Abstract
Evidence demonstrated that glial cells, mainly astrocytes, regulate glutamate uptake, which is regulated by several glutamate transporters. Among these glutamate transporters, glutamate transporter 1 (GLT-1; its human homolog is excitatory amino acid transporter-2) is responsible for the majority of glutamate uptake by glial cells. Cystine-glutamate antiporter (xCT) is another glial protein critical in regulating glutamate transmission. Several studies from our laboratory demonstrated that attenuation of ethanol intkae was associated in part with upregulation of xCT and GLT suggesting the important role of these transporters in the treatment of ethanol dependence. We found recently that β-lactam antibiotic, ampicillin, upregulated GLT-1 expression in the prefrontal cortex (PFC) and nucleus accumbens (NAc) and consequently reduced ethanol intake in alcohol-preferring (P) rats. In this study, we investigated the effects of ampicillin on the expressions of xCT and GLT-1 isoforms (GLT-1a and GLT-1b) as well as on GLAST expression. We found that ampicillin reduced ethanol intake as compared to the saline (control)-treated group. In addition, we found that ampicillin induced upregulation of xCT, GLT-1a, and GLT-1b expressions in both the PFC and NAc, but had no effect on GLAST expression. Our findings provide significant role of ampicillin on upregulating xCT and GLT-1 isoforms expressions, might be suggested as possible tragets for the attenuation of ethanol consumption.
Keywords: Ampicillin, Ethanol Intake, Glutamate, GLT-1a, GLT-1b, xCT, GLAST
Introduction
Ethanol dependence is a public health issue. Existing treatments for ethanol dependence are limited, and finding a neurotransmitter system as a therapeutic target is important [1]. Among the neurotransmitters involved, the glutamatergic system is now well known for its important role in drug dependence, including ethanol [2-4]. There are glutamatergic inputs from the prefrontal cortex (PFC) into the nucleus accumbens (NAc) as well as to other brain reward regions, which are key players in ethanol dependence [for review see refs. [4]]. It is noteworthy that PFC receives glutamatergic projections from other brain regions, including amygdala [5].
Glutamate transmission is regulated by several glutamate transporters; glutamate transporter-1 (GLT1;) is considered the major glutamate transporter responsible for regulating the majority of glutamate uptake [6, 7]. Importantly, GLT-1 is expressed in the mammalian brain primarily in two isoforms, GLT-1a and GLT-1b [8-10]. However, the GLT-1c isoform is expressed less in the brain but it is expressed highly in the retina [11]. GLT-1a is expressed in both neurons and astrocytes, while GLT-1b is expressed only in astrocytes [10, 12]. Glutamate transmission is also regulated by cystine/glutamate exchanger (xCT), another glial glutamate transporter. The xCT system transports anionic cystine inside astrocytes, in exchange with glutamate. Studies from our laboratory have demonstrated that the administration of compounds that upregulate GLT-1 with its isoforms (GLT-1a and GLT-1b) and xCT expressions can reduce ethanol intake and relapse-like ethanol intake in P rats [13-16].
Studies from our laboratory have shown that treatment with ceftriaxone (100 mg/kg), β-lactam antibiotic know to upregulate GLT-1 expression, decreased ethanol intake and attenuated relapse-like ethanol drinking [17-19]. Additionally, we have recently shown that ampicillin at 100 mg/kg treatment reduced ethanol intake and upregulated GLT-1 expression in PFC and NAc [16]. However, the effects of ampicillin at dose of 100 mg/kg on the expressions of xCT, glutamate aspartate transporter (GLAST) and GLT-1 isoforms have not been investigated yet. Thus, we focused in this study to investigate these proteins that have important roles in regulating extracellular glutamate concentrations. It is important to note that, in contrast to ceftriaxone, ampicillin has some clinical revelevaance as it can be given orally to patients. Thus, we have tested ampicillin using our established animal model, P rats.
In this study, using male P rats, we focused on testing the effects of ampicillin on the expressions of xCT, GLT-1a, GLT-1b, and GLAST. The rationale for testing amipicllin is based in recent findings from our laboratory showing that ampicillin reduced ethanol intake in part through upregulatory effect on GLT-1 expression [16]. In order to clarify the mechamism of ampicillin on ethanol reduction, we investigated the effects of this compound on the expressions of xCT and GLAST as well as GLT-1 isoforms (GLT-1a and GLT-1b).
Materials and Methods
Animals
Alcohol-preferring male (P) rats were received from Indiana University, School of Medicine (Indianapolis, IN, USA). P rat is an accepted animal model of alcoholism that displays several behavioral, physiological and neurochemical phenotypes found in alcoholics [20]. Rats were housed in bedded plastic tubs and kept at 21°C, 50% humidity. The Institutional Animal Care and Use Committee of The University of Toledo approved all animal housing and experimental procedures in accordance with guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996). All rats, at the age of 90 days, were individually housed in standard plastic cages and divided into two experimental groups. The control (saline) group received water and food and i.p. injections of 0.9 % saline solution (n=6), and the ampicillin group received 100 mg/kg of the drug (i.p) (n=6).
Behavioral drinking paradigms
At the age of 90 days, designated control (saline) and ampicillin groups were given free choice to food, water and two ethanol concentrations (15% and 30%, v/v) for five weeks. Body weight, water intake and ethanol intake were evaluated three times a week during the last two weeks. Rats selected for the study were required to achieve at least 4 g/kg/day or more of ethanol intake. Body weight, water intake and ethanol intake were measured during Week 4 and Week 5, which served as baseline values. On Week 6, P rats were i.p. injected with either saline or ampicillin (100 mg/kg) daily for five consecutive days. During these five days, animals body weight, water intake and ethanol intake were measured daily. Rats were euthanized by CO2 inhalation and decapitated 24 hours after the last i.p. injections of saline or drug.
Brain tissue harvesting
Brains were removed and immediately frozen on dry ice and stored at −80°C. Brain regions (NAc and PFC) were microdissected with the Leica cryostat apparatus, using stereotaxic coordinates from the Rat Brain Atlas [21]. These brain regions were then immediately stored at −80°C for further immunoblot testing.
Immunoblot assay for detection of xCT, GLT-1a, GLT-1b, and GLAST
Changes in xCT, GLT-1a, GLT-1b, GLAST and GAPDH expressions in the NAc and PFC were determined using immunoblot technique, as described previously (13, 14, 18). In brief, brain tissues were homogenized in a lysis buffer and equal amount of lysed was loaded on 10-20% polyacrylamide gel. Proteins were then transferred electrophoretically onto a PVDF membrane. The membranes were incubated overnight at 4°C with one of the following antibodies: rabbit anti-GLT-1a (1:5,000), rabbit anti-GLT-1b (1:5,000), rabbit anti-xCT (1:1,000 Abecam), rabbit anti-GLAST (1:5,000 Abecam) and mouse anti-GAPDH (1:5,000, Millipore). After washing, the immunoblotting membranes were then incubated with Anti-rabbit IgG (1:3000 Thermo Scientific) or anti-mouse IgG (1:3000 Cell Signaling Technology) for 90 minutes. Membranes were dried and incubated with a chemiluminiscent kit (Super Signal West Pico, Pierce Inc.) for one minute. Membranes were then further dried and exposed to HyBlot CL Films, which were developed using the SRX-101A Film processor; and digitized blots were quantified with MCID software. Data for GLT-1a, GLT-1b, xCT and GLAST expression were represented as a ratio of GAPDH expression in the NAc and PFC.
Statistical analyses
Two way (mixed) ANOVA with repeated measures, followed by Benferroni multiple comparisons, was used for analysis of ethanol intake, water intake, and body weight. Immunoblot data were analyzed using a t-test for comparison between the control and ampicillin groups.. All statistical analyses were based on p<0.05 level of significance.
Results
Effect of ampicillin on ethanol intake
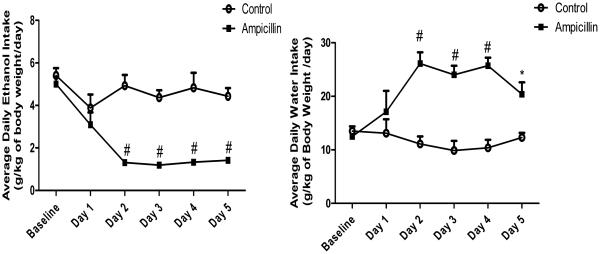
Two-way ANOVA with repeated measures, followed by Benferroni multiple comparisons, demonstrated a significant reduction of ethanol intake in the ampicillin-treated group compared to the control-treated group from day 2 to day 5 (p<0.0001) without significant preference to either 15% or 30% v/v ethanol concentrations. Moreover, mixed ANOVA demonstrated a significant main effect of day [F (1, 5) = 9.398, p<0.0001] and a significant day x treatment interaction [F (1, 5) = 5.934, p<0.001] on ethanol intake (Fig. 1A.). There was also significant increase in water intake in the ampicillin-treated group compared to the control-treated group on day 2 to day 4 (p≤0.0001) and on Day 5 (p<0.05). There were non-significant main effect of day [F (1, 5) = 2.221, p>0.05] and a significant day x treatment interaction [F (1, 5) = 5.979, p<0.001] on water intake (Fig. 1B.). However, we did not reveal any significant effect on body weight between all groups.
Figure 1.
Effects of ampicillin treatment on ethanol and water intake (g/kg/day) in male P rats exposed to five weeks of continuous free choice of ethanol and water Significant difference between treatment groups. A) Effects of ampicillin treatment on ethanol intake (g/kg/day). B) Effects of ampicillin treatment on water intake (g/kg/day). Data are shown as mean ± SEM; (n= 6 for each group), (*p<0.05; #P<0.0001)
Effects of ampicillin on GLT-1a expression in the NAc and PFC
Analysis of immunoblots (Fig. 2A) revealed a significant main effect of ampicillin treatment on GLT-1a expression in the NAc and PFC. An independent t-test analysis of immunoblots demonstrated a significant increase in GLT-1a/GAPDH ratios (100% control-value) in the NAc (p<0.05) and PFC (p<0.05) of the ampicillin-treated group as compared to the control-treated group (Fig. 2B).
Figure 2. Effect of ampicillin on GLT-1a expression in NAc and PFC.
A) Immunoblots for GLT-1a expression and GAPDH as a control loading protein in the NAc and PFC. B) Quantitative t-test analysis of immunoblots showed that ampicillin increased significantly the % ratio of GLT-1a/GAPDH in the NAc and PFC as compared to the saline/control group (100% control-value). Data are shown as mean ± SEM. (*p<0.05). (n= 6 for each group)
Effects of ampicillin on GLT-1b expression in NAc and PFC
We further investigated GLT-1b expression in the NAc and PFC of the ampicillin-treated group. Analysis of immunoblots (Fig. 3A) showed a significant main effect among the ampicillin group on GLT-1b expression in both the NAc and PFC. An independent t-test revealed a significant increase in GLT-1b/GAPDH ratios (100% control-value) in the ampicillin-treated group’s NAc (p<0.05) and PFC (p<0.05) (Fig. 3B).
Figure 3. Effect of ampicillin on GLT-1b expression in NAc and PFC.
A) Immunoblots for GLT-1b expression and GAPDH as a control loading protein in the NAc and PFC. B) Quantitative t-test analysis of immunoblots revealed that ampicillin increased the % ratio of GLT-1b/GAPDH significantly in the NAc and PFC as compared to the control group (100% control-value). Data are shown as mean ± SEM. (*p<0.05). (n= 6 for each group)
Effects of ampicillin on xCT expression in the NAc and PFC
We also investigated the effect of ampicillin on xCT expression (Fig. 4A). An independent t-test analysis of immunoblots revealed increased xCT/GAPDH ratios in the NAc (p<0.05) and PFC (p<0.05) of the ampicillin-treated group as compared to the control-treated group (Fig. 4B).
Figure 4. Effect of ampicillin on xCT expression in NAc and PFC.
A) Immunoblots for xCT expression and GAPDH as a control loading protein in the NAc and PFC. B) Quantitative t-test analysis of immunoblots showed that ampicillin increased the expressions of the % ratio of xCT/GAPDH in the NAc and PFC significantly as compared to the control group (100% control-value). Data are shown as mean ± SEM. (*p<0.05). (n= 6 for each group)
Effects of ampicillin on GLAST expression in the NAc and PFC
We then determined GLAST expression in both the NAc and PFC. We did not observe any changes in GLAST expression in the NAc and PFC (Fig. 5A) of the control or ampicillin-treated groups. An independent t-test analysis did not show any significant effect between the control and ampicillin-treated groups in the NAc ( p > 0.05) and PFC (p >0.05) (Fig. 5B).
Figure 5. Effect of ampicillin on GLAST expression in NAc and PFC.
A) Immunoblots for GLAST expression and GAPDH as a control loading protein in the NAc and PFC. B) Quantitative t-test analysis of immunoblots showed no significant increase in the % ratio GLAST/GAPDH in the NAc and PFC of either the control group (100% control-value) or the treatment group. Data are shown as mean ± SEM. (*p<0.05). (n= 6 for each group)
Discussion
Studies from our laboratory demonstrated that ceftriaxone, β-lactam antibiotic, upregulated GLT-1 and xCT in PFC and NAc [17-19] as well reduced alcohol intake in P rats. It is noteweothry that ceftriaxone can be given clinically through intramuscular or intervenous route. Thus, we tested ampicillin in this study since it has some clinical relevance as it can be administered orally.
It is well known that that ethanol consumption can lead to a marked increase in the extracellular glutamate concentrations [22-24]. It has been reported that ceftriaxone-induced attenuation of ethanol intake and relapse-like ethanol drinking in male P rats is associated in part with upregulation of GLT-1 and its isoforms (GLT-1a and GLT-1b) in the NAc and PFC [13, 15, 17, 19]. The upregulatory effects on GLT-1 could be associated with a decrease in extracellular glutamate concentrations leading to the reduction in ethanol intake. Rothstein and colleagues found that ampicillin upregulated GLT-1 expression [25]. We report here that ampicillin treatment upregulated GLT-1 isoforms in the NAc and PFC, and conseuqenly reduced ethanol intake. Although GLT-1a and GLT-1b are expressed differentially [10, 12], ampicillin treatment was found to increase the expression of both GLT-1 isoforms possibly by a similar mechanism.
We have also investigated the effects of ampicillin on the expression of xCT. xCT has neuroprotecrive effects through modulation of glutathione supply in the brain [26]. Activation of xCT by N-acetylcysteine is associated with restoring glutamate level in the brain. Therefore, glutamate released through xCT can stimulate metabotropic glutamate receptor 2/3 (mGluR2/3) regulating pre-synaptic glutamate release [For review see ref. [27]]. Several studies from our laboratory reported that the increases in xCT and GLT-1 expressions are linked to the attenuation of ethanol consumption in male P rats [14, 17, 18]. In addition, chronic consumption of ethanol can lead to downregulation of xCT expression in the NAc and PFC [13]. Accordingly, downregulation of xCT was also observed in the NAc of a cocaine-seeking animal model [28]. Importantly, we report here that ampicillin has the ability to normalize the expression of xCT in both the NAc and PFC.
We further tested for the effect of ampicillin on GLAST expression, which is co-localized with GLT-1 in astrocytes. We did not observe an upregualtory effect on GLAST expression with ampicillin treatment. This effect is in accordance with a recent finding demonstrating that ceftriaxone treatment did not induce an upregulatory effect on GLAST expression [13, 15].
In summary, the present findings suggest that ampicillin reduced ethanol intake significantly, at least in part through upregulation of xCT, GLT-1a and GLT-1b expressions in both the NAc and PFC. The upregulatory effects of ampicillin on xCT and GLT-1 isoforms may normalize extracellular glutamate concentrations in these brain regions. These data provide ample evidence about the potential therapeutic implications of β-lactam antibiotics such as ampicillin and ceftriaxone for the treatment of alcohol dependence. It is important to note that in contrast to ceftriaxone, ampicillin is a drug that can be given orally, thus it has clinical relevance for its use in alcohol dependence. Studies are warranted to determine the effects of oral administration of this compound on ethanol intake as well as on the expressions of xCT, GLT-1, GLT-1 isoforms and GLAST.
Highlights.
> Ampicillin upregulated xCT and GLT-1 isoforms in both NAc and PFC.
> Ampicillin reduced ethanol intake in male P rats.
> GLAST expression is not changed following treatment with ampicillin.
Acknowledgments
This work was supported by Award Number R01AA019458 (Y.S.) and Award Number AA13522 from the National Institutes on Alcohol Abuse and Alcoholism. The authors thank Dr. J. Rothstein and Dr. P. Rosenberg for providing us with GLT-1a and GLT-1b antibodies, respectively. The authors would like to thank Dr. P.S.S. Rao for his technical assistance.
Abbreviations
- (GLT-1)
Glutamate transporter-1
- (PFC)
nucleus accumbens (NAc) and prefrontal cortex
- (CEF)
ceftriaxone
- (P) rats
alcohol-preferring
- (xCT)
Cystine-glutamate antiporter
- (GLAST)
glutamate aspartate transporter
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011 Nov;12(11):670–84. doi: 10.1038/nrn3110. PubMed PMID: 22011682. Pubmed Central PMCID: 3408029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Jul 22;29(29):9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. PubMed PMID: 19625514. Pubmed Central PMCID: 2737464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–73. doi: 10.1016/j.neuropharm.2008.07.011. PubMed PMID: 18675832. Pubmed Central PMCID: 3280337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem. 2012;19(30):5148–56. doi: 10.2174/092986712803530511. PubMed PMID: 22680643. Pubmed Central PMCID: 3486951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McDonald AJ. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. The Journal of comparative neurology. 1996 Feb 12;365(3):367–79. doi: 10.1002/(SICI)1096-9861(19960212)365:3<367::AID-CNE3>3.0.CO;2-2. PubMed PMID: 8822176. [DOI] [PubMed] [Google Scholar]
- [6].Jensen AA, Fahlke C, Bjorn-Yoshimoto WE, Bunch L. Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr Opin Pharmacol. 2014 Nov 5; doi: 10.1016/j.coph.2014.10.008. PubMed PMID: 25466154. [DOI] [PubMed] [Google Scholar]
- [7].Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001 Sep;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. PubMed PMID: 11369436. [DOI] [PubMed] [Google Scholar]
- [8].Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, et al. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002 Mar 15;22(6):2142–52. doi: 10.1523/JNEUROSCI.22-06-02142.2002. PubMed PMID: 11896154. Pubmed Central PMCID: 2849837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, et al. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 Feb 4;24(5):1136–48. doi: 10.1523/JNEUROSCI.1586-03.2004. PubMed PMID: 14762132. Pubmed Central PMCID: 2849838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Berger UV, DeSilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. The Journal of comparative neurology. 2005 Nov 7;492(1):78–89. doi: 10.1002/cne.20737. PubMed PMID: 16175560. Pubmed Central PMCID: 3676901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rauen T, Wiessner M, Sullivan R, Lee A, Pow DV. A new GLT1 splice variant: cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochemistry international. 2004 Dec;45(7):1095–106. doi: 10.1016/j.neuint.2004.04.006. PubMed PMID: 15337309. [DOI] [PubMed] [Google Scholar]
- [12].Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, et al. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009 Sep 15;162(4):1055–71. doi: 10.1016/j.neuroscience.2009.03.048. PubMed PMID: 19328838. [DOI] [PubMed] [Google Scholar]
- [13].Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology (Berl) 2014 Oct;231(20):4049–57. doi: 10.1007/s00213-014-3545-y. PubMed PMID: 24687412. Pubmed Central PMCID: 4176549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, et al. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014;8:366. doi: 10.3389/fnbeh.2014.00366. PubMed PMID: 25400560. Pubmed Central PMCID: 4214358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rao PS, Saternos H, Goodwani S, Sari Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 2015 Jan 27; doi: 10.1007/s00213-015-3868-3. PubMed PMID: 25619881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rao PS, Goodwani S, Bell RL, Wei Y, Boddu SH, Sari Y. Effects of ampicillin, cefazolin and cefoperazone treatments on GLT1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience. 2015 Mar 24; doi: 10.1016/j.neuroscience.2015.03.038. PubMed PMID: 25813713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011 May-Jun;46(3):239–46. doi: 10.1093/alcalc/agr023. PubMed PMID: 21422004. Pubmed Central PMCID: 3104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rao PS, Sari Y. Effects of ceftriaxone on chronic ethanol consumption: a potential role for xCT and GLT1 modulation of glutamate levels in male P rats. J Mol Neurosci. 2014 Sep;54(1):71–7. doi: 10.1007/s12031-014-0251-5. PubMed PMID: 24535561. Pubmed Central PMCID: 4127185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol. 2013 Jun;27(6):541–9. doi: 10.1177/0269881113482529. PubMed PMID: 23518814. Pubmed Central PMCID: 3657312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behavior genetics. 2002 Sep;32(5):363–88. doi: 10.1023/a:1020266306135. PubMed PMID: 12405517. [DOI] [PubMed] [Google Scholar]
- [21].Paxinos G, Watson C. 6th Academic Press/Elsevier; Amsterdam ; Boston : 2007. The rat brain in stereotaxic coordinates. [Google Scholar]
- [22].Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, De Witte P, et al. Neuro-inflammation induced in the hippocampus of ‘binge drinking’rats may be mediated by elevated extracellular glutamate content. Journal of neurochemistry. 2009;111(5):1119–28. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- [23].Ding ZM, Engleman EA, Rodd ZA, McBride WJ. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcohol Clin Exp Res. 2012 Apr;36(4):633–40. doi: 10.1111/j.1530-0277.2011.01665.x. PubMed PMID: 22017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008 Apr;32(4):617–31. doi: 10.1111/j.1530-0277.2008.00620.x. PubMed PMID: 18341649. [DOI] [PubMed] [Google Scholar]
- [25].Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005 Jan 6;433(7021):73–7. doi: 10.1038/nature03180. PubMed PMID: 15635412. [DOI] [PubMed] [Google Scholar]
- [26].Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006 Oct 11;26(41):10514–23. doi: 10.1523/JNEUROSCI.3178-06.2006. PubMed PMID: 17035536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behavioural pharmacology. 2010;21(5-6):514. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010 Jan 1;67(1):81–4. doi: 10.1016/j.biopsych.2009.07.018. PubMed PMID: 19717140. Pubmed Central PMCID: 2795043. [DOI] [PMC free article] [PubMed] [Google Scholar]