Abstract
Relationships between 1,25-dihydroxyvitamin D (1,25(OH)2D) and skeletal outcomes are uncertain. We examined the associations of 1,25(OH)2D with bone mineral density (BMD), BMD change, and incident fractures in a cohort of older men and compared them with those of 25-hydroxyvitamin D (25OHD). The study population included 1000 men (aged 74.6 ± 6.2 years) in the Osteoporotic Fractures in Men (MrOS) study, of which 537 men had longitudinal dual-energy X-ray absorptiometry (DXA) data (4.5 years of follow-up). A case-cohort design and Cox proportional hazards models were used to test the association between vitamin D metabolite levels and incident nonvertebral and hip fractures. Linear regression models were used to estimate the association between vitamin D measures and baseline BMD and BMD change. Interactions between 25OHD and 1,25(OH)2D were tested for each outcome. Over an average follow-up of 5.1 years, 432 men experienced incident nonvertebral fractures, including 81 hip fractures. Higher 25OHD was associated with higher baseline BMD, slower BMD loss, and lower hip fracture risk. Conversely, men with higher 1,25(OH)2D had lower baseline BMD. 1,25(OH)2D was not associated with BMD loss or nonvertebral fracture. Compared with higher levels of calcitriol, the risk of hip fracture was higher in men with the lowest 1,25(OH)2D levels (8.70 to 51.60 pg/mL) after adjustment for baseline hip BMD (hazard ratio [HR] = 1.99, 95% confidence interval [CI] 1.19–3.33). Adjustment of 1,25(OH)2D data for 25OHD (and vice versa) had little effect on the associations observed but did attenuate the hip fracture association of both vitamin D metabolites. In older men, higher 1,25(OH)2D was associated with lower baseline BMD but was not related to the rate of bone loss or nonvertebral fracture risk. However, with BMD adjustment, a protective association for hip fracture was found with higher 1,25(OH)2D. The associations of 25OHD with skeletal outcomes were generally stronger than those for 1,25(OH)2D. These results do not support the hypothesis that measures of 1,25(OH)2D improve the ability to predict adverse skeletal outcomes when 25OHD measures are available.
Keywords: 1,25-DIHYDROXYVITAMIN D; CALCITRIOL; FRACTURE; BONE MINERAL DENSITY (BMD); 25-HYDROXYVITAMIN D
Introduction
Vitamin D deficiency is usually defined by serum 25-hydroxyvitamin D (25OHD) levels. Lower 25OHD has been shown to correlate with faster bone loss at the hip,(1,2) a higher risk of falls,(3,4) and a higher risk of major osteoporotic fractures,(5) including hip fracture.(5,6) In contrast, limited data have shown an inverse relationship between 1,25-dihydroxyvitamin D (1,25(OH)2D, calcitriol) and bone mineral density (BMD)(7,8) and no relationship with bone loss.(2) There are no data on 1,25(OH)2D levels and fracture risk in older men, as previous reports of this association focused on older women.(9,10) The relationship between 1,25(OH)2D and bone health has been challenging to establish because until recently there has not been a sensitive and reliable assay for 1,25(OH)2D.(11)
Studying the relationships between 1,25(OH)2D and bone health outcomes in longitudinal observational studies can contribute to a better understanding of the biological mechanisms through which vitamin D affects bone. Moreover, it is important to establish which marker of vitamin D status, 25OHD or 1,25(OH)2D, is better correlated with clinical outcomes such as BMD, BMD change, and fracture so that adequate levels of these can be identified and targeted for better bone health. We sought to examine how calcitriol levels are associated with bone turnover markers (BTMs), calciotropic hormones, BMD (lumbar spine and total hip), BMD change, and fractures (nonvertebral and hip) in a large cohort of elderly, community-dwelling men and contrasted these relationships to those of 25OHD. We previously showed that 25OHD was related to hip (but not nonspine) fractures and rates of bone loss.(1,6) In the current study, we expand this work to simultaneously examine both 25OHD and 1,25(OH)2D in the same population and their relationship to multiple skeletal outcomes.
Materials and Methods
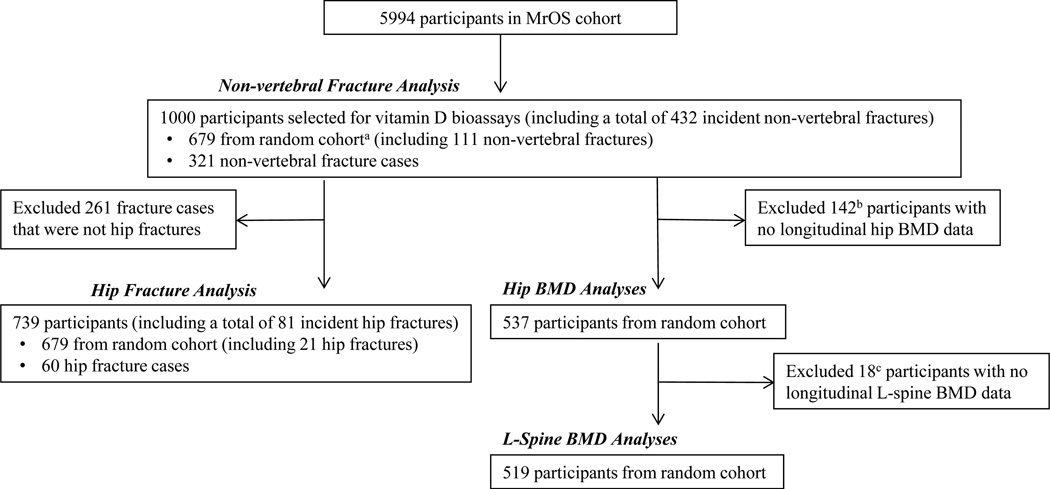
The study design and cohort characteristics of the Osteoporotic Fractures in Men Study (MrOS) have been previously described.(12,13) Briefly, 6 clinical sites in the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA) recruited 5994 community-dwelling men between March 2000 and April 2002 for a study on musculoskeletal aging. To participate, men had to be aged ≥65 years, able to walk unassisted, and be without bilateral hip replacements. The Institutional Review Board at each center approved the study, and written consent was obtained from all participants. Fig. 1 describes the case-cohort design for the vitamin D and skeletal outcomes study. One thousand men from the MrOS study (321 incident nonvertebral fracture cases and 679 randomly selected participants) were included in the nonvertebral fracture analysis (total of 432 incident nonvertebral fractures; 111 fractures from the random cohort and an additional 321 fractures from outside the random cohort). After excluding 261 fracture cases that were not hip fractures, 739 participants remained and were included in the hip fracture analysis (total of 81 incident hip fractures; 21 fractures from the random cohort and an additional 60 fractures from outside the random cohort). The initially selected random cohort (n = 679) was used for the baseline and longitudinal BMD analyses after excluding participants who were missing follow-up BMD data. For other MrOS projects, measures of bone and mineral metabolism were performed on randomly selected participants. For men included in the current analyses, values were available for intact parathyroid hormone (iPTH, n = 675), fibroblast growth factor-23 (FGF-23, n = 437), serum type I collagen N-propeptide (PINP, n = 493), and urinary C-terminal cross-linked telopeptide of type I collagen (α-CTX, n = 491; β-CTX, n = 490).
Fig. 1.
Case-cohort design for the MrOS vitamin D and skeletal outcomes study. aUsed previously obtained bone turnover marker and calciotropic hormone levels from random cohort. bEleven refused, 5 terminated, 57 deceased, 7 missing BMD (1 baseline, 6 follow-up), 62 responded to questionnaire by mail (no clinic visit). cOne terminated, 1 responded to questionnaire by mail (no clinic visit), 16 missing L-spine BMD percent change since baseline (see Materials and Methods).
Study measures
Fasting morning blood samples were collected at baseline (2000–2002), and serum was prepared and stored at −70°C until thawed for assays. Serum was obtained in approximately equal numbers in all of the four seasons. Spot sample from second-voided morning urine was also collected from each participant at baseline and stored at −70°C until thawed for assays.(13) All biochemical measures described below were performed using serum, except urinary CTX.
25OHD
25OHD was measured at the Mayo Medical Laboratories in Rochester, MN, using LC-MS/MS after prior derivatization.(14) The lower limit of quantification (LLQ) was 4 ng/mL for 25OHD2 and 2 ng/mL for 25OHD3. Aliquots of a single-serum pool were included in alternate assay runs. Using the pooled serum, the interassay coefficients of variation (CVs) for 25OHD2 and 25OHD3 were both 4.4%, and the intra-assay CVs were 6.4% and 4.9%, respectively.(14,15) This assay does not cross-react with 24-hydroxy- or 26-hydroxy-derivatives. It does cross-react with 3-epi-25-hydroxyvitamin D. However, the concentration of this metabolite in adults has been reported to be very low.
1,25(OH)2D
Total 1,25(OH)2D was measured at the University of Leuven in Belgium, using LC-MS/MS without derivatization.(8) The LLQ was 4.3 pg/mL for 1,25(OH)2D2 and 6 pg/mL for 1,25(OH)2D3. Interassay CV of pooled serum at low and high serum concentrations, respectively, were 10.1% for serum with mean concentration of 7.16 pg/mL and 5.9% for serum with mean concentration of 55.8 pg/mL.(8) This assay does not cross-react with 24-hydroxy- or 26-hydroxy-metabolites(11) but does cross-react with 3-epi-1,25-dihydroxyvitamin D. Because the concentration of 3-epi-25-hydroxyvitamin D in adults is very low, it is likely that the concentration of 3-epi-1,25-dihydroxyvitamin D is also very low; therefore, interference is probably negligible.
Bone turnover markers and calciotropic hormones
As previously described,(16) bone formation was assessed with serum PINP (Roche Diagnostics, Mannheim, Germany) including both trimeric and monomeric forms. Intra- and interassay CVs for this assay are <4.4% in this laboratory. Alpha (α-CTX; Alpha CrossLaps ELISA, Nordic Bioscience Diagnostics, Herlev, Denmark)(17) and beta (β-CTX; Elecsys 2010 automatic analyzer, Roche Diagnostics)(18) CTX were used to measure bone resorption with intra- and interassay CVs for both isomers <10%. Serum iPTH was measured in duplicate using a Scantibodies immunoradiometric assay (Scantibodies Laboratory, Santee, CA, USA) at Columbia University (normal range in serum defined by MrOS data set, 10 to 46 pg/mL; laboratory normal in EDTA plasma 10 to 66 pg/mL(19)] as described by Curtis and colleagues.(20) Results of duplicate measures were averaged. Duplicate pooled serum controls were included in every other assay run. Using the pooled serum, the interassay CV was 8.4%, and the intra-assay CV was 5.7%. Measurement of intact FGF-23 using a second-generation polyclonal goat antibody ELISA (Millipore, Billerica, MA, USA) has been previously described.(21) The lowest limit of detection was 3.3 pg/mL with an intra- and interassay CV <11%, which was similar to the manufacturer’s reports.
Measurement of BMD
BMD measurements in the MrOS study were performed at baseline (2000–2002) and at a second visit (2005–2006) using Hologic QDR 4,500-W densitometer (Hologic Inc., Waltham, MA, USA) at the femoral neck, total hip, and lumbar spine (L-spine).(13,22) A central quality-control lab, certification of dual-energy X-ray absorptiometry (DXA) operators, and standardized procedures for scanning were used to ensure reproducibility of DXA measurements at all six clinical sites such that the precision of DXA scans at the spine and hip is 1% to 2%.(23) BMD measurements between 2005 and 2006 were missing for approximately 10% of the participants in the longitudinal BMD cohort and, therefore, DXA from an earlier visit (2002–2005) performed as a part of ancillary studies within MrOS was used, but average follow-up time (~4.5 years) was not dramatically different. DXA at the L-spine was not performed as a part of the protocol for one of the interim study visits and, therefore, the 16 men who had longitudinal hip BMD data used from this visit are missing L-spine data (Fig. 1). The rate of change in BMD at the hip and L-spine were expressed as an annualized percentage of the initial value as percentage change in BMD per year.(1)
Assessment of fractures
Incident fracture events were reported by participants at 4-month intervals on brief mailed questionnaires.(24) Subsequently, study physicians centrally adjudicated reported fractures from medical records. For this analysis, fracture types were defined as all nonspine fractures and hip fractures. Pathologic fractures were excluded. During follow-up, next of kin were contacted for men with unreturned questionnaires who could not be reached by telephone. All incident fracture cases as of June 2007 were used for this case-cohort study; therefore, the average time of follow-up for hip and nonvertebral fractures was 5.3 and 5.1 years, respectively (range 0 to 6.8 years for both).
Falls
Incident falls were reported by participants at 4-month intervals on brief mailed questionnaires. For this analysis, we used falls that occurred in the first year after the baseline visit in those participants who were not lost to follow-up. Only 1 participant in the random cohort (n = 679) was missing falls data because his enrollment occurred after the first year’s questionnaires had been mailed out. As previously reported,(25) men who reported at least one fall on any questionnaire in the year of follow-up were classified as having fallen. To evaluate recurrent falling, men were also classified as falling at least twice (compared with none or once) based on the sum of the numbers of falls reported on the questionnaires during the 1-year follow-up.
Vitamin D supplement use
Supplemental vitamin D intake was assessed at baseline with the Block Food Frequency questionnaire.(26–28)
Other measures(13)
Questionnaires were administered at baseline to obtain information regarding smoking history, alcohol consumption, self-reported health status, and demographic factors. The Physical Activity Score for the Elderly (PASE)(29) was used to assess physical activity. Participants’ ability to rise from a chair without using their arms was determined. Walking speed was determined by timing completion of a 6-meter course performed at the participant’s usual walking speed.(13) Standard balance beam or digital scales were used to obtain weight (kg) and Harpenden stadiometers for height (cm) that was then used to calculate body mass index (BMI) as kg/m2. Serum creatinine was measured using a Roche COBAS Integra 800 automated analyzer (Roche Diagnostics) at the Veterans Affairs Clinical Laboratory in Portland, OR, on baseline serum that had been previously thawed. Renal function was expressed as estimated glomerular filtration rate (eGFR) in mL/min/1.73m2 using a standardized serum creatinine-based formula.(1,30)
Statistical analysis
Each vitamin D measure was centered and standardized. Means and standard deviations (SDs) were derived from the random sample. We assessed the linearity of associations using restricted cubic spline models.(31) Cox proportional hazards models were then used to accommodate the case-cohort design and test the association between each vitamin D metabolite measurement and 1) time to first nonvertebral fracture and 2) time to first hip fracture with base model adjusted for age, site, race, season, height, weight, and physical activity. Separate linear regression models were used to estimate the association between each vitamin D measure with baseline L-spine, femoral neck, and total hip BMD and change in BMD at all sites with base models adjusted for age, race, site, season, height, weight, and physical activity. For BMD change, the base model was also adjusted for a baseline longitudinally standardized BMD at the corresponding site. Additional multivariable models adjusted for health status, smoking, alcohol, and inability to rise from chair. To assess for confounding by renal function, we added eGFR to the multivariable models in all analyses to determine if the coefficients change by 10% or more. Variables most likely to affect the above associations were used in base and multivariable models and parallel those used in previous MrOS analyses for 25OHD and skeletal outcomes.(1,6) We previously evaluated the relationships between vitamin D metabolites in this cohort and found that the amount of supplementation was minimal and did not significantly affect the associations of vitamin D metabolites.(15) Therefore, these analyses were not adjusted for vitamin D supplement use. Models that included both 25OHD and 1,25(OH)2D were generated for each outcome. Interactions of 25OHD (by quartile) by 1,25(OH)2D (quartile) were tested. Linear regression was used to examine the associations among each vitamin D metabolite and bone turnover markers and calciotropic hormones. These associations were adjusted for weight and age. Nonlinearity of the association of each vitamin D metabolite was assessed graphically and by testing a squared term for vitamin D in the linear regression model. All analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Baseline characteristics of the participants are shown in Table 1. Men included in these analyses had a mean age of 74.6 (±6.2) years at the time of enrollment and most were white (92.0%). The majority reported excellent or good health status (84.3%) and had an estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73m2 (84.2%). Those who sustained a hip fracture were older (79.8 versus 74.6 years) compared with overall case cohort. Men with a nonvertebral fracture were also older and had more falls in the first year of follow-up but were otherwise similar to the case cohort (Table 1). The case cohort used for these analyses (n = 1000) was similar to the overall MrOS cohort (n = 5994, Table 1) but had slightly more falls. As expected, at baseline the 142 men who were excluded from the BMD loss analysis because of lack of follow-up data were, on average, 3.2 ± 5.8 years older, sicker (fewer reporting good or excellent health status and had more falls), less physically active (lower PASE score), and more likely to report vitamin D supplement use compared with the overall MrOS cohort (n = 5994, data not shown).
Table 1.
Baseline Characteristics, Mean (±SD) or n(%)
| MrOS cohort (n = 5994) |
Case cohort (n =1000)c |
Nonvertebral fx cases (n=432) |
Hip fx cases (n=81) |
Hip BMD analyses (n=537) |
|
|---|---|---|---|---|---|
| Age (years) | 73.7 (5.9) | 74.6 (±6.2) | 75.5 (±6.4) | 79.8 (±5.9) | 73.4 (±5.8) |
| Race | |||||
| White | 5362 (89.5) | 920 (92.0) | 407 (94.2) | 77 (95.1) | 491 (91.4) |
| African American | 244 (4.1) | 26 (2.6) | 5 (1.2) | 2 (2.5) | 16 (3.0) |
| Asian | 126 (2.1) | 22 (2.2) | 9 (2.1) | 1 (1.2) | 12 (2.2) |
| Hispanic | 191 (3.2) | 23 (2.3) | 10 (2.3) | 1 (1.2) | 11 (2.1) |
| Other | 71 (1.2) | 9 (0.9) | 1 (0.2) | 0 (0.0) | 7 (1.3) |
| Total hip BMD(g/cm2)a | 0.95 (±0.1) | 0.93 (±0.1) | 0.89 (±0.2) | 0.79 (±0.1) | 0.95 (±0.1) |
| Falls in the 1st year of follow-upa | |||||
| 0 falls | 4457 (74.6) | 666 (66.7) | 272 (63.0) | 52 (64.2) | 408 (76.1) |
| 1 fall | 1052 (17.6) | 209 (20.9) | 101 (23.4) | 17 (21.0) | 87 (16.2) |
| ≥2 falls | 467 (7.8) | 124 (12.4) | 59 (13.7) | 12 (14.8) | 41 (7.7) |
| PASE scorea | 146.5 (±68.3) | 145.32 (±68.6) | 142.64 (±72.5) | 121.44 (±65.4) | 150.76 (±65.9) |
| Walk speed (m/s)a | 1.20 (±0.23) | 1.17 (±0.24) | 1.17 (±0.25) | 1.12 (±0.33) | 1.22 (±0.22) |
| Excellent or good health statusa | 5135 (85.7) | 842 (84.3) | 363 (84.0) | 66 (81.5) | 467 (87.1) |
| Chair stand without using armsa | 5865 (98.4) | 970 (97.6) | 413 (96.5) | 74 (93.7) | 529 (98.7) |
| Current smoker | 206 (3.4) | 30 (3.0) | 15 (3.5) | 4 (4.9) | 13 (2.4) |
| Alcohol use (≥7 drinks/wk) | 1549 (25.9) | 243 (24.3) | 98 (22.7) | 15 (18.5) | 137 (25.51) |
| Height (cm)a | 174.1 (±6.8) | 174.1 (±7.1) | 173.6 (±7.2) | 172.5 (±6.2) | 174.7 (±6.8) |
| Weight (kg) | 83.1 (±13.3) | 83.0 (±13.5) | 82.2 (±14.0) | 78.8 (±12.8) | 83.5 (±12.9) |
| BMI (kg/m2) | 27.4 (3.8) | 27.3 (±3.9) | 27.2 (±4.1) | 26.5 (±3.8) | 27.3 (±3.7) |
| Kidney function (eGFRmL/min/1.73m2)a | |||||
| <30 | 28 (0.5) | 5 (0.5) | 1 (0.3) | 0 (0.00) | 5 (0.9) |
| 30–59 | 931 (16.8) | 145 (15.3) | 56 (13.8) | 14 (18.4) | 93 (17.3) |
| ≥60 | 4574 (82.7) | 801 (84.2) | 350 (86.0) | 62 (81.6) | 439 (81.8) |
| Vitamin D supplement usea | 3529 (59.3) | 582 (58.6) | 243 (56.8) | 40 (50.6) | 317 (59.4) |
| Serum vitamin D measuresb | |||||
| Total 25(OH)D(ng/mL) | 24.9 (±7.8) | 24.5 (±7.7) | 20.9 (±7.7) | 25.4 (±7.8) | |
| Total 1,25(OH)2D(pg/mL) | 63.9 (±18.0) | 64.2 (±18.6) | 59.5 (±16.9) | 65.2 (±17.6) | |
For the MrOS cohort: 1 missing total hip BMD, 2 missing height, 3 missing PASE, 1 missing health status, 38 missing eGFR, 6missing vitamin D supplement use, 18 missing falls, 34 missing chair stands without using arms.
No data for overall MrOS cohort (n = 5994) because vitamin D measures were not obtained from everyone.
This cohort includes incident fracture cases (n = 321) and random sample (n = 679).
Associations of vitamin D measures with bone turnover markers and calciotropic hormones
Average levels of 25OHD and 1,25(OH)2D were 24.9 ng/mL and 63.9 pg/mL, respectively. 25OHD was moderately positively correlated with 1,25(OH)2D (r = 0.37, p < 0.01) and weakly negatively correlated with PTH (r = −0.22, p < 0.01). Levels of 25OHD were not associated with FGF-23, PINP, or α- or β-CTX concentrations (Table 2). There was a weak, but significant, positive association between 1,25(OH)2D and β-CTX but other 1,25(OH)2D associations were not significant (Table 2). Nonlinearity was not identified in the associations, except for 25OHD and PTH, which had a nonlinear relationship (p for nonlinearity 0.002).
Table 2.
Associations Between Bone Turnover Markers and Calciotropic Hormones With Vitamin D Metabolites
| β (95% CI) for standardized 25(OH)D | β (95% CI) for standardized 1,25(OH)2D | |
|---|---|---|
| PINP (ng/mL) | −0.30 (−2.58, 1.97) | −0.31 (−2.56, 1.93) |
| β-CTX (ng/mL) | −0.09 (−1.37, 1.19) | 1.55 (0.30, 2.80)b |
| α-CTX (µg/L) | 0.07 (−0.40, 0.53) | 0.43 (−0.03, 0.88) |
| PTH (pg/mL) | −3.42 (−4.85, −1.98)a | −1.11(−2.52, 0.30) |
| FGF-23 (pg/mL) | −0.37 (−1.84, 1.10) | −0.35 (−1.74, 1.04) |
Adjusted for age and weight;
p< 0.01;
p< 0.05.
Interactions of vitamin D metabolites and effect of eGFR adjustment
There were no interactions between 25OHD and 1,25(OH)2D in any of the analyses described below (all p ≥ 0.26; data not shown). Adjusting for eGFR in the multivariable models of all analyses (baseline BMD, BMD change, and fractures) did not change the associations with vitamin D metabolites (results not shown), which was expected because the majority (>80%) of the analytical cohort had an eGFR ≥60 mL/min/1.73m2.
Baseline BMD (Table 3)
Table 3.
Associations of Baseline BMD (g/cm2; Presented as Least Square Means [95% CI]) With Quartiles of 25OHD and 1,25(OH)2D
| 25OHD (ng/mL) | Q1(3.13–20.90) n=144 | Q2 (20.91–25.90) n=171 | Q3 (26.00–31.00) n=113 | Q4 (31.10–55.80) n=109 | p trend | βf (95% CI) per SD increase in 25OHD |
| Total hip | ||||||
| Base modela | 0.93 (0.91, 0.95) | 0.94 (0.92, 0.96) | 0.97 (0.94, 0.99) | 0.96 (0.94, 0.99) | 0.013 | 0.02 (0.005, 0.03)d |
| Multib | 0.93 (0.91, 0.95) | 0.94 (0.92, 0.96) | 0.96 (0.94, 0.99) | 0.96 (0.94, 0.98) | 0.022 | 0.01 (0.003, 0.03)e |
| Multib + Dc | 0.92 (0.90, 0.94) | 0.94 (0.92, 0.96) | 0.97 (0.94, 0.99) | 0.97 (0.95, 0.99) | 0.002 | 0.02 (0.01, 0.03)d |
| Lumbar spine | ||||||
| Base modela | 1.12 (1.08, 1.17) | 1.13 (1.09, 1.16) | 1.21 (1.16, 1.25) | 1.18 (1.13, 1.23) | 0.012 | 0.03 (0.006, 0.05)e |
| Multib | 1.12 (1.08, 1.17) | 1.13 (1.09, 1.17) | 1.20 (1.16, 1.25) | 1.18 (1.13, 1.23) | 0.022 | 0.03 (0.003, 0.05)e |
| Multib + Dc | 1.11 (1.06, 1.15) | 1.13 (1.09, 1.17) | 1.21 (1.16, 1.25) | 1.20 (1.15, 1.24) | 0.002 | 0.04 (0.02, 0.07)d |
| 1,25(OH)2D (pg/mL) | Q1 (8.70–51.60)n=121 | Q2 (51.70–62.00)n=135 | Q3 (62.10–75.10)n=143 | Q4 (75.20–142.00)n=138 | p trend | βg (95% CI) per SD increase in 1,25(OH)2D |
| Total hip | ||||||
| Base modela | 0.97 (0.95, 0.99) | 0.96 (0.93, 0.98) | 0.94 (0.92, 0.96) | 0.93 (0.91, 0.95) | 0.006 | −0.01 (−0.02, −0.0002)e |
| Multib | 0.97 (0.95, 1.00) | 0.95 (0.93, 0.97) | 0.94 (0.92, 0.96) | 0.92 (0.90, 0.94) | 0.001 | −0.01 (−0.03, −0.004)d |
| Multib + Dc | 0.98 (0.96, 1.00) | 0.95 (0.93, 0.97) | 0.94 (0.92, 0.96) | 0.92 (0.90, 0.94) | <0.001 | −0.02 (−0.03, −0.01)d |
| Lumbar spine | ||||||
| Base modela | 1.21 (1.16, 1.25) | 1.15 (1.11, 1.20) | 1.15 (1.11, 1.19) | 1.11 (1.06, 1.15) | 0.002 | −0.03 (−0.05, −0.005)e |
| Multib | 1.22 (1.17, 1.27) | 1.15 (1.11, 1.20) | 1.15 (1.11, 1.19) | 1.10 (1.06, 1.14) | <0.001 | −0.03 (−0.06, −0.01)d |
| Multib + Dc | 1.23 (1.19, 1.28) | 1.15 (1.11, 1.19) | 1.15 (1.11, 1.19) | 1.09 (1.05, 1.14) | <0.001 | −0.05 (−0.07, −0.02)d |
Adjusted for age, race, site, season, physical activity, height, and weight.
Adjusted for age, race, site, season, physical activity, height, weight, health status, smoking, alcohol, and inability to rise from chair.
Adjusted for other vitamin D measure; that is, the 25OHD association is adjusted for 1,25(OH)2D and vice versa.
p<0.01.
p<0.05.
Units for β: g/cm2 per standard deviation increase in 25OHD.
Units for β: g/cm2 per standard deviation increase in 1,25(OH)2D.
In base and multivariable models, baseline BMD was associated with both vitamin D metabolites to a similar degree but in opposite directions. At baseline, for each SD increase in 25OHD, BMD was 0.02 g/cm2 higher at the hip (p for β < 0.01) and 0.04 g/cm2 higher at the L-spine (p for β < 0.01) in multivariable models adjusted for 1,25(OH)2D. Conversely, for each SD increase in 1,25(OH)2D, BMD was 0.02 g/cm2 lower at the hip (p for β < 0.01) and 0.05 g/cm2 lower at the L-spine (p for β < 0.01) at baseline in multivariable models adjusted for 25OHD. Data for the femoral neck were similar to the total hip for both baseline BMD and BMD change (data not shown).
Bone loss (Table 4)
Table 4.
Mean Annualized Percent Change, % (95% CI) in BMD by Quartile of 25OHD or 1,25(OH)2D
| 25OHD (ng/mL) | Q1(3.13−20.90) n=144 | Q2 (20.91–25.90) n=171 | Q3 (26.00–31.00) n=113 | Q4 (31.10–55.80) n=109 | p trend | βe (95% CI) per SD increase in 25OHD |
| Total hip | ||||||
| Base modela | −0.68 (−0.83, −0.52) | −0.45 (−0.59, −0.31) | −0.30 (−0.47, −0.12) | −0.51 (−0.68, −0.33) | 0.066 | 0.10 (0.009, 0.18)d |
| Multib | −0.66 (−0.82, −0.50) | −0.46 (−0.59, −0.32) | −0.30 (−0.47, −0.13) | −0.50 (−0.68, −0.33) | 0.087 | 0.09 (0.005, 0.18)d |
| Multi + Dc | −0.67 (−0.83, −0.52) | −0.46 (−0.60, −0.32) | −0.29 (−0.46, −0.12) | −0.49 (−0.68, −0.31) | 0.094 | 0.10 (0.01, 0.20)d |
| Lumbar spine | ||||||
| Base modela | 0.92 (0.30, 1.54) | 1.27 (0.72, 1.81) | 1.30 (0.63, 1.97) | 1.57 (0.87, 2.27) | 0.198 | 0.34 (0.001, 0.69)d |
| Multib | 0.94 (0.32, 1.57) | 1.23 (0.68, 1.77) | 1.25 (0.58, 1.92) | 1.61 (0.91, 2.32) | 0.200 | 0.34 (−0.006, 0.69) |
| Multi + Dc | 0.90 (0.26, 1.54) | 1.23 (0.68, 1.78) | 1.26 (0.58, 1.93) | 1.67 (0.94, 2.39) | 0.161 | 0.45 (0.07, 0.82)d |
| 1,25(OH)2D (pg/mL) | Q1 (8.70–51.60)n=121 | Q2 (51.70–62.00)n=135 | Q3 (62.10–75.10)n=143 | Q4 (75.20–142.00)n=138 | p trend | βf (95% CI) per SD increase in 1,25(OH)2D |
| Total hip | ||||||
| Base modela | −0.49 (−0.66, −0.33) | −0.63 (−0.79, −0.47) | −0.38 (−0.53, −0.23) | −0.47 (−0.63, −0.31) | 0.351 | 0.03 (−0.05, 0.12) |
| Multib | −0.43 (−0.60, −0.26) | −0.65 (−0.81, −0.49) | −0.39 (−0.54, −0.24) | −0.48 (−0.64, −0.32) | 0.724 | 0.004 (−0.08, 0.09) |
| Multi + Dc | −0.38 (−0.55, −0.21) | −0.66 (−0.82, −0.50) | −0.41 (−0.57, −0.26) | −0.49 (−0.65, −0.33) | 0.945 | −0.03 (−0.12, 0.06) |
| Lumbar spine | ||||||
| Base modela | 1.32 (0.66, 1.98) | 1.30 (0.67, 1.92) | 1.28 (0.68, 1.87) | 1.11 (0.49, 1.72) | 0.654 | −0.15 (−0.47, 0.18) |
| Multib | 1.30 (0.62, 1.99) | 1.29 (0.66, 1.91) | 1.22 (0.61, 1.82) | 1.16 (0.54, 1.79) | 0.741 | −0.12 (−0.46, 0.21) |
| Multi + Dc | 1.40 (0.70, 2.10) | 1.30 (0.67, 1.92) | 1.23 (0.62, 1.84) | 1.05 (0.41, 1.70) | 0.507 | −0.28 (−0.63, 0.08) |
Adjusted for age, race, site, season, physical activity, height, weight, and corrected longitudinal BMD adj (of that region).
Adjusted for age, race, site, season, physical activity, height, weight, corrected longitudinal BMD adj (of that region), health status, smoking, alcohol, and inability to rise from chair.
Adjusted for other vitamin D measure; that is, the 25OHD association is adjusted for 1,25(OH)2D and vice versa.
p<0.05.
Units for β: % mean annualized percent change per standard deviation increase in 25OHD.
Units for β: % mean annualized percent change per standard deviation increase in 1,25(OH)2D.
In all models, higher 25OHD was associated with less bone loss at the hip even after adjustment for 1,25(OH)2D (β = 0.10% mean annualized percent change per standard deviation increase in 25OHD, p for β < 0.05 in multivariable model adjusted for 1,25(OH)2D). Men in the lowest quartile of 25OHD had the greatest rate of bone loss at the hip (−0.66% mean annualized percent change in the multivariable model, Table 4). At the L-spine, each SD increase in 25OHD was associated with slower bone loss (β = 0.45% mean annualized percent change per standard deviation increase in 25OHD, p for β < 0.05 in multivariable model adjusted for 1,25(OH)2D), but this trend became nonsignificant when 25OHD was analyzed by quartiles (Table 4). It is unclear if the apparent gain in BMD found at the L-spine is because of true accrual of bone mass and/or artifact from increasing degenerative changes. There were no significant associations between 1,25(OH)2D and rate of bone loss at the hip or L-spine (Table 4).
Fractures (Tables 5 and 6)
Table 5.
Association of Nonvertebral Fracture (Fx) With Quartiles of 25OHD or 1,25(OH)2D (HR, 95% CI) (n = 1000)
| Fx (n) | Base modela | Model 2b | Model 3c | Model 4d | |
|---|---|---|---|---|---|
| 25OHD (ng/mL) | |||||
| Per SD increase in vitamin D | 0.97 (0.87, 1.09) | 1.01 (0.90, 1.13) | 1.02 (0.90, 1.15) | 1.01 (0.90, 1.13) | |
| Q1 (3.13–20.90) | 131 | Referent | Referent | Referent | Referent |
| Q2 (20.91–25.90) | 118 | 0.90 (0.70, 1.16) | 0.93 (0.72, 1.21) | 0.94 (0.72, 1.22) | 0.94 (0.73, 1.22) |
| Q3 (26.00–31.00) | 106 | 1.10 (0.84, 1.44) | 1.17 (0.89, 1.53) | 1.18 (0.90, 1.54) | 1.17 (0.90, 1.54) |
| Q4 (31.10–55.80) | 76 | 0.95 (0.70, 1.29) | 1.04 (0.77, 1.41) | 1.05 (0.77, 1.44) | 1.04 (0.77, 1.41) |
| p for trend | 0.84 | 0.43 | 0.39 | 0.43 | |
| 1,25(OH)2D (pg/mL) | |||||
| Per SD increase in vitamin D | 1.02 (0.92, 1.13) | 0.99 (0.89, 1.10) | 0.99 (0.88, 1.10) | 0.99 (0.89, 1.10) | |
| Q1 (8.70–51.60) | 108 | Referent | Referent | Referent | Referent |
| Q2 (51.70–62.00) | 98 | 0.90 (0.68, 1.19) | 0.88 (0.66, 1.16) | 0.87 (0.66, 1.15) | 0.88 (0.67, 1.16) |
| Q3 (62.10–75.10) | 116 | 1.04 (0.79, 1.36) | 0.97 (0.74, 1.27) | 0.95 (0.72, 1.25) | 0.97 (0.74, 1.27) |
| Q4 (75.20–142.00) | 110 | 1.02 (0.77, 1.35) | 0.94 (0.71, 1.25) | 0.93 (0.69, 1.25) | 0.94 (0.71, 1.25) |
| p for trend | 0.68 | 0.86 | 0.70 | 0.83 | |
Base model adjusted for age, race, site, season, physical activity, height, and weight.
Model 2 = Base model adjusted for baseline hip BMD.
Model3 = Model 2 adjusted for other vitamin D measure; that is, the 25OHD association is adjusted for 1,25(OH)2D and vice versa.
Model 4 = Model 2 adjusted for incident falls in the first year of follow-up.
Table 6.
Hip Fracture (Fx) and Association With Quartiles of 25OHD or 1,25(OH)2D(HR, 95% CI) (n =739)
| Fx (n) | Base modela | Model 2b | Model 3c | Model 4d | |
|---|---|---|---|---|---|
| 25OHD (ng/mL) | |||||
| Per SD increase in vitamin D | 0.69 (0.52, 0.91)e | 0.69 (0.52, 0.93)f | 0.75 (0.54, 1.02) | 0.69 (0.51, 0.93)f | |
| Q1 (3.13–20.90) | 44 | Referent | Referent | Referent | Referent |
| Q2 (20.91–25.90) | 13 | 0.38 (0.20, 0.72) | 0.38 (0.20, 0.73) | 0.41 (0.21, 0.78) | 0.38 (0.20, 0.72) |
| Q3 (26.00–31.00) | 15 | 0.69 (0.37, 1.26) | 0.67 (0.36, 1.27) | 0.72 (0.37, 1.39) | 0.67 (0.36, 1.27) |
| Q4 (31.10–55.80) | 8 | 0.45 (0.21, 1.00) | 0.53 (0.23, 1.22) | 0.55 (0.23, 1.31) | 0.52 (0.23, 1.21) |
| p for trend | 0.04 | 0.07 | 0.17 | 0.07 | |
| Q2, Q3, Q4 | Referent | Referent | Referent | Referent | |
| Q1 | 2.05 (1.28,3.29)f | 2.01 (1.24,3.26)f | 1.86 (1.14,3.05)e | 2.03 (1.25,3.29)f | |
| 1,25(OH)2D (pg/mL) | |||||
| Per SD increase in vitamin D | 0.86 (0.66, 1.13) | 0.74 (0.56, 1.00)f | 0.82 (0.60, 1.12) | 0.74 (0.56, 1.00)f | |
| Q1 (8.70–51.60) | 31 | Referent | Referent | Referent | Referent |
| Q2 (51.70–62.00) | 15 | 0.64 (0.34, 1.22) | 0.49 (0.25, 0.95) | 0.51 (0.26, 1.00) | 0.49 (0.25, 0.94) |
| Q3 (62.10–75.10) | 19 | 0.77 (0.43, 1.39) | 0.51 (0.28, 0.95) | 0.55 (0.30, 1.04) | 0.50 (0.27, 0.94) |
| Q4 (75.20–142.00) | 16 | 0.79 (0.42, 1.50) | 0.50 (0.25, 1.00) | 0.61 (0.30, 1.27) | 0.51 (0.26, 1.01) |
| p for trend | 0.47 | 0.05 | 0.11 | 0.05 | |
| Q2, Q3, Q4 | Referent | Referent | Referent | Referent | |
| Q1 | 1.37 (0.85, 2.19) | 1.99 (1.19,3.33)f | 1.80 (1.07,3.04)e | 2.01 (1.20,3.36)f | |
Base model adjusted for age, race, site, season, physical activity, height, and weight.
Model 2 = Base model adjusted for baseline hip BMD.
Model 3 = Model 2 adjusted for other vitamin D measure; that is, the 25OHD association is adjusted for 1,25(OH)2D and vice
Model 4 = Model 2 adjusted for incident falls in the first year of follow-up.
p< 0.01.
p< 0.05.
Restricted cubic splines and plots based on Cox proportional hazard regression models showed linear associations of both 25OHD and 1,25(OH)2D with hip fracture (p for nonlinearity = 0.30 and 0.25, respectively) and nonvertebral fracture risk (p for nonlinearity = 0.69 and 0.25, respectively). No threshold level of either metabolite was identified. However, as described below and in Table 6, the largest proportion of fracture cases fell into the lowest quartile of each vitamin D metabolite. Therefore, hazard ratios (HRs) for the lowest quartile compared with the upper three quartiles are also reported.
The risk of nonvertebral fracture was not associated with 25OHD or 1,25(OH)2D in base or multivariable analyses (all nonsignificant with HR per SD increase in each vitamin D measure from 0.97 to 1.02; Table 5).
Both vitamin D metabolites were significantly lower in those who sustained a hip fracture compared with those who did not (25OHD 20.9 ng/mL versus 25.2 ng/mL, p < 0.001 and 1,25(OH)2D 59.5 pg/mL versus 64.3 pg/mL, p = 0.02).
The risk of hip fracture was approximately 30% lower (HR = 0.69, 95% confidence interval [CI] 0.52–0.91) per SD increase in 25OHD (Table 6, base model). When adjusted for baseline BMD and falls, the magnitude of this protective effect was preserved (Table 6, model 4). When the associations with fracture were examined as a function of 25OHD quartiles, the risk tended to be lower in men with higher 25OHD values than in those in the lowest quartile in all models, but the association was significant only in those with 25OHD levels 20.91 to 25.90 ng/mL. When the higher quartiles were used as the referent group, those in the lowest quartile (25OHD 3.13 to 20.90 ng/mL) had a significantly increased risk of hip fracture in all models (Table 6). Adjusting for 1,25(OH)2D in analyses of the associations between 25OHD and fractures did not affect the nonvertebral results but did attenuate the hip fracture protection (Tables 5 and 6, model 3).
When examined as a continuous variable, men with higher 1,25(OH)2D tended to have a lower risk of hip fracture, but the association was significant only after BMD adjustment (Table 6). When compared with men in the lowest quartile of 1,25(OH)2D, those with higher 1,25(OH)2D levels had a lower risk of hip fracture in the BMD-adjusted model (model 2). Further adjustment for 25OHD somewhat attenuated that association (Table 6, model 3). Compared with the higher quartiles, those in the lowest quartile of 1,25(OH)2D (8.70 to 51.60 pg/mL) were at significantly increased risk of hip fracture, comparable in magnitude to that found in the lowest quartiles of 25OHD, in all models except for the base model (Table 6). The associations between 1,25(OH)2D and hip fracture were not affected by adjustment for falls or walk speed (walk speed data not shown). Because of the low number of hip fractures, the 1,25(OH)2D–hip fracture association could not be examined by quartiles (or tertiles) of baseline BMD. However, although most participants with hip fracture had low total hip BMD, very few (n = 7) participants with hip fracture had total hip BMD above the median of the BMD distribution for the study. These 7 participants had 1,25(OH)2D below the median.
Discussion
As previously reported, higher levels of 25OHD were associated with higher baseline BMD, slower bone loss at the hip, and fewer hip (but not nonvertebral) fractures in older men. On the other hand, men with higher 1,25(OH)2D levels had lower BMD at baseline, whereas paradoxically tending to have a lower risk of hip fracture, after adjustment for baseline BMD. Additionally, levels of 1,25(OH)2D were not associated with bone loss or the risk of nonvertebral fractures. The associations of 25OHD and 1,25(OH)2D with baseline BMD and BMD change were independent of each other. These results do not support the hypothesis that measures of 1,25(OH)2D improve the ability to predict adverse skeletal outcomes when 25OHD measures are available.
The inverse relationship between 1,25(OH)2D and baseline BMD identified in the MrOS cohort is similar to that identified in the CARDIA study(7) and EMAS cohort.(8) It is possible that this inverse relationship is related to the weak, but significant, positive correlation found between 1,25(OH)2D and urinary β-CTX, a result similar to that reported in EMAS.(8) However, if this correlation represents greater bone resorption among men with higher 1,25(OH)2D, we would have expected to see greater hip BMD loss in men with higher 1,25(OH)2D, but we did not. There was also no association between 1,25(OH)2D and hip BMD loss in a large cohort of community-dwelling elderly white women.(2) Higher levels of bone resorption markers have been associated with higher rates of bone loss and fracture,(32) but those results have not been consistent.(33) It is also possible that this weak association between 1,25(OH)2D and increased bone resorption only manifests as BMD loss over a long period of time (potentially reflected in this analysis by the baseline BMD reflecting cumulative BMD loss over a lifetime) and the duration of our longitudinal BMD analysis (~4.5 years) was not long enough to capture the change in BMD. This is a potential explanation that unifies the relationships between 1,25(OH)2D and bone resorption, BMD, and BMD change, and our hope is that this can be investigated in future studies.
This is the first analysis to describe the relationship between calcitriol and fracture in older men. In the current study, the hip fracture risk appears to be greatest for the lowest quartile of 1,25(OH)2D (8.70 to 51.60 pg/mL). The association in the lowest quartile was significant only after adjusting for baseline BMD and might be driven by the very few hip fracture cases that had relatively high BMD but low 1,25(OH)2D. In contrast, a previous study in older women(10) found an increased risk of hip but not vertebral fracture with low 1,25(OH)2D levels that was present both before and after BMD adjustment. In the current study, the association between 25OHD and hip fracture was unchanged by BMD adjustment; however, previous studies suggested that the hip fracture protection found with higher 25OHD levels is largely mediated by BMD.(6) These discordant results could be attributable to non-BMD–related effects of 25OHD on fracture protection and/or the possibility that baseline BMD inadequately captures all the BMD-related ways 25OHD is protective of hip fracture. FGF-23 inhibits formation of calcitriol and has been associated with fracture in some, but not all, studies.(21,34,35) However, FGF-23 is unlikely to be a confounder in the vitamin D–hip fracture relationships identified in this study because it was not associated with either vitamin D metabolite.
Our results support the importance of 25OHD for bone health: Higher 25OHD was associated with higher BMD at baseline and slower bone loss at the hip, both of which are favorable for decreasing the risk of hip fracture. In contrast, the lower BMD at baseline and lack of association with rate of bone loss observed with calcitriol seems to contradict a skeletally mediated hip fracture protection afforded by higher 1,25(OH)2D levels. Therefore, it seems likely that, similar to 25OHD, there may be non-BMD–related pathways by which 1,25(OH)2D may be protective of hip fracture.
Positive effects of 1,25(OH)2D on muscle function may be a partial explanation for our finding of an association with hip fracture after BMD adjustment. Although most literature on the effects of vitamin D on muscle focuses on 25OHD, there is evidence for muscle effects of 1,25(OH)2D with both genomic and nongenomic actions. The presence of the vitamin D receptor (VDR) in adult muscle is disputed(36–38) because the protein could not be identified when using specific antibodies.(39) However, more recent studies suggest VDR in adult mouse muscle could be identified when using hyperosmolar lysis buffer to release VDR from tight binding to DNA.(40) Older women receiving calcitriol were found to have slower rates of decline in physical performance tests(41) and improved lower-extremity strength and walking distance.(42) On the other hand, no reduction in fracture risk has been observed with calcitriol supplementation(43) and, in this analysis, adjusting for falls and walk speed did not significantly impact the fracture results. But falls are difficult to accurately ascertain,(44) and it is possible that an effect of 1,25(OH)2D on falls was not adequately captured by our assessment. Moreover, the effects of 1,25(OH)2D on muscle may be complex. For instance, the effects may be sex, race, or age specific and may be dependent on VDR polymorphisms and/or PTH-mediated effects.(36)
In general, 25OHD appears to be more consistently and strongly associated with skeletal outcomes compared with 1,25(OH)2D. The magnitude of the association between baseline BMD and the vitamin D metabolites are similar but in opposite directions, such that higher 25OHD and lower 1,25(OH)2D are associated with higher BMD at baseline. Higher levels of both vitamin D metabolites appear to be associated with a lower risk of hip fracture. Attenuation of the protective association of both 25OHD and 1,25(OH)2D when they are simultaneously included in a model is owing to their correlation with each other (r = 0.37, p < 0.01). However, they remain statistically significant, suggesting that both vitamin D metabolites are independently related to hip fracture. Although these analyses are insufficient for establishing the role of 1,25(OH)2D measurements in clinical practice, early evidence suggests that the relationships between 25OHD and skeletal outcomes are stronger than those of 1,25(OH)2D. Because 1,25(OH)2D is mainly regulated as to maintain serum calcium and phosphate homeostasis and may have beneficial or negative effects on bone, a complex relationship between 1,25(OH)2D and bone mass or turnover is to be expected.(45) Future studies should evaluate the predictive abilities and cost/benefit ratio of routine measurement of calcitriol levels in clinical settings.
Strengths of this study include its large sample size and the standardized collection of incident fractures and longitudinal BMD data. In addition, we used sensitive and specific LC-MS/MS assays that provide precise assessments of both 25OHD and 1,25(OH)2D levels. We also recognize that there are several limitations to these data. This analysis was performed in a predominantly white cohort of older men and results may differ in those of different age, sex, or ethnicity. Additionally, despite the large sample size, this cohort may have been underpowered to detect small effect sizes and associations between vitamin D levels and baseline BMD, the rate of BMD change, and fractures. It is possible that the associations between 25OHD, 1,25(OH)2D, and fracture risk are different in men with more severe vitamin D deficiency. However, the very low number of hip fracture cases in men with these levels precluded meaningful analyses. Additionally, falls were ascertained at 4-month intervals and therefore are subject to misclassification, and these analyses use a single serum sample at one time point and may not be representative of calcitriol status over time, particularly because calcitriol has a relatively short half-life. Finally, these analyses do not address the issue of the effects of free vitamin D levels; future research should evaluate how vitamin D binding protein and free vitamin D affect these associations, if at all.
In summary, in this predominantly white cohort of older men, higher 1,25(OH)2D levels were associated with lower baseline BMD and were not associated with rates of BMD change or nonvertebral fracture. Men with higher 1,25(OH)2D levels had a lower risk of subsequent hip fracture only after adjustment for BMD. The associations of 1,25(OH)2D with skeletal outcomes were generally weaker than those with 25OHD. These results do not support the hypothesis that measures of 1,25(OH)2D improve the ability to predict adverse skeletal outcomes when 25OHD measures are available.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. This study was supported in part by an independent investigator grant (SRA-12-009) from Merck & Co., Inc. CMS is supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant T32DK007674-20. CGL receives support from a VA Clinical Science Research and Development (CSR&D) Career Development Award, project number 5IK2CW000729-02.
Footnotes
Disclosures
CMS, PS, CGL, SRC, IJ, JAC, RB, DV, and CMN state that they have no conflicts of interest. ESO consults for and has received research support from Merck, Lilly, and Amgen.
Authors’ roles: Study design: CMN and ESO. Study conduct: SRC, JAC, and ESO. Data collection: SRC, JAC, and ESO. Data analysis: PS and CMN. Data interpretation: CMS, PS, ESO, CMN, DV, JAC, and CGL. Drafting manuscript: CMS. Revising manuscript content: CMS, PS, ESO, CMN, DV, RB, JAC, and CGL. Approving final version of manuscript: CMS, ESO, CMN, PS, DV, RB, IJ, and CGL. CMN takes responsibility for the integrity of data analysis.
References
- 1.Ensrud KE, Taylor BC, Paudel ML, et al. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94(8):2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone K, Bauer DC, Black DM, Sklarin P, Ensrud KE, Cummings SR. Hormonal predictors of bone loss in elderly women: a prospective study. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1998;13(7):1167–1174. doi: 10.1359/jbmr.1998.13.7.1167. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28(5):997–1006. doi: 10.1002/jbmr.1828. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Parimi N, Ensrud KE, et al. Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J Bone Miner Res. 2010;25(3):545–553. doi: 10.1359/jbmr.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiyoshi A, Polgreen LE, Hurley DL, Gross MD, Sidney S, Jacobs DR., Jr A cross-sectional association between bone mineral density and parathyroid hormone and other biomarkers in community-dwelling young adults: the CARDIA study. J Clin Endocrinol Metab. 2013;98(10):4038–4046. doi: 10.1210/jc.2013-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderschueren D, Pye SR, O’Neill TW, et al. Active vitamin D (1,25-dihydroxyvitamin D) and bone health in middle-aged and elderly men: the European Male Aging Study (EMAS) J Clin Endocrinol Metab. 2013;98(3):995–1005. doi: 10.1210/jc.2012-2772. [DOI] [PubMed] [Google Scholar]
- 9.Lips P, van Ginkel FC, Jongen MJ, Rubertus F, van der Vijgh WJ, Netelenbos JC. Determinants of vitamin D status in patients with hip fracture and in elderly control subjects. Am J Clin Nutr. 1987;46(6):1005–1010. doi: 10.1093/ajcn/46.6.1005. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339(11):733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 11.Casetta B, Jans I, Billen J, Vanderschueren D, Bouillon R. Development of a method for the quantification of 1alpha,25(OH)2-vitamin D3 in serum by liquid chromatography tandem mass spectrometry without derivatization. Eur J Mass Spectrom (Chichester, Eng) 2010;16(1):81–89. doi: 10.1255/ejms.1024. [DOI] [PubMed] [Google Scholar]
- 12.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Orwoll E, Nielson CM, Marshall LM, et al. Vitamin D deficiency in older men. J Clin Endocrinol Metab. 2009;94(4):1214–1222. doi: 10.1210/jc.2008-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson CM, Nielson CM, Shrestha S, et al. Higher 25(OH)D2 is associated with lower 25(OH)D3 and 1,25(OH)2D3. J Clin Endocrinol Metab. 2014;99(8):2736–2744. doi: 10.1210/jc.2014-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer DC, Garnero P, Harrison SL, et al. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. J Bone Miner Res. 2009;24(12):2032–2038. doi: 10.1359/JBMR.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lhoste Y, Garnero P. Validation report for the measurements of degradation products of C-terminal telopeptides of type I collage in human urine (U-Alpha-CTX-I) (ALPHA CrossLaps, Nordic Bioscience Diagnostics) Synarc. 2007 [Google Scholar]
- 18.Garnero P, Lhoste Y. Validation report for the measurements of type I collagen peptide in urine sample (U-CTX-I Elecsys) (Beta CrossLaps, ELECSYS 2010 System, Roche Diagnostics) Synarc. 2005 [Google Scholar]
- 19.Boudou P, Ibrahim F, Cormier C, Chabas A, Sarfati E, Souberbielle JC. Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90(12):6370–6372. doi: 10.1210/jc.2005-0715. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JR, Ewing SK, Bauer DC, et al. Association of intact parathyroid hormone levels with subsequent hip BMD loss: the Osteoporotic Fractures in Men (MrOS) Study. J Clin Endocrinol Metab. 2012;97(6):1937–1944. doi: 10.1210/jc.2011-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane NE, Parimi N, Corr M, et al. Association of serum fibroblast growth factor 23 (FGF23) and incident fractures in older men: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res. 2013;28(11):2325–2332. doi: 10.1002/jbmr.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin MH, Zmuda JM, Barrett-Connor E, et al. Race/ethnic differences in associations between bone mineral density and fracture history in older men. Osteoporos Int. 2014;25(3):837–845. doi: 10.1007/s00198-013-2503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288(15):1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 24.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22(2):211–219. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 25.Parsons JK, Mougey J, Lambert L, et al. Lower urinary tract symptoms increase the risk of falls in older men. BJU Int. 2009;104(1):63–68. doi: 10.1111/j.1464-410X.2008.08317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 28.Shannon J, Shikany JM, Barrett-Connor E, et al. Demographic factors associated with the diet quality of older US men: baseline data from the Osteoporotic Fractures in Men (MrOS) study. Public Health Nutr. 2007;10(8):810–818. doi: 10.1017/S1368980007258604. [DOI] [PubMed] [Google Scholar]
- 29.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 31.Daniels NA, Nielson CM, Hoffman AR, Bauer DC Osteoporotic Fractures in Men Study. Sex hormones and the risk of incident prostate cancer. Urology. 2010;76(5):1034–1040. doi: 10.1016/j.urology.2010.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melton LJ, 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12(7):1083–1091. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 33.Bauer DC, Sklarin PM, Stone KL, et al. Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res. 1999;14(8):1404–1410. doi: 10.1359/jbmr.1999.14.8.1404. [DOI] [PubMed] [Google Scholar]
- 34.Mirza MA, Karlsson MK, Mellstrom D, et al. Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res. 2011;26(4):857–864. doi: 10.1002/jbmr.263. [DOI] [PubMed] [Google Scholar]
- 35.Jovanovich A, Buzkova P, Chonchol M, et al. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Clin Endocrinol Metab. 2013;98(8):3323–3331. doi: 10.1210/jc.2013-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceglia L, Simpson RU. Vitamin D and skeletal muscle function. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3rd ed. San Diego, CA: Academic Press/Elsevier; 2011. [Google Scholar]
- 37.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260(15):8882–8891. [PubMed] [Google Scholar]
- 38.Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, DeLuca HF. Is the vitamin D receptor found in muscle? Endocrinology. 2011;152(2):354–363. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 40.Girgis CM, Mokbel N, Minn Cha K, et al. The Vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014 doi: 10.1210/en.2014-1016. en20141016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. J Steroid Biochem Mol Biol. 2004;89–90(1–5):497–501. doi: 10.1016/j.jsbmb.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 42.Verhaar HJ, Samson MM, Jansen PA, de Vreede PL, Manten JW, Duursma SA. Muscle strength, functional mobility and vitamin D in older women. Aging. 2000;12(6):455–460. doi: 10.1007/BF03339877. [DOI] [PubMed] [Google Scholar]
- 43.Avenell A, Gillespie WJ, Gillespie LD, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227. doi: 10.1002/14651858.CD000227.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Hannan MT, Gagnon MM, Aneja J, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am J Epidemiol. 2010;171(9):1031–1036. doi: 10.1093/aje/kwq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisman JA, Bouillon R. Vitamin D: direct effects of vitamin D metabolites on bone: lessons from genetically modified mice. Bonekey Rep. 2014;3:499. doi: 10.1038/bonekey.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]