Abstract
Objective
Estradiol enhances vasodilation in healthy women, but vascular effects of the phytoestrogen genistein are still under investigation. Insulin resistance (IR) compromises microvascular function. We therefore examined the interaction of estradiol, genistein, and IR on microvascular vasodilatory responsiveness.
Methods
We hypothesized that estradiol and genistein increase microvascular vasodilation in healthy women (control, n=8, 23±2 yr, BMI 25.9±2.9 kg/m2) but not in women with IR (n=7, 20±1 yr, BMI 27.3±3.0 kg/m2). We used the cutaneous circulation as a model of microvascular vasodilatory function. We determined cutaneous vascular conductance (CVC) with laser Doppler flowmetry and beat-to-beat blood pressure during local cutaneous heating (42°C) with estradiol or genistein microdialysis perfusions. Because heat induced vasodilation is primarily an NO mediated response, we examined microvascular vasodilation with and without L-NMMA.
Results
In control women, estradiol enhanced CVC (94.4±2.6 % vs. saline 81.6±4.2 % CVCmax, P<0.05), which was reversed with L-NMMA (80.9±7.8 % CVCmax, P<0.05), but genistein did not affect vasodilation. Neither estradiol nor genistein altered CVC in IR, although L-NMMA attenuated CVC during genistein.
Conclusions
Our study does not support improved microvascular responsiveness during genistein exposure in healthy young women, and demonstrates that neither estradiol nor genistein improve microvascular vasodilatory responsiveness in women with IR.
Key terms: cutaneous microdialysis, vascular reactivity, phytoestrogens, nitric oxide, estrogen
INTRODUCTION
Estrogens increase blood flow in many vascular beds. In particular, 17β-estradiol (E2) enhances vasodilation by activating endothelial nitric oxide synthase (eNOS) to rapidly produce nitric oxide (NO), as elegantly reviewed by Kim et al (33). Recently, the cardiovascular benefits of estradiol have been subject to considerable debate (2, 48). Despite mechanistic studies supporting beneficial cardiovascular effects of estradiol, large-scale clinical trials have demonstrated estrogen-associated increases in the risk of thrombolytic events in women over 60 and in women with underlying or established cardiovascular disease (2, 48). Thus, many women cannot or do not wish to take estrogens and seek alternatives such as phytoestrogens.
Phytoestrogens are plant-derived compounds that can induce physiological effects similar to those of estrogens. Phytoestrogens are used to treat some menopausal symptoms, including vasomotor symptoms, and do not appear to increase the risk of carcinogenic outcomes associated with unopposed estrogens. Indeed, certain phytoestrogens may even provide protection against some gynecological cancers (44). The most commonly used phytoestrogen is genistein (GEN). As with estrogens, GEN may enhance vasodilation, improve endothelial function, and lower the risk of cardiovascular disease (8, 15). For example, in women with coronary heart disease, GEN elicits greater vasodilation in isolated subcutaneous vessels compared to E2 (10). In healthy men, GEN causes NO-dependent vasodilation in forearm resistance vessels comparable in magnitude to the vasodilatory actions of E2 (59). Therefore, the beneficial cardiovascular effects of GEN may be similar to, or even more favorable than, those of E2 (10).
The American Diabetes Association indicates that approximately 35% of Americans are pre-diabetic including individuals with insulin resistance (IR). Moreover, independent of progression to diabetes, IR increases cardiovascular disease risk in both men and women. The primary physiological action of insulin is to facilitate glucose uptake by tissues, but insulin also modulates the sympathetic nervous system and mediates vasodilation by increasing NO release from endothelial cells (55). As such, IR is often associated with endothelial dysfunction and peripheral vascular disease, thereby increasing hypertension and cardiovascular disease risks. The endothelial dysfunction associated with IR is evident in the peripheral microcirculation (45) and related in part to compromised NO bioavailability and eNOS signaling (46, 55). Given the extent to which E2-mediated vasodilation depends on NO and eNOS signaling, the vasodilatory actions of estrogens and phytoestrogens may be compromised in women with IR.
The cutaneous circulation is an easily accessible vascular bed, and is frequently used as a model to examine changes in peripheral microcirculatory function in humans (22, 35) because the same systems that contribute to regulating vascular smooth muscle, vascular tone, and endothelial function [i.e. NO and prostaglandins (11, 57)] operate in the skin vasculature (12, 13, 21, 22, 28–30, 40). In addition, local heating of the skin induces vasodilation that is largely mediated by the endothelium and may be more sensitive in detecting microvascular dysfunction compared to iontophoresis of acetylcholine in patients with CVD (1, 23, 32). Cutaneous microdialysis (described below) is a minimally invasive technique that can explore mechanisms regulating vascular function in vivo and in humans, permitting local drug delivery without whole body exposure (22). This paradigm is of particular interest because vascular dysfunction is a systemic process that often begins in the microcirculation, and the mechanisms and degree of dysfunction in the cutaneous microvasculature reflect those in other microvascular beds (1, 9, 23, 32). Impaired vasomotor function in the microvascular circulation is a key feature of cardiovascular disease risk (1, 23, 32) associated with insulin resistance and hypertension (26, 45). The purpose of this study was to determine if either E2 or GEN exposure enhances microvascular vasodilatory responses in women with IR. We hypothesized that E2 and GEN enhance local heating-induced cutaneous microvascular vasodilation in healthy controls but not in women with IR.
MATERIALS and METHODS
Subjects and study design
Fifteen women participated in this study. Women were between 18–30 years of age, non-smoking, and taking no medication as indicated by a standard medical history. All of the women were sedentary or moderately active. Women completed two experimental visits: 1) oral glucose tolerance test (OGTT) to determine insulin resistance, and 2) microvascular vasodilatory assessment to determine microvascular responsiveness. We compared women with and without IR (as determined by the OGTT, see below). We focused on this IR group and not diabetics because IR can precede diabetes and is an important interventional state. All women gave written informed consent to participate in the study, which conformed to the guidelines contained in the Declaration of Helsinki and had prior approval by the Human Investigation Committee of Yale School of Medicine.
Oral glucose tolerance test (OGTT)
In order to assess insulin resistance, all women underwent a three-hour oral glucose tolerance test (OGTT). Women reported to the laboratory in the morning after an overnight fast. They provided a urine sample to determine hydration status and underwent an over-the-counter pregnancy test. Women were seated in a semi-recumbent position in a modified dental chair, and an IV catheter was placed in the left arm. After a 30-minute rest period, a blood sample was taken for fasting measures of plasma glucose and insulin. Women then consumed a 75 mg glucose beverage (Orangedex; Custom Laboratories, Baltimore, MD), and venous blood samples were drawn every 30 minutes to analyze plasma glucose and insulin concentrations. The area under the curve (AUC) for insulin concentration during the 180-minute period was used to determine insulin resistance (Table 1). Women were placed into control versus IR groups based on the spread of the data, with half above and half below the median of the area under the curve for insulin (see table below). Importantly, the responses of the women in each group were consistent with insulin and glucose responses typically associated with their respective groups (7).
Table 1.
Subject Characteristics
| CONTROL | IR | |
|---|---|---|
| Age, yr | 23 ± 2 | 20 ± 1 |
| Weight, kg | 60.4 ± 4.5 | 76.9 ± 7.7 |
| Height, cm | 1.56 ± 0.08 | 1.69 ± 0.03 |
| BMI, kg/m2 | 25.9 ± 2.9 | 27.3 ± 2.9 |
| P[Gl], mg/100 ml | 81.2 ± 2.3 | 85.3 ± 3.6 |
| P[Ins], µU/ml | 4.8 ± 1.1 | 10.0 ± 1.9* |
| Glucose, mg/100 ml AUC | 20,331 ± 727 | 20,167 ± 1,028 |
| Insulin, µU/ml AUC | 5,900 ± 457 | 10,933 ± 1,253* |
Subject characteristics in Control and women with insulin resistance (IR) taken at rest on the day of the oral glucose tolerance test (OGTT). Fasting plasma concentrations of glucose (P[Gl]) and insulin (P[Ins]), and area under the curve (AUC) during the OGTT.
Different from Control. Differences were considered significant at P < 0.05.
Microvascular vasodilatory assessment
In the present investigation, we used the cutaneous circulation as a model to explore the impact of E2 and GEN on microvascular vasodilatory responsiveness. To control for the variability in endogenous E2 exposure across the menstrual cycle, all experiments were performed during the early follicular phase (days 1–6). No women were taking hormonal treatments of any kind. Studies were conducted in the morning after an overnight fast in an environmental chamber (Ta = 28°C). Women were seated in a semi-recumbent position (modified dental chair) with an IV catheter placed in the left arm to obtain a blood sample for the analysis of serum concentrations of E2 (S[E2]) and progesterone (S[P4]). Following the blood collection, under sterile conditions, five microdialysis fibers each with a 30kDa cutoff (Basi, Inc. West Lafayette, IN) were placed just below the surface of the skin on the dorsal side of the right forearm as previously described (60, 61). After fiber insertion, we perfused 0.9% saline through all five fibers (2 µL/min) for at least 90 minutes following fiber insertion to permit trauma recovery. Red blood cell flux was then measured using laser Doppler flowmetry (Doppler Monitor, PF 5020 LDPM Unit, Perimed AB, Stockholm, Sweden) and used as an index of skin blood flow (SkBF). We measured beat-by-beat blood pressure on the left middle finger throughout the experiment (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands).
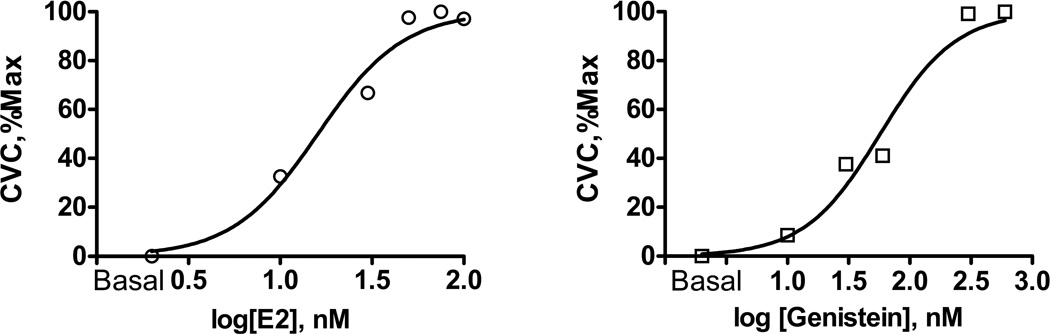
We measured resting baseline SkBF for 10 minutes with skin temperature clamped at 32°C. Following the baseline measurement, we perfused the following substances through five separate fibers: 0.9% saline; E2 (75 nM); E2 in combination with the nitric oxide synthase (NOS) inhibitor NG-monomethyl-L-arginine (L-NMMA, 10 mM); GEN (200 nM); and GEN in combination with L-NMMA (10 mM) at 5µL/min for 45 minutes. All compounds were purchased from Sigma-Aldrich and perfusion solution prepared by the Investigational Drug Service at Yale-New Haven Hospital. Because E2 and GEN are not water soluble, 0.01M of each compound was first dissolved in 100% ethanol. This solution was then diluted in normal saline for microdialysis perfusions at the desired concentrations (see below). The final concentration of ethanol is very small and had no effect on vasodilatory function; separate pilot studies in our laboratory indicated similar basal CVC (ethanol 0.29 vs. saline 0.24 au/mmHg) and vasodilatory responses to local heating (ethanol 1.1 vs. saline 1.7 au/mmHg) during perfusions of 0.01M ethanol in saline compared to saline alone. We attribute the differences in heating response to site-to-site variation in SkBF (a function of anatomical differences in blood vessel density at each skin site), but in any event, these data indicate that ethanol did not induce a vasodilation in these small amounts. The doses of E2 (molecular weight = 272.38) and GEN (molecular weight = 270.2) were determined from pilot studies conducted in our laboratory on a subset of women using dose-response curves (Prism 5.0, Graphpad Inc, San Diego, California) generated with five incremental doses of E2 (10, 30, 50, 75, 100 nM) and five incremental doses of GEN (10, 30, 60, 300, 600 nM). In order to induce vasodilation and determine the impact of E2 and GEN these experiments were conducted during local heating. Infused E2 and GEN concentrations were log transformed, and % CVCmax was normalized within each probe with the largest value of the data set at 100% and lowest value of the data set at 0% (vasodilation), and then plotted using nonlinear regression with a variable slope (62). Normalizing the % CVCmax to a maximum value of 100% (highest concentration of E2 or GEN) and the minimum value 0% (lowest concentration of E2 or GEN) enabled the comparison of the dose-response curves on a similar scale and is useful when comparing curve position (62). Dose-response curves are presented in Figure 1.
Figure 1.
Dose-response curves generated with five incremental doses of estradiol (E2; Left: 10, 30, 50, 75, 100 nM) and five incremental doses of genistein (GEN; Right: 10, 30, 60, 300, 600 nM).
Following these initial 45-minute hormone perfusions we increased the temperature of all Doppler probes to 42°C for 30–45 minutes until we achieved a plateau in SkBF (60). Local heating of the skin induces microvascular dilation that is largely endothelial-dependent (22, 31, 40). This plateau was measured for five minutes, after which we perfused sodium nitroprusside (SNP; 28 nM, an endothelial independent vasodilator) at a rate of 10 µL/min while increasing the temperature of the Doppler probes to 43°C to elicit maximal vasodilation (60).
Blood analysis
An aliquot was transferred to a tube without anticoagulant for the determination of S[E2], and S[P4]. The samples were centrifuged, frozen immediately and stored at −80°C until analysis. Serum [E2] and S[P4] were measured using competitive binding radioimmunoassay methods. Intra- and inter-assay coefficients of variation for the mid-range standards for S[E2] (180±13.1 pg•ml−1) were 2.7% and 4.3% (Siemens Healthcare Diagnostics, Los Angeles, CA, USA), for S[P4] (3.5±0.2 ng•ml−1) were 2.4% and 2.6% (Siemens Healthcare Diagnostics). Glucose samples were analyzed in duplicate immediately after withdrawal using a glucose/lactate analyzer (Yellow Springs Instruments, Yellow Springs OH). Insulin was measured in duplicate by competitive binding radioimmunoassay (Siemens Healthcare Diagnostics) with intra- and inter-assay coefficients of variation for the midrange standard (34.6µlU/ml) of 2.43% and 3.26%, respectively.
Data analysis and Statistics
Microvascular SkBF data were collected at 1000 Hz using Powerlab (ADInstruments, Bella Vista, NSW, Australia), and expressed as cutaneous vascular conductance (CVC), calculated by dividing SkBF by mean arterial blood pressure (Finometer) over the five-minute plateau period. We compared the plateau phase of local heating (to 42°C) across hormonal exposure between controls and women with IR using a 2-way ANOVA. In order to account for site to site variation in SkBF (a function of anatomical differences in blood vessel density at each skin site), all data are expressed as a percent of maximal CVC (%CVCmax) achieved during the perfusion of SNP concomitant with local heating to 43°C.
Sample size calculation
Sample size calculations were based on our primary outcome variable of interest, %CVCmax. The desired statistical test is two-sided and we assumed an α level of 0.01 to account for multiple comparisons. We used an effect size of 1.935 (6 subjects/group) (52). Given 6 per group and alpha=0.01, this effect size allowed us 80% power (0.843) for ANOVA to differentiate these changes from chance (14) (© G-Power 3.1).
RESULTS
Based on the AUC during the 180 minute OGTT, 8 women were classified as controls and 7 women as insulin resistant (Table 1). Fasting serum insulin concentrations and the AUC for insulin was elevated in the IR group (by design). All other baseline subject characteristics were similar between groups (Table 1).
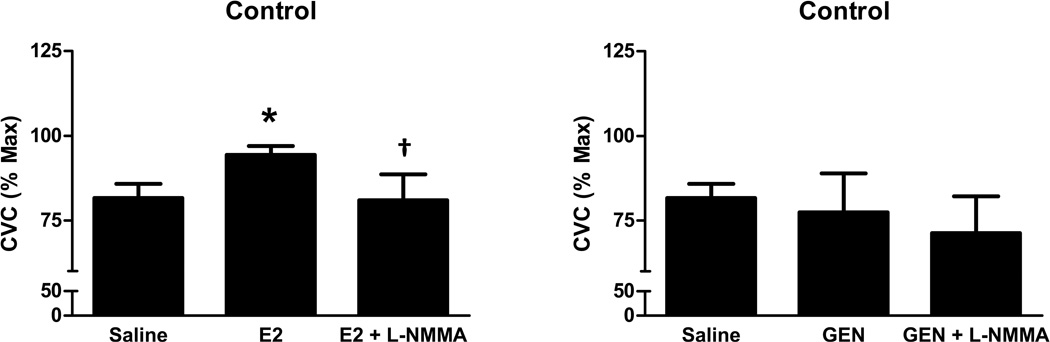
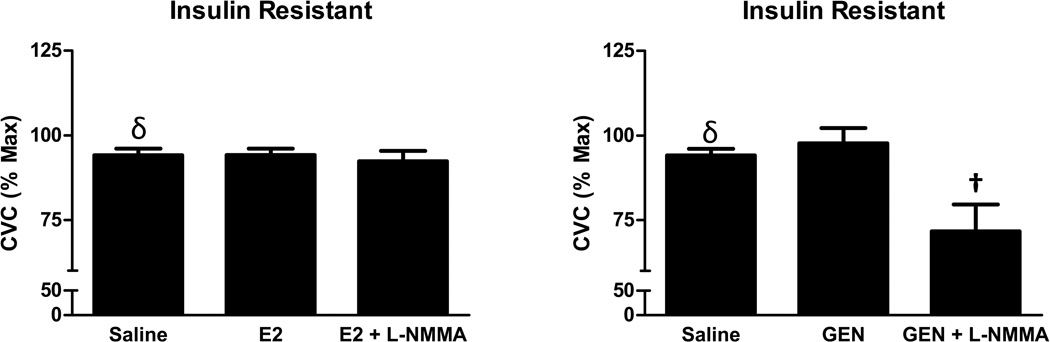
On the day of microdialysis testing blood analysis of ovarian hormones confirmed that women were in the early follicular phase of the menstrual cycle, (S[E2], Control: 39.2±5.7 pg/ml, IR: 34.2±4.7 pg/ml; S[P4], Control: 1.9± 0.9 ng/ml, IR 2.2 ± 1.3 ng/ml) with no differences between the groups in either hormone concentration. The SkBF increased during heating with saline perfusion in both groups of women, and this increase was higher in IR (P=0.04), indicating that overall microvascular responsiveness was preserved in women with IR. In controls only, E2 perfusion enhanced microvascular dilation induced by local heating (Figure 2), and L-NMMA reversed this E2 effect. In contrast, GEN had no impact on microvascular dilation in controls (Figure 2). In women with IR, neither E2 nor GEN increased microvascular dilation, although NO blockade attenuated the heating-induced vasodilation during GEN perfusion (Figure 3). Mean arterial blood pressure was similar between groups as was maximal vasodilation achieved with SNP combined with heating (data not shown).
Figure 2.
Microvascular vasodilatory responses to estradiol (E2; left) and genistein (GEN, right) in healthy control women. Data are presented as mean ± SEM. * Different from Saline. †Different from E2. Differences were considered significant at P < 0.05.
Figure 3.
Microvascular vasodilatory responses to estradiol (E2; left) and genistein (GEN, right) in women with insulin resistance. Data are presented as mean ± SEM. δ Different from Controls. †Different from GEN. Differences were considered significant at P < 0.05.
DISCUSSION
This is the first investigation to examine the direct effects of E2 and GEN exposure on peripheral cutaneous microvascular vasodilatory function in humans, both with and without IR. Our most important findings were that although E2 enhanced microvascular dilation in healthy young women, E2 had no vasodilatory effect in the skin microvasculature of young women with IR. Furthermore, GEN exposure did not impact microvascular responses in healthy controls or women with IR. These findings have important clinical meaning because women use both E2 and GEN with the expectation that these substances will improve cardiovascular health, or they will at least attenuate cardiovascular decline with aging. Our study does not support improved microvascular responsiveness during GEN exposure in healthy young women, and demonstrates that neither E2 nor GEN improve microvascular vasodilatory responsiveness in women with IR. The latter is a particularly important clinical finding because women with IR are at especially high risk for endothelial dysfunction and peripheral microvascular disease. Thus, E2-mediated vasodilation in women may depend on insulin resistance, and GEN appears to have little impact on peripheral microvascular function. Finally, our findings confirmed that NO is an important mediator of the E2 effects on the endothelium in the microvasculature. These findings extend earlier data indicating that E2 exposure improves conduit arterial function in both young and postmenopausal women (6, 39). The poor E2-mediated vascular responsiveness in women with IR suggests either lower NO bioavailability, functional changes in estrogen receptor (ER) expression, or changes in downstream mechanisms associated with NO-mediated vasodilation in IR. Our data cannot distinguish which of the mechanisms play the most important role in the impaired response to E2 in women with IR, but certainly suggest areas for future research both on physiological and molecular levels.
Estradiol binds primarily to the ERα receptor in the vasculature, stimulating eNOS and NO activation and leading to rapid vasodilation (19). Furthermore, estrogens modulate insulin sensitivity, likely through ERα receptors (47, 54). Indeed, a reduction of NO bioavailability contributes to impaired endothelial function in men with IR (27, 41). Our young women with IR did not appear to exhibit impaired endothelial function, but inhibiting NO during E2 perfusions had minimal effect on vasodilation. Therefore, it is possible that young women with IR have lower NO bioavailability, but that compensatory mechanisms are up-regulated to maintain endothelial function. However, we would still expect to see an increase in vasodilation during E2 perfusion, whether it is NO or non-NO mediated. Thus, consistent with previous findings (37), we propose that low ERα receptor signaling may contribute to the increased risk of IR and metabolic syndrome (37). In support of this hypothesis, ERα knockout mice exhibit endothelial dysfunction (49), glucose intolerance and IR (18) compared to wild type mice, whereas glucose tolerance and insulin sensitivity are unchanged in ERβ knockouts (5). Furthermore, E2 can promote re-endothelialization via the ERα receptor but not the ERβ receptor in mice (3). Given the lack of response to direct perfusion of E2 in women with IR, we propose that there is a functional down-regulation of either ERα expression or transcriptional activity associated with IR (42). Future studies can determine whether IR per se modulates ER expression in various tissues, or whether a change in estrogen status (either hyper- or hypo- estrogenic) drives the onset of IR or the comprised cardiovascular function associated with IR.
The vasodilatory effects of GEN are still unclear. Chronic GEN administration (6 months, oral) improved flow-mediated dilation, blood lipids and cholesterol profiles as well as insulin sensitivity in postmenopausal women (24, 53, 58). However, GEN administration of either 8-weeks (50) or 2-weeks (17) did not improve endothelial function in postmenopausal women. Our data are the first to examine the effects of acute GEN exposure on microvascular function in young women, and indicate that GEN has little impact on microvascular dilation in young women with or without IR. These findings are in contrast to those in middle-aged or older adults, where intra-arterial infusions of GEN enhanced endothelial mediated dilation in middle-aged men and premenopausal women as measured by plethysmography (59). We speculate that GEN may attenuate age-related declines in endothelial function, but has little impact in young adults. Interestingly, we did observe a significant reduction in vasodilation with NO blockade during GEN exposure only in IR women that was not observed during E2 perfusion, suggesting GEN may alter the mechanisms for NO mediated dilation. Because GEN may have a greater binding affinity for ERβ compared to 17β-estradiol (E2) (34), we propose that either greater eNOS activation or NO bioavailability associated with GEN in women with IR may be related to alterations in ER expression. Consistent with these proposed mechanisms, GEN elicited greater vasodilation compared to E2 in postmenopausal women with underlying cardiovascular disease, and this enhanced vasodilation is associated with upregulation of ERβ receptors in the vasculature of these vessels (10). Changes in the ERα/β ratio may also explain the lack of vasodilation during E2 perfusion in our IR group. Thus, GEN may be more efficacious compared to E2 to improve cardiovascular function in women with underlying disease because of its’ affinity for the ERβ receptors in the vasculature (10). The contrasting responses to E2 (ERα) and GEN (ERβ) suggest that selective targeting of ERs may have important therapeutic implications for women’s cardiovascular health (10).
Insulin is an important vasoactive substance that can modulate both NO and ET-1 (55). Indeed, prior studies demonstrate impaired endothelial function in adults with IR, and endothelial function has important implications for blood pressure regulation and hypertension. Recently, impaired microvascular endothelial function in the cutaneous circulation has been demonstrated in women with IR, when adjusting differences in waist circumference between the two groups (45). We were surprised that heat-induced microvascular vasodilation in our group of women with IR was not attenuated, and in fact, was actually higher compared to control women. Because of redundant mechanisms mediating the local heating response, it is possible that additional mechanisms (such as potassium channels) or other compensatory mechanisms are up-regulated in women with insulin resistance, contributing to this preserved blood flow response. For example, acetylcholine-induced vasodilation was similar in control and hypercholesterolemic rabbits, but blockade of calcium-dependent potassium channels significantly reduced vasodilation in the hypercholesterolemic group only (43), suggesting a compensatory upregulation of additional non-NO mechanisms to preserve endothelial function. Similarly, microvascular vasodilation to acetylcholine and sodium nitroprusside were preserved in young adults with obesity or metabolic syndrome, whereas vasodilatory responsiveness to prostacyclin were impaired (36). Our findings of preserved microvascular function are consistent with these (36), although the reason for the contrasting findings among other studies is not clear from our data. It is possible that microvascular effects of these risk factors are additive (obesity and IR) to impair vasodilation. Our study was not powered to look at the independent contributions of CVD risk factors on impaired microvascular function, and was primarily focused on examining the effects of IR per se. Future studies can explore the link between additional risk factors such as family history of disease and birth weight, along with examining the duration of IR.
Limitations
The women in our study were instrumented with five microdialysis fibers, and it would be stressful to the subject to insert more within each session. Thus, we were unable to include perfusion of L-NMMA alone, and recognize this as a limitation to the interpretation of our findings. Because there are redundant mechanisms involved in mediating the vasodilatory response to local skin heating, it is it likely that other non-NO mechanisms may be involved in mediating the responses we observed. Although NO plays a primary role in the vasodilatory response to local heating, it is plausible that E2 may have NO independent actions, which we cannot rule out in the absence of a separate L-NMMA site. For example, blockade of NO in addition to calcium-activated potassium channels almost completely abolished the vasodilation during local skin heating (4) indicating a role for potassium channels in mediating cutaneous vasodilation; E2-associated relaxation is partially mediated through potassium channels in isolated mesenteric artery rings (56). Future studies can address the role of potassium channels in mediating E2 associated vasodilation in the skin microcirculation. Moreover, we recognize that both norepinephrine and neuropeptide Y play a role in mediating cutaneous vasodilation during local skin heating (20), and that both pathways are modulated by E2 (25, 51). Finally, we cannot rule out a potential role of oxidative stress, as reactive oxygen species are also involved in the local heating response (38). Because oxidative stress can be apparent before any signs of endothelial dysfunction in adults with insulin resistance (16), it is possible that although vasodilatory function was preserved in women with IR, oxidative stress is still present. However, since E2 is generally known to attenuate oxidative stress, this cannot explain the lack of response observed during E2 perfusions in the women with IR, which may suggest an issue with estrogen receptors as highlighted above. The lack if E2 vasodilation observed in women with IR may also be due to a ‘ceiling effect’ since the vasodilation during saline alone was augmented. However, we still would have expected to see an increase in vasodilation with E2; 7 of the 8 women in the control group demonstrated a 10–15% increase in vasodilation during E2 whereas only 3 women in the IR group had an increase in vasodilation (~6%) and 4 women had no change with E2. Although this is the first study to examine the influence of reproductive hormones on vascular function in young women with IR, identifying the precise mechanisms involved remain an important and interesting area for future investigation.
In summary, our data are the first to show direct E2 perfusion increased cutaneous microvascular dilation in healthy women, but not in women with IR. Further, our data show that GEN did not influence microvascular dilation in young women with or without IR. Although women use phytoestrogens assuming a level of cardiovascular benefit, our data do not support a role for GEN in improving microvascular responsiveness in young women, and suggest little impact on endothelial function. Finally, IR attenuated microvascular responsiveness to E2, suggesting women with IR may not receive the same cardiovascular benefits as those presumed for healthy women taking estrogen.
Perspectives
In women, the prevalence of both cardiovascular disease and IR increase with age, particularly after menopause. Our data show little impact of E2 or GEN on microvascular vasodilation in younger women with IR, but clearly similar studies need to be conducted in postmenopausal women. These data support the practice of determining insulin sensitivity in women before prescribing estrogens or phytoestrogens to improve cardiovascular health.
Acknowledgements
We gratefully acknowledge Cheryl Leone, MS for technical assistance, Osama Abdelghany, PharmD, BCOP, for microdialysis drug guidance and preparations, and the subjects for their time.
Grants
This research was supported by National Heart, Lung, and Blood Institute Grant R21-HL-109822 (NSS).
ABBREVIATIONS
- E2
Estradiol
- GEN
Genistein
- L-NMMA
NG-monomethyl-L-arginine
- CVC
Cutaneous vascular conductance
- IR
Insulin resistance
- C
Control women
- BMI
Body mass index
- NO
Nitric oxide
- eNOS
endothelial nitric oxide synthase
- OGTT
Oral glucose tolerance test
- AUC
Area under the curve
- S[E2]
serum estradiol
- S[P4]
serum progesterone
- SkBF
Skin Blood Flow
- SNP
sodium nitroprusside
- ER
estrogen receptor
- CVD
cardiovascular disease
Footnotes
Author Contributions
MMW and NSS performed the experiments; NSS analyzed the data; MMW and NSS interpreted the results of the experiments; MMW prepared the figures; HST and NSS contributed to the conception and design of the research; MMW drafted the manuscript; MMW, HST, and NSS edited and revised the manuscript; MMW, HST, and NSS approved the final version of the manuscript.
DISCLOSURES: The authors report no conflicts of interest.
REFERENCES
- 1.Agarwal SC, Allen J, Murray A, Purcell IF. Laser Doppler assessment of dermal circulatory changes in people with coronary artery disease. Microvasc Res. 2012;84:55–59. doi: 10.1016/j.mvr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 3.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 4.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol. 2012;590:3523–3534. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 6.Bush DE, Jones CE, Bass KM, Walters GK, Bruza JM, Ouyang P. Estrogen replacement reverses endothelial dysfunction in postmenopausal women. Am J Med. 1998;104:552–558. doi: 10.1016/s0002-9343(98)00117-x. [DOI] [PubMed] [Google Scholar]
- 7.Ciampelli M, Leoni F, Cucinelli F, Mancuso S, Panunzi S, De Gaetano A, Lanzone A. Assessment of insulin sensitivity from measurements in the fasting state and during an oral glucose tolerance test in polycystic ovary syndrome and menopausal patients. J Clin Endocrinol Metab. 2005;90:1398–1406. doi: 10.1210/jc.2004-0410. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson TB. Soy, soy phytoestrogens and cardiovascular disease. J Nutr. 2002;132:566S–569S. doi: 10.1093/jn/132.3.566S. [DOI] [PubMed] [Google Scholar]
- 9.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens. 2011;26:56–63. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 10.Cruz MN, Agewall S, Schenck-Gustafsson K, Kublickiene K. Acute dilatation to phytoestrogens and estrogen receptor subtypes expression in small arteries from women with coronary heart disease. Atherosclerosis. 2008;196:49–58. doi: 10.1016/j.atherosclerosis.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Davidge ST, Zhang Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ Res. 1998;83:388–395. doi: 10.1161/01.res.83.4.388. [DOI] [PubMed] [Google Scholar]
- 12.Davison JL, Short DS, Wilson TE. Effect of local heating and vasodilation on the cutaneous venoarteriolar response. Clin Auton Res. 2004;14:385–390. doi: 10.1007/s10286-004-0223-x. [DOI] [PubMed] [Google Scholar]
- 13.Durand S, Tartas M, Bouye P, Koitka A, Saumet JL, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol. 2004;561:811–819. doi: 10.1113/jphysiol.2004.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 15.Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini Rev Med Chem. 2012;12:149–174. doi: 10.2174/138955712798995020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopaul NK, Manraj MD, Hebe A, Lee Kwai Yan S, Johnston A, Carrier MJ, Anggard EE. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia. 2001;44:706–712. doi: 10.1007/s001250051679. [DOI] [PubMed] [Google Scholar]
- 17.Hale G, Paul-Labrador M, Dwyer JH, Merz CN. Isoflavone supplementation and endothelial function in menopausal women. Clin Endocrinol (Oxf) 2002;56:693–701. doi: 10.1046/j.1365-2265.2002.01533.x. [DOI] [PubMed] [Google Scholar]
- 18.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hisamoto K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2005;70:382–387. doi: 10.1016/j.steroids.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol (1985) 2008;105:233–240. doi: 10.1152/japplphysiol.90412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–H1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 23.I Jzerman R, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest. 2003;33:536–542. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 24.Irace C, Marini H, Bitto A, Altavilla D, Polito F, Adamo EB, Arcoraci V, Minutoli L, Di Benedetto A, Di Vieste G, de Gregorio C, Gnasso A, Corrao S, Licata G, Squadrito F. Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur J Clin Invest. 2013;43:1025–1031. doi: 10.1111/eci.12139. [DOI] [PubMed] [Google Scholar]
- 25.Jackson DN, Ellis CG, Shoemaker JK. Estrogen modulates the contribution of neuropeptide Y to baseline hindlimb blood flow control in female Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1351–R1357. doi: 10.1152/ajpregu.00420.2009. [DOI] [PubMed] [Google Scholar]
- 26.Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 2007;22:252–260. doi: 10.1152/physiol.00012.2007. [DOI] [PubMed] [Google Scholar]
- 27.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol. 2008;93:158–163. doi: 10.1113/expphysiol.2007.039172. [DOI] [PubMed] [Google Scholar]
- 28.Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 29.Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 30.Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 31.Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H123–H129. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 2008;115:295–300. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 33.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73:864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 35.Kuliga KZ, McDonald EF, Gush R, Michel C, Chipperfield AJ, Clough GF. Dynamics of microvascular blood flow and oxygenation measured simultaneously in human skin. Microcirculation. 2014;21:562–573. doi: 10.1111/micc.12136. [DOI] [PubMed] [Google Scholar]
- 36.Limberg JK, Harrell JW, Johansson RE, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Microvascular function in younger adults with obesity and metabolic syndrome: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2013;305:H1230–H1237. doi: 10.1152/ajpheart.00291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manrique C, Lastra G, Habibi J, Mugerfeld I, Garro M, Sowers JR. Loss of Estrogen Receptor alpha Signaling Leads to Insulin Resistance and Obesity in Young and Adult Female Mice. Cardiorenal Med. 2012;2:200–210. doi: 10.1159/000339563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol (1985) 2011;111:20–26. doi: 10.1152/japplphysiol.01448.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol. 2008;294:H1630–H1637. doi: 10.1152/ajpheart.01314.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 41.Murphy C, Kanaganayagam GS, Jiang B, Chowienczyk PJ, Zbinden R, Saha M, Rahman S, Shah AM, Marber MS, Kearney MT. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27:936–942. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 42.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Najibi S, Cowan CL, Palacino JJ, Cohen RA. Enhanced role of potassium channels in relaxations to acetylcholine in hypercholesterolemic rabbit carotid artery. Am J Physiol. 1994;266:H2061–H2067. doi: 10.1152/ajpheart.1994.266.5.H2061. [DOI] [PubMed] [Google Scholar]
- 44.Neill AS, Ibiebele TI, Lahmann PH, Hughes MC, Nagle CM, Webb PM. Dietary phyto-oestrogens and the risk of ovarian and endometrial cancers: findings from two Australian case-control studies. Br J Nutr. 2014;111:1430–1440. doi: 10.1017/S0007114513003899. [DOI] [PubMed] [Google Scholar]
- 45.Pienaar PR, Micklesfield LK, Levitt NS, Gooding K, Shore AC, Goedecke JH, Gill JM, Lambert EV. Insulin resistance is associated with lower acetylcholine-induced microvascular reactivity in nondiabetic women. Metab Syndr Relat Disord. 2014;12:178–184. doi: 10.1089/met.2013.0126. [DOI] [PubMed] [Google Scholar]
- 46.Prior JO, Quinones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111:2291–2298. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 47.Ropero AB, Alonso-Magdalena P, Quesada I, Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73:874–879. doi: 10.1016/j.steroids.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 49.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99:2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simons LA, von Konigsmark M, Simons J, Celermajer DS. Phytoestrogens do not influence lipoprotein levels or endothelial function in healthy, postmenopausal women. Am J Cardiol. 2000;85:1297–1301. doi: 10.1016/s0002-9149(00)00759-1. [DOI] [PubMed] [Google Scholar]
- 51.Song D, Yuen VG, Yao L, McNeill JH. Chronic estrogen treatment reduces vaso-constrictor responses in insulin resistant rats. Can J Physiol Pharmacol. 2006;84:1139–1143. doi: 10.1139/y06-061. [DOI] [PubMed] [Google Scholar]
- 52.Sprung VS, Cuthbertson DJ, Pugh CJ, Daousi C, Atkinson G, Aziz NF, Kemp GJ, Green DJ, Cable NT, Jones H. Nitric oxide-mediated cutaneous microvascular function is impaired in polycystic ovary sydrome but can be improved by exercise training. J Physiol. 2013;591:1475–1487. doi: 10.1113/jphysiol.2012.246918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Squadrito F, Altavilla D, Morabito N, Crisafulli A, D'Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP, Squadrito G. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–347. doi: 10.1016/s0021-9150(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 54.Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, Shizuta Y. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol. 2003;176:237–246. doi: 10.1677/joe.0.1760237. [DOI] [PubMed] [Google Scholar]
- 55.Tousoulis D, Tsarpalis K, Cokkinos D, Stefanadis C. Effects of insulin resistance on endothelial function: possible mechanisms and clinical implications. Diabetes Obes Metab. 2008;10:834–842. doi: 10.1111/j.1463-1326.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 56.Tsang SY, Yao X, Chan HY, Wong CM, Chen ZY, Au CL, Huang Y. Contribution of K+ channels to relaxation induced by 17beta-estradiol but not by progesterone in isolated rat mesenteric artery rings. J Cardiovasc Pharmacol. 2003;41:4–13. doi: 10.1097/00005344-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 58.Villa P, Costantini B, Suriano R, Perri C, Macri F, Ricciardi L, Panunzi S, Lanzone A. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: relationship with the metabolic status. J Clin Endocrinol Metab. 2009;94:552–558. doi: 10.1210/jc.2008-0735. [DOI] [PubMed] [Google Scholar]
- 59.Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17beta-estradiol. Circulation. 2001;103:258–262. doi: 10.1161/01.cir.103.2.258. [DOI] [PubMed] [Google Scholar]
- 60.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab. 2013;305:E818–E825. doi: 10.1152/ajpendo.00343.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wenner MM, Taylor HS, Stachenfeld NS. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol. 2011;589:4671–4679. doi: 10.1113/jphysiol.2011.216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenner MM, Wilson TE, Davis SL, Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol (1985) 2011;111:1703–1709. doi: 10.1152/japplphysiol.00780.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]