Abstract
Estrogen receptor alpha (ERα) has been implicated in bone’s response to mechanical loading in both males and females. ERα in osteoblast lineage cells is important for determining bone mass, but results depend on animal sex and the cellular stage at which ERα is deleted. We demonstrated previously that when ERα is deleted from mature osteoblasts and osteocytes in mixed background female mice, bone mass and strength are decreased. However, few studies exist examining the skeletal response to loading in bone cell-specific ERαKO mice. Therefore, we crossed ERα floxed (ERαfl/fl) and osteocalcin-Cre (OC-Cre) mice to generate animals lacking ERα in mature osteoblasts and osteocytes (pOC-ERαKO) and littermate controls (LC). At 10 weeks of age the left tibia was loaded in vivo for two weeks. We analyzed bone mass through microCT, bone formation rate by dynamic histomorphometry, bone strength from mechanical testing, and osteoblast and osteoclast activity by serum chemistry and immunohistochemistry. ERα in mature osteoblasts differentially regulated bone mass in males and females. Compared to LC, female pOC-ERαKO mice had decreased cortical and cancellous bone mass, while male pOC-ERαKO mice had equal or greater bone mass than LC. Bone mass results correlated with decreased compressive strength in pOC-ERαKO female L5 vertebrae, and with increased maximum moment in pOC-ERαKO male femora. Female pOC-ERαKO mice responded more to mechanical loading, while the response of pOC-ERαKO male animals was similar to their littermate controls.
Keywords: genetic animal model, sex steroids, osteoblasts, osteoporosis
INTRODUCTION
Sex hormones are important regulators of bone mass in males and females. During puberty estrogens inhibit, while androgens stimulate, periosteal bone formation in humans, contributing to generally higher bone mass in males (1). The decline in estrogen associated with menopause is a primary contributor to post-menopausal osteoporosis in females (2–4). In men sex hormone levels also decline with age and correlate with gradual bone loss (5–7). Estrogen signaling in bone occurs primarily through two estrogen receptors, ERα and ERβ. Although both receptors are important in bone, a point mutation in ERα caused unfused growth plates and osteoporosis in a single reported human case (8). Since then, the role of ERα in skeletal health in both males and females has become a major focus of research (9–11).
To better understand the role of estrogen in bone cells, global ERα knockout (ERαKO) and cell-specific ERαKO mice that remove ERα at specific points in the osteoblast-osteocyte lineage were developed, with conflicting outcomes concerning the cortical and cancellous bone status in males and females. With global deletion of ERα, cancellous and cortical tibial bone mineral density increased in females, but cortical and cancellous bone mass decreased in males (12–16). However, systemic effects that include altered hormone levels and body weight differences confound these results in global knockouts (14, 16). When ERα was removed from osteoblast progenitors or precursors, using Prx1- or Osx-Cre mice, respectively, cortical bone mass decreased in females and young males, while cancellous bone was unaffected (17). ERα deletion in mature osteoblasts (OC-Cre) decreased cortical and cancellous bone mass (18, 19), but bone mass was unchanged with deletion in committed osteoblasts in females (Col1a1-Cre) (17). Bone mass in young and growing male mice was unaffected by ERα deficiency in osteoblasts in both targeted knockouts (18, 19). Finally, when ERα was removed from osteocytes (Dmp-1-Cre), female and male mice exhibited no change in or decreased bone mass, respectively (20, 21).
Bone is mechanosensitive. Bone mass can increase in response to dynamic loading, but decreases with disuse in adult animals (22, 23). In vivo tibial and ulnar mechanical loading rodent models are established methods for studying the adaptive response of cancellous and cortical bone to controlled, dynamic bone loading (24–26). Although ERα has been implicated in bone mechanotransduction (27, 28), the results were conflicting when the skeletal response to mechanical loading was examined in different bone cell-specific ERαKO mice. ERα in females had no effect on bone’s anabolic response to mechanical loading when removed at the osteocyte stage of the lineage (20). In Prx-1-ERαKO and Osx-ERαKO mice, loading-induced periosteal expansion was reduced, while there was no difference in cortical adaptation to load in Col1-ERαKO mice (29). In male animals, the response to mechanical loading has not been reported in any bone cell-specific ERαKO mouse. In all these models, the cancellous bone response to applied loading has not been reported.
No study to date has investigated cancellous and cortical bone adaptation to mechanical loading in male and female mice generated using the OC-Cre to remove ERα at the stage of mature osteoblasts and osteocytes (pOC-ERαKO). To generate these animals and littermate controls we crossbred OC-Cre and ERα floxed mice. At 10 weeks of age, we subjected the left tibiae to two weeks of in vivo mechanical loading, with the right limb as an internal control, and analyzed bone mass and architecture through microCT, dynamic histomorphometry and immuno-histochemistry (IHC). In addition, we examined bone mass, morphology and strength of L5 vertebrae and femoral midshafts in LC and targeted animals. We hypothesized that ERα deficiency in mature osteoblasts and osteocytes would decrease bone mass in both female and male mice, and that the response to mechanical loading would be attenuated in pOC-ERαKO mice. Our results did not fully support the hypothesis and revealed a more complex situation.
METHODS
Generation of osteoblast-specific ERαKO mice
pOC-ERαKO and littermate control (LC) mice were bred and validated as previously described (19). Briefly, mice containing a transgene encoding Cre recombinase driven by the human osteocalcin promoter (OC-Cre, provided by Dr. Thomas Clemens, The Johns Hopkins University, Baltimore, MD) (30, 31) were crossed with mice in which exon 3 of the DNA-binding domain of the ERα gene (Esr1) was flanked by loxP sequences (ERαfl/fl, provided by Dr. Kahn, University of Cincinnati, Cincinnati, OH) (32). Prior to generation of pOC-ERαKO, ERαfl/fl mice were inbred to be >99% pure C57Bl/6 by speed congenics (DartMouse Speed Congenic Core Facility, Geisel School of Medicine at Dartmouth, Hanover, NH). All mice were genotyped as described (19). Mice were housed 3–5 per cage with ad libitum access to food and water. All animal procedures were approved by Cornell University’s IACUC.
In vivo tibial mechanical loading
At ten weeks of age, single element strain gauges (EA-06-015LA-120, Micromeasurements) were surgically attached to the tibial midshafts of female and male LC and pOC-ERαKO mice (n=5–6 per genotype). A series of axial cyclic compressive loads (−2 to −12N) were applied to the left and right tibiae in our custom tibial loading device. Bone stiffness was calculated from the load and strain data as previously described (33) and used to calculate the peak load required to induce 1200 microstrain (με) at the tibial midshaft during compressive axial tibial loading; strains at this location are well-characterized. Bone stiffness was similar among pOC-ERαKO and LC male and female mice (0.00671 ± 0.0010 N/με LC females, 0.00763 ± 0.00068 N/με pOC-ERαKO females, 0.00760 ± 0.00029 N/με LC males, 0.00767 ± 0.00016 N/με pOC-ERαKO males). A peak load of −9.0N was applied to all animals in the subsequent loading experiment.
The left tibiae of male and female LC and pOC-ERαKO mice (n=12–14 per group) were loaded in compression in vivo for 2 weeks (33). In brief, a cyclic compressive load was applied at a rate of 4Hz for 1200 cycles per day, 5 days per week, in a triangular waveform with a peak load of −9.0N. A dwell of 100ms at −0.5N was maintained between successive load cycles, and the dwell-to-peak time was 75ms, corresponding to a strain rate of 0.0016ε/s at the tibial midshaft cortex. This methodology is well established in the literature (26, 33, 34).
Mass and serum marker measurements
Three days after the last day of mechanical loading, mice were euthanized via isoflurane overdose and cardiac puncture. Blood was stored overnight at 4°C and centrifuged at 2,000rpm for 20min to separate serum. Serum was assayed (n=8–10 per group) for estrogen (E2, CalBiotech EW180S-100, Spring Valley, CA), insulin-like growth factor 1 (IGF-1, ALPCO 22-IGF-R21, Salem, NH), osteocalcin (OC, ALPCO 31-50-1300), tartrate-resistant acid phosphatase 5b (TRAP5b, IDS SB-TR103, Scottsdale, AZ), and pro-collagen I N-terminal peptide (PINP, MyBioSource 703389). Body mass and female uterine and ovarian masses were recorded at euthanasia.
Microcomputed tomography
Right femora and L5 vertebrae were wrapped in PBS-soaked gauze and stored at −20°C prior to microCT scanning at 15um resolution (μCT35, Scanco Medical AG, Switzerland; 55kVp, 145μA, 600ms integration time). Mineralized tissue was separated from non-mineralized tissue using gender- and bone-specific thresholds. The cancellous core and cortical shell of the vertebrae were analyzed as previously described (19). For the femur, a cortical volume of interest extending 0.5 mm, centered at the midshaft, was analyzed.
At euthanasia, left and right tibiae were stored overnight in 4% paraformaldehyde or 70% ethanol and then scanned in 70% ethanol at 15μm voxel resolution (μCT35, Scanco Medical AG, Switzerland; 55kVp, 145μA, 600ms integration time). For each tibia, the metaphyseal cancellous core and cortex were manually separated and analyzed. The metaphyseal region was defined from ~0.5mm distal to the growth plate and extending distally 10% of total tibial length (19). The cortical midshaft was analyzed as previously described (19). The tibial midshaft was chosen to allow direct comparison to our previous work (19, 33), because this location corresponds to the site of in vivo tibial strain gauge calibration, and because strains at the cortical midshaft are well established across vertebrates (35).
Cancellous bone outcome measures were bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and cancellous tissue mineral density (cn.TMD). Cortical bone outcome measures were cortical area (Ct.Ar), marrow area (Ma.Ar, tibial and femoral midshaft only), maximum and minimum moments of inertia (IMAX, IMIN), cortical thickness (Ct.Th), and cortical tissue mineral density (ct.TMD).
Dynamic histomorphometry
Ten and three days before euthanasia, mice (n=6–7 per group) received injections of calcein (30mg/kg IP). After microCT scanning, tibiae were embedded in acrylosin and sectioned by the Bone Histology/Histomorphometry Laboratory (Yale University Department of Orthopaedics and Rehabilitation, New Haven, CT). Both transverse sections of the tibial midshaft and longitudinal sections of the tibial metaphysis were analyzed to measure single and double fluorescent labels on bone surfaces (2 slides per animal, OsteomeasureXP v3.2.1.7, Osteometrics, Decatur, GA). Measurable outcomes were mineralizing surface (MS), mineral apposition rate (MAR), bone formation rate (BFR), and woven bone area (Wo.Ar) at the periosteal and endosteal surfaces of the tibial midshaft and cancellous metaphysis according to ASBMR standards (36). Woven bone regions were excluded from double label analyses.
Histology
Left and right tibiae not used for dynamic histomorphometry were decalcified in 10% EDTA for two weeks, processed, and embedded in paraffin (n=6–7 per group). Tibiae were sectioned longitudinally at 6μm using a rotary microtome (Leica RM2255, Germany). Sections were stained for TRAP and pro-collagen I as previously described (19). The number of positively-stained osteoclasts (TRAP) or osteoblasts (pro-collagen I) in the cancellous metaphysis was quantified and normalized to bone surface (2 slides/animal, OsteomeasureXP v3.2.1.7). Growth plate thickness was quantified from sections stained with Safranin O/Fast Green/Alcian Blue by averaging five evenly spaced lines (2 slides per mouse, n=6 mice/group, OsteomeasureXP v3.2.1.7).
Mechanical testing
Prior to mechanical testing, femora and L5 vertebrae were thawed to room temperature and kept moist with PBS. Femora were tested in three-point bending to failure, and vertebrae were tested in compression to failure in the cranial-caudal direction as previously described (858 Mini Bionix, MTS, Eden Prairie, MN) (19). Whole bone strength and stiffness were determined from the load-displacement data for bending and compression.
Statistics
For serum, bone lengths, in vivo bone stiffness, vertebral and femoral microCT, and mechanical testing data, a one-way ANOVA was used for each sex. To compare the loaded and control tibiae for tibial microCT, dynamic histomorphometry, histology and IHC data, a repeated measures ANOVA with interaction was used on the absolute values for each sex with a Tukey HSD post-hoc test performed when the interaction term was significant. The between-subject factor was genotype, and the within-subject factor was loaded (left) vs. control (right) limb. All statistical comparisons were made between sex-matched pOC-ERaKO and LC; females and males were not compared directly. Significance was set at p<0.05. Increases and decreases among factors are reported as percentages in the text only when the absolute values from the statistical tests were significantly different.
RESULTS
Physical characterization of pOC-ERαKO mice
We generated and examined male and female pOC-ERαKO and LC. Because the global ERαKO mouse possessed systemic effects that confound the role of ERα in bone alone (13, 14, 16, 37), we measured body weight, crown/rump length, ovarian and uterine weight (females only), tibia and femur length, and serum levels of E2 and IGF-1 (Supplementary Fig 1, Supplementary Table 1). All outcome measures were similar between pOC-ERαKO and LC within each sex, except for femoral length, which was greater in pOC-ERαKO males vs. LC.
Female pOC-ERαKO mice exhibit decreased bone mass
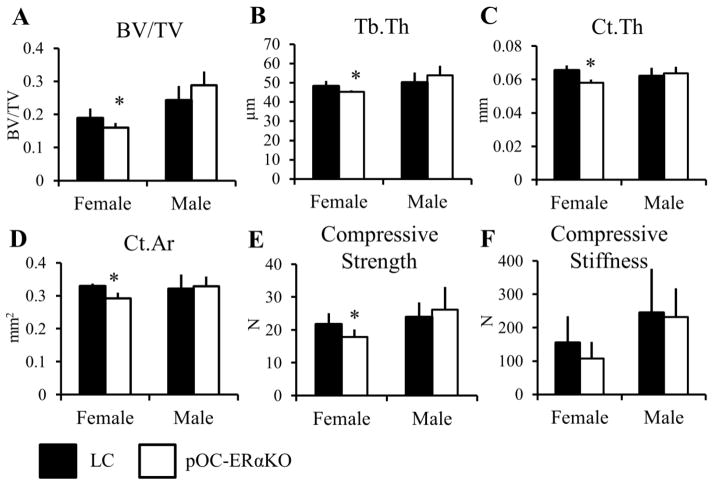
To assess changes in bone structure and geometry, microCT analysis was performed on the L5 vertebrae, femoral midshafts, and proximal and mid-diaphyseal control tibiae (Table 1, Table 2). Cancellous BV/TV was lower in pOC-ERαKO female mice by 16% in the vertebral body and by 25% in the tibial metaphysis control limbs, due to lower Tb.Th in the vertebra (−6.2%), and due to increased Tb.Sp in the tibia (+28%) (Figure 1, Table 2). Analogously, cortical bone at these two cortico-cancellous sites was also affected, but to a lesser extent than the cancellous tissues. In the vertebral shell, both Ct.Ar (−11%) and Ct.Th (−11%) were lower in the knockouts compared to LC. From compression testing, the lower bone mass found in both cortical and cancellous regions of the pOC-ERαKO vertebra correlated with lower compressive strength (−18%), but compressive stiffness was unchanged. The tibial metaphyseal cortical shell was 9.3% thinner in female pOC-ERαKO mice.
Table 1.
Cancellous and cortical bone were differentially affected in 10-week-old pOC-ERαKO females and males measured by microCT in the vertebral body, vertebral shell, and femoral midshaft.
| Female | Male | |||||
|---|---|---|---|---|---|---|
| LC | pOC-ERαKO | LC | pOC-ERαKO | |||
| Vertebral Body | BV/TV | 0.189 ± 0.029 | 0.160 ± 0.015* | 0.243 ± 0.042 | 0.288 ± 0.042 | |
| Tb.Th (μm) | 48.3 ± 2.7 | 45.3 ± 0.85* | 50.2 ± 5.1 | 53.9 ± 5.0 | ||
| Tb.Sp (μm) | 225 ± 21 | 277 ± 16 | 175 ± 13 | 158 ± 10* | ||
| Tb.N | 4.40 ± 0.37 | 4.40 ± 0.27 | 5.49 ± 0.28 | 5.96 ± 0.29* | ||
| cn.TMD (mg HA/cc) | 632 ± 10 | 639 ± 7.6 | 646 ± 22 | 653 ± 17 | ||
|
| ||||||
| Vertebral Shell | Ct.Ar (mm2) | 0.329 ± 0.0084 | 0.292 ± 0.017* | 0.321 ± 0.043 | 0.329 ± 0.029 | |
| Ct.Th (μm) | 65.5 ± 3.0 | 58.0 ± 2.0* | 62.1 ± 4.9 | 63.6 ± 4.0 | ||
| IMAX (mm4) | 0.142 ± 0.0088 | 0.139 ± 0.014 | 0.136 ± 0.019 | 0.141 ± 0.020 | ||
| IMIN (mm4) | 0.0297 ± 0.0026 | 0.0284 ± 0.0025 | 0.0333 ± 0.0073 | 0.0338 ± 0.0044 | ||
| ct.TMD (mg HA/cc) | 791 ± 7.5 | 787 ± 8.1 | 795 ± 6.3 | 798 ± 9.0 | ||
|
| ||||||
| Femoral Midshaft | Ct.Ar (mm2) | 0.726 ± 0.027 | 0.675 ± 0.021* | 0.934 ± 0.088 | 1.06 ± 0.097* | |
| Ma.Ar (mm2) | 0.890 ± 0.046 | 0.908 ± 0.053 | 1.17 ± 0.096 | 1.24 ± 0.13 | ||
| Ct.Th (mm) | 0.180 ± 0.0040 | 0.167 ± 0.0095* | 0.193 ± 0.0077 | 0.202 ± 0.011 | ||
| IMAX (mm4) | 0.195 ± 0.020 | 0.175 ± 0.015* | 0.351 ± 0.063 | 0.430 ± 0.072* | ||
| IMIN (mm4) | 0.107 ± 0.0077 | 0.103 ± 0.0072 | 0.167 ± 0.029 | 0.206 ± 0.039* | ||
| ct.TMD (mg HA/cc) | 945 ± 8.9 | 933 ± 9.1* | 925 ± 8.2 | 930 ± 6.8 | ||
Data are mean ± SD. BV/TV, bone volume fraction; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; cn.TMD, cancellous tissue mineral density; Ct.Ar, cortical area; Ma.Ar, marrow area; Ct.Th, cortical thickness; IMAX and IMIN, maximum and minimum moments of inertia; ct.TMD, cortical tissue mineral density
pOC-ERαKO different from LC, p<0.05 by one-factor ANOVA for each sex
Table 2.
Female 10-week-old pOC-ERαKO mice responded more to 2 weeks of tibial compression than LC while male pOC-ERαKO mice responded similarly to LC, measured by microCT in the proximal tibia and tibial midshaft.
| Female | Male | |||||
|---|---|---|---|---|---|---|
| LC | pOC-ERαKO | LC | pOC-ERαKO | |||
| Cancellous Metaphysis | BV/TV | Control | 0.0779 ± 0.019b | 0.0584 ± 0.0095c | 0.121 ± 0.041b | 0.162 ± 0.037*a |
| Loaded | 0.111 ± 0.014†a | 0.115 ± 0.015†a | 0.143 ± 0.030ab | 0.156 ± 0.016*ab | ||
|
|
||||||
| Tb.Th (μm) | Control | 48.4 ± 2.4c | 46.4 ± 2.2*c | 46.0 ± 5.2c | 52.4 ± 5.6*b | |
| Loaded | 67.3 ± 4.4†b | 74.2 ± 4.2*†a | 58.7 ± 3.0†a | 61.0 ± 5.7*†a | ||
|
|
||||||
| Tb.Sp (μm) | Control | 323 ± 35 | 413 ± 41* | 227 ± 28 | 225 ± 36 | |
| Loaded | 327 ± 32 | 436 ± 52* | 225 ± 31 | 230 ± 36 | ||
|
|
||||||
| Tb.N | Control | 3.16 ± 0.36 | 2.52 ± 0.23* | 4.46 ± 0.49 | 4.55 ± 0.63 | |
| Loaded | 3.09 ± 0.26 | 2.39 ± 0.25* | 4.41 ± 0.61 | 4.41 ± 0.59 | ||
|
|
||||||
| cn.TMD (mg HA/cc) | Control | 767 ± 13 | 757 ± 14 | 765 ± 9.1 | 774 ± 15 | |
| Loaded | 798 ± 12† | 791 ± 15† | 796 ± 11† | 794 ± 13† | ||
|
| ||||||
| Cortical Shell Metaphysis | Ct.Ar (mm2) | Control | 0.964 ± 0.092b | 0.866 ± 0.050b | 1.11 ± 0.12 | 1.18 ± 0.14 |
| Loaded | 1.28 ± 0.097†a | 1.27 ± 0.049†a | 1.31 ± 0.084† | 1.39 ± 0.11† | ||
|
| ||||||
| Ct.Th (mm) | Control | 0.141 ± 0.010b | 0.128 ± 0.0049*c | 0.134 ± 0.013 | 0.141 ± 0.015 | |
| Loaded | 0.179 ± 0.011†a | 0.174 ± 0.0090*†a | 0.156 ± 0.0076† | 0.158 ± 0.0082† | ||
|
| ||||||
| IMAX (mm4) | Control | 0.373 ± 0.055 | 0.339 ± 0.032 | 0.553 ± 0.086 | 0.613 ± 0.11* | |
| Loaded | 0.534 ± 0.070† | 0.540 ± 0.047† | 0.670 ± 0.063† | 0.771 ± 0.12*† | ||
|
| ||||||
| IMIN (mm4) | Control | 0.301 ± 0.040b | 0.268 ± 0.035*c | 0.421 ± 0.061b | 0.440 ± 0.061b | |
| Loaded | 0.433 ± 0.061†a | 0.429 ± 0.037*†a | 0.530 ± 0.064†a | 0.598 ± 0.085†a | ||
|
| ||||||
| ct.TMD (mg HA/cc) | Control | 833 ± 23 | 816 ± 19 | 817 ± 11 | 815 ± 14 | |
| Loaded | 843 ± 20† | 883 ± 12† | 827 ± 17 | 815 ± 7.8 | ||
|
| ||||||
| Cortical Midshaft | Ct.Ar (mm2) | Control | 0.560 ± 0.029c | 0.534 ± 0.026c | 0.769 ± 0.080 | 0.823 ± 0.053* |
| Loaded | 0.717 ± 0.034†b | 0.755 ± 0.042†a | 0.791 ± 0.049† | 0.834 ± 0.052*† | ||
|
|
||||||
| Ma.Ar (mm2) | Control | 0.333 ± 0.017 | 0.348 ± 0.021 | 0.432 ± 0.029b | 0.450 ± 0.024* ab | |
| Loaded | 0.317 ± 0.028† | 0.325 ± 0.027† | 0.420 ± 0.030b | 0.465 ± 0.031*a | ||
|
|
||||||
| Ct.Th (mm) | Control | 0.205 ± 0.0095b | 0.194 ± 0.0062b | 0.241 ± 0.018 | 0.249 ± 0.012 | |
| Loaded | 0.253 ± 0.011†a | 0.258 ± 0.013†a | 0.246 ± 0.008 | 0.248 ± 0.0072 | ||
|
|
||||||
| IMAX (mm4) | Control | 0.0603 ± 0.0054c | 0.0598 ± 0.0065*c | 0.121 ± 0.022 | 0.141 ± 0.017* | |
| Loaded | 0.0926 ± 0.0098†b | 0.105 ± 0.0089*†a | 0.127 ± 0.017† | 0.1474 ± 0.020*† | ||
|
|
||||||
| IMIN (mm4) | Control | 0.0505 ± 0.0034b | 0.0470 ± 0.0046b | 0.0849 ± 0.014 | 0.0936 ± 0.010* | |
| Loaded | 0.0646 ± 0.0064†a | 0.0687 ± 0.0057†a | 0.0883 ± 0.010† | 0.0985 ± 0.012*† | ||
|
|
||||||
| ct.TMD (mg HA/cc) | Control | 1020 ± 16 | 1020 ± 8.4 | 1040 ± 12 | 1050 ± 17 | |
| Loaded | 1010 ± 18† | 1010 ± 12† | 1040 ± 11 | 1050 ± 10 | ||
Data are mean ± SD. BV/TV, bone volume fraction; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; cn.TMD, cancellous tissue mineral density; Ct.Ar, cortical area; Ma.Ar, marrow area; Ct.Th, cortical thickness; IMAX and IMIN, maximum and minimum moments of inertia; ct.TMD, cortical tissue mineral density
pOC-ERαKO different from LC,
Loaded tibia different from Control, p<0.05 by repeated measures ANOVA with interaction for each sex.
Groups not sharing the same letter are significantly different by Tukey HSD post-hoc where a > b > c. Post-hoc performed when ANOVA interaction term was significant.
Figure 1.
Vertebral cancellous and cortical bone morphology and strength were differentially affected in 12-week-old pOC-ERαKO females and males compared to LC. (A) Vertebral body BV/TV was decreased due to (B) decreased Tb.Th in female knockouts compared to LC. (A) In male pOC-ERαKO mice, BV/TV in the vertebral shell was similar between genotypes. (C) The vertebral shell had thinner cortices in female pOC-ERaKO mice compared to LC, resulting in (D) decreased Ct.Ar. Vertebral shell Ct.Ar and Ct.Th in pOC-ERaKO male mice were not different from LC. The cortical and cancellous morphology changes seen in female and male pOC-ERαKO mice contributed to (E) a decrease in compressive strength in vertebral compression tests in females, but no change in compressive strength in male knockouts or (F) in compressive stiffness in either sex.
BV/TV, bone volume fraction; Tb.Th, trabecular thickness; Ct.Ar, cortical area; Ct.Th, cortical thickness. Data are mean ± SD, n=8–12 per group. *pOC-ERαKO different from LC, p<0.05 by one-way ANOVA for each sex.
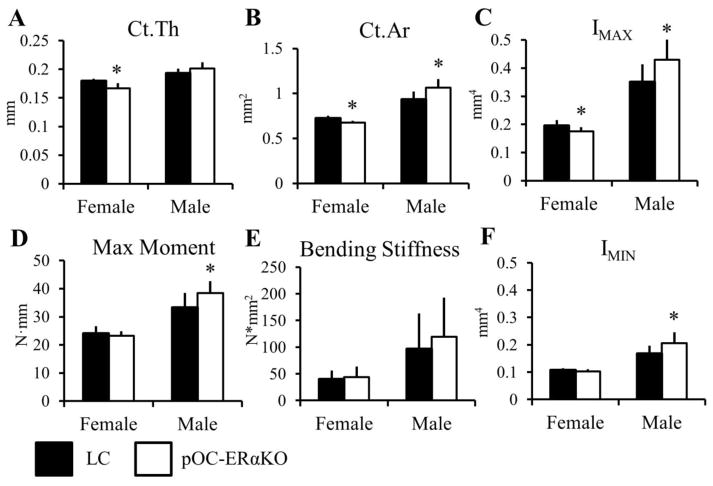
At the tibial midshaft, the non-loaded right limbs in pOC-ERαKO female mice showed no difference from LC mice in Ct.Ar, Ct.Th, IMAX, IMIN, or other parameters (Table 2). At the femoral midshaft, however, Ct.Ar (−7.0%) and Ct.Th (−7.3%) were lower, as were IMAX and ct.TMD (−10% and −1.3%, respectively) (Figure 2, Table 1). These changes in bone geometry and architecture at the femoral midshaft did not result in changes in either maximum moment or bending stiffness in three-point bending tests in targeted mice compared to LC.
Figure 2.
Femoral midshaft bone morphology and strength were differentially affected in 12-week-old pOC-ERαKO females and males compared to LC. Female pOC-ERαKO mice had (A) decreased Ct. Th, (B) decreased Ct.Ar, and (C) decreased IMAX compared to LC. (D,E) Maximum moment and bending stiffness were not different between genotypes in females from 3-point bending mechanical tests. Male pOC-ERαKO mice exhibited an opposite bone phenotype compared to LC than that found in females. (B) Ct.Ar, (C) IMAX, and (F) IMIN were all increased in male pOC-ERαKO mice, which resulted in (D) increased maximum moment in 3-point bending tests.
Ct.Ar, cortical area; Ct.Th, cortical thickness; IMAX and IMIN, maximum and minimum moments of inertia. Data are mean ± SD, n=8–12 per group. *pOC-ERαKO different from LC, p<0.05 by one-way ANOVA for each sex.
Male pOC-ERαKO mice exhibit increased bone mass
The bone phenotype seen in male pOC-ERαKO mice compared to their littermate controls was opposite to that seen in females (Table 1, Table 2). In the vertebral body, Tb.Sp was lower in knockouts (−9.9%) while Tb.Th remained unchanged, but these changes did not result in an overall alteration in BV/TV (Figure 1). In the right tibial metaphysis, cancellous BV/TV was greater by 34%, due largely to increased Tb.Th (+14%) (Table 2). In the cortical shell of the tibial metaphysis, pOC-ERαKO mice had larger IMAX (+15%) compared to LC, but unchanged Ct.Ar, IMIN, Ct.Th, and ct.TMD. The cortical shell of the vertebrae was unaffected by ERα deletion in osteoblasts and osteocytes. Compressive strength and stiffness were not different in pOC-ERαKO males compared to LC, reflecting the cortical bone mass and geometry at that site.
At two purely cortical regions, the right femoral and tibial midshafts, cortical bone mass was significantly greater in male pOC-ERαKO mice. In the femur, Ct.Ar (+14%), IMAX (+23%), and IMIN (+23%) were larger, which were reflected in greater maximum bending moment (+15%) but with no change in whole bone stiffness from bending tests (Figure 2). Similarly, at the right tibial midshaft, male pOC-ERαKO mice had larger Ct.Ar (+6.9%), IMAX (+16%), and IMIN (+10%).
ERα in osteoblasts and osteocytes suppresses the anabolic response to mechanical loading in female mice
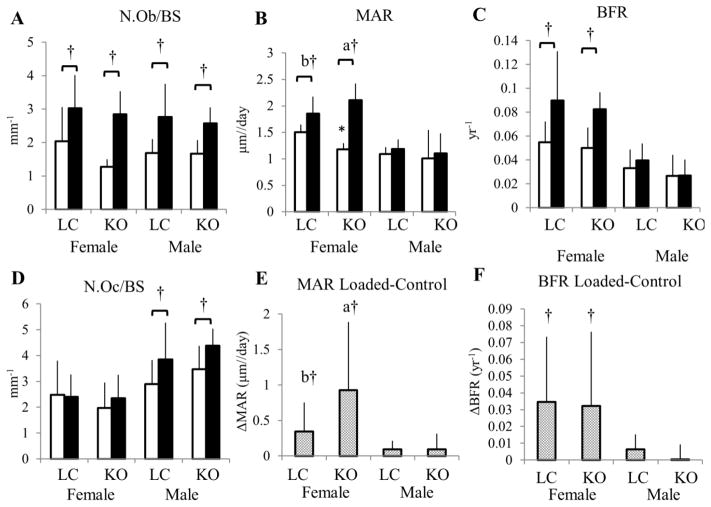
At the tibial metaphysis, cancellous bone responded robustly to mechanical loading in both LC and gene-deleted females (Figure 3). BV/TV, Tb.Th, and cn.TMD were increased after two weeks of loading (Table 2). Matrix-secreting osteoblast activity from pro-collagen-I IHC normalized to bone surface was increased in loaded versus control tibiae (Figure 4, Figure 5), as expected from new bone formation due to loading. Osteoclast number measured by TRAP histology normalized to bone surface was not affected by loading or by genotype (Figure 4). Tibial growth plate thickness increased with loading, and was increased in control limbs of knockouts compared to control limbs of LC (Table 3).
Figure 3.
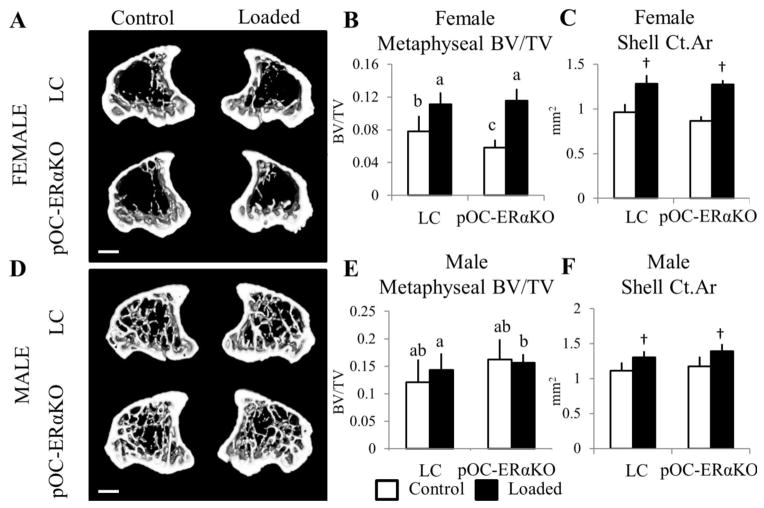
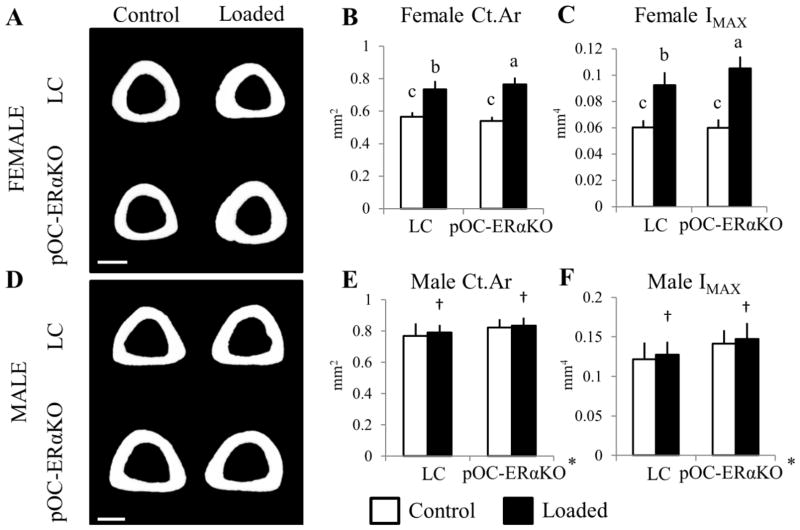
Tibial metaphyseal bone mass was reduced in pOC-ERαKO female mice but increased in pOC-ERαKO male mice, and pOC-ERαKO female mice responded more to 2 weeks of tibial compression. Representative transverse 3D microCT reconstructions (0.51mm thick) of the tibial metaphysis in (A) female and (D) male 12-week-old LC (top) and pOC-ERaKO mice (bottom) after 2 weeks of left tibial loading. (B) BV/TV of cancellous bone in female pOC-ERaKO mice was 25% lower in the unloaded right tibia compared to LC. After two weeks of tibial loading, BV/TV increased more (+97%) in pOC-ERaKO mice than in LC mice (+43%). (E) BV/TV was increased in the tibial metaphysis of male pOC-ERaKO compared to LC, but 2 weeks of loading did not alter BV/TV in left vs. right limbs for either genotype. (C,F) Area of the cortical shell increased similarly between genotypes within each sex after loading, and Ct.Ar was unaffected by ERα deletion in both sexes.
BV/TV, bone volume fraction; Ct.Ar, cortical area. Data are mean ± SD, n=12–14 per group. †Loaded tibia different from Control, p<0.05 by repeated measures ANOVA with interaction for each sex. Bars not sharing same letter are significantly different from one another from Tukey HSD post-hoc only when interaction term (load*genotype) was significant. Scale bar = 1.0mm.
Figure 4.
(A) Two weeks of loading increased matrix-secreting N.Ob/BS in the cancellous tibial metaphysis in both genotypes and sexes. In female mice, (B,E) MAR and (C,F) BFR were increased with loading, and MAR increased more in pOC-ERαKO than in LC mice (B,E). In male mice, MAR and BFR were not affected by tibial loading. (D) N.Oc/BS increased after loading in male but not female mice.
Matrix-secreting N.Ob/BS, number of osteoblasts staining positively for pro-collagen I normalized to bone surface; N.Oc/BS, number of osteoclasts staining positively for TRAP normalized to bone surface; MAR, mineral apposition rate; BFR, bone formation rate. Data are mean ± SD, n=6–7 per group. *pOC-ERαKO different from LC, †Loaded tibia different from Control, p<0.05 by repeated measures ANOVA with interaction for each sex. Bars not sharing same letter are significantly different from one another from Tukey HSD post-hoc only when interaction term (load*genotype) was significant.
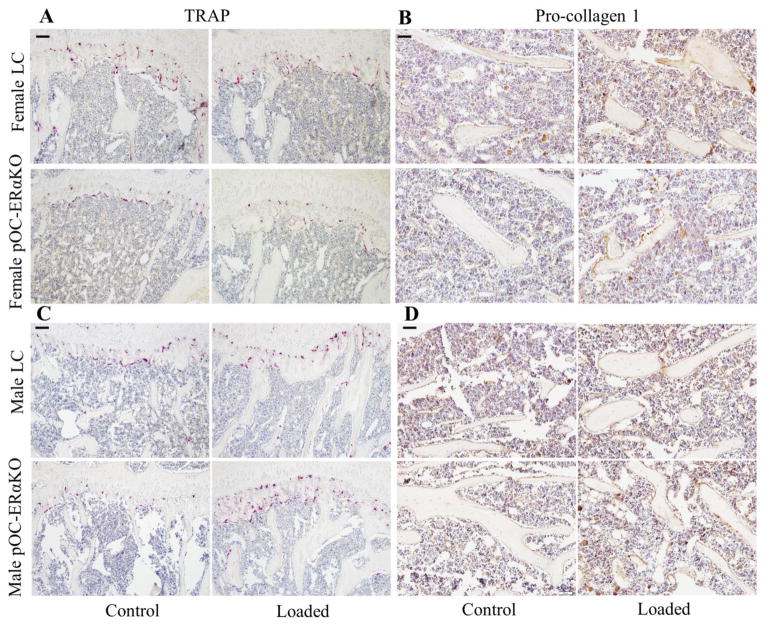
Figure 5.
Representative IHC and histology images for sagittal sections of the proximal tibiae of female (top) and male (bottom) LC and pOC-ERαKO mice in loaded and control limbs. (A) N.Oc/BS was not altered by genotype or loading in female mice. (B) N.Ob/BS increased with 2 weeks of in vivo tibial loading similarly in both genotypes. (C,D) Osteoclast number and osteoblast activity normalized to bone surface both increased after 2 weeks of in vivo tibial mechanical loading in male pOC-ERαKO and LC mice.
Matrix-secreting N.Ob/BS, number of osteoblasts staining positively for pro-collagen I normalized to bone surface; N.Oc/BS, number of osteoclasts staining positively for TRAP normalized to bone surface. Scale bar = 80μm for A,C; 20μm for B,D.
Table 3.
Two weeks of daily tibial loading increased cancellous, periosteal, and endosteal MAR and BFR of 10-week-old female pOC-ERαKO and LC mice. Dynamic histomorphometry, histology and immunohistochemistry data for tibia in female and male pOC-ERαKO and LC mice
| Female | Male | ||||
|---|---|---|---|---|---|
| LC | pOC-ERαKO | LC | pOC-ERαKO | ||
| Cn.MS | Control | 0.210 ± 0.033bc | 0.231 ± 0.040c | 0.276 ± 0.046 | 0.250 ± 0.059 |
| Loaded | 0.325 ± 0.041†ab | 0.279 ± 0.034†a | 0.320 ± 0.025† | 0.279 ± 0.086† | |
|
|
|||||
| Cn.MAR (μm/day) | Control | 1.51 ± 0.14b | 1.18 ± 0.12*b | 1.09 ± 0.13 | 1.01 ± 0.53 |
| Loaded | 1.85 ± 0.33†ab | 2.11 ± 0.31†a | 1.19 ± 0.18 | 1.10 ± 0.38 | |
|
|
|||||
| Cn.BFR (yr−1) | Control | 0.0550 ± 0.017 | 0.0502 ± 0.017 | 0.0331 ± 0.016 | 0.0266 ± 0.018 |
| Loaded | 0.0897 ± 0.041† | 0.0396 ± 0.014† | 0.0825 ± 0.014 | 0.0270 ± 0.013 | |
|
| |||||
| Es.MS | Control | 0.587 ± 0.029 | 0.551 ± 0.186 | 0.357 ± 0.19 | 0.195 ± 0.12* |
| Loaded | 0.556 ± 0.16 | 0.380 ± 0.10 | 0.312 ± 0.19† | 0.137 ± 0.059*† | |
|
|
|||||
| Es.MAR (μm/day) | Control | 0.825 ± 0.19 | 0.710 ± 0.37 | 0.438 ± 0.37 | 0.320 ± 0.37 |
| Loaded | 2.08 ± 0.57† | 1.47 ± 0.43† | 0.529 ± 0.72 | 0.143 ± 0.35 | |
|
|
|||||
| Es.BFR (yr−1) | Control | 0.0669 ± 0.014b | 0.0605 ± 0.038*b | 0.0241 ± 0.017 | 0.0106 ± 0.015 |
| Loaded | 0.198 ± 0.076†a | 0.105 ± 0.037*†a | 0.0323 ± 0.045 | 0.00282 ± 0.007 | |
|
|
|||||
| Es.Wo.Ar (mm2) | Control | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Loaded | 0.0105 ± 0.015† | 0.0223 ± 0.014† | 0.00 ± 0.0 | 0.00 ± 0.0 | |
|
| |||||
| Ps.MS | Control | 0.105 ± 0.060 | 0.0827 ± 0.032 | 0.0839 ± 0.045 | 0.211 ± 0.17 |
| Loaded | 0.226 ± 0.081† | 0.260 ± 0.11† | 0.431 ± 0.23† | 0.413 ± 0.15† | |
|
|
|||||
| Ps.MAR (μm/day) | Control | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.147 ± 0.36 |
| Loaded | 2.34 ± 0.19† | 1.70 ± 0.91† | 1.02 ± 0.95† | 0.775 ± 0.48† | |
|
|
|||||
| Ps.BFR (yr−1) | Control | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00596 ± 0.015 |
| Loaded | 0.0964 ± 0.019† | 0.0774 ± 0.040† | 0.0552 ± 0.053 | 0.0270 ± 0.019 | |
|
|
|||||
| Ps.Wo.Ar (mm2) | Control | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Loaded | 0.140 ± 0.028† | 0.166 ± 0.049† | 0.00525 ± 0.012 | 0.00 ± 0.0 | |
|
| |||||
| N.Ob/BS (mm−1) | Control | 2.48 ± 1.0 | 1.27 ± 0.23 | 1.68 ± 0.42 | 1.67 ± 0.41 |
| Loaded | 3.02 ± 1.0† | 2.84 ± 0.69† | 2.77 ± 0.99† | 2.57 ± 0.48† | |
|
|
|||||
| N.Oc/BS (mm−1) | Control | 2.48 ± 1.3 | 1.97 ± 0.98 | 2.90 ± 0.93 | 3.47 ± 0.91 |
| Loaded | 2.41 ± 0.87 | 2.35 ± 0.91 | 3.85 ± 1.4† | 4.38 ± 0.66† | |
|
|
|||||
| GP.Th (μm) | Control | 107 ± 8.5 | 119 ± 2.7* | 106 ± 15 | 119 ± 10 |
| Loaded | 178 ± 5.0† | 198 ± 17*† | 187 ± 15† | 180 ± 34† | |
Data are mean ± SD. Cn, cancellous; Es, endosteal; Ps, periosteal; MS, mineralizing surface; MAR, mineral apposition rate; BFR, bone formation rate; N.Ob/BS and N.Oc/BS, number of matrix-secreting osteoblasts positively-stained for procollagen 1 or positively-stained osteoclasts with TRAP normalized to bone surface; GP.Th, growth plate thickness
pOC-ERαKO different from LC,
Loaded tibia different from Control, p<0.05 by repeated measures ANOVA with interaction for each sex.
Groups not sharing the same letter are significantly different by Tukey HSD post-hoc where a > b > c. Post-hoc performed when ANOVA interaction term was significant.
Both BV/TV and Tb.Th increased significantly more in response to mechanical loading in mice lacking ERα in mature osteoblasts and osteocytes than in LC. BV/TV increased 97% in pOC-ERαKO and only 43% in LC, due to increased Tb.Th (+60%, +39%, respectively). Although BV/TV was lower in knockout mice in the control limbs compared to LC, Tb.Th was not different between control right tibiae. Dynamic histomorphometry results showed that MAR in the cancellous metaphysis increased more in pOC-ERαKO females in response to 2 weeks of loading than in LC mice, analogous to the increased loading response seen in the microCT data (Table 3, Figure 4, Supplementary Fig 2). MS and BFR increased similarly with loading in both genotypes.
Similar to the cancellous region of the tibial metaphysis, Ct.Ar, Ct.Th, IMAX, IMIN, and ct.TMD in the cortical shell of the tibial metaphysis increased in response to mechanical loading. Both IMIN and Ct.Th were decreased in the control tibiae of pOC-ERαKO female mice compared to LC, and these two parameters responded significantly more to mechanical loading in pOC-ERαKO mice. IMIN increased 64% in pOC-ERαKO but only 44% in LC. Ct.Th increased 37% in knockouts but only 27% in LC. Ct.Ar responded similarly to mechanical loading as LC and was not different in right control limbs.
At the tibial midshaft, female pOC-ERαKO mice responded more to mechanical loading than LC despite having similar bone architecture in contralateral limbs (Figure 6). Ct.Ar increased more in pOC-ERαKO mice (+41%) than in LC (+28%), as did IMAX (+76% in pOC-ERαKO and +53% in LC) (Table 2). Ct.Th, IMIN, and ct.TMD increased similarly between genotypes with two weeks of tibial loading. New bone formed on both the periosteal and endosteal surfaces of the tibial midshaft, as indicated by increased MS, MAR, and BFR at the periosteum and increased MAR and BFR at the endosteum (Table 3, Supplementary Fig 2). As such, Ma.Ar decreased with loading (−4.9% pOC-ERαKO, −6.5% LC). Of note, woven bone formation was present at the tibial midshaft of both genotypes of female mice in response to loading, but not in the metaphysis. MicroCT measurements at the tibial midshaft included both woven and lamellar bone, while the histomorphometric data (MS, MAR and BFR) excluded woven bone.
Figure 6.
Tibial midshaft cortical bone mass was similar between pOC-ERαKO and LC mice, but knockouts responded more to 2 weeks of tibial compression; male pOC-ERαKO had increased cortical bone mass but responded similarly to loading as LC mice. Representative transverse 3D microCT reconstructions (45um thick) of the tibial midshaft in (A) female and (D) male 12-week-old LC (top) and pOC-ERaKO mice (bottom) after 2 weeks of left tibial loading. (B,C) Although Ct.Ar was not different between LC and pOC-ERaKO female mice in the right, control limb, after 2 weeks of tibial loading, Ct.Ar increased more in pOC-ERaKO mice (+41%) vs. LC (+28%), as did IMAX. (E,F) Male pOC-ERaKO mice had increased Ct.Ar and IMAX at the tibial midshaft in the right unloaded limbs, and both genotypes showed a similar increase in Ct.Ar and IMAX after 2 weeks of mechanical loading.
Ct.Ar, cortical area; IMAX, maximum moment of inertia. Data are mean ± SD, n=12–14 per group. *pOC-ERαKO different from LC, †Loaded tibia different from Control, p<0.05 by repeated measures ANOVA with interaction for each sex. Bars not sharing same letter are significantly different from one another from Tukey HSD post-hoc only when interaction term (load*genotype) was significant. Scale bar = 0.5mm.
Global measures of bone formation and osteoblast activity, such as serum P1NP and OC levels, were not different between genotypes (Supplementary Fig. 1), consistent with local measures of matrix production by osteoblasts in the cancellous metaphysis (matrix secreting N.Ob/BS) and dynamic histomorphometry measurements at both the cancellous metaphysis and tibial midshaft. The latter data differed only in MAR, which was decreased in the cancellous metaphysis of control tibiae of knockout animals.
Response to mechanical loading is unchanged in male pOC-ERαKO mice
After 2 weeks of tibial loading in male mice, cancellous trabeculae thickened (+16% pOC-ERαKO, +28% LC) and cn.TMD increased (+2.6% pOC-ERαKO, +4.0% LC) in the loaded limb compared to the contralateral limb in both genotypes in the tibial metaphysis (Table 2). Overall BV/TV did not increase with loading as in previous similar experiments with male C57Bl/6 mice, although Tb.Th did increase (26, 38) (Figure 3). Activity of matrix-secreting osteoblasts and osteoclast number normalized to bone surface, indicative of increased turnover, were both increased in loaded versus contralateral tibial metaphyses, as was tibial growth plate thickness (Table 3, Figure 4). Cancellous MS increased in pOC-ERαKO and LC with loading, but MAR and BFR were unchanged (Table 3, Figure 4, Supplementary Fig 2).
In the cortical shell of the metaphysis, Ct.Ar, IMAX, and IMIN increased in response to 2 weeks of mechanical loading compared to control limbs. Despite pOC-ERαKO mice having higher bone mass in the cancellous metaphysis and increased IMAX in the cortical shell metaphysis, LC and pOC-ERαKO showed similar responses to in vivo tibial loading.
At the cortical tibial midshaft, Ct.Ar increased 1.4% in pOC-ERαKO with loading and 2.8% in LC (Figure 6). Most new bone formation reflected periosteal expansion, as shown through increased periosteal MS and MAR, but no change in marrow area (Table 3). However, endosteal MS increased with loading in both genotypes, while IMAX and IMIN also were higher. Cortical geometry in pOC-ERαKO male mice responded similarly to mechanical loading as LC mice.
Serum levels of PINP and TRAP5b were similar between genotypes, analogous to local activity of matrix-secreting osteoblasts and osteoclast number, but serum levels of OC were decreased in male pOC-ERαKO mice in the face of higher bone mass (Supplementary Fig 1).
DISCUSSION
We generated 10-week-old female and male mice lacking ERα in mature osteoblasts and osteocytes by breeding ERαfl/fl and OC-Cre mice. Serum measurements, bone lengths and growth plate analyses revealed that systemic effects were not present in females and limited in males, who showed increased femur length. When ERα was removed in mature osteoblasts and osteocytes, cancellous and cortical bone mass were lower at most sites in females; in contrast, pOC-ERαKO male mice had increased cancellous and cortical bone mass. When mice were subjected to two weeks of in vivo mechanical tibial loading, female pOC-ERαKO mice formed more bone in response to loading than LC. In contrast, genotype did not affect the skeletal response to mechanical loading in males.
Although osteoblast-specific ERαKO mice do not have the confounding systemic effects of global ERαKO mice, the possibility of other compensating mechanisms cannot be ruled out. When both ERα and ERβ are present, their functions are hypothesized to have minimal overlap. However, in global ERαKO mice, ERβ may mediate gene transcription normally controlled by ERα (39). ERβ has also been implicated in the skeletal response to mechanical loading and could compensate for lack of ERα (12, 40).
ERα in osteoblasts and osteocytes clearly has a role in bone mass accrual and its response to mechanical loading. Our previous work and that of Määttä et al. found similarly reduced bone mass in female mice when ERα was deleted in mature osteoblasts and osteocytes using the OC-Cre-driven promoter (18, 19). Määttä et al. did not find genotype differences in males, except at 6 months of age, at which time tibial BV/TV and Tb.N were lower in knockouts. Genetic background can profoundly affect bone structure and mass (41, 42). Our mice were backcrossed fully to a C57Bl/6 background, whereas the background of those studied by Määttä, et al. was not reported. Of note, our current study examined 10-week-old mice, whereas the Määttä study analyzed older mice at 3.5, 6 and 12 months of age. We have shown previously that age at the time of loading influences bone adaptation and does so differently in the cortical mid-diaphysis and cancellous metaphysis (43). We chose to examine 10-week-old mice that are still growing and whose proximal tibia contains a substantial volume of cancellous bone that is responsive to loading. Including skeletally mature and aged mice in future studies will provide a more complete picture of the role of ERα in osteoblasts and osteocytes.
During puberty, sex steroids promote bone growth and are major contributors to sexual skeletal dimorphism in humans (1). Late in puberty, estrogen suppresses periosteal apposition and leads to growth plate fusion in both sexes (44, 45). Adult males have greater bone mass due to a prolonged puberty and high testosterone levels that increase periosteal apposition (46). In pOC-ERαKO male mice, because the growth-suppressive effects of estrogen do not act on mature osteoblasts or osteocytes via ERα, the stimulating effects of testosterone on bone growth may be enhanced and might help to explain the higher bone mass phenotype found in these animals. Females lacking an estrogenic response via ERα in mature osteoblasts and osteocytes may accrue bone mass during growth more slowly resulting in the decreased bone mass found in female pOC-ERαKO mice.
Our findings indicate that ERα mediates mechanosensitivity in bone of female mice. We found that both cortical and cancellous adaptation to load were greater in pOC-ERαKO female mice but unchanged in pOC-ERαKO male mice compared to sex-matched controls. At the cancellous metaphysis, the effect of the reduced bone mass on the tissue strains is unclear. With similar loading on less bone mass, the strains could be increased, producing an increased adaptive response; alternately, the reduced bone mass could reduce load sharing and reduce the strain in the tissue. Our results are in contrast to other studies examining the role of ERα in mechanotransduction. For example, in osteoblast cultures, cells lacking ERα responded less to mechanical strain (47). Similarly, the cortical response to mechanical loading was decreased in global ERαKO female mice and in female mice with ERα deleted at the osteoblast progenitor and osteoblast precursor stages compared to controls (12, 29, 48). In mice with ERα deleted at the committed osteoblast stage or the osteocyte stage, the cortical response to mechanical loading was unchanged in female mice (20, 29). However, other researchers have indicated that estrogen and/or ERα may inhibit the skeletal response to mechanical loading, in agreement with our work. Kondoh et al. performed hindlimb unloading in Dmp1-ERαKO female mice and showed an accelerated loss of bone mass compared to littermate controls (21). Furthermore, whole body vibration increased periosteal and endosteal perimeters in OVX rat tibiae but not in sham control tibiae (49). Based on these prior results and our data, we conclude that the absence of ERα in mature osteoblasts and/or osteocytes of female mice did not reduce, but maintained or enhanced sensitivity to the loading environment in ERα-deficient bone (45).
The formation of woven bone in response to mechanical loading at both the periosteal and endosteal surfaces of the tibial midshaft in female pOC-ERαKO and LC mice is a limitation of this study, as we aimed to analyze lamellar bone formation from skeletal loading. A peak load of −9.0N was required to produce 1200με at the midshaft cortex, and similar load levels have been used previously for mouse tibial loading (33, 34). Following mechanical loading, the amount and variety of differentially expressed genes depends on whether lamellar or woven bone is being generated (50). The possible effects of woven vs. lamellar bone on data interpretation at the cortex from the current study cannot be discounted. Findings at the tibial midshaft of this study cannot be directly compared to other studies investigating lamellar bone formation at the tibial midshaft. The similar increases in MS, MAR and BFR at the periosteum between genotypes were unexpected given that the microCT data showed that Ct.Ar and IMAX increased more in response to loading in pOC-ERαKO vs. LC female mice. However, the presence of woven bone prevented quantification of single- and double-labeling along ~25% of the periosteal surface, thus the differences in loading response in pOC-ERαKO females may have been obscured. Both woven and lamellar bone were included in microCT measures, whereas woven bone regions were excluded from the histomorphometric analyses.
Female pOC-ERαKO also responded more to mechanical loading in the proximal tibia, where woven bone was not present and where Ct.Ar was similar between genotypes, indicating that ERα does regulate the skeletal response to mechanical loading during accrual of lamellar bone. Bone mass in the cancellous proximal tibia of pOC-ERαKO female mice was lower than that of LC mice. Because load levels were normalized for a target strain level at the cortical midshaft, a site at which bone mass and stiffness were similar between genotypes, we cannot distinguish whether the increased response to mechanical load in the cancellous tissues of the female pOC-ERαKO was caused purely by genotypic effects or a combination of genotype and different strains in the cancellous tissues of the knockouts relative to the LC.
Male pOC-ERαKO mice responded similarly to loading as LC mice in the cancellous metaphysis, the cortical shell of the proximal tibia, and the tibial midshaft. Although Tb.Th increased with loading in both genotypes in the cancellous metaphysis, surprisingly overall BV/TV was unchanged. One possible explanation for the dampened loading response in the cancellous metaphysis could be the group-housing of our male mice. Recently, group-housed males had an attenuated response to mechanical loading compared to single-housed males that was attributed to cage fighting (51). However, those mice were recently purchased and housed together, whereas the mice in the current study were group housed for months after breeding in-house, and thus less likely to fight. Also, this same loading protocol increased cancellous bone mass in recently purchased 10-week-old C57Bl/6 male mice previously (33). Furthermore, the current loading study did produce an anabolic response to loading in male mice at both cortical sites analyzed. The lack of change of BV/TV in the proximal tibia with loading in the male animals is unexpected and requires further investigation.
Use of a cre-recombinase driven by the OC promoter (pOC) has been widely used in the literature (31). Although the OC-Cre mouse has been a tool to knockout specific receptors and proteins in mature osteoblasts and subsequent osteocytes, cre expression has been detected in the growth plate (52). Previously in our mixed background strain of female pOC-ERαKO mice, we found no differences in growth plate thickness or in tibial or femoral lengths compared to LC (19). However, in the current animals, on a pure C57Bl/6 background, female pOC-ERαKO mice had thicker tibial growth plates but no differences in tibial or femoral bone length. In contrast, while growth plate thickness was the same in pOC-ERαKO males, femoral length was increased but tibial length was unaffected. Määttä et al. found increased tibial lengths in male pOC-ERαKO mice at 3 months of age (18). These results are difficult to interpret, as mouse growth plates never fuse. Thus, OC-Cre expression in the growth plate may affect growth plate thickness and bone length. However, overall body mass and crown/rump length were not altered in pOC-ERαKO mice of either sex.
Because declining sex hormones contribute greatly to osteoporosis in the elderly, especially post-menopausal women who have severely decreased estrogen levels, recent research has focused on understanding the role of estrogen signaling via its receptors in bone (9, 53). Here ERα in mature osteoblasts and osteocytes differentially regulated bone mass in males and females. Removing ERα increased the skeletal response to mechanical loading at cortical and cancellous sites in females, but did not affect skeletal adaption to physical stimuli in male mice. Further research should emphasize elucidating the cellular mechanisms and signaling pathways involved in estrogen signaling in bone, which may provide valuable insight into the pathogenesis of osteoporosis.
Supplementary Material
Acknowledgments
Financial support:
National Institutes of Health Grants R01-AG028664 and P30-AR046121 (MCHM), NSF GRFP (KMM and NHK), and F32-AR054676 (RPM).
We acknowledge Funmi Adebayo, Frank Ko, and Cornell CARE Staff for their assistance. We thank Dr. Thomas Clemens for providing OC-Cre mice and Dr. Sohaib Khan for providing ERαfl/fl mice.
Footnotes
All authors state that they have no conflicts of interest
Supplementary material is being submitted with this work
Authors’ roles: Study design: KMM, FPR, MCHM. Study conduct: KMM, NHK, DBB. Data collection: KMM, NHK, GS, DBB. Data analysis: KMM. Data interpretation: KMM, MCHM, FPR. Drafting manuscript: KMM. Revising manuscript content: KMM, MCHM, FPR, RPM. Approving final version of manuscript: all authors. KMM takes responsibility for the integrity of the data analysis.
Contributor Information
Katherine M. Melville, Email: kmp242@cornell.edu.
Natalie H. Kelly, Email: nhk28@cornell.edu.
Gina Surita, Email: gs486@cornell.edu.
Daniel B. Buchalter, Email: dbb77@cornell.edu.
John C. Schimenti, Email: jcs92@cornell.edu.
Russell P. Main, Email: rmain@purdue.edu.
F. Patrick Ross, Email: RossF@hss.edu.
Marjolein C. H. van der Meulen, Email: mcv3@cornell.edu.
References
- 1.Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol. 2010;207(2):127–34. doi: 10.1677/JOE-10-0209. [DOI] [PubMed] [Google Scholar]
- 2.Richelson LS, Wahner HW, Melton LJ, 3rd, Riggs BL. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. N Engl J Med. 1984;311(20):1273–5. doi: 10.1056/NEJM198411153112002. [DOI] [PubMed] [Google Scholar]
- 3.Riggs BL. Endocrine causes of age-related bone loss and osteoporosis. Novartis Found Symp. 2002;242:247–59. discussion 60–4. [PubMed] [Google Scholar]
- 4.Eastell R. Role of oestrogen in the regulation of bone turnover at the menarche. J Endocrinol. 2005;185(2):223–34. doi: 10.1677/joe.1.06059. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–64. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callewaert F, Boonen S, Vanderschueren D. Sex steroids and the male skeleton: a tale of two hormones. Trends Endocrinol Metab. 2010;21(2):89–95. doi: 10.1016/j.tem.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553–60. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 9.Price JS, Sugiyama T, Galea GL, Meakin LB, Sunters A, Lanyon LE. Role of endocrine and paracrine factors in the adaptation of bone to mechanical loading. Curr Osteoporos Rep. 2011;9(2):76–82. doi: 10.1007/s11914-011-0050-7. [DOI] [PubMed] [Google Scholar]
- 10.Vico L, Vanacker JM. Sex hormones and their receptors in bone homeostasis: insights from genetically modified mouse models. Osteoporos Int. 2010;21(3):365–72. doi: 10.1007/s00198-009-0963-5. [DOI] [PubMed] [Google Scholar]
- 11.Khosla S. Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1226–35. doi: 10.1093/gerona/gls163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol. 2004;182(2):193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- 13.Vidal O, Lindberg M, Savendahl L, Lubahn DB, Ritzen EM, Gustafsson JA, Ohlsson C. Disproportional body growth in female estrogen receptor-alpha-inactivated mice. Biochem Biophys Res Commun. 1999;265(2):569–71. doi: 10.1006/bbrc.1999.1711. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol. 2001;171(2):229–36. doi: 10.1677/joe.0.1710229. [DOI] [PubMed] [Google Scholar]
- 15.Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci U S A. 2000;97(10):5474–9. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikka V, Peng Z, Hentunen T, Risteli J, Elo T, Vaananen HK, Harkonen P. Estrogen responsiveness of bone formation in vitro and altered bone phenotype in aged estrogen receptor-alpha-deficient male and female mice. Eur J Endocrinol. 2005;152(2):301–14. doi: 10.1530/eje.1.01832. [DOI] [PubMed] [Google Scholar]
- 17.Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, Onal M, Xiong J, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123(1):394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maatta JA, Buki KG, Gu G, Alanne MH, Vaaraniemi J, Liljenback H, Poutanen M, Harkonen P, Vaananen K. Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. FASEB J. 2012 doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 19.Melville KM, Kelly NH, Khan SA, Schimenti JC, Ross FP, Main RP, van der Meulen MC. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Miner Res. 2014;29(2):370–9. doi: 10.1002/jbmr.2082. [DOI] [PubMed] [Google Scholar]
- 20.Windahl SH, Borjesson AE, Farman HH, Engdahl C, Moverare-Skrtic S, Sjogren K, Lagerquist MK, Kindblom JM, Koskela A, Tuukkanen J, Divieti Pajevic P, Feng JQ, Dahlman-Wright K, Antonson P, Gustafsson JA, Ohlsson C. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci U S A. 2013;110(6):2294–9. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondoh S, Inoue K, Igarashi K, Sugizaki H, Shirode-Fukuda Y, Inoue E, Yu T, Takeuchi JK, Kanno J, Bonewald LF, Imai Y. Estrogen receptor alpha in osteocytes regulates trabecular bone formation in female mice. Bone. 2014;60:68–77. doi: 10.1016/j.bone.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavolina JM, Evans GL, Harris SA, Zhang M, Westerlind KC, Turner RT. The effects of orbital spaceflight on bone histomorphometry and messenger ribonucleic acid levels for bone matrix proteins and skeletal signaling peptides in ovariectomized growing rats. Endocrinology. 1997;138(4):1567–76. doi: 10.1210/endo.138.4.5040. [DOI] [PubMed] [Google Scholar]
- 23.Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204–8. [PubMed] [Google Scholar]
- 24.Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling. Bone. 1991;12(2):73–9. doi: 10.1016/8756-3282(91)90003-2. [DOI] [PubMed] [Google Scholar]
- 25.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37(6):810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36(6):1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Lee KC, Lanyon LE. Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev. 2004;32(2):64–8. doi: 10.1097/00003677-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich PJ, Noble BS, Jessop HL, Stevens HY, Mosley JR, Lanyon LE. The effect of in vivo mechanical loading on estrogen receptor alpha expression in rat ulnar osteocytes. J Bone Miner Res. 2002;17(9):1646–55. doi: 10.1359/jbmr.2002.17.9.1646. [DOI] [PubMed] [Google Scholar]
- 29.Iyer S, Kim H, Ucer SS, Bartell S, Warren A, Crawford J, Skinner R, Dallas M, Johnson M, Weinstein RS, Jilka RL, O’Brien CA, Almeida M, Manolagas SC. ERα Signaling in Osterix1 and Prx1 Expressing Cells, Respectively, Mediates the Anabolic Effect of Mechanical Loading in the Murine Periosteum and the Protective Effects of Estrogens on Endocortical Resorption. J Bone Miner Res. 2013;28(S1) [Google Scholar]
- 30.Clemens TL, Tang H, Maeda S, Kesterson RA, Demayo F, Pike JW, Gundberg CM. Analysis of osteocalcin expression in transgenic mice reveals a species difference in vitamin D regulation of mouse and human osteocalcin genes. J Bone Miner Res. 1997;12(10):1570–6. doi: 10.1359/jbmr.1997.12.10.1570. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–12. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 32.Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(37):14718–23. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, van der Meulen MC. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol (1985) 2010;109(3):685–91. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holguin N, Brodt MD, Sanchez ME, Kotiya AA, Silva MJ. Adaptation of tibial structure and strength to axial compression depends on loading history in both C57BL/6 and BALB/c mice. Calcif Tissue Int. 2013;93(3):211–21. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984;107(2):321–7. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- 36.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9(11):1441–54. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 38.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Bone mass is preserved and cancellous architecture altered due to cyclic loading of the mouse tibia after orchidectomy. J Bone Miner Res. 2008;23(5):663–71. doi: 10.1359/JBMR.080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17(2):203–8. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 40.Castillo AB, Triplett JW, Pavalko FM, Turner CH. Estrogen receptor-beta regulates mechanical signaling in primary osteoblasts. Am J Physiol Endocrinol Metab. 2014;306(8):E937–44. doi: 10.1152/ajpendo.00458.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18(5):397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 42.Wergedal JE, Sheng MH, Ackert-Bicknell CL, Beamer WG, Baylink DJ. Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross-sectional geometry and bone density. Bone. 2005;36(1):111–22. doi: 10.1016/j.bone.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, van der Meulen MC. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011;49(3):439–46. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakley GK, Schutte HD, Jr, Hannon KS, Turner RT. Androgen treatment prevents loss of cancellous bone in the orchidectomized rat. J Bone Miner Res. 1991;6(4):325–30. doi: 10.1002/jbmr.5650060403. [DOI] [PubMed] [Google Scholar]
- 45.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 46.Turner RT, Colvard DS, Spelsberg TC. Estrogen inhibition of periosteal bone formation in rat long bones: down-regulation of gene expression for bone matrix proteins. Endocrinology. 1990;127(3):1346–51. doi: 10.1210/endo-127-3-1346. [DOI] [PubMed] [Google Scholar]
- 47.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424(6947):389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 48.Saxon LK, Galea G, Meakin L, Price J, Lanyon LE. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology. 2012;153(5):2254–66. doi: 10.1210/en.2011-1977. [DOI] [PubMed] [Google Scholar]
- 49.Rubinacci A, Marenzana M, Cavani F, Colasante F, Villa I, Willnecker J, Moro GL, Spreafico LP, Ferretti M, Guidobono F, Marotti G. Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int. 2008;82(4):316–26. doi: 10.1007/s00223-008-9115-8. [DOI] [PubMed] [Google Scholar]
- 50.McKenzie JA, Bixby EC, Silva MJ. Differential gene expression from microarray analysis distinguishes woven and lamellar bone formation in the rat ulna following mechanical loading. PLoS One. 2011;6(12):e29328. doi: 10.1371/journal.pone.0029328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meakin LB, Sugiyama T, Galea GL, Browne WJ, Lanyon LE, Price JS. Male mice housed in groups engage in frequent fighting and show a lower response to additional bone loading than females or individually housed males that do not fight. Bone. 2013;54(1):113–7. doi: 10.1016/j.bone.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borjesson AE, Lagerquist MK, Liu C, Shao R, Windahl SH, Karlsson C, Sjogren K, Moverare-Skrtic S, Antal MC, Krust A, Mohan S, Chambon P, Savendahl L, Ohlsson C. The role of estrogen receptor alpha in growth plate cartilage for longitudinal bone growth. J Bone Miner Res. 2010;25(12):2690–700. doi: 10.1002/jbmr.156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.