Abstract
Background
Poor adherence to prevention regimens for gel-based anti-HIV-1 microbicides has been a major obstacle to more effective pre-exposure prophylaxis. Concern persists that the antiretroviral drug containing microbicides might promote development of antiretroviral resistance.
Methods
Using in vitro transwell systems and a humanized mouse model of HIV-1 sexual transmission, we examined, as candidate microbicides, antibodies targeting the heterodimeric leukocyte function associated antigen 1 (LFA-1), a non-virally encoded protein acquired by the virus that also plays a critical role cell movement across endothelial and epithelial barriers. LFA-1 specific single domain variable regions from alpaca heavy-chain only antibodies (VHH) were identified and evaluated for their ability to inhibit HIV-1 transmission in the in vitro transwell system.
Results
Monoclonal antibodies targeting the CD11a and CD18 components of LFA-1 significantly reduced cell-free and cell-associated HIV-1 transmission in the in vitro transwell culture system and prevented virus transmission in the humanized mouse model of vaginal transmission. The broadly neutralizing monoclonal antibody b12 was unable to block transmission of cell-free virus. CD11a-specific VHH were isolated and expressed and the purified variable region protein domains reduced in vitro transepithelial transmission with an efficacy comparable to that of the CD11a monoclonal antibody.
Conclusions
Targeting integrins acquired by HIV-1 during budding and which are critical to interactions between epithelial cells and lymphocytes can reduce viral movement across epithelial barriers and prevent transmission in a humanized mouse model of sexual transmission. VHH capable of being produced by transformed bacteria can significantly reduce transepithelial virus transmission in in vitro model systems.
Introduction
HIV-1 prevention studies analyzing the prophylactic use of antiretroviral agents have demonstrated varying levels of efficacy, with adherence to prophylactic regimens being a major source of failure1–3. Additionally, whether antiretroviral-based microbicides are effective against vaginal transmission of cell-associated HIV, which is readily found in seminal and vaginal fluids4–6, is unknown.
The role of the β-integrin leukocyte function-associated antigen-1 (LFA-1) and its counter-receptor Intercellular Adhesion Molecule-1 (ICAM-1) in movement of cells across endothelial and epithelial barriers is well-described7–16. ICAM-1 is expressed on both cervical and vaginal epithelium17, potentially facilitating transmigration of HIV-1 infected lymphocytes and monocytes.
LFA-1 is a heterodimer consisting of an alpha chain, CD11a, and a beta chain, CD18. CD11a contains the conserved 200 amino acid I-domain, which is responsible for ligand binding18,19. Both ICAM-1 and LFA-1 have been demonstrated to be acquired by the HIV-1 virion as it buds from infected cells20–23. In the current study we have examined the potential efficacy of targeting this interaction to interrupt sexual HIV-1 transmission.
Materials and Methods
Cell lines and antibodies
HT-3 cervical epithelial cells were obtained from the American Type Tissue Collection (ATCC, Manassas, VA). Jβ2.7 LFA-1+ and LFA-1- Jurkat cells were kindly provided by Catarina Hioe (NYU, New York, NY). Anti-CD18 monoclonal antibody (Mab), H52, was a gift from Dr. James Hildreth (University of California, Davis, Davis, CA). Anti-CD11a Mab (38) was purchased from Abcam (Cambridge, MA). The broadly neutralizing anti-gp120 Mab, b12,24 was kindly provided by Dr. Dennis Burton (The Scripps Institute, La Jolla, CA). IgG Isotype control was purchased from Becton Dickinson (Franklin, Lakes, NJ). FITC conjugated goat anti-mouse IgG was purchased from Jackson ImmunoResearch Laboratories (Westgrove, PA). Anti-T7 tail fiber Mab was purchased from Novagen (San Diego, CA). Anti-His Mab was purchased from GE Healthcare Biosciences (Pittsburgh, PA). HRP-conjugated goat anti-mouse IgG was purchased from Sigma-Aldrich (St. Louis, MO).
Flow Cytometry
Jβ2.7 cells were stained with anti-CD11a (1:1000) in 3% BSA (Sigma-Aldrich, St. Louis, MO) in PBS at 4°C for 1 hr. Cells were washed twice with cold PBS and FITC conjugated goat anti-mouse IgG was added to cells at 4°C for 30 min. Cells were washed twice, resuspended in 1% paraformaldehyde and analyzed using the Becton Dickinson FACSCalibur and the Cellquest program (BD Biosciences, San Jose, CA). Data were analyzed using Flojo software (Ashland, OR).
Human cervical epithelial transwell cultures
HIV-1 infected human PBMC were prepared as previously described25. For epithelial cell transwell cultures, 600 μl cMcCoy’s media was added to wells in a 24 well tissue culture plate (Becton Dickinson, Franklin Lakes, NJ). HT-3 cells were plated at 5 × 105 cells/well in 12-mm diameter transwell inserts with a pore size of 3μm (Millipore, Billerica, MA) and placed into each well. Transwells were maintained at 37°C, 5% CO2. Media was replaced with cRPMI every two days. Confluency of the cervical epithelial monolayer was confirmed by monitoring the permeability to horse radish peroxidase (HRP, Sigma-Aldrich, St. Louis, MO); cells were considered confluent when ≤ 1% of the HRP could be recovered from the basal compartment.
Cell-associated HIV-1 in vitro transmigration studies
Transmigration studies were performed as previously described25.
Cell-free HIV-1 in vitro transmigration studies
1 × 106 PHA stimulated huPBMC in cRPMI with 10 u/mL IL-2 were infected with 105 TCID50 HIV-1BaL, originally grown in primary human monocyte/macrophage cell culture (Advanced Biotechnologies, Columbia, MD). After one week HIV-1 (2.6 ng p24) from these cultures was mixed with the indicated antibodies for 1 hr at 37°C and then placed into the apical chamber of transwells.
Jβ2.7 LFA-1+ and LFA-1- control cells were transfected with pNL4-3 by electroporation. Two ng p24 LFA-1+ or LFA-1- HIV-1 obtained from these cell cultures was added to the apical side of cervical epithelial monolayers and incubated at 37°C. For both sets of experiments p24 levels in the lower chamber were determined after 24 hr.
Cell-associated HIV-1 transmission in HuPBL-SCID mouse models
The HuPBL-SCID mouse model, with known patterns of human cell distribution26, was used as previously described25. Reconstituted and progestin-treated mice were intravaginally administered 10 μl of the indicated antibodies prepared in PBS and 1% BSA (Sigma-Aldrich, St. Louis, MO) 5 min before vaginal administration of 1 × 106 HIV-1BaL infected HuPBMCs. The five minute pre-challenge interval for antibody administration was selected to mimic a setting in which constitutively-produced VHH would be present. Two weeks later, HuPBMCs were recovered from the peritoneal cavity of euthanized mice by cold PBS lavage.
Recovered cells were cultured with 1 × 106 PHA-stimulated normal PBMCs in cRPMI and 10 U/mL IL-2 for one week. HIV-1 positivity was determined by p24 ELISA of supernatant and p17 gag PCR amplification from cultured cells27.
CD11a I-domain production
CD11a I-domain/pET20 was kindly provided by Timothy Springer (Harvard Medical School, Boston, MA). The sequence contained two mutations (F265S/F292G) that conferred increased affinity to ICAM-1. The DNA construct was transformed into BL21 DE3 cells (New England Biolabs, Ipswich, MA) and colonies grown on LB agar overnight at 37°C. Selected colonies were grown in 800 ml LB media to an OD of 0.8 before being induced with 1 mM IPTG for 4 hr at 37°C. Cultures were centrifuged and pellets were stored in −20°C. CD11a I-domain purification was previously described19.
Alpaca PBMC isolation and cDNA preparation
Alpacas from a local breeder were housed at a large animal facility maintained by Johns Hopkins University. PBMC obtained from Ficoll Hypaque centrifugation of peripheral blood was stored in 106 cells/mL aliquots in RNAlater (Ambion, Norwalk, CT) at −80°C. Thawed cells were resuspended in 1 mL of TRIzol Reagent (invitrogen). RNA was extracted in chloroform, precipitated with isopropanol, washed in ethanol, dissolved in DEPC treated water (Quality Biological, Gaithersburg, MD) and stored at −80°C. First-strand cDNA synthesis was performed using SuperScriptIII first strand cDNA synthesis reverse transcription kit (Invitrogen, Grand Island, NY) according to the manufacturer’s protocol. cDNA was stored at −20°C.
VHH cloning and T7 VHH library construction
VHH isolation was performed as previously described28. A T7 VHH library was produced using a cloning and packaging kit provided by Novagen (San Diego, CA) following the manufacturer’s protocol. Briefly, the vhh was ligated into T7Select10-3b arms and packaged into T7 phage. The resulting library was amplified in BLT5403 E. coli for the inserted vhh gene to be expressed on the viral capsid.
Biopanning and VHH selection
CD11a I-domain (0.5 μg/ml) was immobilized overnight at 4°C in a 96 well immunoplate (NUNC, Thermo Fisher Scientific, Waltham, MA) and blocked with 5% milk in TBS for 2 hours at RT. 1010 phage from the phage library was added to each well overnight at 4° C. Wells were washed 12X with 1X TBS with .1% Tween-20 (TBS-T) followed by 12 washes with TBS. Bound phage were eluted with 1% SDS in TBS and added to BLT5403 E.coli for further amplification. Amplified phage were used for another round of biopanning. Phage eluted in the final step were added to BLT5403 E.coli and plated onto LB agar plates for plaque purification.
Individual phage were screened against CD11a I-domain for further selection using a phage ELISA. Briefly, CD11a I-domain was immobilized overnight at 4° in a 96 well immunoplate (NUNC, Thermo Fisher Scientific, Waltham, MA). The following steps were performed at room temperature. Wells were blocked with 2% BSA in PBS for 1 hour. Phage were added directly to each well for 2 hr. Wells were washed 5X with 1X PBS and .05% Tween-20 (PBS-T) between each step in the assay. Bound phage were detected by the addition of anti-T7 tail fiber Mab for 1 hr. followed by the addition of HRP conjugated anti-mouse IgG Mab for 1 hr. ABTS Peroxidase Substrate System (KPL, Gaithersburg, MD) was added for up to 30 minutes and plates were read at 405 nm in a Synergy HT plate reader (Biotek, Winooski, VT). VHH binding to CD11a was screened using the same protocol except that 0.5 μg of the indicated antibody or VHH was added to the microwell instead of phage.
Soluble VHH production
VHH sequences were subcloned into the pET47b plasmid (Novagen, San Diego, CA) using primers included in the T7 cloning kit provided by Novagen. Vhh/pET47b plasmids were transformed into E. coli BL21 DE3 (New England Biolabs, Ipswich, MA). Cultures were grown overnight at 37°C, diluted into 800 mL of LB Broth (LB), grown to an OD of 0.8, induced with IPTG at a concentration of 1mM and grown for an additional 4 hr. Culture pellets were stored at −20°C. Pellets were thawed on ice and resuspended in 10 ml lysis buffer (100 mM NaH2PO4, 10mM Tris Base, 6M GuHCl, 10mM Imidazole, pH 8.0) and placed in −80° for 30 min to lyse cells. The frozen lysis mixture was thawed at room temperature, 30 ml lysis buffer was added and lysis continued at room temperature for 2 hr. Mixtures were centrifuged at 14000 rpm for 30 min at 4°C. Supernatants were added to 1 mL Ni-NTA agarose (Qiagen, Germantown, MD) and rocked for 1 hr at room temperature. The mixture was added to Poly-Prep chromatography columns (Bio-Rad, Hercules, CA). Agarose was washed with 2 column volumes of denaturing buffer (100 mM NaH2PO4, 150 mM NaCl, 8 M Urea, 20 mM Imidazole, pH 8.0) followed by 7 column volumes of renaturing buffer (50 mM NaH2PO4, 500 mM NaCl, 20 mM Imidazole). His-VHH was eluted with 3 mL elution buffer (50 mM NaH2PO4, 500 mM NaCl, 250 mM Imidazole, pH 8.0). The eluted protein was dialyzed overnight in 1X PBS.
Soluble VHH ELISA
CD11a I-domain (0.5 μg/ml) was immobilized overnight at 4°C in a 96 well immunoplate (NUNC, Thermo Fisher Scientific, Waltham, MA). The following steps were performed at room temperature, with 5× washes occurring between each incubation step. Wells were blocked with 2% BSA in PBS for 1 hr. His-VHH was added to each well for 1 hr, followed by the addition of anti-His Mab for 1 hr. HRP conjugated anti-mouse was added for 1 hr. followed by addition of the ABTS Peroxidase Substrate (KPL, Gaithersburg, MD) for up to 30 minutes. Plates were read at 405nm in a Synergy HT plate reader (Biotek, Winooski, VT).
Statistics
A one-way analysis of variance with Bonferroni correction was used to determine the significance of differences in p24 concentrations. A Fisher exact test was used to evaluate the significance of differences in infection rates among challenged mice. Correlation data were determined using pwcorr. All statistical analyses employed Stata software (College Station, TX).
Results
Antibodies against CD11a and CD18 prevent the transmigration of cell-associated HIV-1 across a cervical epithelial monolayer
Anti-CD11a (38), anti-CD18 (H52), or isotype control mouse IgG1 were assayed as described for their ability to block migration of infected PBMC. At concentrations of 0.02 μM and .004 μM, both anti-CD11a, 38, which specifically binds to the I-domain and blocks ligand binding, and anti-CD18, H52, which binds to the hybrid region of CD18 and prevents LFA-1 activation29,30, significantly reduced both transmigration of PBMCs across the cervical epithelial transwell and HIV-1 p24 levels in the lower chamber of the transwell compared to the IgG controls (p<0.001) (Fig. 1A, 1B). There was a significant correlation (r=0.72, p=0.001) between reduction in cell levels and p24.
Figure 1. Effect of Mab against LFA-1 on transmission of cell-associated HIV-1 across an epithelial monolayer.

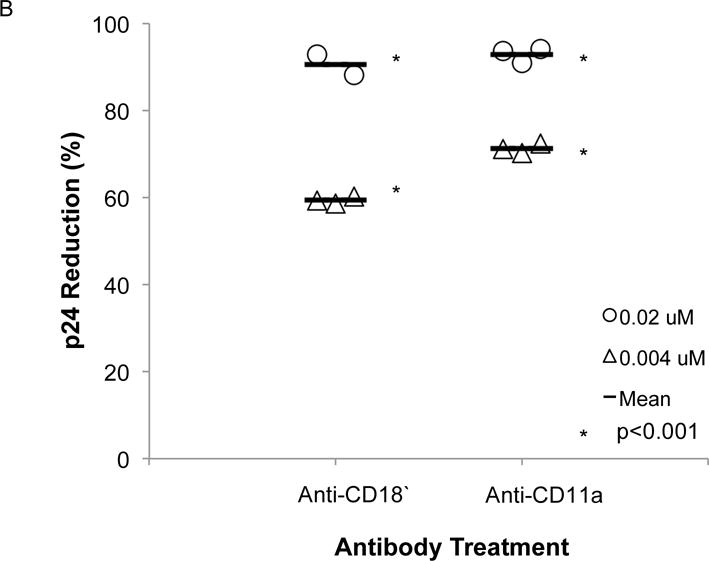
Mouse anti-human CD11a (38), mouse anti-human CD18 (H52), or mouse IgG1 at concentrations of 0.02 μM or 0.004 μM were mixed with 5 × 105 HIV-1-infected PBMCs 1 hr prior to the addition of the mixture to the apical side of HT-3 cervical monolayers grown on permeable transwell supports. After 24h (A) cells in the basal compartment were counted and (B) p24 concentrations in the basal compartment were measured and percent reduction was determined compared to control IgG. All groups treated with a Mab had significantly lower number of cells and of p24 concentrations in the basal chamber compared to the IgG control group. The reduction in cell number significantly correlated (r=0.73, p=0.001) with the reduction in p24 concentration.
Targeting LFA-1 reduces cell-free HIV-1 transmission
Host-derived LFA-1 is reportedly incorporated into the viral membrane during budding20–23. To evaluate the role of host-derived LFA-1 on migration of cell-free virus across epithelial surfaces, we produced HIV-1 from Jβ2.7 cells lacking LFA-1 (Fig. 2A), and from Jβ2.7 cells constitutively expressing LFA-1 (Fig. 2B). Virus from these two sources in equivalent concentrations (2 ng in 1 ml) was added to the apical side of cervical epithelial transwells and viral transmission to the basilar compartment was assayed after 24 hr. Overall transmission of free virus across the epithelial barrier was inefficient with less than 3% of the LFA+ HIV-1 in the apical compartment reaching the basilar compartment. Nevertheless, compared to LFA-1+ HIV-1, LFA-1-HIV-1 had a significant reduction (35%) in p24 concentrations attained in the basilar compartment (p=0.0009) (Fig. 2C).
Figure 2. HIV-1 From LFA-1 Expressing Cells Crosses Epithelium with Greater Efficiency than HIV-1 from Cells Lacking LFA-1 Expression.
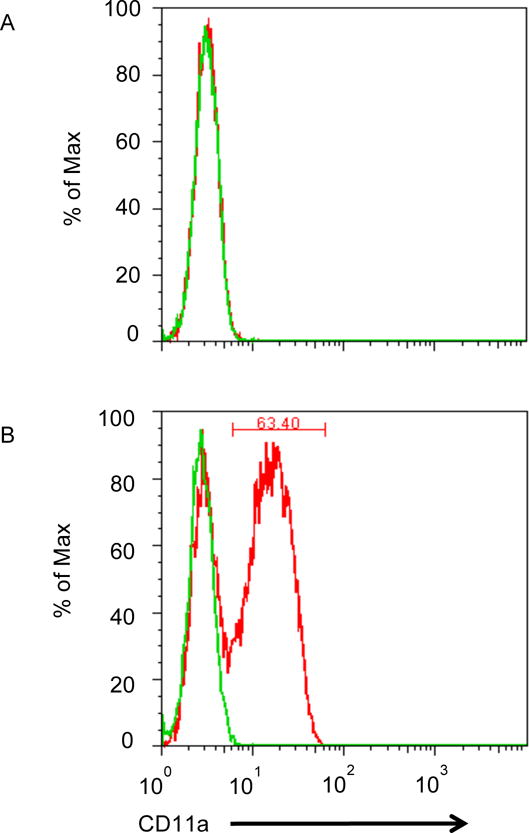
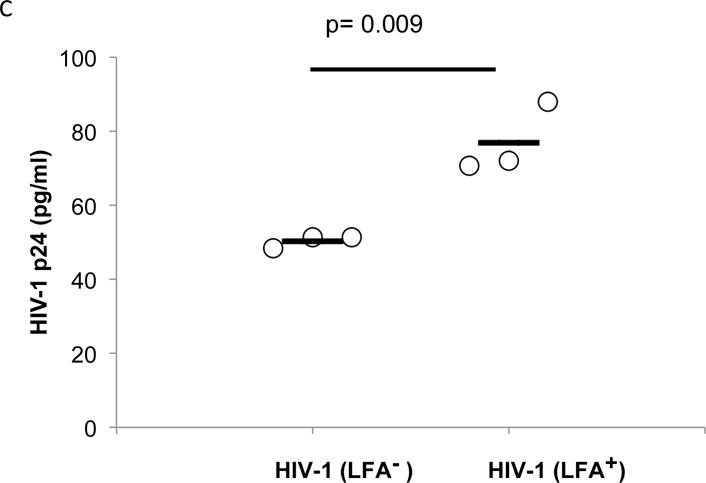
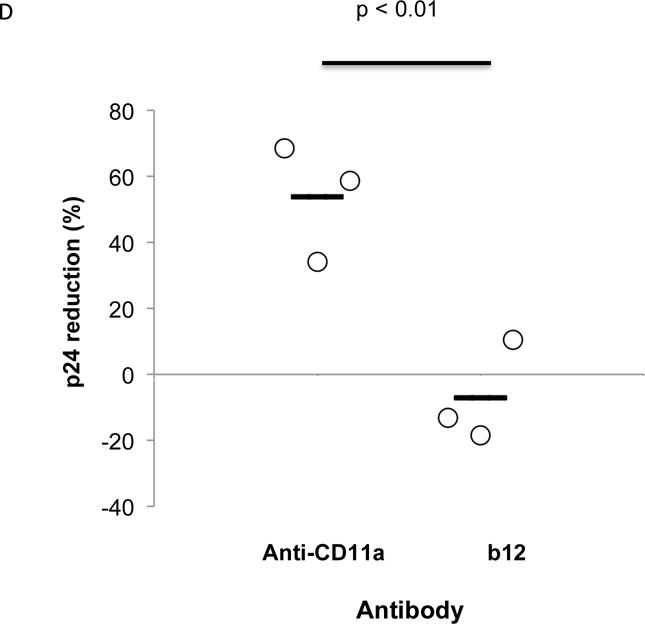
Jβ2.7 LFA-1- and Jβ2.7 LFA-1+ cells were used to produce HIV-1. The expression or absence of CD11a on the cells was tested by flow cytometry. A) Jb2.7 LFA-1- did not express CD11a. B) 63.40% of Jβ2.7 LFA-1+ cells expressed CD11a. For producing HIV-1, plasmid NL4-3 was transfected into these cells by electroporation. The production of HIV-1 was detected by viral p24. Based on p24 determinations, a 100 μl solution containing 2000 pg of HIV-1 was added to the apical compartment of the transwell. C) After 24 hours, HIV-1 transmisson was evaluated by detecting viral p24 in the basal chamber. The virus produced from Jβ2.7 (LFA-1+) showed significantly greater transepithelial transmission compared to that from Jβ2.7 (LFA-1-), p=0.009. D) HIV virions produced from PBMC infection were mixed with mouse anti-human CD11a (38), anti-gp120 (b12), or mouse IgG1 at a concentration of 0.1 μM 1 hour prior to their addition to the apical side of transwell inserts. After 24 hours basal p24 levels were measured and percent reduction compared to IgG control were calculated. Reduction in p24 levels compared to the IgG1 control was observed only in anti-CD11a treated groups (p < 0.01).
In a related experiment, we then examined the comparative ability of anti-CD-11a antibody or the broadly neutralizing gp120-specific b12 Mab24 at 0.1 μM concentration to block transmission of PBMC derived HIV-1 (Fig. 2D). Virus (2.6 ng p24) was incubated with 1 ml. of the indicated antibodies for one hour prior to the application of the mixture to the apical side of cervical epithelial transwells. Again free virus transmission across the epithelial barrier was inefficient with less than 5% of the added virus reaching the basilar compartment in the group treated with IgG. However, after 24h p24 concentrations in the lower chambers were significantly reduced (54%) in the anti-CD11a treated group compared to control IgG1 treated groups (p < 0.01). Interestingly, the broadly neutralizing b12 antibody did not reduce cell free virus transmission across the epithelial barrier when compared to IgG.
In vivo protective efficacy of anti-CD11a and anti-CD18 in the HuPBL-SCID Mouse Model
We next evaluated the ability of anti-CD11a and anti-CD18 Mab to protect Hu-PBL- SCID mice from vaginal HIV-1 transmission. Mice received 10 μl of either 5 or 20 μg/ml of the antibodies intravaginally five minutes prior to challenge with 106 HIV-1 infected PBMCs obtained from a source that differed from the originally transplanted PBMC (Table 1). Cell-associated transmission was evaluated because cell-free virus cannot be transmitted in this model system. Two weeks following challenge, the mice were euthanized, and human PBMC were recovered from the peritoneal cavity and assayed for HIV-1 infection as described in the Methods. In separate experiments, both anti-CD11a (5 μg/ml) and anti- CD18 (20 μg/ml) completely protected against HIV-1 infection (Table 1, p<0.01 compared to IgG controls).
Table 1.
Ability Of Monoclonal Antibodies Targeting Either the CD11a or CD18 Components of LFA-1 To Block Cell-Associated HIV-1 Transmission In A Hu-PBL SCID Mouse Model
| Treatment | Concentration (μg/mL) | HIV-1 positive Mice/Total Challenged | P-value |
|---|---|---|---|
| Mouse IgG1 Isotype | |||
| Control | 20 | 7/10 | – |
| Human Anti-CD11a | 20 | 1/10 | p = 0.02 |
| Human Anti-CD11a | 5 | 0/10 | p < 0.01 |
|
| |||
| Mouse IgG1 Isotype | |||
| Control | 20 | 6/7 | – |
| Human Anti-CD18 | 20 | 0/8 | P < 0.01 |
Selection of VHH against the CD11a I-domain from a T7 phage library
An alternative method for delivering antibodies would be their constitutive production and secretion by the transformed bacterial flora of the vagina, which consists primarily of bacteria of the genus Lactobacillus. Studies from other laboratories have shown that lactobacilli can constitutively express and secrete VHH31–35.
To address the feasibility of developing biologically active VHH against the CD11a I-domain, we screened an alpaca PBMC-derived VHH T7 phage display library. We selected for VHH against the CD11a I-domain rather than CD18 due to the availability of soluble recombinant and conformationally correct CD11a I-domain19,36. We performed two rounds of phage biopanning against recombinant CD11a I-domain. Eighty individual phage plaques recovered from the biopanning process were evaluated for binding in a phage ELISA. Of the 80, five had binding to the CD11a I-domain that was significantly above that of the control (data not shown). Sequencing of the complementarity-determining regions of the 5 VHH indicated they were all unique (Table 3). The VHH from these five phage were cloned into an E. coli BL21 bacterial expression vector for soluble VHH expression and purification. Only two of the purified VHH, VHH55 and VHH60, differed significantly from the IgG control (p<0.001, Figure 3A) by CD11a I-domain ELISA. In this analysis VHH60 had significantly greater binding than VHH55 (p=0.01, Figure 3A), but those two VHH and the anti-CD11a antibody all differed significantly from the IgG control (p<0.001).
Figure 3. VHH against the CD11a I-domain reduces in vitro cell-associated HIV-1 transmission.
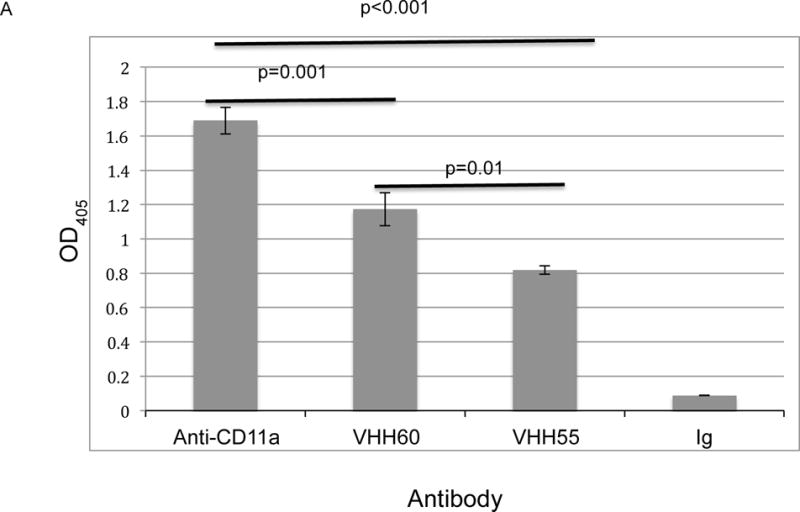
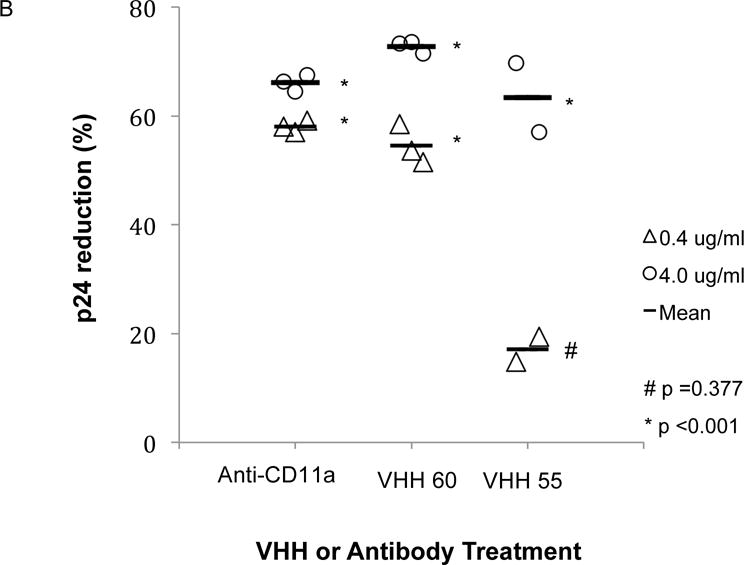
(A) ELISA measurement of binding of 0.5 μg of different VHH or anti- CD11a Mab. The relative binding affinity, as measured by OD405, indicated that VHH55 bound with significantly less affinity than either anti-CD11a or VHH60, although all bound with significantly greater affinity than the IgG control (p<0.001). Not shown in the figure were three additional VHH included in the experiment, all of which had OD405 levels below 0.26, which did not differ significantly from the IgG control (p=1.000). (B) VHH 55 and VHH 60, normal IgG or an anti-CD11a Mab (38) were mixed with 5 × 105 HIV-1 infected, PHA activated PBMCs 1 hr prior to application to the apical side of HT-3 cervical epithelial transwell inserts. After transmigration was allowed to occur for 24 hr, basal side p24 concentrations were measured and the percent reduction compared to control IgG was determined. This experiment is representative of three experiments in which the reduction in HIV-1 transmission to the basal compartment resulting from co-incubation with VHH 60 at 0.4 or 4.0 μg/ml differed significantly from the IgG control. Compared to the the IgG control VHH 60 at a concentration of 0.4 μg/ml reduced p24 concentrations in the three separate experiments by 74%, 58% (this experiment) and 51%. VHH 55 significantly reduced p24 concentrations at a concentration of 4.0 μg/ml but significant reduction was lost at 0.4 μg/ml concentration.
We then evaluated the efficacy of VHH55 and VHH60 to prevent transmigration of HIV-1-infected PBMCs in the in vitro transwell assay. The indicated VHH, anti-CD11a, or a mouse IgG1 isotype control were mixed with HIV-1-infected PBMCs 1h prior to their addition to the apical side of cervical epithelial transwells. As seen in Figure 3B, after 24 hours VHH 55, VHH 60 and the anti-CD11a Mab at concentrations of 4 μg/ml significantly reduced transmitted HIV-1 p24 compared to the IgG control (p<0.001). At the lower concentrations of 0.4 μg/ml, only VHH 60 and the anti-CD11a Mab achieved significant reduction of transmitted p24 compared to the IgG control (p<0.001). At this concentration VHH 55 was less effective and did not significantly differ from the IgG negative control (p=0.377).
Discussion
In these studies we have demonstrated that antibodies to the heterodimeric components of LFA-1 are capable of reducing both cell-associated and cell-free HIV-1 transmission in an in vitro transwell system. The observed in vitro reduction in cell- associated transepithelial transmission of HIV-1 of 51% (0.4 μg/ml) and 72% (4 μg/ml), correlated with nearly complete interruption of HIV-1 transmission in an in vivo model of vaginal transmission in Hu-PBL-SCID mice. The current studies indicate a specific role for LFA-1 in initial events leading to sexual transmission of cell-associated virus in an in vivo model system.
Our studies in mice were facilitated by the ability of human LFA-1 to bind murine ICAM-1; however, the converse is not true37. In addition, anti-human CD11a and anti- human CD18 do not cross-react with mouse LFA-1; therefore the in vivo protection we observed reflects the role of human LFA-1 on the transmission of HIV-1 infected human cells. Previous studies from our group have demonstrated that human PBMC inoculated vaginally can be detected in periaortic lymph nodes of Hu-PBL-SCID mice within four hours of inoculation. However, human PBMCs injected peritoneally migrate and engraft into peritoneal mesentery with few cells migrating to the spleen, and none populating the vaginal tract26.
We have also studied the effect of antibodies against LFA-1 on cell-free viral transmission. As it buds from host cells, HIV-1 acquires surface expressed adhesion molecules and integrins, including LFA-138, which has been shown to increase HIV-1 infection of T-cells22. The mechanism by which cell-free HIV-1 might cross the layer(s) of epithelial cells lining the vagina and cervix is not established and several relevant mechanisms might contribute to this process, including disruption of cell junctions by HIV-1 envelope protein39, engagement of ICAM-1 signaling pathways by LFA-111, or by transcytosis through epithelium, although the relevance of this latter model is controversial40–42. However, the finding that HIV-1 produced from LFA-1 deficient cells had reduced transmigration compared to HIV-1 produced from cells expressing LFA-1 suggests that interactions between LFA-1 and cell adhesion molecules on cervical epithelial cells can play a role in HIV-1 transmission.
That b12 did not reduce recovery of virus from the basilar chamber of transwells indicates that viral proteins involved in neutralization are distinct from those that play a role in viral movement across an epithelial cell barrier. Cell-free virus transmission could not be evaluated in our in vivo Hu-PBL SCID model system because, in the absence of susceptible human cells in the perivaginal region, transvaginal infection cannot be established in this model system.
It has previously been shown that antibodies against CD11a of LFA-1 prevent the transmigration of monocyte associated HIV-1 under pro-inflammatory conditions and that LFA-1 was interacting in these studies with ICAM-2 and ICAM-3, but not ICAM-143. However, lymphocytes, which were the predominant cell in our transmission studies (data not shown), account for the majority of cell-associated HIV-1 found in seminal fluid4. In the non-inflammatory setting of our studies, current and previous findings25 clearly implicate LFA-1 and ICAM-1 in the cellular transmission process.
CD11a is also expressed on dendritic cells that play a role in conception, in transmission of sexually transmitted infections and in the initiation of adaptive immune responses within the genitourinary tract44. While activity against dendritic cells could theoretically interfere with the development of adaptive immune responses, adaptive responses have already evolved to be attenuated within the GU tract {Clark, 2013 #790}, presumably to reduce the risk of rejection of the fetus and sperm.
One mechanism by which antibodies might be introduced into the female genitourinary tract to prevent HIV-1 transmission would be their constitutive secretion by the commensal bacterial flora of that site, primarily different species of Lactobacilli. The pH stability of VHH might prove particularly important in this setting, as will their ease of expression in conformationally correct form by bacteria, especially as compared to the commonly employed single chain variable region antibody constructs (scFv)45. Constitutive expression of VHH at sufficient concentrations would obviate any concerns about the half-life of these molecules within the genitourinary tract. Bacterial expression and secretion of VHH has been demonstrated to be protective against pathogen challenge in multiple model systems31,33–35,45. Lactobacilli cannot sustain colonization within the murine genitourinary tract during diestrus, the phase most favorable to HIV-1 infection in our mouse model46, but in the human setting, in which colonization with lactobacilli uniformly occurs in healthy women, the potential efficacy of VHH could be evaluated. Our in vitro studies indicated that VHH 60 achieved inhibition of cell-associated HIV-1 transmission that was equivalent to that observed with the anti-CD11a Mab that was effective in vivo.
Previous studies have shown that VHH are not immunogenic and do not disrupt the integrity of the epithelial layer47,48, reducing their likely exposure to the underlying lymphoid tissue. Colonization by transformed lactobacilli might permit stable, constitutive expression of VHH targeting CD11a, reducing the need for frequent applications, while providing protection that would be transparent to all users and effective against both cell-associated and cell-free virus.
Table 2.
CDR sequences of the five VHH with highest anti-CD11a reactivity by ELISA
| CDR1 | CDR2 | CDR3 | |
|---|---|---|---|
| VHH 52 | KSTLDEYS | ISASGFSI | AADRWL-------VCRGRQTTDFNT |
| VHH 55 | GSALTYYT | LSSFQGRT | |
| AAQASWTADSVQTMCDEMAPREYDI VHH 56 GNDFSIHN ISS- | |||
| GGTT | KAEIVT------TPPPWYRETQFDV | ||
| VHH 58 | GSLSSINV | ISSGTST | NLDIT--------TTTMWLSQAY-- |
| VHH 60 | GFSLENKP | ISSTGDET | AVYLGG------GNCLSSLGHDY-- |
Acknowledgments
Sources of Funding: This work was supported by grants T32AI007417 and R21AI079794 from the National Institute of Allergy and Infectious Diseases
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEnery R. Oral tenofovir arm of VOICE trial discontinued early. IAVI Rep. 2011 Sep-Oct;15(5):21. [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. The Journal of infectious diseases. 1997 Oct;176(4):960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 5.Iversen AK, Larsen AR, Jensen T, et al. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. The Journal of infectious diseases. 1998 May;177(5):1214–1220. doi: 10.1086/515266. [DOI] [PubMed] [Google Scholar]
- 6.John GC, Nduati RW, Mbori-‐Ngacha D, et al. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. The Journal of infectious diseases. 1997 Jan;175(1):57–62. doi: 10.1093/infdis/175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etienne Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM 1 coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. Journal of immunology. 2000 Sep 15;165(6):3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 8.Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM1 via a rho–dependent pathway. Journal of immunology. 1999 Mar 1;162(5):2964–2973. [PubMed] [Google Scholar]
- 9.Reiss Y, Hoch G, Deutsch U, Engelhardt B. T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur J Immunol. 1998;28(10):3086–3099. doi: 10.1002/(SICI)1521-4141(199810)28:10<3086::AID-IMMU3086>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Rosseau S, Selhorst J, Wiechmann K, et al. Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. Journal of immunology. 2000;164(1):427–435. doi: 10.4049/jimmunol.164.1.427. [DOI] [PubMed] [Google Scholar]
- 11.Porter JC, Hall A. Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009 Feb;23(2):492–502. doi: 10.1096/fj.08-115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. Journal of immunology. 1998;161(10):5755–5761. [PubMed] [Google Scholar]
- 13.Pryce G, Male D, Campbell I, Greenwood J. Factors controlling T-cell migration across rat cerebral endothelium in vitro. J Neuroimmunol. 1997;75(1–2):84–94. doi: 10.1016/s0165-5728(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 14.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. Journal of immunology. 1995 Apr 15;154(8):4099–4112. [PubMed] [Google Scholar]
- 15.Walton LJ, Thornhill MH, Farthing PM. VCAM-1 and ICAM-1 are expressed by Langerhans cells, macrophages and endothelial cells in oral lichen planus. J Oral Pathol Med. 1994;23(6):262–268. doi: 10.1111/j.1600-0714.1994.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 16.Herold S, von Wulffen W, Steinmueller M, et al. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. Journal of immunology. 2006 Aug 1;177(3):1817–1824. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- 17.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999 Feb;96(2):272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JO, Rieu P, Arnaout MA, Liddington R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18) Cell. 1995 Feb 24;80(4):631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 19.Legge GB, Kriwacki RW, Chung J, et al. NMR solution structure of the inserted domain of human leukocyte function associated antigen–1. J Mol Biol. 2000 Feb 4;295(5):1251–1264. doi: 10.1006/jmbi.1999.3409. [DOI] [PubMed] [Google Scholar]
- 20.Bastiani Lallos L, Cecilia D, Fenyo EM, Laal S, Zolla-Pazner S. HIV phenotype correlates with the relative amounts of lymphocyte function-related molecule 1 (LFA-1) and major histocompatibility complex (MHC) class II in the virion envelope. AIDS. 2000 Jul 28;14(11):1523–1531. doi: 10.1097/00002030-200007280-00008. [DOI] [PubMed] [Google Scholar]
- 21.Orentas RJ, Hildreth JE. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9(11):1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 22.Liao Z, Roos JW, Hildreth JE. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res Hum Retroviruses. 2000 Mar 1;16(4):355–366. doi: 10.1089/088922200309232. [DOI] [PubMed] [Google Scholar]
- 23.Meerloo T, Sheikh MA, Bloem AC, et al. Host cell membrane proteins on human immunodeficiency virus type 1 after in vitro infection of H9 cells and blood mononuclear cells. An immune-electron microscopic study. The Journal of general virology. 1993 Jan;74(Pt 1):129–135. doi: 10.1099/0022-1317-74-1-129. [DOI] [PubMed] [Google Scholar]
- 24.Zwick MB, Parren PW, Saphire EO, et al. Molecular features of the broadly neutralizing immunoglobulin G1 b12 required for recognition of human immunodeficiency virus type 1 gp120. Journal of virology. 2003 May;77(10):5863–5876. doi: 10.1128/JVI.77.10.5863-5876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chancey CJ, Khanna KV, Seegers JF, et al. Lactobacilli-expressed single-chain variable fragment (scFv) specific for intercellular adhesion molecule 1 (ICAM-1) blocks cell-associated HIV-1 transmission across a cervical epithelial monolayer. Journal of immunology. 2006 May 1;176(9):5627–5636. doi: 10.4049/jimmunol.176.9.5627. [DOI] [PubMed] [Google Scholar]
- 26.Khanna KV, Whaley KJ, Zeitlin L, et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. The Journal of clinical investigation. 2002 Jan;109(2):205–211. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu T, Corey L, Hwangbo Y, et al. Persistence of extraordinarily low levels of genetically homogeneous human immunodeficiency virus type 1 in exposed seronegative individuals. Journal of virology. 2003 Jun;77(11):6108–6116. doi: 10.1128/JVI.77.11.6108-6116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maass DR, Sepulveda J, Pernthaner A, Shoemaker CB. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs) J Immunol Methods. 2007 Jul 31;324(1–2):13–25. doi: 10.1016/j.jim.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Shamkhani A, Law SK. Expression of the H52 epitope on the beta2 subunit is dependent on its interaction with the alpha subunits of the leukocyte integrins LFA-1, Mac-1 and p150,95 and the presence of Ca2+ Eur J Immunol. 1998 Oct;28(10):3291–3300. doi: 10.1002/(SICI)1521-4141(199810)28:10<3291::AID-IMMU3291>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 30.Tan SM, Robinson MK, Drbal K, van Kooyk Y, Shaw JM, Law SK. The N-terminal region and the mid-region complex of the integrin beta 2 subunit. J Biol Chem. 2001 Sep 28;276(39):36370–36376. doi: 10.1074/jbc.M102392200. [DOI] [PubMed] [Google Scholar]
- 31.Hultberg A, Tremblay DM, de Haard H, et al. Lactobacillli expressing llama VHH fragments neutralise Lactococcus phages. BMC Biotechnol. 2007;7:58. doi: 10.1186/1472-6750-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pant N, Hultberg A, Zhao Y, et al. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. The Journal of infectious diseases. 2006 Dec 1;194(11):1580–1588. doi: 10.1086/508747. [DOI] [PubMed] [Google Scholar]
- 33.Pant N, Marcotte H, Hermans P, et al. Lactobacilli producing bispecific llama-derived anti-rotavirus proteins in vivo for rotavirus-induced diarrhea. Future Microbiol. 2011 May;6(5):583–593. doi: 10.2217/fmb.11.32. [DOI] [PubMed] [Google Scholar]
- 34.Kruger C, Hu Y, Pan Q, et al. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat Biotechnol. 2002 Jul;20(7):702–706. doi: 10.1038/nbt0702-702. [DOI] [PubMed] [Google Scholar]
- 35.Kruger C, Hultberg A, van Dollenweerd C, Marcotte H, Hammarstrom L. Passive immunization by lactobacilli expressing single-chain antibodies against Streptococcus mutans. Mol Biotechnol. 2005 Nov;31(3):221–231. doi: 10.1385/MB:31:3:221. [DOI] [PubMed] [Google Scholar]
- 36.Shimaoka M, Xiao T, Liu JH, et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003 Jan 10;112(1):99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston SC, Dustin ML, Hibbs ML, Springer TA. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. Journal of immunology. 1990;145(4):1181–1187. [PubMed] [Google Scholar]
- 38.Gilbert MT, Rambaut A, Wlasiuk G, Spira TJ, Pitchenik AE, Worobey M. The emergence of HIV/AIDS in the Americas and beyond. Proc Natl Acad Sci U S A. 2007 Nov 20;104(47):18566–18570. doi: 10.1073/pnas.0705329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS pathogens. 2010 Apr;6(4):e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3(1):42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 41.Bobardt MD, Chatterji U, Selvarajah S, et al. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. Journal of virology. 2007 Jan;81(1):395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dezzutti CS, Guenthner PC, Cummins JE, Jr, et al. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. The Journal of infectious diseases. 2001 Apr 15;183(8):1204–1213. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- 43.Carreno MP, Chomont N, Kazatchkine MD, et al. Binding of LFA-1 (CD11a) to Intercellular Adhesion Molecule 3 (ICAM-3; CD50) and ICAM-2 (CD102) Triggers Transmigration of Human Immunodeficiency Virus Type 1-Infected Monocytes through Mucosal Epithelial Cells. Journal of virology. 2002;76(1):32–40. doi: 10.1128/JVI.76.1.32-40.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. Journal of immunology. 2012 Mar 1;188(5):2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 45.Martin MC, Pant N, Ladero V, et al. Integrative expression system for delivery of antibody fragments by lactobacilli. Appl Environ Microbiol. 2011 Mar;77(6):2174–2179. doi: 10.1128/AEM.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Gregorio PR, Juarez Tomas MS, Santos V, Nader–Macias ME. Beneficial lactobacilli: effects on the vaginal tract in a murine experimental model. Antonie van Leeuwenhoek. 2012 Nov;102(4):569–580. doi: 10.1007/s10482-012-9752-9. [DOI] [PubMed] [Google Scholar]
- 47.Cortez-Retamozo V, Backmann N, Senter PD, et al. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004 Apr 15;64(8):2853–2857. doi: 10.1158/0008-5472.can-03-3935. [DOI] [PubMed] [Google Scholar]
- 48.Coppieters K, Dreier T, Silence K, et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006 Jun;54(6):1856–1866. doi: 10.1002/art.21827. [DOI] [PubMed] [Google Scholar]


