Abstract
Adenoviruses have been clinically tested as anti-cancer therapies but their utility has been severely limited by rapid, systemic cytokine release and consequent inflammatory toxicity. Here we describe a new approach to tackling these dangerous side effects. Using human ovarian cancer cell lines as well as malignant epithelial cells harvested from the ascites of women with ovarian cancer, we show that tumour cells do not produce cytokines in the first 24 hours following in vitro infection with the oncolytic adenovirus dl922-947. In contrast, dl922-947 does induce inflammatory cytokines at early time points following intraperitoneal (IP) delivery in mice with human ovarian cancer IP xenografts. In these animals, cytokines originate predominantly in murine tissues, especially in macrophage-rich organs such as the spleen. We use a non-replicating adenovirus to confirm that early cytokine production is independent of adenoviral replication. Using β3 integrin knockout mice injected intraperitoneally with dl922-947 and β3 null murine peritoneal macrophages we confirm a role for macrophage cell surface β3 integrin in this dl922-947-induced inflammation. We present new evidence that co-administration of a cyclic RGD-mimetic specific inhibitor of β3 integrin significantly attenuates the cytokine release and inflammatory hepatic toxicity induced by dl922-947 in an IP murine model of ovarian cancer. Importantly, we find no evidence that β3 inhibition compromises viral infectivity and oncolysis in vitro or anticancer efficacy in vivo. By enabling safe, systemic delivery of replicating adenoviruses, this novel approach could have a major impact on the future development of these effective anti-cancer agents.
Keywords: Adenovirus, β3-integrin, ovary, cancer, inflammation
Introduction
Oncolytic adenoviruses replicate selectively within infected cancer cells, causing cell death. Viruses target immunity to infected malignant cells, whilst oncolysis activates T-cells by releasing tumour-specific antigens (reviewed in (1,2)). The potential of oncolytic adenoviruses as an anti-cancer therapy has repeatedly been demonstrated and one virus, H101 (3), has been licensed in China. A consistent and worrying feature of systemic viral therapy is the cytokine release that occurs rapidly after viral administration. These cytokines cause dose-limiting inflammatory toxicities, which can be severe (4-8) and have hindered further investigation and clinical development of these promising anti-cancer agents.
During adenoviral infection, the viral fibre terminal knob protein anchors cell surface Coxsackie Adenovirus Receptor (CAR). Arg-Gly-Asp (RGD) motifs in penton proteins at the base of the viral fibre also bind cell surface integrins, predominantly αvβ3 and αvβ5. Virions are internalised and transported along microtubules to the nucleus where viral replication takes place. Oncolytic viruses replicate selectively within cells with matched genetic defects. E1A-CR2 deleted adenoviruses including dl922-947 (9) and Δ24 (10) target cells with Rb checkpoint abnormalities, a defining feature of ovarian cancer (11,12). In a recent phase I trial, an E1A-CR2 deleted adenovirus induced stable disease in 14 of 19 patients with advanced gynaecological cancers (13).
Ovarian cancer, in particular the high grade serous subtype, spreads by seeding across peritoneal surfaces, producing ascites that is rich in macrophages and inflammatory cytokines. This local inflammation has been implicated in tumour promotion (14), and clinical trials have suggested that inhibition of the cytokine TNF-α may have therapeutic potential (15). We have shown that siltuximab (CNTO328), an inhibitory monoclonal antibody to another cytokine, IL-6, induces anti-tumour responses in patients with relapsed ovarian cancer (16). Since tumour spread outside the peritoneum is rare, ovarian cancer is amenable to therapies delivered by the intraperitoneal (IP) route. In oncolytic virotherapy, IP rather than intravenous (IV) delivery can reduce systemic, cytokine-induced toxicity but local inflammation still causes severe abdominal side effects (17). Although various mechanisms have been described by which viruses initiate inflammation, the interplay between viruses and the microenvironment of the peritoneal cavity in ovarian cancer is poorly understood and the extent to which inflammation helps or hinders viral oncolytic efficacy remains unclear.
We have previously shown that the replication-selective, E1A-CR2 deleted adenovirus, dl922-947, has more potent anti-cancer activity in vitro than wild-type adenovirus (18). In female nude mice with intraperitoneal human ovarian cancer xenografts, which recapitulate the cytokine and macrophage-rich peritoneal environment of human ovarian cancer, survival was significantly prolonged by IP dl922-947 but, similar to the human experience with these agents, high levels of ascitic cytokines (unpublished) and marked hepatic toxicity were observed (18). We previously attempted to modulate these inflammatory side effects by combining oncolytic adenovirus with inhibitors of TNF-α. Although this improved viral efficacy, liver toxicity still occurred (19).
In non-tumour bearing mice, wild-type adenovirus 5 (Ad5-WT) has been shown to promote inflammation via viral RGD motifs binding β3 integrins on macrophages, including hepatic kupffer cells and splenic marginal zone macrophages (20). Cytokine expression was attenuated with an RGD-deleted adenovirus (20). We now describe a novel, clinically applicable approach to control the inflammatory side effects of oncolytic adenoviruses through pharmacological inhibition of β3 integrins in ovarian cancer. In line with the accumulated evidence from clinical trials, we confirm that oncolytic adenovirus causes rapid, systemic cytokine induction in vivo. In agreement with previously published data, we observed that cytokines originate predominantly in macrophage-rich supporting tissues rather than malignant epithelial cells and that cytokine expression and release was dependent on β3 integrins.
In a murine ovarian cancer model, we show for the first time that virus-induced systemic cytokines and hepatic toxicity can be reduced by pharmacological inhibition of β3 with the cyclic RGD-mimetic peptide, H2574, without compromising anticancer activity. H2574, also described as EMD 66203, c(RGDfV), is widely used pre-clinically as a specific inhibitor of β3 integrins. H2574 inhibits binding of vitronectin to αvβ3 integrin with an IC50 of 0.0025μM, whilst inhibition of αvβ5 is minimal (reviewed in (21). We used H2574 to maximise the clinical applicability of our findings since its N-methylated analogue, Cilengitide (EMD 121974, c(RGD-N(Me)V-), is already in clinical use. In contrast to H2574, Cilengitide does have inhibitory activity for αvβ5 but this is still more than 100 fold lower than that for αvβ3 (IC50 = 140nM compared to 1nM for αvβ3 (22)). The novel pharmacological approach we describe here should enable the safe delivery of many viral anticancer agents and thus facilitate further clinical testing of many more of these promising therapies.
Materials and Methods
Cell lines, viruses and chemicals
SKOV3ip1 cells (23) were obtained from Cancer Research UK (Clare Hall, UK). OVCAR4 (24) were obtained from Dr R. Camalier (NCI-Frederick, MD, USA). Murine WT, β3- and hβ3+ endothelial cells were transformed with Polyoma Middle T Antigen (obtained from Dr S. Robinson, University of East Anglia (25)). SKOV3ip1 and OVCAR4 cells were cultured in DMEM plus 1% penicillin and streptomycin and 10% FCS and underwent 16 locus STR verification (DNA Diagnostics Centre, London, UK. 10/6/14). Immortalised endothelial cells were grown in 1:1 DMEM (low glucose) and Ham’s F12 containing 20% FCS, 50mg Heparin (Sigma-Aldrich, Dorset, UK), 100ml GlutaMAX (Gibco, Paisley, UK), 1% penicillin and streptomycin and 100mg endothelial mitogen (AbD-Serotec).
dl922-947 (9) and dlCR2-pIX-dsRed (26) are already described. Ad-GFP is an E1-deleted, non-replicating, type-5 adenovirus, expressing GFP under the CMV promoter. H2574 was purchased from Bachem (Bubendorf, Switzerland). Cell survival was assessed by MTT assay (18).
RNA extraction, reverse transcription and quantitative-PCR
Cultured cells and 30mg murine tissue were lyzed with Trizol (Invitrogen, Paisley, UK) and homogenised by pipetting (cultured cells) or with a T25 Ultratissue homogenizer (tissue). After 5min incubation at room temperature (RT), 200μl chloroform was added per 1ml of Trizol. Samples were mixed, incubated (2min, RT) and centrifuged (15min, 1400rpm, 4°C). RNA was precipitated from the aqueous upper phase with 500μl isopropanol at −20°C for 20min. The sample was then applied to a RNeasy mini column (Qiagen, Manchester, UK) according to the manufacturer’s instructions. 1μg RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies, Paisley, UK) (25°C, 10min; 37°C, 120min; 85°C, 5min). cDNA was subjected to qPCR using Taqman Gene Expression Assay primers (Life Technologies, Paisley, UK). Expression was normalised to 18s RNA.
Protein extraction and cytokine ELISA
Cell supernatants were collected over time post adenovirus. Tissues were lyzed in 600μl lysis buffer (150mM NaCL, 20mM TrisBase, 1mM EDTA, 1mM EGTA, 1% Triton X-100) with Complete Protease Inhibitor cocktail tablets (Roche Diagnostics, Welwyn, UK) and homogenized with a T25 Ultratissue homogenizer. After 30min incubation on ice, samples were centrifuged (15min, 13,000rpm, 4°C) and protein in the lower liquid phase diluted to 5mg/ml. Cytokines were measured using the Mesoscale Discovery System multiplex plates (Mesoscale Discovery, Rockville, MD, USA).
Immunofluorescence
Cells were grown on poly-L-lysine coated coverslips and fixed in 3.7% formaldehyde (10mins, RT). Cells were washed, permeabilised in 0.5% Triton for 10min and then blocked in 10% goat serum (1hour, RT). Cells were incubated with primary antibody (EpCam 1:500 [AbCam ab20160]) for 1hour at RT in 0.2% PBS-Tween+3% BSA, washed and then incubated with secondary antibody (Alexa Fluor 488 goat anti-mouse IgG [Molecular Probes, Invitrogen]) for 1hour at RT. Coverslips were stained with DAPI (Invitrogen) and mounted onto glass slides.
Viral internalisation in murine peritoneal macrophages: PCR
Peritoneal macrophages were harvested from WT and β3−/− mice as described in Animal Models. 5×105 murine peritoneal cells were seeded in 6cm plates. After overnight incubation, cells were washed in cold RPMI+1% BSA and infected with dl922-947 (MOI 10) on ice for 1hour. Unbound virus was removed by washing in cold RPMI. Cells were then incubated at 37°C for 1hour. DNA was harvested from cell pellets using a QIampDNA extraction kit (Qiagen, Manchester, UK). Hexon was quantified by qPCR against a standard curve of viral particles (5′-GGTGGCCATTACCTTTGACTCT-3′, 3′-GGGTAAGCAGGCGGTCATT-5′, probe:[6FAM]CTGTCAGCTGGCCTGG [TAM]).
Viral internalisation in murine peritoneal macrophages: Confocal microscopy
Peritoneal macrophages were harvested from WT and β3−/− mice as described in Animal Models. 5×105 murine peritoneal cells were seeded overnight on poly-L-lysine coated coverslips. After overnight incubation, cells were washed in cold RPMI+1% BSA and infected with dlCR2-pIX-dsRed (50,000vp/cell) on ice for 1hour. Unbound virus was removed by washing in cold RPMI. Cells were fixed in cold 95% ethanol and 5% acetic acid (10min, RT) or the infection was continued at 37°C for a further 1hour prior to washing and fixing. Fixed cells were blocked with 3% BSA for 30min and then incubated at RT for 60min in anti-CD11b-AlexaFluor488 (1:100 [eBioscience, Hatfield, UK]). Cells were stained with DAPI (1:10,000, 1mg/ml [Invitrogen]), washed and mounted onto glass slides.
Confocal microscopy
Confocal was performed with an inverted Zeiss LSM 510 META laser-scanning microscope with a Plan Apochromat 63x/1.4 Oil objective. DAPI was detected with a 405nm laser. Alexa Fluor 488 with a 488nm laser and dsRed with a 543nm laser. Images were acquired in the x,y,z direction with a line average of 4. Z-sections were acquired at optimum interval levels with sections of 0.43μm. maximal intensity Z-projections were assembled with the LSM5 Image browser software.
Flow cytometry in murine peritoneal cells
Peritoneal macrophages were harvested from WT and β3−/− mice as described in Animal Models. For adenoviral receptors, cells were blocked with Fc fragments for 15min before addition of conjugated antibodies (CAR-PE (Millipore, Watford, UK), β3-PE (eBioscience, Hatfield, UK), β5-PE (eBioscience, Hatfield, UK) and IgG-PE (eBioscience, Hatfield, UK)) in PBS+1% BSA for 1hour on ice in the dark. Samples were analysed on a BD FACSCalibur (BectonDickinson, Oxford, UK).
To quantify macrophage cell surface markers using multichannel flow cytometry, cells were incubated with a viability dye (eBioscience, Hatfield, UK) for 30min in the dark on ice and then blocked with Fc fragments for 15min. Conjugated antibodies (CD45-FITC (eBioscience, Hatfield, UK), CD11b-eFluor450 (eBioscience, Hatfield, UK), mMR-PE/CD206 (BioLegend, London, UK), Ly-6C-APC (eBioscience, Hatfield, UK), Ly-6G-AlexaFluor700 (eBioscience, Hatfield, UK), CsfR1/CD115 (eBioscience, Hatfield, UK) and F4/80-PE-Cy5 (eBioscience, Hatfield, UK)) were added for 15min on ice in the dark. Cells were washed and fixed in 2% formaldehyde. Samples were analysed on a BD LSRFortessa (BectonDickinson, Oxford, UK).
Animal models
Experiments complied with National Cancer Research Institute guidelines and were conducted under UK government Home Office personal (70/19108) and project (70/7263) licenses. Local approval was obtained from Queen Mary University of London (PAC60-3). β3−/− mice (B6.129S2-Itgb3tm1Hyn/JSemJ) and WT controls (obtained from Prof K. Hodivala-Dilke, Barts Cancer Institute) (27) were bred inhouse and used between 6 and 8 weeks old. Intraperitoneal (IP) ovarian cancer xenografts were created by inoculating 5×106 SKOV3ip1 cells IP in 6 week old CD1nu/nu female mice. For most experiments, 1010 particles of dl922-947 were injected IP in 400μl PBS. For virus efficacy, dl922-947 (5×109 particles) was injected daily for 5 days in 400μl PBS. 5mg H2574 was dissolved in DMSO, diluted in H2O and then injected IP (total volume 400μl) or into an Alzet pump (Cupertino, CA, USA) (total volume 210μl). Alzet pumps were inserted subcutaneously and removed two weeks later under isofluorane inhalation anaesthesia. Mice were assessed for weight, general health and accumulation of ascites and were killed according to UK Home Office guidelines.
At necropsy, murine peritoneal macrophages were harvested by peritoneal lavage with 10mls cold PBS. Samples were treated with Red Blood Cell Lysis Buffer (Sigma-Aldrich, Dorset, UK), washed and cultured in RPMI, 1% penicillin and streptomycin and 10% FCS. Murine blood was obtained by cardiac puncture. Serum liver function was analysed at the Royal Veterinary College (Hertfordshire, UK) and cytokines were quantified with the Mesoscale Discovery System. Murine tissue was macrodissected at postmortem and either snap frozen in liquid nitrogen or fixed in 10% formaldehyde. 4μm sections were cut from formalin-fixed, paraffin-embedded tissues. All pathology and immunohistochemistry slides were interpreted and scored by a pathologist blind to the experimental groups.
Patient samples
Patient samples were collected with informed consent, via the Barts Gynae Tissue Bank (Research Ethics Committee: 10/H0304/14). Ascites was obtained from women with ovarian cancer undergoing therapeutic drainage at St Bartholomew’s Hospital (London, UK). Ascites was centrifuged (500g, 15min) and supernatant stored at −80°C. Cells were cultured in RPMI and 25% autologous supernatant.
Statistics
Prism 6.0 (GraphPad, San Diego, CA) was used for statistical analysis. Data are presented as mean ± SD throughout and the unpaired t-test was used for all experiments except for analysis of changes over time for which two-way Anova was used. p<0.05 is considered statistically significant.
Results
Oncolytic adenovirus does not induce early cytokine production in ovarian cancer cells
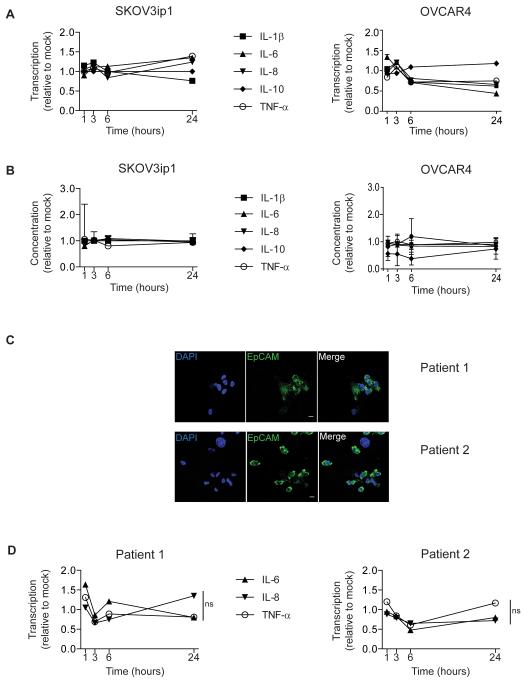
To explore the kinetics of early adenovirus-induced cytokine release, we used SKOV3ip1 cells (high β3, low CAR), because we had previously shown that these cells can produce high levels of TNF-α and IL-6 (19). Cytokine production by SKOV3ip1 cells was negligible for the first 24 hours after dl922-947 infection (Fig.1A and B). Infecting cells with Ad5-WT or higher viral doses did not increase cytokine protein release compared to dl922-947 MOI 10 (Supplementary Fig.S1). To demonstrate that this observation was not unique, we evaluated another ovarian cancer cell line: OVCAR4 (high β3, moderate CAR). We again found no increase in cytokines in the first 24 hours (Fig.1A and B). We also investigated tumour cells harvested from the ascites of two women with (high grade serous) ovarian cancer (Fig.1C). In these ascitic cells, transcription of IL-6, IL-8 and TNF-α did not increase above baseline for the first 24 hours after ex vivo infection with dl922-947 (Fig.1D).
Fig.1. Adenovirus does not cause rapid cytokine induction in ovarian cancer cells in vitro.
A+B SKOV3ip1 and OVCAR4 cells were infected with dl922-947 (MOI 10) or vehicle for 1-24 hours. Cytokine mRNA expression (A) and cytokine protein in cell supernatants (B) were compared to mock. Experiments were repeated three times and mean±SD is shown.
C Confocal microscopy of EpCAM positive malignant cells harvested from the ascites of two ovarian cancer patients (Scale=10μm)
D Malignant cells from two ovarian cancer patients were infected with dl922-947 (MOI 10) or vehicle. Cytokine mRNA expression was compared to mock. Samples were analysed in triplicate and mean±SD is shown. (ns=non-significant).
Oncolytic adenovirus induces cytokines in murine tissues at early time points
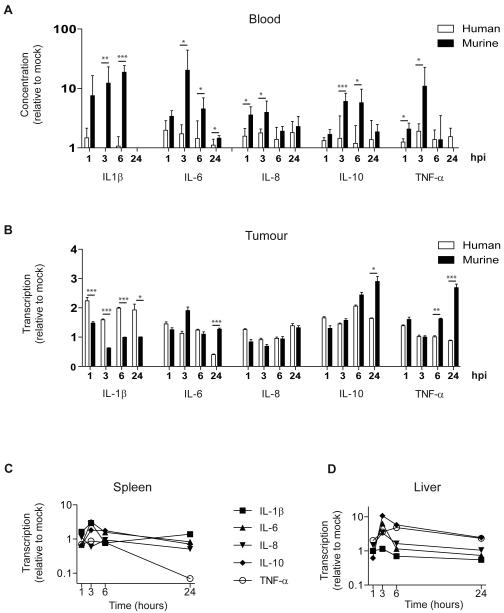
To model the adenovirus-induced inflammatory response in more clinically relevant models, we created intraperitoneal SKOV3ip1 xenografts in female nude mice. Mice with established tumours received a single intraperitoneal (IP) injection of dl922-947 (1010 particles). Cytokines were quantified in blood, intraperitoneal tumours, spleen and liver (Fig.2). All cytokines tested increased in the blood within 1 hour (Fig.2A). Using species-specific reagents, we found that circulating cytokines originated predominantly in murine tissues rather than the human xenografted SKOV3ip1 cells. This was true for all cytokines (Fig.2A). To determine the origin of these cytokines, mRNA was quantified in excised tissues. Although adenovirus did not induce early cytokine release in SKOV3ip1 cells in vitro, human cytokine mRNA was identified in intraperitoneal SKOV3ip1 tumours (Fig.2B). In a separate experiment, cytokine mRNA correlated closely with cytokine protein isolated from the same IP tumours (Supplementary Fig.S2). This suggests that cytokine production by tumour cells is enhanced by interaction with the tumour stroma or in this case the murine component of the xenograft. Tumour stroma appears also to be an important source of inflammatory cytokines since murine cytokine mRNA in excised tumours exceeded human mRNA for several cytokines (IL-6, IL-10 and TNF-α), (Fig.2B). Murine spleen and liver were also rich sources of inflammatory cytokines. Although the profiles of individual cytokines differed in the two organs, cytokines peaked in both spleen and liver at 3 hours and had largely returned to baseline 24 hours after adenovirus (Fig.2C and D).
Fig.2. Adenovirus causes rapid cytokine induction in murine tissues.
Female CD1nu/nu mice with intraperitoneal SKOV3ip1 tumours were injected IP with dl922-947 (1010 particles). Samples were analysed in triplicate and data are presented as mean±SD (n=3-4).
A. Cytokine protein in murine serum using mouse and human specific plates shown relative to 0 hours. Mouse and human cytokines are compared (p<0.05, **p<0.01, ***p<0.001). hpi=hours post-infection.
B. qRT-PCR analysis of tumour. Expression at 1, 3, 6 and 24 hours is shown relative to expression at 0 hours. Mouse and human cytokines are compared (*p<0.05, **p<0.01, ***p<0.001). hpi=hours post-infection.
C+D qRT-PCR analysis of spleen (C) and liver (D). Cytokines at 1, 3, 6 and 24 hours are shown relative to 0 hours post IP dl922-947.
Cytokine induction correlates with viral presence
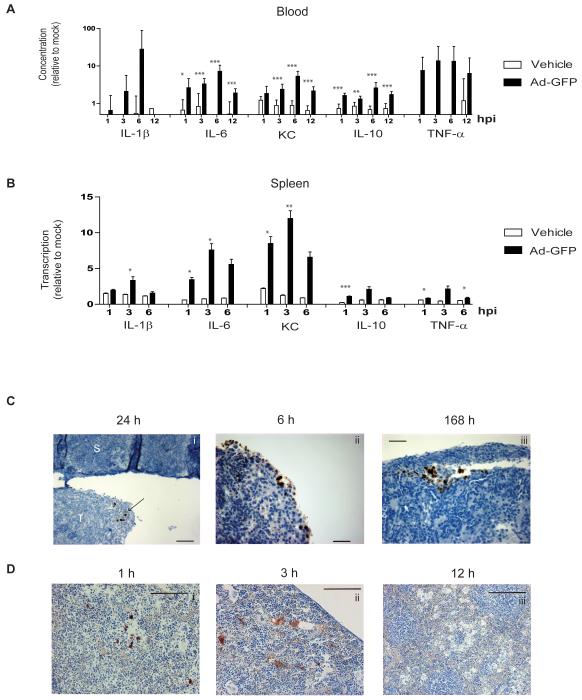
The adenoviral replication cycle takes at least 24 hours and is severely attenuated in murine cells (28,29). Our observations that in vivo cytokine release originates in murine tissue and occurs at early time points following IP injection imply that adenovirus-induced cytokine release is independent of viral replication. To test this, cytokines were analysed following a single IP injection of a non-replicating virus (Ad-GFP, 1010 particles) in mice with mature IP SKOV3ip1 xenografts. In the circulation (Fig.3A), and spleen (Fig.3B), murine cytokine levels were significantly greater following Ad-GFP compared to PBS control. All circulating cytokines tested peaked 6 hours after IP injection. Likewise, splenic cytokine production also increased over the first 6 hours, suggesting that adenoviral replication is not responsible for inducing cytokines in murine tissues at early time points.
Fig.3. Adenovirus-induced cytokine production correlates with viral presence.
Female CD1nu/nu mice with intraperitoneal SKOV3ip1 tumours were injected IP with the non-replicating virus, Ad-GFP (1010 particles), or with vehicle. (n=3-5). hpi=hours post-infection.
A Cytokine protein in murine serum relative to 0 hours. Ad-GFP and vehicle are compared. Samples were analysed in triplicate and mean±SD is shown (*p<0.05, **p<0.01, ***p<0.001). KC=keratinocyte chemoattractant.
B. qRT-PCR analysis of spleen. Expression at 1, 3, and 6 hours is shown relative to 0 hours. Ad-GFP and vehicle are compared. Samples were analysed in triplicate and mean±SD is shown (*p<0.05, **p<0.01, ***p<0.001). KC=keratinocyte chemoattractant.
C+D. Female CD1nu/nu mice with intraperitoneal SKOV3ip1 tumours were injected IP with dl922-947 (1010 particles). Representative images are shown following immunohistochemistry for adenoviral E1A in IP tumours (C: T=tumour, S=spleen. Scale=50μm.) and Hexon protein in murine spleen (D: Scale=50μm (i and ii) or 100μm (iii).
To explore this further, spleen and tumour were analysed by immunohistochemistry following a single dose of dl922-947 in mice bearing SKOV3ip1 xenografts (Fig.3C and D). E1A is the first adenoviral protein to be expressed. As expected, we only found E1A expression in human tumour tissues and never in murine tissues (Fig.3Ci). In contrast to cytokines, which were first seen at 1 hour and had resolved by 24 hours, E1A was first identified at 6 hours (Fig.3Cii) and persisted for up to a week (Fig.3Ciii and Supplementary Fig.S3). Hexon, the major structural protein of the adenoviral coat is indicative of viral presence rather than active replication. In the spleen, discrete hexon staining was first seen 1 hour post-adenoviral injection (Fig.3Di). By 3 hours, the staining was more diffuse and widespread (Fig.3Dii) and by 12 hours it had resolved (Fig.3Diii) mirroring the kinetics of cytokine release. These findings suggest that cytokines are induced by adenoviral presence rather than replication.
Cytokine induction by oncolytic adenovirus is β3 integrin-dependent
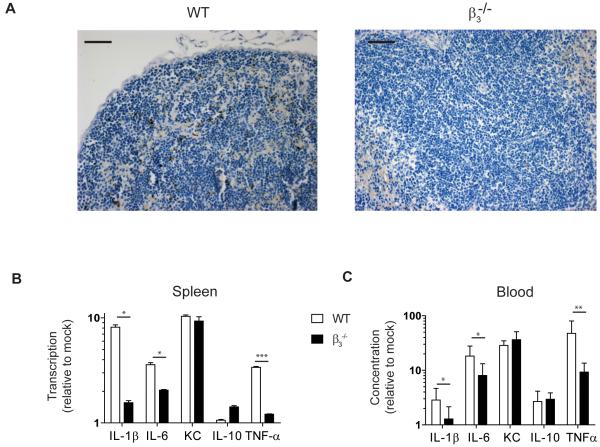
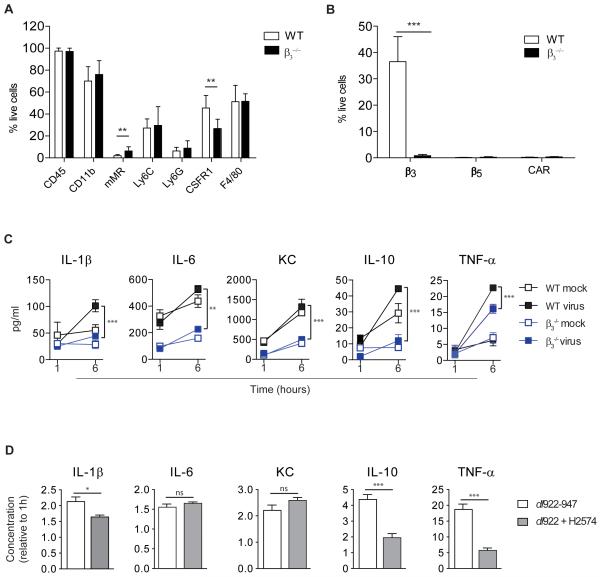
In non-tumour bearing mice, wild-type adenovirus induces cytokines via β3 integrin-expressing macrophages (20). Having shown that marked cytokine production occurs in macrophage-rich tissues such as liver and spleen, we explored the role of β3 integrins following oncolytic adenovirus in the malignant setting. First, we injected WT and β3−/− mice with a single dose of dl922-947 IP (1010 particles). Immunohistochemistry identified hexon in the spleens of WT mice three hours post-injection (Fig.4A). In contrast, no hexon was identified in the spleens of β3−/− animals (Fig.4A and Supplementary Fig.S4). Cytokine transcription was significantly higher in the spleens of WT compared to β3−/− mice (Fig.4B), indicating that β3-mediated viral uptake induces splenic cytokine transcription. This was most marked for IL-1β (p<0.05), IL-6 (p<0.05) and TNF-α (p<0.001). There was no difference in baseline circulating cytokines in the two genotypes but adenovirus-induced cytokines were significantly higher in the circulation of WT compared to β3−/− animals (p<0.05 IL-1β and IL-6; p<0.01 TNF-α) (Fig.4C). Although cytokine induction was attenuated in β3−/− compared to WT animals, some cytokines were induced in β3−/− mice, most notably KC (keratinocyte chemoattractant: murine homologue of IL-8), confirming that β3 is not the only mediator of the virus-induced inflammatory response.
Fig.4. Adenovirus-induced cytokine release is dependent on β3 integrins.
WT and β3−/− female mice were injected IP with dl922-947 (1010particles) or with vehicle (n=4-7).
A. Hexon immunohistochemistry in murine spleens. Scale=50μm.
B. qRT-PCR analysis of murine spleen. Expression at 3 hours is shown relative to 0 hours and WT and β3−/− mice are compared. Samples were analysed in triplicate and data are presented as mean±SD (*p<0.05, ***p<0.001). KC=keratinocyte chemoattractant
C. Cytokine protein in murine serum post. Concentration at 3 hours is shown relative to 0 hours. WT and β3−/− mice are compared. Samples were analysed in triplicate and data are presented as mean±SD (*p<0.05, **p<0.01). KC=keratinocyte chemoattractant
Since ovarian cancer is predominantly a disease of the peritoneum, we investigated peritoneal cells in WT and β3−/− mice. In both genotypes, these cells showed strong expression of CD11b (WT:70.1±13.1% of live cells, β3−/−:76.1±12.6%) and F4/80 (WT:51.3±14.9% of live cells, β3−/−: 51.9±6.8%) indicating that they were predominantly macrophages (Fig.5A). As expected, expression of β3 was significantly higher in peritoneal cells from WT compared to β3−/− mice (p<0.001) but β5 and CAR were negligible in both genotypes (Fig.5B). Peritoneal cells were infected ex vivo with dl922-947 or vehicle and cytokine production measured 6 hours later (Fig.5C). With the exception of KC, in both genotypes the addition of virus significantly increased cytokine production compared to control (WT: IL-6, p<0.01; IL-1β, IL-10, TNF-α, p<0.001 and β3−/−: IL-10, p<0.01; IL-1β, IL-6, TNF-α, p<0.001). However, release of all cytokines over time following adenovirus was significantly attenuated in β3−/− compared to WT cells (IL-6, p<0.01; IL-1β, KC, IL-10, TNF-α, p<0.001).
Fig.5. Cytokine release by murine peritoneal macrophages is β3 integrin-dependent.
Peritoneal cells from female WT and β3−/− mice were pooled for each genotype and assessed by multichannel FACS. Experiments were carried out in triplicate and data are presented as mean±SD (n=4-24).
A. Expression of macrophage cell surface markers (**p<0.01).
B. Expression of the adenoviral receptors, CAR, β3 and β5 (***p<0.001).
C. Murine peritoneal cells were treated ex vivo with dl922-947 (10,000vp/cell) or vehicle. Cytokine protein in supernatants was quantified at 1 and 6 hours. Change over time was compared in WT and β3−/− peritoneal cells. (**p<0.01, ***p<0.001). KC=keratinocyte chemoattractant
D. Murine peritoneal cells were pooled and treated ex vivo with dl922-947 (10,000vp/cell) alone or in combination with the β3 inhibitor, H2574 (20μM). Cytokine protein is shown at 6 hours relative to 1 hour (*p<0.05, ***p<0.001, ns=non-significant). KC=keratinocyte chemoattractant
We then tested whether cytokine release could be reduced by pharmacological inhibition of β3 integrin. Peritoneal cells from WT mice were treated ex vivo with dl922-947 together with the cyclic RDG-mimetic inhibitor of β3 integrin, H2574. The addition of H2574 caused a significant reduction in IL-1β (p<0.05), IL-10 (p<0.001) and TNF-α (p<0.001) 6 hours post-treatment (Fig.5D).
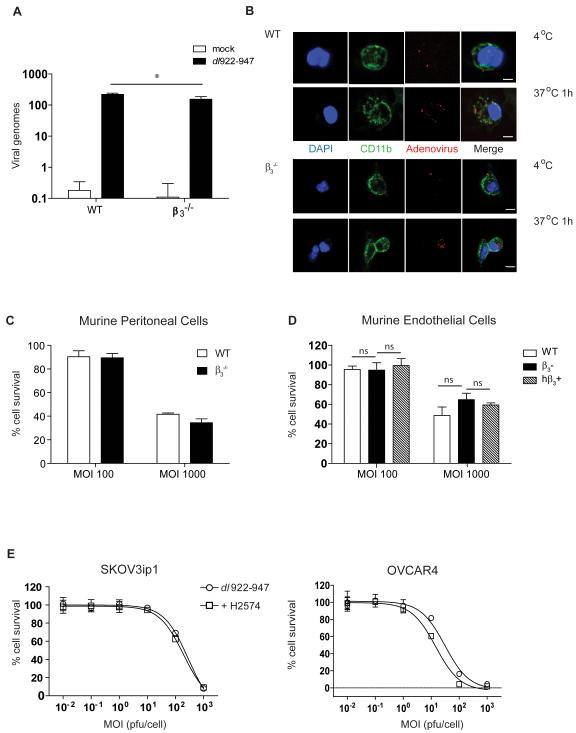
β3 integrin is not essential for adenoviral cytotoxicity
αvβ3 and αvβ5 integrins are known to act as secondary receptors during adenoviral infection. Pharmacological inhibition of these integrins might therefore have the undesirable effect of reducing adenoviral infectivity and thus anti-cancer activity. In murine peritoneal cells infected ex vivo with dl922-947, PCR for adenoviral hexon showed that, whilst adenovirus was internalised by β3−/− cells, this was lower than in WT cells (Fig.6A). To explore this, we used a fluorescent capsid-labelled adenovirus, dlCR2-pIX-dsRed (26) and demonstrated adenovirus at the cell surface after infection at 4°C followed by viral internalisation when cells were warmed to 37°C in both genotypes (Fig.6B).
Fig.6. β3 integrin expression is not essential for adenoviral cytotoxicity.
A. qPCR for adenoviral hexon in pooled peritoneal cells from female WT and β3−/− mice following infection with dl922-947 (MOI 10) or vehicle. Experiments were repeated three times and mean±SD is shown. (*p<0.05).
B. Pooled peritoneal cells from female WT and β3−/− mice were infected with dlCR2-pIX-dsRed on ice, warmed to 37°C for 1 hour, stained with DAPI and CD11b and analysed by confocal microscopy. Scale=10μm.
C. Pooled peritoneal cells from female WT and β3−/− mice were treated with dl922-947 and cell survival quantified by MTT 72 hours later. Experiments were repeated three times and data are presented as mean±SD.
D. Endothelial cells from WT and β3−/− mice were transformed using polyoma middle T antigen and human β3 integrin was over-expressed in β3-cells (hβ3+). Adenoviral cytotoxicity was analysed by MTT assay at 120 hours post-infection with dl922-947. Experiments were repeated three times and data are presented as mean±SD (ns=non-significant).
E. SKOV3ip1 and OVCAR4 cells were treated with dl922-947 alone or in combination with the β3 inhibitor, H2574 (2μM) and cell survival quantified by MTT at 120 hours. Experiments were repeated three times and data are presented as mean±SD.
Next we tested whether integrins were required for adenoviral cytotoxicity in murine peritoneal cells from β3−/− and WT mice (Fig.6C). We also used immortalised endothelial cells from β3−/− and WT mice, as well as immortalised endothelial cells created from β3−/− mice, which over-express human β3 (hβ3+)(Fig.6D). In all cells, cytotoxicity was not influenced by β3 integrin expression. We then investigated whether β3 inhibition reduced adenoviral cytotoxicity in human cancer cells. Two ovarian cancer cell lines, with high β3 integrin expression: SKOV3ip1 (low CAR) and OVCAR4 (moderate CAR), were treated with dl922-947 together with H2574. The addition of H2574 did not impair viral cytotoxicity in either cell line (Fig.6E). Together, these experiments confirm that inhibition of adenoviral uptake was not a concern, supporting further testing of the dl922-947 and H2574 combination in vivo.
β3 inhibition reduces adenoviral inflammatory toxicity in a murine model of ovarian cancer
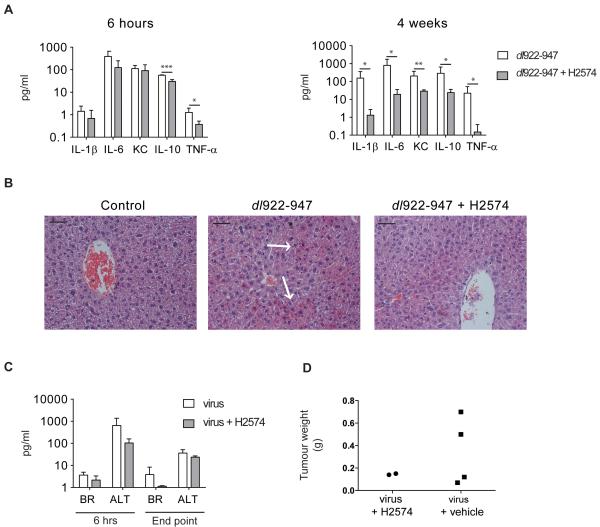
Female CD1nu/nu mice with SKOV3ip1 xenografts were treated with a single IP dose of dl922-947 (1010 particles) and H2574 (5mg). In a separate experiment, mice received our standard therapeutic viral dosing regimen of 109 particles IP daily for 5 days (18) together with a subcutaneous pump infusing H2574 (5mg/2weeks). Vehicle controls were used for all experiments. In the single dose experiment, mice were sacrificed 6 hours after treatment, whilst mice with subcutaneous pumps were sacrificed either at 4 weeks or when they reached UK Home Office end point. At 6 hours cytokine levels were lower in mice that had received H2574, reaching statistical significance for all cytokines by four weeks (Fig.7A).
Fig.7. β3 inhibition reduces inflammatory toxicity in vivo.
Female CD1nu/nu mice (n=3-5) with IP SKOV3ip1 tumours were co-treated with a single dose of dl922-947 (1010 particles) and H2574 (5mg) IP and sacrificed 6 hours later or co-treated with dl922-947 (109 particles IP) days 1-5 and H2574 (5mg) via subcutaneous pump for two weeks and sacrificed at 4 weeks.
A. Cytokines in murine serum were analysed 6 hours and 4 weeks post-treatment by Mesoscale. Mean±SD (*p<0.05, **p<0.01, ***p<0.001). KC=keratinocyte chemoattractant
B. H+E staining of murine livers 6 hours post-IP dl922-947 and H2574. Slides were reviewed by a pathologist who was blind to the treatment groups. Representative images are shown. Arrows indicate eosinophilic degeneration. Scale=50μm.
C. Liver function tests on murine serum 6 hours post-IP treatment and when mice reached UK Home Office end point. BR=serum bilirubin, ALT=alanine transaminase. (Mean±SD)
D. Weights of macro-dissected IP tumours when mice reached UK Home Office end point. (Mean±SD)
To determine whether this H2574-induced reduction in circulating cytokines correlated with diminished inflammatory toxicities, livers were examined. As early as 6 hours post-injection, eosinophilic degeneration, indicative of viral hepatotoxicity, was apparent in dl922-947 treated mice but not in mice treated with dl922-947 and H2574 combined (Fig.7B). Liver function tests upheld this observation since both bilirubin and alanine transaminase were reduced by co-administration of dl922-947 with H2574 (Fig.7C), although this did not reach statistical significance. This experiment was not powered to detect differences in survival. However as an indication of anti-tumour efficacy, we weighed all residual peritoneal tumour in the small number of mice that reached legal limits. In accordance with our in vitro findings, the combination of dl922-947 and H2574 did not reduce anticancer efficacy compared to dl922-947 alone (Fig.7D).
Discussion
Systemic inflammation induced by oncolytic adenoviruses is a major limitation to the clinical development of these promising anti-cancer agents. Here we show in primary malignant cells, ovarian cancer cells lines, genetically modified mice and ovarian cancer xenografts that the oncolytic adenovirus, dl922-947, causes rapid induction of inflammatory cytokines that originate primarily in macrophage-rich host tissues. We confirm that cytokine release and the associated inflammatory hepatic toxicity is β3 integrin-dependent and show here for the first time that these toxicities can be controlled by pharmacological inhibition of β3 integrins without obviously compromising anti-cancer efficacy.
Ovarian cancer is characterised by a tumour-promoting network of cytokines (14). We previously showed that oncolytic adenoviruses, including dl922-947, induce TNF-α in ovarian cancer cells between 24 and 72 hours (19). However, the clinical experience with systemic adenovirus suggests that cytokine release occurs much earlier (5,6). Most notably, in one highly publicised death, the patient had already developed advanced symptoms of systemic inflammation 18 hours after virus administration and interestingly, serum TNF-α was not initially elevated (4). When we inhibited TNF-α with RNAi or with anti-TNF-α antibodies in murine ovarian cancer xenografts, virus-induced hepatic toxicity was not reduced (19). These observations imply that clinically problematic inflammation occurs early after systemic virus and that TNF-α may not be the initiating cytokine.
In ovarian cancer cell lines in vitro, we did not observe an increase in cytokines in the first 24 hours following adenovirus. In contrast, the very early cytokine production seen in murine xenografts more accurately reproduced the human experience and was associated with the inflammatory hepatotoxicity and elevation of liver enzymes that is well described following adenovirus infection (30-33). The differences we observed in ovarian cancer cells in vitro and in vivo implicate tumour/stroma interactions in the initiation of this inflammatory response. In support of this, adenoviral induction of pro-inflammatory cytokines has been shown to be augmented when malignant epithelial cells are co-cultured with macrophages in vitro (34). In tumour-bearing mice we found that virus-induced cytokines arose predominantly in murine tissues rather than the implanted human tumour cells. The cell types that are responsible for innate responses to adenovirus such as macrophages and dendritic cells, (33,35,36) are present at high levels in liver, spleen and peritoneum and are therefore likely to be a major source of the systemic inflammatory cytokines reported here.
Our finding that adenoviral cytokine induction is not dependent on viral replication and gene expression is also in keeping with a large body of evidence, acquired in non-tumour bearing organisms, implicating a range of potential viral sensors as initiators of the innate immune response (20,37,38). In addition to the interaction of viral RGD with β3 integrins previously reported with Ad5-WT in the non-malignant setting (20), internalised viral DNA is sensed by toll-like receptors, which stimulate dendritic cells and macrophages to release type I interferon. Viral DNA has also been shown to cause an inflammatory response in macrophages via maturation of IL-1β within a cytosolic complex known as the inflammasome (38).
The existence of these varied mechanisms explains our observation that, whilst inhibition of β3 integrins reduces inflammatory cytokine release, it does not completely abrogate the cytokine response. Nonetheless, the interaction between adenoviral coat RGD and β3 integrins on the macrophage surface is particularly attractive for targeted therapeutic manipulation. Mutant RGD adenoviruses have been used to abrogate viral binding to β3 integrin (20) but the need to create RGD mutant versions of all clinically applicable oncolytic adenoviruses is problematic in terms of clinical development. Others have attempted to manipulate the inflammatory response with immunosuppressive agents such as cyclophosphamide (39) and cyclosporine (40). Both of these drugs have myriad other effects, including a paradoxical immunostimulatory effect of cyclophosphamide (41). Pharmacological inhibition of integrins offers a more specific, versatile and clinically applicable strategy that could be applied to a range of viral agents.
To our knowledge, this is the first investigation of a specific pharmacological strategy to inhibit integrin binding and the first evaluation of this approach in murine cancer models. Given the role of αvβ3 as a secondary adenoviral receptor, we were reassured to find that β3 null cells could still be infected and that oncolysis was not impaired by the absence of β3. In ovarian cancer, which is largely a disease of the peritoneum, our demonstration of a reduction in cytokine production by β3 null murine peritoneal cells is particularly clinically relevant.
αvβ3 is up-regulated on tumour blood vessels (42). Several drugs targeting this pathway are currently undergoing testing as anticancer therapies (43) and cilengitide, the most widely studied inhibitor of αvβ3 (44), has shown some clinical activity in glioblastoma (45,46). In ovarian cancer, a small case series identified β3 integrin expression in normal ovarian epithelium and low grade tumours but in poorly differentiated cancers, expression was lost (47). In another series, β3 integrin was associated with a favourable prognosis in ovarian cancer (48) and since more than 80% of human ovarian tumours are high grade, poor prognostic malignancies, it is not surprising that cilengitide has not been clinically tested in ovarian cancer.
In this first report of combination therapy with an oncolytic adenovirus and H2574, a cilengitide-like inhibitor of β3 integrin, we significantly reduced the inflammatory cytokines and hepatotoxicity caused by systemic adenovirus. Importantly, in vitro and in the small numbers of mice tested, the anti-cancer activity of dl922-947 did not appear to be compromised by the addition of H2574. In summary, oncolytic adenoviruses have proven anticancer activity and combination with β3 integrin inhibitors could enable safer delivery and facilitate further clinical development of these promising agents.
Supplementary Material
Acknowledgements
We thank Kairbaan Hodivala-Dilke for β3−/− mice, Stephen Robinson for murine endothelial cell lines, Kate English, Sue Rodway and Geoff Grant at the Royal Veterinary College for analysing murine liver function and Susana Godinho for help with confocal images. We thank George Elias, Linda Hammond, Guglielmo Rosignoli, Vipul Bhakta, Julie Andow and Hagen Schmidt for technical assistance. This manuscript was prepared with help and advice from Ian Hart, Kairbaan Hodivala-Dilke, Sarah Martin, Paulo Ribeiro and Tyson Sharp. The work was supported by Cancer Research UK (A13034) and The Barts and The London Charity (467/1307).
Footnotes
Authors disclose no conflicts of interest
References
- 1.Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Human gene therapy. 2009;20(10):1119–32. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19(6):1008–16. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7(2):141–8. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 4.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Molecular genetics and metabolism. 2003;80(1-2):148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14(1):107–17. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer research. 2002;62(21):6070–9. [PubMed] [Google Scholar]
- 7.Zeimet AG, Marth C. Why did p53 gene therapy fail in ovarian cancer? Lancet Oncol. 2003;4(7):415–22. doi: 10.1016/s1470-2045(03)01139-2. [DOI] [PubMed] [Google Scholar]
- 8.Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Human gene therapy. 2004;15(1):35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- 9.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6(10):1134–9. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 10.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–60. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 11.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer cell. 2002;2(2):103–12. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 12.TCGA C Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KH, Dmitriev IP, Saddekni S, Kashentseva EA, Harris RD, Aurigemma R, et al. A phase I clinical trial of Ad5/3-Delta24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecologic oncology. 2013;130(3):518–24. doi: 10.1016/j.ygyno.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer research. 2007;67(2):585–92. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhusudan S, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, et al. Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer. J Clin Oncol. 2005;23(25):5950–9. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 16.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(18):6083–96. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8(2):89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 18.Lockley M, Fernandez M, Wang Y, Li NF, Conroy S, Lemoine N, et al. Activity of the adenoviral E1A deletion mutant dl922-947 in ovarian cancer: comparison with E1A wild-type viruses, bioluminescence monitoring, and intraperitoneal delivery in icodextrin. Cancer research. 2006;66(2):989–98. doi: 10.1158/0008-5472.CAN-05-2691. [DOI] [PubMed] [Google Scholar]
- 19.Salako MA, Kulbe H, Ingemarsdotter CK, Pirlo KJ, Williams SL, Lockley M, et al. Inhibition of the inflammatory cytokine TNF-alpha increases adenovirus activity in ovarian cancer via modulation of cIAP1/2 expression. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19(3):490–9. doi: 10.1038/mt.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31(1):110–21. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10(10):753–68. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Bottcher S, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. European journal of cancer. 2003;39(7):917–26. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 23.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer research. 1993;53(4):891–8. [PubMed] [Google Scholar]
- 24.Hamilton TC, Young RC, Ozols RF. Experimental model systems of ovarian cancer: applications to the design and evaluation of new treatment approaches. Semin Oncol. 1984;11(3):285–98. [PubMed] [Google Scholar]
- 25.Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, et al. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. The Journal of biological chemistry. 2009;284(49):33966–81. doi: 10.1074/jbc.M109.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingemarsdotter CK, Baird SK, Connell CM, Oberg D, Hallden G, McNeish IA. Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer. Oncogene. 2010;29(45):6051–63. doi: 10.1038/onc.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. The Journal of clinical investigation. 1999;103(2):229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young AM, Archibald KM, Tookman LA, Pool A, Dudek K, Jones C, et al. Failure of translation of human adenovirus mRNA in murine cancer cells can be partially overcome by L4-100K expression in vitro and in vivo. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20(9):1676–88. doi: 10.1038/mt.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallden G, Hill R, Wang Y, Anand A, Liu TC, Lemoine NR, et al. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Molecular therapy: the journal of the American Society of Gene Therapy. 2003;8(3):412–24. doi: 10.1016/s1525-0016(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 30.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. Journal of virology. 2004;78(10):5368–81. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. Journal of virology. 2005;79(12):7478–91. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler H, Machemer T, Philopena J, Wen SF, Quijano E, Ramachandra M, et al. Acute hepatotoxicity of oncolytic adenoviruses in mouse models is associated with expression of wild-type E1a and induction of TNF-alpha. Virology. 2004;328(1):52–61. doi: 10.1016/j.virol.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. Journal of virology. 1997;71(11):8798–807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BH, Kushwah R, Wu J, Ng P, Palaniyar N, Grinstein S, et al. Adenoviral vectors stimulate innate immune responses in macrophages through cross-talk with epithelial cells. Immunology letters. 2010;134(1):93–102. doi: 10.1016/j.imlet.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Molecular therapy: the journal of the American Society of Gene Therapy. 2001;3(5 Pt 1):697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 36.Korst RJ, Mahtabifard A, Yamada R, Crystal RG. Effect of adenovirus gene transfer vectors on the immunologic functions of mouse dendritic cells. Molecular therapy: the journal of the American Society of Gene Therapy. 2002;5(3):307–15. doi: 10.1006/mthe.2002.0538. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452(7183):103–7. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 39.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer research. 2005;65(24):11255–8. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 40.Smakman N, van der Bilt JD, van den Wollenberg DJ, Hoeben RC, Borel Rinkes IH, Kranenburg O. Immunosuppression promotes reovirus therapy of colorectal liver metastases. Cancer Gene Ther. 2006;13(8):815–8. doi: 10.1038/sj.cgt.7700949. [DOI] [PubMed] [Google Scholar]
- 41.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(2 Pt 1):644–53. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 42.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8(5):724–30. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 43.Ruegg C, Alghisi GC. Vascular integrins: therapeutic and imaging targets of tumor angiogenesis. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2010;180:83–101. doi: 10.1007/978-3-540-78281-0_6. [DOI] [PubMed] [Google Scholar]
- 44.Smith JW. Cilengitide Merck. Curr Opin Investig Drugs. 2003;4(6):741–5. [PubMed] [Google Scholar]
- 45.Carter A. Integrins as target: first phase III trial launches, but questions remain. Journal of the National Cancer Institute. 2010;102(10):675–7. doi: 10.1093/jnci/djq186. [DOI] [PubMed] [Google Scholar]
- 46.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(13):1651–7. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carreiras F, Denoux Y, Staedel C, Lehmann M, Sichel F, Gauduchon P. Expression and localization of alpha v integrins and their ligand vitronectin in normal ovarian epithelium and in ovarian carcinoma. Gynecologic oncology. 1996;62(2):260–7. doi: 10.1006/gyno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 48.Kaur S, Kenny HA, Jagadeeswaran S, Zillhardt MR, Montag AG, Kistner E, et al. {beta}3-integrin expression on tumor cells inhibits tumor progression, reduces metastasis, and is associated with a favorable prognosis in patients with ovarian cancer. The American journal of pathology. 2009;175(5):2184–96. doi: 10.2353/ajpath.2009.090028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.