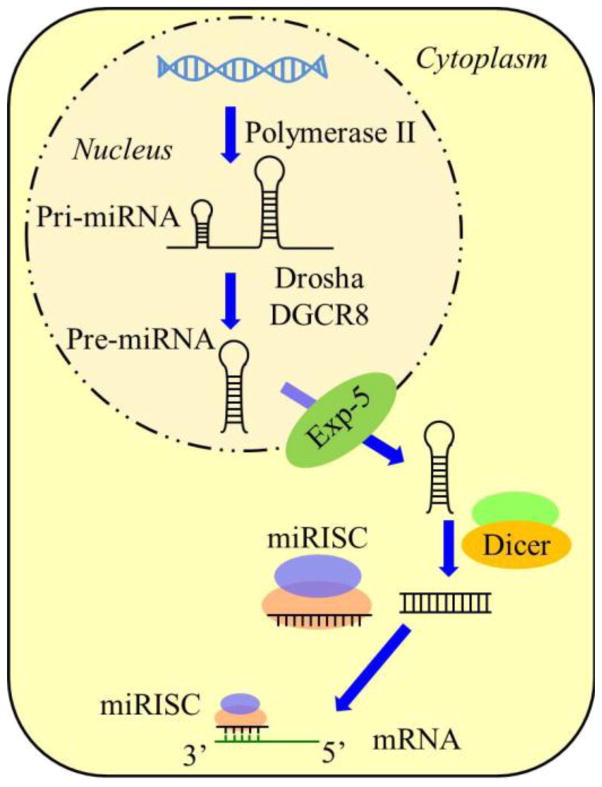
As a post-transcriptional gene regulator, miRNA is small non-coding single-strand endogenous RNA with length of 20–24 nucleotides (nt) [12]. It is derived from the precursor transcript called primary miRNA (pri-miRNA) which contains stem-loop structures with length of hundreds to thousands nt. Pri-miRNA is further processed in the nucleus by DGCR8 and ribonuclease Drosha into 70 to 100 nt long hairpin structure, called pre-miRNA [13–15]. The pre-miRNA is then translocated into the cytoplasm by a shuttle system containing the Exportin 5 and its cofactor Ran. In the cytoplasm it is further processed by Dicer, an RNase III-like enzyme, into approximately 22 nt double-strand miRNA duplex (Fig. 1) [16, 17]. As the mature miRNA strand loads onto the miRNA-induced silencing complex (miRISC), the passenger strand (also denoted as miRNA*) is released and subsequently degraded [18, 19].
Figure 1.
Schematic representation of the biogenesis of miRNA. Following loop formation, the pri-miRNA is processed by DGCR8 and Drosha into pre-miRNA. The pre-miRNA is exported from the nucleus by Exportin 5 before maturation by Dicer. Then the miRNA duplex is unwound and the mature strand remains within the miRISC complex which further binds to the 3′ untranslated region of mRNA.
The mature miRNA strand in the miRISC can recognize the target mRNA by binding of the seed sequence (position 2 to 8 from the 5′ end) of the mature miRNA strand to the 3′ untranslated region of mRNA. This specific interaction generally results in deadenylation, decapping, translation inhibition and eventually degradation of the target mRNA [20, 21]. However, in rare cases, the translation could be promoted rather than repressed by the miRNA [22]. Unlike the short interfering RNA (siRNA), complementarity between miRNA and mRNA does not require perfect pairing, which means a single miRNA could simultaneously recognize hundreds of target mRNAs and an mRNA could be regulated by multiple miRNAs [23]. The complex miRNA regulation network has profound impact on the expression of an abundance of proteins, and eventually important ramifications on many developmental and cellular processes.
Recently, the identification of stem-cell-specific miRNA has brought the stem cell and miRNA fields together [6, 7, 24]. It suggests that miRNA therapy could impact regenerative medicine. A great obstacle to the progression of miRNA therapy is delivery of miRNA to the desired cell types, tissues or organs. Because of the negative charge of miRNA, it cannot penetrate the cellular membrane by passive endocytosis. Furthermore, rapid clearance of naked miRNA in the blood stream renders its systemic delivery highly inefficient. So the successful application of miRNA largely depends on the development of effective delivery platforms [25]. The aim of this review is to capture the state-of-art of miRNA delivery systems and highlight their design consideration from the regenerative medicine perspective.
2. MicroRNA therapeutic approaches
It is now clear that miRNA plays an important role in directing stem cell fate by fine-tuning the levels of various factors. Ex vivo manipulation of the miRNA level in stem cells is a viable strategy for regenerative applications. Depending on the expression status of the target miRNA, the miRNA therapy could be separated into miRNA replacement therapy which up-regulates miRNA expression and miRNA inhibition therapy which down-regulates miRNA expression.
2.1. miRNA replacement therapy
The levels of one or more miRNAs of stem cells will change significantly during differentiation. Overexpression of the target miRNA would be a viable strategy to enhance this differentiation process. The miRNA replacement therapy could be performed in two ways. The first one is delivery of miRNA mimics that are double-strand oligonucleotides containing the same sequence as the mature endogenous miRNA. Because the miRNA mimics possess the same structure with the miRNA duplex, they enter the miRISC complex and affect the target mRNA [26]. Although single-strand RNA molecules could also work as miRNA mimics, the high potency of double-strand miRNA mimics (100 to 1000 fold higher than single-strand miRNA mimics) [27] makes them much better candidates.
However, subtle difference in physico-chemical properties between DNA and RNA renders the optimization of miRNA delivery not so straightforward; one cannot assume that an efficient DNA delivery system is also efficient for miRNA delivery. The persistence length of DNA is about 50 nm [28], making a typical pDNA of several Kb a flexible molecule amenable to condensation by polycations. In contrast, the persistence length of RNA is about 70 nm (260 nt) [29], and it is also a stiffer molecule more resistant to efficient condensation. So just like siRNA polyplexes, miRNA polyplexes tend to be larger and have fewer options of cellular uptake; for instance, they are not typically internalized via the clatherin-mediated endocytic pathway [30–32].
The second mode of miRNA activation is delivery of synthetic miRNA precursor mimics or miRNA-expressing DNAs which could be incorporated into the viral vectors [33]. This strategy possesses the advantage of sustained generation of miRNA, which is especially important for regeneration application. However, since pri-miRNA is processed in the nucleus, nuclear targeting is required for delivery of pri-miRNA. On the other hand, delivery of pri-miRNA might saturate the RNA machinery, resulting in off-target effects that are undesirable [34].
2.2. miRNA inhibition therapy
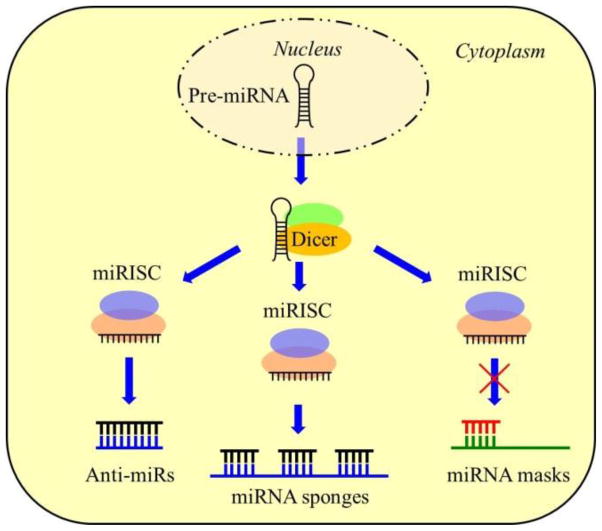
In contrast to the miRNA replacement therapy, the miRNA inhibition therapy aims to block the miRNA repression of protein expression. Several methods have been developed to inhibit miRNA functions through disruption of the miRISC complex (Fig. 2). The most straightforward method is to use anti-miRNA oligonucleotide (AMO), which is complementary to the miRNA mature strand, to inhibit interactions between miRISC and its target mRNA. To achieve effective inhibition, several independent chemical modifications have been adopted to improve the affinity and stability of AMOs [35, 36].
Figure 2.
Strategies of miRNA inhibition therapy. Anti-miRs contain sequences that are complementary to the miRNA mature strands and act as competitive inhibitors. MiRNA sponges are DNA sequences with multiple binding sites to the miRNA. They could inhibit a panel of miRNAs. MiRNA masks could selectively block specific mRNA pathway.
AntagomiRs are the first miRNA inhibitors demonstrated to work in mammals [37]. They contain 2′-O-methyl-modified ribose sugars (2′-OMe), a terminal phosphorothioate linkage instead of a natural phosphate linkage, and a cholesterol group at the 3′ end. Although AntagomiRs have shown higher stability than AMOs and effective inhibition of specific miRNA in several tissues, the high dose required for effective inhibition hinders their application.
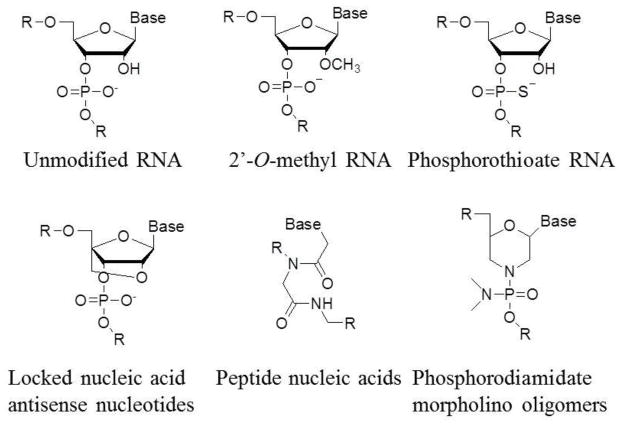
Locked nucleic acid (LNA) antisense nucleotides are modified with an extra bridge connecting the 2′ oxygen and 4′ carbon (Fig. 3) [38]. The locked ribose conformation increases the resistance to many endonucleases and the RNA melting temperature significantly. Lower dose of LNA-anti-miRs required for miRNA inhibition makes them more suitable for regenerative medicine applications.
Figure 3.
Chemical modifications of AMOs. 2′-O-methyl RNA is modified by changing the 2′ hydroxy group of the ribose into methoxy group; phosphorothioate RNA introduces the sulfur substitution of a non-bridging oxygen to make a phosphorothioate linkage between nucleotides; locked nucleic acid links 2′, 4′ of the ribose by methylene bridge to form a bicyclic nucleotide; peptide nucleic acids (PNAs) substitute a peptide bond at the 1′ amide linkage to the base. Phosphorodiamidate morpholino oligomers (PMOs) contain morpholine rings instead of deoxyribose rings that are linked through phosphorodiamidate groups instead of phosphates.
Peptide nucleic acids (PNAs) are synthetic polymers with a structure similar to that of nucleic acids and function like nucleic acids with high specificity, and additional benefit of stability [39, 40]. Different from RNA, the backbone of PNA is N-(2-aminoethyl)-glycine. Because of the non-charge nature of PNAs, transfection agents are usually not required for delivery of PNAs. Cell penetrating peptides can be linked with PNAs to enhance their delivery using standard peptide chemistry.
MiRNA sponges are DNA sequences with multiple binding sites to the miRNA. They are usually expressed by plasmid DNAs encoding the miRNA sponges to saturate the miRISC complex. Different miRNA binding sites could be introduced into miRNA sponges to inhibit a family of miRNAs. Viral vectors could be used to deliver miRNA sponges to a number of hard-to-transfect cells. However, before applying miRNA sponges clinically, the expression of transcripts must be precisely controlled to avoid the unwanted side effects [41, 42].
The miRNA inhibition strategies mentioned above always sequester all the functions of target miRNA. Because a single miRNA could regulate expression of many genes, this inhibition strategy may lead to unwanted side effects. So miRNA masks are developed to minimize the off-target effects. MiRNA masks are designed to selectively block specific mRNA pathway, consequently only the specific protein expression is inhibited. But the clear profile of miRNA target genes is required.
3. Therapeutic prospect of microRNA in regenerative medicine
Since the discovery of microRNAs (lin-4 and let-7) playing a crucial role in the development of C. elegans, their role in the mammalian system has been expanding into development, metabolism, proliferation, and apoptosis [43]. More importantly, microRNA plays an important role in the differentiation and proliferation of stem cells. Unlike siRNA, miRNA is an endogenous RNA. So either activation or inhibition strategy can be used to up-regulate or down-regulate miRNA expression during the development and differentiation process to achieve therapeutic effects.
3.1 MiRNA in bone regeneration
There are two fundamentally distinct processes for bone formation. Direct differentiation of mesenchymal stem cells (MSCs) into osteoblasts produces intramembranous bones such as calvarial bone. Differentiaton of MSCs toward chondrocytes via endochondral ossification produces a cartilaginous template, which contributes to the longitudinal growth of long bones [44]. Depletion of miRNAs by conditional deletion of the miRNA-processing endoribonuclease Dicer enzyme reveals the role of miRNAs in these two processes [45, 46].
MiRNAs are involved in multiple pathways for promoting osteoblast differentiation [47]. Runx2 is a transcription factor essential for osteoblastogenesis. The miRNA cluster 23a~27a~24–2 could repress the osteoblast differentiation by targeting the SATB2 protein, a co-regulator of Runx2. Conversely, Runx2 could inhibit expression of those miRNAs by a feedforward mechanism to de-repress SATB2, resulting in promotion of osteoblast differentiation [48]. However, miR-27a in the cluster could downregulate Hoxa10, an activator of Runx2. It further results in repression of Runx2. During the mineralization of bone formation, miR-23a could repress Runx2 to attenuate osteoblast maturation. A second miRNA regulatory loop is induced by the BMP2-Runx2 pathway. In the BMP2-induced osteogenesis of ST2 stromal cells, Runx2 increases the expression of miR-2861~miR-3960 cluster by binding to their promoter. MiR-2861 and miR-3960 maximize Runx2 activity to drive differentiation by targeting two Runx2 inhibitors, Hdac5 and Hoxa2, respectively [49]. Thus, fine-tuning the miRNA levels spatially and temporally could be a delicate way for bone regeneration. To illustrate the importance of miRNA in osteogenesis, introduction or inhibition of several miRNAs including miR-31 [50], miR-26a [51, 52] and miR-29b [52] for calvarial defect repair has been evaluated in vivo.
In addition to intramembranous bone formation, miRNA also participates in the endochondral ossification process [53]. In vitro studies have identified several miRNAs related to the MSC chondrogenic differentiation, including negative regulators miR-145 and miR-449a [54, 55] and positive regulator miR-23b [56]. MiR-449a could repress expression of SOX9, the essential transcription factor for chondrogenesis, leading to delayed progression of chondrogenesis while positive regulator could promote chondrogenic differentiation of human MSCs by inhibiting protein kinase A (PKA) signaling [55].
Expression of miR-140 is predominantly limited to cartilage [57]. The positive function of miR-140 in craniofacial development and endochondral bone formation has been proved both in mouse and zebrafish models [58, 59]. MiR-140 represses two negative effectors including histone deactylase 4 (HDAC4; a known inhibitor of chondrocyte hypertrophy) and a splicing factor which reduces BMP signaling [59].
3.2 MiRNA in wound healing
The wound healing process in adults is a highly orchestrated physiological process involving keratinocytes, fibroblasts, endothelial cells, macrophages and platelets [60]. It is regulated by a complex signaling network of molecules including several miRNAs [61]. It can be divided into three overlapping phases: inflammation, proliferation and remodeling. The migration, proliferation and differentiation of keratinocytes in the proliferation phase are the key steps in wound healing. The keratinocytes fill in the gap created by the wound and restore the integrity of the skin [62]. Inhibition of miR-198, which could switch off the expression of follistatin-like 1 protein, has proved useful to enhance the keratinocyte migration and proliferation [63]. Due to the complexity of wound healing process, simply activation or inhibition of miRNA expression may not be enough. For instance, although miR-21 could promote keratinocyte migration and re-epithelialization [64], overexpression of miR-21 would also induce inhibition of epithelialization and granulation tissue formation in a rat wound model [65].
The maturation phase is important for wound contraction and scar formation. It is characterized by ECM adjustment, remodeling of collagen from type III to type I, and the replacement of granulation tissue by scar tissue. MiR-29b is a positive effector in this process by directly targeting ECM genes, such as fibronectin, collagen type I, and collagen type III. Topical application of miR-29b to mouse wound has proved effective to improve the collagen type III/I ratio, increase the activity of matrix metalloproteinase 8, and enhance scarless wound healing [66].
3.3. MiRNA in skeletal muscle and cardiac regeneration
Although skeletal muscle has a remarkable capacity to respond to pathological stress and regenerate upon injury, severe traumatic injuries associated with significant muscle tissue loss and gaps could not be repaired endogenously, such as volumetric muscle loss (VML) injuries. Similar to the wound healing process, skeletal muscle repair can also be roughly divided into three stages: demolition stage, repair stage and remodeling stage [67]. During this process, satellite cells (resident mononuclear stem cells) serve as the dominant contributor to muscle repair [68]. In vivo study establishes that miR-206 promotes satellite cell differentiation during skeletal muscle regeneration in response to injury [69], via a mechanism of repressing a set of negative regulators including Pax7, Notch3, and Igfbp5 [70]. Besides, overexpression of miR-206 could upregulate the expression of various growth factors by inhibition of connexin 43 (CX43) and histone deacetylase 4 (HDAC4) [71]. MiR-26a also promotes differentiation of myoblasts [72]. It targets the transcription factors Smad1 and Smad4, which are critical for the TGF-b/BMP pathway. Inhibition of miR-26a after injury could prevent myogenesis and delay muscle regeneration [73].
Unlike skeletal muscle, repair of cardiac damage through myocardial regeneration is limited because of the low proliferative rate of cardiomyocytes during adult life [74]. MiRNA can activate cardiomyocyte proliferation and promote mammalian cardiac repair in vivo [75–77]. The miR-15 family, including miR-195, miR-15a, miR-15b, miR-16 and miR-497, plays key role of postnatal cardiomyocyte mitotic arrest by targeting multiple cell cycle regulators, including the checkpoint kinase 1 (Chek1) [78]. Through large scale screening of miRNA, miR-590 and miR-199a have been identified as the promotors of cardiomyocyte proliferation in both neonatal and adult animals [79]. They also show the ability to stimulate remarkable cardiac regeneration in the murine myocardial infarction model.
Another way to repair cardiac damage is to stimulate the differentiation of cardiac stem cells (CSCs) into cardiomyocyte [75]. MiR-499 is reported as a negative regulator of Sox6 and Rod1 and promotor of CSC differentiation [80, 81]. Most importantly, miR-499-overexpressed hCSCs show enhanced cardiac differentiation and regeneration abilities. Injection of these cells into the infarcted mouse heart leads to a 2.1-fold greater regenerated myocyte mass and better preservation of the left ventricular (LV) function than injection of control hCSCs.
3.4. MiRNA in angiogenesis
Angiogenesis is one of the most important parameters to optimize in tissue engineering such as bone repair and wound healing [52, 82]. During angiogenesis, endothelial cells are induced to proliferate, migrate out of an existing vessel, differentiate, and assemble to form branches of tubules capable of carrying blood and its constituents. Mice with endothelial-selective Dicer inactivation have an impaired angiogenic response to limb ischemia, proving that miRNAs are important for angiogenesis [83–85]. Overexpression of miR-503 in endothelial cells results in impaired cell proliferation, migration, and ability to form networks by targeting the cell division cycle 25 homolog A and cyclin E1 [86]. Corresponding in vivo study shows that local miR-503 inhibition could correct diabetes mellitus-induced impairment of post-ischemic angiogenesis and recover the blood flow in diabetic mice. Cardiac endothelial cells enriched miR-24 could inhibit transcription factor GATA2 and the p21-activated kinase PAK4, resulting in endothelial cell apoptosis, impaired endothelial capillary network formation, and cell sprouting [87]. Blocking of endothelial miR-24 leads to improved myocardial angiogenesis and cardiac function in the mouse myocardial infarction model [88].
Due to the pathogenic relationship between vascularity and tumor, the use of miRNA to promote angiogenesis might increase the risk of oncogenesis [89]. For instacne, the miR-17–92 cluster (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92–1) improves angiogenesis both in vitro and in vivo [90]. However, this cluster has also been identified as tumor-promoting miRNA oncomiR-1. Therefore, the use of miRNA for angiogenesis must be judiciously applied [91].
3.5. MiRNA in neurogenesis
The poor regenerative potential of the nervous system has stimulated various research strategies to induce neuronal regeneration using reprogrammed neuronal cells. The main obstacle in neuronal reprogramming is the low neuroblasts differentiation yield of adult stem cells. MiRNA plays essential roles in neurogenesis [92–94], particularly the ones (miR-9, -124, -128, -132, -134, -138) that are specifically or richly expressed in the brain and spinal cord [95]. Among them, miR-124 and miR-9/9* could induce direct conversion of fibroblasts into neuronal-like cells [96–98]. Upon neuronal differentiation, BAF53a (subunit composition of ATP-dependent BAF chromatin remodeling complex) is replaced by its homolog BAF53b. Ectopic expression of miR-124 and miR-9* induces the down-regulation of BAF53a, allowing the incorporation of BAF53b and neurogenesis. MiR-124 could also inhibit small carboxy-terminal domain phosphatase 1 (SCP1; also known as CTDSP1 [99], a component of the RE1-silencing transcription factor (REST)) and polypyrimidine tract binding protein 1 (PTBP1; a repressor of neuron-specific splicing) [100] while miR-9 targets TLX, a highly conserved orphan nuclear receptor that is critical for neural stem cell self-renewal [101]. Notch signaling is one of the key pathways regulating neuronal development and expansion of neural progenitors. MiR-124 also targets several components of the Notch signaling cascade including Notch ligand Jag1 [102] and the Notch down-stream effector Sox9 [103] to increase neurogenesis. In addition, miR-9 regulates the Hes gene family members [104].
Repair of the central nervous system is much more challenging than that of the peripheral nervous system. It is largely due to the failure of remyelination by oligodendrocytes, which do not proliferate in response to injury [105]. Microarray analyses have identified a series of microRNAs that are developmentally regulated during maturation of oligodendrocytes [106]. MiR-219 and miR-338 discovered in this study promote oligodendrocyte differentiation by repressing transcription factors Sox6 and Hes5, the antagonists of oligodendrocyte maturation [107].
4. MicroRNA delivery systems for regenerative medicine
It is now widely recognized that effective delivery system is the key to miRNA development. Primary stem cells are more difficult to transfect than immortalized cell lines, therefore requiring a more efficient miRNA delivery system for regenerative medicine applications. Tissue regeneration or development can also be a lengthy process. Besides the obvious requirements of low cytotoxicity and high transfection efficiency, an ideal miRNA delivery system for regenerative medicine should be able to deliver the miRNA at specific tissue or organ in a local and sustained manner.
In the context of this review, miRNA delivery systems could be simply divided into systemic or scaffold-mediated delivery. Systemic or bolus gene delivery systems for DNA or siRNA delivery have been well studied and expertly reviewed in the past two decades [34, 113–115]. Numerous viral and non-viral vectors with high transfection efficiency, good biocompatibility, and even high targeting efficiency have been developed. Those vectors might be directly adaptable for miRNA delivery. However, despite the promise of systematic delivery systems they are intended mostly for cancer therapy or genetic diseases [26, 27, 116], and may not meet the requirement of high and sustained miRNA levels for regenerative medicine applications.
Scaffold-mediated delivery by encapsulating or immobilizing the miRNA in or onto the tissue engineering scaffold addresses such requirement for regenerative medicine applications. Scaffold-mediated delivery offers a three-dimensional (3D) distribution of miRNA at target tissue for more controlled, localized transfection. Furthermore, the miRNA could be released in accordance with the degradation of the scaffold to achieve a transgene expression kinetics matching the profile of tissue regeneration. By carefully choosing the scaffold fabrication and sterilization process, loss of miRNA activity could also be minimized. It is an emerging approach with limited report, but the promise is stimulating chemical, biochemical, and mechanical innovations to design miRNA-functionalized scaffolds for tissue regeneration.
4.1. Systemic miRNA delivery systems
4.1.1. Viral vectors
Various viral vectors can now transfect the majority of cell types [116, 117]. The major drawback of miRNA is its relative short half-life due to the susceptibility to degradation by RNase. They only exert a transient silencing effect because miRNA concentration decreases with cell division, although gene construct design can mitigate this transient effect. The high transduction efficiency and sustained transgene expression of viral vectors render them attractive for miRNA delivery both in vitro and in vivo (Table 2). Safety issues such as potential immunogenicity and insertional mutagenesis remain the main barriers for clinical translation of viral vectors [116, 118].
Table 2.
Representative viral miRNA delivery vectors for regenerative medicine
| Viral vectors | Target miRNA | Cell type | Therapeutic approaches | MiRNA level variation | Potential application | Ref. |
|---|---|---|---|---|---|---|
| Adenovirus | MiR-375 | Alveolar epithelial cell | Replacement | ~17 fold increase | Lung recovery | [135] |
| Adeno-assoc iated virus | MiR-26a | Myoblast | Inhibition | About 75% knockdown | Myogenesis | [73] |
| Retrovirus | MiR-138 | MEFs | Replacement | >1000 fold increase | Cellular reprogramming | [123] |
| Retrovirus | MiR-205 | Hair follicle stem cells | Inhibition | Nearly 100% knockdown | Skin recovery | [136] |
| Retrovirus | MiR-106b; miR-93; miR-25 | NSPCs | Replacement | miR-106b: ~18 fold; miR-93: ~15 fold; miR-25: ~30 fold | Neurogenesis | [125] |
| Lentivirus | MiR-424 | hDPCs | Inhibition | 70% knock down | Osteogenesis | [128] |
| Lentivirus | MiR-31 | Bone marrow stromal stem cells | Inhibition | ~90% knock down | Osteogenesis | [51] |
| Lentivirus | MiR-346 | hBMSCs | Replacement | 5.5 fold increase | Osteogenesis | [137] |
| Lentivirus | MiR-153; miR-181a/a*; miR-324-5p/3p | Human neural stem cell | Replacement | 10~300 fold increase | Neurogenesis | [138] |
| Lentivirus | MiR-1 | MSCs | Replacement | ~300 fold increase | Cardiogenesis | [129] |
| Lentivirus | MiR-143; miR-145 | Corneal epithelial progenitor cells | Replacement | MiR-143: 2487 fold increase; miR-145: 876 fold increase | Vasculogenesis | [139] |
| Baculovirus | MiR-26a; miR-29b; miR-148b;miR-196a | hASCs | Replacement | miR-26a: ~5 fold; miR-29b: ~5 fold; miR-148b: ~8 fold increase; miR-196a: ~3 fold increase | Osteogenesis | [134] |
Adeno-associated virus (AAV) is a non-enveloped virus from the Parvoviridae family with single-strand DNA genomes of 4.7 kb. To date, 12 primate serotypes (AAV1-12) have been identified [119]. AAV vectors are the smallest known viral vectors. However, they are ideal for the delivery of miRNA because of smaller size of miRNA compared to pDNA. Furthermore, their non-pathogenicity in humans is attractive. Because rAAV9 vector has shown high affinity for myocardium, it is frequently used for delivering miRNA-related therapeutic molecules to the heart [120]. Mauro Giacca et al. [79] used rAAV9 to deliver miR-590 and miR-199a effectively to the heart of neonatal mice by both intraperitoneal and intracardiac injection. The former resulted in ~240-fold increase of miR-590 expression and 3.5-fold increase of miR-199a expression comparing to control rAAV9 vector. The latter was even more effective, which led to over 1000-fold increase of miR-590 expression and 13-fold increase of miR-199a expression. One single intracardiac injection of rAAV9-miR-590 or rAAV9-miR-199a could boost the normally ineffective myocardial repair and restore the cardiac function. The left ventricular ejection fraction (LVEF) increased from 38% to 58% (rAAV9-miR-590) or 52% (rAAV9-miR-199a), fractional shortening (LVFS) increased from 19% to 31% (rAAV9-miR-590) or 26% (rAAV9-miR-199a), and end-systolic anterior wall thickness (LVAW) increased from 0.75 mm to 1.12 mm (rAAV9-miR-590) or 0.95 mm (rAAV9-miR-199a) after 60 days. The infarct size also decreased from 32% LV to 14% LV (rAAV9-miR-590) or 13% LV (rAAV9-miR-199a) after 60 days.
Retroviruses are enveloped viruses that carry two copies of the single-strand RNA genome of ~10 kb. Upon cell entry, the RNA is copied by the reverse transcriptase enzyme into double-strand DNA. It stably integrates into one of the host chromosomes, guaranteeing long-term expression of inserted therapeutic genes. Viral protein synthesis is not required for retroviral entry and genome integration, therefore all viral genes can be replaced with foreign sequences [121, 122].
Retrovirus vectors are frequently used for cellular reprogramming because of their ability to simultaneously deliver four full-length genes (i.e., either Oct4-Sox2-c-Myc-Klf4 or Oct4-Sox2-Nanog-Lin28) into targeted somatic cells. So they have also been used to deliver miRNA with or without those genes to generate induced pluripotent stem cells (iPSCs). Using a retrovirus vector as a carrier, Ye et al. [123] delivered miR-138 together with Oct4, Sox2, and Klf4 (OSK) to OG-mouse embryonic fibroblast (MEFs) to promote the iPSCs generation. The delivery of miR-138 reduced the level of p53 by 60% and promoted the reprogramming with 3 fold higher efficiency than using OSK alone. Moreover, single miRNA delivery by retrovirus vector may be enough to achieve cellular reprogramming. For instance, human embryo stem cell-like pluripotent stem cells can be generated by retroviral delivery of miR-302s into human cancer cell lines including Colo and PC3 cells at extremely high transfection efficiency (~100%) [124]. Retrovirus-based constructs can also deliver multiple miRNAs. To study the importance of miRNA gene cluster miR-106b~25 (miR-106b, miR-93, and miR-25) in neurogenesis, researchers delivered a retrovirus vector encoding the cluster to neural stem/progenitor cells (NSPCs) [125]. As a result, the expression of miR-106b, miR-93, and miR-25 was increased by 16, 11, and 30 fold, respectively, compared with empty retrovirus. In addition, Tuj1-positive (a marker of neurons) cells in the NSPCs infected by miR-106b~25 retroviruses were increased by 2.6 fold comparing with the NSPCs infected by empty retrovirus, indicating that delivery of miR-106b~25 could enhance neurogenesis effectively.
Lentivirus forms a subgroup of retrovirus that can integrate the exogenous gene into the host genome [126], enabling a stable miRNA expression and a long-term silencing effect. Unlike retroviruses, lentiviruses favor integration within introns of active transcriptional units, which limits their potential to cause insertional oncogenesis [127][66], so they are more frequently used in miRNA delivery for regenerative medicine research in recent years.
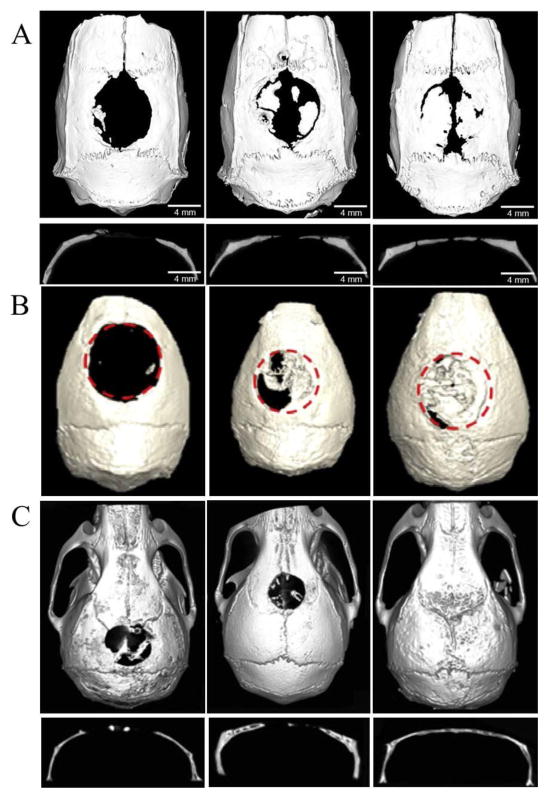
To explore whether miR-424 could regulate the differentiation of human dental pulp cells (hDPCs) into vascular lineage, both miR-424 and anti-miR-424 were delivered into hDPCs by lentivirus vector with over 70% transfection efficiency [128]. As a result, the miR-424 level was elevated more than 3 fold in miR-424 overexpression cells relative to empty vector-transfected cells and reduced by nearly 70% in miR-424 knockdown cells, respectively. The protein expression of endothelial markers (vWF and KDR) was enhanced by miR-424 knockdown. This study proves that miR-424 plays a negative role in regulating endothelial differentiation of hDPCs; consequently the inhibition of miR-424 expression in hDPCs promotes endothelial differentiation. Transfection of mesenchymal stem cells (MSCs) was also achieved by miR-1 lentiviral vector with efficiency over 90% [129]. Overexpression of miR-1 inhibited the Hes-1 by about 25% at day 1, over 50% at day 7 and over 60% at day 14, resulting in promoted differentiation of MSCs into the cardiac lineage. Lentivirus vectors were also used to deliver anti-miR-31 into adipose tissue-derived stem cells (ASCs) [130] or bone marrow stem cells (BMSCs) [50] for bone regeneration. The inhibition of miR-31 in BMSCs increased the expression of Runx2, Opn and Ocn nearly 3 fold from day 4 to 21, which indicated osteogenic differentiation [50]. Then the poly(glycerol sebacate) (PGS) scaffold seeded with transduced BMSCs was implanted into the 8 mm critical-sized calvarial defects in rats for bone regeneration. Micro-CT showed that knockdown of miR-31 could increase new bone formation; the ratio of new bone volume relative to the tissue volume (BV/TV) was significantly higher than the control group (22.18±3.39 % vs 41.82 ±6.54 %) at week 8 (Fig. 4A).
Figure 4.
Bone regeneration through different miRNA treatment. (A) Coronal and sagittal views of the harvested skulls by micro-CT at week 8 post-implantation (left: PGS scaffold, middle: anti-miR-negative/BMSCs/PGS, right: anti-miR-31/BMSCs/PGS). Adapted and reprinted with permission from Ref. [50]. (B) Micro-CT of nude mice at week 12 after surgery. (left: hASCs/scaffolds, middle: Bac-FLPo/Bac-FCBW/hASCs/scaffolds, right: Bac-FLPo/Bac-FCBW/Bac-miR-148b/hASCs/scaffolds). Adapted and reprinted with permission from Ref. [51]. (C) Micro-CT of live mice at week 12 after surgery. (left: BMSCs/hydrogel, middle: miR-negative/BMSCs/hydrogel, right: miR-26a/BMSCs/hydrogel). Adapted and reprinted with permission from Ref. [52].
Besides the common viral vectors, baculoviral vector is being increasingly investigated among various viral gene delivery systems. Baculoviruses are insect viruses in nature that can transduce a wide variety of stem cells including ASCs and BMSCs at high efficiency [131]. Unlike retroviruses, baculoviruses neither replicate inside the transduced mammalian cells nor integrate into host chromosomes. Importantly, baculoviruses are non-pathogenic to humans and baculoviral DNAs degrade in the mammalian cells over time [132]. Although rarely reported as miRNA carriers, the high transfection efficiency and low toxicity of baculoviruses would render them promising miRNA vectors for regenerative medicine [133, 134]. Liao et al [51] transduced human adipose derived stem cells (hASCs) with baculovirus vectors expressing human miR-26a, miR-29b, miR-148b and miR-196a respectively to stimulate the osteogenesis, in which mature miRNA level of miR-26a, miR-29b, miR-148b and miR-196a increased 5 fold, 4.8 fold, 7.9 fold and 3.4 fold at day 1. However, Bac-miR148b and Bac-miR196a induced more calcium deposition and higher expression of osteopontin at day 14, revealing that miR-148b and miR-196a expression triggered more potent osteogenesis than miR-26a and miR-29b. Implantation of the hASCs transfected with miR-148b-expressing and bone morphogenetic protein 2 (BMP-2)-expressing baculovirus vectors (Bac-FLPo/Bac-FCBW) into critical-sized (4 mm in diameter) calvarial bone defects in nude mice accelerated and potentiated the bone healing and remodeling, filling ~94% of defect area and ~89% of defect volume of native calvaria-like flat bone in 12 weeks (Fig. 4B). This study shows that baculoviruses can deliver miRNA into stem cells efficiently in vitro.
4.1.2. Lipid-based delivery systems
Lipid-based systems are among the most commonly used non-viral vectors in vitro [35, 140]. In comparison to viral transduction, nonviral delivery systems such as lipid-based (lipoplex) and polymer-based (polyplex) systems impose a lower degree of genetic perturbation and may be more beneficial to the clinical application of stem cell differentiation. Many cationic lipid-based systems can be selected from a number of commercially available products (Table 3), including Lipofectamine® [139, 141, 142], siPORT™ [143], Oligofectamine™ [144], MaxSuppressor™ In Vivo RNA-LANCEr II [76], HiPerFect [77], Lullaby [145], DharmaFECT® [146], SilentFect™ [101] and NeuroPorter™ [147]. Many investigations have validated the possible delivery of miRNA by lipoplexes. However, safe and efficacious delivery in vivo has yet to be achieved due to toxicity and nonspecific uptake.
Table 3.
Representative lipid-based miRNA delivery systems for regenerative medicine
| Transfection reagents | Target miRNA | Cell type | Therapeutic approaches | MiRNA level variation | Potential application | Ref. |
|---|---|---|---|---|---|---|
| HiPerFect | MiR-146b-5 p; miR-23b; miR-99a | Human neural stem cells | Replacement | MiR-146b-5p: ~65 fold increase; miR-23b: 30 fold increase; miR-99a: 25 fold increase | Neurogenesis | [149] |
| DharmaFECT® | MiR-198 | keratinocytes | Replacement | >3 fold increase | Promoting wound healing | [63] |
| SiPORT™ NeoFX™ | MiR-21 | MSCs | Replacement | ~600 fold increase | Osteogenesis | [150] |
| SiPORT™ NeoFX™ | MiR-335-5p | Primary calvarial osteoblasts | Replacement | Transfection efficiency ~40% | Osteogenesis | [151] |
| SiPORT™ NeoFX™ | MiR-17 | human periodontal ligament tissue-derived mesenchymal stem cells | Replacement | >4000 fold increase | Osteogenesis | [152] |
| SiPORT™ NeoFX™ | MiR-1; miR-499 | human cardiomyocyte progenitor cells | Replacement | miR-1: increase to 8 ΔΔ Ct; miR-499: increase to 12 ΔΔCt | Cardiomyogenesis | [153] |
| Lipofectamine® 2000 | MiR-1 | ESC | Inhibition | ~90% down regulation of ESC differentiation | Osteogenesis | [154] |
| Lipofectamine® 2000 | MiR-375 | 3T3-L1 adipocyte | Inhibition | ~80% knockdown | Adipogenesis | [155] |
| Lipofectamine® RNAi-MAX | MiR-720 | hDPCs | Replacement | ~180 fold increase | Osteogenesis | [148] |
| Lipofectamine® RNAiMAX | MiR-682 | Myogenic progenitor cell | Inhibition | ~50% knockdown | Myogenesis | [156] |
Sundaram et al. [63] used DharmaFECT® to deliver miR-198 mimics to the keratinocytes. Delayed keratinocyte immigration and scratch-wound closure were observed, indicating the negative role of miR-198 in the wound healing process. Consequently, inhibition of miR-198 might offer a different strategy to improve non-healing chronic diabetic ulcers. Commercialized lipid-based transfection reagents have also been used to transfect stem cells. Gain- and loss-of-function studies were performed by transfecting dental pulp cells with miR-720 mimics and miR-720 inhibitor using Lipofectamine® RNAi-MAX [148], resulting in about 50% decrease of NANOG mRNA level and promoting odontogenic differentiation.
4.1.3. Polymer-based delivery systems
Numerous cationic polymers have been synthesized for DNA and/or siRNA delivery for different therapeutic purposes [157, 158]. Surprisingly, polymer-mediated miRNA delivery is rarely reported, especially in the field of regenerative medicine. Compared with lipoplexes, polyplexes enjoy a higher degree of versatility via variation in molecular weight, molecular structure, and composition. Development of controlled polymerization such as atom transfer radical polymerization (ATRP), reversible addition-fragmentation chain transfer polymerization (RAFT) and ring-opening polymerization (ROP) offers a powerful tool to synthesize gene carriers with controlled size, well-defined structure and diverse functionalities. For instance, stimuli-sensitive moities such as pH-sensitive, redox-sensitive, or enzyme-sensitive linkages can be incorporated into the gene carrier to facilitate site-specific degradation or intracellular unpacking [158]. A wide choice of functional groups also simplifies ligand conjugation for tissue- and cell-specific delivery [39].
Polyplexes rely on electrostatic interaction between the polycation and the nucleic acid to form a nanocomplex. Polyethylenimine (PEI) is one of the most widely used and studied polycations for gene delivery [159]. The ‘proton sponge’ effect of PEI causes influx of protons and water into the endosome, which could swell and eventually disrupt the endosome to release the polyplexes to cytoplasm. However, high cytotoxicity prevents its clinical application. A promising modification to lower the cytotoxicity is to crosslink low molecular weight PEI through a disulfide linkage [158, 160]. Further introduction of rabies virus glycoprotein (RVG) enabled the carrier to specifically target to the nicotinic acetylcholine receptors on neuronal cells [158]. In vitro delivery of miR-124a, a neuron-specific miRNA that can potentially promote neurogenesis, to Neuro2a cells, revealed efficient intracellular uptake as early as 1 h after transfection and 90% knockdown of luciferase reporter activity. For in vivo studies, mannitol was used to facilitate transport across the blood-brain barrier and overcome the size limitation of PEI vector. Analysis of fluorescence signals revealed considerable accumulation of Cy5.5-miRNA in the brain, which represented a promising delivery system for in vivo neurogenesis, although the functional activity of miR-124a to promote neurogenesis remained to be investigated.
Poly(lactide-co-glycolide) (PLGA) is a biodegradable, FDA-approved copolyester that has been widely used for drug delivery. It can encapsulate miRNA by physical entrapment. The ability to protect nucleic acids from degradation and prolonged release render them attractive for regenerative medicine application. Surface modification of PLGA nanoparticles such as pegylation or ligand decoration provides further options to control their pharmacodynamics. Saltzman et al. [161] showed that PLGA nanoparticles formed by double emulsion could load modified miRNA inhibitor with a neutral backbone (PMO and PNA) with high efficiency while the encapsulate efficiency of ssDNA or DNA-cation polyplexes by the PLGA nanoparticles was quite low. Modification of the PLGA nanoparticles with cell-penetrating peptides could significantly improve their intracellular uptake efficiency. In vitro study showed that delivery of anti-miR-155 PNAs and PMOs to KB cells could achieve ~60% knockdown. In vivo study further demonstrated that PLGA nanoparticles encapsulating anti-miR-155 PNA could accumulate in the pre-B-cell tumors through enhanced permeability and retention (EPR) effect [39]. After treatment with 1.5 mg/kg anti-miR-155 PNA loaded in PLGA nanoparticles for 5 days, the pre-B cell tumors had an approximately 50% decrease in growth relative to the control.
Other than synthetic polymers such as PEI and PLGA, natural polymers including peptides and polysaccharides have also been reported as miRNA carriers. Chitosan (CS) is one of the most reported natural polymeric gene carriers [162–164]. It has beneficial qualities such as low toxicity, low immunogenicity, biodegradability, as well as pH-adjustable cationic charge density to form polyelectrolyte complexes with negatively charged nucleotides. Deng et al. [165] demonstrated that nanocomplexes formed by hyaluronic acid and chitosan (HA-CS NPs) could encapsulate not only the miR-34a mimics but also doxorubicin (DOX) effectively. Delivery of miR-34a mimics to the human breast cancer cells MDA-MB-231 resulted in more than 60% Bcl-2 knockdown. Co-delivery of DOX and miR-34a mimics led to lower IC50 than free DOX or HA-CS NPs loaded with DOX alone, indicating the synergistic effect between DOX and miR-34a on cell apoptosis. Not only triggering apoptosis, the HA-CS NPs-mediated miR-34a delivery could also inhibit the migration of breast cancer cells via targeting Notch-1 signal. In vivo study showed that the co-delivery of DOX and miR-34a could suppress the growth of MDA-MB-231 solid tumors over 25 days. This miRNA delivery system should also be effective for delivery of miRNA to stem cells.
Another type of nature-derived polymer, cell-penetrating peptide (CPP), has already been tested for stem cell transfection [166]. CPP could significantly improve cellular uptake of various therapeutic molecules both in vitro and in vivo. Suh et al. [167] reported that a non-toxic, arginine-rich CPP called low molecular weight protamine (LMWP) could effectively transfect miR-29b into hMSCs and induce osteogenic differentiation. The cell penetration nature of LMWP promoted cellular uptake as early as 0.5 h post-transfection. After 5 h of treatment, the transfection efficiency of miR-29b using LMWP was 6.5 fold higher than that using a commercialized cationic lipid: Lipofectamine® RNAi MAX. A panel of factors, including HDAC4, CTNNBIP1 and DUSP2 inhibits osteoblastic differentiation. Down-regulating these factors to 50%, 70% and 40%, respectively, by CPP-mediated delivery of miR-29b led to significant calcium deposition for up to 14 days. Not only the enhanced mineralization, but accompanied increase in osteogenic markers indicated the potency of miR-29b in enhancing osteogenesis.
4.2. Scaffold-based delivery systems
Proliferation and differentiation of cells depend on the exogenous signals including diffusible factors, extracellular matrix (ECM) proteins, cell-cell interactions, cell-substrate interactions, as well as biomechanical cues [168, 169]. An ideal regenerative medicine system should mimic the natural microenvironment and provide all those exogenous signals at the appropriate time and location to promote the functional tissue regeneration. By providing a structural support with optimized mechanical properties, surface nanotopography and surface chemistry, a scaffold plays an important role in regenerative medicine applications. However, a typical scaffold cannot provide the appropriate biochemical cues. Controlled release functions of drug and gene delivery incorporated into a scaffold design can overcome this limitation [170, 171].
4.2.1. Advantages of scaffold-based delivery systems
A major challenge of regenerative medicine is how to mimic the complex and dynamic microenvironment for tissue development. Although there are only limited reports on scaffold-based miRNA delivery, studies on using scaffolds for DNA/siRNA delivery have demonstrated the promise for regenerative medicine. First of all, gene transfer from a matrix offers more controlled, localized transfection compared with bolus delivery. By entrapping plasmid DNA in highly porous PLGA scaffold, Shea et al. showed that the pDNA released from the scaffold would be principally taken up by the surrounding cells at the implant site [172]. Secondly, delivery from scaffolds can achieve a more prolonged transgene expression over bolus delivery. By encapsulating siRNA/CPP complexes within PCLEEP fibers, Chew et al. [173] achieved sustained release of siRNA up to at least 28 days, which was 2 to 3 times longer than conventional bolus delivery. Finally, both the viral and non-viral vectors could be encapsulated into the scaffold to minimize the unwanted degradation or immune reaction [126, 174]. Immune response is the most important challenge for viral vectors. The scaffold could protect the vectors from neutralizing immune complexes and mitigate the immune response due to inflammatory cytokines released by immune cells. Sailaja et al. [175] showed that encapsulation of adenovirus vectors in alginate microspheres would greatly reduce the immune response of mice. As a result, high transfection efficiency was achieved while the bolus delivery of adenovirus vectors led to significantly reduced transgene expression in immunized animals primarily due to immune responses against the vectors. Chemical modification could also be incorporated into the scaffold to suppress the immune response. Ligands or antibodies that induce T-cell apoptosis through Fas signaling can be incorporated into the scaffold to provide local immunosuppression [174].
With all those advantages, scaffold-mediated gene delivery has been extensively reviewed previously [168, 170, 176, 177]. To date, scaffold-based miRNA delivery to stem cells remains an area of immense research opportunities. There are generally two ways to prepare scaffold-based miRNA delivery systems: one is encapsulation of miRNA into the scaffold, the other is immobilization of miRNA onto the scaffold surface.
4.2.2. Scaffold encapsulation
Among the materials used for scaffold construction, hydrogels are attractive because of their highly hydrated, tissue-like nature. By adding the miRNA before the crosslinking process, hydrogels encapsulated with miRNA could be easily prepared since the mild crosslinking condition usually would not alter the bioactivity of miRNA. Both natural and synthetic polymers could be used as hydrogels for tissue engineering with distinct advantages. Hydrogels can be fabricated ex vivo for subsequent implantation, or can be formed in situ through minimal invasive surgery. Degradation of hydrogels can be tailored to occur through a variety of mechanisms including enzymolysis and hydrolysis. The mesh size, ion exchange, and the strength of crosslinking interactions could also influence the degradation rate of hydrogels. Since the release of encapsulated miRNA occurs through a combination of hydrogel degradation and payload diffusion, long-term controlled release could be achieved through manipulating the physicochemical properties of the hydrogels.
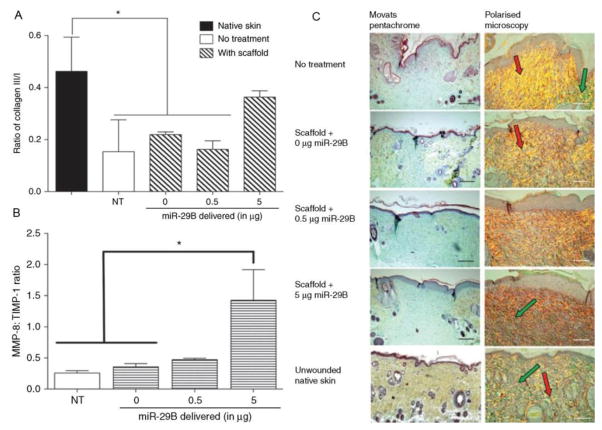
Monaghan et al. [66] used the four-arm poly(ethylene glycol)-terminated succinimidyl glutarate (4S-StarPEG) crosslinked collagen type I encapsulated with miR-29b for amelioration of the wound healing process. The release rate was tuned by the crosslink density in an inversely proportional manner. The miR-29b and collagen scaffold showed synergistic effect during the wound healing process. The collagen type III : collagen type I ratio is an important indicator of injury recovery because the ratio would decrease significantly after traumatic injury. Collagen alone could promote restoration of the natural balance between collagen type III and collagen type I. However, addition of miR-29b further pushed this ratio toward the value in normal tissues (Fig. 5). This ratio increased with time up to 14 days, suggesting the sustained release of miR-29b. In vivo study showed a dose-dependent increase of granulation tissue and decrease of wound contraction through delivery of miR-29b, indicating a better wound healing. Upon the delivery of miR-29b, expressions of a number of proteins have been regulated including the matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMPs). The increase of MMP-8 : TIMP-1 ratio was in accordance with the high collagen type III : collagen type I ratio in the wound healing process because MMP-8 would break down collagen type I.
Figure 5.
(A) Delivery of miRNA-29b increased the ratio of collagen type III-like fibers to collagen type I-like fibers within the wound bed. (B) Transfection of miRNA-29b could increase the ratio of MMP-8 to TIMP-1 in excised tissue at day 28. NT indicates an excisional wound with no treatment applied. (C) The first column showed Russel-Movat’s pentachrome staining of wound bed sections at day 28. The granulation tissue is represented by blue/cyan and the original skin collagen represented by yellow. The second column shows the polarized light images of picrosirius red-stained wound bed sections. The presence of collagen type III-like fibers could be seen as green stained fibers and the collagen type I-like fibers as yellow and red-stained fibers. Collagen type-I like fibers are highlighted with red arrows within some sections and collagen type-III like fibers are highlighted with green arrows. Scale bar = 100 μm. Adapted and reprinted with permission from Ref. [133].
Supramolecular hydrogels formed by dipeptide Gly-Ala linked with biphenyl-substituted tetrazole (Tet-GA) also have been used as matrix to entrap and deliver miRNA [178]. Tet-GA could form stable and biocompatible gel at neutral pH and entrap both miRNA and living cells in 3D manner. In comparison of 2D vs 3D configuration, transfection of HepG2 cells by naked miR-122 mimics was successful only in the hydrogel, showing ~80% transfection and a 10 fold increase of miR122 expression over the control.
Li et al. [52] used the commercialized hydrogel named HyStem-HP which contained thiol-modified HA, thiol-modified heparin, thiol-modified gelatin, and a thiol-reactive crosslinker to deliver miR-26a for bone regeneration. The authors proved that increasing endogenous miR-26a in vivo could augment bone repair through coordinating processes of endogenous angiogenesis and osteogenesis processes. Delivery of miR-26a through the hydrogel resulted in an 8-fold miR-26a level elevation compared with control at 24 h post-transfection. The level increased to 70 fold on day 11. In the in vivo study, hydrogels loaded with agomiR-26a hBMMSCs were implanted into 5-mm critical-sized calvarial bone defects in nude mice. Twelve weeks post-implantation analysis showed that the miR-26a delivery led to nearly complete repair of the defect while implantation of the control hydrogel only resulted in moderate bone regeneration (~40%) (Fig. 4C). At the same time, micro-CT angiography analysis and immunofluorescence staining for CD31 showed that vascular volume of the treated group significantly increased. The levels of vascular endothelial growth factor (VEGF) and osteogenesis gene marker Runx2 of the treated group were also higher than that of the control group. These results confirmed that sustained delivery of miR-26a not only enhanced bone formation but also accelerated vascularization.
Hydrophobic polymers can also be used to encapsulate miRNA or siRNA [179]. This in principle can be done in the fabrication of fibrous scaffolds, which has been widely used as tissue grafts such as skin, vascular, cartilage, bone, and nerve grafts [180, 181]. Electrospinning is an effective and popular technique to produce fibers with diameter between hundreds of nanometers and several micrometers [180, 182]. The porous nature of fibrous scaffold provides a 3D matrix with high specific surface area for cell proliferation and nutrient transport. The topography of nanofiber could influence the migration and differentiation of stem cells [183–185]. It would be reasonable to hypothesize that a combination of nanofiber topography and miRNA delivery could modulate tissue development on a fibrous scaffold. However, despite many reports on the application of fibrous scaffolds for DNA or siRNA delivery, there has been no report on the use of miRNA entrapment for tissue engineering. Owing to the structural similarity between miRNA and siRNA, we can learn from the promising fibrous scaffold-based siRNA delivery systems discussed below.
SiRNA/polymer mixed solution has been used to encapsulate siRNA into the electrospun fibers by blend electrospinning technique [186, 187]. Chew et al. have used this technique to encapsulate siRNA/CPP complexes in electrospun PCLEEP fibers with uncompromised bioactivity [172]. A sustained release of siRNA was obtained for at least 28 days. Although the bolus delivery of siRNA could efficiently down-regulate the expression of collagen type I (COL1A1) at day 3, the COL1A1 level returned to normal at day 5. On the contrary, the scaffold-mediated delivery showed continuous down-regulation of COL1A1 level up to day 14. Transfection reagent (TransIT-TKO) and two CPPs (MPG and CADY) have been evaluated and TKO exhibited the best performance both in vitro and in vivo. The knockdown efficiency of PCLEEP fibers encapsulated with TKO/siCOL1A1 complexes was 50.5% at day 14, compared with 26.7% and 46.7% for siCOL1A1/MPG and siCOL1A1/CADY samples, respectively. Excessive deposition of collagen matrix would result in formation of fibrous capsule surrounding implants. In vivo study showed that the thickness of fibrous capsule formed around the PCLEEP fibers encapsulated with TKO/siCOL1A1 complexes was only about 60 μm at week 2 while that of the control sample was 80 μm. The fibrous capsule thickness further decreased to less than 30 μm at week 4.
4.2.3. Surface immobilization
Since the fabrication and sterilization process might inactivate miRNA, surface immobilization after scaffold fabrication offers an alternative for scaffold-mediated delivery. Additionally, the surface immobilization is especially suitable for bone implants and intravascular stents made from metals such as titanium (Ti) and stainless steel. Both the nonspecific interactions including hydrophobic/ionic interaction and specific interactions such as antigen-antibody and biotin-avidin interactions have been used to immobilize nucleic acid for substrate-based gene delivery. Lyophilization is the simplest way to immobilize nucleic acid or nanocomplex onto the scaffold surface. Both miR-29b and antimiR-138 lipoplexes (Lipofectamine® 2000) have been immobilized on microporous titanium oxide surface by lyophilization [188]. Through this technique, 1000 nm thick lipoplexes could be coated onto Ti surface and the transfection efficiency for MSC could reach up to 90%. Leveraging on the osteogenic potential of miR-29b and antimiR-138, Ti surface functionalized with these lipoplexes could be used as bone implant to enhance osteogenesis. The expression levels of osteogenic genes including ALP, BMP, OCN, COL1, OSX and Runx2 of MSC transfected by antimiR-138 and miR-29b functionalized surfaces were much higher than the control up to day 14. The enhanced collagen secretion and bone extracellular matrix (ECM) mineralization also proved that the Ti implants functionalized with miR-29b and antimiR-138 enhanced the osteogenic differentiation of MSC in vitro.
Ionic interaction is another technique to immobilize nucleic acids onto a surface due to their anionic sugar back bone. Poly(caprolactone) (PCL) fiber could be first coated with polydopamine (PD) to allow subsequent immobilization of siRNAs [189]. After adsorption of RE-1 silencing transcription factor (REST) siRNA onto PD-modified electrospun PCL nanofibers (PD-PCL scaffold), sustained REST knockdown in neural stem/progenitor cells (NPCs) was achieved up to 5 days. Comparing with the 2D PD-modified PCL film (PD-PCL film), the PD-PCL scaffold exhibited higher cumulative siRNA release and higher REST knockdown efficiency. Most importantly, siREST PD-PCL scaffold showed not only enhanced NPC neuronal commitment than the fiber control, but also significantly higher commitment than siREST PD-PCL film. It suggests that siREST together with the nanofiber topography has a positive influence on the neurogenesis of the seeded NPCs.
Layer-by-layer (LbL) technology is another approach to immobilize genes onto the scaffold surface through ionic interaction. Because of the small size of miRNAs/siRNAs, formation of multilayered polyelectrolyte films by LbL self-assembly of siRNA and polycations would result in inefficient ionic interaction and high initial burst release [190]. An alternative is to form multilayered polyelectrolyte films by siRNA/polycation polyplexes and polyanions [191, 192]. Dimitrova et al. [192] used PEI/siRNA polyplexes and HA/CS complexes to form multilayered polyelectrolyte films on the polylysine (PLL) and polyglutamic acid (PGA) films. With the degradation of HA by hyaluronidase, siRNA could be sustainably released from the film and efficiently inhibited HCV replication and infection in hepatocyte-derived cells over 12 days. Presumably LbL immobilization of polyplexes instead of naked siRNA would be advantageous because of higher transfection efficiency of the former. Although immobilization has proved effective in most studies, Shea et al. [193] showed that immobilization of PEI/DNA polyplexes onto the HA-collagen hydrogels with or without the biotin-neutravidin binding would not make a significant difference in transfection efficiency.
Antigen-antibody interaction was proved to be an efficient way to immobilize adenovirus vectors and plasmid DNA, especially for the gene-eluting stent application [194–196]. Probably due to complexity and high cost of antibody introduction, this approach has yet to be applied for miRNA delivery.
4.3. Scaffold properties
The properties of a scaffold may also affect the potency of scaffold-based gene delivery systems. Since the stiffness [197], nanotopography [198] and surface chemistry [175, 199] of scaffolds could have significant influence on cell behavior, a combination of those factors could potentially enhance the transfection efficiency of miRNA delivery systems. Mooney et al. showed that the stiffness of a scaffold could impact the DNA/PEI polyplex transfection efficiency [197]. Increase of scaffold stiffness would enhance the cell endocytosis of polyplexes, facilitate unpacking of the pDNA, and ultimately increase the transfect efficiency. The effect of scaffold stiffness depends on the cell type [200]. Increase in scaffold stiffness would increase the transfection efficiency to fibroblasts as their cell area and nuclear aspect ratio would change significantly with substrate stiffness. However, the transfectability of BMSCs and myoblasts were not so sensitive to scaffold stiffness. In the case of scaffold-based delivery systems, the influence of scaffold stiffness might be more complicated. Segura et al. [201] tried gene delivery to MSC through matrix metalloproteinase (MMP)-degradable HA hydrogel scaffolds. The study showed that softer hydrogel (0.1 vs 1.7 kPa) would increase the transfection efficiency. The mechanism underlying this phenomenon may be multi-factorial and warrants further studies. For instance, the higher crosslinking density used to create the tougher hydrogel may also decrease the hydrogel degradability and the subsequent DNA/PEI polyplex release.
Structure and nanotopography of a scaffold would also influence the transfection efficiency of gene delivery systems. Chew et al. [189] showed that dopamine-modified PCL nanofibers would achieve higher REST knockdown in NPC than dopamine-modified PCL nanofilms. Shea et al. [202] fabricated macroporous PEG hydrogel by first embedding gelatin microspheres into the gel during the hydrogel formation, followed by dissolution of the gelatin microspheres to create interconnected pores. The lentivirus encapsulated inside the hydrogel maintained its activity during the whole fabrication process. They have proved that increase of the macroporosity would increase cell penetration and HT-1080 cells would be transfected throughout the hydrogel, while non-macroporous PEG hydrogels could only transfect cells in the adjacent host tissues. Most importantly, in the in vivo study, delivery of lentivirus encoding for VEGF increased vascularization throughout the macropores of the hydrogel.
Using the self-assembled monolayer (SAM) technology, Shea et al. showed that the surface chemistry has a significant influence on the transfection efficiency of the surface-immobilized DNA nanocomplexes [199, 203]. Generally, the scaffold with a hydrophilic surface would result in higher transfection efficiency than one with a hydrophobic surface. Transfection efficiency of anionic carboxylic-acid-terminated SAM was two times higher than the hydroxyl-terminated SAM. Introduction of oligo(ethylene glycol) groups to the carboxylic acid-terminated SAM would further increase the transfection efficiency to five fold. Not only the surface functional groups, the proteins immobilized on the scaffold surface would also alter the endocytosis pathway and increase the transfection efficiency. PEI/DNA polyplexes immobilized on fibronectin-coated scaffold exhibited improved transfection efficiency and their endocytic pathway was also changed from clathrin-mediated endocytosis to caveolae-mediated endocytosis [204]. Interestingly, bolus delivery of PEI/DNA polyplexes to NIH/3T3 cells seeded on the same surface would not exhibit the same phenomenon.
5. Closure
MiRNA delivery has proved a promisng approach to direct stem cell fate. Manipulating specific miRNA levels inside stem cells through replacement therapy or inhibition therapy, a number of studies have demonstrated success in augmenting osteogenesis, reducing fibrosis, and improving wound healing among many other tissue engineering applications. MiRNA for regenerative medicine is still an evolving topic. However, evidence so far suggests that it will be a fertile research direction.
Like in many other gene therapies, effective miRNA delivery holds the key to eventual clinical translation. Although viral vectors pose long term safety concerns, the approval of adeno-associated viral vectors (AAV) for treating patients with familial hyperchylomicronemia in Europe shows that viral delivery is a viable approach for applications with a high benefit-to-risk ratio. Since AAV-mediated RNA interference therapy has proved effective in many studies, there is reason to believe that this approach would warrant investigation. Baculoviral vector might also offer opportunities for safe and effective miRNA delivery.
While nonviral vectors may be safer, their intrinsic limitations of low and transient transgene expression must be overcome for many tissue engineering applications. The issue of low transfection efficiency may or may not be fatal since the interference of certain pathways upstream may already have a profound effect on phenotypic development of stem cells. The issue of transient expression may be compensated by innovations of controlled release technologies. For example, sustained and local delivery of miRNA polyplexes or lipoplexes by hydrogels to the seeded or encapsulated cells can lead to prolonged miRNA expression. Scaffolds-based miRNA delivery also presents another opportunity to rescue the nonviral approach.
Although scaffolds-based miRNA delivery for regenerative medicine is still in its infancy, it is eliciting excitement. Tissue engineering scaffolds encapsulated with miRNA not only can function as a substrate for cell delivery, proliferation, differentiation, and tissue development but also provide essential and complementary factors for stem cells to differentiate towards specific linkage. Reports on scaffold-based miRNA delivery have shown particular promise for osteogenesis as well as wound healing. As innovations developed for RNAi therapy inspire miRNA delivery, we should see increasing application of miRNA therapy impacting regenerative medicine.
Table 1.
Representative in vivo studies of miRNA therapy for regenerative medicine
| Target miRNA | Means of delivery | Therapeutic approaches | Observations | Application | Ref. |
|---|---|---|---|---|---|
| MiR-31 | Poly(glycerol sebacate) scaffold | Inhibition | Percentage of new bone area after 8 weeks increased from 35% to 61% | Osteogenesis | [50] |
| MiR-148b | Baculovirus | Replacement | Percentage of new bone area after 12 weeks increased from 61% to 94% | Osteogenesis | [51] |
| MiR-26a | HyStem-HP™ hydrogel | Replacement | Percentage of new bone area after 12 weeks increased from 40% to ~100% and accelerated vascularization | Osteogenesis and angiogenesis | [52] |
| MiR-138 | Hydroxyapatite-tricalcium phosphate scaffold | Inhibition | Total bone volume increased 2.2-fold in implants treated with the antimiR-138 compared with miR control | Osteogenesis | [108] |
| MiR-29b | Atelocollagen/PEO scaffold | Replacement | MMP-8: TIMP-1 ratio 3-fold higher than treated with scaffold alone | Promoting wound healing | [66] |
| MiR-21 | Direct injection of plasmid expressing miR-21 | Replacement | Wound contraction increased over 30% compared to untreated wound | Promoting wound healing | [109] |
| MiR-1, miR-133; miR-206 | Atelocollagen | Replacement | The number of centronucleated myofibres in the miRNA group increased 136% than that in the control group; capillary density in the miRNA group increased over 30% than that in the control group | Myogenesis and angiogenesis | [110] |
| MiR-302 | MaxSuppressor™ In Vivo RNA-LANCEr II | Replacement | Fibrosis size of hearts decreased over 60% after 50 days compared to treated with control miRNA | Cardiomyogenesis | [76] |
| MiR-590; miR-199a | Adeno-associated virus | Replacement | Infarct size of miRNA treated group decreased about 50% at 12 days and 60 days after myocardial infarction | Cardiomyogenesis | [79] |
| MiR-34a | RiboJuice™ | Inhibition | Infarct cell death in heart treated with anti-miR-34a decreased 75 % compared to control group after myocardial infarction | Cardiomyogenesis | [77] |
| MiR-132 | PLGA polymer nanoparticles | Replacement | Over 10-fold increase in the number of microvessels per square millimeter compared to negative control | Angiogenesis | [111] |
| MiR-134 | Adeno-associated virus | Replacement | 13% reduction of total dendritic length compared to control vector | Dendritogenesis | [112] |
Acknowledgments
We acknowledge funding support by NIH (UH3 TR000505-03), AOSpine Foundation, Guangdong Innovative and Entrepreneurial Research Team Program (Grant No. 2013S086), National Science Foundation of China (51403243), and the China Postdoctoral Science Foundation (2014M552262).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Chen R, Mooney D. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 3.Giatsidis G, Venezia ED, Bassetto F. The role of gene therapy in regenerative surgery: Updated insights. Plast Reconstr Surg. 2013;131:1425–1435. doi: 10.1097/PRS.0b013e31828bd153. [DOI] [PubMed] [Google Scholar]
- 4.Mellott A, Forrest ML, Detamore M. Physical non-viral gene delivery methods for tissue engineering. Ann Biomed Eng. 2013;41:446–468. doi: 10.1007/s10439-012-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: Challenges and recent progress. Nat Rev Genet. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lüningschrör P, Hauser S, Kaltschmidt B, Kaltschmidt C. MicroRNAs in pluripotency, reprogramming and cell fate induction. BBA-Mol Cell Res. 2013;1833:1894–1903. doi: 10.1016/j.bbamcr.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell. 2010;7:36–41. doi: 10.1016/j.stem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Small EM, Olson EN. Pervasive roles of micrornas in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoshgoo N, Kholdebarin R, Iwasiow BM, Keijzer R. MicroRNAs and lung development. Pediatr Pulm. 2013;48:317–323. doi: 10.1002/ppul.22739. [DOI] [PubMed] [Google Scholar]
- 10.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of micrornas. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III drosha initiates microrna processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 14.Denli AM, Tops BBJ, Plasterk RHA, Ketting RF, Hannon GJ. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Lee Y, Yeom K-H, Kim Y-K, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 18.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 19.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: MicroRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 23.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 24.Gangaraju VK, Lin H. Micrornas: Key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzman-Villanueva D, El-Sherbiny IM, Herrera-Ruiz D, Vlassov AV, Smyth HDC. Formulation approaches to short interfering RNA and microRNA: Challenges and implications. J Pharm Sci. 2012;101:4046–4066. doi: 10.1002/jps.23300. [DOI] [PubMed] [Google Scholar]
- 26.Muthiah M, Park I-K, Cho C-S. Nanoparticle-mediated delivery of therapeutic genes: Focus on miRNA therapeutics. Exp Opin Drug Del. 2013;10:1259–1273. doi: 10.1517/17425247.2013.798640. [DOI] [PubMed] [Google Scholar]
- 27.Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–1126. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagerman PJ. Investigation of the flexibility of DNA using transient electric birefringence. Biopolymers. 1981;20:1503–1535. doi: 10.1002/bip.1981.360200710. [DOI] [PubMed] [Google Scholar]
- 29.Shah SA, Brunger AT. The 1.8 å crystal structure of a statically disordered 17 base-pair RNA duplex: Principles of RNA crystal packing and its effect on nucleic acid structure. J Mol Biol. 1999;285:1577–1588. doi: 10.1006/jmbi.1998.2385. [DOI] [PubMed] [Google Scholar]
- 30.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 31.Lee S-Y, Huh MS, Lee S, Lee SJ, Chung H, Park JH, Oh Y-K, Choi K, Kim K, Kwon IC. Stability and cellular uptake of polymerized siRNA (poly-siRNA)/polyethylenimine (PEI) complexes for efficient gene silencing. J Control Release. 2010;141:339–346. doi: 10.1016/j.jconrel.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Richards Grayson A, Doody A, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res. 2006;23:1868–1876. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- 33.Terasawa K, Shimizu K, Tsujimoto G. Synthetic pre-miRNA-based shRNA as potent RNAi triggers. J Nucleic Acids. 2011;2011 doi: 10.4061/2011/131579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daka A, Peer D. RNAi-based nanomedicines for targeted personalized therapy. Adv Drug Deliver Rev. 2012;64:1508–1521. doi: 10.1016/j.addr.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruberti F, Barbato C, Cogoni C. Targeting microRNAs in neurons: Tools and perspectives. Exp Neurol. 2012;235:419–426. doi: 10.1016/j.expneurol.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 37.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 38.Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, Orum H, Kauppinen S, Kenny PJ, Wahlestedt C. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A. 2009;106:3507–3512. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM, Slack FJ. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A. 2012;109:E1695–E1704. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabani MM, Abreu-Goodger C, Williams D, Lyons PA, Torres AG, Smith KGC, Enright AJ, Gait MJ, Vigorito E. Efficient inhibition of miR-155 function in vivo by peptide nucleic acids. Nucleic Acids Res. 2010;38:4466–4475. doi: 10.1093/nar/gkq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat Meth. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebert MS, Sharp PA. MicroRNA sponges: Progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L, Hannon GJ. Micrornas: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 44.Lu C-H, Chang Y-H, Lin S-Y, Li K-C, Hu Y-C. Recent progresses in gene delivery-based bone tissue engineering. Biotechnol Adv. 2013;31:1695–1706. doi: 10.1016/j.biotechadv.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Gaur T, Hussain S, Mudhasani R, Parulkar I, Colby JL, Frederick D, Kream BE, van Wijnen AJ, Stein JL, Stein GS, Jones SN, Lian JB. Dicer inactivation in osteoprogenitor cells compromises fetal survival and bone formation, while excision in differentiated osteoblasts increases bone mass in the adult mouse. Dev Biol. 2010;340:10–21. doi: 10.1016/j.ydbio.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nie X, Wang Q, Jiao K. Dicer activity in neural crest cells is essential for craniofacial organogenesis and pharyngeal arch artery morphogenesis. Mech Dev. 2011;128:200–207. doi: 10.1016/j.mod.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassan MQ, Gordon JAR, Beloti MM, Croce CM, Wijnen AJv, Stein JL, Stein GS, Lian JB. A network connecting Runx2, Satb2, and the miR-23a~27a~24–2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci U S A. 2010;107:19879–19884. doi: 10.1073/pnas.1007698107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu R, Liu W, Li H, Yang L, Chen C, Xia Z-Y, Guo L-J, Xie H, Zhou H-D, Wu X-P, Luo X-H. A Runx2/miR-3960/miR-2861 regulatory feedback loop during mouse osteoblast differentiation. J Biol Chem. 2011;286:12328–12339. doi: 10.1074/jbc.M110.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng Y, Bi XP, Zhou HF, You ZW, Wang YD, Gu P, Fan XQ. Repair of critical-sized bone defects with anti-miR-31-expressing bone marrow stromal stem cells and poly(clycerol sebacate) scaffolds. Eur Cells Mater. 2014;27:13–25. doi: 10.22203/ecm.v027a02. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y-H, Chang Y-H, Sung L-Y, Li K-C, Yeh C-L, Yen T-C, Hwang S-M, Lin K-J, Hu Y-C. Osteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148b. Biomaterials. 2014;35:4901–4910. doi: 10.1016/j.biomaterials.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Fan L, Liu S, Liu W, Zhang H, Zhou T, Wu D, Yang P, Shen L, Chen J, Jin Y. The promotion of bone regeneration through positive regulation of angiogenic – osteogenic coupling using microRNA-26a. Biomaterials. 2013;34:5048–5058. doi: 10.1016/j.biomaterials.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 53.Shang J, Liu H, Zhou Y. Roles of microRNAs in prenatal chondrogenesis, postnatal chondrogenesis and cartilage-related diseases. J Cell Mol Med. 2013;17:1515–1524. doi: 10.1111/jcmm.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang B, Guo HF, Zhang YL, Chen L, Ying DJ, Dong SW. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting sox9. PloS One. 2011;6 doi: 10.1371/journal.pone.0021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paik S, Jung HS, Lee S, Yoon DS, Park MS, Lee JW. MiR-449a regulates the chondrogenesis of human mesenchymal stem cells through direct targeting of lymphoid enhancer-binding factor-1. Stem Cells Dev. 2012;21:3298–3308. doi: 10.1089/scd.2011.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ham O, Song B-W, Lee S-Y, Choi E, Cha M-J, Lee CY, Park J-H, Kim I-K, Chang W, Lim S, Lee CH, Kim S, Jang Y, Hwang K-C. The role of microRNA-23b in the differentiation of msc into chondrocyte by targeting protein kinase a signaling. Biomaterials. 2012;33:4500–4507. doi: 10.1016/j.biomaterials.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi T, Lu J, Cobb BS, Rodda SJ, McMahon AP, Schipani E, Merkenschlager M, Kronenberg HM. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc Natl Acad Sci U S A. 2008;105:1949–1954. doi: 10.1073/pnas.0707900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eberhart JK, He X, Swartz ME, Yan Y-L, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA mirn140 modulates pdgf signaling during palatogenesis. Nat Genet. 2008;40:290–298. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, Takada S, Lotz MK, Ueno-Kudo H, Asahara H. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–1185. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enoch S, Leaper DJ. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y, Wang Y, Chen K, Youliang Wang, keping Chen. The role of microRNA in cutaneous wound healing. J Biol. 2014;31:81–84. [Google Scholar]
- 62.Peng L-H, Fung K-P, Leung P-C, Gao J-Q. Genetically manipulated adult stem cells for wound healing. Drug Discov Today. 2011;16:957–966. doi: 10.1016/j.drudis.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Sundaram GM, Common JEA, Gopal FE, Srikanta S, Lakshman K, Lunny DP, Lim TC, Tanavde V, Lane EB, Sampath P. ‘see-saw’ expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature. 2013;495:103–106. doi: 10.1038/nature11890. [DOI] [PubMed] [Google Scholar]
- 64.Yang X, Wang J, Guo SL, Fan KJ, Li J, Wang YL, Teng Y. MiR-21 promotes keratinocyte migration and re-epithelialization during wound healing. Int J Biol Sci. 2011;7:685–690. doi: 10.7150/ijbs.7.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, Kirsner RS, Jimenez JJ, Leslie C, Tomic-Canic M. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem. 2012;287:29324–29335. doi: 10.1074/jbc.M112.382135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monaghan M, Browne S, Schenke-Layland K, Pandit A. A collagen-based scaffold delivering exogenous microRNA-29b to modulate extracellular matrix remodeling. Mol Ther. 2014;22:786–796. doi: 10.1038/mt.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Järvinen TAH, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: Biology and treatment. Am J Sports Med. 2005;33:745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 68.Lepper C, Partridge TA, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu N, Williams AH, Maxeiner JM, Bezprozvannaya S, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MicroRNA-206 promotes skeletal muscle regeneration and delays progression of duchenne muscular dystrophy in mice. J Clin Invest. 2012;122:2054–2065. doi: 10.1172/JCI62656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCarthy JJ. MicroRNA-206: The skeletal muscle-specific myomir. BBA-Gene Regul Mech. 2008;1779:682–691. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson C, Catoe H, Werner R. MiR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006;34:5863–5871. doi: 10.1093/nar/gkl743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong CF, Tellam RL. MicroRNA-26a targets the histone methyltransferase enhancer of zeste homolog 2 during myogenesis. J Biol Chem. 2008;283:9836–9843. doi: 10.1074/jbc.M709614200. [DOI] [PubMed] [Google Scholar]
- 73.Dey BK, Gagan J, Yan Z, Dutta A. MiR-26a is required for skeletal muscle differentiation and regeneration in mice. Genes Dev. 2012;26:2180–2191. doi: 10.1101/gad.198085.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Amerongen MJ, Engel FB. Features of cardiomyocyte proliferation and its potential for cardiac regeneration. J Cell Mol Med. 2008;12:2233–2244. doi: 10.1111/j.1582-4934.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu G, Huang Z-P, Wang D-Z. MicroRNAs in cardiac regeneration and cardiovascular disease. Sci China Life Sci. 2013;56:907–913. doi: 10.1007/s11427-013-4534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, Snitow M, Morley M, Li D, Petrenko N, Zhou S, Lu M, Gao E, Koch WJ, Stewart KM, Morrisey EE. A microRNA-hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015;7:279ra38. doi: 10.1126/scitranslmed.3010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, Horrevoets AJG, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Muller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 78.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam Y-J, Matkovich SJ, Dorn GW, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eulalio A, Mano M, Ferro MD, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 80.Wilson KD, Hu S, Venkatasubrahmanyam S, Fu J-D, Sun N, Abilez OJ, Baugh JJA, Jia F, Ghosh Z, Li RA, Butte AJ, Wu JC. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: Role for miR-499. Circ-Cardiovasc Gene. 2010;3:426–435. doi: 10.1161/CIRCGENETICS.109.934281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogórek B, Ferreira-Martins J, Arranto C, D’Amario D, del Monte F, Urbanek K, D’Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Eming SA, Brachvogel B, Odorisio T, Koch M. Regulation of angiogenesis: Wound healing as a model. Prog Histochem Cytochem. 2007;42:115–170. doi: 10.1016/j.proghi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Suárez Y, Fernández-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kane NM, Thrasher AJ, Angelini GD, Emanueli C. Concise review: MicroRNAs as modulators of stem cells and angiogenesis. Stem Cells. 2014;32:1059–1066. doi: 10.1002/stem.1629. [DOI] [PubMed] [Google Scholar]
- 85.Landskroner-Eiger S, Moneke I, Sessa WC. MiRNAs as modulators of angiogenesis. CSH Perspect Med. 2013;3 doi: 10.1101/cshperspect.a006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caporali A, Meloni M, Völlenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, Agami R, le Sage C, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 87.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JTG, Sohn-Lee C, Loyer X, Soutschek J, Brand T, Tuschl T, Heineke J, Martin U, Schulte-Merker S, Ertl G, Engelhardt S, Bauersachs J, Thum T. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 88.Meloni M, Marchetti M, Garner K, Littlejohns B, Sala-Newby G, Xenophontos N, Floris I, Suleiman MS, Madeddu P, Caporali A, Emanueli C. Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther. 2013;21:1390–1402. doi: 10.1038/mt.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y, Gao D-Y, Huang L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv Drug Deliver Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 91.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a myc-activated microrna cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji F, Lv X, Jiao J. The role of microRNAs in neural stem cells and neurogenesis. J Genet Genomics. 2013;40:61–66. doi: 10.1016/j.jgg.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 93.Perruisseau-Carrier C, Jurga M, Forraz N, McGuckin C. MiRNAs stem cell reprogramming for neuronal induction and differentiation. Mol Neurobiol. 2011;43:215–227. doi: 10.1007/s12035-011-8179-z. [DOI] [PubMed] [Google Scholar]
- 94.Papagiannakopoulos T, Kosik KS. MicroRNA-124: Micromanager of neurogenesis. Cell Stem Cell. 2009;4:375–376. doi: 10.1016/j.stem.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Stappert L, Roese-Koerner B, Brüstle O. The role of microRNAs in human neural stem cells, neuronal differentiation and subtype specification. Cell Tissue Res. 2015;359:47–64. doi: 10.1007/s00441-014-1981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton Stuart A, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, Jiang L, Cai Z, Sun H, Zhang K, Zhang Y, Chen J, Fu X-D. Direct conversion of fibroblasts to neurons by reprogramming ptb-regulated microRNA circuits. Cell. 152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Visvanathan J, Lee S, Lee B, Lee JW, Lee S-K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-Mrna splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng L-C, Pastrana E, Tavazoie M, Doetsch F. MiR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through notch signaling pathway. PloS One. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonev B, Stanley P, Papalopulu N. MicroRNA-9 modulates Hes1 ultradian oscillations by forming a double-negative feedback loop. Cell Rep. 2012;2:10–18. doi: 10.1016/j.celrep.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Svaren J. MicroRNA and transcriptional crosstalk in myelinating glia. Neurochem Int. 2014;77:50–57. doi: 10.1016/j.neuint.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi Q-S, Xin M, Wang F, Appel B, Lu QR. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eskildsen T, Taipaleenmaki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S, Kassem M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci U S A. 2011;108:6139–6144. doi: 10.1073/pnas.1016758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang T, Feng Y, Sun H, Zhang L, Hao L, Shi C, Wang J, Li R, Ran X, Su Y, Zou Z. Mir-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol. 2012;181:1911–1920. doi: 10.1016/j.ajpath.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 110.Nakasa T, Ishikawa M, Shi M, Shibuya H, Adachi N, Ochi M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med. 2010;14:2495–2505. doi: 10.1111/j.1582-4934.2009.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Devalliere J, Chang WG, Andrejecsk JW, Abrahimi P, Cheng CJ, Jane-wit D, Saltzman WM, Pober JS. Sustained delivery of proangiogenic microRNA-132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J. 2014;28:908–922. doi: 10.1096/fj.13-238527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christensen M, Larsen LA, Kauppinen S, Schratt G. Recombinant adeno-associated virus-mediated microRNA delivery into the postnatal mouse brain reveals a role for miR-134 in dendritogenesis in vivo. Front Neural Circuit. 2010;3:16. doi: 10.3389/neuro.04.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim S-S, Garg H, Joshi A, Manjunath N. Strategies for targeted nonviral delivery of siRNAs in vivo. Trends Mol Med. 2009;15:491–500. doi: 10.1016/j.molmed.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lai W-F. Cyclodextrins in non-viral gene delivery. Biomaterials. 2014;35:401–411. doi: 10.1016/j.biomaterials.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raemdonck K, Martens TF, Braeckmans K, Demeester J, De Smedt SC. Polysaccharide-based nucleic acid nanoformulations. Adv Drug Deliv Rev. 2013;65:1123–1147. doi: 10.1016/j.addr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 116.Liu YP, Berkhout B. MiRNA cassettes in viral vectors: Problems and solutions. BBA-Gene Regul Mech. 2011;1809:732–745. doi: 10.1016/j.bbagrm.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X, Godbey WT. Viral vectors for gene delivery in tissue engineering. Adv Drug Deliver Rev. 2006;58:515–534. doi: 10.1016/j.addr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 118.Couto LB, High KA. Viral vector-mediated RNA interference. Curr Opin Pharmacol. 2010;10:534–542. doi: 10.1016/j.coph.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 119.Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskämper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss H-P, Hajjar RJ, Poller WC. Long-term cardiac-targeted rna interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pagès JC, Bru T. Toolbox for retrovectorologists. J Gene Med. 2004;6:S67–S82. doi: 10.1002/jgm.498. [DOI] [PubMed] [Google Scholar]
- 122.ELLIS James, YAO S. Retrovirus silencing and vector design : Relevance to normal and cancer stem cells? Curr Gene Ther. 2005;5:367–373. doi: 10.2174/1566523054546233. [DOI] [PubMed] [Google Scholar]
- 123.Ye D, Wang G, Liu Y, Huang W, Wu M, Zhu S, Jia W, Deng A-M, Liu H, Kang J. MiR-138 promotes induced pluripotent stem cell generation through the regulation of the p53 signaling. Stem Cells. 2012;30:1645–1654. doi: 10.1002/stem.1149. [DOI] [PubMed] [Google Scholar]
- 124.Lin S-L, Chang DC, Chang-Lin S, Lin H, Wu DTS, Chen DT, Ying S-Y. MiR-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA. 2008;14:2115–2124. doi: 10.1261/rna.1162708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A. The microRNA cluster miR-106b similar to 25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging-Us. 2011;3:108–124. doi: 10.18632/aging.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seidlits SK, Gower RM, Shepard JA, Shea LD. Hydrogels for lentiviral gene delivery. Exp Opin Drug Del. 2013;10:499–509. doi: 10.1517/17425247.2013.764864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi LS, Benedicenti F, Ambrosi A, Di Serio C, Doglioni C, von Kalle C, Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotech. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 128.Liu W, Gong Q, Ling J, Zhang W, Liu Z, Quan J. Role of miR-424 on angiogenic potential in human dental pulp cells. J Endodont. 2014;40:76–82. doi: 10.1016/j.joen.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 129.Huang F, Tang L, Fang ZF, Hu XQ, Pan JY, Zhou SH. MiR-1-mediated induction of cardiogenesis in mesenchymal stem cells via downregulation of Hes-1. Biomed Res Int. 2013 doi: 10.1155/2013/216286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deng Y, Zhou H, Zou D, Xie Q, Bi X, Gu P, Fan X. The role of miR-31-modified adipose tissue-derived stem cells in repairing rat critical-sized calvarial defects. Biomaterials. 2013;34:6717–6728. doi: 10.1016/j.biomaterials.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 131.Lo W-H, Hwang S-M, Chuang C-K, Chen C-Y, Hu Y-C. Development of a hybrid baculoviral vector for sustained transgene expression. Mol Ther. 2009;17:658–666. doi: 10.1038/mt.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen C-Y, Lin C-Y, Chen G-Y, Hu Y-C. Baculovirus as a gene delivery vector: Recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol Adv. 2011;29:618–631. doi: 10.1016/j.biotechadv.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin C-Y, Chang Y-H, Lin K-J, Yen T-C, Tai L, Chen C-Y, Lo W-H, Hsiao I-T, Hu Y-C. The healing of critical-sized femoral segmental bone defects in rabbits using baculovirus-engineered mesenchymal stem cells. Biomaterials. 2010;31:3222–3230. doi: 10.1016/j.biomaterials.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 134.Lin C-Y, Chang Y-H, Kao C-Y, Lu C-H, Sung L-Y, Yen T-C, Lin K-J, Hu Y-C. Augmented healing of critical-size calvarial defects by baculovirus-engineered mscs that persistently express growth factors. Biomaterials. 2012;33:3682–3692. doi: 10.1016/j.biomaterials.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Huang C, Reddy Chintagari N, Bhaskaran M, Weng T, Guo Y, Xiao X, Liu L. MiR-375 regulates rat alveolar epithelial cell trans-differentiation by inhibiting Wnt/β-catenin pathway. Nucleic Acids Res. 2013;41:3833–3844. doi: 10.1093/nar/gks1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang D, Zhang Z, O’Loughlin E, Wang L, Fan X, Lai EC, Yi R. MicroRNA-205 controls neonatal expansion of skin stem cells by modulating the PI(3)K pathway. Nat Cell Biol. 2013;15:1153–1163. doi: 10.1038/ncb2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Q, Cai J, Cai X-h, Chen L. MiR-346 regulates osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by targeting the Wnt/β-catenin pathway. PloS One. 2013;8:e72266. doi: 10.1371/journal.pone.0072266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stappert L, Borghese L, Roese-Koerner B, Weinhold S, Koch P, Terstegge S, Uhrberg M, Wernet P, Brüstle O. MicroRNA-based promotion of human neuronal differentiation and subtype specification. PloS One. 2013;8:e59011. doi: 10.1371/journal.pone.0059011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee SK-W, Teng Y, Wong H-K, Ng T-K, Huang L, Lei P, Choy K-W, Liu Y, Zhang M, Lam DSC, Yam GHF, Pang C-P. MicroRNA-145 regulates human corneal epithelial differentiation. PloS One. 2011;6:e21249. doi: 10.1371/journal.pone.0021249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 141.Wahlquist C, Jeong D, Rojas-Munoz A, Kho C, Lee A, Mitsuyama S, van Mil A, Jin Park W, Sluijter JPG, Doevendans PAF, Hajjar RJ, Mercola M. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–535. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang H-M, Martinez NJ, Thornton JE, Hagan JP, Nguyen KD, Gregory RI. Trim71 cooperates with microRNAs to repress cdkn1a expression and promote embryonic stem cell proliferation. Nat Commun. 2012;3:923. doi: 10.1038/ncomms1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J, Huang WC, Wu YH, Hou JF, Nie Y, Gu HY, Li J, Hu SS, Zhang H. MicroRNA-193 pro-proliferation effects for bone mesenchymal stem cells after low-level laser irradiation treatment through inhibitor of growth family, member 5. Stem Cells Dev. 2012;21:2508–2519. doi: 10.1089/scd.2011.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 146.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krichevsky AM, Sonntag K-C, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hara ES, Ono M, Eguchi T, Kubota S, Pham HT, Sonoyama W, Tajima S, Takigawa M, Calderwood SK, Kuboki T. MiRNA-720 controls stem cell phenotype, proliferation and differentiation of human dental pulp cells. PloS One. 2013;8:e83545. doi: 10.1371/journal.pone.0083545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stevanato L, Sinden J. The effects of microRNAs on human neural stem cell differentiation in two- and three-dimensional cultures. Stem Cell Res Ther. 2014;5:49. doi: 10.1186/scrt437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y, Tang L, Ding Y, Jin Y. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28:559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- 151.Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res. 2011;26:1953–1963. doi: 10.1002/jbmr.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29:1804–1816. doi: 10.1002/stem.728. [DOI] [PubMed] [Google Scholar]
- 153.Sluijter JPG, van Mil A, van Vliet P, Metz CHG, Liu J, Doevendans PA, Goumans M-J. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscl Throm Vas. 2010;30:859–868. doi: 10.1161/ATVBAHA.109.197434. [DOI] [PubMed] [Google Scholar]
- 154.Xie C, Huang H, Sun X, Guo Y, Hamblin M, Ritchie RP, Garcia-Barrio MT, Zhang J, Chen YE. MicroRNA-1 regulates smooth muscle cell differentiation by repressing kruppel-like factor 4. Stem Cells Dev. 2011;20:205–210. doi: 10.1089/scd.2010.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ling H-Y, Wen G-B, Feng S-D, Tuo Q-H, Ou H-S, Yao CH, Zhu B-Y, Gao Z-P, Zhang L, Liao D-F. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin Exp Pharmacol P. 2011;38:239–246. doi: 10.1111/j.1440-1681.2011.05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Y, Gelfond J, McManus LM, Shireman PK. Temporal microRNA expression during in vitro myogenic progenitor cell proliferation and differentiation: Regulation of proliferation by miR-682. Physiol Genomics. 2011;43:621–630. doi: 10.1152/physiolgenomics.00136.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Boese AS, Majer A, Saba R, Booth SA. Small RNA drugs for prion disease: A new frontier. Exp Opin Drug Dis. 2013;8:1265–1284. doi: 10.1517/17460441.2013.818976. [DOI] [PubMed] [Google Scholar]
- 158.Son S, Namgung R, Kim J, Singha K, Kim WJ. Bioreducible polymers for gene silencing and delivery. Accounts Chem Res. 2011;45:1100–1112. doi: 10.1021/ar200248u. [DOI] [PubMed] [Google Scholar]
- 159.Höbel S, Aigner A. Polyethylenimines for siRNA and miRNA delivery in vivo. WIREs Nanomed Nanobi. 2013;5:484–501. doi: 10.1002/wnan.1228. [DOI] [PubMed] [Google Scholar]
- 160.Hwang DW, Son S, Jang J, Youn H, Lee S, Lee D, Lee Y-S, Jeong JM, Kim WJ, Lee DS. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic micro RNA. Biomaterials. 2011;32:4968–4975. doi: 10.1016/j.biomaterials.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 161.Cheng CJ, Saltzman WM. Polymer nanoparticle-mediated delivery of microRNA inhibition and alternative splicing. Mol Pharm. 2012;9:1481–1488. doi: 10.1021/mp300081s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Raftery R, Brien F, Cryan S-A. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules. 2013;18:5611–5647. doi: 10.3390/molecules18055611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nitta S, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14:1629–1654. doi: 10.3390/ijms14011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliver Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 165.Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, Xiao X, Yang Y, Sheng W, Wu Y, Zeng Y. Hyaluronic acid-chitosan nanoparticles for co-delivery of miR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35:4333–4344. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 166.Li H, Zheng X, Koren V, Vashist YK, Tsui TY. Highly efficient delivery of siRNA to a heart transplant model by a novel cell penetrating peptide-dsRNA binding domain. Int J Pharm. 2014;469:206–213. doi: 10.1016/j.ijpharm.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 167.Suh JS, Lee JY, Choi YS, Chong PC, Park YJ. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation. Biomaterials. 2013;34:4347–4359. doi: 10.1016/j.biomaterials.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 168.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliver Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang Y, Leong KW. Nanoscale surfacing for regenerative medicine. WIREs Nanomed Nanobi. 2010;2:478–495. doi: 10.1002/wnan.74. [DOI] [PubMed] [Google Scholar]
- 170.Monaghan M, Pandit A. RNA interference therapy via functionalized scaffolds. Adv Drug Deliver Rev. 2011;63:197–208. doi: 10.1016/j.addr.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 171.Cam C, Segura T. Matrix-based gene delivery for tissue repair. Curr Opin Biotech. 2013;24:855–863. doi: 10.1016/j.copbio.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jang J-H, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: Transgene expression and cellular transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rujitanaroj P-o, Jao B, Yang J, Wang F, Anderson JM, Wang J, Chew SY. Controlling fibrous capsule formation through long-term down-regulation of collagen type I (COL1A1) expression by nanofiber-mediated siRNA gene silencing. Acta Biomater. 2013;9:4513–4524. doi: 10.1016/j.actbio.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Su J, Hu B-H, Lowe WL, Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31:308–314. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.O’Rorke S, Keeney M, Pandit A. Non-viral polyplexes: Scaffold mediated delivery for gene therapy. Prog Polym Sci. 2010;35:441–458. [Google Scholar]
- 177.Wang C-HK, Pun SH. Substrate-mediated nucleic acid delivery from self-assembled monolayers. Trends Biotechnol. 2011;29:119–126. doi: 10.1016/j.tibtech.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 178.Li J, Kooger R, He M, Xiao X, Zheng L, Zhang Y. A supramolecular hydrogel as a carrier to deliver microRNA into the encapsulated cells. Chem Commun. 2014;50:3722–3724. doi: 10.1039/c4cc00156g. [DOI] [PubMed] [Google Scholar]
- 179.Nelson CE, Kim AJ, Adolph EJ, Gupta MK, Yu F, Hocking KM, Davidson JM, Guelcher SA, Duvall CL. Tunable delivery of siRNA from a biodegradable scaffold to promote angiogenesis in vivo. Adv Mater. 2014;26:607–614. doi: 10.1002/adma.201303520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meinel AJ, Germershaus O, Luhmann T, Merkle HP, Meinel L. Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications. Eur J Pharm Biopharm. 2012;81:1–13. doi: 10.1016/j.ejpb.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 181.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B-Rev. 2011;17:349–364. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomed. 2013;8:2997–3017. doi: 10.2147/IJN.S43575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chew SY, Mi R, Hoke A, Leong KW. The effect of the alignment of electrospun fibrous scaffolds on schwann cell maturation. Biomaterials. 2008;29:653–661. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yim EKF, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299–1306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang Y, Kulangara K, Lam RTS, Dharmawan R, Leong KW. Effects of topographical and mechanical property alterations induced by oxygen plasma modification on stem cell behavior. ACS Nano. 2012;6:8591–8598. doi: 10.1021/nn301713d. [DOI] [PubMed] [Google Scholar]
- 186.Cao H, Jiang X, Chai C, Chew SY. RNA interference by nanofiber-based siRNA delivery system. J Control Release. 2010;144:203–212. doi: 10.1016/j.jconrel.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 187.Ji W, Sun Y, Yang F, van den Beucken JJP, Fan M, Chen Z, Jansen J. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm Res. 2011;28:1259–1272. doi: 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu K, Song W, Zhao L, Liu M, Yan J, Andersen MØ, Kjems J, Gao S, Zhang Y. MicroRNA functionalized microporous titanium oxide surface by lyophilization with enhanced osteogenic activity. ACS Appl Mater Inter. 2013;5:2733–2744. doi: 10.1021/am400374c. [DOI] [PubMed] [Google Scholar]
- 189.Low WC, Rujitanaroj P-O, Lee D-K, Messersmith PB, Stanton LW, Goh E, Chew SY. Nanofibrous scaffold-mediated rest knockdown to enhance neuronal differentiation of stem cells. Biomaterials. 2013;34:3581–3590. doi: 10.1016/j.biomaterials.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 190.Bechler SL, Lynn DM. Characterization of degradable polyelectrolyte multilayers fabricated using DNA and a fluorescently-labeled poly(β-amino ester): Shedding light on the role of the cationic polymer in promoting surface-mediated gene delivery. Biomacromolecules. 2012;13:542–552. doi: 10.1021/bm2016338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mehrotra S, Lee I, Chan C. Multilayer mediated forward and patterned siRNA transfection using linear-PEI at extended N/P ratios. Acta Biomater. 2009;5:1474–1488. doi: 10.1016/j.actbio.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dimitrova M, Affolter C, Meyer F, Nguyen I, Richard DG, Schuster C, Bartenschlager R, Voegel J-C, Ogier J, Baumert TF. Sustained delivery of siRNAs targeting viral infection by cell-degradable multilayered polyelectrolyte films. Proc Natl Acad Sci U S A. 2008;105:16320–16325. doi: 10.1073/pnas.0800156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26:1575–1584. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Levy RJ, Song C, Tallafragada S, DeFelice S, Hinson JT, Vyavahare N, Connolly J, Ryan K, Li Q. Localized adenovirus gene delivery using antiviral IgG complexation. Gene Ther. 2001;8:659–667. doi: 10.1038/sj.gt.3301452. [DOI] [PubMed] [Google Scholar]
- 195.Zhang L-H, Luo T, Zhang C, Luo P, Jin X, Song C-X, Gao R-L. Anti-DNA antibody modified coronary stent for plasmid gene delivery: Results obtained from a porcine coronary stent model. J Gene Med. 2011;13:37–45. doi: 10.1002/jgm.1529. [DOI] [PubMed] [Google Scholar]
- 196.Ma GL, Wang Y, Fishbein I, Yu M, Zhang LH, Alferiev IS, Yang J, Song CX, Levy RJ. Anchoring of self-assembled plasmid DNA/anti-DNA antibody/cationic lipid micelles on bisphosphonate-modified stent for cardiovascular gene delivery. Int J Nanomed. 2013;8:1029–1035. doi: 10.2147/IJN.S40077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kong HJ, Liu J, Riddle K, Matsumoto T, Leach K, Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 198.Adler AF, Leong KW. Emerging links between surface nanotechnology and endocytosis: Impact on nonviral gene delivery. Nano Today. 2010;5:553–569. doi: 10.1016/j.nantod.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pannier AK, Wieland JA, Shea LD. Surface polyethylene glycol enhances substrate-mediated gene delivery by nonspecifically immobilized complexes. Acta Biomater. 2008;4:26–39. doi: 10.1016/j.actbio.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chu C, Kong H. Interplay of cell adhesion matrix stiffness and cell type for non-viral gene delivery. Acta Biomater. 2012;8:2612–2619. doi: 10.1016/j.actbio.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 201.Gojgini S, Tokatlian T, Segura T. Utilizing cell-matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels. Mol Pharm. 2011;8:1582–1591. doi: 10.1021/mp200171d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shepard JA, Virani FR, Goodman AG, Gossett TD, Shin S, Shea LD. Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis. Biomaterials. 2012;33:7412–7421. doi: 10.1016/j.biomaterials.2012.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pannier AK, Anderson BC, Shea LD. Substrate-mediated delivery from self-assembled monolayers: Effect of surface ionization, hydrophilicity, and patterning. Acta Biomater. 2005;1:511–522. doi: 10.1016/j.actbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bengali Z, Rea JC, Shea LD. Gene expression and internalization following vector adsorption to immobilized proteins: Dependence on protein identity and density. J Gene Med. 2007;9:668–678. doi: 10.1002/jgm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]