Abstract
The human polyomavirus JC (JCV) infects glial cells in immunosuppressed individuals leading to progressive multifocal leukoencephalopathy (PML). JCV can also infect neurons in patients with JCV granule cell neuronopathy and JCV encephalopathy. CD8-positive T-cells play a crucial role in viral containment and outcome in PML but whether CD8-positive T-cells can also recognize JCV-infected neurons is unclear. We used immunohistochemistry to determine the prevalence of T-cells in neuron-rich areas of archival brain samples from 77 patients with JCV CNS infections and 94 control subjects. Neurons predominantly sustained a restrictive infection with expression of JCV regulatory protein T antigen (T Ag), while glial cells were productively infected and expressed both T Ag and the capsid protein VP1. T-cells were more prevalent near JCV-infected cells with intact nuclei expressing both T Ag and VP1 compared to those expressing either protein alone. CD8-positive T-cells also colocalized more with JCV-infected glial cells than with JCV-infected neurons. Major histocompatibility complex I (MHC I) expression was upregulated in JCV-infected areas but could only be detected in rare neurons interspersed with infected glial cells. These results suggest that isolated neurons harboring restrictive JCV-infection do not upregulate MHC I and thus may escape recognition by CD8-positive T-cells.
Keywords: CD8-positive T-cells, Immune response, JCV encephalopathy, JCV granule cell neuronopathy, JC virus, Major histocompatibility complex I, Neurons, Progressive multifocal leukoencephalopathy (PML)
INTRODUCTION
The polyomavirus JC (JCV) infects most healthy adults without causing disease. In immunosuppressed individuals, however, JCV can reactivate and causes lytic infection of oligodendrocytes and astrocytes leading to the CNS demyelinating disease progressive multifocal leukoencephalopathy (PML) (1). JCV can also infect granule cell neurons of the cerebellum causing JCV granule cell neuronopathy (JCV GCN) (2–6). JCV GCN is characterized clinically by a cerebellar syndrome and imaging reveals cerebellar atrophy (7). Furthermore, JCV can also infect cortical pyramidal neurons in JCV encephalopathy (JCVE), which presents with cognitive dysfunction and lesions restricted to the cortical gray matter (GM) on magnetic resonance imaging (MRI) (8–11). We have shown that JCV-infected granule cell neurons and cortical pyramidal neurons can also be found in up to half of the cases of PML (5, 12); thus, they constitute an important and previously overlooked site of JCV infection.
JCV encodes 6 proteins: the regulatory small t and large T antigens (T Ag) are expressed early in the viral cycle, whereas the capsid proteins VP1, VP2, VP3 and the agnoprotein are expressed at a later stage prior to viral assembly. Mature viral particles, which do not encompass t or T Ag, contain 72 pentamers of VP1 protein. Hence, detection of T Ag by immunohistochemistry (IHC) in the absence of VP1 suggests an early or restricted infection whereas detection of VP1 indicates a full replication cycle and the presence of mature viral particles, which are formed in the nuclei of infected cells. Productively infected glia express both JCV T Ag and VP1 but cerebellar granule cell and cortical pyramidal neurons appear mostly to sustain a restrictive infection, as demonstrated by the predominance of T Ag over VP1 expression in those cells (5, 12).
CD8-positive cytotoxic T-cells play a crucial role in the containment of JCV and in the clinical outcome of PML (13–21). These cells are present in PML lesions and colocalize with JCV-infected glial cells (22). CD8-positive T-cells use their T-cell receptor to recognize virus-infected cells that present viral peptides on major histocompatibility class I (MHC I) molecules located on cell surfaces. Once this recognition has occurred, CD8-positive T-cells secrete toxins including perforins and granzymes that punch holes in the membrane of the virus-infected cells and ultimately destroy them.
Although virtually all nucleated cells express MHC I molecules, but whether this is also the case for neurons and other cells of the CNS is a subject of debate (23–28), in part because the CNS has long been considered to be an immune privileged site (29, 30). The absence of MHC I expression might shelter infected neurons from immune recognition (23). To determine whether JCV-infected neurons can be recognized and thereby possibly destroyed by the cellular immune response in the CNS, we studied JCV T Ag and VP1 expression in the brains of a large population of HIV-infected and HIV-seronegative patients with PML, JCV GCN and JCVE. We determined the abundance of CD8-positive T-cells in areas containing JCV-infected neurons and glia. We then characterized patterns of MHC I expression in JCV-infected neurons and glia to understand whether JCV-infected neurons may elude detection by the cellular immune response.
MATERIALS AND METHODS
Availability of Samples with Potential JCV-infected Neurons
A total of 334 blocks from the brains of 77 patients with JCV CNS infections were selected for this study. Of those patients, 41 had blocks with a cerebral cortical PML component, 15 had blocks with a cerebellar JCV GCN component, 20 had blocks with both cortical cerebral and cerebellar components, and 1 was a JCVE patient with lesions restricted to the cortical GM. In addition, 127 blocks from 94 HIV-positive and –negative patients without JCV-related diseases were used as controls (Table 1).
Table 1.
JC Virus-related Diseases and Control Patient Samples
| HIV Serology | HIV- positive |
HIV- negative* |
Total | HIV- positive controls |
HIV- negative controls |
Total |
|---|---|---|---|---|---|---|
| JCV Disease | PML, JCV GCN |
PML, JCV GCN, JCVE |
||||
| Number of patients | 61 | 16 | 77 | 52 | 42 | 94 |
| Number of blocks | 206 | 128 | 334 | 70 | 57 | 127 |
HIV, human immunodeficiency virus infection; JCV, JC virus; PML, progressive multifocal leukoencephalopathy; GCN, granular cell neuronopathy; JCVE, JCV encephalopathy.
Conditions predisposing to JCV-associated brain diseases in HIV-negative patients included: chronic lymphocytic leukemia (n = 4), multiple sclerosis treated with natalizumab (n = 1), idiopathic T-cell lymphocytopenia (n = 1), transplant recipient (n = 1), polycythemia vera and myelofibrosis (n = 1), polymyositis (n = 1), rheumatoid arthritis (n = 1), brain tumor distant from PML lesions (n = 1), other tumor (n = 2) and 3 patients for which information was not available. Controls: HIV-positive or HIV-negative control subjects.
IHC and Immunofluorescence Staining
The antibodies used are listed in Table 2. Single and double IHC and immunofluorescence (IFA) staining assays were performed as previously described (8, 12, 22, 31). In multiple staining, primary antibodies from different species (mouse monoclonal, rabbit, chicken and sheep polyclonal) were used with appropriately matched (species and isotype) secondary antibodies. The secondary antibodies were conjugated either to alkaline phosphatase, horseradish peroxidase, glucose oxidase or to Alexa Fluor 350, 488, 568 & 647 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Single and double IHC were performed with the Mouse IgG and/or Rabbit IgG Vectastain Elite ABC Kits (Vector Laboratories, Burlingame, CA), according to the manufacturer’s instructions. Other kits and chromogens were also used (22, 31). To limit steric interference, triple to quintuple IHC were done with the ImmPRESS™ polymerized reporter enzyme staining system (Vector Laboratories), according to the manufacturer’s instruction. Because those multiple IHC assays included primary and secondary antibodies from more than 5 different species we had to substitute ImmPRESS™ species-specific buffers with general antibody diluents (S0809 or S3022) (Dako, Carpinteria, CA) to avoid non-specific deposits. Sections were mounted in VectaMount Permanent Mounting Medium (H-5000, Vector Laboratories).
Table 2.
Antibodies Used for Immunohistochemistry
| Antigen; product name | Host | Isot. | Target | Source |
|---|---|---|---|---|
| PAB597 | m | NA | JCV VP1 | |
| SV40 T Ag (v-300); sc-20800 | r | IgG | JCV T Ag | Santa Cruz Biotechnology, Santa Cruz, CA |
| MAP-2; HM-2; M4403 | m | IgG1 | Neurons | Sigma, St-Louis, MO |
| MAP-2; ab5622 | r | IgG | Neurons | Chemicon, Temecula, CA |
| MAP-2; LS-B290 | ch | IgY | Neurons | Lifespan Bioscience, Seattle, WA |
| MAP-2; ab5392 | ch | IgY | Neurons | Abcam, Cambridge, MA |
| NeuN (FOX3); ab104225 | r | IgG | Neurons | Abcam |
| NeuN (FOX3); ABN90P | gp | IgG | Neurons | EMD Millipore, Billerica, MA |
| GFAP; M0761; 6F2 | m | IgG1 | Astrocytes | Dako, Carpinteria, CA |
| GFAP; Z0334 | r | IgG | Astrocytes | Dako |
| GFAP; ab4674 | ch | IgY | Astrocytes | Abcam |
| GFAP; ab90601 | sh | IgG | Astrocytes | Abcam |
| GFAP; 173004 | gp | IgG | Astrocytes | Synaptic Systems, Göttingen, DE |
| CNPase; C5922; 11–5B | m | IgG1 | Myelin/Oligo | Sigma |
| CNPase; ab18527 | r | IgG | Myelin/Oligo | Abcam |
| CNPase; ab50739 | ch | IgY | Myelin/Oligo | Abcam |
| CNP; HPA023266 | r | IgG | Myelin/Oligo | Sigma |
| CD8; 1A5; VP-C325 | m | IgG1 | CD8 | Vector Laboratories, Burlingame, CA |
| CD8 (144B); ab17147 | m | IgG1 | CD8 | Abcam |
| CD3; CD3–12; OASA11552 | rat | IgG | CD3 | Aviva Systems Biology, San Diego, CA |
| CD3; CD3–12; MCA1477 | rat | IgG | CD3 | AbD Serotec, Raleigh, NC |
| CD68; M0814; KP1 | m | IgG1 | Monocytes/ Macrophages |
Dako |
| Granzyme B (11F1); VP-G812 | m | IgG2a | Granzyme B | Vector Laboratories |
| Perforin (5B10); VP-P967 | m | IgG1 | Perforin | Vector Laboratories |
| MHC class I (F-3), sc-55582 | m | IgG2a | MHC class I | Santa Cruz Biotechnology |
| MHC class I (H-300), sc-25619 | r | IgG | MHC class I | Santa Cruz Biotechnology |
| A (EP1395Y); ab52922HLA- | r | IgG | MHC class I | Abcam |
ch, chicken; CNP and CNPase, CNP, 2’,3’-cyclic-nucleotide 3’-phosphodiesterase; GFAP, glial fibrillary acidic protein; JCV, JC virus; m, mouse; MAG, myelin-associated glycoprotein; MAP-2, microtubule-associated protein 2; MOG, myelin-oligodendrocyte glycoprotein; oligo, oligodendrocytes; r, rabbit; SV40 T Ag, Simian virus 40 T antigen.
Negative controls for IHC and IFA included omission of the primary antibodies and the use of isotype-matched controls, as well as sections known to be free of JCV-infected cells.
Availability of Samples Containing JCV-infected Neurons
The first step of our selection process among samples from patients with JCV-related brain diseases (Table 1) was to identify blocks containing JCV-infected cells, expressing either JCV T Ag and/or the major capsid protein VP1, in areas known to contain neurons (neuronal area) as determined by the presence of cells harboring a neuronal phenotype and expressing neuronal markers in by IHC and IFA staining. Those areas included the gray-white matter junction (GWJ) and/or GM, either in the hemispheric cortex, the basal ganglia or nuclei of the brainstem, the cerebellar granular layer (GL) or molecular layer (ML).
We have shown in previous studies that oligodendrocytes and astrocytes in the neuron-rich areas are commonly infected by JCV in the brains of patients with PML (5, 12). Therefore, we first sought to identify the samples that also contained JCV-infected neurons expressing either the JCV T Ag or the VP1 capsid protein. We hypothesized that the immune response might be different Against JCV-infected neurons that are relatively isolated in the GM in contrast to those that are intermixed with or near JCV-infected glial cells of the white matter (WM). Therefore, we analyzed isolated neuronal areas (GM and ML) separately from neuronal areas near WM (GWJ, GL).
Selection of Regions of Interest (ROI) for Semiquantitative Studies
JCV lesions characterization was done as previously described (5, 12). We previously showed there is colocalization between CD8-positive T-cells, PML lesions (demyelination) and JCV-infected cells in most PML patients, irrespective of the underlying disease associated with PML (22).
Selection of the blocks for semiquantitative analyses was done using a triple IHC procedure followed by a first set of panoramic pictures of the regions of interest (ROI) of the block characterizing the distribution of the CD8-positive and CD3-positive, CD8-negative (presumed CD4-positive) T-cells, and JCV-infected cells expressing T Ag (5, 22). The ROI in patients with JCV CNS infection included 1) areas with JCV-infected cells of neuronal phenotype only that were located in the cerebral or cerebellar GM, and 2) areas with JCV-infected cells of glial and neuronal phenotype, located in GWJs. The ROI had to be uniform or at least representative of the JCV-infected part of the section. The majority of the ROI comprised at least 10 to up to thousands of JCV-infected cells. After the first panoramas of the ROI were done, the sections were un-mounted in xylene, rehydrated and stained with anti-VP1 Ab. A second set of panoramic pictures of the same ROI showing the distribution of the CD8-positive, CD4-positive T-cells as well as JCV-infected cells expressing T Ag and/or VP1 was performed. The 2 sets of pictures were compared and, when necessary, combined in an Adobe Photoshop picture comprising 2 layers allowing characterization of CD8-positive and CD8-negative T-cells as well as T Ag and/or VP1-expressing cells. Additional multiple IHC and IFA, including at least 1 neuronal marker (microtubule-associated protein-2 [MAP-2], NeuN), a T cell marker (CD3, CD8, Granzyme B, Perforin) and a JCV marker (T Ag, VP1) were done to identify the type of cells infected (neurons vs. glia) in each of the ROI analyzed.
Evaluation of the Number of JCV-infected Cells and T-Cells
Parenchymal T-cell frequencies were estimated in the ROI according to a semiquantitative scale (i.e. absent, <1 cell/mm2; rare, 1–25 cells/mm2; common, 26–250 cells/mm2; numerous, >250 cells/mm2) based on the observations from a previous quantitative study (22). Similarly, JCV-infected cells frequencies were estimated with the following scale: absent, <1 cell/mm2; rare, 1–5 cells/mm2 and/or <1% area covered by JCV-infected cells; common, 6–50 cells/mm2 and/or >1%–24% area covered by JCV-infected cells; numerous, >50 cells/mm2 and/or >25% area covered by JCV-infected cells.
Evaluation of MHC I Level or Upregulation
MHC I (α chain of the MHC heterodimer) expression levels were estimated in the ROI with the following semiquantitative scale: 0, not detectable; 1, detectable but not clearly upregulated vs. other cells or non-specific background; 2, highly upregulated. The pattern of expression of β-2-microglobulin was tested in 10 patients and was found to be similar to that of the α chain.
Statistical Analyses
Patient proportions were analyzed by the Fisher exact test. Significant differences (p < 0.05) are shown on the graphs.
RESULTS
Availability of Samples Containing JCV-infected Neurons
JCV-infection of any cell present in the various neuronal areas was found in 58/77 patients (75%); the remaining 19 (25%) had JCV infection restricted to the WM in all available blocks. None of the 94 controls had any evidence of JCV-infection.
In the GWJ and GL areas mixed with glial cells, we found T Ag and VP1 expression independently in neurons of 47% and 17% of the patients, respectively (Table 3). In the GM and ML areas away from the JCV-infected WM, we detected T Ag and VP1 expression independently in neurons of 36% and 10% of the patients, respectively. These data confirm and expand our previous observations (12) and indicate that JCV-infected neurons express significantly more JCV T Ag than JCV VP1 protein in all neuronal areas (Table 3). There were no differences in the frequency of those cells in the GWJ and GL close to JCV-infected WM compared to the GM and ML, i.e. neurons more remote from the WM. The number of JCV-infected neurons varied among the patients. None of the controls without JCV CNS infection had neurons expressing those JCV proteins (Table 3).
Table 3.
Frequency of Patients and Control Subjects with JCV-infected Neurons Expressing T Ag and/or VP1 in Neuronal Areas
| JCV-positive Neurons |
Patients with JCV CNS infection | Controls | |||
|---|---|---|---|---|---|
| Antigen | T Ag | VP1 | Fisher Exact test | T Ag | VP1 |
| GWJ, GL | 35/74 (47%) | 12/70 (17%) | p < 0.001 | 0/90 | 0/78 |
| GM, ML | 25/70 (36%) | 7/70 (10%) | p < 0.001 | 0/78 | 0/78 |
(GWJ, gray-white matter junction; GL, cerebellar granular layer; GM, gray matter; ML, cerebellar molecular layer; JCV, JC virus; N, number tested); T Ag, JCV regulatory protein T antigen.
T-Cells Expressing Perforin and Granzyme B Are Present in Neuronal Areas Harboring JCV Infection
To determine whether T-cells could recognize JCV-infected neurons, a subset of 73 patients with JCV CNS infection and 48 control subjects were used to study the presence of T-cells in the neuronal areas. T-cells colocalized with JCV-infected cells and with PML lesions in 56 of those patients (Table 4). In patients with PML, they were mostly present around JCV-infected cells in the parenchyma of the WM and in contiguous neuronal areas including the GWJ (Fig. 1A1). Rare T-cells were mostly associated with vessels in neuronal areas away from the WM (Fig. 1A2), even when JCV infected cells were present. In a patient with JCVE, T-cells were present mostly in the GM around JCV-infected cells (Fig. 1B). In JCV GCN patients, T cells were present around JCV-infected cells at the border of the WM and GL (Fig. 1C, D). Altogether, T-cells were present in neuronal areas of 56/73 (77%) of patients. The numbers of T-cells varied in different patients. T-cells were absent in the parenchyma of all but 1 of the 48 control HIV-infected patients (2%) (Table 4).
Table 4.
Frequency of Patients and Control Subjects with T-Cells, Granzyme B and Perforin Expression in Neuronal Areas
| Immunohistochemical marker |
Patients with JCV CNS infection | Controls |
|---|---|---|
| T-cells | 56/73 (77%) | 1/48* (2%) |
| Granzyme B | 12/12 (100%) | NA |
| Perforin | 11/13 (85%) | NA |
NA, not available.
One in a patient with HIV encephalopathy.
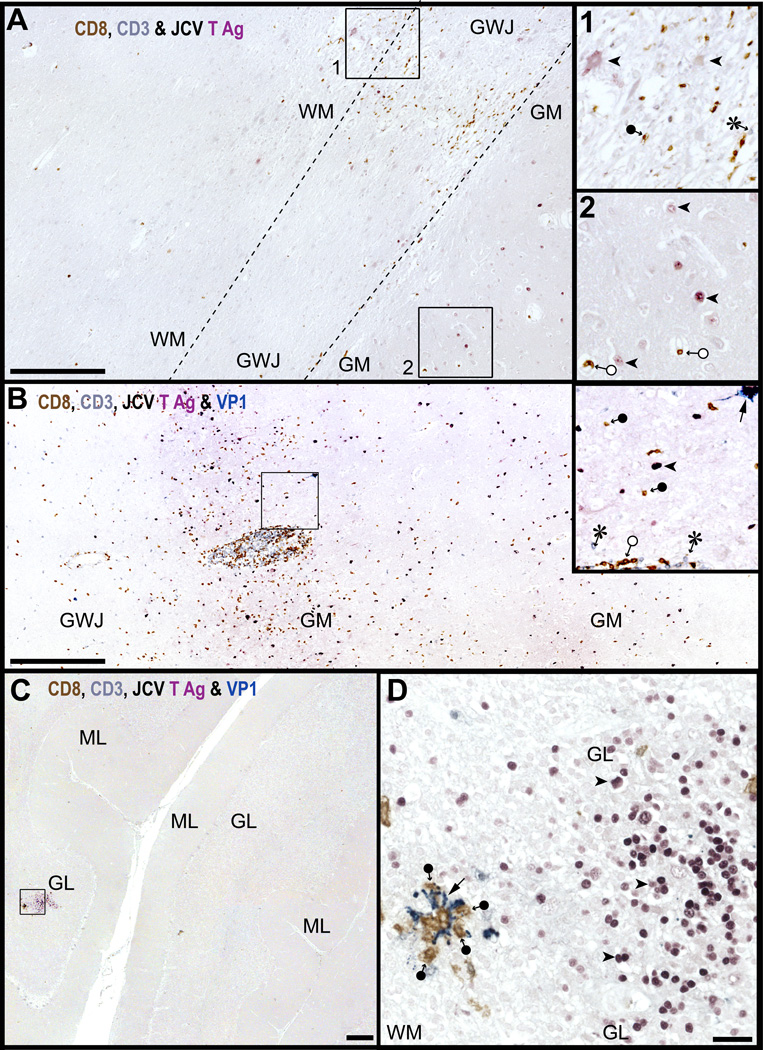
Figure 1.
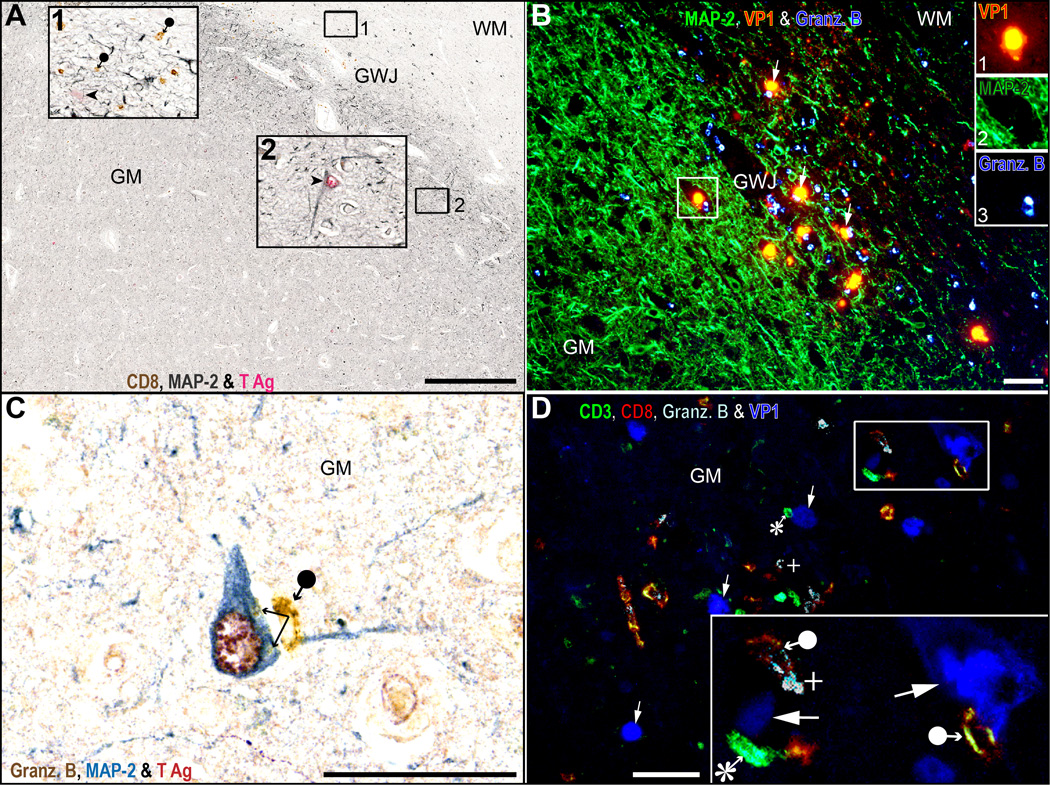
T-cells are present in white matter (WM), gray-white junction (GWJ), gray matter (GM), granule cell layer (GL) and molecular layer (ML) areas harboring JCV-infected cells. (A–D) Triple (A) and quadruple (B–D) immunostaining for CD8 (brown), CD3 (grey blue), T antigen (T Ag, purple), and VP1 (cyan-blue) show that numerous CD8-positive T-cells (brown, filled circle-arrow) are present in the vicinity of JC virus (JCV)-infected cells expressing T Ag (purple, arrowheads) in partially burnt out lesions located in the GWJ (A) square 1, magnified in inset 1 of an HIV-seropositive patient with progressive multifocal leukoencephalopathy. Rare presumed CD4-positive T-cells (CD3-positive/CD8-negative, grey blue, asterisk in inset 1) are also present. The PML lesions extend partially in the GM (A, square 2, magnified in inset 2) where cells expressing T Ag can be seen (purple, arrowheads) as well as a few CD8-positive T-cells located in the vessels (brown, empty circle-arrows, inset 2). In (B), the presence of CD8-positive and frequent presumed CD4-positive T-cells in the gray matter of a JCV encephalopathy (JCVE) patient harboring numerous JCV-infected cells expressing T Ag and VP1. Numerous CD8-positive T-cells in the parenchyma and blood vessels (brown, filled and empty circle-arrows, respectively), presumed CD4-positive T-cells (grey blue, asterisk) and JCV-infected cells expressing T Ag (purple, arrowhead) or VP1 (cyan-blue, arrow) are visible in the inset. An isolated cerebellar lesion at the border of the white matter (WM) and granule layer (GL) of a JCV granule cell neuronopathy (JCV GCN) patient with chronic lymphocytic leukemia (C) and the magnification of the square in (D) show that CD8-positive T-cells (brown, filled circle-arrows) are present in the parenchyma in areas with JCV-infected cells expressing T Ag (purple, arrowheads) and/or VP1 (cyan-blue, arrows). Aggregates of CD8 on JCV-infected cells expressing VP1 are also evident. Scale bars: A-C, 500 µm. D, 50 µm.
T-cells producing granzyme B and perforin were present in 100% and 85% of 2 sub-samples of 12 and 13 patients, respectively, with JCV CNS infection (Table 4). Those results suggest that activated, cytotoxic T-cells colocalize with JCV-infected cells in neuronal areas of the CNS.
T-cells in the Neuronal Areas Are More Frequently Found Near JCV-Infected Cells with Intact Nuclei Expressing T Ag and VP1 vs. Those Expressing Only 1 of the Proteins
T-lymphocytes are the major effectors of the cellular immune response against JCV. To determine whether T-cells colocalize similarly with cells harboring early/restrictive infection or with productively infected cells in neuronal areas, we studied the presence of CD3-positive T-cells by multiple IHC stains in lesions with JCV-infected cells expressing T Ag (irrespective or VP1 expression) and in lesions with JCV-infected cells expressing VP1 (irrespective of T Ag expression) in 2 sub-samples of 38 and 45 patients with JCV CNS infection (Fig. 2). There was no significant difference between the numbers of patients harboring T-cells in lesions containing T Ag vs. VP1.
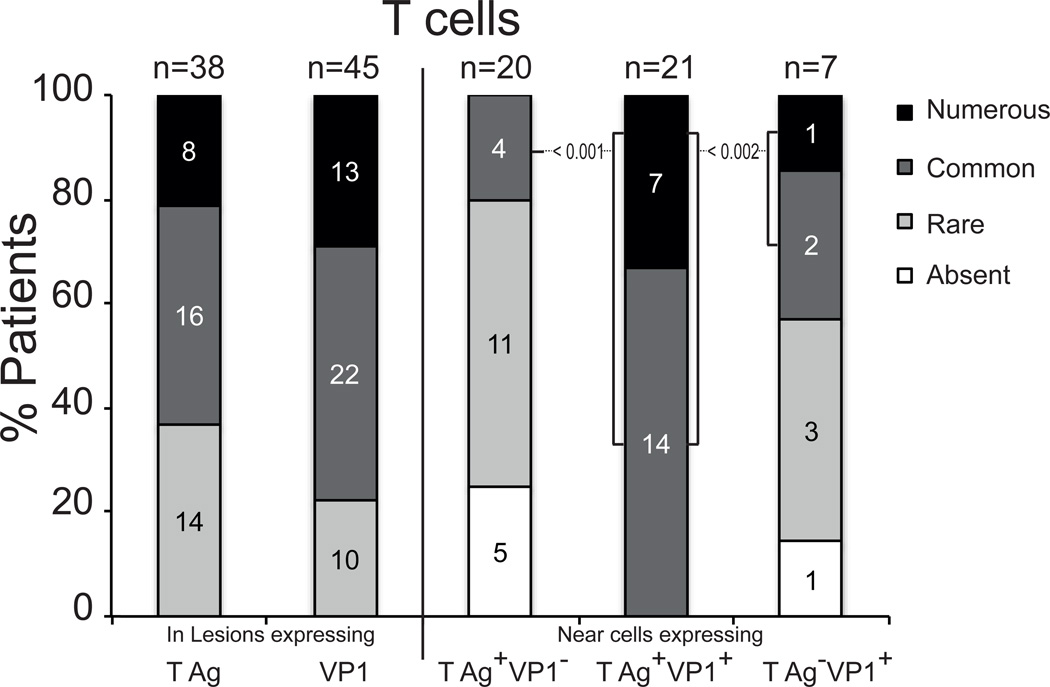
Figure 2.
Frequency of T-cells in JC virus (JCV)-infected neuronal areas expressing JCV T antigen (T Ag) and / or VP1. Numbers of patients with various amounts of T cells in lesions expressing T Ag irrespective of VP1 or expressing VP1 irrespective of T Ag, as determined by IHC are shown (left columns). In a subset of cases, the number of patients with various numbers of T cells present near T Ag-positive/VP1-negative cells, T Ag-positive/VP1-positive cells and T Ag-negative/VP1-positive cells, as determined by immunofluorescence, is shown (right columns). When significant, probabilities of the Fisher Exact tests are shown. The brackets show the group of patients with common and numerous T cells, based on the pattern of T Ag and VP1 expression.
We then performed multiple IFA stains to differentiate cells expressing T Ag alone, T Ag and VP1 together and VP1 alone in 20, 21 and 7 patients with JCV CNS infection (Fig. 2). JCV-infected cells expressing T Ag alone colocalized with common or numerous T-cells in 4/20 (20%) of the patients. In few patients who had cells expressing VP1, but not T Ag, the cells usually were in more advance stage of lysis, did not have intact nuclei, and harbored VP1 in their cytoplasm (Fig. 2). Those cells colocalized with common or numerous T cells in 3/7 (43%) of patients. On the contrary, JCV-infected cells expressing both T Ag and VP1 colocalized with common and numerous T-cells in 21/21 (100%) patients (p < 0.001 and p < 0.002) (Fig. 2). These results suggest that T-cells colocalize with productively infected cells, and that this colocalization is at its peak when JCV-infected cells are in the pre-lytic stage and still have intact nuclei.
We have previously shown that the majority of T lymphocytes in PML lesions are CD8-positive T-cells (22). Figure 3 shows CD8-positive T-cells near JCV-infected cells (regardless of their phenotype) expressing T Ag alone, T Ag and VP1 together, and VP1 alone.
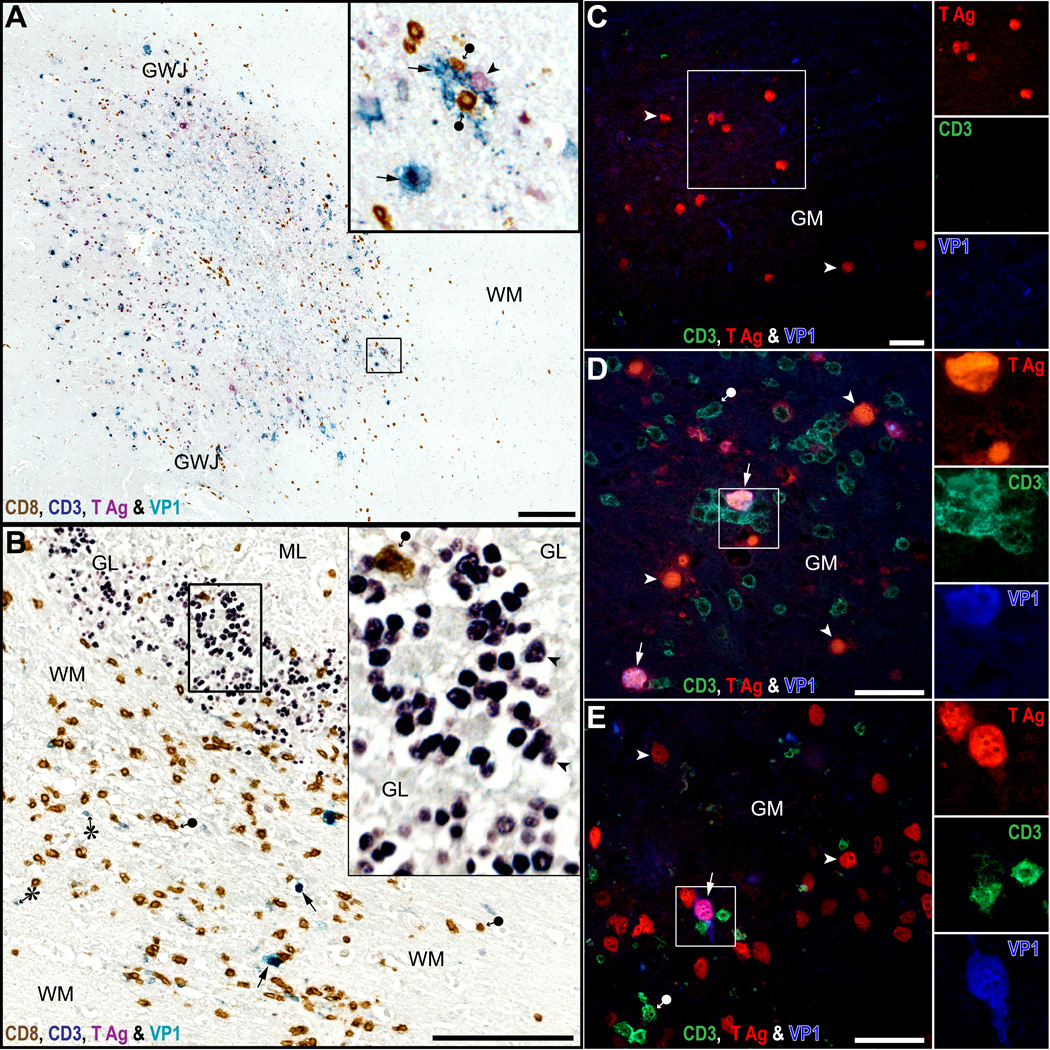
Figure 3.
CD8-positive T-cells aggregate mostly around cells with intact nuclei, expressing JC virus (JCV) T antigen (T Ag) and VP1. (A, B) Quadruple immunostaining for CD8 (brown), CD3 (grey blue), T Ag (purple) and VP1 (cyan-blue) done on an HIV-seronegative PML patient with chronic lymphocytic leukemia (A) and an HIV-seropositive PML patient (B). In PML lesions of the cerebral gray-white junction (GWJ) and gray matter (GM) (A, inset), where JCV-infected cells expressing VP1 are present (cyan-blue, arrows), there are numerous parenchymal CD8-positive T-cells (brown, black circle arrow). They are located mostly at the leading edge of infection where newly infected cells have still intact nuclei and express T Ag alone (purple, arrowhead) or T Ag and VP1 (cyan-blue, arrows). In JCV-infected cerebellum (B), only rare CD8-positive T-cells (brown, filled circle-arrow) colocalize in the GL with hundreds of JCV-infected cells expressing T Ag (inset, purple, arrowheads), while more numerous CD8-positive T-cells and some CD4-positive T-cells (grey-blue, asterisk) colocalize in the contiguous WM with a few JCV-infected cells expressing VP1 (cyan-blue, arrow). (C–E) Triple immunofluorescence (IFA) staining for CD3, T Ag and VP1 done in the cerebrum of an HIV-seronegative PML patient (C), an HIV seropositive PML patient (D) and a JCV encephalopathy (JCVE) patient (E). The IFA immunostains confirm that T-cells are absent in an area with T Ag only (C), whereas T-cells are mostly present in areas where JCV-infected cells have intact nuclei with co-expression of T Ag and VP1 (pink, arrows in squares in D and E). Separate color channels are shown in insets. Scale bars: A, B, 250 µm; C-E, 100 µm.
CD8-Positive T-Cells Colocalize with JCV-Infected Glia in Neuronal Areas But Not with JCV-Infected Neurons
To determine whether CD8-positive T-cells colocalize with JCV-infected neurons, the presence of JCV-infected neurons remote from the JCV-infected glia, was analyzed in 65 patients. One third of the patients had isolated JCV-infected neurons in the GM and half of the patients had areas with JCV-infected neurons in GM intermixed with JCV-infected glial cells. The quantity of CD8-positive T-cells present in the vicinity of isolated JCV-infected neurons and those mixed with glial cells was analyzed in sub-samples of 18 and 26 patients. CD8-positive T-cells colocalize less with isolated JCV-infected neurons (absent in 14/18, 78%) than with JCV-infected neurons mixed with JCV-infected glial cells (absent in 3/26, 11%) (Fig. 4). Aggregates of CD8-positive T-cells were never seen around JCV-infected neurons in areas containing JCV-infected glia in a subset of 18 cases, but they could be seen around JCV-infected glial in 14 of those cases (Fig. 4). Examples of CD8-positive T-cells found in neuronal areas are shown in Figure 5. Granzyme and perforin could be detected occasionally in CD8-positive T-cells in direct apposition with a neuron expressing JCV T Ag intermixed with glia, consistent with cytolytic viral clearance (32). Overall, these results suggest that CD8-positive T-cells are more numerous in areas containing JCV-infected neurons if those areas also contain JCV-infected glia.
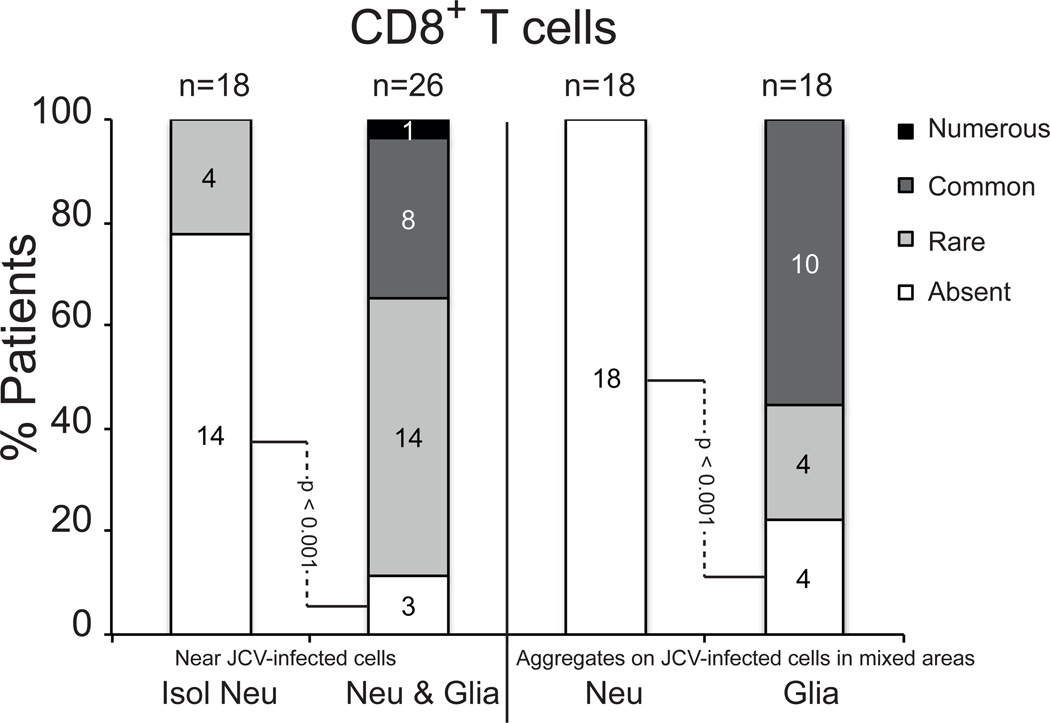
Figure 4.
Frequency of CD8-positive (CD8+) T-cells near JC virus (JCV)-infected neurons and/or glial cells in neuronal areas. Numbers of patients with various amounts of CD8-positive T-cells near or aggregating around JCV-infected neurons and glia are shown. Isol Neu, isolated JCV-infected neurons; Neu & Glia, intermix of JCV-infected neurons and JCV-infected glia; Neu, JCV-infected neurons; Glia, JCV-infected glia.
Figure 5.
CD8-positive T-cells colocalize with JC virus (JCV)-infected glia rather than neurons in mixed areas. (A) Triple immunostaining for CD8 (brown), microtubule-associated protein-2 ([MAP-2], grey blue) and T antigen (T Ag) (purple) of the cerebrum of a HIV-seropositive patient with progressive multifocal leukoencephalopathy (PML). CD8-positive T-cells (brown, black circle arrow) are common in the gray-white junction (GWJ) area (inset 1) in the vicinity of JCV-infected glia (MAP-2-negative) expressing T Ag (purple, arrowhead). Inset 2 shows that the isolated JCV-infected cells, which express T Ag alone (purple, arrowheads) and are located in the area free of CD8-positive T-cells of the gray matter (GM), are in fact neurons (grey blue, arrowheads MAP-2-positive). (B) Triple immunofluorescence (IFA) staining for VP1 (inset 1), MAP-2 (inset 2) and granzymes B (inset 3) in the cerebrum of an HIV-seropositive PML patient shows that the CD8-positive T-cells of the GWJ produce granzyme B when they are in contact with productively JCV-infected glia (MAP-2-negative, arrows). (C) Triple immunostaining for granzymes B (brown), MAP-2 (grey blue) and T Ag (purple) of the cerebrum of a JCV encephalopathy (JCVE) patient showing a T-cell (black circle arrow) releasing its granzyme B (double arrow) in a JCV-infected neuron. (D) Quadruple IFA for CD3, CD8, granzyme B and VP1 done on the same patient shown in (C) show numerous CD3-positive/CD8-positive T-cells (yellow-red, filled circle arrows) producing granzyme B (white, plus sign) as well as some presumed CD4-positive T-cells (CD3-positive/CD8-negative, asterisk) devoid of granzyme B near VP1-expressing cells. A granzyme-producing CD3-positive/CD8-positive T-cell (full circle arrow) apposed to a JCV-infected cell of neuronal phenotype expressing VP1 (arrow) is visible at the right of the inset. WM, white matter. Scale bars: A, 1 mm; B–D, 50 µm.
MHC I Is Upregulated In Infiltrates and Parenchymal Cells In JCV-Infected Areas Compared to Uninfected Areas
Because MHC I is necessary for antigen presentation to CD8-positive T-cells, we analyzed whether cells showed higher expression of MHC I in the areas of JCV infection of 19 patients and outside of those areas in 21 patients and 1 control subject. Cells expressing high levels of MHC I were present in the JCV-infected parenchyma of the 19 patients but were absent or restricted to the blood vessels in uninfected areas or in the control subject. Furthermore, some patients with JCV-CNS infection also had numerous cellular infiltrates expressing high levels of MHC I in the brain parenchyma, blood vessels and perivascular cuffs, including CD8-positive T-cells and CD68-positive macrophages (Fig. 6).
Figure 6.
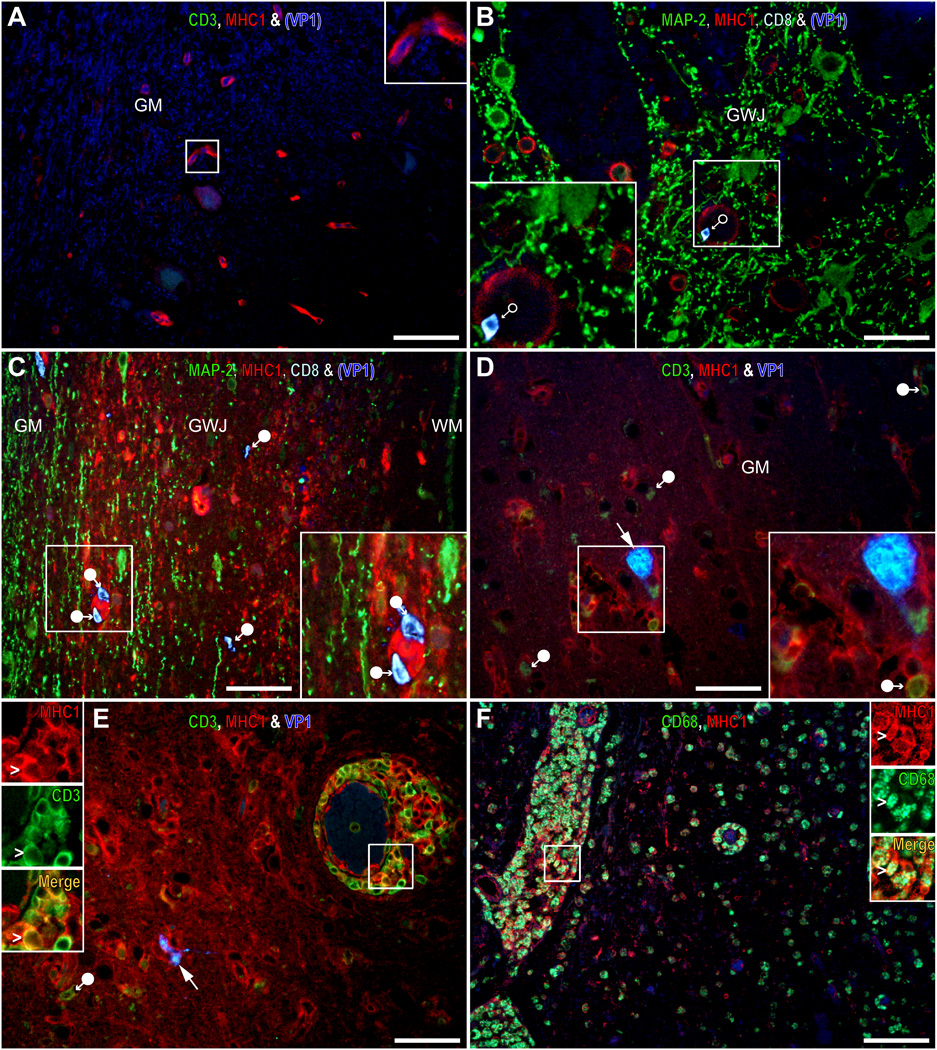
Major histocompatibility complex class I (MHC I) is upregulated in infiltrates and in parenchymal cells in JC virus (JCV)-infected areas. (A–F) Multiple immunofluorescence (IFA) staining images of CD3, MHC I and VP1 (A, D, E), microtubule-associated protein-2 (MAP-2), MHC I, CD8 and VP1 (B, C), and CD68 and MHC I (F). A typical uninfected area in the temporal lobe of an HIV-seropositive patient with progressive multifocal leukoencephalopathy (PML) are shown in (A) and (B). In those areas, cells expressing high level of MHC I are rare and restricted to vessels, parenchymal CD3-positive (A) and CD8-positive T-cells (B) are absent and only rare CD8-positive T-cells (B, empty circle arrow) are seen in blood vessels. Conversely, numerous cells expressing high levels of MHC I (red) are present in lesions of an HIV-seronegative patient with miliary PML (C) and a JCV encephalopathy (JCVE) patient (D), that harbor parenchymal CD8-positive (C) or CD3-positive (D) T-cells (filled circle arrows) close to JCV-infected cells expressing VP1 (arrow, and inset). Some of the cells expressing a high level of MHC I are infiltrates in the parenchyma and perivascular cuffs as shown by double positive for CD3-positive T-cells and MHC I in a JCVE patient (white, E, inset) and for CD68-positive macrophages and MHC I in a pediatric HIV-seropositive PML patient (white, F, inset). Scale bars: 50 µm.
MHC I Is More Frequently Upregulated in JCV-Infected Neurons in Areas with Concomitant Glial Infection
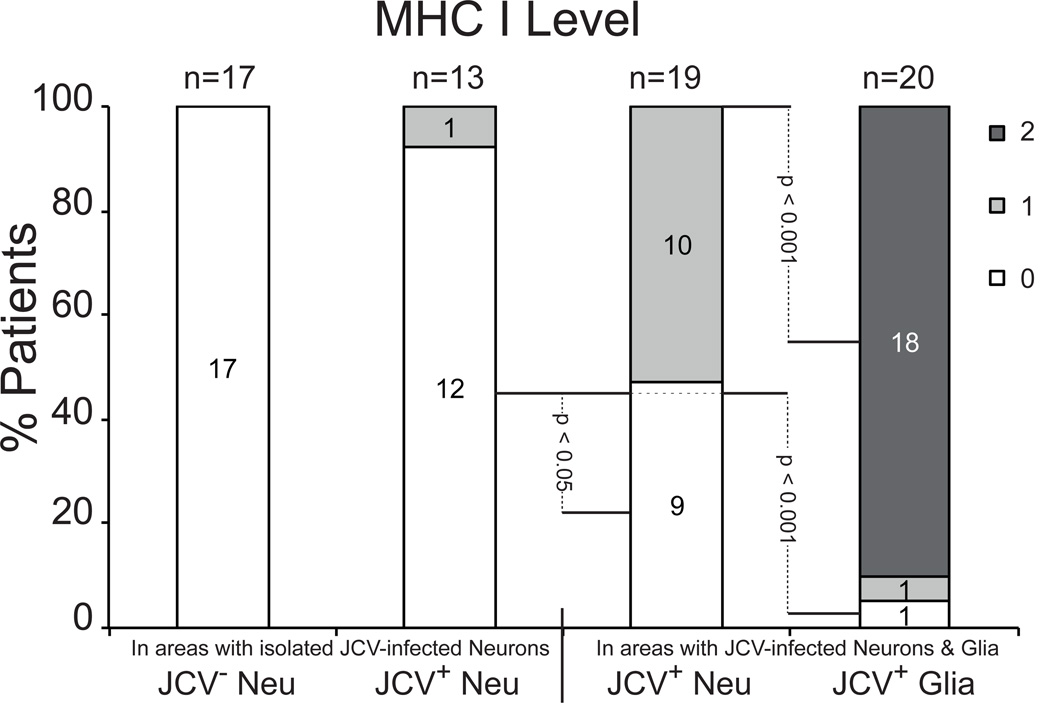
To determine whether JCV-infected neurons upregulate MHC I expression, levels of MHC I immunoreactivity were quantified in uninfected neurons, isolated JCV-infected neurons and JCV-infected neurons mixed with JCV-infected glia (Fig. 7). The following semiquantitative scale was used: 0, undetected; 1, detected; 2, upregulated. None (0%) of the patients had MHC I upregulation in uninfected neurons. Only 1/13 (8%) of the patients with isolated JCV-infected neurons compared to 10/19 (53%) of those with JCV-infected neurons mixed with JCV-infected glia had detectable MHC I staining (p = 0.01). Conversely, 18/20 (90%) of patients with JCV-infected glial cells showed clear upregulation of MHC I immunoreactivity (Fig. 7).
Figure 7.
Major histocompatibility complex class I (MHC I) expression levels in neurons and glia. JCV Neu, JC virus un-infected neuron; JCV+ Neu, JCV-infected neuron; JCV+ Glia, JCV-infected glia. 0, no upregulation of MHC class I; 1; possible, weak upregulation of MHC class I, 2; robust upregulation of MHC class I.
Apposition of Parenchymal Cells Upregulating MHC I May Allow Recognition of JCV-Infected Neurons by CD8-Positive T-Cells
We investigated whether JCV-infected neurons could potentially also be recognized by the immune system through MHC I expression of surrounding parenchymal cells. The majority of isolated JCV-infected neurons were not surrounded by parenchymal cells with upregulated MHC I (Fig. 8A). However, MHC I was frequently upregulated in JCV-infected glial cells next to JCV-infected neurons (Fig. 8B). Occasionally, parenchymal cells with upregulated MHC I were found to be apposed to JCV-infected or uninfected neurons (Fig. 8C, D). In some cases, the presence of MHC I-positive parenchymal cells surrounding JCV-infected neurons appeared to enable recognition of the neurons by CD8-positive T-cells (Fig. 8C). Aggregates of CD8-positive T-cells were only seen around JCV-infected glia expressing high level of MHC I.
Figure 8.
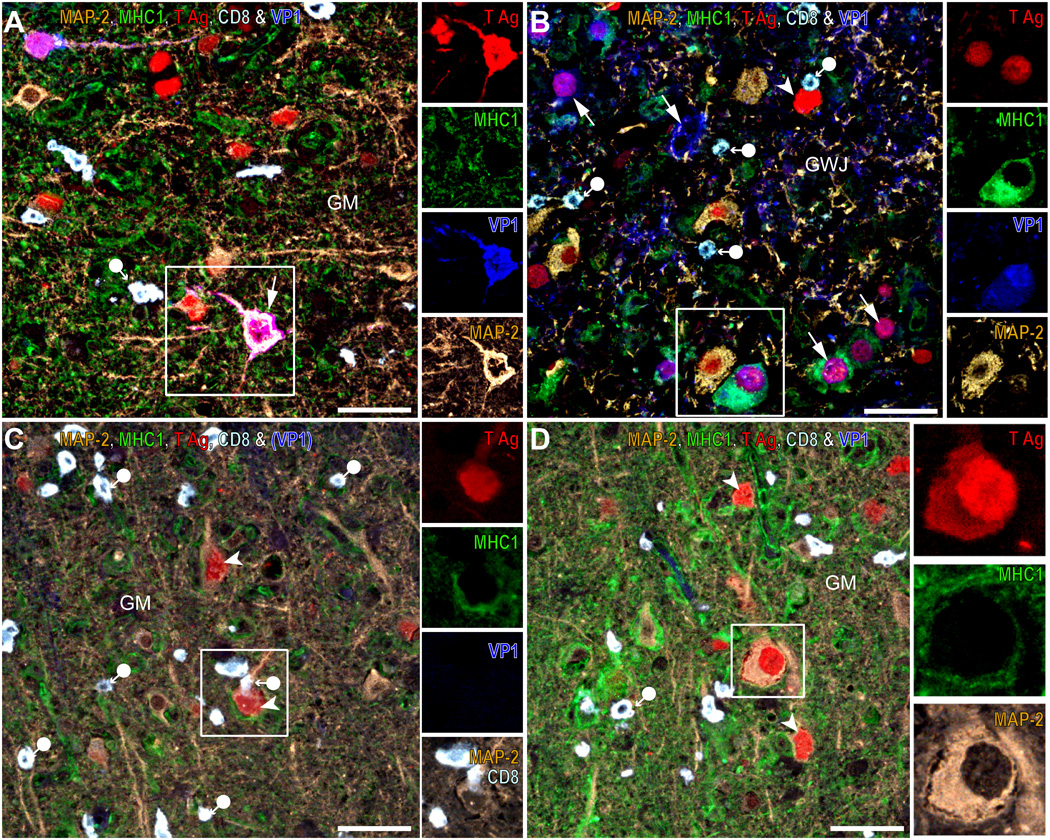
Apposition of parenchymal cells upregulating major histocompatibility complex class I (MHC I) may allow recognition of JC virus (JCV)-infected neurons by CD8-positive T-cells. (A–D) Quadruple and quintuple color immunohistochemistry/immunofluorescence (IHC/IFA) staining for microtubule-associated protein-2 (MAP-2, color inverted or Alexa 488), MHC I (Alexa 488 or 568), T antigen (T Ag) (Alexa 568), CD8 (white-blue) and VP1 (Alexa 350, blue) done on a JCV encephalopathy (JCVE) patient (A, C, D) and a pediatric HIV-seropositive progressive multifocal leukoencephalopathy (PML) patient (B). In (A), a highly JCV-infected area (T Ag, red, arrowheads and T Ag plus MAP-2, purple, arrows) of the gray matter (GM) in the JCVE patient shows that JCV-infected or un-infected neurons are expressing no or a low level of MHC I, despite the presence of numerous parenchymal CD8-positive T-cells (white-blue, filled circle arrow) and other cells expressing MHC I (green). As shown in the inset in (A) and its separate color channels, there is no colocalization of MHC I (green) with T Ag (red), VP1 (blue) and MAP-2 (brown) in a JCV-infected neuron expressing both T Ag and VP1. In (B), an area of the gray-white junction (GWJ) of the PML patient containing numerous JCV infected cells expressing T Ag (red, arrowhead), VP1 (blue, arrows) or both (purple, arrows) and containing CD8-positive T-cells (pale blue, filled circle arrows) show a JCV-infected glial cell (MAP-2-negative) of oligodendrocyte phenotype, expressing both T Ag and VP1 (purple, arrow, inset) and high levels of MHC I (green) near a JCV-infected neuron (brown, inset) expressing T Ag but not MHC I. In (C), a JCV-infected (T Ag-positive) neuron (brown, arrowhead, inset), that is MHC I-negative but is surrounded by cells expressing high levels of MHC I, is recognized by a CD8-positive T-cell (white, filled circle-arrow). In (D), cells and their processes expressing high levels of MHC I can be apposed against or even embed uninfected or JCV-infected neurons (inset), which do not express MHC I. Scale bars: 50 µm.
CD8, MAP2, T Ag, MHC I and VP1 quadruple and quintuple staining were done to analyze the frequencies of CD8-positive T-cells expressing perforin and granzyme in direct contact with a JCV-infected neuron with detectable MHC I expression; those could only be found rarely in the JCVE patient.
DISCUSSION
The present study indicates that JCV may escape recognition by the immune system by several mechanisms. As demonstrated previously for WM lesions of PML (22), we show that T-cells with cytotoxic phenotype expressing perforin and granzyme are present in neuronal areas harboring JCV infection. This result suggests that geographic localization of neurons in GM and at the GWJ is not protective and that T-cells are readily able to patrol those areas. However, the nature of JCV infection of neurons differs than that of glia in an important way. Whereas oligodendrocytes and astrocytes, which have dividing and differentiating abilities, can sustain a productive, lytic infection with expression of both early regulatory T Ag and late viral capsid protein VP1, neurons (which are by and large non-dividing cells) mostly develop an early or restrictive infection with predominant expression of T Ag over VP1 (5, 12).
Similarly, we observed here that CD8-positive T-cells present in neuron-rich areas colocalized in greater numbers with JCV-infected cells harboring intact nuclei expressing T Ag and VP1 vs. those expressing T Ag only. Because both T Ag and VP1 can be recognized by CD8-positive cytotoxic T lymphocytes (14–16, 33), these results suggest that the presence of both early and late proteins may be more immunogenic than either one alone. Indeed, cells harboring only VP1 had similar numbers of T-cells around them compared to those expressing only T Ag; however, cells expressing VP1 only were less commonly found in neuronal areas and were at a more advanced stage of infection and cytolysis, presumably caused by terminal and lytic infection by JCV.
We further studied the tropism of CD8-positive T-cells in areas with isolated JCV-infected neurons compared to areas containing both infected neurons and glia. CD8-positive T-cells were rarely found near isolated neurons and were more numerous in mixed areas. These results suggest that isolated JCV-infected neurons (which mostly sustain a restrictive infection by JCV) may be able to escape detection by the cellular immune response. Even in mixed areas, aggregates of CD8-positive T-cells were commonly found around JCV-infected glia but never around JCV-infected neurons.
A key requirement for effective recognition of virus-infected cells by CD8-positive T-cells is presentation of the viral epitope peptides by MHC I molecules on the surface of the infected cells. Whereas JCV-infected glial cells frequently demonstrated a high upregulation of MHC I expression (as shown previously in WM lesions of PML [34]), this was never seen in isolated JCV-infected neurons but more frequently observed in areas with mixed glial and neuronal infection. The presence of a pro-inflammatory milieu permitting the functional upregulation of MHC I in neurons has been demonstrated in experimental and animal models as well as within lesions of multiple sclerosis (MS) (35–37). In the present study, MHC I was upregulated in cellular infiltrates and parenchymal cells in JCV-infected areas vs. uninfected areas. Furthermore, we observed that apposition of parenchymal cells upregulating MHC I around JCV-infected neurons might allow recognition of those cells by CD8-positive T-cells. In some cases, this recognition led to bystander destruction of the JCV-infected neuron, as demonstrated by expression of perforins and granzyme by the T-cells. It is possible that this vicarious upregulation of MHC I from parenchymal cells around infected neurons may be a mechanism of defense consistent with experimental systems (38, 39).
Our study has several limitations. First, all material samples were collected postmortem and the dissemination of lymphocytes in the tissue of terminal PML cases may be different from what may be seen ex-vivo in brain biopsies. However, our investigations focused on areas of active JCV infection and did not include burnt out lesions. Because the diagnosis of PML is usually established based on clinical and radiological signs and confirmed by JCV PCR in CSF, biopsies are rarely performed and, when they are available, biopsy samples are usually too small to carry out the multiple analyses performed in this study. Second, our patients were both HIV-seropositive and seronegative. Concomitant infection of the brain by HIV might also attract T lymphocytes. However, we observed previously that HIV p24 expression in HIV encephalitis (HIVE) patients is mostly associated with perivascular cuffs similarly to what is observed in simian immunodeficiency virus-infected monkeys (22, 40), and that in PML patients with HIVE, HIV p24 expression (found in perivascular HIVE lesions) did not colocalize with JCV protein expression, which is found in parenchymal PML lesions. Furthermore, HIV has never been shown to infect neurons. We also did not see differences in the patterns of T-cell distribution between HIV-seropositive without encephalitis and seronegative patients with JCV CNS infection (22). Lastly, we did not address the potential capacity for CD8-positive T cells to mediate non-cytolytic viral clearance from neurons via the release of soluble antiviral mediators such as interferon-γ, which should be determined in future studies.
The findings that JCV can infect isolated neurons in a restrictive, non-lytic fashion and that those cells may escape detection from the cellular immune response due to their lack of MHC I expression shed new light on JCV pathogenesis in the CNS. It is possible that neurons may be the first cells of the CNS parenchyma to be infected by JCV. The recent demonstration that JCV can cause meningeal infection (41) supports the view that the virus could spread to cortical cells first, prior to infecting glia at the GWJ and in the WM. If that is the case, chronic infection of cortical pyramidal or granule cell neurons might cause neurologic dysfunction, although absence of cytolysis may preclude detection of the lesions on MRI.
These findings are of particular importance in the setting of expanding use of immunomodulatory drugs for treatment of inflammatory diseases such as MS. Indeed, a number of those medications, including natalizumab, dimethylfumarate, fingolimod, efalizumab and rituximab, have been associated with the development of PML and/or JCV GCN (31, 42–55). Whether incipient neuronal infection exist in those patients and cause neurologic dysfunction may warrant further investigation. Finally, recognition of JCV-infected neurons by the immune system may also play a role in the context of immune reconstitution inflammatory syndrome (IRIS). Indeed, the first 2 cases of natalizumab-associated JCV GCN described in MS patients developed IRIS in their cerebellum after discontinuation of the drug, determined by a T-cell infiltrate on biopsy in 1 case (45, 46) and by contrast enhancement on MRI in the other (48). In those instances, it is possible that concomitant infection of glial cells, or apposition of parenchymal cells to JCV-infected neurons may have triggered this inflammatory response.
Altogether, our findings also provide an important insight in the role of JCV in the development of seizures. Indeed, up to one third of PML patients suffer from seizures, which have been associated with IRIS and cortical involvement by JCV (56, 57). Moreover, seizures occurred in 53% of MS patients with PML who developed IRIS after discontinuation of natalizumab (58). Because seizures originate in the cerebral cortex, further investigation into the role of JCV-infection of neurons in epileptogenesis is warranted.
Acknowledgments
This study was supported by NIH grants NS047029 and NS074995 to IJK. The funding agencies had no input in the investigations or analysis of the results.
Footnotes
Conflict of Interest: Dr. Koralnik receives royalties for chapters in the online textbook UpToDate. Ms. Batson and Dr. Wüthrich declare that they have no competing interests.
REFERENCES
- 1.Gheuens S, Wüthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- 2.Du Pasquier RA, Corey S, Margolin DH, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV-positive individual. Neurology. 2003;61:775–782. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- 3.Koralnik IJ, Wüthrich C, Dang X, et al. JC virus granule cell neuronopathy: A novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57:576–580. doi: 10.1002/ana.20431. [DOI] [PubMed] [Google Scholar]
- 4.Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol. 2006;87:2533–2537. doi: 10.1099/vir.0.81945-0. [DOI] [PubMed] [Google Scholar]
- 5.Wüthrich C, Cheng YM, Joseph JT, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2009;68:15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang X, Vidal JE, Oliveira AC, et al. JC virus granule cell neuronopathy is associated with VP1 C terminus mutants. J Gen Virol. 2012;93:175–183. doi: 10.1099/vir.0.037440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijburg MT, van Oosten BW, Murk JL, et al. Heterogeneous imaging characteristics of JC virus granule cell neuronopathy (GCN): a case series and review of the literature. J Neurol. 2015;262:65–73. doi: 10.1007/s00415-014-7530-5. [DOI] [PubMed] [Google Scholar]
- 8.Wüthrich C, Dang X, Westmoreland S, et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol. 2009;65:742–748. doi: 10.1002/ana.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang X, Wüthrich C, Gordon J, et al. JC virus encephalopathy is associated with a novel agnoprotein-deletion JCV variant. PLoS One. 2012;7:e35793. doi: 10.1371/journal.pone.0035793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis LC, Norton E, Dang X, et al. Agnogene deletion in a novel pathogenic JC virus isolate impairs VP1 expression and virion production. PLoS One. 2013;8:e80840. doi: 10.1371/journal.pone.0080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis LC, Koralnik IJ. JC virus nucleotides 376–396 are critical for VP1 capsid protein expression. J Neurovirol. 2014 doi: 10.1007/s13365-014-0278-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wüthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2012;71:54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Pasquier RA, Clark KW, Smith PS, et al. JCV-specific cellular immune response correlates with a favorable clinical outcome in HIV-infected individuals with progressive multifocal leukoencephalopathy. J Neurovirol. 2001;7:318–322. doi: 10.1080/13550280152537175. [DOI] [PubMed] [Google Scholar]
- 14.Koralnik IJ, Du Pasquier RA, Letvin NL. JC virus-specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol. 2001;75:3483–3487. doi: 10.1128/JVI.75.7.3483-3487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koralnik IJ, Du Pasquier RA, Kuroda MJ, et al. Association of prolonged survival in HLA-A2-positive progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J Immunol. 2002;168:499–504. doi: 10.4049/jimmunol.168.1.499. [DOI] [PubMed] [Google Scholar]
- 16.Du Pasquier RA, Kuroda MJ, Schmitz JE, et al. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1(p36) in patients with proven or possible progressive multifocal leukoencephalopathy. J Virol. 2003;77:11918–11926. doi: 10.1128/JVI.77.22.11918-11926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Pasquier RA, Kuroda MJ, Zheng Y, et al. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 18.Du Pasquier RA, Autissier P, Zheng Y, et al. Presence of JC virus-specific CTL in the cerebrospinal fluid of PML patients: rationale for immune-based therapeutic strategies. AIDS. 2005;19:2069–2076. doi: 10.1097/01.aids.0000194804.97164.86. [DOI] [PubMed] [Google Scholar]
- 19.Lima MA, Marzocchetti A, Autissier P, et al. Frequency and phenotype of JC virus-specific CD8-positive T lymphocytes in the peripheral blood of patients with progressive multifocal leukoencephalopathy. J Virol. 2007;81:3361–3368. doi: 10.1128/JVI.01809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73:1551–1558. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gheuens S, Bord E, Kesari S, et al. Role of CD4-positive and CD8-positive T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011;85:7256–7263. doi: 10.1128/JVI.02506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wüthrich C, Kesari S, Kim WK, et al. Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: co-localization of CD8(+) T cells with JCV-infected glial cells. J Neurovirol. 2006;12:116–128. doi: 10.1080/13550280600716604. [DOI] [PubMed] [Google Scholar]
- 23.Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- 24.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 25.Huh GS, Boulanger LM, Du H, et al. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redwine JM, Buchmeier MJ, Evans CF. In vivo expression of major histocompatibility complex molecules on oligodendrocytes and neurons during viral infection. Am J Pathol. 2001;159:1219–1224. doi: 10.1016/S0002-9440(10)62507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melzer N, Meuth SG, Wiendl H. CD8+ T cells and neuronal damage: direct and collateral mechanisms of cytotoxicity and impaired electrical excitability. FASEB J. 2009;23:3659–3673. doi: 10.1096/fj.09-136200. [DOI] [PubMed] [Google Scholar]
- 28.Chevalier G, Suberbielle E, Monnet C, et al. Neurons are MHC class I-dependent targets for CD8 T cells upon neurotropic viral infection. PLoS Pathog. 2011;7:e1002393. doi: 10.1371/journal.ppat.1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aarli JA. The immune system and the nervous system. J Neurol. 1983;229:137–154. doi: 10.1007/BF00313738. [DOI] [PubMed] [Google Scholar]
- 30.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Wüthrich C, Popescu BF, Gheuens S, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in a patient with multiple sclerosis: a postmortem study. J Neuropathol Exp Neurol. 2013;72:1043–1051. doi: 10.1097/NEN.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nature Immunol. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Trofe J, Gordon J, et al. BKV and JCV large T antigen-specific CD8+ T cell response in HLA A*0201+ kidney transplant recipients with polyomavirus nephropathy and patients with progressive multifocal leukoencephalopathy. J Clin Virol. 2008;42:198–202. doi: 10.1016/j.jcv.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achim CL, Wiley CA. Expression of major histocompatibility complex antigens in the brains of patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 1992;51:257–263. doi: 10.1097/00005072-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Vass K, Lassmann H. Intrathecal application of interferon gamma Progressive appearance of MHC antigens within the rat nervous system. Am J Pathol. 1990;137:789–800. [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann H, Cavalie A, Jenne DE, et al. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 37.Hoftberger R, Aboul-Enein F, Brück W, et al. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14:43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G, Herlyn M. Information sharing and collateral damage. Trends Mol Med. 2005;11:350–352. doi: 10.1016/j.molmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 39.McPherson SW, Heuss ND, Roehrich H, et al. Bystander killing of neurons by cytotoxic T cells specific for a glial antigen. Glia. 2006;53:457–466. doi: 10.1002/glia.20298. [DOI] [PubMed] [Google Scholar]
- 40.Kim WK, Corey S, Chesney G, et al. Identification of T lymphocytes in simian immunodeficiency virus encephalitis: Distribution of CD8+ T cells in association with central nervous system vessels and virus. J Neurovirol. 2004;10:315–325. doi: 10.1080/13550280490505382. [DOI] [PubMed] [Google Scholar]
- 41.Agnihotri SP, Wüthrich C, Dang X, et al. A fatal case of JC virus meningitis presenting with hydrocephalus in a human immunodeficiency virus-seronegative patient. Ann Neurol. 2014;76:140–147. doi: 10.1002/ana.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 43.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 44.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 45.Dang X, Koralnik IJ. Gone over to the dark side: Natalizumab-associated JC virus infection of neurons in cerebellar gray matter. Ann Neurol. 2013;74:503–505. doi: 10.1002/ana.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schippling S, Kempf C, Buchele F, et al. JC virus granule cell neuronopathy and GCN-IRIS under natalizumab treatment. Ann Neurol. 2013;74:622–626. doi: 10.1002/ana.23973. [DOI] [PubMed] [Google Scholar]
- 47.van Oosten BW, Killestein J, Barkhof F, et al. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368:1658–1659. doi: 10.1056/NEJMc1215357. [DOI] [PubMed] [Google Scholar]
- 48.Agnihotri SP, Dang X, Carter JL, et al. JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene. Neurology. 2014;83:727–732. doi: 10.1212/WNL.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalkias S, Dang X, Bord E, et al. JC virus reactivation during prolonged natalizumab monotherapy for multiple sclerosis. Ann Neurol. 2014;75:925–934. doi: 10.1002/ana.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dang L, Dang X, Koralnik IJ, et al. JC polyomavirus granule cell neuronopathy in a patient treated with rituximab. JAMA neurology. 2014;71:487–489. doi: 10.1001/jamaneurol.2013.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giovannoni G, Naismith RT. Natalizumab to fingolimod washout in patients at risk of PML: when good intentions yield bad outcomes. Neurology. 2014;82:1196–1167. doi: 10.1212/WNL.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 52.Killestein J, Vennegoor A, van Golde AE, et al. PML-IRIS during fingolimod diagnosed after natalizumab discontinuation. Case Rep Neurol Med. 2014;2014:307872. doi: 10.1155/2014/307872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoppe M, Thoma E, Liebert UG, et al. Cerebellar manifestation of PML under fumarate and after efalizumab treatment of psoriasis. J Neurol. 2014;261:1021–1024. doi: 10.1007/s00415-014-7311-1. [DOI] [PubMed] [Google Scholar]
- 54.Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2015 doi: 10.1007/s13365-015-0316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheremata W, Brown AD, Rammohan KW. Dimethyl fumarate for treating relapsing multiple sclerosis. Expert Opin Drug Saf. 2015;14:161–170. doi: 10.1517/14740338.2015.977251. [DOI] [PubMed] [Google Scholar]
- 56.Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–264. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- 57.Khoury MN, Alsop DC, Agnihotri SP, et al. Hyperintense cortical signal on magnetic resonance imaging reflects focal leukocortical encephalitis and seizure risk in progressive multifocal leukoencephalopathy. Ann Neurol. 2014;75:659–669. doi: 10.1002/ana.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2013;84:1068–1074. doi: 10.1136/jnnp-2013-304897. [DOI] [PMC free article] [PubMed] [Google Scholar]