Abstract
IMPORTANCE
Research has identified improved biomarkers of acute kidney injury (AKI). Cystatin C (CysC) is a better glomerular filtration rate marker than serum creatinine (SCr) and may improve AKI definition.
OBJECTIVE
To determine if defining clinical AKI by increases in CysC vs SCr alters associations with biomarkers and clinical outcomes.
DESIGN, SETTING, AND PARTICIPANTS
Three-center prospective cohort study of intensive care units in New Haven, Connecticut, Cincinnati, Ohio, and Montreal, Quebec, Canada. Participants were 287 patients 18 years or younger without preoperative AKI or end-stage renal disease who were undergoing cardiac surgery. The study dates were July 1, 2007, through December 31, 2009.
EXPOSURES
For biomarker vs clinical AKI associations, the exposures were first postoperative (0–6 hours after surgery) urine interleukin 18, neutrophil gelatinase – associated lipocalin, kidney injury molecule 1, and liver fatty acid–binding protein. For clinical AKI outcome associations, the exposure was Kidney Disease: Improving Global Outcomes AKI definition (based on SCr or CysC).
MAIN OUTCOMES AND MEASURES
Clinical AKI, length of stay, and length of mechanical ventilation. We determined areas under the receiver operating characteristic curve and odds ratios for first postoperative biomarkers to predict AKI.
RESULTS
The SCr-defined vs CysC-defined AKI incidence differed substantially (43.6% vs 20.6%). Percentage agreement was 71% (κ = 0.38); stage 2 or worse AKI percentage agreement was 95%. Interleukin 18 and kidney injury molecule 1 discriminated for CysC-defined AKI better than for SCr-defined AKI. For interleukin 18 and kidney injury molecule 1, the areas under the receiver operating characteristic curve were 0.74 and 0.65, respectively, for CysC-defined AKI, and 0.66 and 0.58, respectively, for SCr-defined AKI. Fifth (vs first) quintile concentrations of both biomarkers were more strongly associated with CysC-defined AKI. For interleukin 18 and kidney injury molecule 1, the odds ratios were 16.19 (95% CI, 3.55–73.93) and 6.93 (95% CI, 1.88–25.59), respectively, for CysC-defined AKI vs 6.60 (95% CI, 2.76–15.76) and 2.04 (95% CI, 0.94–4.38), respectively, for SCr-defined AKI. Neutrophil gelatinase–associated lipocalin and liver fatty acid–binding protein associations with both definitions were similar. The CysC definitions and SCr definitions were similarly associated with clinical outcomes of resource use.
CONCLUSIONS AND RELEVANCE
Compared with the SCr-based definition, the CysC-based definition is more strongly associated with urine interleukin 18 and kidney injury molecule 1 in children undergoing cardiac surgery. Consideration should be made for defining AKI based on CysC in clinical care and future studies.
Acute kidney injury (AKI) occurs in approximately 40% of children undergoing cardiac surgery and is a risk factor for morbidity and mortality.1 Such injury leads to several complications, including fluid and electrolyte disturbances, nutrition provision difficulties, and drug metabolism disorders.1,2 Acute kidney injury treatment is limited because of a lack of clinical trials. This is in part because the main AKI diagnostic test, serum creatinine (SCr), is suboptimal, rising late in the course of the disease and delaying treatment evaluation and application within the narrow AKI therapeutic window.3,4 Research on new biomarkers for early AKI diagnosis has aimed to achieve more timely AKI treatment for use in clinical care and clinical trials.5–7 Nevertheless, the current reference standard for comparing new kidney injury biomarkers remains SCr rise, applied in AKI definitions.4,8,9 In steady state, SCr is not a precise marker of glomerular filtration rate (GFR).10,11 Therefore, acute SCr change (which happens with AKI) could exaggerate this imprecision and may not accurately reflect corresponding acute GFR change. A suboptimal AKI reference standard (eg, SCr) thereby also contributes to lower novel biomarker diagnostic performance.
Cystatin C (CysC) is a more accurate GFR estimate than SCr and is more diagnostic of chronic kidney disease.12–14 Unlike SCr, CysC concentrations are unaffected by muscle mass or sex, although some medications or conditions may independently influence CysC concentrations (eg, corticosteroids and thyroid disease).15,16 In AKI and non-AKI settings, CysC has been found to be associated with increased mortality or cardiovascular events,17–20 and CysC may be a more sensitive marker of contrast-induced AKI in adults.21–23 Because CysC is a better marker of GFR, it is reasonable to surmise that CysC may better detect acute GFR changes with AKI. Several studies21,24,25 have used acute CysC change to define AKI using a definition similar to the SCr-defined AKI definition. However, the CysC change for AKI definition has not been extensively validated in large studies to our knowledge.
Because there is no gold standard AKI test for comparison, we evaluated CysC rise for defining AKI by studying CysC-defined AKI associations with renal injury biomarkers and clinical outcomes. We hypothesized that CysC rise is superior to SCr rise for defining AKI and would thus be more strongly associated with new AKI biomarkers and clinical outcomes.
Methods
Design and Participants
This analysis used pediatric data from the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) study,1 a 3-center prospective cohort study of children 18 years or younger undergoing cardiac surgery. The participating centers were Yale Children’s Hospital, Montreal Children’s Hospital, and Cincinnati Children’s Hospital Medical Center. The studydates were July 1, 2007, through December 31, 2009. Children undergoing surgical procedures with a Risk Adjustment in Congenital Heart Surgery 1 (RACHS-1)26 category of at least 2 were favored for recruitment. Exclusion criteria were the presence of end-stage renal disease or preoperative AKI (SCr rise ≥50% from baseline). This analysis excludes patients younger than 1 month (because of challenges in defining AKI in neonates27,28) and children in whom preoperative SCr, CysC, and height data were not available. Sites obtained institutional ethics board approvals before study initiation, and parental written informed consent was obtained for all participants.
Study Procedures
Patients were recruited during preoperative evaluations, within 1 month of surgery. Blood and urine samples were collected before surgery or with anesthesia induction. After surgery, blood and urine were collected within 6 hours of intensive care unit (ICU) arrival (referred to as first postoperative or 0–6 hours after surgery) and then daily for up to 5 days. Urine was collected fresh from the bladder catheter urinometer if present using cotton balls in patients wearing diapers or by clean catch in older children without a catheter. Biospecimens were centrifuged (3000g for 10 minutes), and urine supernatant and plasma were aliquoted and stored at −80°C until analyte measurement.
Data Collection
Variables collected included age, sex, race, RACHS-1 category,26 cardiopulmonary bypass time, and preoperative medications (including angiotensin-converting enzyme inhibitors and diuretics). The RACHS-1 contains 6 categories differentiating surgical mortality risk based on procedure. Height was recorded to calculate preoperative SCr-estimated GFR (eGFR).29 Preoperative GFR was classified as normal or abnormal based on normative values for age.30
AKI Outcome Definitions
We studied SCr-defined AKI (hereafter SCr-AKI) and CysC-defined AKI (hereafter CysC-AKI) to compare their associations with first postoperative biomarkers and clinical outcomes. The SCr-AKI definition was based on the internationally accepted Kidney Disease: Improving Global Outcomes31 criteria. Stage 1 is at least a 50% SCr rise from baseline within 7 days or a 0.3-mg/dL rise within 48 hours, stage 2 is SCr doubling, and stage 3 is SCr tripling requiring dialysis or an eGFR of less than 35 mL/min/1.73 m2 at any time (to convert creatinine level to micromoles per liter, multiply by 88.4). We did not use the pediatric risk, injury, failure, loss, and end-stage renal disease definition32 because the required eGFR equations have not been extensively validated in young infants. The CysC-AKI was defined by applying the Kidney Disease: Improving Global Outcomes definition but using CysC change instead of SCr change similar to what has been done by others21,33,34 (ie, definitions of stage 1, stage 2, and stage 3 above).
Clinical Outcomes
We evaluated length of ICU and hospital stay (LOS) and length of mechanical ventilation (LOV). There were fewer than 5 deaths, so we did not evaluate mortality as an outcome.
Analyte Measurements
Preoperative and postoperative SCr was measured at each institution’s laboratory (by either modified Jaffe or enzymatic assay) and were isotope dilution mass spectrometry traceable.35 The CysC was measured by nephelometry (BN-II; Siemens) at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory, for which the coefficient of variation was 1.1%. Urine biomarkers were measured as previously described36,37 at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory (reported as concentration per milliliter and per urine creatinine). Briefly, urine neutrophil gelatinase–associated lipocalin (NGAL) and interleukin 18 (IL-18) were measured using an assay (ARCHITECT; Abbott Diagnostics) with coefficients of variation of 5% and 8%, respectively.36 Kidney injury molecule 1 (KIM-1) and liver fatty acid–binding protein (L-FABP) were measured in multiplex format (Sekisui Diagnostics LLC) based on capture antibodies bound to 96-well plates (Multi-Assay; Me-soScale Discovery), biotinylated antibodies detection, and electrochemical signal detection of streptavidin (Sulfo-Tag; Meso-Scale Discovery) using an imaging system (Sector Imager 2400; MesoScale Discovery).37 The intraassay coefficient of variation is 10% or less for both KIM-1 and L-FABP assays. Individuals performing assays were blinded to clinical data.
Statistical Analysis
Percentage agreement and κ statistic were calculated to evaluate agreement between CysC-AKI and SCr-AKI definitions (presence or absence of AKI and classification across AKI severity stages). Continuous variable comparisons across groups were performed using orthogonal contrasts in analysis of variance, and categorical variables were compared using χ2 test or Fisher exact test. Comparisons were made across the following 4 SCr- AKI and CysC-AKI definition combinations: no AKI by SCr or CysC, SCr-AKI only, CysC-AKI only, and AKI by SCr and CysC. Biomarker analyses focused on first postoperative (0–6 hours) urine biomarkers because our group has previously shown that all acute biomarker rises begin at this time point and demonstrate the strongest diagnostic characteristics for AKI.37,38 To evaluate first postoperative urine biomarkers to predict postoperative SCr-AKI or CysC-AKI, biomarker concentrations were categorized into quintile groups (first quintile is the lowest, and fifth quintile is the highest), and logistic regression was used to estimate unadjusted odds ratios (ORs) and 95% CIs of each quintile to predict AKI relative to the first biomarker quintile group. The areas under the receiver operating characteristic curve and 95% CIs of first postoperative biomarkers to predict AKI were also calculated. To evaluate associations of SCr-AKI and CysC-AKI with LOS and LOV, univariable and multivariable Poisson regression was used, controlling for age, sex, site, white race, preoperative eGFR, RACHS-1 category greater than 2, and cardiopulmonary bypass time exceeding 120 minutes. The selection of these other variables was based on work from our group’s previous studies1,38 elucidating a postoperative AKI predictive model. We performed analyses using software programs (SAS, version 9.2; SAS Institute Inc and R, version 2.12.1; R Foundation for Statistical Computing).
Results
Patients and Comparison of AKI Definitions
In total, 311 children were recruited. One patient was excluded from analysis because of missing preoperative height, and 23 patients were excluded for missing preoperative CysC, resulting in an analysis cohort of 287 children. As summarized in Table 1, patients who had AKI defined by both SCr and CysC had an overall worse risk profile for AKI vs patients who only had AKI defined by SCr, including lower preoperative GFR and longer cardiopulmonary bypass time and aortic cross-clamp time. Patients with AKI by both SCr and CysC also had the longest LOS and LOV of all AKI definition methods and tended to have a more severe SCr-AKI severity pattern (higher stages) than patients with only SCr-AKI. No patients received preoperative corticosteroids or nonsteroidal anti-inflammatory drugs. Five patients received postoperative dialysis.
Table 1.
Patient Characteristics and Outcomes by Acute Kidney Injury (AKI) Definition Methods in 287 Children Undergoing Cardiac Surgery
| Variable | No AKI by SCr or CysC (n = 154) |
SCr-AKI Only (n = 74) |
CysC-AKI Only (n = 8) |
AKI by SCr and CysC (n = 51) |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Age, mean (SD), y | 4.8 (4.7) | 3.0 (4.1) | 5.4 (5.1) | 1.9 (3.8)a |
| Male sex, No. (%) | 88 (57.1) | 46 (62.2) | 6 (75.0) | 19 (37.3)a,b |
| Nonwhite race, No. (%) | 27 (17.5) | 10 (13.5) | 1 (12.5) | 15 (29.4) |
| Preoperative SCr-estimated GFR, mean (SD), mL/min/1.73 m2 | 90.3 (20.7) | 104.3 (28.3) | 79.2 (15.6) | 73.0 (23.0)a,b |
| Abnormal preoperative GFR, No. (%)c | 46 (29.9) | 6 (8.1) | 4 (50.0) | 17 (33.3)a,b |
| Preoperative CysC, median (25th-75th percentiles), mg/L | 0.71 (0.61–0.86) | 0.81 (0.66–0.96) | 0.59 (0.44–0.65) | 0.84 (0.66–1.17)a |
| Prior cardiac surgery, No. (%) | 57 (37.0) | 36 (48.6) | 5 (62.5) | 17 (33.3) |
| Elective surgery, vs urgent, No. (%) | 143 (92.9) | 71 (95.9) | 7 (87.5) | 45 (88.2) |
| Preoperative medication, No. (%) | ||||
| Loop diuretic | 34 (22.1) | 27 (36.5) | 2 (25.0) | 23 (45.1) |
| Angiotensin-converting enzyme inhibitor | 22 (14.3) | 17 (23.0) | 3 (37.5) | 8 (15.7) |
| Risk Adjustment in Congenital Heart Surgery 1 category, No. (%)d | (n = 153) | (n = 73) | ||
| 1 | 17 (11.1) | 0 | 0 | 0 |
| 2 | 76 (49.7) | 35 (47.9) | 4 (50.0) | 24 (47.1) |
| 3 | 58 (37.9) | 34 (46.6) | 4 (50.0) | 21 (41.2) |
| 4 | 2 (1.3) | 4 (5.5) | 0 | 6 (11.8)a |
| Cardiopulmonary bypass time, mean (SD), min | 88.9 (47.6) | 114.7 (67.4) | 111.5 (54.4) | 149.5 (77.9)a,b |
| Aortic cross-clamp time, mean (SD), min | 42.1 (39.3) | 44.8 (48.6) | 53.1 (74.5) | 69.9 (51.3)a,b |
| Site, No. (%) | ||||
| Cincinnati, Ohio | 113 (73.4) | 41 (55.4) | 6 (75.0) | 37 (72.5) |
| Montreal, Quebec, Canada | 27 (17.5) | 18 (24.3) | 2 (25.0) | 9 (17.6) |
| New Haven, Connecticut | 14 (9.1) | 15 (20.3) | 0 | 5 (9.8) |
| Outcomes | ||||
| SCr-AKI, No. (%) | ||||
| No AKI | 154 (100) | 0 | 8 (100) | 0 |
| Stage 1 | 0 | 65 (87.8) | 0 | 16 (31.4) |
| Stage 2 | 0 | 8 (10.8) | 0 | 3 (5.9) |
| Stage 3 | 0 | 1 (1.4) | 0 | 32 (62.7) |
| In-hospital death | 0 | 2 (2.7) | 0 | 2 (3.9) |
| Length, median (IQR), d | ||||
| ICU stay | 2 (1–3) | 3 (2–5) | 3 (2–3) | 4 (3–7)a,e |
| Hospital stay | 4 (3–6) | 6 (4–10) | 5 (6–7) | 8 (6–15)a,e |
| Mechanical ventilation | 1 (0–1) | 1 (1–3) | 1 (0–1) | 2 (1–4)a,e |
Abbreviations: CysC, cystatin C; CysC-AKI, CysC-defined AKI; GFR, glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; SCr, serum creatinine; SCr-AKI, SCr-defined AKI.
Statistically significant difference across the 4 groups (P < .05, analysis of variance).
Statistically significant difference between SCr-AKI only group vs AKI by SCr and CysC group (P < .005, correction for multiple comparisons).
Defined as less than 90 mL/min/1.73 m2 if at least 2 years old, less than 76 if 1.5 to younger than 2 years, less than 74 if 1 to younger than 1.5 years, less than 65 if 8 months to younger than 1 year, and less than 58 if 3 to younger than 8 months.30
It was not possible to categorize this in 2 individuals (one in column 1 and another in column 2). Therefore, the group frequencies do not add up to column totals.
Longer in AKI by SCr and CysC group vs SCr-AKI only group (P = .05).
As summarized in Table 2 and Table 3, AKI incidence defined by any SCr criteria was more than double that of AKI incidence defined by any CysC criteria (125 of 287 [43.6%] vs 59 of 287 [20.6%]). Percentage agreement between any SCr-AKI and CysC-AKI was 71% (κ = 0.38, SE = 0.05). There was 95% agreement (κ = 0.78, SE = 0.05) between SCr-AKI and CysC-AKI stage 2 or worse. Percentage agreement between definitions for staging AKI severity was 70% (κ = 0.61, SE = 0.05). The highest discrepancy was in stage 1 AKI classification: of 81 children with stage 1 SCr-AKI, 65 were classified as having no CysC-AKI. None of the patients fulfilled SCr-AKI criteria only by the 0.3-mg/dL rise for the baseline criterion. Acute kidney injury defined by CysC-AKI also tended to be detected somewhat later after surgery compared with by SCr-AKI (mean [SD] and median of 2.02 [1.38], 2 days vs 1.54 [0.86], 1 day after surgery).
Table 2.
Comparison of Acute Kidney Injury (AKI) Severity by CysC-AKI Staging vs SCr-AKI Staging
| SCr-AKI Staging |
CysC-AKI Staging | ||||
|---|---|---|---|---|---|
| No AKI | 1 | 2 | 3 | Total | |
| No AKI | 154a | 7 | 1 | 0 | 162 |
| 1 | 65 | 14a | 2 | 0 | 81 |
| 2 | 8 | 1 | 2a | 0 | 11 |
| 3 | 1 | 2 | 0 | 30a | 33 |
| Total | 228 | 24 | 5 | 30 | 287b |
Abbreviations: CysC-AKI, cystatin C–defined AKI; SCr-AKI, serum creatinine–defined AKI.
Patients for whom there is agreement in AKI staging between the 2 definitions.
κ Statistic (SE) for AKI severity staging is 0.611 (0.045) (P < .001).
Table 3.
Comparison of Acute Kidney Injury (AKI) Stage 2 or Worse by CysC-AKI Staging vs SCr-AKI Staging
| SCr-AKI Stage 2 Staging |
CysC-AKI Stage 2 Staging | ||
|---|---|---|---|
| No AKI or Stage 1 |
Stage 2 or 3 |
Total | |
| No AKI or stage 1 | 240a | 3 | 243 |
| Stage 2 or 3 | 12 | 32a | 44 |
| Total | 252 | 35 | 287b |
Abbreviations: CysC-AKI, cystatin C–defined AKI; SCr-AKI, serum creatinine–defined AKI.
Patients for whom there is agreement in AKI staging between the 2 definitions.
κ Statistic (SE) for AKI stage 2 or worse classification is 0.78 (0.05) (P < .001).
Comparison of First Postoperative Urinary Biomarker Associations Between SCr-AKI and CysC-AKI
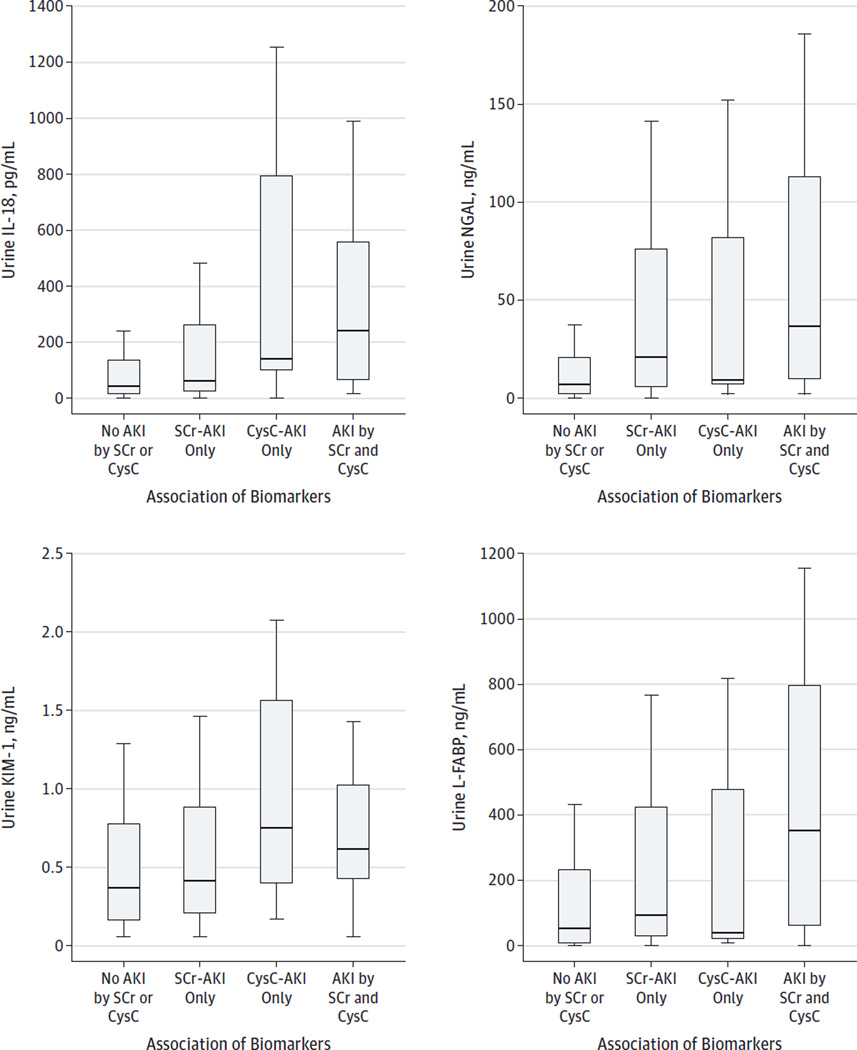
The Figure 1 shows that the highest concentrations of NGAL, IL-18, and L-FABP were found in patients with AKI by both SCr and CysC definitions, while KIM-1 was highest in patients with AKI by CysC only. Biomarkers per milliliter concentrations are shown. As summarized in Table 4, IL-18 and KIM-1 were more strongly associated with CysC-AKI than with SCr-AKI. For example, the fifth quintile IL-18 group had an OR of 16.19 (95% CI, 3.55–73.93) vs the first quintile for CysC-AKI compared with an OR of 6.60 (95% CI, 2.76–15.76) vs the first quintile for SCr-AKI. The IL-18 was diagnostic for CysC-AKI with an area under the receiver operating characteristic curve of 0.74 vs 0.66 for SCr-AKI. Similar comparative areas under the receiver operating characteristic curve were found for urine KIM-1 but with lower magnitude. For NGAL and L-FABP, magnitudes of association between biomarkers and AKI were similar, regardless of AKI definition. There was no appreciable difference in AKI vs non-AKI biomarker concentrations for biomarkers sampled within 2 vs 2 to 6 hours after surgery.
Figure. Association of Biomarkers (Expressed per Milliliter of Urine Concentration) Across 4 Serum Creatinine (SCr) and Cystatin C (CysC) Acute Kidney Injury (AKI) Categories.
Four boxplots (middle line is the median, and upper and lower stems are 95th and 5th percentiles, respectively) of first postoperative biomarker concentrations across 4 groups of patients. From left to right in each graph, the groups did not fulfill any criteria for AKI by SCr or CysC, only fulfilled AKI criteria by SCr but not CysC criteria, only fulfilled AKI criteria by CysC but not SCr criteria, and fulfilled both SCr and CysC for AKI. IL-18 indicates interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver fatty acid–binding protein; and NGAL, neutrophil gelatinase–associated lipocalin.
Table 4.
Association of Urine Biomarkers With Serum Creatinine (SCr) and Cystatin C (CysC) Acute Kidney Injury (AKI) Definitionsa
| Urine Biomarker | Quintile (Range) | Value (95% CI) | |||
|---|---|---|---|---|---|
| SCr-AKI | CysC-AKI | ||||
| Unadjusted Odds Ratio | AUROC | Unadjusted Odds Ratio | AUROC | ||
| Raw Data | |||||
| IL-18, pg/mL | 1 (<16) | 1 [Reference] | 0.66 (0.59–0.72) | 1 [Reference] | 0.74 (0.67–0.81) |
| 2 (16–42) | 3.86 (1.63–9.15) | 3.18 (0.61–16.58) | |||
| 3 (43–109) | 3.59 (1.51–8.53) | 3.78 (0.74–19.2) | |||
| 4 (110–316) | 4.44 (1.87–10.52) | 14.59 (3.19–66.67) | |||
| 5 (>317) | 6.60 (2.76–15.76) | 16.19 (3.55–73.93) | |||
| NGAL, ng/mL | 1 (<3) | 1 [Reference] | 0.69 (0.63–0.75) | 1 [Reference] | 0.66 (0.59–0.74) |
| 2 (3–7) | 2.39 (1.02–5.61) | 2.12 (0.60–7.54) | |||
| 3 (8–17) | 2.21 (0.94–5.22) | 3.47 (1.04–11.57) | |||
| 4 (18–69) | 6.05 (2.59–14.14) | 3.84 (1.16–12.70) | |||
| 5 (>69) | 7.36 (3.12–17.4) | 6.68 (2.09–21.35) | |||
| KIM-1, ng/mL | 1 (<0.15) | 1 [Reference] | 0.58 (0.51–0.65) | 1 [Reference] | 0.65 (0.57–0.72) |
| 2 (0.16–0.34) | 0.90 (0.41–1.98) | 2.89 (0.72–11.60) | |||
| 3 (0.35–0.60) | 1.76 (0.82–3.80) | 5.78 (1.55–21.57) | |||
| 4 (0.61–0.96) | 1.89 (0.88–4.08) | 6.34 (1.71–23.53) | |||
| 5 (>0.97) | 2.04 (0.94–4.38) | 6.93 (1.88–25.59) | |||
| L-FABP, ng/mL | 1 (<14) | 1 [Reference] | 0.66 (0.59–0.72) | 1 [Reference] | 0.68 (0.60–0.76) |
| 2 (14–50) | 1.39 (0.60–3.18) | 1.43 (0.42–4.83) | |||
| 3 (51–154) | 3.15 (1.41–7.04) | 3.02 (0.99–9.21) | |||
| 4 (155–422) | 1.63 (0.72–3.69) | 1.43 (0.42–4.83) | |||
| 5 (>423) | 7.32 (3.15–17.01) | 7.5 (2.58–21.77) | |||
| Corrected for Urine Creatinine | |||||
| IL-18, pg/mg of creatinine | 1 (<0.81) | 1 [Reference] | 0.66 (0.59–0.72) | 1 [Reference] | 0.74 (0.67–0.80) |
| 2 (0.82–1.91) | 2.39 (1.02–5.59) | 0.98 (0.19–5.08) | |||
| 3 (1.92–5.53) | 3.43 (1.48–7.95) | 3.31 (0.85–12.95) | |||
| 4 (5.55–18.00) | 4.88 (2.11–11.30) | 9.55 (2.64–34.49) | |||
| 5 (>18.00) | 5.87 (2.52–13.70) | 10.60 (2.94–38.23) | |||
| NGAL, ng/mg of creatinine | 1 (<0.12) | 1 [Reference] | 0.69 (0.62–0.75) | 1 [Reference] | 0.67 (0.59–0.74) |
| 2 (0.12–0.40) | 2.05 (0.87–4.83) | 4.23 (1.11–16.07) | |||
| 3 (0.40–0.93) | 2.57 (1.10–6.00) | 3.31 (0.85–12.95) | |||
| 4 (0.93–4.68) | 6.51 (2.79–15.20) | 5.75 (1.55–21.32) | |||
| 5 (>4.68) | 6.82 (2.91–16.00) | 9.07 (2.50–32.89) | |||
| KIM-1, ng/mg of creatinine | 1 (<0.01) | 1 [Reference] | 0.59 (0.53–0.66) | 1 [Reference] | 0.65 (0.57–0.72) |
| 2 (0.01–0.02) | 2.00 (0.90–4.43) | 1.67 (0.51–5.45) | |||
| 3 (0.02–0.03) | 2.15 (0.97–4.76) | 3.02 (1.00–9.16) | |||
| 4 (0.03–0.04) | 2.48 (1.13–5.48) | 2.44 (0.79–7.58) | |||
| 5 (>0.04) | 3.08 (1.39–6.80) | 5.13 (1.76–15.01) | |||
| L-FABP, ng/mg of creatinine | 1 (<0.58) | 1 [Reference] | 0.65 (0.58–0.71) | 1 [Reference] | 0.67 (0.59–0.75) |
| 2 (0.58–2.43) | 1.76 (0.78–3.96) | 1.17 (0.37–3.72) | |||
| 3 (2.43–8.30) | 1.90 (0.84–4.26) | 1.56 (0.52–4.74) | |||
| 4 (8.30–24.91) | 2.93 (1.31–6.53) | 2.0 (0.68–5.84) | |||
| 5 (>24.91) | 5.27 (2.33–11.93) | 5.69 (2.09–15.49) | |||
Abbreviations: AUROC, area under the receiver operating characteristic curve; CysC-AKI, cystatin C–defined AKI; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver fatty acid–binding protein; NGAL, neutrophil gelatinase–associated lipocalin; SCr-AKI, serum creatinine–defined AKI.
The quintile groups represent increasing biomarker concentration groups. Odds ratios represent risk for developing AKI compared with the first quintile group.
Comparison of Clinical Outcome Associations Between AKI Definitions
Overall associations between SCr-AKI and CysC-AKI and the clinical outcomes were similar (Table 5). For both AKI definitions, LOS and LOV were longer in patients with AKI after adjustment for covariates. The univariate association of longer LOS and LOV in patients with AKI by both SCr and CysC (vs SCr-AKI only) in Table 1 remained statistically significant (P < .001 for all) after covariate adjustments.
Table 5.
Acute Kidney Injury (AKI) Associations With Lengths of Intensive Care Unit (ICU) Stay, Hospital Stay, and Mechanical Ventilation for Each AKI Definition
| AKI Definition | Median (Interquartile Range), d | ||
|---|---|---|---|
| Length of ICU Stay |
Length of Hospital Stay |
Length of Mechanical Ventilation |
|
| Serum Creatinine KDIGO AKI | |||
| Yes | 3 (2–5) | 7 (5–13) | 2 (1–3) |
| No | 2 (1–3) | 4 (3–6) | 1 (0–2) |
| Unadjusted P value | <.001 | <.001 | <.001 |
| Adjusted P valuea | <.001 | <.001 | <.001 |
| Cystatin C KDIGO AKI | |||
| Yes | 4 (3–5) | 7 (5–15) | 2 (1–3) |
| No | 2 (1–3) | 5 (3–7) | 1 (0–2) |
| Unadjusted P value | <.001 | <.001 | <.001 |
| Adjusted P valuea | <.001 | <.001 | .008 |
Abbreviation: KDIGO AKI, Kidney Disease: Improving Global Outcomes AKI definition (based on serum creatinine or cystatin C).
Adjusted for age (per year), sex, site, white race, preoperative estimated glomerular filtration rate, cardiopulmonary bypass time exceeding 120 minutes, and Risk Adjustment in Congenital Heart Surgery 1 category greater than 2.
Discussion
The use of CysC first emerged in nephrology research as a more accurate alternative to SCr for estimating GFR because of its desirable properties, including constant production, lack of association with muscle mass, and absence of renal tubular secretion.15,16 Several studies12,13,29 have shown that, especially in children, CysC-based equations are more accurate for estimating GFR and more sensitive in detecting low GFR. Similar to the study of urine renal tubular injury biomarkers, CysC in the AKI literature has mostly been examined as an early biomarker of AKI, being shown to rise before SCr does and to predict later clinical evidence of AKI as defined by SCr.39–42 It has been purported that CysC concentration may be less affected than SCr concentration by volume status.43 Our group previously found in this study cohort that immediate postoperative CysC concentration predicted the development of mild and more severe (stage 2) postoperative AKI.34 However, CysC remains a marker of GFR and not of direct renal tubular injury. We hypothesized that, if CysC is a better marker of steady-state GFR than SCr is, it may also be a more accurate marker of GFR changes with AKI and therefore a superior way to define AKI in clinical trials, biomarker validation stud-ies, and AKI outcome studies. We found in this study that AKI defined by acute CysC rise was more strongly associated than SCr-AKI with 2 AKI urine biomarkers of kidney injury (KIM-1 and IL-18). This is important to understand because several studies19,21,24,25 have used CysC to define AKI. The implication is that studies using CysC change to define AKI would have different conclusions about the diagnostic ability of KIM-1 and IL-18. Association of CysC-AKI with urine NGAL was not stronger than that of SCr-AKI with urine NGAL. It is possible that urine NGAL is sensitive to AKI seen with intravascular volume depletion, which is commonly associated with acute SCr rise after surgery. Biomarker concentrations were highest in patients with both SCr-AKI and CysC-AKI. This suggests that information from both SCr and CysC change may be useful in future studies or in clinical care in which specificity of AKI diagnosis is most desirable (eg, a new drug trial, determination of preoperative risk, or decision to stop treatment with a nephrotoxic antibiotic). This should be evaluated in prospective studies with adequate sample size to evaluate AKI by both SCr and CysC as an outcome.
Several North American and European centers have also begun using CysC for renal evaluation in clinical care. Therefore, it is important to understand implications of acute CysC rise in the common clinical setting of AKI in hospitalized patients. Although agreement in the presence of any AKI was only moderate between SCr-AKI and CysC-AKI, agreement between identifying stage 2 CysC-AKI and SCr-AKI was very high. This may suggest that CysC rise may be more specific for denoting the presence of true and more severe acute renal tubular injury. We also found that time to first diagnosis of CysC-AKI occurred later than time to first diagnosis of SCr-AKI (mean, 2 days vs 1 day). This may lead to the conclusion that CysC-AKI occurs later and thus that CysC is not an early AKI biomarker. However, many patients who developed SCr-AKI had stage 1 SCr-AKI but no CysC-AKI. It is possible that patients classified as having SCr-AKI immediately after surgery did not have actual renal tubular injury but simply had immediate postoperative SCr rise due to creatinine production or secretion or metabolism. This is supported by the stronger association of CysC-AKI with some of our studied renal injury biomarkers. Therefore, simply because on average CysC-AKI was detected later than SCr-AKI does not necessarily mean that it is a later AKI biomarker. Rather, our findings suggest that CysC-AKI is likely a more specific AKI biomarker. Moreover, the focus of this work was to determine if CysC is more useful for defining AKI (eg, to validate early AKI urine biomarkers) and not to evaluate CysC for early AKI diagnosis. In the clinical setting, if CysC is more specific for true AKI (ie, renal tubular injury), then CysC might be helpful to distinguish patients who are at higher risk for complications such as fluid overload and drug nephrotoxicity and who may require more intensive renal monitoring. Patients with both SCr-AKI and CysC-AKI had longer LOS and LOV, again supporting that the inclusion of CysC in determining if AKI is present increases association of AKI with outcomes. However, we did not find that CysC-AKI by itself was more strongly associated with LOS or LOV than SCr-AKI was. Therefore, as has been shown in the literature deriving GFR equations,11,13 in which the most accurate way to estimate GFR is by combining SCr and CysC data, the same may be true in AKI. Using both biomarkers increases the chance of identifying patients who have significant AKI. Future larger studies should evaluate whether a combined SCr and CysC AKI definition is most strongly associated with clinical outcomes, including mortality.
This study had limitations. The sample size precluded us from performing subgroup analyses to evaluate associations for more severe AKI development (dialysis) and mortality outcomes. Also, we did not collect the required data to incorporate the urine output criteria for defining AKI and were unable to explore if SCr-AKI vs CysC-AKI definitions are associated with different urine output indexes. Studies44,45 have shown that, by correcting for SCr dilution using fluid balance measures, the diagnosis of AKI using SCr might be more specific, but we were not able to evaluate this. Our findings may not be applicable to nonpediat-ric cardiac surgery populations. Centers that do not have the availability of CysC measurement or that do not routinely measure CysC would not be able to evaluate CysC in clinical care without having routine preoperative CysC measurement available. We used new AKI biomarkers to evaluate the validity of CysC to define AKI, which assumes that these biomarkers have been validated for AKI diagnosis. The 4 studied biomarkers have not been extensively validated in other patient populations. However, we selected biomarkers that we know from our group’s prior work are diagnostic of SCr-AKI in this specific cohort.37,38 Moreover, in our group’s previous research,46 only urine IL-18 and KIM-1 were most strongly independently associated with long-term mortality, adding credence to our findings that these 2 biomarkers are more strongly associated with CysC-AKI vs SCr-AKI.
Our study also had several strengths. Data were collected prospectively, and we had CysC values available at all time points with SCr values available (including before surgery), allowing for a true comparison. Our evaluation of AKI definitions with both biomarkers and clinical outcomes allowed for a comprehensive examination of defining AKI by CysC. Moreover, our work builds on previous studies that have used CysC to define AKI but have not evaluated the CysC association with outcomes or markers of renal tubular injury.
Conclusions
In conclusion, defining AKI by CysC appears to lead to stronger associations with some biomarkers, without significantly affecting clinical outcome associations. This suggests that using CysC to define AKI in clinical trials or AK outcome studies is valid. Future research should specifically determine the extent to which incorporating CysC into clinical care leads to changes in patient management and should evaluate association of biomarkers with CysC-AKI in other patient populations.
At a Glance.
The current diagnostic test for acute kidney injury (AKI), serum creatinine, is suboptimal because it rises only late in the course of the disease.
Cystatin C is a more accurate measure of glomerular filtration rate.
In children undergoing cardiac surgery, cystatin C was more strongly associated with urine interleukin 18 and kidney injury molecule 1 biomarkers of AKI than was serum creatinine.
Using cystatin C to define AKI in clinical trials or AKI outcome studies appears valid.
Future research should specifically determine the extent to which incorporating cystatin C into clinical care leads to changes in patient management.
Acknowledgments
Funding/Support: Funding to the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory allows for biomarker measurements at cost. Urine biomarker assays were donated by Abbott Diagnostics (interleukin 18 and neutrophil gelatinase–associated lipocalin) and by Sekisui Diagnostics LLC (kidney injury molecule 1 and liver fatty acid–binding protein). This research was supported by grant R01HL-085757 from the National Heart, Lung, and Blood Institute (Dr Parik). Dr Zappitelli was supported by a Career Salary Award from the Fonds de Recherche du Québec– Santé. Dr Coca has been supported by Career Development Award K23DK080132 from the National Institutes of Health. Dr Devarajan is supported by grant P50DK096418 from the National Institutes of Health. Dr Parikh is supported by grant K24DK090203 from the National Institutes of Health. Drs Zappitelli, Coca, Devarajan, and Parikh are members of the National Institutes of Health–sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Consortium (funded by grant U01DK082185).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Thiessen-Philbrook and Parikh had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zappitelli, Krawczeski, Devarajan, Parikh.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Zappitelli, Greenberg, Krawczeski, Devarajan, Parikh.
Critical revision of the manuscript for important intellectual content: Zappitelli, Greenberg, Coca, Li, Thiessen-Philbrook, Bennett, Devarajan, Parikh.
Statistical analysis: Zappitelli, Thiessen-Philbrook, Parikh.
Obtained funding: Devarajan, Parikh.
Administrative, technical, or material support: Zappitelli, Krawczeski, Bennett.
Study supervision: Zappitelli, Krawczeski, Bennett, Devarajan, Parikh.
Conflict of Interest Disclosures: Dr Devarajan reported being a coinventor on the neutrophil gelatinase-associated lipocalin patent. Dr Parikh reported being a coinventor on the interleukin 18 patent. No other disclosures were reported.
Group Information: A list of the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium investigators is available at http://patr.yale.edu/projects/tribe.aspx#page2.
Additional Contributions: Primary study coordinators Isabel A. Butrymowicz, MD, CCRP (Yale University School of Medicine) and Ana Palijan, PhD (McGill University Health Centre) contributed to study processes and performance. No compensation was provided.
REFERENCES
- 1.Li S, Krawczeski CD, Zappitelli M, et al. TRIBE-AK Consortium. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39(6):1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24(2):253–263. doi: 10.1007/s00467-008-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Society of Nephrology. American Society of Nephrology renal research report. J Am Soc Nephrol. 2005;16(7):1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative Workgroup. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koyner JL, Parikh CR. Clinical utility of biomarkers of AKI in cardiac surgery and critical illness. Clin J Am Soc Nephrol. 2013;8(6):1034–1042. doi: 10.2215/CJN.05150512. [DOI] [PubMed] [Google Scholar]
- 6.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury. Nephrol Dial Transplant. 2013;28(2):254–273. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury. Kidney Int. 2008;73(9):1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 8.Levin A, Kellum JA, Mehta RL. Acute Kidney Injury Network (AKIN). Acute kidney injury: toward an integrated understanding through development of a research agenda. Clin J Am Soc Nephrol. 2008;3(3):862–863. doi: 10.2215/CJN.04841107. [DOI] [PubMed] [Google Scholar]
- 9.James M, Bouchard J, Ho J, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):673–685. doi: 10.1053/j.ajkd.2013.02.350. [DOI] [PubMed] [Google Scholar]
- 10.Zappitelli M, Joseph L, Gupta IR, Bell L, Paradis G. Validation of child serum creatinine–based prediction equations for glomerular filtration rate. Pediatr Nephrol. 2007;22(2):272–281. doi: 10.1007/s00467-006-0322-0. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4(11):1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 12.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18(10):981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 13.Zappitelli M, Parvex P, Joseph L, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48(2):221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grubb AO. Cystatin C: properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of sglomerular filtration rate? Clin Chem. 2002;48(5):699–707. [PubMed] [Google Scholar]
- 17.Ruan ZB, Zhu L, Yin YG, Chen GC. Cystatin C, N-terminal probrain natriuretic peptides and outcomes in acute heart failure with acute kidney injury in a 12-month follow-up. J Res Med Sci. 2014;19(5):404–409. [PMC free article] [PubMed] [Google Scholar]
- 18.Shlipak MG, Weekley CC, Li Y, Hansson LO, Larsson A, Whooley M. Comparison of cardiovascular prognosis by 3 serum cystatin C methods in the Heart and Soul Study. Clin Chem. 2011;57(5):737–745. doi: 10.1373/clinchem.2010.158915. [DOI] [PubMed] [Google Scholar]
- 19.Spahillari A, Parikh CR, Sint K, et al. TRIBE-AK Consortium. Serum cystatin C- versus creatinine-based definitions of acute kidney injury following cardiac surgery: a prospective cohort study. Am J Kidney Dis. 2012;60(6):922–929. doi: 10.1053/j.ajkd.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waheed S, Matsushita K, Astor BC, Hoogeveen RC, Ballantyne C, Coresh J. Combined association of creatinine, albuminuria, and cystatin C with all-cause mortality and cardiovascular and kidney outcomes. Clin J Am Soc Nephrol. 2013;8(3):434–442. doi: 10.2215/CJN.04960512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briguori C, Visconti G, Rivera NV, et al. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121(19):2117–2122. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 22.Ebru AE, Kilic A, Korkmaz FS, et al. Is cystatin-C superior to creatinine in the early diagnosis of contrast-induced nephropathy? a potential new biomarker for an old complication. J Postgrad Med. 2014;60(2):135–140. doi: 10.4103/0022-3859.132317. [DOI] [PubMed] [Google Scholar]
- 23.Tanaga K, Tarao K, Nakamura Y, et al. Percutaneous coronary intervention causes increase of serum cystatin C concentration even in the patients with a low risk of contrast-induced nephropathy. Cardiovasc Interv Ther. 2012;27(3):168–173. doi: 10.1007/s12928-012-0106-3. [DOI] [PubMed] [Google Scholar]
- 24.Ricci Z, Luciano R, Favia I, et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase–associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15(3):R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation. 2012;126(25):3008–3016. doi: 10.1161/CIRCULATIONAHA.112.103317. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins KJ, Gauvreau K. Center-specific differences in mortality: preliminary analyses using the Risk Adjustment in Congenital Heart Surgery (RACHS-1) method. J Thorac Cardiovasc Surg. 2002;124(1):97–104. doi: 10.1067/mtc.2002.122311. [DOI] [PubMed] [Google Scholar]
- 27.Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? what do we need to learn? Pediatr Nephrol. 2009;24(2):265–274. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atiyeh BA, Dabbagh SS, Gruskin AB. Evaluation of renal function during childhood. Pediatr Rev. 1996;17(5):175–180. doi: 10.1542/pir.17-5-175. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidney Disease; Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. [Accessed March 3, 2015];Kidney Int Suppl. 2013 3(1):1–150. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012 _CKD_GL.pdf. [Google Scholar]
- 31.Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. [Accessed March 3, 2015];Kidney Int. 2012 (suppl 2):1–138. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI %20Guideline.pdf.
- 32.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 33.Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 34.Zappitelli M, Krawczeski CD, Devarajan P, et al. TRIBE-AKI Consortium. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80(6):655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers GL, Miller WG, Coresh J, et al. National Kidney Disease Education Program Laboratory Working Group. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 36.Koyner JL, Garg AX, Coca SG, et al. TRIBE-AK Consortium. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23(5):905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh CR, Thiessen-Philbrook H, Garg AX, et al. TRIBE-AK Consortium. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parikh CR, Devarajan P, Zappitelli M, et al. TRIBE-AK Consortium. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22(9):1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiessling AH, Dietz J, Reyher C, Stock UA, Beiras-Fernandez A, Moritz A. Early postoperative serum cystatin C predicts severe acute kidney injury following cardiac surgery: a post-hoc analysis of a randomized controlled trial. J Cardiothorac Surg. 2014;9(1):10. doi: 10.1186/1749-8090-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan SB, Liu GL, Yu ZQ, Pan P. Urinary KIM-1, IL-18 and Cys-C as early predictive biomarkers in gadolinium-based contrast-induced nephropathy in the elderly patients. Clin Nephrol. 2013;80(5):349–354. doi: 10.5414/CN107829. [DOI] [PubMed] [Google Scholar]
- 41.Ataei N, Bazargani B, Ameli S, et al. Early detection of acute kidney injury by serum cystatin C in critically ill children. Pediatr Nephrol. 2014;29(1):133–138. doi: 10.1007/s00467-013-2586-5. [DOI] [PubMed] [Google Scholar]
- 42.Krawczeski CD, Vandevoorde RG, Kathman T, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5(9):1552–1557. doi: 10.2215/CJN.02040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soto K, Coelho S, Rodrigues B, et al. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol. 2010;5(10):1745–1754. doi: 10.2215/CJN.00690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu KD, Thompson BT, Ancukiewicz M, et al. National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39(12):2665–2671. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–667. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coca SG, Garg AX, Thiessen-Philbrook H, et al. TRIBE-AK Consortium. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol. 2014;25(5):1063–1071. doi: 10.1681/ASN.2013070742. [DOI] [PMC free article] [PubMed] [Google Scholar]