Abstract
Objective
To assess surface APRIL (a proliferation-inducing ligand; CD256) expression by circulating myeloid cells in rheumatoid arthritis (RA) and to determine its relationship to disease activity.
Methods
Peripheral blood mononuclear cells (PBMC) and plasma were obtained from patients with RA and healthy donors. PBMC were stained for flow cytometry to detect surface APRIL and blood cell markers to identify circulating myeloid cell subsets. Based on CD14 and CD16 phenotypes, monocyte subsets described as classical (CD14+CD16−), intermediate (CD14+CD16+), and nonclassical (CD14loCD16+) were identified. Levels of surface APRIL expression were measured by flow cytometry and median fluorescence intensity was used for comparisons. Levels of soluble APRIL in the plasma were determined by ELISA. Disease activity was measured by the Disease Activity Score in 28 joints.
Results
In patients with RA, total myeloid cells showed expression of surface APRIL that correlated with disease activity and with plasma APRIL levels observed in these patients. In healthy donors, classical monocytes were composed of > 80% of circulating monocytes. However, in patients with RA, the intermediate and nonclassical subsets were elevated and made up the majority of circulating monocytes. In contrast to healthy donors, where high levels of surface APRIL were only observed in nonclassical monocytes, patients with RA showed high levels of surface APRIL expression by all circulating monocyte subsets.
Conclusion
Surface APRIL is elevated in circulating myeloid cells in patients with RA where it is highly correlated with disease activity. Patients with RA also showed skewing of monocytes toward subsets associated with secretion of tumor necrosis factor-α and/or interleukin 1β.
Keywords: APRIL, TNFSF13, monocytes, rheumatoid arthritis, inflammation, autoimmunity
Rheumatoid arthritis (RA) is a systemic B cell-mediated autoimmune disease dominated by autoantibodies that recognize intracellular and extracellular antigens1,2. These autoantibodies result in chronic systemic immune responses that target the synovium, cartilage, and bone, resulting in joint damage3. During inflammatory synovitis, immune cells infiltrate the joint and produce cytokines4. Stimulation by cytokines induces B cells at different stages of development to proliferate and differentiate into antibody-producing plasma cells, thus continuing the cycle of chronic inflammation in RA5,6,7.
Studies of B cell-mediated autoimmune disease implicate the cytokine APRIL (a proliferation-inducing ligand) as a potential disease mediator. APRIL has been shown to support B cell development and survival in mice and humans5. APRIL is a member of the tumor necrosis factor (TNF) superfamily and is secreted by monocytes8, dendritic cells9, macrophages10, neutrophils, myelocytes8, astrocytes11, adipocytes12, and activated T and B cells13. Elevated levels of APRIL have been measured in the serum and synovial fluid of patients with RA14,15. In addition, fibroblast-like synoviocytes (FLS) have been shown to secrete APRIL in RA, but not osteoarthritis16. Novel surface forms of APRIL have been reported in human cell lines derived from lymphoid17 and myeloid malignancies18. In addition, surface APRIL has been observed by microscopy in synovial macrophages from patients with RA18.
The effects of APRIL are dependent on the receptor that it binds. APRIL has 2 receptors: (1) TACI (the transmembrane activator, calcium modulator and cyclophilin ligand interactor receptor), and (2) BCMA (the B cell maturation antigen receptor). TACI is expressed in B cells19 while BCMA expression has been reported in plasma cells and on FLS from patients with RA16. Binding of APRIL to the TACI or BCMA receptor leads to increased B cell or plasma cell survival, respectively20.
Monocytes exist as a heterogeneous population in the blood of healthy individuals and 3 subsets have been identified based on the expression of surface CD14 and CD16. Classical monocytes (CD14+CD16−) encompass the majority of circulating monocytes (~90%). Intermediate monocytes (CD14+CD16+) have been described as proinflammatory monocytes21,22. Nonclassical monocytes (CD14loCD16+) are also called patrolling monocytes and make up the minority subset in the circulating monocyte pool23. Classical monocytes are excellent phagocytes and produce interleukin 6 (IL-6) and IL-8 in response to bacterial pathogens. Intermediate monocytes produce the proinflammatory cytokines TNF-α and IL-1β24. Nonclassical monocytes exhibit vascular patrolling activity, poor phagocytic ability, and secrete proinflammatory cytokines TNF-α and IL-1β in response to Toll-like receptor (TLR) 7 and TLR8 stimulation. These nonclassical/patrolling monocytes are increased in active RA and are present in the glomerular vessels of patients with systemic lupus erythematosus (SLE) with lupus nephritis25,26,27,28,29.
Increases in serum levels of soluble APRIL, and in specific myeloid cell populations, have been associated with RA. A novel surface form of APRIL and its expression to myeloid cells and RA18 have been identified. However, expression of surface APRIL by monocyte subsets in healthy individuals and its relationship to RA are unknown. In our study, we sought to compare surface APRIL expression in circulating myeloid cells in both normal and autoimmune patients, and to determine whether the expression of surface APRIL was related to plasma levels of soluble APRIL and disease activity in patients with RA. We showed that surface APRIL was elevated on circulating myeloid cells and correlated with disease activity in RA. In healthy donors, surface APRIL was only observed in nonclassical monocytes that make up ~5% of circulating monocytes. In contrast, all monocyte subsets showed high levels of surface APRIL in patients with RA. While intermediate and nonclassical subsets made up < 20% of circulating monocytes in healthy donors, they were composed of the majority of the circulating monocytes in patients with RA.
MATERIALS AND METHODS
Subjects
Subjects were enrolled in our study and gave informed consent under a protocol approved by the Loma Linda University Institutional Review Board, Loma Linda, California, USA. All patients with RA were from the Loma Linda University Division of Rheumatology Clinic or Beaver Medical Group Rheumatology Clinic, Redlands, California, USA. All patients had a clinical diagnosis of RA and met the 1987 American College of Rheumatology (ACR) criteria30. Disease Activity Score in 28 joints (DAS28) was used as a measure of RA disease activity (Table 1). The total patient population was 26 (23 women and 3 men) with a mean age of 55.2 ± 15.6 years. Normal peripheral blood (PB) from anonymous adult donors was obtained from Leukopak leukocyte filters (Fenwal Laboratories) and donated by the Blood Processing and Quality Control from LifeStream. Normal plasma was purchased from Tennessee Blood Services.
Table 1.
Clinical characteristics of the RA population.
| Patient | Sex/Age, Yrs | Disease Duration, Yrs |
RF | DAS28 | Treatment | Assay |
|---|---|---|---|---|---|---|
| 1 | M/58 | 15 | Positive | 0.77 | none | ELISA, FC |
| 2 | F/47 | 10 | Positive | 1.40 | MTX | FC |
| 3 | F/57 | 15 | Positive | 1.53 | MTX, ETN | FC |
| 4 | F/66 | 24 | Positive | 1.56 | LEF | ELISA, FC |
| 5 | F/26 | 12 | Negative | 2.09 | ADA | FC |
| 6 | F/38 | 9 | Negative | 2.30 | MTX, RTX | FC |
| 7 | F/61 | 26 | Negative | 2.30 | MTX, ETN | FC |
| 8 | F/42 | 11 | Positive | 2.35 | ETN | ELISA, FC |
| 9 | F/64 | 5 | Positive | 2.35 | MTX, HCQ | ELISA, FC |
| 10 | F/42 | 4 | Negative | 2.66 | ADA | FC |
| 11 | F/62 | 1 | Positive | 2.72 | MTX, PRED | ELISA, FC |
| 12 | F/64 | 6 | Negative | 2.80 | MTX, HCQ | FC |
| 13 | F/43 | 27 | Positive | 2.82 | MTX, ADA | FC |
| 14 | F/56 | 28 | Positive | 3.34 | MTX, PRED | ELISA, FC |
| 15 | F/75 | 15 | NR | 3.57 | MTX, IFX | FC |
| 16 | F/75 | 15 | NR | 3.57 | MTX, IFX | FC |
| 17 | M/46 | 1 | Positive | 3.70 | LEF | ELISA, FC |
| 18 | F/47 | 25 | Negative | 3.80 | naproxen | FC |
| 19 | F/21 | 3 | Positive | 4.35 | none | ELISA, FC |
| 20 | F/33 | 1 | Negative | 4.58 | HCQ | FC |
| 21 | F/70 | 4 | Negative | 4.73 | MTX, HCQ | ELISA, FC |
| 22 | F/75 | 5 | Positive | 4.75 | MTX | ELISA, FC |
| 23 | F/61 | 6 | Positive | 4.99 | MTX, ADA | ELISA, FC |
| 24 | F/56 | 29 | Positive | 5.13 | MTX, PRED, LEF | ELISA, FC |
| 25 | M/60 | 4 | Positive | 6.95 | none | FC |
| 26 | F/68 | 10 | NR | NR | MTX | ELISA, FC |
RA: rheumatoid arthritis; RF: rheumatoid factor; DAS28; 28-joint Disease Activity Score; NR: not reported; FC: flow cytometry; MTX: methotrexate; ETN: etanercept; LEF: leflunomide; ADA: adalimumab; RTX: rituximab; HCQ: hydroxychloroquine; PRED: prednisone; IFX: infliximab.
Sample preparation
Blood samples were collected in tubes containing acid citrate dextrose from Becton, Dickinson and Company. Whole blood samples were centrifuged at 1500 rpm for 15 min to remove plasma. Peripheral blood mononuclear cells (PBMC) were obtained by red blood cell (RBC) lysis using an ammonium chloride-based RBC lysis buffer in both patient with RA and normal PB samples.
Soluble cytokine quantification
Plasma levels of APRIL were determined by a sandwich ELISA (Human APRIL Platinum ELISA, Bender MedSystems GmbH). ELISA were performed according to the manufacturer’s instructions on freshly thawed plasma. The ELISA plates were read at 450 nm absorbance using the uQuant 96 well plate reader (Bio-Tek Instruments Inc.). A standard curve was run in triplicate for each plate and samples were run in triplicate. APRIL plasma levels were calculated using a linear model in Bio-Tek software KCJunior, version 1.6.
Flow cytometry
PBMC from RA and healthy donors were stained with monoclonal antibodies in phosphate buffered saline (PBS) for 30 min in the dark at 4°C. Cells were then washed with PBS and stained with Fixable Viability Dye eFluor 450 (eBioscience) to distinguish between living and dead cells and incubated for 30 min at 4°C in the dark. Cells were subsequently washed and fixed with a 1% paraformaldehyde solution before analysis using the MACSQuant Analyzer (Miltenyi Biotec). The following isotype control antibodies were used: mouse IgG1 FITC, mouse IgG1 PE–Cy7 (eBioscience); mouse IgG1 APC-Cy7, rat IgG2b PerCP-Cy5.5 (Biolegend); and mouse IgG1 PE, mouse IgG1 APC (Miltenyl Biotech Inc.). The following antihuman antibodies were used: CD256 PE (clone T3-6), CD66b PerCP-Cy5.5 (clone G10F5), CD1a APC (clone HI149), CD16 APC-Cy7 (clone 3G8; Biolegend); and CD14 PE-CY7 (clone 61D3; eBioscience). Compensation beads (anti-mouse Ig k compensation beads; BD Biosciences) and cells stained with Fixable Viability Dye eFluor 450 were used for machine compensation settings. Flow cytometry alignment particles were used as an instrument settings control for each experiment (Spher Ultra Rainbow Fluorescent Particles). Flow cytometry data analysis was performed using Flowjo data analysis software (TreeStar). Relative surface APRIL expression for patients with RA was obtained by normalizing the APRIL median fluorescence intensity (MFI) of each patient sample to the mean APRIL MFI obtained for normal samples (n > 11) that were stained with the same antibody combinations and collected with the same instrument settings as the patient with RA samples.
Statistical analysis
Statistical differences were determined with the nonparametric Mann-Whitney U 2–tailed tests using GraphPad PRISM software (GraphPad). Correlation analysis was performed using the Spearman 1-tailed test. Differences were considered statistically significant for p ≤ 0.05. Grubb’s Test was used to exclude significant outliers. The mean and 95% CI are shown in each figure.
RESULTS
Surface APRIL expression is elevated in circulating myeloid cells in patients with RA
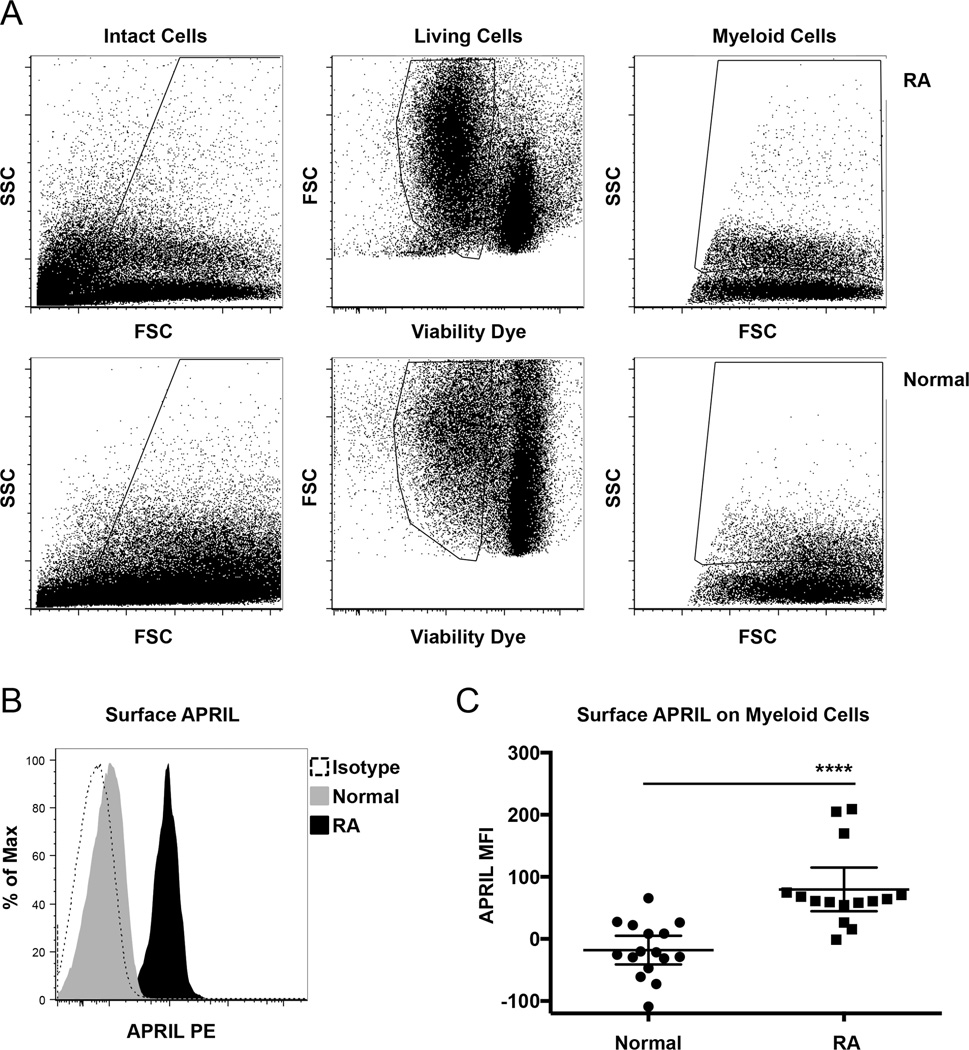
Multiple blood cells have been shown to produce and secrete APRIL8,9,10,11,12,13. Surface APRIL expression has been reported in malignant myeloid cell lines17 and in macrophages in synovial tissues in patients with RA16,31. However, to our knowledge, expression of APRIL by circulating myeloid cells has not been reported. We used multicolor flow cytometry to determine whether surface APRIL was expressed in peripheral blood myeloid cells in patients with RA and healthy donors. For flow cytometry analysis, intact PBMC were gated based on forward (FSC) and side (SSC) light scatter (Figure 1A, left panels). These cells were further gated to identify living cells (cells that were negative for viability dye; Figure 1A, middle panels). From intact living cells, myeloid cells were identified based on size (indicated by FSC) and granularity (indicated by SSC; Figure 1A, right panel for gate).
Figure 1.
Surface APRIL expression is elevated in RA. A. PBMC from healthy donors and patients with RA were stained for flow cytometry to detect surface APRIL and with viability dye. FSC and SSC light scatter and intact cell gate are shown (left panels). From these, living cells were gated (middle panels). Total myeloid cells were gated from living intact cells based on FSC and SSC (right panels). B. Representative histograms show surface APRIL and isotype staining in gated myeloid cells. C. Graphed is the MFI of surface APRIL staining in gated myeloid cells from 16 healthy donors and 15 patients with RA. **** p ≤ 0.0001. APRIL: a proliferation- inducing ligand; RA: rheumatoid arthritis; PBMC: peripheral blood mononuclear cells; FSC: forward light scatter; SSC: side light scatter; MFI: median fluorescence intensity.
Surface APRIL was clearly detectable in gated myeloid cells from all patients with RA as compared to isotype controls (Figure 1B). In contrast, the majority of healthy donor samples showed surface APRIL staining that was similar or slightly higher than isotype controls (Figure 1B). A graph comparing the MFI of surface APRIL staining in gated myeloid cells from 16 healthy donors and 15 patients with RA is shown in Figure 1C. These data show that surface APRIL is elevated in myeloid cells in patients with RA compared to controls (p ≤ 0.0001).
Surface APRIL expression correlates with plasma levels of soluble APRIL in patients with RA
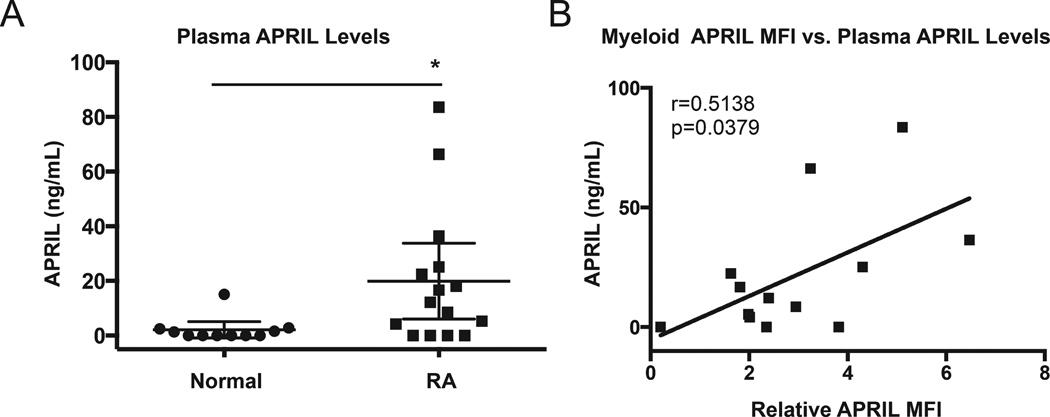
Elevated levels of soluble APRIL have been reported in the serum of patients with RA16,32,33; therefore, we wanted to determine whether there was a correlation between the surface APRIL expression and the levels of soluble APRIL circulating in the plasma of patients with RA. Plasma APRIL levels were measured in 15 patients with RA and 11 healthy donors by ELISA. Plasma levels of APRIL were increased in patients with RA as compared to healthy donors (19.92 ± 25.02 vs 2.12 ± 4.44 ng/ml; Figure 2A). These data confirm previous reports that soluble APRIL is elevated in RA16,32,33.
Figure 2.
Level of surface APRIL in myeloid cells correlates with soluble APRIL in the plasma of patients with RA. A. Soluble APRIL in plasma from healthy donors (n = 11) and patients with RA (n = 15) was determined by ELISA. B. Surface APRIL expression by myeloid cells from patients with RA was determined by flow cytometry. MFI of surface APRIL staining in patients with RA was normalized to that of healthy donors to determine the relative APRIL MFI. The Spearman ρ test indicates that relative surface APRIL expression in myeloid cells is highly correlated with plasma APRIL levels in 13 patients with RA. * p ≤ 0.05. APRIL: a proliferation-inducing ligand; RA: rheumatoid arthritis; MFI: median fluorescence intensity.
Next we evaluated the relationship between surface APRIL expression and plasma levels of soluble APRIL in patients with RA. Plasma APRIL levels and surface APRIL expression in myeloid cells (gated as shown in Figure 1A) were determined in patients with RA. Patient characteristics are shown in Table 1. Relative surface APRIL expression was calculated by normalizing the APRIL MFI for each patient sample to the mean APRIL MFI obtained from healthy donor samples. Spearman ρ analysis showed a positive correlation between relative surface APRIL expression by myeloid cells and plasma APRIL levels in patients with RA (r = 0.514, p = 0.038; Figure 2B).
Surface APRIL expression correlates with increased disease activity in RA
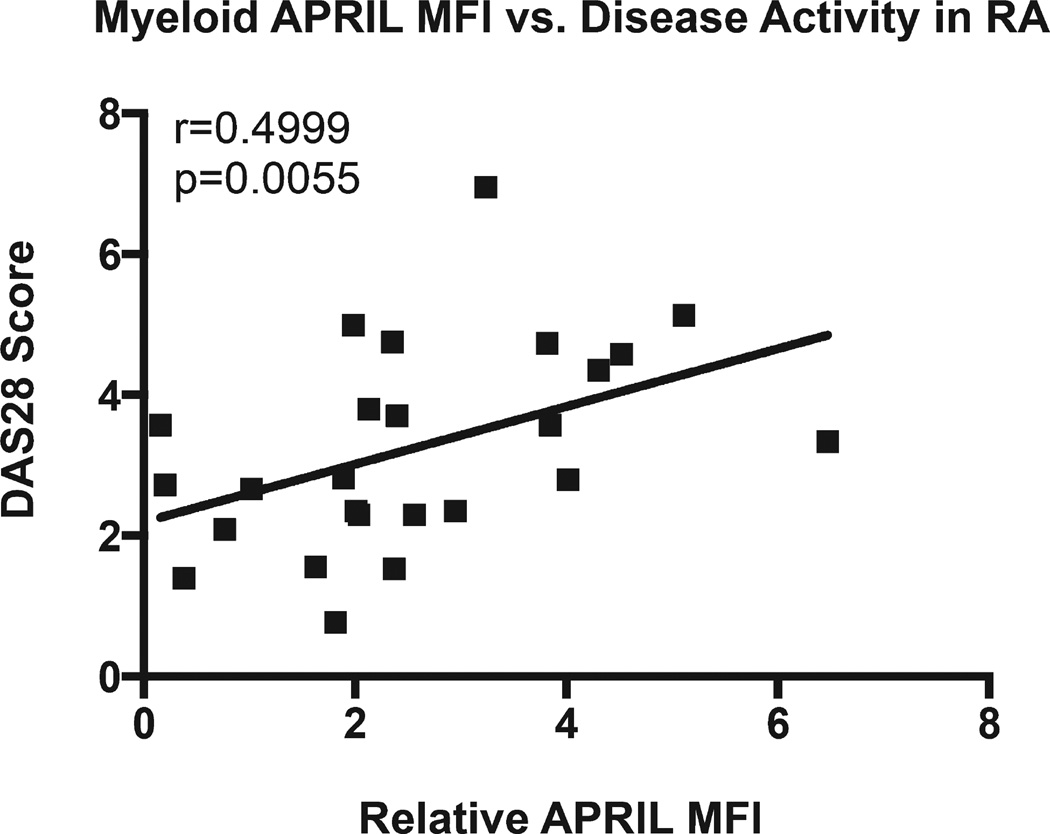
Soluble APRIL has been shown to correlate with disease activity in lupus nephritis, SLE, and juvenile idiopathic arthritis15,34,35. Increased soluble APRIL levels have been reported in patients with active RA disease14. We evaluated the relationship between surface APRIL expression in myeloid cells and disease activity in RA. The MFI for the expression of surface APRIL was calculated as described above. DAS28, as found in Table 1, was used to determine the disease activity in our cohort of patients with RA. In Figure 3, Spearman ρ analysis shows that relative surface APRIL expression by myeloid cells in patients with RA positively correlates with DAS28 (r = 0.499, p = 0.006). Spearman ρ analysis of soluble APRIL did not show a correlation with disease activity in the patients with RA we studied, although their levels of soluble APRIL were elevated as compared to healthy donors. These data show that surface APRIL expression by myeloid cells in patients with RA correlates with disease activity as indicated by DAS28 score.
Figure 3.
Level of surface APRIL in myeloid cells correlates with disease activity in patients with RA. Surface APRIL expression by myeloid cells from patients with RA was determined by flow cytometry. MFI of surface APRIL staining in patients with RA was normalized to that of healthy donors to determine the relative APRIL MFI. The Spearman ρ test indicates that relative surface APRIL expression in myeloid cells is highly correlated with disease activity as determined by DAS28 in 25 patients with RA. APRIL: a proliferation-inducing ligand; RA: rheumatoid arthritis; MFI: median fluorescence intensity; DAS28: 28-joint Disease Activity Score.
Frequency of intermediate and nonclassical monocytes are increased in the circulating monocyte pool of patients with RA
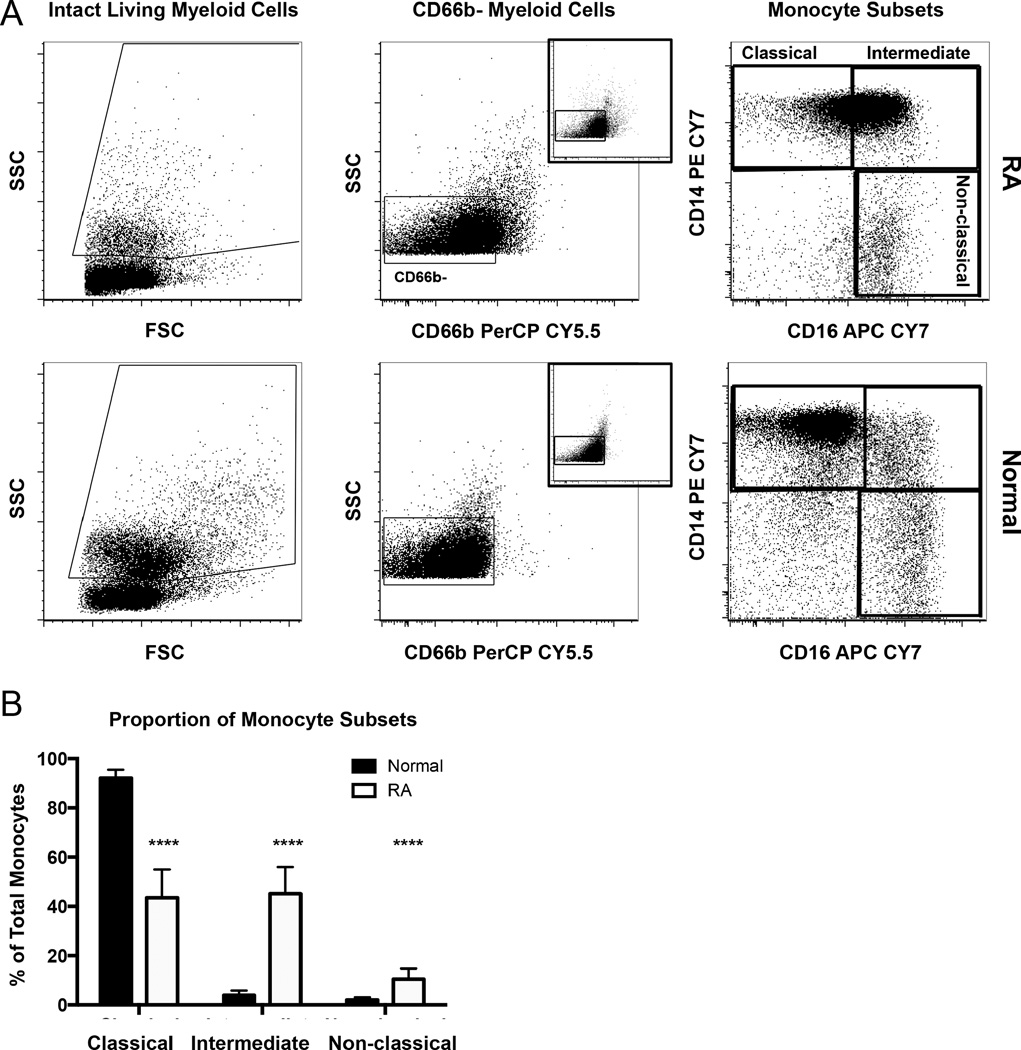
Given the relationship that we observed between surface APRIL in circulating myeloid cells and disease activity in patients with RA, we sought to identify the subsets of myeloid cells responsible for increased surface APRIL expression. Circulating myeloid cells include monocytes as well as very short-lived neutrophils. Previous reports showed that monocytes were associated with inflammation and that a subset of these monocytes, described as nonclassical (CD14loCD16+), was increased in patients with active RA24,26. We used flow cytometry to compare the frequency of classical, intermediate, and nonclassical monocyte subsets among circulating myeloid cells in healthy donors and our RA cohort. PBMC samples were stained with antibodies to detect CD66b, CD14, and CD16. Cells within myeloid light scatter (Figure 4A, left panels) were gated from intact, living PBMC as shown in Figure 1A. Monocytes were distinguished from short-lived granulocytes (< 1% of living cells in all samples) based on granularity (indicated by SSC) and the absence of the granulocyte marker CD66b (gated as shown in Figure 4A, middle panels). The coexpression pattern of CD14 and CD16 was used to phenotypically identify myeloid cells that have been described as classic (CD14+CD16−), intermediate (CD14+CD16+), and nonclassical (CD14loCD16+) monocytes23 (Figure 4A, right panels).
Figure 4.
The circulating monocyte pool is skewed toward intermediate and nonclassical monocytes in RA. A. PBMC from patients with RA and normal donors were stained for flow cytometry to detect CD66b, CD14, and CD16. Myeloid cells were gated (left panels) as in Figure 1. Expression of CD66b versus SSC was plotted, and monocytes were gated based on low side scatter and absence of CD66b (middle panels). Co-staining with CD14 and CD16 was used to identify 3 subsets of monocytes (right panels). B. The percentage of each subset within the total monocyte pool in healthy donors (n = 16) and patients with RA (n = 15). **** p ≤ 0.0001. RA: rheumatoid arthritis; PBMC: peripheral blood mononuclear cells; SSC: side light scatter.
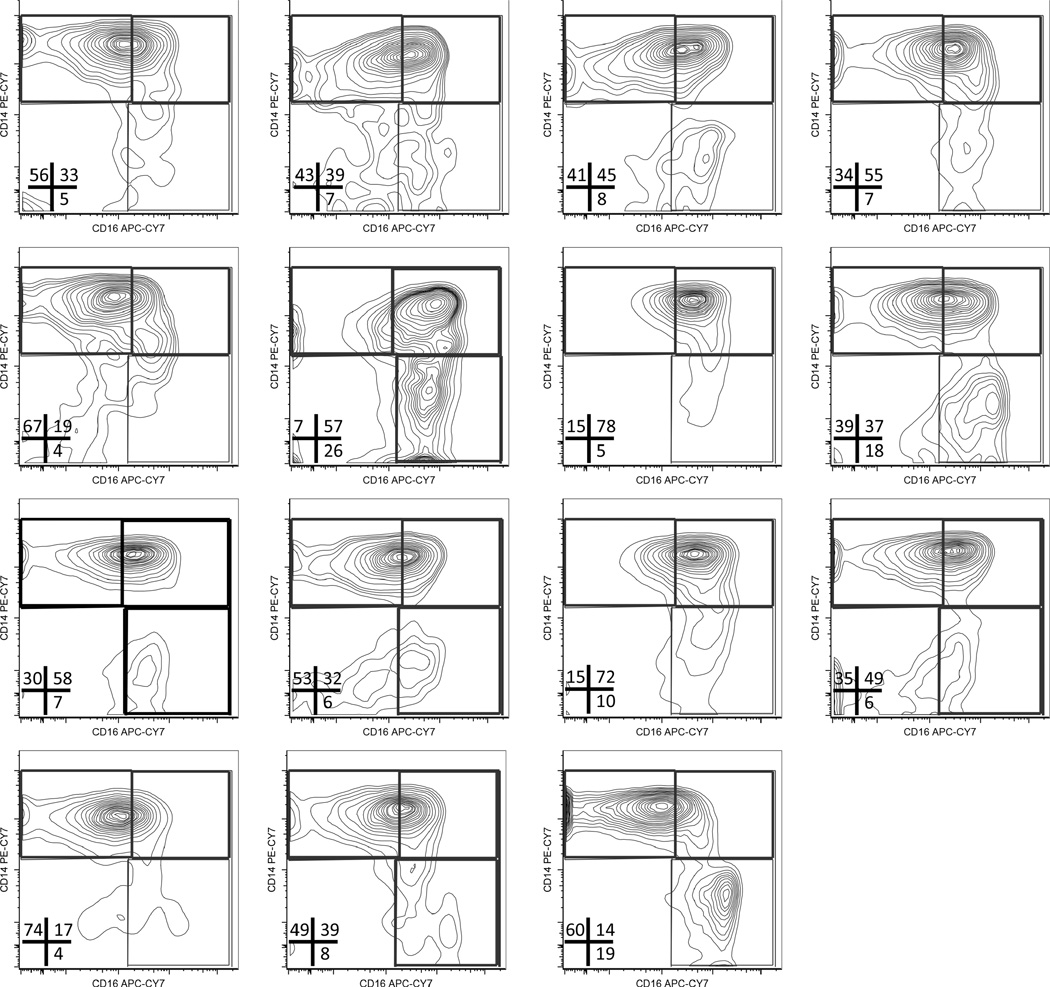
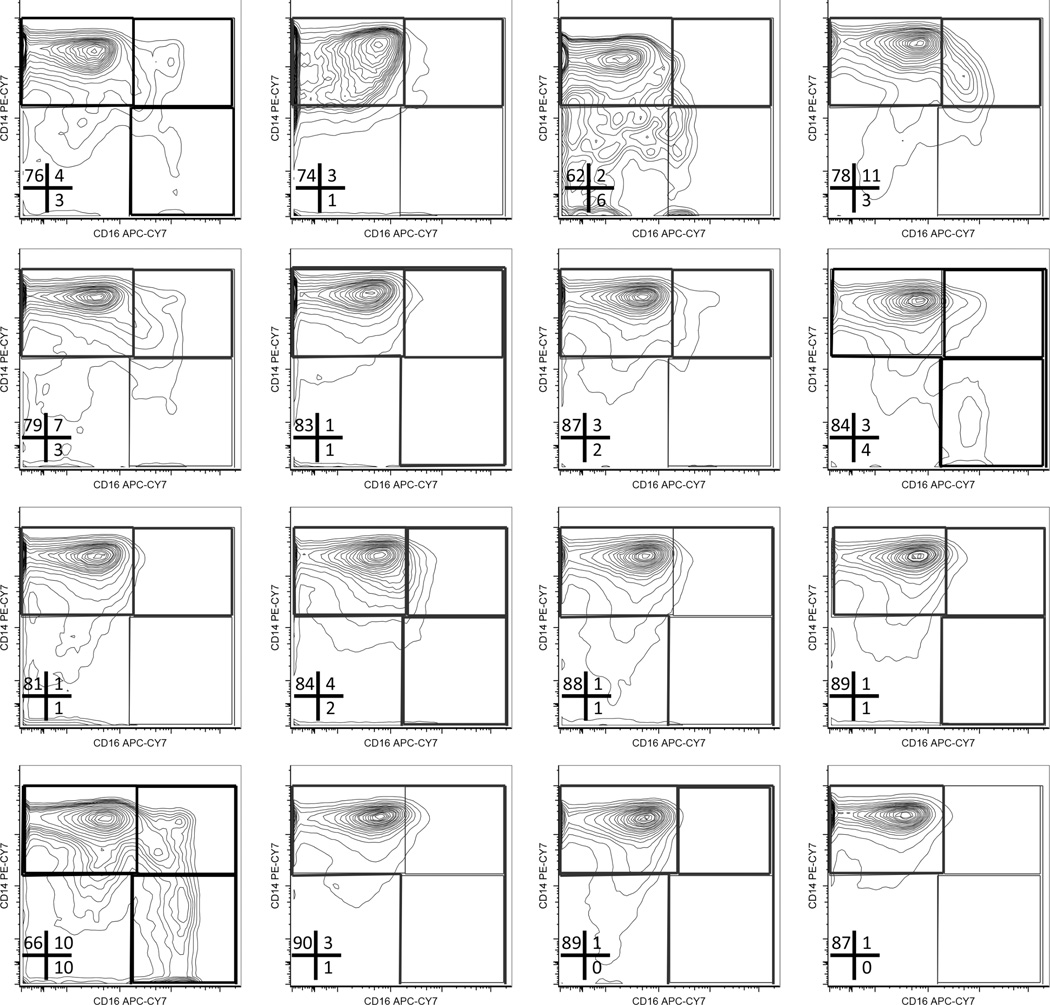
Flow cytometry plots of monocytes in RA samples show a clear increase in CD16 expression that results in a shift from predominantly classical monocytes (CD14+CD16−) to inflammatory intermediate (CD14+CD16+) and nonclassical (CD14loCD16+) monocytes. This shift (Figure 4A, right panels) was observed in 12 out of 15 patients with RA. (The CD14 vs CD16 plots for monocyte subsets in each normal and RA sample are shown in Appendix 1 and Appendix 2.)
APPENDIX 1.
Monocyte subsets in patients with RA. Flow cytometry plots showing CD14 versus CD16 co-staining in gated monocytes in PBMC from patients with RA (n = 15). The frequency of gated monocyte for each subset is indicated. RA: rheumatoid arthritis; PBMC: peripheral blood mononuclear cells.
APPENDIX 2.
Monocyte subsets in healthy donors. Flow cytometry plots showing CD14 versus CD16 co-staining in gated monocytes in PBMC from healthy donors (n = 16). The frequency of gated monocyte for each subset is indicated. PBMC: peripheral blood mononuclear cells.
A comparison of the frequency of each monocyte subset within the total circulating monocyte pool revealed that the proportion of intermediate and nonclassical monocytes was increased (~8- and ~4-fold, respectively), while the proportion of classical monocytes was reduced in patients with RA compared to healthy controls (Figure 4B). These data show that the pool of circulating monocytes in patients with RA is skewed toward proinflammatory, intermediate, and nonclassical subsets.
Surface APRIL expression is elevated in PB monocyte subsets
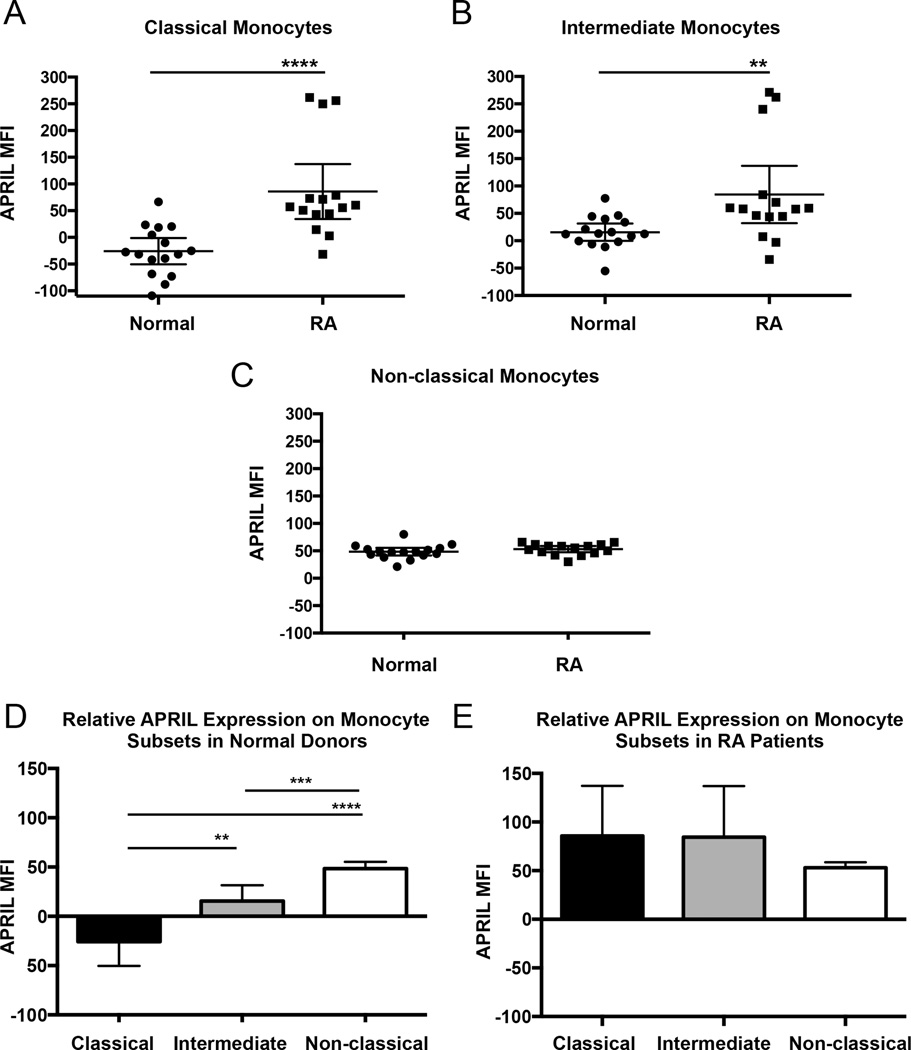
Surface APRIL expression has been identified in monocytic leukemia (THP-1) and monocytic lymphoma (U937) cell lines and in primary macrophages from patients with RA16,31. Our data in Figure 1C show surface APRIL expression in some myeloid cells in healthy samples, and that this expression is elevated in patients with RA. We sought to determine normal surface APRIL expression in monocyte subsets and how this compares to patients with RA. We evaluated the expression of surface APRIL in monocyte subsets gated as described in Figure 4A. Our data show that in patients with RA, levels of surface APRIL are elevated in classical and intermediate monocytic subsets as compared to healthy donors (Figures 5A–B). APRIL levels were, on average, increased 3-fold in classic monocytes and 6-fold in intermediate monocytes as compared to healthy donors. However, there was no significant difference in APRIL expression when nonclassical monocytes in patients with RA and healthy donors were compared (Figure 5C). These data show that in patients with RA, surface APRIL is upregulated in classical and intermediate monocytes as compared to healthy donors.
Figure 5.
Surface APRIL is elevated in classical and intermediate monocytes in patients with RA. PBMC from patients with RA and healthy donors were stained for flow cytometry and monocyte subsets were gated as in Figure 4. The MFI of APRIL staining on monocytes in the (A) classical, (B) intermediate, and (C) nonclassical subsets in patients with RA (n = 15) and healthy donors (n = 16) is plotted. D. A comparison of surface APRIL among monocyte subsets in healthy donors (n = 16) and (E) patients with RA (n = 15). ** p ≤ 0.01. *** p ≤ 0.001. **** p ≤ 0.0001. APRIL: a proliferation-inducing ligand; RA: rheumatoid arthritis; PBMC: peripheral blood mononuclear cells; MFI: median fluorescence intensity.
To gain insights into the potential role of surface APRIL in normal monocytes, we compared expression across the classical, intermediate, and nonclassical subsets (Figure 5D). In healthy donors, we found that surface APRIL was undetectable in classical monocytes, and expressed at very low levels on intermediate monocytes and at high levels on nonclassical monocytes (Figure 5D). In contrast, patients with RA showed similar high levels of surface APRIL across all monocyte subsets (Figure 5E). Taken together, these data suggest that in RA, all monocyte subsets express high levels of surface APRIL normally found only on nonclassical monocytes.
DISCUSSION
Surface forms of APRIL have been identified in cell lines from lymphoid17 and myeloid malignancies18, as well as macrophages in the synovial tissue of patients with RA31. Therefore, we sought to determine whether circulating myeloid cells in patients with RA express surface APRIL. We show that circulating myeloid cells in patients with RA express surface APRIL (Figures 1B–C) and this surface APRIL expression positively correlates with disease activity in our RA cohort (Figure 3). Thus, to the best of our knowledge, our study is the first to show that surface APRIL is expressed in circulating myeloid cells in patients with RA and that levels of surface APRIL are highly correlated with disease activity.
Next we sought to determine which myeloid cell subsets are responsible for elevated surface APRIL in RA. Previous reports have shown that the frequency of nonclassical monocytes was higher in patients with active RA26,27,28. We show that both nonclassical and intermediate monocyte subsets are increased in patients with RA (Figure 4B). Although these subsets make up less than 20% of the circulating monocyte population in healthy donors, in patients with RA, they represent the majority of circulating monocytes (Figure 4B). Intermediate monocytes are believed to represent a subset of monocytes that are able to rapidly mature and differentiate into tissue macrophages because of their expression of CD1636. They also migrate in response to the chemokine CX3CL137, which is elevated in patients with RA38. Thus, the pool of monocytes in patients with RA is skewed toward intermediate and nonclassical monocytes, populations of monocytes known to produce the inflammatory cytokines TNF-α and/or IL-1β25, cytokines important in the pathogenesis of RA.
Our evaluation of monocyte subsets from healthy donors showed that staining for surface APRIL was not above background in the classical monocytes. Low levels of surface APRIL were observed in intermediate monocytes, while high levels of surface APRIL were only detected in nonclassical monocytes in healthy donors (Figure 5D). In contrast, surface APRIL was expressed at high levels on all monocyte subsets in patients with RA (Figure 5E). Overall, our data suggest that in patients with RA, a large fraction of monocytes take on features characteristic of the proinflammatory monocyte subsets (Appendix 1–2). Specifically, monocytes in patients with RA show elevated levels of CD16 (Figure 4A), a feature characteristic of intermediate and nonclassical monocytes, as well as high levels of surface APRIL, which in healthy donors was only found in nonclassical monocytes (Figures 5D–E).
Surface APRIL has the potential to contribute to RA by acting either as a ligand or as a receptor during interactions with TACI and BCMA. TACI and BCMA are receptors for soluble APRIL. TACI is expressed in normal and malignant B cells, while BCMA is expressed in plasmablasts, plasma cells, and in FLS cells in RA joints16,17,39,40,41,42,43. Cell-to-cell contact between monocytes that express surface APRIL and B lineage cells that express TACI or BCMA have the potential to contribute to RA. It is currently unclear whether surface APRIL gives the same prosurvival signals that soluble APRIL produces in B lineage cells. This will be an important area for future studies.
Surface APRIL has also been shown to act as a signaling receptor when stimulated by interaction with TACI or BCMA. Monocytic leukemia cells expressing surface APRIL produced both IL-8 and matrix metalloprotease 9 when cocultured with B cells that expressed either TACI or BCMA18. Because FLS and plasma cells in the RA joint express BCMA, it is possible that cell-to-cell contact results in BCMA-induced surface APRIL signals in myeloid cells and the secretion of proinflammatory cytokines that ultimately leads to joint destruction.
Alternative APRIL splice forms give rise to surface APRIL44. APRIL is classically processed intracellularly within the Golgi apparatus and is then secreted from the cell45. An intergenic splice form exists that is generated from the combination of exons 1–6 of TNF-related weak inducer of apoptosis (TWEAK) and exons 2–6 of APRIL, producing TWE-PRIL46. The TWE-PRIL splice form has the transmembrane domain of TWEAK and the trimeric form of APRIL as an uncleavable membrane-bound protein46. TWE-PRIL and other alternative splice forms of APRIL may lead to the surface expression of APRIL44. We show that surface APRIL is elevated in circulating myeloid cells in patients with RA. The function of surface APRIL in the pathology of rheumatic diseases has not been elucidated and will be a focus for future studies.
The implication of APRIL in B cell-mediated autoimmune diseases led to the recent development of atacicept and other drugs currently in the research stage47. Atacicept antagonizes APRIL as well as another cytokine, B cell activating factor (BAFF), and is currently in phase III clinical trials for the treatment of SLE48. APRIL and BAFF have overlapping effects in B cell survival and maintenance; therefore, this drug provides promise in targeting multiple autoreactive B cell subsets, including plasma cells, by preventing APRIL and/or BAFF from activating TACI, BAFF receptor, and BCMA. We speculate that atacicept could also lead to the removal of monocyte populations that aberrantly express surface APRIL.
In 2 phase II clinical trials for RA (August I and II), atacicept reduced rheumatoid factor antibody levels, mature B cells, and plasma cells in patients with inadequate response to either methotrexate or anti-TNF therapy49,50. However, these trials failed to meet the primary ACR20-C-reactive protein response endpoint49,50, possibly because of patient selection criteria. Future trials with atacicept may prove effective in treating patient cohorts with elevated expressions of APRIL/BAFF. Data in Figure 3 indicate that ~50% of our RA cohort showed moderately elevated surface APRIL, and ~30% had surface APRIL that is more than 3-fold higher than that of healthy donors, suggesting that atacicept may be effective only in a subset of patients.
APRIL and BAFF have been shown to be particularly elevated in very early RA (VERA), suggesting that atacicept14,51 may prove beneficial in patients with newly diagnosed RA. While all of our patients had established RA, the positive correlation we saw between plasma APRIL and surface APRIL in myeloid cells suggests that the surface APRIL levels may be even higher in VERA. Our findings suggest that surface APRIL could provide an easily detectable biomarker and be a useful selection criterion for the administration of atacicept. Surface APRIL expression, together with monocyte subset skewing, might provide a prognostic indicator of patient response to atacicept or other drugs that target APRIL47.
APRIL is expressed in circulating myeloid cells in patients with RA and positively correlates with the DAS28 measurement of disease activity. In patients with RA, circulating monocytes are skewed toward subsets associated with inflammation and all monocyte subsets show the high levels of surface APRIL normally found only on nonclassical monocytes. Further studies are needed to determine the mechanisms responsible for surface APRIL expression and its role in RA pathogenesis.
ACKNOWLEDGMENT
We thank the members of the Payne Research laboratory for their helpful discussions and critical review of the manuscript. We also thank the nurses, fellows, and physicians in the Loma Linda University Division of Rheumatology and at the Beaver Medical Group Rheumatology Clinic for assisting with sample collection.
Dr. K.J. Payne is supported by the US National Institute of General Medical Sciences and the National Institute of Health Disparities and Minority Health of the US National Institutes of Health, under award numbers 2 R25 GM060507 and P20MD006988.
REFERENCES
- 1.Dörner T, Burmester GR. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol. 2003;15:246–252. doi: 10.1097/00002281-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Dörner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond B, Bloom O, Al Abed Y, Kowal C, Huerta PT, Volpe BT. Moving towards a cure: blocking pathogenic antibodies in systemic lupus erythematosus. J Intern Med. 2011;269:36–44. doi: 10.1111/j.1365-2796.2010.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takakubo Y, Tamaki Y, Hirayama T, Iwazaki K, Sasaki K, Sasaki A, et al. Inflammatory immune cell responses and Toll-like receptor expression in synovial tissues in rheumatoid arthritis patients treated with biologics or DMARDs. Clin Rheumatol. 2013;32:853–861. doi: 10.1007/s10067-013-2209-3. [DOI] [PubMed] [Google Scholar]
- 5.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 6.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, et al. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–3092. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Matthes T, Dunand-Sauthier I, Santiago-Raber ML, Krause KH, Donze O, Passweg J, et al. Production of the plasma-cell survival factor a proliferation-inducing ligand (APRIL) peaks in myeloid precursor cells from human bone marrow. Blood. 2011;118:1838–1844. doi: 10.1182/blood-2011-01-332940. [DOI] [PubMed] [Google Scholar]
- 9.Hardenberg G, Planelles L, Schwarte CM, van Bostelen L, Le Huong T, Hahne M, et al. Specific TLR ligands regulate APRIL secretion by dendritic cells in a PKR-dependent manner. Eur J Immunol. 2007;37:2900–2911. doi: 10.1002/eji.200737210. [DOI] [PubMed] [Google Scholar]
- 10.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell—dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 11.Thangarajh M, Masterman T, Hillert J, Moerk S, Jonsson R. A proliferation-inducing ligand (APRIL) is expressed by astrocytes and is increased in multiple sclerosis. Scand J Immunol. 2007;65:92–98. doi: 10.1111/j.1365-3083.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- 12.Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, et al. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol. 2009;183:5948–5956. doi: 10.4049/jimmunol.0901186. [DOI] [PubMed] [Google Scholar]
- 13.Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007;179:5947–5957. doi: 10.4049/jimmunol.179.9.5947. [DOI] [PubMed] [Google Scholar]
- 14.Moura RA, Cascão R, Perpétuo I, Canhão H, Vieira-Sousa E, Mourão AF, et al. Cytokine pattern in very early rheumatoid arthritis favours B-cell activation and survival. Rheumatology. 2011;50:278–282. doi: 10.1093/rheumatology/keq338. [DOI] [PubMed] [Google Scholar]
- 15.Hegazy M, Darwish H, Darweesh H, El-Shehaby A, Emad Y. Raised serum level of APRIL in patients with systemic lupus erythematosus: correlations with disease activity indices. Clin Immunol. 2010;135:118–124. doi: 10.1016/j.clim.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Nagatani K, Itoh K, Nakajima K, Kuroki H, Katsuragawa Y, Mochizuki M, et al. Rheumatoid arthritis fibroblast-like synoviocytes express BCMA and are stimulated by APRIL. Arthritis Rheum. 2007;56:3554–3563. doi: 10.1002/art.22929. [DOI] [PubMed] [Google Scholar]
- 17.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–688. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Kim WJ, Suk K, Lee WH. Cell to cell interaction can activate membrane-bound APRIL which are expressed on inflammatory macrophages. Immune Netw. 2010;10:173–180. doi: 10.4110/in.2010.10.5.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SK, Arendt BK, Darce JR, Wu X, Jelinek DF. A role for BLyS in the activation of innate immune cells. Blood. 2006;108:2687–2694. doi: 10.1182/blood-2005-12-017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimberley FC, van der Sloot AM, Guadagnoli M, Cameron K, Schneider P, Marquart JA, et al. The design and characterization of receptor-selective APRIL variants. J Biol Chem. 2012;287:37434–37446. doi: 10.1074/jbc.M112.406090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smeekens SP, van de Veerdonk FL, Joosten LA, Jacobs L, Jansen T, Williams DL, et al. The classical CD14++ CD16− monocytes, but not the patrolling CD14+ CD16+ monocytes, promote Th17 responses to Candida albicans. Eur J Immunol. 2011;41:2915–2924. doi: 10.1002/eji.201141418. [DOI] [PubMed] [Google Scholar]
- 22.Tallone T, Turconi G, Soldati G, Pedrazzini G, Moccetti T, Vassalli G. Heterogeneity of human monocytes: an optimized four-color flow cytometry protocol for analysis of monocyte subsets. J Cardiovasc Transl Res. 2011;4:211–219. doi: 10.1007/s12265-011-9256-4. [DOI] [PubMed] [Google Scholar]
- 23.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 24.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 25.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, et al. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–2586. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- 27.Baeten D, Boots AM, Steenbakkers PG, Elewaut D, Bos E, Verheijden GF, et al. Human cartilage gp-39+,CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000;43:1233–1243. doi: 10.1002/1529-0131(200006)43:6<1233::AID-ANR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Hepburn AL, Mason JC, Davies KA. Expression of Fcgamma and complement receptors on peripheral blood monocytes in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology. 2004;43:547–554. doi: 10.1093/rheumatology/keh112. [DOI] [PubMed] [Google Scholar]
- 29.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–677. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 30.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 31.Lee SM, Jeon ST, Suk K, Lee WH. Macrophages express membrane bound form of APRIL that can generate immunomodulatory signals. Immunology. 2010;131:350–356. doi: 10.1111/j.1365-2567.2010.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan SM, Xu D, Roschke V, Perry JW, Arkfeld DG, Ehresmann GR, et al. Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis Rheum. 2003;48:982–992. doi: 10.1002/art.10860. [DOI] [PubMed] [Google Scholar]
- 33.Dong W, Li X, Liu H, Zhu P. Infiltrations of plasma cells in synovium are highly associated with synovial fluid levels of APRIL in inflamed peripheral joints of rheumatoid arthritis. Rheumatol Int. 2009;29:801–806. doi: 10.1007/s00296-008-0773-7. [DOI] [PubMed] [Google Scholar]
- 34.Treamtrakanpon W, Tantivitayakul P, Benjachat T, Somparn P, Kittikowit W, Eiam-Ong S, et al. APRIL, a proliferation-inducing ligand, as a potential marker of lupus nephritis. Arthritis Res Ther. 2012;14:R252. doi: 10.1186/ar4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gheita TA, Bassyouni IH, Emad Y, el-Din AM, Abdel-Rasheed E, Hussein H. Elevated BAFF (BLyS) and APRIL in juvenile idiopathic arthritis patients: relation to clinical manifestations and disease activity. Joint Bone Spine. 2012;79:285–290. doi: 10.1016/j.jbspin.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–1467. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 37.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yano R, Yamamura M, Sunahori K, Takasugi K, Yamana J, Kawashima M, et al. Recruitment of CD16+ monocytes into synovial tissues is mediated by fractalkine and CX3CR1 in rheumatoid arthritis patients. Acta Med Okayama. 2007;61:89–98. doi: 10.18926/AMO/32882. [DOI] [PubMed] [Google Scholar]
- 39.Zhao LD, Li Y, Smith MF, Jr, Wang JS, Zhang W, Tang FL, et al. Expressions of BAFF/BAFF receptors and their correlation with disease activity in Chinese SLE patients. Lupus. 2010;19:1534–1549. doi: 10.1177/0961203310375268. [DOI] [PubMed] [Google Scholar]
- 40.Ozcan E, Rauter I, Garibyan L, Dillon SR, Geha RS. Toll-like receptor 9, transmembrane activator and calcium-modulating cyclophilin ligand interactor, and CD40 synergize in causing B-cell activation. J Allergy Clin Immun. 2011;128:601–609. doi: 10.1016/j.jaci.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 43.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 44.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 45.López-Fraga M, Fernández R, Albar JP, Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pradet-Balade B, Medema JP, López-Fraga M, Lozano JC, Kolfschoten GM, Picard A, et al. An endogenous hybrid mRNA encodes TWE-PRIL, a functional cell surface TWEAK-APRIL fusion protein. EMBO J. 2002;21:5711–5720. doi: 10.1093/emboj/cdf565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Hou Y, Jiang J, Zhao Q, Zhong W, Wang W, et al. Pharmacokinetics, pharmacodynamics, and tolerability of single ascending doses of RCT-18 in Chinese patients with rheumatoid arthritis. Clin Pharmacokinet. 2014;53:1033–1044. doi: 10.1007/s40262-014-0175-9. [DOI] [PubMed] [Google Scholar]
- 48.Daridon C, Burmester GR, Dörner T. Anticytokine therapy impacting on B cells in autoimmune diseases. Curr Opin Rheumatol. 2009;21:205–210. doi: 10.1097/BOR.0b013e32832a0760. [DOI] [PubMed] [Google Scholar]
- 49.van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2011;63:1782–1792. doi: 10.1002/art.30372. [DOI] [PubMed] [Google Scholar]
- 50.Genovese MC, Kinnman N, de La Bourdonnaye G, Pena Rossi C, Tak PP. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum. 2011;63:1793–1803. doi: 10.1002/art.30373. [DOI] [PubMed] [Google Scholar]
- 51.Moura RA, Canhão H, Polido-Pereira J, Rodrigues AM, Navalho M, Mourão AF, et al. BAFF and TACI gene expression are increased in patients with untreated very early rheumatoid arthritis. J Rheumatol. 2013;40:1293–1302. doi: 10.3899/jrheum.121110. [DOI] [PubMed] [Google Scholar]