Abstract
Importance
Injection drug use is the primary mode of transmission for hepatitis C virus (HCV) infection. Prior studies suggest opioid agonist therapy may reduce incidence of HCV among people who inject drugs, however, little is known about its effects in younger injectors.
Objective
To evaluate whether opioid agonist therapy was associated with a lower incidence of HCV in a cohort of young adult injectors.
Design
Observational cohort study conducted January 2000 through August 2013 with quarterly interviews and blood samples.
Setting
San Francisco community outreach.
Participants
Young adult injectors (<30 years old) who were anti-HCV negative.
Exposure(s)
Recent (within past 3 months) substance use treatment: no treatment, non-opioid agonist forms of treatment, or opioid agonist therapy (methadone or buprenorphine) detoxification or maintenance.
Main Outcome(s) and Measure(s)
Incident HCV infection documented with a new positive HCV RNA result and/or a positive anti-HCV result. Cumulative HCV incidence rates and 95% confidence intervals were calculated assuming a Poisson distribution. Cox Proportional Hazards models were fit adjusting for age, gender, race, years of injection drug use, homelessness and incarceration.
Results
Baseline characteristics of the sample (n=552) were: median age 23 (IQR: 20–26), 32% female, 73% Caucasian, 40% did not graduate high school, and 69% were homeless. Over the observation period of 680 person-years (py), there were 171 incident cases of HCV (incidence rate=25.1/100 py; 95% CI: 21.6–29.2). The rate ratio was significantly lower for participants who reported recent maintenance opioid agonist therapy (0.31; 95% CI: 0.14–0.65), but not for those who reported recent non-opioid agonist forms of treatment (0.63; 95% CI: 0.37–1.08), or opioid agonist detoxification (1.45; 95% CI: 0.80–2.69). After adjustment for other covariates, maintenance opioid agonist therapy was associated with lower relative hazards for becoming infected with HCV over time (AHR=0.39; 95% CI: 0.18–0.87).
Conclusions and Relevance
In this cohort of young adults who inject drugs, recent maintenance opioid agonist therapy was associated with lower incidence of HCV. Maintenance treatment with methadone or buprenorphine for opioid use disorders may be an important strategy to prevent spread of HCV among young injectors.
Keywords: HCV, PWID, injection drug use, methadone, buprenorphine
Background
Injection drug use is the primary mode of transmission for hepatitis C virus (HCV) infection,1, 2 accounting for at least half of all documented new infections, a figure which is likely to be a significant underestimate.1, 3, 4 HCV is endemic among persons who inject drugs, with estimates of prevalence ranging 60–90%.5 Although newer medications for HCV offer potential for cure with fewer side effects, treatment will come at great financial cost. Furthermore, major barriers to HCV treatment for individuals who inject drugs exist and will not be easily overcome.6 Therefore, there is still a critical need for interventions that can prevent new HCV infections in this group. Providing maintenance opioid agonist therapy with methadone or buprenorphine for opioid use disorders is one strategy for reducing injection drug use and the spread of HCV. Maintenance opioid agonist therapy may facilitate injection cessation and thus reduce risk for HCV acquisition,7 however, treatment adherence can fluctuate among injectors, and not all treatment programs require complete abstinence.8 Two studies, a meta-analysis and pooled analysis study reported reduced HCV incidence in association with opioid agonist therapy, with reductions ranging from 40% to 60%.8, 9 However, the studies in those two analyses were conducted in populations of older patients and prisoners, and they predated the approval of buprenorphine for the treatment of opioid dependence in 2002.
Little is known about the effects of opioid agonist therapy in preventing HCV in younger persons who inject drugs, and those treated in the era of buprenorphine. Younger injectors are an important population to target as they are at the core of the HCV epidemic.10–15 Incidence rates are highest among new injectors, among which it is estimated that a quarter will become infected after two years of injecting.16 Despite the fact that younger injectors are a group at high risk for complications such as HCV and HIV, studies suggest that access to treatment for substance use disorders, especially methadone maintenance therapy, in young persons is limited.17 However, from 2002 to 2007, total numbers of buprenorphine prescriptions have increased from approximately 50,000 to 5.7 million, and young adults aged 21–30 are the age group most frequently receiving prescriptions.18 People seeking opioid agonist therapy for opioid use disorders may prefer treatment with buprenorphine over methadone,19 as it can be prescribed by non-specialist physicians in office-based settings, dispensed by wide networks of community pharmacies, and self-administered without daily observed dosing by treatment program staff. However, with less supervision, buprenorphine patients also have opportunities to interrupt their treatment to engage in illicit opioid use. For these reasons, studies that include young injectors, especially those treated with buprenorphine, are needed to determine the current effectiveness of maintenance opioid agonist therapy in reducing new HCV infections in real-world settings.
The purpose of this study was to assess whether opioid agonist therapy was associated with a lower incidence of HCV in an observational cohort of young adults who inject drugs in San Francisco. Participants in this prospective cohort underwent systematic testing for HCV and reported information about substance use treatment every 3 months, providing a unique opportunity to study the relationship between opioid agonist therapy and HCV incidence. We hypothesized that self-reported treatment with maintenance opioid agonist therapy (either methadone or buprenorphine) would be associated with lower incidence of HCV.
Methods and Materials
Study Sample and Design
This study analysis used observational data from the UFO Study, a prospective study of young adult injectors in San Francisco designed to assess factors associated with incident HCV infection. Details of its study design and methods have been published previously.7, 14 In brief, participants were eligible if they were under age 30, reported injecting drugs in the prior month, spoke English as their primary language, and if recruited in 2003 or later, did not plan to travel outside of San Francisco within the next three months as high rates of travel complicating follow-up were noted in the early period of recruitment.7 The UFO Study recruited, screened, and enrolled eligible injectors (negative for HCV antibody (anti-HCV) or HCV RNA at baseline screening) for participation in prospective follow-up over three separate waves, beginning in January 2000, February 2003, and May 2010, respectively, through September 2012. Intermittent pauses in enrollment occurred in 2002, 2005, and 2009 due to funding lapses. In all waves, HCV testing and behavioral questionnaires were administered quarterly among HCV negative participants. The behavioral questionnaire, administered at quarterly intervals during follow-up, queried participants regarding demographic factors, risk exposures (for example, types of drugs used, frequency of injection, and injection equipment sharing), and preventive behaviors (for example, use of syringe exchange programs, and condom use). Although some questions were modified slightly over the different waves, the focus was on quantitative assessment of exposures associated with injection drug use, HCV and HIV infections throughout all time periods.
The study conducted active outreach with participants using contact information that was updated at each follow-up visit, including phone, e-mail, social and familial contacts as well as street-based neighborhood searches where participants indicated they usually stayed. Since 2007, the study followed participants (including collection of serology and exposure information) who were incarcerated in the San Francisco jail if their stay was over 30 days. Follow-up consisted of monthly “check-ins” and quarterly study visits that included structured interviews and blood testing conducted at a community-based clinical research site located in the Tenderloin area of San Francisco for the past 9 years (sites were located in other neighborhoods, including the Mission, the Polk and Haight Ashbury districts prior to 2005). The study provided all participants with HIV and HCV prevention counseling, access to sterile injection equipment, and referrals as needed or requested for medical care, substance use treatment, and HCV care if new infections were detected. For this study, the sample was restricted to participants who were anti-HCV negative at enrollment and who had two or more study follow-up visits.
Study Outcomes
The primary outcome was incident HCV infection. Incident HCV was defined as: (1) a new positive HCV RNA and/or anti-HCV test result following a previously documented anti-HCV negative test; or (2) a positive HCV RNA test concomitant with a negative anti-HCV test, which was considered an incident acute HCV infection. Quarterly HCV testing included anti-HCV testing by enzyme immunoassay (EIA) (HCV EIA 2.0, Abbott Laboratories, Abbott Park, IL, or EIA-3, Ortho Clinical Diagnostics, Raritan NJ, and HCV RIBA™ 3.0 Test System (Novartis Vaccines & Diagnostics, Emeryville, CA.), as well as HCV RNA testing using transcription mediated amplification (TMA) technique (dHCV TMA assay component of the Procleix HIV-1/HCV assay, Gen-Probe Inc., San Diego, CA) to detect early HCV infection.14, 20
Study Predictors
The primary predictor of interest was receipt of treatment for an opioid use disorder, based on subject self-report from quarterly interviews. We categorized recent treatment responses into: no treatment, non-opioid agonist forms of treatment, opioid agonist detoxification, and maintenance opioid agonist therapy. Non-opioid agonist forms of treatment could include any non-medication assisted treatment such as 12-step groups, counseling and alternative treatment (acupuncture, etc.). Recent opioid agonist therapy was defined as treatment with either buprenorphine or methadone anytime within the past year at baseline screening interview, past 3 months at quarterly interviews in waves 1 and 3, and the past week for wave 2 participants (the time frame of the questions asked regarding opioid agonist therapy in wave 2 was shortened as the study added questions examining very recent behaviors associated with drug treatment program attendance). Participants who reported receiving multiple categories of treatment were classified hierarchically, such that if both maintenance and detoxification were reported, we classified as maintenance. If both detoxification and non-opioid agonist forms of treatment were reported, we classified as detoxification. For waves 1 and 3 of the study, participants were asked specifically about use of opioid agonist therapy for “detoxification” versus “maintenance” treatment. For wave 2, open-ended responses describing the type of substance use treatment were grouped into non-opioid agonist treatment, maintenance opioid agonist therapy, and opioid agonist detoxification; participants in wave 2 who responded that they were treated with opioid agonist therapy but did not specify further were labeled as maintenance. For this reason (and for the differing time frame for the question for wave 2), we conducted additional exploratory analyses restricting data from waves 1 and 3 only. We adjusted for the following covariates (which were selected a priori) in multivariate models: age at baseline, gender, non-white race, baseline number of years of injection drug use, homelessness and incarceration within the previous three months.
Statistical Analysis
Baseline characteristics of the sample were assessed using simple tabulations and calculation of measures of central tendency (means and medians) and statistical dispersion (standard deviation (SD) and interquartile range (IQR)). Cumulative HCV incidence rates by treatment status (no treatment, non-opioid agonist forms of treatment, opioid agonist detoxification, maintenance opioid agonist therapy) were calculated using person-time of observation, and 95% confidence intervals for the rates were calculated assuming a Poisson distribution. Treatment reported at the time of the last HCV-negative blood sampling was used for nonseroconverters; treatment reported at the time of the first HCV-positive blood sampling was used for seroconverters. Occurrence dates of infection were imputed as the midpoint of the interval between the dates of the last observed HCV-negative test result and either the first HCV RNA-positive or first anti-HCV positive test result (with or without concurrent HCV RNA detection). For 88 of 171 incident infections, HCV RNA was detected in the acute window prior to antibody seroconversion. For these cases, the date of infection was 30 days prior to the first positive TMA test result. This date is used because the period in which HCV RNA is detectable but HCV antibody is not detectable is, on average, 60 days.20, 21 Survival time was defined as time from study enrollment to date of HCV infection. Subjects entered into the analysis at the baseline visit and remained until date of HCV infection, or were censored at August 21, 2013, or last interview date. Censoring at last visit could occur for various reasons, including loss to follow-up and death. Cox proportional hazards models were fit to evaluate the association between treatment category and incident HCV adjusting for potential confounders (age, gender, non-white race, number of years of injection drug use, homelessness and incarceration). Treatment, homelessness and incarceration were treated as time-varying covariates in the Cox models. Multiple imputation with chained equations was performed to impute the values of predictors for visits where only laboratory testing, and not behavioral data, was collected. Imputed values were obtained for a total of 11 observations: (1) 4 (0.7%) regular quarterly interviews and (2) 7 interim visits. Additional exploratory analyses adjusted for number of days injected in the past month, to assess whether frequency of injection might be a potential mediator of relationships between opioid agonist therapy and HCV incidence, and also adjustment past month use of needle-syringe exchange programs. Adjusted hazard ratios (HR) and 95% confidence intervals are reported. Spearman correlations were used to evaluate potential collinearity between independent variables and covariates. All analyses were conducted using two-sided tests and a significance level of 0.05. Cox models were checked for violation of the proportional hazards assumption by assessing scaled Schoenfeld residuals and log-minus-log survival plots for patterns of non-proportionality. Sensitivity analyses were conducted restricting analysis to data from waves 1 and 3 since the questionnaire format differed slightly in wave 2. All analyses were conducted with Stata 11.2 (College Station, TX).
Results
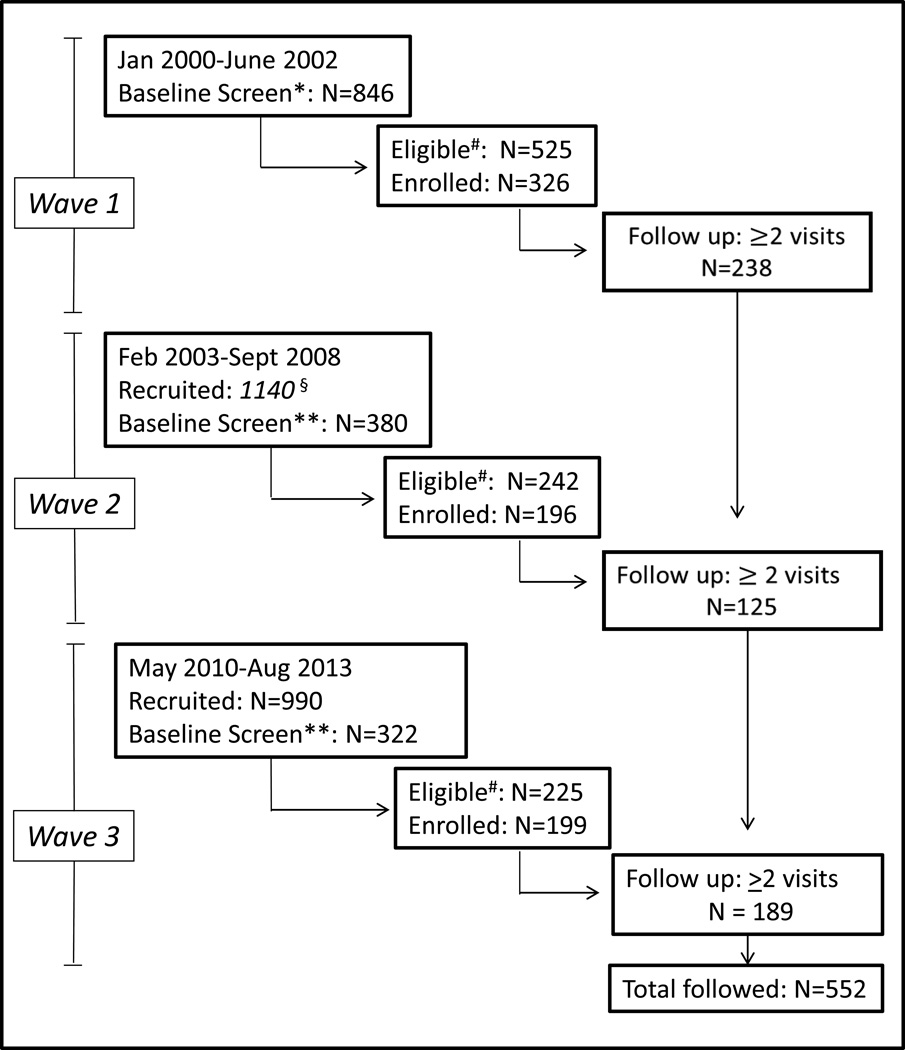
Between January 2000 and August 2013 a total of 1548 participants were screened, 992 (64%) met eligibility criteria, 721 (73%) were enrolled and 552 (77%) were followed (Figure 1). Participants who were enrolled and followed compared to participants who were lost to follow-up did not differ in terms of gender, race/ethnicity, years of education, or years injecting. Compared to enrolled participants who were lost to follow-up, enrolled participants who were followed were older (median age=23 vs. 22, p<0.01), more likely to inject every day in the past month (33% vs. 20%; p<0.01, more likely to use non-injection methamphetamine (64% vs. 53%, p<0.01), more likely to have been in substance use treatment (18% vs. 10%), to have received mental health counseling in the past 3 months (26% vs. 16%, p<0.01), and less likely to have been incarcerated in the past 3 months (27% vs. 38%, p<0.01). Characteristics of the sample (n=552) at study enrollment are shown in Table 1. The median age was 23 (IQR: 20–26); two-thirds (68%) of participants were Caucasian men, 40% reported they did not graduate high school, 69% were homeless or unstably housed in the past 3 months, and 27% had been incarcerated in the past 3 months. The median years injecting was 3.6 (IQR: 1.5–6.6); 34% were daily injectors and most (60%) reported heroin as the drug they had “most often used” in the past month. The majority (82%) reported no substance use treatment in the prior year, and 4% reported recent opioid agonist treatment in the prior year.
Figure 1. UFO Study Cohort Participation: Waves 1,2,3 (2000–2013).
Wave 1 eligibility for baseline screening: age <30 years, active injection drug use in prior month. No records were kept of the number recruited for Wave 1.
** Additional eligibility criterion for waves 2 and 3: no plans to travel in next 3 months. Number recruited is an estimate based on records kept starting in 2006.
# Eligible for this analysis: anti-HCV negative on baseline screening.
Table 1.
Selected demographic and behavioral characteristics at study enrollment among young adult injectors followed (N=552) in the UFO Cohort Study, San Francisco, CA, 2000 – 2013.
| Characteristic | No. | % |
|---|---|---|
| Overall | 552 | 100.0 |
| Study wave | ||
| 2000 – 2002 | 238 | 43.1 |
| 2003 – 2009 | 125 | 22.6 |
| 2010 – 2013 | 189 | 34.2 |
| Age, years | ||
| 15 – 18 | 16 | 2.9 |
| 18 – 24 | 340 | 61.6 |
| 25 – 30 | 196 | 35.5 |
| Gender | ||
| Female | 176 | 31.9 |
| Male | 376 | 68.1 |
| Education (n=549) | ||
| Less than high school | 218 | 39.7 |
| High school or more | 331 | 60.3 |
| Race (n=550) | ||
| Caucasian | 402 | 73.1 |
| Non-Caucasian | 148 | 26.9 |
| Homeless, past 3 months | ||
| No | 170 | 30.8 |
| Yes | 382 | 69.2 |
| Sexual behavior | ||
| Femalea | 176 | 31.9 |
| Heterosexual male | 214 | 38.8 |
| MSM | 162 | 29.3 |
| HIV Positive (n=475) | ||
| No | 454 | 95.6 |
| Yes | 21 | 4.4 |
| Age of first drug injection, years (n=551) | ||
| <18 | 227 | 41.2 |
| 18 – 19 | 115 | 20.9 |
| ≥20 | 209 | 37.9 |
| Duration injecting, years (n=551) | ||
| <3 | 170 | 30.9 |
| 3 – 5 | 187 | 33.9 |
| ≥6 | 194 | 35.2 |
| Injected every day, past month | ||
| No | 368 | 66.7 |
| Yes | 184 | 33.3 |
| Drug injected most days, past month | ||
| Speed/methamphetamine | 183 | 33.2 |
| Heroin/heroin mix | 330 | 59.8 |
| Other | 39 | 7.1 |
| Injected alone, past 3 months (n=551) | ||
| No | 248 | 45.0 |
| Yes | 303 | 55.0 |
| Recent syringe exchangeb | ||
| No | 124 | 22.5 |
| Yes | 438 | 77.5 |
| Ever overdosed (n=548) | ||
| No | 362 | 66.1 |
| Yes | 186 | 33.9 |
| Overdose, past 3 months (n=551) | ||
| No | 481 | 87.3 |
| Yes | 70 | 12.7 |
| Incarcerated, past 3 months (n=549) | ||
| No | 400 | 72.9 |
| Yes | 149 | 27.1 |
| Recent drug treatment (n=551)c | ||
| None | 454 | 82.4 |
| Non-OAT | 46 | 8.4 |
| OAT detoxification | 28 | 5.1 |
| OAT maintenanced | 23 | 4.2 |
Abbreviations: OAT, opioid agonist therapy
Female included women who had sex with men, as well as women who had sex with other women.
Time frame is past 30 days in wave 1 and past 3 months for waves 2 and 3.
Time frame is past year at baseline and past 3 months at follow-up in wave 1. Time frame is past week. for participants surveyed in wave 2, and past 3 months for wave 3.
Includes OAT unspecified for wave 2 only.
The study observation period totaled 680 person-years (py), during which there were 171 incident cases of HCV for an estimated incidence rate of 25.1/100 py (95% CI: 21.6–29.2). Participants completed a median of 3 behavioral interviews (IQR: 2 – 5), and the median interval between interviews was 93 days (IQR: 56 – 131). Participants who reported maintenance opioid agonist therapy in the past 3 months had lower incidence of HCV compared to those who reported no treatment in the past 3 months (Table 2). The rate ratio (RR) was significantly lower for participants who reported recent maintenance opioid agonist therapy (0.31; 95% CI: 0.14–0.65), but not for those who reported non-opioid agonist forms of treatment (0.63; 95% CI: 0.37–1.08), or opioid agonist detoxification (1.45; 95% CI: 0.80–2.69), compared to no treatment. Cox proportional hazards models which were adjusted for age, gender, race/ethnicity, years injecting, recent incarceration, and homelessness, demonstrated that maintenance opioid agonist therapy was independently associated with significantly lower relative hazards for becoming infected with HCV over time (AHR=0.39; 95% CI: 0.18–0.87) (Table 3). A model fit to examine mediation, with adjustment for frequency of injecting (number of days injecting in the past month), showed that the association became attenuated (AHR=0.59; 95% CI: 0.27–1.26). On the other hand, adjustment for use of needle-syringe exchange program had no substantive impact on the maintenance opioid agonist therapy effect (AHR=0.39; 95% CI: 0.18–0.85).
Table 2.
Incident HCV infection and type of drug treatment programs attended in young adult injectors followed (N=552) in the UFO Cohort Study, San Francisco, CA, 2000 – 2013.
| Baseline characteristic | Incident HCV N |
PYO | Incidence per person year (95% CI)c |
Rate Ratio (95% CI) |
p-value |
|---|---|---|---|---|---|
| Overall | 171 | 680 | 25.1 (21.6, 29.2) | ||
| Drug treatment, past 3 monthsa | |||||
| None | 138 | 488 | 28.2 (23.9, 33.4) | 1 | |
| Non-OAT | 15 | 84 | 17.9 (10.8, 29.6) | 0.63 (0.37, 1.08) | 0.09 |
| OAT detox | 11 | 27 | 41.1 (22.8, 74.2) | 1.45 (0.80, 2.69) | 0.23 |
| OAT maintenanceb | 7 | 81 | 8.6 (4.1, 18.1) | 0.31 (0.14, 0.65) | <0.01 |
Abbreviations: OAT, opioid agonist therapy; PYO, person-years of observation
Time frame is past year at baseline and past 3 months at follow-up in wave 1. Time frame is past week for participants surveyed in wave 2, and past 3 months for wave 3.
Includes OAT unspecified for wave 2 only.
Incidence was calculated using behavior or characteristic at the last HCV-negative time period. (uninfected during follow-up) or the first HCV-positive risk period (incident infections).
Table 3.
Multivariate Cox proportional hazards model of independent predictors of incident HCV infection in young adult injectors followed (N=552) in the UFO Cohort Study, San Francisco, CA, 2000 – 2013.
| Characteristic | AHRc | 95% CI | P |
|---|---|---|---|
| Drug treatment, past 3 monthsa | |||
| None | Referent | Referent | |
| Non-OAT | 0.71 | 0.41, 1.20 | 0.20 |
| OAT detox | 1.39 | 0.73, 2.67 | 0.32 |
| OAT maintenanceb | 0.39 | 0.18, 0.87 | 0.02 |
| Age, years | 0.99 | 0.94, 1.04 | 0.66 |
| Duration injecting, years | 1.03 | 0.98, 1.07 | 0.24 |
| Gender | |||
| Female | 1.00 | Referent | |
| Male | 0.72 | 0.52, 1.00 | 0.05 |
| Race/ethnicity | |||
| Caucasian | 1.00 | Referent | |
| Non-Caucasian | 1.17 | 0.82, 1.67 | 0.37 |
| Homeless, past 3 months | |||
| No | 1.00 | Referent | |
| Yes | 1.22 | 0.86, 1.74 | 0.26 |
| Incarcerated, past 3 months | |||
| No | 1.00 | Referent | |
| Yes | 1.58 | 1.12, 2.23 | <0.01 |
Abbreviations: OAT, opioid agonist therapy; AHR, adjusted hazard ratio; CI, confidence interval.
Time frame is past year at baseline and past 3 months at follow-up in wave 1. Time frame is past week for participants surveyed in wave 2, and past 3 months for wave 3.
Includes OAT unspecified for wave 2 only.
For time-dependent covariates, HRs were calculated using time-dependent Cox proportional-hazards regression.
In the sensitivity analyses restricting analyses to waves 1 and 3 (for which participants were directly queried about detoxification versus maintenance so that no “unspecified” responses remained) the results did not differ substantively. Again, the HCV incidence was lower among participants who reported recent maintenance opioid agonist therapy compared to those on no treatment (RR 0.37; 95% CI: 0.14–1.02, p=0.05), but HCV incidence was not lower for those subjects who reported recent opioid agonist detoxification (RR 1.77; 95% CI: 0.95, 3.32, p=0.07).
Discussion
In this study of young adult injectors, we found that maintenance opioid agonist therapy (either methadone or buprenorphine) for opioid use disorders was associated with more than a 60% reduction in HCV incidence over time compared to no treatment.
These results are in concordance with prior studies conducted in other populations. A meta-analysis by Hagan et al.,8 which included 8 studies published between 1996–2009, reported a pooled relative risk of 0.60 (95% CI: 0.35–1.03) for incident HCV associated with opioid agonist therapy. A pooled analysis of 6 UK studies by Turner et al. also reported that receipt of OAT was significantly associated with lower relative odds for seroconversion (aOR 0.41; 95% CI: 0.21–0.82).9 Our additional analyses adjusting for past month injection drug use suggest that maintenance opioid agonist therapy reduces HCV incidence in part by decreasing the frequency of injection, which will also lower risk of acquiring HIV and other blood borne pathogens.22 Our additional finding of a higher incidence of HCV among patients who reported recent opioid agonist detoxification compared to those who reported no treatment is novel. Studies have demonstrated high relapse rates when buprenorphine and methadone are discontinued,23, 24 suggesting that detoxification is a less effective treatment strategy than maintenance treatment. Studies also have demonstrated an increased risk of opioid overdose when patients relapse after premature detoxification or periods of abstinence (e.g., incarceration),25, 26 and it may be that they also engage in injecting behaviors that put them at higher risk of HCV acquisition in this period.
This is the first study of the effects of opioid agonist therapy on HCV to be conducted in young adults who inject drugs, and as such it extends the literature by demonstrating the potential benefits of maintenance opioid agonist therapy in reducing the incidence of HCV infections in this age group. Young injectors are a major driving force in the epidemic of HCV in the U.S.A. and Canada, and therefore are an important target for prevention.15 Buprenorphine is an efficacious treatment for youth with opioid use disorders.24, 27 In spite of this, young adults who inject drugs often encounter significant barriers to receiving opioid agonist therapy for the treatment of opioid use disorders,17 which is reflected in the general low rates of self-reported use of methadone and buprenorphine in this study. Young adults are typically characterized as having short addiction histories for which opioid agonist maintenance therapy is considered excessive as federal regulations concerning patient admission criteria to methadone maintenance treatment (42 CFR 8.12 (e)) stipulate that a person be addicted at least 1 year before admission for treatment and that a person under 18 years of age have had two documented unsuccessful attempts at short-term detoxification or drug-free treatment within a 12-month period.28 Rules differ from state to state regarding whether an adolescent may obtain substance use disorder treatment without parental consent; however, in California non-emancipated minors seeking methadone treatment must obtain parental consent and also pre-approval for treatment by the Department of Alcohol and Drug Programs (ADP) Narcotic Treatment Program Licensing Branch.29 Although only 16 (2.9%) were minors at the time of the study, almost half (41%) of all participants reported that they started injecting drugs at <18 years of age and may have benefited from early initiation of opioid agonist therapy. In addition to these unique barriers for young injectors, there are known barriers to opioid agonist therapy for all patients with opioid use disorders including insufficient providers, treatment facilities and insurance coverage for medications. Also, motivation to seek treatment may be lower among young adult injectors who typically have fewer co-morbidities related to their substance use disorders.
Given that studies have shown frequent HCV seroconversion within the first few years of initiating injection drug use,16 and evidence from this study that maintenance opioid agonist therapy is associated with decreased HCV incidence among young adult injectors, opioid treatment guidelines and regulations that defer opioid agonist therapy for young adults with opioid use disorders may warrant reconsideration. Furthermore, in keeping with the concern that the risk of opioid overdose increases following cessation of opioid agonist therapy,26, 30, 31 these study results support the view of many addiction experts that maintenance opioid agonist therapy, rather than detoxification, is a safer and more effective strategy for preventing serious medical complications of opioid addiction in young adult injectors.32
This study has some limitations. There were only a modest number of participants who reported being treated with opioid agonist therapy, particularly buprenorphine; therefore, we could not analyze the effects of buprenorphine and methadone separately. An additional limitation of the study is that opioid agonist therapy was defined by self-report and not confirmed by treatment episode data. It is possible that some participants might report misinformation about treatment status (social desirability bias). Also, no specific duration of treatment was used to differentiate detoxification versus maintenance, and therefore there may have been some overlap. We assume these types of misclassification would bias to the null, in which case our results would be an underestimate of the true effect. An additional limitation is that the questionnaires for one wave of data collection only provided open-ended responses on substance use treatment; therefore, some subjects were missing data on whether recent opioid agonist therapy was for detoxification or maintenance. Furthermore, there was a difference in the time frame of the questions asked regarding opioid agonist therapy between waves 1 and 3 and wave 2, which only captured very recent (past week) attendance. This should in theory bias our results to the null, which strengthens our findings. Also, sensitivity analyses were performed excluding data from wave 2, and results were not substantively changed. A major strength of the study is the repeated and accurate ascertainment of the outcome measure of HCV incidence. This is the only study of which we are aware that performed systematic testing, including HCV viremia, at regular intervals in order to measure true incidence of HCV.
In summary, among a cohort of young adult injectors, we found that report of recent treatment with opioid agonist maintenance therapy was associated with lower incidence of HCV. Our results suggest that treatment for opioid use disorders with maintenance opioid agonist therapy can reduce transmission of HCV in young adults who inject drugs, and should be offered as an important component of comprehensive strategies for primary HCV prevention.
Acknowledgements
The authors received support from the National Institutes of Health - National Institute on Drug Abuse grants R01DA016017 and K23DA027367, National Institute on Alcohol and Alcoholism grant K24AA022586. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The National Institutes of Health did not contribute to the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. We also acknowledge support from the UCSF CTSI (NIH UL1 RR024131) and the UCSF Liver Center (NIH P30 DK026743).
The authors would like to acknowledge the helpful contributions from colleagues: Drs. Michael P. Busch and Leslie Tobler at Blood Systems Research Institute for ongoing laboratory expertise, and Dr. Stephen Shiboski for statistical consultation. We thank Marlene Lira for help with manuscript preparation and submission. The study would not be possible without the leadership of Ms. Alya Briceno, and the UFO Study staff and volunteers for their dedicated research assistance and support. We thank the San Francisco Department of Public Health for their ongoing commitment to the health of the young people who participate; their contributions, including preventive vaccines and primary care for participants is invaluable. We thank our community partners at the Housing and Urban Health Clinic, Homeless Youth Alliance, San Francisco Needle Exchange and San Francisco AIDS Foundation. Last but not least, we especially acknowledge the participation of all the UFO Study participants without whom this research and the knowledge we gain to help prevent HCV would not be possible. This study is dedicated to the memory of Dr. Leslie Tobler who dedicated herself to furthering expert laboratory research methods to the fields of viral hepatitis and many other blood borne infections.
Footnotes
The authors do not have financial conflicts of interest to declare. All authors contributed significantly (see below) and have approved of the manuscript.
Author contributions
Study concept and design: Tsui, Page and Evans
Acquisition of data: Page, Lum and Hahn
Analysis and interpretation of the data: Tsui, Page, and Evans
Drafting of the manuscript: Tsui and Evans
Critical revision of the manuscript for important intellectual content: Page, Lum and Hahn
Obtained funding: Page, Hahn and Lum
References
- 1.Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch. Intern. Med. 2011 Feb 14;171(3):242–248. doi: 10.1001/archinternmed.2010.511. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance for Acute Viral Hepatitis - United States, 2006. MMWR; 2008. Centers for Disease Control and Prevention. [Google Scholar]
- 3.Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin. Infect. Dis. 2012 Jul;55(Suppl 1):S3–S9. doi: 10.1093/cid/cis393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edlin BR, Carden MR. Injection drug users: the overlooked core of the hepatitis C epidemic. Clin. Infect. Dis. 2006 Mar 1;42(5):673–676. doi: 10.1086/499960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011 Aug 13;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruggmann P. Accessing Hepatitis C patients who are difficult to reach: it is time to overcome barriers. J. Viral Hepat. 2012 Dec;19(12):829–835. doi: 10.1111/jvh.12008. [DOI] [PubMed] [Google Scholar]
- 7.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J. Infect. Dis. 2002 Dec 1;186(11):1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 8.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J. Infect. Dis. 2011 Jul 1;204(1):74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011 Nov;106(11):1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Use of enhanced surveillance for hepatitis C virus infection to detect a cluster among young injection-drug users - New York, November 2004–April 2007. Morbidity and Mortality Weekly Report. 2008;57:517–521. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002–2009. Morbidity and Mortality Weekly Report. 2011;60:537–541. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Notes from the Field: Hepatitis C Virus Infections Among Young Adults — Rural Wisconsin, 2010. Morbidity and Mortality Weekly Report. 2012 May 18;61(19):358–358. 2012. [PubMed] [Google Scholar]
- 13.Christian WJ, Hopenhayn C, Christian A, McIntosh D, Koch A. Viral hepatitis and injection drug use in Appalachian Kentucky: a survey of rural health department clients. Public Health Rep. 2010 Jan-Feb;125(1):121–128. doi: 10.1177/003335491012500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J. Infect. Dis. 2009 Oct 15;200(8):1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin. Infect. Dis. 2013 Aug;57(Suppl 2):S32–S38. doi: 10.1093/cid/cit300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am. J. Epidemiol. 2008 Nov 15;168(10):1099–1109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadland SE, Kerr T, Li K, Montaner JS, Wood E. Access to drug and alcohol treatment among a cohort of street-involved youth. Drug Alcohol Depend. 2009 Apr 1;101(1–2):1–7. doi: 10.1016/j.drugalcdep.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene P. Outpatient Drug Utilization Trends for Buprenorphine Years 2002–2009. [Accessed: 2014-03-24];2010 http://buprenorphine.samhsa.gov/bwns/2010_presentations_pdf/09_Greene_508.pdf. (Archived by WebCite® at http://www.webcitation.org/6OJVKGwyg). Accessed March 24, 2014.
- 19.Gryczynski J, Jaffe JH, Schwartz RP, et al. Patient perspectives on choosing buprenorphine over methadone in an urban, equal-access system. Am. J. Addict. 2013 May-Jun;22(3):285–291. doi: 10.1111/j.1521-0391.2012.12004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page-Shafer K, Pappalardo BL, Tobler LH, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J. Clin. Microbiol. 2008 Feb;46(2):499–506. doi: 10.1128/JCM.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busch MP. Insights into the epidemiology, natural history and pathogenesis of hepatitis C virus infection from studies of infected donors and blood product recipients. Transfus. Clin. Biol. 2001 Jun;8(3):200–206. doi: 10.1016/s1246-7820(01)00125-2. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000 Mar 8;283(10):1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 24.Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008 Nov 5;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison--a high risk of death for former inmates. N. Engl. J. Med. 2007 Jan 11;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seal KH, Kral AH, Gee L, et al. Predictors and Prevention of Nonfatal Overdose Among Street-Recruited Injection Heroin Users in the San Francisco Bay Area, 1998–1999. [2001/11/01];Am. J. Public Health. 2001 91(11):1842–1846. doi: 10.2105/ajph.91.11.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch. Gen. Psychiatry. 2005 Oct;62(10):1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- 28.Center for Substance Abuse Treatment. Federal Guidelines for Opioid Treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 29.CSAM Committee on Treatment of Opioid Dependence. Guidelines for Physicians Working in California Opioid Treatment Programs. [Accessed January 2, 2014];2008 http://www.csamasam.org/sites/default/files/CSAMOTPGuideline21Apr09.pdf. (Archived by WebCite® at http://www.webcitation.org/6MKQ5hD5k). [Google Scholar]
- 30.Davoli M, Bargagli AM, Perucci CA, et al. Risk of fatal overdose during and after specialist drug treatment: the VEdeTTE study, a national multi-site prospective cohort study. Addiction. 2007;102(12):1954–1959. doi: 10.1111/j.1360-0443.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 31.Fischer B, Brissette S, Brochu S, et al. Determinants of overdose incidents among illicit opioid users in 5 Canadian cities. CMAJ. 2004 Aug 3;171(3):235–239. doi: 10.1503/cmaj.1031416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. [2009/10/01];Curr. Psychiatry Rep. 2009 11(5):360–363. doi: 10.1007/s11920-009-0054-5. [DOI] [PubMed] [Google Scholar]