Abstract
Detection of donor-specific anti-HLA antibodies (DSA) has been associated with graft rejection in all forms of transplantation. The mechanism by which DSA increase the risk of graft failure remains unclear. We hypothesized that complement-binding DSA are associated with engraftment failure in hematopoietic stem-cell transplantation and analyzed 122 haploidentical transplant recipients tested prospectively for DSA. Retrospective C1q testing was done on 22 allosensitized recipients. Twenty-two of 122 patients (18%) had DSA, 19 of which were females (86%). Seven patients with DSA (32%) rejected the graft. Median DSA level at transplant for patients who failed to engraft was 10,055 MFI versus 2,065 MFI for those who engrafted (p=0.007). Nine patients with DSA were C1q positive in the initial samples with median DSA level 15,279 MFI (range 1,554-28,615), compared with 7 C1q negative patients with median DSA level 2,471 MFI (665-12,254) (p=0.016). Of 9 patients, who tested positive for C1q in the initial samples, 5 patients remained C1q positive at time of transplant [all with high DSA levels (median 15,279, range 6,487-22,944)] and experienced engraftment failure, while 4 patients became C1q negative pre-transplant and all engrafted the donor cells (p=0.008). In conclusion, patients with high DSA levels (> 5,000 MFI) and complement-binding antibodies (C1q positive) appear to be at much higher risk of primary graft failure. C1q should be assessed in patients with DSA prior to hematopoietic stem-cell transplantation. Reduction of DSA to non-complement binding levels might prevent engraftment failure in hematopoietic stem cell transplantation.
Keywords: Donor-specific anti-HLA antibodies, complement-binding DSA, C1q, graft rejection, hematopoietic stem cell transplantation, desensitization, buffy coat
Introduction
Allosensitization is a common problem in both solid organ and hematopoietic stem-cell transplantation (HSCT).(1, 2) Approximately 50% of all patients requiring a transplant could become allosensitized and develop anti-HLA antibodies, and up to 30% of patients might have donor-specific anti-HLA antibodies (DSA) which pose a threat to organ rejection or graft failure (GF) in HSCT.(3, 4) Our group initially showed that DSA are associated with primary GF in HSCT with mismatched donors.(5, 6) While a clear association between DSA and GF in HSCT has been subsequently demonstrated,(7-11) the mechanism by which DSA may cause GF in HSCT remains unclear.
Activation of the complement cascade has been shown in allosensitized recipients of solid organ transplantation and has been suspected in animal models of HSCT.(12, 13) The classical pathway of the complement cascade is activated when the antigen-antibody complex binds C1q, initiates activation of other complement components resulting in the formation of membrane attack complex, which in turn causes cell lysis with apoptosis and clearance of the targeted cells.(14, 15)
In HSCT, DSA that target donor HLA antigens present on the surface of hematopoietic progenitor cells and antigen-antibody complexes may bind C1q, activate the complement cascade and cause destruction of the donor cells resulting in allograft rejection. C1q testing was developed to assess complement cascade activation in allosensitized recipients of solid organ transplants;(16, 17) however, whether complement cascade activation represents a mechanism which mediates graft rejection in HSCT remains unclear.
Here we hypothesized that complement-binding DSA might be associated with primary GF in HSCT, and assessed the joint impact of DSA and C1q activation in a cohort of allosensitized recipients.
Methods
Patients
One hundred and twenty-two consecutive patients received a haploidentical stem-cell transplant at the University of Texas MD Anderson Cancer Center (MDACC) between 09/2005 - 09/2013, 21 (17%) with T cell depletion (CD34+ selection), and 101 (83%) using a T cell replete bone marrow graft and post-transplantation cyclophosphamide, tacrolimus and mycophenolate for GVHD prevention as previously reported by us.(18, 19) Patients were tested prospectively between 2008-2013, while a small number of patients (treated before 2008) were tested retrospectively for the presence of DSA in the pre-transplant specimens. Retrospective C1q testing was done in bank serum samples for all patients with DSA.
DSA testing
Pre-transplant sera of all patients were tested prospectively for anti-HLA class I and class II antibodies using multianalyte bead assays performed on the Luminex platform including LABScreen® PRA, LABScreen® Mixed methods for screening; the binding level of donor-specific antibody was determined by the LABScreen® Single Antigen bead assay (One Lambda), Part of Thermo Fisher Scientific (Canoga Park, California, USA) per manufacturer's instructions and results were expressed as mean fluorescence intensity (MFI). Briefly, 5 μl of mixed beads, HLA class I and class II single antigen beads were added to 20 μl of sample serum, and incubated for 30 min at room temperature (RT) in the dark with gentle shaking. After washing with wash buffer three times, 100 μl of goat anti-human IgG secondary antibody conjugated with R-phycoerythrin (PE) was added and the samples were incubated in the dark for 30 min at RT. After washing three times, the samples were read on Luminex-based LABScan™ 100 flow analyzer. Antibody specificity and binding level were analyzed and determined through HLA Visual or HLA Fusion software from the manufacturer.
C1q testing
Complement-binding antibodies were detected retrospectively for patients with DSA using the C1q assay as reported by Chen G, et al.(16) The complement component (C1q) bound by the antigen-antibody complex was detected with an R-PE labeled anti-C1q antibody. Fluorescence intensity was measured using Luminex-based LABScan™ 100 flow analyzer. DSA specificity and binding level were determined by the C1qScreen™ assay per manufacturer's instructions [One Lambda, Part of Thermo Fisher Scientific (Canoga Park, California, USA)]. Briefly, 5 μl of human C1q and 5 μl of HLA class I and class II single antigen beads were added to 5 μl of heat-inactivated sample serum and incubated for 20 min in dark at RT, followed by adding 5 μl of R-PE labeled anti-C1q antibody and incubation for 20 min in dark at RT. The samples were read and C1q specific antibody specificity and binding levels were analyzed and determined.
Treatment of patients with DSA prior to transplantation
Twelve patients with DSA (55%) received desensitization treatment prior to transplant with alternate day plasma exchange (PE) × 3 (1-1.5 × plasma volume) replaced with either fresh frozen plasma or with albumin, starting one week prior to admission for transplantation/beginning of conditioning chemotherapy, followed by one dose of rituximab (Rituxan™) 375mg/m2 the next day after completion of PE, followed one day later by one dose of intravenous gammaglobulin (IVIG) 1 g/kg × 1 (PE/R/IVIG).(5) In addition, 5 patients received a buffy coat infused on day -1, prepared from the same haploidentical donor.
Buffy coat preparation
Bone Marrow stem cell donors underwent two autologous phlebotomies within 30 days of the scheduled bone marrow harvest. On both occasions, 500 ml of whole blood was collected in citrate-phosphate-dextrose adenine (CPDA-1) collection bags. The buffy coat was prepared from the second phlebotomy performed two days prior to the marrow stem cell infusion (Day-2). The second autologous whole blood unit was separated by centrifugation within 8 hours of collection into packed red cells and plasma containing the buffy coat layer. The plasma component then underwent a second centrifugation to separate the buffy coat. The final red cells and buffy coat volume consisted of approximately 40-50 ccs which was then cross-matched, irradiated and ready for infusion to the patient. All donors were consented and tested for infectious disease markers in accordance with the current American Association of Blood Banks and FDA guidelines.
Statistical methods
Summary statistics were computed for all patients, and within specific subgroups. Categorical variables were summarized by frequencies and percentages and their associations assessed using either Fisher's exact test(20) or the Fisher-Freeman-Halton test(21). Continuous variables were summarized by median and range (minimum, maximum) and their associations with categorical variables assessed using the Wilcoxon rank sum test.(22) GF was defined as the patient either (i) not engrafting, (ii) experiencing delayed engraftment beyond day 28 post-transplant or (iii) neutrophil recovery with autologous reconstitution verified by chimerism. Associations between GF and patient covariates were assessed by fitting a Bayesian logistic regression model for the probability of GF as a function of numerical DSA value within each C1Q status subgroup, type of pre-transplant T-cell depletion (TCD = T-cell depleted versus TCR = T-cell replete), age, gender, race, and diagnosis. A similar model, including only DSA value within each C1Q status as covariates, was fit in the subgroup of 17 patients who received TCD. In each fitted Bayesian model, the expression Pr(β > 0 | Data) is the posterior probability that the coefficient of the associated variable is positive. Values either >.99 or <.01 may be interpreted as highly significant, values in the ranges .95 - .99 or .01 – 0.05 may be interpreted as significant, and values in the ranges .90 - .94 or .06 – 0.10 may be interpreted as moderately significant.
Overall survival (OS) was computed from date of HSCT to date of last known vital sign for those groups categorized at or before HSCT date. For GF status, which was assessed after the date of stem-cell infusion, OS was determined using a landmark analysis where one month after HSCT was defined as the landmark time. Patients that did not experience engraftment within one month of HSCT were categorized as GF. Patients alive at the last follow-up date were administratively censored. Time to engraftment was computed from date of HSCT to date of engraftment or administratively censoring at either last follow-up date or date of delayed engraftment. The Kaplan-Meier method(23) was used to estimate unadjusted distributions of OS and time to engraftment and the log-rank test(24) was used to assess differences between groups, where appropriate.
All statistical analyses were performed using SAS 9.3 for Windows (Copyright © 2011 by SAS Institute Inc., Cary, NC). All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
Baseline characteristics of transplant recipients
Of 122 patients who received a haploidentical transplant and were tested for anti-HLA antibodies, 22 (18%) were found to have DSA in the initial samples. The median DSA level in the initial sample for all patients was 6,040 MFI (range 85-28,615), while the median DSA level at transplant was 4,667 MFI (range 614-22,944). Patient characteristics overall, and by DSA status, are summarized in Table 1. From the entire cohort of patients, 19/58 (33%) of female patients were allosensitized versus 3/64 (5%) males (p<0.0001). Similarly, a much higher percentage of DSA positive patients were females (86%) compared with those who were DSA negative (39%) (p<0.0001). In addition, females had higher DSA levels than males; median 7,858 MFI versus 864 MFI, respectively (p=0.021).
Table 1.
| Measure | DSA | |||
|---|---|---|---|---|
| All (N=122) |
Yes (N=22) |
No (N=100) |
p-valuea | |
| Gender, n (%) | ||||
| Male | 64 (52) | 3 (14) | 61 (61) | <0.0001 |
| Female | 58 (48) | 19 (86) | 39 (39) | |
|
| ||||
| Age at transplant (years) | ||||
| Median | 42.0 | 40.5 | 43.0 | 0.64b |
| Range | (18.0, 67.0) | (20.0, 63.0) | (18.0, 67.0) | |
|
| ||||
| Race/Ethnicity, n (%) | ||||
| White | 52 (43) | 8 (36) | 44 (44) | 0.48c |
| Black | 29 (24) | 8 (36) | 21 (21) | |
| Hispanic | 2 (2) | 0 | 2 (2) | |
| Other | 39 (32) | 6 (27) | 33 (33) | |
|
| ||||
| Diagnosis, n (%) | ||||
| AML/MDS | 71 (59) | 16 (76) | 55 (55) | 0.0211c |
| ALL | 14 (12) | 1 (5) | 13 (13) | |
| CLL/lymphoma | 18 (15) | 0 | 18 (18) | |
| CML/MPD | 15 (12) | 2 (10) | 13 (13) | |
| Other | 3 (2) | 2 (10) | 1 (1) | |
| Missing | 1 | 1 | 0 | |
|
| ||||
| Transplant type, n (%) | ||||
| TCD | 21 (17) | 5 (23) | 16 (16) | 0.53 |
| TCR | 101 (83) | 17 (77) | 84 (84) | |
|
| ||||
| C1q status at transplant, n (%) (DSA patients only) | ||||
| Positive | 5 (24) | 5 (24) | - | - |
| Negative | 16 (76) | 16 (76) | - | |
| Missing | 1 | 1 | - | |
|
| ||||
| Treatment, n (%) (DSA patients only) | ||||
| None | 10 (45) | 10 (45) | - | - |
| Desensitization alone | 7 (32) | 7 (32) | - | |
| Desensitization + Buffy Coat | 5 (23) | 5 (23) | - | |
|
| ||||
| Number of loci having antibodies (DSA patients only) | ||||
| 1 | 12 (60) | 12 (60) | - | - |
| > 1 | 8 (40) | 8 (40) | - | |
| Not evaluable | 2 | 2 | - | |
|
| ||||
| Outcomes | ||||
|
| ||||
| Graft failure, n (%) | ||||
| Yes | 11 (9) | 7 (32) | 4 (4) | 0.0006 |
| No | 109 (91) | 15 (68) | 94 (96) | |
| Early death | 2 | 0 | 2 | |
|
| ||||
| Overall survival (months), median (95%CI) | 14.1(9.1, 25.6) | 8.9(3.8, 24.7) | 16.0(9.6, NE) | 0.14d |
Fisher's exact test.
Wilcoxon rank sum test.
Fisher-Freeman-Halton test.
Log-rank test.
Abbreviation: NE = not estimated.
There were no other significant differences between the DSA positive and negative groups except that a significantly higher percentage of patients with DSA were diagnosed with AML/MDS (76% vs. 55%).
Impact of DSA and C1Q status on engraftment
GF was initially assessed for all patients as well as by DSA status (Table 1). Two patients died early and were not evaluated for GF. Overall 11 of 120 evaluable patients (9%) experienced GF. Consistent with what we have previously reported,(5) a significantly higher proportion of DSA positive patients in this study experienced GF (7/22, 32%) compared with DSA negative patients (4/98, 4%), (p < 0.001). In patients who were allosensitized, all the GF events occurred in those with DSA levels > 5,000 MFI at transplant (p=0.004, Table 2A). The median DSA level at transplant for patients who engrafted versus those who rejected the graft was 2,065 MFI versus 10,055 MFI, respectively (p=0.007).
Table 2.
| Covariate | Graft Failure | Fisher's exact test p-value | |
|---|---|---|---|
| Yes (N=7) |
No (N=15) |
||
| C1q at transplant, n (%) | |||
| Positive | 5 (100) | 0 | 0.0003 |
| Negative | 1 (6) | 15 (94) | |
| Non-evaluable | 1 | 0 | |
|
| |||
| Treatment, n (%) | |||
| None | 3 (30) | 7 (70) | 0.14a |
| Desensitization alone | 4 (57) | 3 (43) | |
| Desensitization with buffy coat | 0 | 5 (100) | |
Fisher-Freeman-Halton test.
Time to engraftment was assessed by DSA status (Figure 1A) as well as by DSA level (Figure 1B). The median time to engraftment for all patients was 18 days. A significant difference in time to engraftment was noted for DSA status (p=0.004). Although the difference in median time to engraftment for DSA positive patients compared with DSA negative patients was very small (19 days versus 18 days), the percentage of patients experiencing engraftment was lower for DSA positive patients (68%) compared with DSA negative patients (96%). Within the allosensitized patients, the median time to engraftment for those with DSA levels ≤ 5,000 MFI was 16 days, however, for patients with DSA levels > 5,000 MFI, the median time to engraftment was not reached since less than 50% of those patients engrafted.
Figure 1.
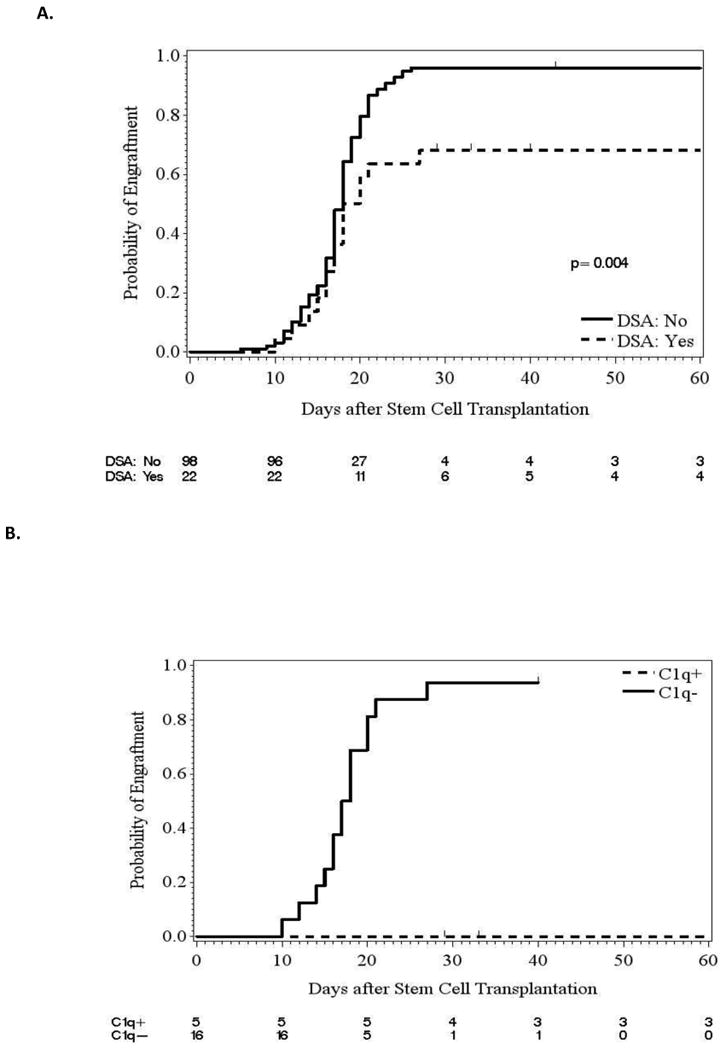
Joint impact of DSA and C1q on engraftment for patients with donor-specific anti-HLA antibodies
All patients with DSA and available serum were tested for the presence of C1q, both in the initial serum samples and after receiving treatment/before infusion of stem cells from the donor. Sixteen patients (73%) had serum available for testing in the initial samples and 21 patients (95%) had serum available in the subsequent samples for C1q evaluation. At the time of initial testing, 9/16 (56%) were C1q positive patients, with a median DSA level of 15,279 MFI (range 1,554-28,615) compared with 7 C1q negative patients who had a median DSA level of 2,471 MFI (664-12,254) (p=0.016).
At the time of transplant, 5 of the 9 patients who were positive at initial testing remained C1q positive. All 4 patients who were initially C1q positive and became C1q negative at transplant experienced engraftment of donor cells. A significantly higher percentage of C1q positive patients experienced GF compared with C1q negative patients (p<0.001, Table 2). The median time to engraftment for C1q negative patients was 18 days; however, for C1q positive patients the median time to engraftment was not reached since less than 50% of those patients engrafted.
As shown by Figure 4, the distributions of numerical DSA value in the C1Q+ and C1Q- patients were very different, with on average much higher DSA values in the C1Q+ patients. Due to this strong association between DSA and C1Q status, to assess effects of these variables and other patient covariates on the probability of GF, Prob(GF), a Bayesian logistic regression model was fit including terms for DSA within each C1Q status subgroup, type of pre-transplant T-cell depletion (TCD versus TCR with post-Cy), age, gender, race, and diagnosis. The fitted model, summarized in Table 3, shows that higher DSA was significantly associated with a larger Prob(GF) in C1Q+ patients, with Pr(β > 0 | Data) = .95, and was moderately significantly associated with a smaller Prob(GF) in C1Q- patients. No other covariates had a significant effect on Prob(GF).
Figure 4.
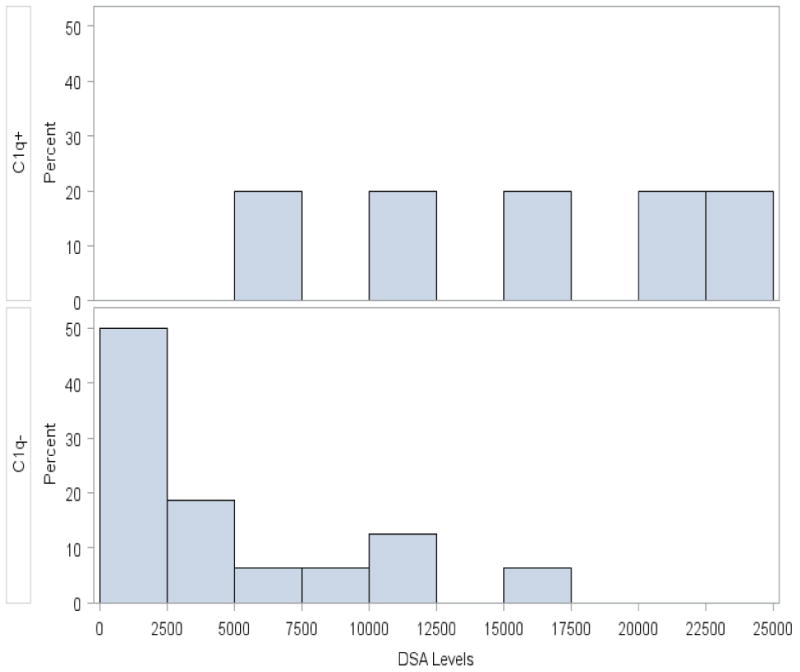
Table 3.
Fitted Bayesian logistic regression model assessing covariate effects on the probability of graft failure in 21 DSA+ patients for whom C1Q status was evaluated, where 6 patients suffered graft failure.
| Posterior Quantities | |||||
|---|---|---|---|---|---|
| Variable | Mean of β | SD of β | 95% Credible Interval | Pr(β > 0 | Data) | |
| Intercept | -0.408 | 1.906 | -4.069 | 3.302 | -- |
| log(DSA)*[DSA+/C1q+] | 1.920 | 1.252 | -0.353 | 4.502 | 0.95 |
| log(DSA)*[DSA+/C1q- ] | -1.373 | 1.005 | -3.324 | 0.557 | 0.079 |
| TCD type vs. TCR type | -0.539 | 1.704 | -3.826 | 2.873 | 0.382 |
| Age at SCT | 0.008 | 0.075 | -0.132 | 0.164 | 0.564 |
| Male vs. Female | -0.997 | 1.682 | -4.306 | 2.315 | 0.280 |
| White vs. Other | 1.711 | 1.539 | -1.351 | 4.661 | 0.872 |
| AML/MDS diagnosis vs. Other | 0.755 | 1.585 | -2.176 | 3.969 | 0.679 |
SD = standard deviation.
The joint effect of DSA and C1Q status was assessed similarly in the subgroup of 17 DSA+ patients who received TCR, but without additional covariates included in the model due to the small subsample size. The fitted model, summarized in Table 4, shows that the significant association of higher DSA with a larger Prob(GF) in C1Q+ patients persisted in this subgroup, with Pr(β > 0 | Data) = .98. It thus appears that the deleterious effect of higher DSA in C1Q + patients may be general, and not dependent on type of GVHD prophylaxis.
Table 4.
Fitted Bayesian logistic regression model assessing the probability of graft failure in the subgroup of 17 DSA+ patients who received T-cell replete grafts.
| Posterior Quantities | |||||
|---|---|---|---|---|---|
| Variable | Mean of β | SD of β | 95% Credible Interval | Pr(β > 0 | Data) | |
| Intercept | -0.293 | 1.829 | -3.863 | 3.336 | -- |
| log(DSA)*DSA+/C1q+ | 1.845 | 1.170 | -0.240 | 4.212 | 0.98 |
| log(DSA)*DSA+/C1q- | -0.737 | 0.620 | -1.956 | 0.475 | 0.113 |
SD = standard deviation.
Treatment of patients with donor-specific anti-HLA antibodies and transplant outcomes
We initially adopted a multimodality treatment strategy similar to that employed in solid organ transplantation, in an attempt to decrease the antibody levels prior to the beginning of transplant conditioning chemotherapy and achieve engraftment in allosensitized recipients using the PE/R/IVIG regimen,(5) as described in the Methods section. Because this treatment appeared to be only partially effective, we added infusion of an irradiated buffy coat prepared 24-48 hours in advance from a unit of donor peripheral blood and infused one day before the infusion of stem cells, hypothesizing that infusion of HLA antigens from the same donor would block complement-binding antibodies, clear C1q and achieve engraftment of donor cells.
Of 22 patients with DSA, 10 (45%) received no desensitization treatment while 12 patients (55%) received desensitization treatment with PE/R/IVIG alone (n=7) or the same treatment plus the addition of buffy coat infused on day -1 (n=5). In the untreated group, 2/6 C1q positive patients, remained C1q positive at transplant and experienced graft failure (one patient had no serum left for testing, had high DSA levels of 11,283 MFI in the initial sample and experienced graft failure), 5/7 patients in the desensitization alone group were C1q positive in initial samples, 3 remained positive at transplant and all experienced engraftment failure, while in the buffy coat group 2/5 patients were C1q positive, both became negative prior to stem cell infusion and achieved engraftment of donor cells. In summary, all 5 patients who remain C1q positive after treatment/before transplant experienced engraftment failure, while all 4 patients who became C1q negative after treatment/before transplant engrafted the donor cells. Interestingly, we did not observe significant changes in antibody levels during treatment including with buffy coat before the stem cell infusion (data not shown) These results suggested that reduction to non-complement binding DSA should be the goal of treatment rather than clearing of the non-complement binding DSA, which appear to clear more slowly in the immediate post-transplant period and became undetectable in all patients within the first few weeks after transplant, similar with prior experience.(25)
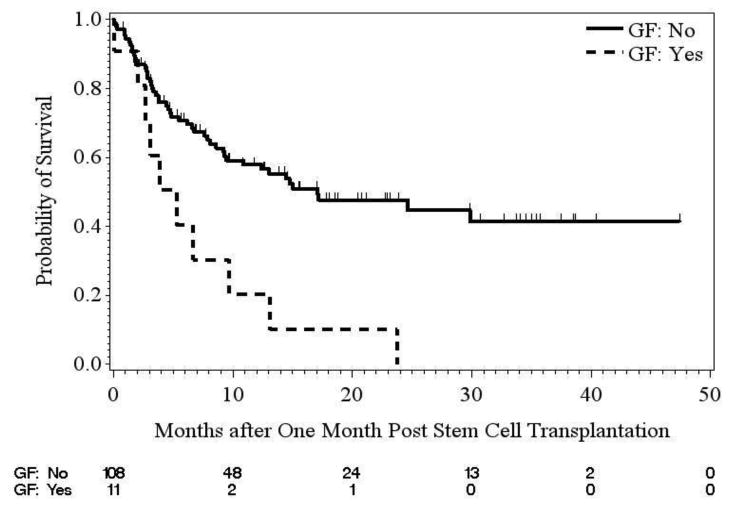
Assessment of Overall Survival
OS was assessed by DSA and C1q combined status (data not shown), GF status (using a landmark analysis, Figure 2B), and by treatment group for patients with DSA (Figure 1S). The median OS one month post-transplant for patients who experienced GF was shorter compared with those who did not experience GF (5.3 months vs. 17.1 months). Finally, median OS for DSA patients who did not receive treatment was 5.3 months compared with 8.9 months for DSA patients receiving desensitization alone, and 13.4 months for patients receiving desensitization plus buffy coat.
Figure 2.
Discussion
We hypothesized that complement activation plays an important role in the development of graft failure and investigated the role of C1q, the first component of the classical complement pathway, in the development of primary graft failure in allosensitized hematopoietic stem-cell transplant recipients. Although demonstrated in a limited number of patients, a high association between complement-binding DSA and engraftment was observed. Previous work by Chen and Tyan showed that there is no complete correlation between C1q and IgG MFI levels, indicating an effect associated with the ability of DSA to fix complement.(16) These results also suggest a complement-dependent cytotoxicity rather than antibody-dependent cell mediated cytotoxicity (ADCC) mechanism to primary graft failure in transplantation, however, potentiation by ADCC cannot be excluded(13, 26), and imply that at least a reduction of DSA to non-complement binding levels prior to transplant is of paramount importance in achieving engraftment in these highly allosensitized individuals.
As we have previously shown(6), a high risk population is middle aged multiparous females who become allosensitized through exposure to foreign HLA antigens during pregnancy.(6) Updated results in this larger series of haploidentical transplant recipients confirmed those findings. A third of all female patients requiring a haploidentical transplant had DSA, and the great majority of allosensitized recipients were females (90%), with high MFI levels. Male recipients had much lower MFI levels, and none were C1q positive, suggesting that this is a much lower risk population. Although GF can occur with lower MFI levels, it is evident now that female recipients are at much higher risk to become allosensitized and pregnancy produces a much higher risk for allosensitization than transfusion of blood products.
This is the first study to examine the association between complement-binding DSA and risk of primary GF in patients with HSCT. We observed significantly higher probability of graft failure in patients with higher DSA levels and C1q positivity (Table 3 and Figure 3), and this effect also was seen in the subgroup of 17 T-cell replete haploidentical transplant patients. Our results indicate that 1) Presence of a positive C1q assay is associated with engraftment failure in patients with higher DSA; and 2) The goal of treatment should be clearing of the complement fixing antibodies before infusion of stem cells. A positive C1q assay was significantly associated with highly allosensitized patients (high DSA levels) suggesting that activation of the complement pathway likely causes apoptosis of hematopoietic progenitor cells.(27) Although the number of patients with complement-binding DSA was relatively small, the high risk of GF seen in patients who had high DSA levels and were C1q positive, suggest that this is a very high risk condition which should be avoided. Upfront testing of C1q in patients with DSA is necessary, as our data suggests that clearance of C1q is needed to obtain engraftment of donor cells. A two-step cost effective approach may be to screen for DSA patients with mismatched transplants and test for C1q patients who are allosensitized. Half of the patients with high DSA levels (> 5,000 MFI) tested negative for C1q and their risk for rejection was much lower. In fact only one patient in this category rejected the graft (MFI 6,265.78). It remains unclear if complement pathway gets activated in these patients or not and future studies will attempt to elucidate this aspect.
Figure 3.
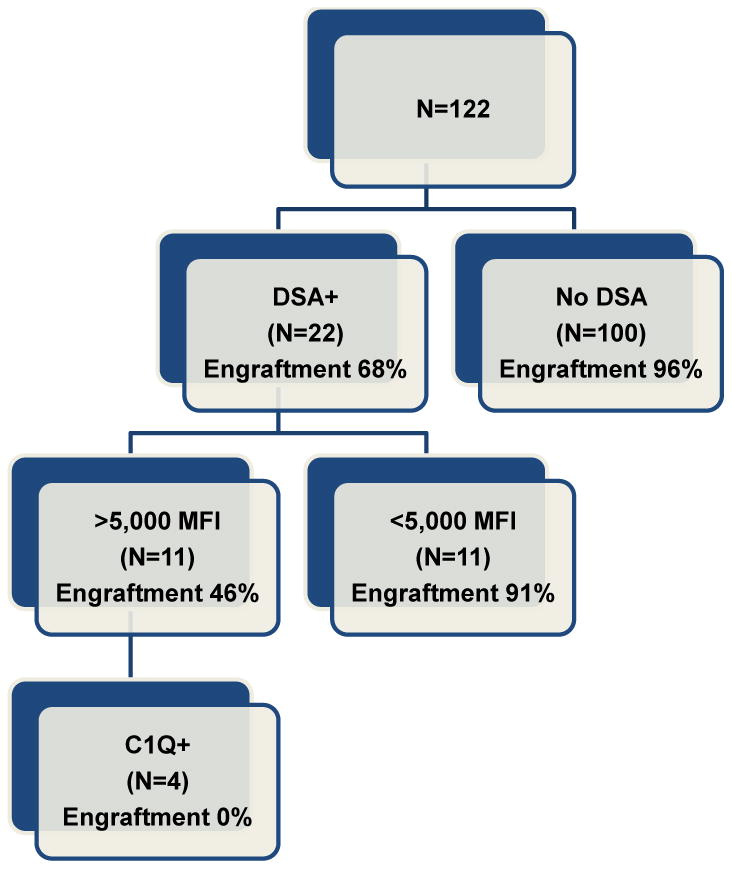
In contrast with reports from a different institution,(28, 29) our experience suggested that treatment with plasma exchange, rituximab and IVIG is only partially effective in treating allosensitized recipients of hematopoietic stem-cell transplantation, similar with the experience reported in solid organ transplantation.(30, 31) A novel approach developed by our group has focused on blocking the DSA with HLA antigens from the donor using a buffy coat prepared from the donor cells (using HLA antigen of the donor). Peripheral blood mononuclear cells express both HLA class I and II on cell surface, and seem to be an ideal source for HLA antigens, as compared with RBC or platelets, which may express only partially HLA antigens limited to mostly HLA class I.(32) In our experience, all patients with complement-binding antibodies and high DSA levels (> 5,000 MFI) were broadly allosensitized against both HLA class I and class II antigens thus a blood product capable of reliably binding all antigens would be needed.
Our results are in line with experience from solid organ transplantation.(33) Taken together, these data suggest that complement mediated rejection plays a major role in antibody-mediated graft rejection and that C1q should be tested routinely along with DSA levels prior to transplant. In addition, it appears that C1q negativity should be the goal of treatment, at least in C1q positive patients, and high DSA levels may not clear right away from circulation. Infusion of donor HLA antigens in the form of a buffy coat is a promising approach for the treatment of C1q positive patients and should be further explored in prospective clinical trials.
Supplementary Material
Key Points.
- Patients with complement-binding DSA (C1q+) are at highest risk of primary graft failure in hematopoietic stem-cell transplantation
- Blocking complement activation prior to transplantation may prevent engraftment failure in this high risk pati
Acknowledgments
This study was supported by an MDACC Institutional Research Grant (to SOC) and MDACC research fund (#110560) (to KC). We also acknowledge the support of the Trujillo family, by means of the Jose M. Trujillo endowment in memory of Dr. Jose M. Trujillo, a pioneer of laboratory medicine, through Dr. Elizabeth A. Wager.
Footnotes
Authorship contribution: SOC contributed with data collection, interpretation of results and wrote the paper; PFT contributed with statistical analysis, interpretation of statistical results, and reviewed, revised, and approved the manuscript; DRM contributed statistical analysis, interpretation of statistical results, revisions to the manuscript, and approved the manuscript; THB contributed to antibody testing and results interpretation, reviewed and approved the manuscript; YC contributed to data collection and data analysis, reviewed and approved the manuscript; PK and GR contributed with data collection, reviewed and approved the manuscript; AAL and DAY contributed to antibody testing, reviewed and approved the manuscript; UP, BL, FA, VAK contributed with clinical treatment, reviewed and approved the manuscript, QM contributed with data interpretation, reviewed and approved the manuscript, MFV contributed with data collection and interpretation, reviewed and approved the manuscript; REC contributed with data interpretation, reviewed, edited and approved the manuscript; KC contributed with data collection, interpretation and manuscript writing.
Conflict of Interest: The authors declare no potential conflict of interests.
References
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. The New England journal of medicine. 1969;280(14):735–9. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 2.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. The New England journal of medicine. 1989;320(4):197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 3.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69(3):319–26. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Everly MJ. Donor-specific anti-HLA antibody monitoring and removal in solid organ transplant recipients. Clinical transplants. 2011:319–25. [PubMed] [Google Scholar]
- 5.Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88(8):1019–24. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciurea SO, Thall PF, Wang X, Wang SA, Hu Y, Cano P, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118(22):5957–64. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–46. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- 8.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704–8. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691–7. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone marrow transplantation. 2012;47(4):508–15. doi: 10.1038/bmt.2011.131. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri A, Rocha V, Masson E, Labopin M, Cunha R, Absi L, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Societe Francophone d'Histocompatibilite et d'Immunogenetique (SFHI) and Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) analysis. Haematologica. 2013;98(7):1154–60. doi: 10.3324/haematol.2012.077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, et al. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108(10):3611–9. doi: 10.1182/blood-2006-04-017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor PA, Ehrhardt MJ, Roforth MM, Swedin JM, Panoskaltsis-Mortari A, Serody JS, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109(3):1307–15. doi: 10.1182/blood-2006-05-022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205(4-5):395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- 15.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Human immunology. 2011;72(10):849–58. doi: 10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nature reviews Immunology. 2012;12(6):431–42. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 18.Ciurea SO, Saliba R, Rondon G, Pesoa S, Cano P, Fernandez-Vina M, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone marrow transplantation. 2010;45(3):429–36. doi: 10.1038/bmt.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(12):1835–44. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher R. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87–94. [Google Scholar]
- 21.Freeman GHHJ, H J. Note on exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38(141):141–9. [PubMed] [Google Scholar]
- 22.Randles RWD. Introduction to the Theory of Nonparametric Statistics. John Wiley; 1979. [Google Scholar]
- 23.Kaplan EL, M P. Nonparametric estimator from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;60:163–70. [PubMed] [Google Scholar]
- 25.Fasano RM, Mamcarz E, Adams S, Donohue Jerussi T, Sugimoto K, Tian X, et al. Persistence of recipient human leucocyte antigen (HLA) antibodies and production of donor HLA antibodies following reduced intensity allogeneic haematopoietic stem cell transplantation. British journal of haematology. 2014;166(3):425–34. doi: 10.1111/bjh.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghebrehiwet B, Medicus RG, Muller-Eberhard HJ. Potentiation of antiboty-dependent cell-mediated cytotoxicity by target cell-bound C3b. Journal of immunology (Baltimore, Md : 1950) 1979;123(3):1285–8. [PubMed] [Google Scholar]
- 27.Lee JW, Gersuk GM, Kiener PA, Beckham C, Ledbetter JA, Deeg HJ. HLA-DR-triggered inhibition of hemopoiesis involves Fas/Fas ligand interactions and is prevented by c-kit ligand. Journal of immunology (Baltimore, Md : 1950) 1997;159(7):3211–9. [PubMed] [Google Scholar]
- 28.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. The New England journal of medicine. 2011;365(4):318–26. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 29.Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(4):647–52. doi: 10.1016/j.bbmt.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marfo K, Lu A, Ling M, Akalin E. Desensitization protocols and their outcome. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(4):922–36. doi: 10.2215/CJN.08140910. [DOI] [PubMed] [Google Scholar]
- 31.Tait BD, Susal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95(1):19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 32.Giles CM, Botto M, King MJ. A study of HLA (Bg) on red cells and platelets by immunoblotting with monoclonal antibodies. Transfusion. 1990;30(2):126–32. doi: 10.1046/j.1537-2995.1990.30290162897.x. [DOI] [PubMed] [Google Scholar]
- 33.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. The New England journal of medicine. 2013;369(13):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


