Abstract
By blocking dopamine and norepinephrine transporters, methylphenidate affects cognitive performance and regional brain activation in healthy individuals as well as those with neuropsychiatric disorders. Resting-state connectivity evaluates the functional integrity of a network of brain regions. Here, we examined how methylphenidate effects resting-state functional connectivity of the dorsal striatum and thalamus, areas each with dense dopaminergic and noradrenergic innervations, as well as global cerebral connectivity. We administered a single, oral dose (45 mg) to 24 healthy adults and compared resting-state connectivity to 24 demographically matched adults who did not receive any medication. The results showed that methylphenidate alters seed-based and global connectivity between the thalamus/dorsal striatum with primary motor cortex, amygdala/hippocampus and frontal executive areas (p<0.05, corrected). Specifically, while methylphenidate at this dosage enhances connectivity to the motor cortex and memory circuits, it dampens prefrontal cortical connectivity perhaps by increasing catecholaminergic signalling past the ‘optimal’ level. These findings advance our understanding of a critical aspect of the multifaceted effects of methylphenidate on brain functions. The results may also facilitate future studies of the aetiology and treatment of neurological and psychiatric disorders that implicate catecholaminergic dysfunction.
Keywords: fMRI, Functional connectivity, methylphenidate, thalamus, striatum
Introduction
By blocking norepinephrine and dopamine transporters, methylphenidate increases the availability of catecholamines, which play a critical role in cognitive functioning (Berridge et al., 2006, 2012; Devilbiss and Berridge, 2006; Spencer et al., 2012). Methylphenidate is a common treatment and improves cognitive performance in people with attention-deficit hyperactivity disorder (Tannock et al., 1989; Aron et al., 2003; Scheres et al., 2003; Broyd et al., 2005; Jonkman et al., 2007). For instance, methyl-phenidate improves the stop signal reaction time in the stop signal task (Aron et al., 2003), and reduces response errors on the go/no-go task (Broyd et al., 2005). Methylphenidate increases medial prefrontal cortical activation and restores the Stroop effect, where the reaction time for interference trials is prolonged compared to noninterference trials (Zang et al., 2005). Methylphenidate also improves cognitive performance in patients with other neurological conditions, including traumatic brain injury (Kim et al., 2006) and Parkinson’s disease (Auriel et al., 2006; Devos et al., 2007; Pollak et al., 2007), suggesting that its cognition enhancing effects could help clinical populations beyond ADHD.
Connectivity analysis of resting-state functional magnetic resonance imaging (fMRI) data characterizes functional integrity of brain networks (Passingham et al., 2002). Specifically, low frequency blood oxygenation level dependent (BOLD) signal fluctuations reflect connectivity between functionally related brain regions (Biswal et al., 1995; Fair et al., 2007; Fox and Raichle, 2007). Studies of this ‘spontaneous’ activity have provided insight into the intrinsic functional architecture of the brain (Fox and Raichle, 2007). For instance, based on the findings that regions with similar functionality tend to correlate in spontaneous BOLD activity, we described functional subdivisions of the medial superior frontal cortex (Zhang and Li, 2012b) and precuneus (Zhang and Li, 2012a) recently. Few studies have examined how catecho-laminergic agents influence cerebral functional connectivity during resting state. In children with ADHD, methylphenidate increased and decreased regional homogeneity, a measure of functional connectivity of local in contrast to surrounding voxels, each in bilateral ventral prefrontal cortex/cerebellar vermis and right parietal/visual cortices (An et al., 2013). Methylphenidate also increased regional homogeneity (Zhu et al., 2013) but has otherwise not been studied for its effects on resting-state functional connectivity in healthy adults.
Midbrain dopaminergic neurons project to the basal ganglia, including the caudate, putamen, pallidum and throughout the cerebral cortex (Bentivoglio and Morelli, 2005). Noradrenergic neurons of the locus coeruleus heavily innervate the thalamus and cerebral cortex (Descarries and Saucier, 1972; O’Donnell et al., 2012). A number of cognitive processes including inhibitory control and behavioural adjustment are mediated by the cortico-striato-thalamic circuitry (Wagner et al., 2006; Diamond and Ahissar, 2007; Urbain and Deschenes, 2007). In an earlier work, we demonstrated a critical role of the thalamus and epithalamus in orchestrating error-related cognitive control (Hendrick et al., 2010; Ide and Li, 2011a, b). Because these cortico-subcortical circuits are regulated by catecholaminergic signalling (Graybiel, 1990; Crawford et al., 1998; Bymaster et al., 2002; Grillner et al., 2005; Andrews and Lavin, 2006; Monchi et al., 2006), we hypothesized that pharmacological manipulation of catecholamine availability would likely result in changes in functional connectivity.
In this exploratory study, we used resting-state fMRI to examine whether and how methylphenidate alters functional connectivity between the striatum and thalamus with the rest of the brain in healthy adults. We also performed an analysis of global connectivity, as an additional measure, to identify the effects of methylphenidate on network functional changes.
Method
Participants
The study was performed under a protocol approved by the Yale Human Investigation and Magnetic Resonance Imaging Safety Committees. Participants were recruited from the greater New Haven area by advertisement, word of mouth and referrals. Written informed consent was obtained from all participants after a full explanation of study procedures. Twenty-five healthy adults (17 females; age 25±6 years; all right-handed) were recruited and compensated for their participation in the study. All participants were admitted as outpatients to the Yale New Haven Hospital, and were without medical, neurological or psychiatric conditions. All denied history of head injury and current use of prescription medications or illicit substances. One subject was eliminated from the study because of a lesion found on the structural brain image. The resulting 24 participants comprised 16 females, with a mean age of 24±4 years – the methylphenidate (MPH) group. Data of a cohort of 24 matched healthy participants (16 females; age 24±4 years) scanned under identical imaging protocols except without being given methylphenidate were used for comparison – the no-MPH group.
On the day of fMRI, participants rested in a recovery room for at least 10 min, during which baseline heart rate, blood pressure and anxiety measurements were taken. An hour prior to fMRI scans a physician examined participants before approving administration of a single 45 mg oral dose of methylphenidate. All participants in the MPH group received methylphenidate, although participants did not know whether they would be receiving methylphenidate or a placebo, according to the protocol and consent. From this time until the beginning of the structural MRI scans (approximately 40 min), heart rate and blood pressure as well as anxiety were monitored every 5 min. These measures were taken approximately every 10 min between sessions during fMRI. At each vital sign reading, participants also marked how anxious they felt on a visual analogue scale from one (not anxious at all) to ten (extremely anxious). Compared to baseline, MPH increased heart rate, systolic blood pressure and anxiety rating, as we reported recently (Farr et al., 2013).
Imaging protocol
Conventional T1-weighted spin-echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin-echo imaging in the axial plane parallel to the AC-PC line with TR=300 ms, TE=2.5 ms, bandwidth=300 Hz/pixel, flip angle=60°, field of view=220×220 mm, matrix= 256×256, 32 slices with slice thickness=4 mm and no gap. Functional, blood oxygenation level dependent (BOLD) signals were then acquired with a single-shot gradient echo echo-planar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with repetition time=2000 ms, echo time=25 ms, bandwidth=2004 Hz/ pixel, flip angle=85°, field of view=220×220 mm, matrix =64×64, 32 slices with slice thickness=4 mm and no gap. Three hundred images were acquired in the resting state run, following four other BOLD runs during which participants performed a stop signal task (Farr et al., 2013). In the resting state scans, participants were instructed to close their eyes but stay awake.
Imaging data pre-processing
Brain imaging data were pre-processed using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, UK), as described in our previous work (Zhang et al., 2012). Briefly, images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per each run from the realigned image volumes. These mean images were co-registered with the high-resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (Friston et al., 1995; Ashburner and Friston, 1999). The normalization parameters determined for the structural volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum.
Additional pre-processing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (Rombouts et al., 2003; Fox et al., 2006; Fair et al., 2007; Fox and Raichle, 2007). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, the white matter and the whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1 Hz contribute to regionally specific BOLD correlations (Cordes et al., 2001). The majority of resting state studies low-pass filtered BOLD signal at a cut-off of 0.08 or 0.1 Hz (Fox and Raichle, 2007). Thus, we applied a temporal band-pass filter (0.009 Hz<f<0.08 Hz) to the time course in order to obtain low-frequency fluctuations (Fox et al., 2006; Fair et al., 2007; Fox and Raichle, 2007).
Seed-based functional connectivity: linear correlations
We used the templates from the Anatomical Automatic Labelling (AAL) atlas for each region of interest- caudate, putamen, pallidum and thalamus (Tzourio-Mazoyer et al., 2002). The BOLD time courses were averaged spatially across all voxels each for the four seed regions. We computed the correlation coefficient between the averaged time course of each mask and the time courses of individual voxels of the brain for individual subjects. To assess and compare the resting state ‘correlograms,’ we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (Jenkins and Watts, 1968; Berry and Mielke, 2000): z=0.5 loge[(1+r)/(1–r)]. The z maps were used in group random effect analyses (Penny et al., 2004) with a two-sample t-test to compare MPH and no-MPH groups.
Global connectivity
Global connectivity was computed as the averaged voxel-to-voxel connectivity across the whole brain (Cole et al., 2010b). Here, we examined the connectivity of individual voxels to the 116 anatomical masks from the AAL atlas (Tzourio-Mazoyer et al., 2002); not to voxels of the whole brain), in order to manage computational load. The BOLD time courses were averaged spatially across the voxels within each of the 116 masks for correlation with the time course of each grey matter voxel, for each individual subject. Because positive and negative connectivities would cancel each other out, global connectivity was computed for positive and negative connectivities separately. Otherwise, for instance, an area with equally strong positive and negative connectivity would exhibit no significant connectivity. We also weighted by the number of voxels of each mask to account for seed size after z transformation. Thus, each correlation coefficient (Pearson’s r) was Fisher’s z transformed and then the weighted averaged z map was obtained for each of the positive and negative connectivity and for each subject. Voxels connected to more of the 116 masks positively or negatively would be more connected globally.
Because individual positive/negative global connectivity maps contain only positive/negative values, all grey matter voxels would show significant connectivity with one sample t-test. We thus applied the ‘top percentage’ threshold (Cole et al., 2010b) to identify voxels with the highest global connectivity and to quantify the connectivity for each group. Thresholds were determined by reducing the p value (i.e. applying a higher threshold) until the desired percentage (e.g. 5%) of the total grey matter voxels remained for each group’s one sample t-test. This analysis identified brain regions that are most connected and allowed us to examine changes in global connectivity as a result of methylphenidate, complementing findings from the seed-based analyses.
For both seed-based and global connectivity, we used two sample t-tests to compare MPH and no-MPH groups and identified voxels that were significant at a corrected threshold. Investigators have argued that the corrected voxel peak threshold of p<0.05, based on the Gaussian random field theory, may be too restrictive and suggested the use of a cluster threshold (Poline et al., 1997; Hayasaka and Nichols, 2003). Thus, we present results that satisfy either peak voxel FWE p<0.05 or a combined threshold of voxel p<0.001, uncorrected and cluster FWE p<0.05.
Results
Seed-based functional connectivity
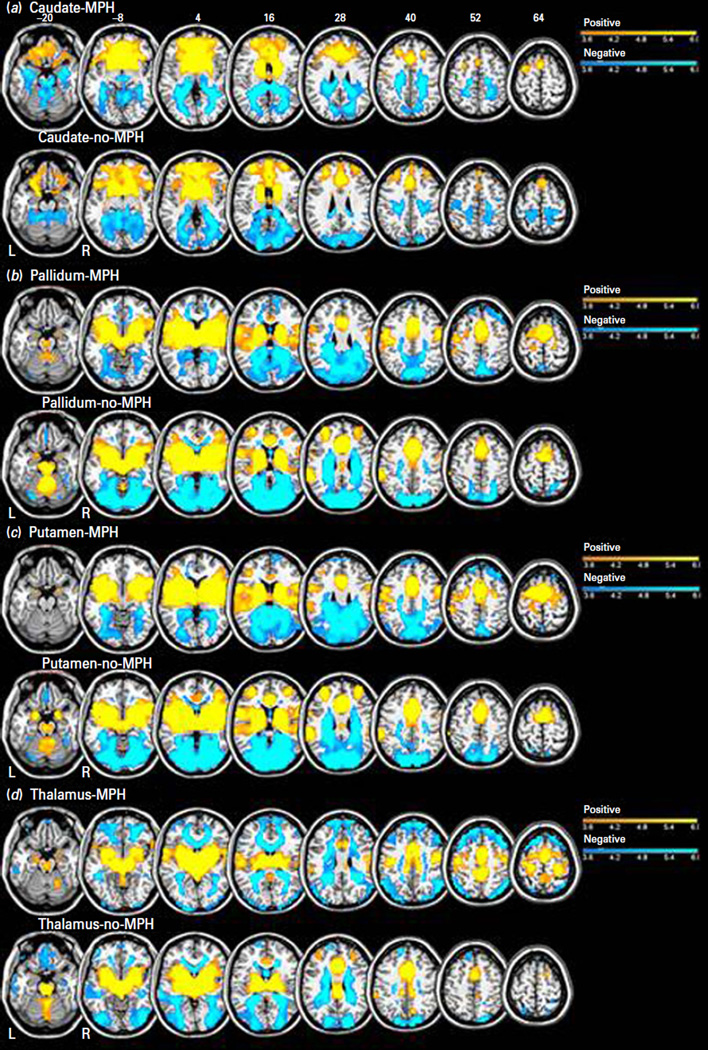
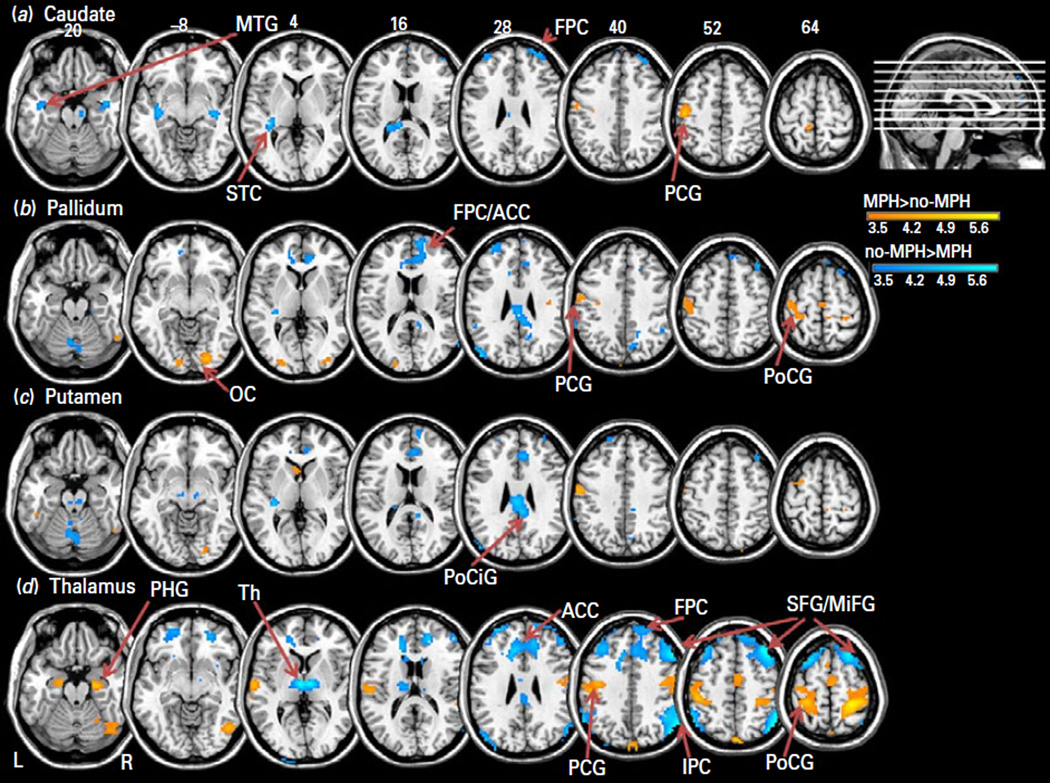
We first examined the right and left caudate, putamen, pallidum and thalamic seed regions separately and found no hemispheric differences in functional connectivity (p<0.001 uncorrected). We thus elected to show and discuss the results from the bilateral seeds for each area of interest. Figure 1 shows the results of one-sample t-tests each for the methylphenidate (MPH) and no-MPH group. The results of two sample t-tests are shown in Fig. 2 and summarized in Table 1. Supplementary Figure S1 shows the effect sizes of each group for all seed-based connectivities that differed between MPH and no-MPH. In the following, we describe structures that share significant connectivity with each seed region in both no-MPH and MPH groups (one-sample t tests) and those that demonstrate significant differences in connectivity (two-sample t test). In the latter case, we highlight whether the differences result from a change in the strength of connectivity or a reversal in the sign of connectivity.
Fig. 1.
One sample t-tests for resting-state functional connectivity with bilateral caudate (a); pallidum (b); putamen (c); or thalamus (d) as the seed region (p<0.001, uncorrected). Warm and cool colour shows positive and negative connectivity. BOLD contrasts are superimposed on a T1 structural image in axial sections from z=−20 to z=64, in neurological orientation. The adjacent sections are 12 mm apart. The colour bar represents voxel T value.
Fig. 2.
Two-sample t-tests showed differences in resting-state functional connectivity with bilateral caudate (a); pallidum (b); putamen (c); or thalamus (d) as the seed region, at p<0.001 uncorrected. MPH>no-MPH (warm colours) and no-MPH>MPH (cool colours). Clusters that met cluster p<0.05, FWE corrected are listed in Table 1 and some of them are labelled here: FPC: fronto-polar cortex; ACC: anterior cingulate cortex; PCG: pre-central gyrus; PoCG: post-central gyrus; OC: occipital cortex; MTG: middle temporal gyrus; STC: superior temporal cortex; PoCiG: posterior cingulate gyrus; PHG: parahippocampal gyrus; Th: thalamus; MFG: medial frontal gyrus; SFG/MiFG: superior frontal gyrus/middle frontal gyrus; IPC: inferior parietal cortex. BOLD contrasts are superimposed on a T1 structural image in axial sections from z=−20 to z=64, in neurological orientation. The adjacent sections are 12 mm apart. The colour bar represents voxel T value.
Table 1.
Brain regions showing significant differences in seed-based functional connectivity between participants who received methylphenidate (MPH) and those who did not (no-MPH); two-sample t test, at voxel p<0.001 uncorrected and cluster-level p<0.05, FWE corrected or voxel p<0.05 FWE corrected
| MNI coordinates (mm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seed ROI | Contrast | MPH | no-MPH | Cluster size (mm3) |
z-score | X | Y | Z | Side | Identified region |
| Caudate | MPH>no-MPH | + | −^ | 2970 | 4.42 | −45 | −19 | 55 | L | Pre-central G |
| no-MPH>MPH | −^ | ++^ | 4023 | 4.49 | 30 | 53 | 34 | R | Fronto-polar C | |
| −^ | +^ | 4725 | 4.46 | −36 | −31 | 1 | L | Superior Temporal G | ||
| −^ | +^ | 4752 | 3.99 | 45 | 2 | −41 | R | Middle Temporal G | ||
| Pallidum | MPH>no-MPH | −^ | −^ | 4617* | 4.8 | 24 | −85 | −11 | R | Inferior Occipital G |
| −^ | −^ | 3.86 | 36 | −85 | −2 | R | Middle Occipital G | |||
| ++^ | − | 3780 | 4.54 | −54 | −10 | 46 | L | Pre-central G | ||
| ++^ | − | 3.53 | −48 | −25 | 49 | L | Post-central G | |||
| + | −^ | 2538 | 3.86 | −30 | −31 | 67 | L | Pre-central G | ||
| no-MPH>MPH | − | ++^ | 36612* | 5.05 | −15 | −49 | −41 | L | Cerebellum | |
| −^ | + | 4131 | 4.15 | 6 | −40 | 25 | R/L | Cingulate G | ||
| −^ | ++^ | 4752 | 1.07 | 12 | 59 | 13 | R | Medial frontal G | ||
| −^ | ++^ | 3.77 | 9 | 38 | 10 | R/L | Anterior cingulate G | |||
| Putamen | MPH>no-MPH | None significant | ||||||||
| no-MPH>MPH | − | + | 6426* | 4.59 | 6 | −40 | 22 | R/L | Posterior cingulate G | |
| −^ | + | 4.41 | 0 | −25 | 28 | R/L | Mid-cingulate G | |||
| +^ | ++^ | 34911 | 4.5 | −12 | −46 | −35 | L | Cerebellum | ||
| − | ++^ | 1782** | 4.9 | 66 | −46 | 34 | R | Supramarginal G | ||
| Thalamus | MPH>no-MPH | ++^ | −^ | 21600* | 5.31 | 27 | −37 | 61 | R | Post-central G |
| ++^ | −^ | 4.32 | 45 | −7 | 34 | R | Pre-central G | |||
| ++^ | −^ | 4.19 | 63 | −7 | 7 | R | Superior Temporal G | |||
| +^ | −^ | 6453* | 4.81 | 39 | −70 | −14 | R | Middle Occipital G | ||
| +^ | −^ | 3.94 | 24 | −58 | −17 | R | Cerebellum | |||
| +^ | −^ | 3.83 | 33 | −64 | −17 | R | Inferior Occipital G | |||
| ++^ | − | 3456* | 4.77 | −63 | −16 | 10 | L | Post-central G | ||
| ++^ | − | 15471 | 4.48 | −45 | −10 | 46 | L | Pre-central G | ||
| ++^ | − | 4.3 | −54 | −19 | 46 | L | Post-central G | |||
| ++^ | +^ | 3213 | 3.75 | 3 | −7 | 61 | R/L | Medial Frontal G | ||
| +^ | −^ | 2592** | 4.81 | 0 | −88 | 46 | R/L | Precuneus | ||
| ++^ | − | 2133** | 4.69 | −21 | −7 | −26 | L | Parahippocampal G | ||
| no-MPH>MPH | −^ | +^ | 54837* | 6.17 | 12 | −85 | −32 | R | Cerebellum | |
| −^ | +^ | 67068* | 5.77 | 39 | 29 | 55 | R | Middle Frontal G | ||
| −^ | +^ | 5.3 | 21 | 26 | 58 | R | Superior Frontal G | |||
| −^ | +^ | 5.22 | 51 | 23 | 43 | R | Middle Frontal G | |||
| −^ | + | 16740* | 5.74 | 57 | −58 | 46 | R | Inferior Parietal G | ||
| +^ | ++^ | 7695* | 5.36 | −6 | −13 | 4 | R/L | Thalamus | ||
| −^ | − | 7749* | 5.1 | −60 | −58 | 43 | L | Inferior Parietal G | ||
Also significant at peak p<0.05, FWE corrected
Only significant at peak p<0.05, FWE corrected; R- right, L- left; G- gyrus; +/++ positively/more positively connected and −/− negatively/more negatively connected by one-sample t-test, with the sign and magnitude of connectivity determined by the effect size of each cluster.
A superscript on +/− indicates significant at p<0.05 for one sample t-test of the effect size for the cluster.
Caudate
Both groups showed positive connectivity of the caudate nucleus with the medial frontal cortex including supplementary motor area (SMA) and pre-SMA, and anterior cingulate cortex, as well as the middle frontal cortex, orbitofrontal cortex, thalamus and basal ganglia. Both groups showed negative connectivity of the caudate with the precuneus, occipital cortices, hippocampus, parahippocampal gyri and cerebellum.
Methylphenidate reversed the negative connectivity between the caudate and left primary motor cortex (PMC) and positive connectivity between the caudate and frontal polar cortex as well as superior/middle temporal gyri, as observed for the no-MPH group (Fig. 2a; Table 1).
Pallidum
Both groups showed positive connectivity of the pallidum with the medial frontal cortex including supplementary motor area (SMA) and pre-SMA, and anterior cingulate cortex, as well as middle frontal cortex, thalamus, basal ganglia and insula. Both groups showed negative connectivity of the pallidum with the precuneus, occipital cortices and the posterior cingulate cortex.
Methylphenidate reversed the negative connectivity between the pallidum and left pre-central and post-central cortices, as observed in the no-MPH group. Methylphenidate reversed the positive connectivity between the pallidum and anterior and posterior cingulate cortices, cerebellum and medial prefrontal cortex. Methylphenidate also decreased negative connectivity of the pallidum with the occipital cortices (Fig. 2b; Table 1).
Putamen
Both groups showed positive connectivity of the putamen with the medial frontal cortex including supplementary motor area (SMA) and pre-SMA, and anterior cingulated cortex, as well as middle/inferior frontal cortices, thalamus, basal ganglia, superior temporal cortex and insula. Both groups showed negative connectivity of the putamen with the precuneus, occipital cortices, parahippocampal gyri and the posterior cingulate cortex.
Methylphenidate reversed the positive connectivity between the putamen and mid/posterior cingulate cortex and right supramarginal gyrus. Methylphenidate also decreased positive connectivity of the putamen to cerebellum (Fig. 2c; Table 1).
Thalamus
Both groups showed positive connectivity of the thalamus with the medial frontal cortex including supplementary motor area (SMA) and pre-SMA, and anterior cingulate cortex, as well as thalamus and basal ganglia. Both groups showed negative connectivity of the thalamus with the occipital and inferior temporal cortex.
Methylphenidate reversed the negative connectivity between the thalamus and a wide array of brain regions, including bilateral pre-central, post-central, and occipital cortices, as well as the superior temporal gyri, parahippocampal gyri and precuneus, as observed for the no-MPH group. Methylphenidate also reversed the positive connectivity between the thalamus and cerebellum, superior/middle frontal gyri and the inferior parietal cortex (Fig. 2d; Table 1).
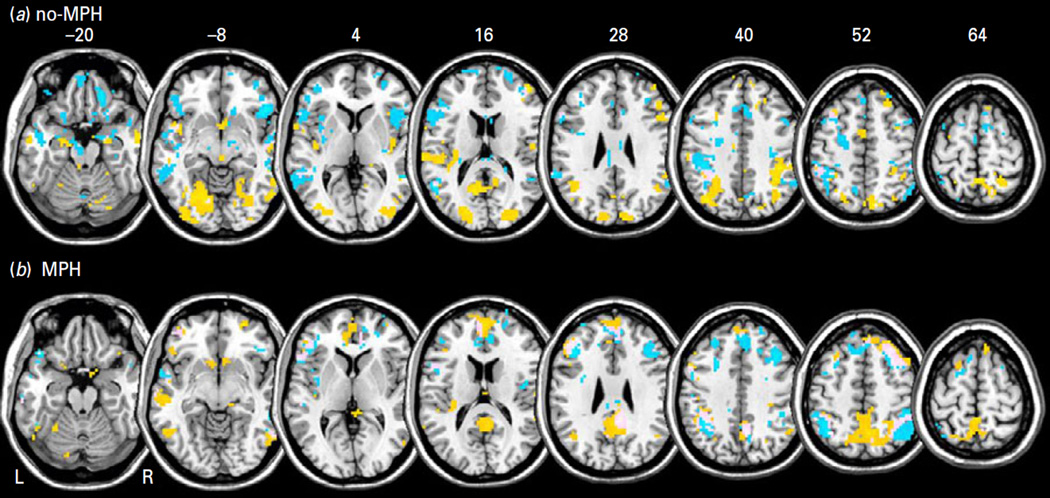
Global connectivity
The results of one-sample t tests for global connectivity are shown in Fig. 3. For both groups, we observed voxels with more positive connectivity in the dorsolateral prefrontal cortex, putamen, visual cortices, precuneus, cuneus and insula, and more negative connectivity with the supplementary motor area, midbrain, temporal cortices, insula, parietal cortices and occipital cortices. This aligns with previous findings of the inferior parietal cortex, inferior frontal cortex and cuneus as being the most connected across a large number of participants (Tomasi and Volkow, 2010).
Fig. 3.
One sample t-tests for global resting-state functional connectivity for (a) no-MPH and (b) MPH group showing the top 5% of voxels. Warm and cool colour shows positive and negative connectivity. BOLD contrasts are superimposed on a T1 structural image in axial sections from z=−20 to z=64, in neurological orientation. The adjacent sections are 12 mm apart.
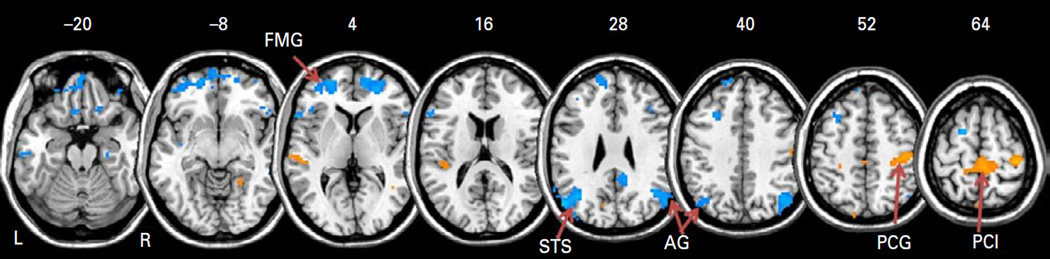
In two-sample t tests, the negative global connectivity of primary motor cortex and supplementary motor cortex decreased in MPH compared to no-MPH group (Fig. 4; Table 2). In contrast, medial prefrontal and parietal cortices showed more negative global connectivity in the MPH as compared to no-MPH group. We did not observe any significant differences in positive global connectivity between the two groups.
Fig. 4.
Two sample t-test shows differences in global resting-state functional connectivity at p<0.001, uncorrected: MPH>no-MPH (warm colours) and no-MPH>MPH (cool colours). BOLD contrasts are superimposed on a T1 structural image in axial sections from z=−20 to z=64, in neurological orientation. The adjacent sections are 12 mm apart. Clusters that met cluster p<0.05, FWE corrected are listed in Table 2 and labelled here: AG: angular gyrus; FMG: frontal marginal gyrus; PCG: pre-central gyrus; PCL: paracentral lobule; STS: superior temporal sulcus.
Table 2.
Summary of significant negative global connectivity differences between participants who received methylphenidate (MPH) and those who did not (no-MPH) at a combined threshold of voxel p<0.001 uncorrected and cluster-level p<0.05, FWE corrected
| MNI coordinates (mm) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Contrast | MPH | no-MPH | Cluster size (mm3) |
z-score | X | Y | Z | Side | Identified region |
| MPH>no-MPH | – | – | 11124 | 4.4 | 39 | −28 | 61 | R | Pre-central G |
| – | – | 4.24 | 9 | −25 | 76 | R | Paracentral lobule | ||
| no-MPH>MPH | – | – | 6210 | 4.53 | −57 | −67 | 22 | L | Superior temporal S |
| – | – | 4.39 | −54 | −73 | 31 | L | Angular G | ||
| – | – | 9990 | 4.33 | −21 | 53 | −2 | L | Frontal marginal G | |
| – | – | 3.96 | 24 | 47 | 4 | R | Middle frontal/anterior cingulate G |
||
| – | – | 5940 | 4 | 42 | −61 | 31 | R | Angular G | |
| – | – | 3.96 | 42 | −67 | 40 | R | Inferior parietal G | ||
| – | – | 3.89 | 54 | −70 | 25 | R | Middle temporal G | ||
R- right, L- left; G- gyrus; S- sulcus; −/− indicates significance of the negative connectivity.
Gender differences in connectivity and correlation with physiological variables
For both seed-based and global connectivity, we performed a full factorial analysis to include gender (32 females, 16 males) as a covariate, in order to examine gender main effects as well as group (MPH vs. no-MPH) by gender interactions. The results showed that, as expected, the group main effects were identical to what we reported. In addition, there were no significant regional brain activations for the gender main effects, at voxel p<0.001, uncorrected and cluster p<0.05 FWE corrected. For the group by gender interaction, there is a single cluster in the area of right superior temporal cortex, secondary somatosensory cortex and insula (x=48, y=−4, z=10, z = 3.93, cluster size=3132 mm3), which showed greater negative global connectivity in men than women with administration of methylphenidate (i.e. [MPH_Men – noMPH_Men]>[MPH_Women – noMPH_Women]).
We also explored correlations between the effect sizes of seed-based as well as global connectivity and percentage changes in SBP, HR and anxiety rating. As shown in Supplementary Table S1, there were few significant correlations at p<0.05, and none of these correlations were significant at a corrected p=0.05/90=0.00055 (with a total of 90 tests).
Discussion
Methylphenidate and thalamic/striatal connectivity to the primary motor cortex
With the exception of putamen, the thalamus/dorsal striatum showed negative resting state functional connectivity with the motor and somatosensory cortices, as observed in the no-MPH group and many previous studies of healthy participants (Baird et al., 2013; Erpelding et al., 2013; Nasrallah et al., 2013; Posner et al., 2013; Werner et al., 2013; Zhou et al., 2013). Methylphenidate alters the functional connectivity from negative to positive between the thalamus/dorsal striatum and somatomotor cortices. Methylphenidate also decreases negative global connectivities of the motor cortex and paracentral lobules. Thus, overall, methylphenidate enhances somatomotor functional connectivity to the thalamus and striatum, in accord with previous studies where levodopa and haloperidol each increased and decreased resting-state and task-related functional connectivity between the motor cortex and striatum in healthy participants (Tost et al., 2010; Cole et al., 2013).
These findings are also consistent with reported effects of methylphenidate and other catecholaminergic agents on motor performance. For instance, methylphenidate increased locomotor activity in mice (Penner et al., 2001). A single dose of methylphenidate improved motor coordination in children with developmental coordination disorder and ADHD (Bart et al., 2013), perhaps compensating for impaired integrity of the white matter connecting the thalamus with primary motor cortex and hippocampus (Xia et al., 2012).
Patients with Parkinson’s disease (PD) demonstrate altered cortical and subcortical activation and functional connectivity (Eidelberg et al., 1994; Huang et al., 2007; Ma and Eidelberg, 2007). Low doses of methylphenidate improved gait and voluntary movement (Auriel et al., 2006; Devos et al., 2007; Kwak et al., 2010), and along with levodopa improved performance on complex hand movements (Nutt et al., 2004) in patients with PD. In an earlier study, adding methylphenidate to levodopa treatment increased peak hand tapping speed in patients with PD compared to levodopa alone (Camicioli et al., 2001). Pridopidine, a dopamine-stabilizing compound, improved motor performance in patients with Huntington’s disease (Investigators, 2013), who showed decreased white matter integrity of the caudate, putamen and primary motor cortex in progression with their motor symptoms (Bohanna et al., 2011). Together, these studies suggested that patients with clinical conditions that implicate catecholaminergic dysfunction show altered motor cortical connectivity and performance that can be ameliorated by methylphenidate.
Methylphenidate and thalamic/striatal connectivity to the hippocampus and amygdala
Methylphenidate increased connectivity between the thalamus and hippocampus, amygdala and visual areas, in addition to the primary motor cortex. The thalamus, amygdala and hippocampus all receive direct noradrenergic projections from the locus coeruleus (Ishikawa and Tanaka, 1977; Talley et al., 1996; Glass et al., 2001), a circuitry known to promote wakefulness and arousal (McBride and Sutin, 1976; McCormick et al., 1991). The thalamus plays a critical role in the detection of, filtering and reorientation to salient stimuli (Petersen et al., 1985; Robinson and Petersen, 1992; Saalmann et al., 2012), and, through projections to the hippocampus, facilitates learning and memory of salient information (Grieve et al., 2000; Casanova et al., 2001).
Methylphenidate increased metabolism/activity in thalamus and hippocampus (Glavin, 1985), and improved working memory (Ramasubbu et al., 2012) as well as decision-making (Schlosser et al., 2009) in healthy adults and/or children with ADHD (Bedard et al., 2007; Bedard and Tannock, 2008; Strand et al., 2012). In rodents, methylphenidate increased noradrenergic metabolism in the thalamus and amygdala (Glavin, 1985) and facilitated spatial memory (Guo et al., 2012) and cue-reward learning (Ferry et al., 1999; Tye et al., 2010). Thalamus showed stronger resting-state connectivity to the amygdala in association with increased autonomic activity and physiological arousal in healthy men (Hermans et al., 2011; Chang et al., 2013). Norepinephrine, which surges during arousal, promotes long-term potentiation at thalamo-amygdalar synapses (Tully et al., 2007), and influences affective (Li and Kirouac, 2008), reward and saliency processing (Baxter and Murray, 2002; Etkin et al., 2006; Murray, 2007; Haber and Knutson, 2010; Linke et al., 2010). Thus, the current findings may provide a neural basis in evaluating this earlier body of work.
Methylphenidate and thalamic/striatal connectivity to regions of executive control
In resting state, the thalamus/dorsal striatum showed positive functional connectivity with many brain regions instrumental to executive control, such as the superior/ middle frontal cortex and medial prefrontal cortex including the SMA, pre-SMA and dorsal anterior cingulate cortex and inferior parietal cortex, as observed in the no-MPH group and many previous studies of healthy participants (Baird et al., 2013; Erpelding et al., 2013; Nasrallah et al., 2013; Posner et al., 2013; Werner et al., 2013; Zhou et al., 2013). In contrast to its effects on motor cortical connectivity, methylphenidate decreases the positive thalamic/striatal connectivity to the frontopolar cortex and some fronto-parietal control regions or alters the connectivity from positive to negative with these brain regions. This is consistent with an earlier work where sulpiride, a dopamine antagonist, enhanced striato-thalamic activity to the dorsolateral prefrontal cortex, while methylphenidate appeared to produce the opposite effects (Honey et al., 2003). Furthermore, methylphenidate increased negative global connectivity of the fronto-parietal cortices. These findings in healthy adults are in contrast with many previous studies of clinical populations, where methylphenidate increased regional activations and connectivities in association with executive functioning (Scheres et al., 2003; Kim et al., 2006; Jonkman et al., 2007; Pollak et al., 2007; Li et al., 2010; Tye et al., 2010; Nandam et al., 2011; Tomasi et al., 2011). For instance, methylphenidate improved working memory and visuospatial attention in patients with traumatic brain injury (Kim et al., 2006), and increased prefrontal activations for cognitive control in cocaine-addicted adults (Li et al., 2010). Although speculative, this contrasting pattern of the effects of methylphe-nidate may reflect the inverted U relationship between level of catecholaminergic signalling and cognitive performance, as postulated earlier (Birnbaum et al., 1999; Arnsten, 2009; Berridge et al., 2012; Rajala et al., 2012). That is, while methylphenidate facilitates cognitive performance in clinical populations who are compromised in catecholaminergic neurotransmission, it dampens performance in healthy adults by increasing catecholamines past the optimal level, as has been observed in dosaging studies of methylphenidate in rodents, non-human primates and humans (Sagvolden et al., 1988; Tannock et al., 1989; Elliott et al., 1997; Rajala et al., 2012).
Conclusions and limitations of the study
To summarize, methylphenidate enhances resting-state functional connectivity of the striatum/thalamus with primary motor cortex and increases negative connectivity with frontal executive regions. Augmented motor cortical connectivity is consistent with the effects of methylphenidate and other catecholaminergic agents in improving motor functions in healthy participants and various clinical populations. Methylphenidate also increases thalamic/striatal connectivity to the hippocampus and amygdala, which may speak to its alerting and memory-enhancing effects. We also speculate that the findings of methylphenidate-elicited decrease in striatal/ thalamic connectivity to prefrontal regions may have to do with individual variation in catecholaminergic signals for optimal cognitive functioning. Together, the influences of methylphenidate on cerebral functioning are multifaceted, an issue that deserves consideration in studies of its use and misuse.
There are a few important limitations to this study. First and most significantly, we did not have a placebo control for the individuals who received methylphenidate. The placebo effect is thus a potential confound for the differences that we observed between the methylphenidate and no-methylphenidate group. Additionally, we did not collect blood samples and assay plasma levels of methylphenidate to control for individual differences in pharmacokinetics. Secondly, methylphenidate influences both dopaminergic and noradrenergic neurotransmission. While there is heavy dopaminergic innervation of the basal ganglia circuitry, the cortical mantle receives both dopaminergic and noradrenergic inputs. Thus, it remains to be determined whether and how blockade of dopaminergic and/or noradrenergic transporters by methylphenidate accounts for the current findings. Furthermore, although the seed regions do not overlap spatially, they are functionally connected. It remains to be examined in future studies whether and how shared and distinct thalamic and striatal connectivities relate to cognitive and affective functions, as influenced by methylphenidate. It is also to be noted that we evaluated global connectivity to the 116 AAL masks and a voxel-wise analysis may reveal a finer pattern of connectivities as influenced by methylphenidate. Similarly, a top percentage threshold limits our analysis to those brain regions that are most connected. It remains to be evaluated whether and how other brain regions are altered in global connectivity. Third, our participants are not assessed for cognitive or motor performance; thus, the functional implications of the current findings need to be re-considered in future work. Fourth, this study involved only healthy adult participants. Thus, the implications of the current results cannot be generalized to patient populations or older adults (Hu et al., 2012, 2013). Fifth, stimulants can potentially influence fMRI blood oxygenation level-dependent (BOLD) signals, which depend on the haemodynamic coupling of neuronal activities and local changes in blood flow and oxygenation. However, a number of earlier studies have suggested that stimulants decreased cortical cerebral blood flow but did not obscure BOLD signals (Gollub et al., 1998; Rao et al., 2000), and that haemodynamic responses were faithfully followed by neuronal responses after their peak effects (at 6 min after administration) on blood flow and volume (Devonshire et al., 2004). Heart rate also had no effect on BOLD signals in one of these studies (Rao et al., 2000) and neither changes in heart rate or blood pressure was correlated to changes in functional connectivities (Supplementary Table 1). Nevertheless, we acknowledge that these physiological variables could potentially confound imaging findings and need to be considered in future experiments that properly quantify these changes in a placebo-controlled setting. Finally, we wish to consider a methodological issue regarding the findings on negative functional connectivity, which has been reported since the very beginning of the resting-state fMRI studies (Biswal et al., 1995). Negative functional connectivity, or anti-correlation, represents negative cross-correlation in spontaneous BOLD signal between two brain regions. It was suggested that global signal regression, a common step of data pre-processing in seed-based connectivity analyses, is a likely cause of anti-correlated functional networks (Murphy et al., 2009; Weissenbacher et al., 2009). However, recent investigations demonstrated that the negative correlations are not an artifact but have biological origins (Fox et al., 2009; Chen et al., 2011; Chai et al., 2012). For instance, negative functional connectivity is associated predominantly with long-range connections and correlates with the shortest path length in the human brain network (Scholvinck et al., 2010; Chen et al., 2011; Schwarz and McGonigle, 2011). Indeed, the negative correlations between brain regions with presumably opposing functional roles have been observed in many different studies (Greicius et al., 2003; Fox et al., 2005; Fransson, 2005; Kelly et al., 2008; Uddin et al., 2009; Chen et al., 2011), including those using independent component analysis, which does not involve global signal regression (Cole et al., 2010a; Zuo et al., 2010; Zhang and Li, 2012c). Furthermore, the existence of negative functional connectivity was also suggested by computational simulations of cerebral network activities in both monkeys and humans (Honey et al., 2007; Izhikevich and Edelman, 2008; Deco et al., 2009) and supported by simultaneous recording of unit activity and local field potentials from task-positive and task-negative (default mode) networks in cats (Popa et al., 2009). Together, these earlier studies suggest functional significance of negative functional connectivity. On the other hand, future work that combines BOLD signal acquisition and electrophysiological recording of neuronal activities is needed to fully understand the effects of the methylphenidate on positive vs. negative functional connectivities (Goense and Logothetis, 2008).
Supplementary Material
Acknowledgments
This study was part of Olivia Farr’s thesis work for the Interdepartmental Neuroscience Program at Yale University and was supported by NIH grants T32 NS07224 (Crair), R01DA023248 (Li), R21AA018004 (Li), K02DA026990 (Li), R01AA021449 (Li), K24 DA017899 (Malison), P20DA027844 (Potenza) and a NARSAD Young Investigator Award (Li). We also thank the staff at the Connecticut Mental Health Center for assistance in medical evaluation of the participants and the Connecticut Department of Mental Health and Addiction Services (DMHAS) for their support. We thank Dr Amy Arnsten for her input to this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Drug Abuse, National Center for Research Resources or the National Institutes of Health.
Footnotes
Supplementary material
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S1461145714000674.
Statement of Interest
None
References
- An L, Cao XH, Cao QJ, Sun L, Yang L, Zou QH, Katya R, Zang YF, Wang YF. Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology. 2013;38:1287–1295. doi: 10.1038/npp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29:15–17. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, Margulies DS. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J Neurosci. 2013;33:16657–16665. doi: 10.1523/JNEUROSCI.0786-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart O, Daniel L, Dan O, Bar-Haim Y. Influence of methylphenidate on motor performance and attention in children with developmental coordination disorder and attention deficit hyperactive disorder. Res Dev Disabil. 2013;34:1922–1927. doi: 10.1016/j.ridd.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Tannock R. Anxiety, methylphenidate response, and working memory in children with ADHD. J Atten Disord. 2008;11:546–557. doi: 10.1177/1087054707311213. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Jain U, Johnson SH, Tannock R. Effects of methylphenidate on working memory components: influence of measurement. J Child Psychol Psychiatry. 2007;48:872–880. doi: 10.1111/j.1469-7610.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Morelli M, editors. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. Amsterdam: Elsevier; 2005. [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, Waterhouse BD. Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic alpha(1) - and alpha(2)-receptors. Biol Psychiatry. 2012;71:467–473. doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW., Jr A Monte Carlo investigation of the Fisher Z transformation for normal and nonnormal distributions. Psychol Rep. 2000;87:1101–1114. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bohanna I, Georgiou-Karistianis N, Sritharan A, Asadi H, Johnston L, Churchyard A, Egan G. Diffusion tensor imaging in Huntington’s disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav. 2011;5:171–180. doi: 10.1007/s11682-011-9121-8. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Johnstone SJ, Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Lawrence CA. The effect of methylphe-nidate on response inhibition and the event-related potential of children with attention deficit/hyperactivity disorder. Int J Psychophysiol. 2005;58:47–58. doi: 10.1016/j.ijpsycho.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/ hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Lea E, Nutt JG, Sexton G, Oken BS. Methylphenidate increases the motor effects of L-Dopa in Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2001;24:208–213. doi: 10.1097/00002826-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Casanova C, Merabet L, Desautels A, Minville K. Higher-order motion processing in the pulvinar. Prog Brain Res. 2001;134:71–82. doi: 10.1016/s0079-6123(01)34006-2. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen G, Xie C, Li SJ. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connectivity. 2011;1:195–206. doi: 10.1089/brain.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010a;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. NeuroImage. 2010b;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Oei NY, Both S, van Gerven JM, Rombouts SA. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. NeuroImage. 2013;78:59–67. doi: 10.1016/j.neuroimage.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Meier TL, Collins RL, Watson JB. Repeated methylphenidate treatment induces behavioral sensitization and decreases protein kinase A and dopamine-stimulated adenylyl cyclase activity in the dorsal striatum. Psychopharmacology (Berl) 1998;136:34–43. doi: 10.1007/s002130050536. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa V, McIntosh AR, Sporns O, Kotter R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci USA. 2009;106:10302–10307. doi: 10.1073/pnas.0901831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Saucier G. Disappearance of the locus coeruleus in the rat after intraventricular 6-hydroxdopamine. Brain Res. 1972;37:310–316. doi: 10.1016/0006-8993(72)90676-2. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Berridge CW. Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J Pharmacol Exp Ther. 2006;319:1327–1335. doi: 10.1124/jpet.106.110015. [DOI] [PubMed] [Google Scholar]
- Devonshire IM, Berwick J, Jones M, Martindale J, Johnston D, Overton PG, Mayhew JE. Haemodynamic responses to sensory stimulation are enhanced following acute cocaine administration. Neuroimage. 2004;22:1744–1753. doi: 10.1016/j.neuroimage.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Devos D, Krystkowiak P, Clement F, Dujardin K, Cottencin O, Waucquier N, Ajebbar K, Thielemans B, Kroumova M, Duhamel A, Destee A, Bordet R, Defebvre L. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Ahissar E. When outgoing and incoming signals meet: new insights from the zona incerta. Neuron. 2007;56:578–579. doi: 10.1016/j.neuron.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S, Fahn S. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- Erpelding N, Sava S, Simons LE, Lebel A, Serrano P, Becerra L, Borsook D. Habenula functional resting-state connectivity in pediatric CRPS. J Neurophysiol. 2013;111(2):239–47. doi: 10.1152/jn.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Hu S, Matuskey D, Zhang S, Abdelghany O, Li CS. The effects of methylphenidate on cerebral activations to Salient Stimuli in healthy adults. Exp Clin Psychopharmacol. 2013;22(2):154–65. doi: 10.1037/a0034465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biol Psychiatry. 1999;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multi-variate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Aicher SA, Milner TA, Pickel VM. Subcellular localization of alpha-2A–adrenergic receptors in the rat medial nucleus tractus solitarius: regional targeting and relationship with catecholamine neurons. J Comp Neurol. 2001;433:193–207. doi: 10.1002/cne.1135. [DOI] [PubMed] [Google Scholar]
- Glavin GB. Methylphenidate effects on activity-stress gastric lesions and regional brain noradrenaline metabolism in rats. Pharmacol Biochem Behav. 1985;23:379–383. doi: 10.1016/0091-3057(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–40. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, Makris N, Guimaraes A, Riorden J, Campbell T, Foley M, Hyman SE, Rosen B, Weisskoff R. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab. 1998;18:724–734. doi: 10.1097/00004647-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve KL, Acuna C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends Neurosci. 2000;23:35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs-roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Guo T, Yang C, Guo L, Liu K. A comparative study of the effects of ABT-418 and methylphenidate on spatial memory in an animal model of ADHD. Neurosci Lett. 2012;528:11–15. doi: 10.1016/j.neulet.2012.08.068. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. NeuroImage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hendrick OM, Ide JS, Luo X, Li CS. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PLoS ONE. 2010;5:e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernandez G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SC, Brown J, Bullmore ET. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chao HH, Winkler AD, Li CS. The effects of age on cerebral activations: internally vs. externally driven processes. Front Aging Neurosci. 2012;4:4. doi: 10.3389/fnagi.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chao HH, Zhang S, Ide JS, Li CS. Changes in cerebral morphometry and amplitude of low-frequency fluctuations of BOLD signals during healthy aging: correlation with inhibitory control. Brain Stru & Func. 2013 doi: 10.1007/s00429-013-0548-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, Dhawan V, Eidelberg D. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. NeuroImage. 2011a;54:455–464. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS. Error-related functional connectivity of the habenula in humans. Front Hum Neurosci. 2011b;5:25. doi: 10.3389/fnhum.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators HSGH. A randomized, double-blind, placebo-controlled trial of pridopidine in Huntington’s disease. Mov Disord. 2013;28:1407–1415. doi: 10.1002/mds.25362. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Tanaka C. Morphological organization of catecholamine terminals in the diencephalon of the rhesus monkey. Brain Res. 1977;119:43–55. doi: 10.1016/0006-8993(77)90090-7. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proc Natl Acad Sci USA. 2008;105:3593–3598. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral analysis and its applications. San Francisco, CA: Holden-Day; 1968. [Google Scholar]
- Jonkman LM, van Melis JJ, Kemner C, Markus CR. Methylphenidate improves deficient error evaluation in children with ADHD: an event-related brain potential study. Biol Psychol. 2007;76:217–229. doi: 10.1016/j.biopsycho.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim YH, Ko MH, Na SY, Park SH, Kim KW. Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: a double-blind placebo-controlled study. Clin Rehabil. 2006;20:24–30. doi: 10.1191/0269215506cr927oa. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Muller ML, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson’s disease. J Neurophysiol. 2010;103:942–949. doi: 10.1152/jn.00197.2009. [DOI] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JL, Rounsaville BJ, Ding YS, Malison RT. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci USA. 2010;107:14455–14459. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Linke J, Kirsch P, King AV, Gass A, Hennerici MG, Bongers A, Wessa M. Motivational orientation modulates the neural response to reward. NeuroImage. 2010;49:2618–2625. doi: 10.1016/j.neuroimage.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Ma Y, Eidelberg D. Functional imaging of cerebral blood flow and glucose metabolism in Parkinson’s disease and Huntington’s disease. Mol Imaging Biol. 2007;9:223–233. doi: 10.1007/s11307-007-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride RL, Sutin J. Projections of the locus coeruleus and adjacent pontine tegmentum in the cat. J Comp Neurol. 1976;165:265–284. doi: 10.1002/cne.901650302. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: a [(11)C] raclopride PET study. NeuroImage. 2006;33:907–912. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, Cummins TD, Garner K, Dean AJ, Kim BN, Nathan PJ, Mattingley JB, Bellgrove MA. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry. 2011;69:902–904. doi: 10.1016/j.biopsych.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Nasrallah FA, Tay HC, Chuang KH. Detection of functional connectivity in the resting mouse brain. NeuroImage. 2013;86:417–24. doi: 10.1016/j.neuroimage.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Carter JH, Sexton GJ. The dopamine transporter: importance in Parkinson’s disease. Ann Neurol. 2004;55:766–773. doi: 10.1002/ana.20089. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–2512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3:606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Penner MR, McFadyen MP, Carrey N, Brown RE. Effects of chronic and acute methylphenidate hydrochloride (Ritalin) administration on locomotor activity, ultrasonic vocalizations, and neuromotor development in 3- to 11-day-old CD-1 mouse pups. Dev Psychobiol. 2001;39:216–228. doi: 10.1002/dev.1047. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes AP, Friston K, editors. Random-effects analysis. London: Academic Press; 2004. [Google Scholar]
- Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. NeuroImage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Pollak L, Dobronevsky Y, Prohorov T, Bahunker S, Rabey JM. Low dose methylphenidate improves freezing in advanced Parkinson’s disease during off-state. J Neural Transm Suppl. 2007;72:145–148. doi: 10.1007/978-3-211-73574-9_17. [DOI] [PubMed] [Google Scholar]
- Popa D, Popescu AT, Pare D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2013;35(6):2852–60. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala AZ, Henriques JB, Populin LC. Dissociative effects of methylphenidate in nonhuman primates: trade-offs between cognitive and behavioral performance. J Cogn Neurosci. 2012;24:1371–1381. doi: 10.1162/jocn_a_00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Singh H, Zhu H, Dunn JF. Methylphenidate-mediated reduction in prefrontal hemodynamic responses to working memory task: a functional near-infrared spectroscopy study. Hum Psychopharmacol. 2012;27:615–621. doi: 10.1002/hup.2258. [DOI] [PubMed] [Google Scholar]
- Rao SM, Salmeron BJ, Durgerian S, Janowiak JA, Fischer M, Risinger RC, Conant LL, Stein EA. Effects of methylphenidate on functional MRI blood-oxygen-level-dependent contrast. Am J Psychiatry. 2000;157:1697–1699. doi: 10.1176/appi.ajp.157.10.1697. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. NeuroImage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Slatta K, Arntzen E. Low doses of methylphenidate (Ritalin) may alter the delay-of-reinforcement gradient. Psychopharmacology (Berl) 1988;95:303–312. doi: 10.1007/BF00181938. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, Sergeant JA. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Schlosser RG, Nenadic I, Wagner G, Zysset S, Koch K, Sauer H. Dopaminergic modulation of brain systems subserving decision making under uncertainty: a study with fMRI and methylphenidate challenge. Synapse. 2009;63:429–442. doi: 10.1002/syn.20621. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, McGonigle J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage. 2011;55:1132–1146. doi: 10.1016/j.neuroimage.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Spencer RC, Klein RM, Berridge CW. Psychostimulants act within the prefrontal cortex to improve cognitive function. Biol Psychiatry. 2012;72:221–227. doi: 10.1016/j.biopsych.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand MT, Hawk LW, Jr, Bubnik M, Shiels K, Pelham WE, Jr, Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. J Abnorm Child Psychol. 2012;40:1193–1207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of alpha 2A–adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci USA. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. NeuroImage. 2011;54:3101–3110. doi: 10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat Neurosci. 2010;13:920–922. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci USA. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Tye LD, Cone JJ, Hekkelman EF, Janak PH, Bonci A. Methylphenidate facilitates learning-induced amygdala plasticity. Nat Neurosci. 2010;13:475–481. doi: 10.1038/nn.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Deschenes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlosser RG. The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44:2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Werner CJ, Dogan I, Sass C, Mirzazade S, Schiefer J, Shah NJ, Schulz JB, Reetz K. Altered resting-state connectivity in Huntington’s Disease. Hum Brain Mapp. 2013;35(6):2852–93. doi: 10.1002/hbm.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Li X, Kimball AE, Kelly MS, Lesser I, Branch C. Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;204:161–167. doi: 10.1016/j.pscychresns.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, Jin Z, Weng XC, Zhang L, Zeng YW, Yang L, Wang YF, Seidman LJ, Faraone SV. Functional MRI in attention-deficit hyperactivity disorder: evidence for hypofrontality. Brain Dev. 2005;27:544–550. doi: 10.1016/j.braindev.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage. 2012a;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Task-related, low-frequency task-residual., and resting state activity in the default mode network brain regions. Front Psychol. 2012b;3:172. doi: 10.3389/fpsyg.2012.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp. 2012c;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22:99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Liu Y, Zhang Z, An N, Yao H, Wang P, Wang L, Zhang X, Jiang T. Impaired functional connectivity of the thalamus in Alzheimer’s disease and mild cognitive impairment: a resting-state FMRI study. Curr Alzheimer Res. 2013;10:754–766. doi: 10.2174/15672050113109990146. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gao B, Hua J, Liu W, Deng Y, Zhang L, Jiang B, Zang Y. Effects of methylphenidate on resting-state brain activity in normal adults: an fMRI study. Neurosci Bull. 2013;29:16–27. doi: 10.1007/s12264-013-1306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.