Synopsis
We have discussed in this chapter ethical issues surrounding the resuscitation of infants who are at great risk to die or survive with significant morbidity. We have introduced data regarding three separate aspects of the morality of resuscitation for these infants – money, outcomes, and prediction. We have demonstrated that there are no credible financial arguments against NICU care for infants born at the border of viability – rather, the NICU is a bargain in terms of dollars devoted to infants who will survive to discharge as opposed to die in hospital. Moreover, the NICU is particularly cost-effective when compared with medical interventions in adults. We have noted that of the four possible outcomes after birth (comfort care, death in the NICU, survival with NDI, survival without NDI), gestational age influences some (death in the NICU) much more than others (percentage of survivors with NDI). Consequently, for parents who view ‘giving their child a chance’ by starting NICU intervention as a worthwhile option, counseling about resuscitation as a function of gestational age appears to have limited support from the data. Finally, we have noted that prediction is possible at four stages of the resuscitation process – before birth (antenatal counseling), in the delivery room, in the NICU while the child remains on the ventilator (when there are ethical alternatives to continued NICU intervention – namely extubation and palliative care), and at the time of discharge. We have presented data suggesting that antenatal and delivery room predictions are inadequately accurate, and prediction at the time of discharge is too late. Rather, we suggest that learning about individual infants from data collected during the trajectory of their own NICU experience (specifically, abnormal head ultrasound and healthcare professional intuitions that the child will ‘die before discharge’) can offer a positive predictive value of >95% for the combined outcome of death or survival with neuro-developmental impairment. This predictive value is worth talking about.
Keywords: resuscitation, neonatal ethics, neonatal outcomes, prognostication, distributive justice
Introduction
Most neonates do not need resuscitation. A bit of drying, a slap on the butt, and they are good to go. Some neonates unexpectedly need resuscitation. Their antenatal histories yield no hint that at the time of birth they will need extra efforts to get them going. There are almost no ethical dilemmas for these infants – they need resuscitation, and receive it. Airway, breathing, compressions, drugs … The details of these interventions are described elsewhere in this volume.
But some babies, predictably, are born at a time when it is not clear whether we should offer, perform, deny, or accede to resuscitative efforts. These babies are born extremely prematurely - at the border of neonatal viability. Resuscitation of these babies provokes passionate debates and disagreements. We will discuss data that underlie these debates here.
The issues that arise for doctors are different from those facing parents or policy makers. If you were a doctor, you might want to know the data on the likelihood that your resuscitative efforts would be successful. How many infants like this one in front of you now responded to resuscitative efforts? Of those that survived, how many were in the hospital for months at enormous expense? How many had terrible long-term outcomes?
If you were a public policy-maker, you might want to know how many such babies were born, how much their treatment costs, both initially and long-term, and how these expenses compare to other public health expenses, either in children or adults. You might want to know if money spent to prevent preterm birth would lead to lower overall costs.
If you were a parent, you want to know the answer to only one question, “what will happen to my baby”? It wouldn't help to know that, of 100 babies like your baby, 50 die and half the survivors have neurocognitive problems. You want to know what will happen to your baby and you want to know as early as possible in the baby's treatment course so that you can make the right decision at the right time.
Are data like these available – data that speak to the morality of neonatal resuscitation from the perspective of what is sometimes called ‘evidence-based ethics’? Yes, data are available, and in this chapter we will discuss three distinct lines of evidence as they bear on the morality of neonatal resuscitation – 1) money; 2) outcomes; 3) predictive ability.
Financial Considerations for Neonatal Resuscitation
Approximately 4 million babies are born each year in the U.S. Approximately 3 million people die. Approximately 1% of 4 million, or 40,000 infants are born each year weighing less than 1000g (extremely low birth weight [ELBW], roughly 28 weeks gestation). 0.6% of the 4 million births (24,000) will die each year, of whom half will die of complications of prematurity. (The other half die of congenital anomalies. Those babies raise a set of complicated issues that are related to, but not identical to, the issues raised by premature babies. In this chapter, we will focus only on premature babies.)
The first financial consideration regarding resuscitation for extremely premature infants deals with the following question – what percentage of NICU resources expended on ELBW infants are devoted to infants who will die in the NICU, as opposed to resources devoted to infants who will survive to be discharged? Figure 1 presents the answer, depicting data from the University of Chicago hospitals [1,2]. At the higher ELBW birth-weights, e.g., >800g, mortality is so low (<20%) that the vast majority of NICU expenses (closely approximated by NICU bed-days) are devoted to infants who survive to discharge.
Figure 1.
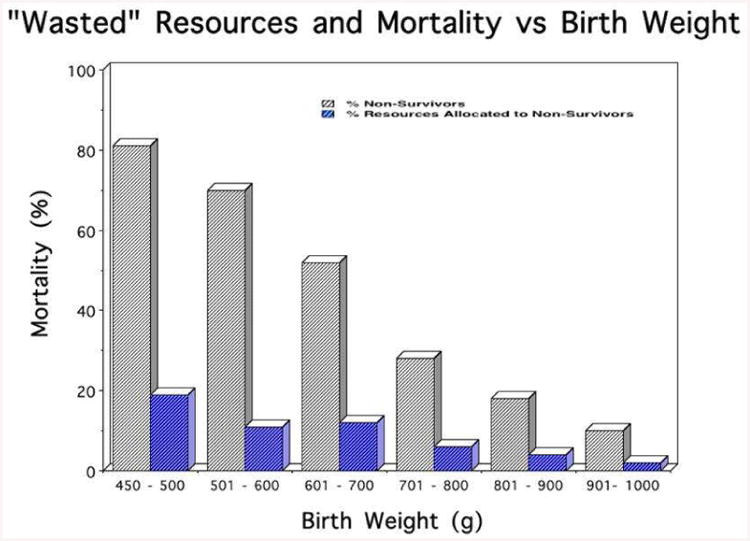
Mortality and Resources devoted to non-survivors as a function of gestational age. At all gestational ages, independent of the likelihood of mortality, more dollars are devoted to infants who will survive than those who will die.
The surprising feature of Figure 1 is that even at the lower end of the ELBW distribution (BW 450 – 600g) where survival is less than 50%, still the large majority of NICU expenses (>80%) are expended on infants who will survive to discharge, as opposed to those infants who will die in the NICU. How can this possibly be? Because doomed infants die relatively quickly (median day of death is <7days); because the smallest and the sickest die the quickest; and because survivors stay in the NICU for a long, long time.
This is not the case in the adult medical intensive care unit. There, the relationship between ultimate survival and overall cost is the inverse of the relationship that holds in the NICU [3,4]. Figure 2 presents comparable data for the NICU and adult medical intensive care unit (MICU), contrasting survival and expenses devoted to non-survivors, for patients with low, moderate, or high risk of dying in the hospital. The data are striking. In the NICU, at every risk of death, only a small percentage of resources are expended on doomed infants – the vast majority of NICU bed-days are occupied by patients who will be discharged, independent of their initial risk of dying.
Figure 2.
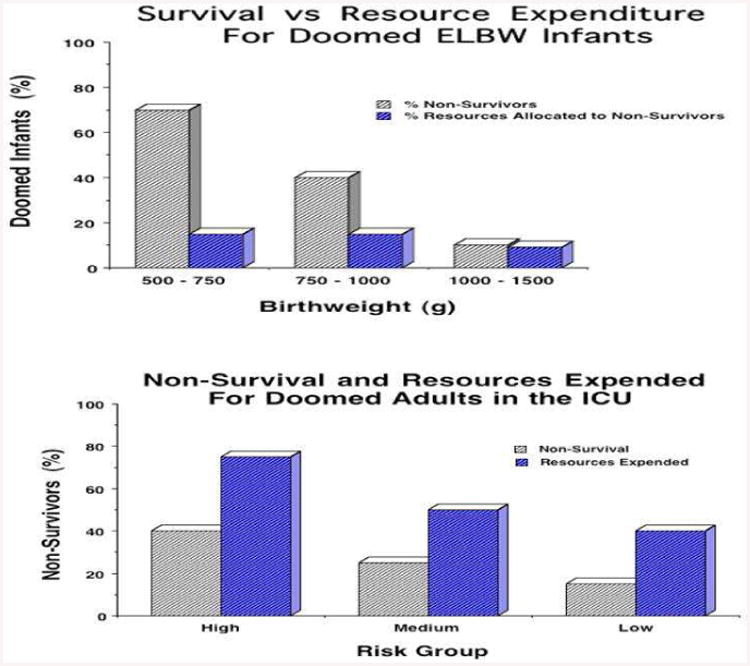
Comparison of mortality and resources devoted to non-survivors for the NICU vs MICU. In contrast to the NICU, for adults in the MICU proportionally more dollars are devoted to patients who will not survive, at all risk groups.
In the adult ICU, the phenomenon is reversed. For every risk group, more money is directed to patients who will die in hospital than to those who will be discharged. The mean and median length of stay for MICU non-survivors is higher than for MICU survivors, at every risk group. Moreover, as was noted previously, of the 3 million people who die in the U.S. each year, only 24,000, or approximately 1%, are neonates. The other 99% of U.S. deaths are adults.
We clearly spend more, both absolutely and relatively, on dying MICU patients than dying NICU patients. This remains true, even if long-term costs of impaired NICU survivors are included (5). NICU costs have been estimated at between $5,000 and $10,000/QALY (quality adjusted life-year), whereas comparable adult procedures cost roughly 10× more (3). The individual patients who stay the longest in the NICU mostly survive, the individual patients who stay longest in the MICU mostly die. And there are 100× more dying adults than dying neonates. Consequently, if policy makers seek evidence-based algorithms for rationing care, starting with the least cost-effective care rather than more cost-effective alternatives, they would look to the MICU, rather than the NICU, as a place to start. Saving 500-gram premature babies is far more cost-effective than saving 85 year-old adults. If any cost-savings at the end-of-life are to be sought, they should be found in adult ICUs, not NICUs.
Outcomes of Neonatal Resuscitation
There are only four possible outcomes after birth of an extremely premature infant – 1) they can not be resuscitated, but rather receive comfort care – these infants all die, and we will not discuss them further here; 2) they can be resuscitated in the delivery room, be admitted to the NICU, but die at some point during their NICU care; 3) they can be resuscitated, survive to NICU discharge, and experience neuro-developmental impairment (NDI, defined in this chapter as either MDI or PDI < 70 on the Bayley-II score); 4) they can be resuscitated, survive to NICU discharge, and be neurologically intact.
Moral calculations of the value of a short trial of NICU even in situations where survival is unlikely depend on the value (positive or negative) assigned to each of these possible outcomes. If survival is the desired outcome then ‘good’ outcomes are calculated as the ratio of all survivors (Groups 3 and 4) to all births (Groups 1 + 2 + 3 + 4). If ‘intact’ survival is the only desirable outcome, then ‘good’ outcomes are the ratio of ‘intact survivors’ (Group 4) to all births (Groups 1 + 2 + 3 + 4). However, one more moral calculation is conceivable. We have found that many parents report that they feel better if they gave their baby a chance, if they opted to try resuscitation, even if it fails, than they feel if they didn't even try. For such parents ‘trying and failing in the NICU’ (Group 2) is better than not trying (Group 1). For such parents, ‘good’ outcomes are calculated as the ratio of ‘intact’ survivors (Group 4) to all survivors (Groups 3 + 4). These differing moral weightings lead to very different conclusions about the ‘worth’ of NICU resuscitation as a function of gestational age for extremely premature infants.
Figure 3 displays a subset of data obtainable from the NICHD calculator published in 2008 [6]. The X-axis presents gestational age divided into SGA and AGA groups, and the Y-axis presents survival. The point to be made is familiar – survival is strongly dependent on GA between 22 – 25 weeks. Figure 4 superimposes intact survival (that is, survival with both MDI and PDI > 70) as a function of GA. The point is clear – intact survival is lower than overall survival, and also depends strongly on GA. Figure 5 superimposes one more outcome – the percentage of NICU survivors who survive ‘intact’. Two points are clear. First, the percentage of survivors who survive ‘intact’ is much higher than the percentage of all births who survive intact. Second, the percentage of intact survivors is relatively independent of gestational age.
Figure 3.
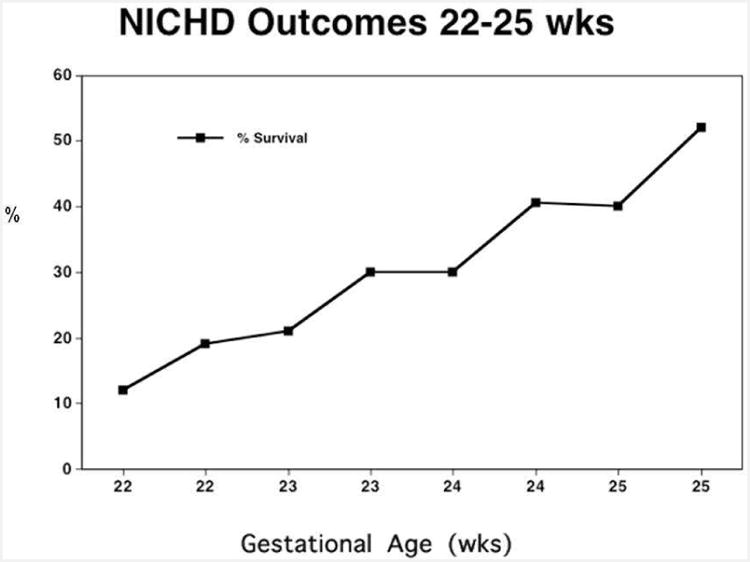
Likelihood of survival vs gestational age for infants weighing <600g from the NICHD on-line calculator. Survival depends strongly on GA.
Figure 4.
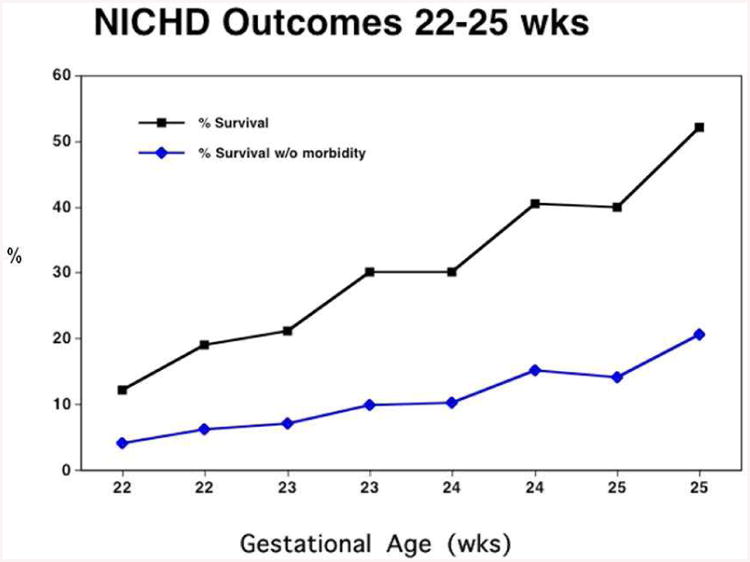
Likelihood of intact survival (survival with out neuro-developmental impairment) vs gestational age for infants weighing <600g from the NICHD on-line calculator. Intact survival depends strongly on GA.
Figure 5.
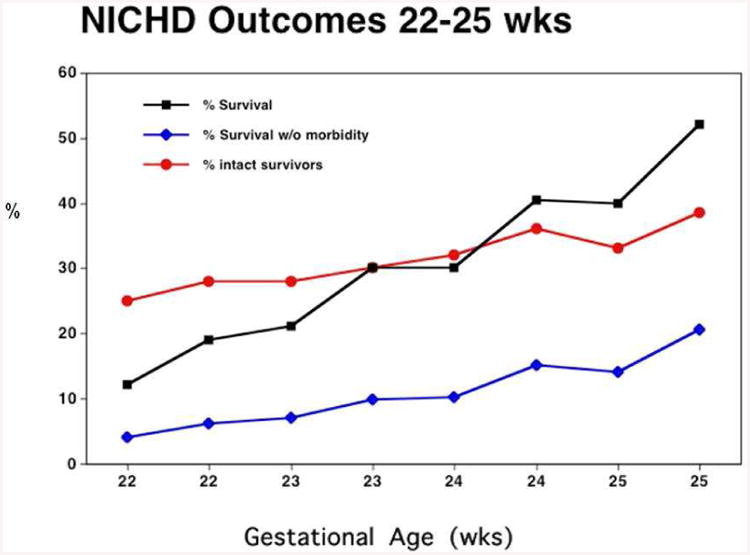
Percentage of survivors who will be neuro-developmentally intact vs gestational age for infants weighing <600g from the NICHD on-line calculator. Percentage of intact survivors does not depend strongly on GA.
This phenomenon can be seen in other countries as well. In Figures 6 and 7, data from Britain [7] and France [8] reveal that outcomes for NICU survivors measured at 6 years of age, and 7-9 years of age, reveal the same lack of dependence on gestational age.
Figure 6.
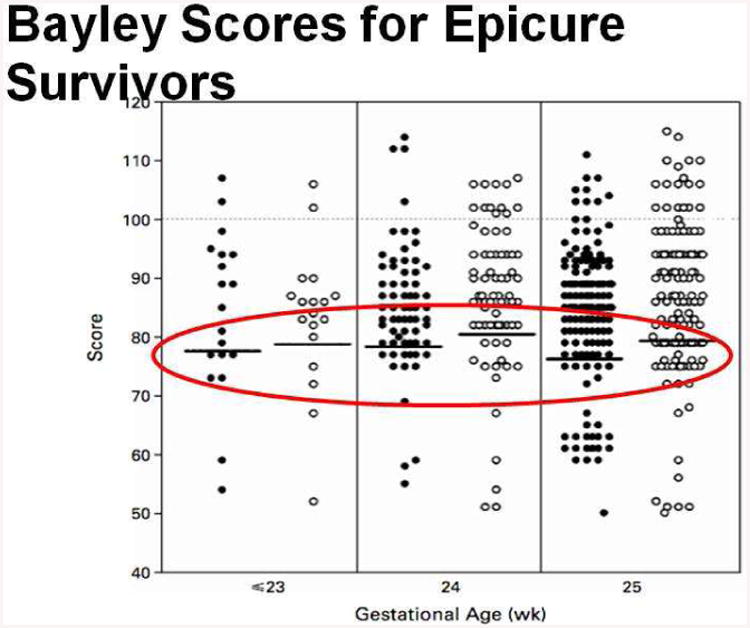
Bayley II scores vs gestational age for infants born in the UK enrolled in the EPICURE study. The scores do not depend strongly on gestational age.
Figure 7.
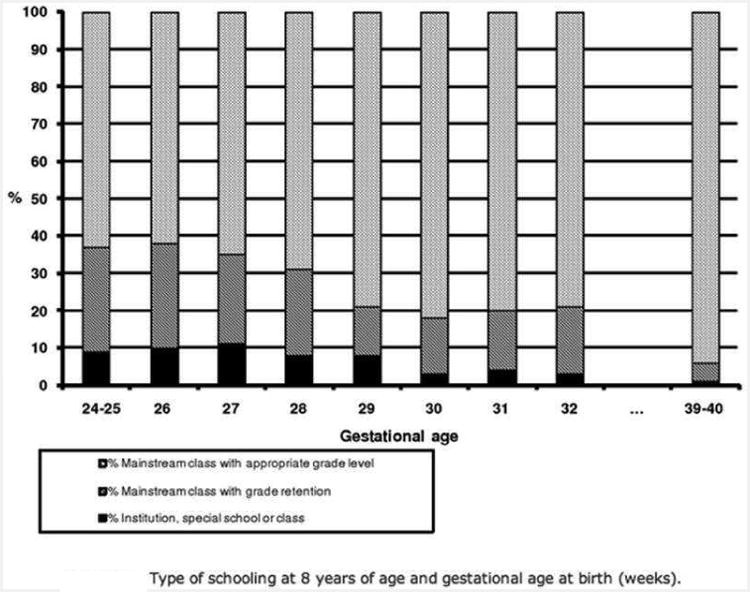
Type of schooling vs gestational age for infants born in France enrolled in the Epipage study. The scores do not depend strongly on gestational age.
In sum, moral calculations of the value of neonatal resuscitation depend strongly on the valence assigned to two different types of dying in the NICU. If dying without resuscitation and dying after failed resuscitation are equivalent, then ‘good’ outcomes vary strongly as a function of gestational age. Many more babies die at lower gestational ages. If, however, dying after resuscitative efforts is better than dying without such efforts, that is, if ‘giving your child a chance’ matters to parents, and if the worst outcome is not death but impaired survival, then the likelihood of a ‘good’ outcome does not depend much on gestational age. At every gestational age between 23 and 26 weeks, the percentage of survivors with neurodevelopmental problems is about the same.
This leads to a problem with no obvious solution. If the major concern is survival of an infant with neuro-developmental impairment, then the gestational age to be most ‘feared’ from the standpoint of neonatal resuscitation is not 23-24 weeks, but rather 25-26 weeks [cf. Figure 8] – precisely because so many more infants will survive as GA increases, while ‘intactness’ of survivors does not vary much as gestational age increases. And, of course, currently we have no ethical alternatives to resuscitation when confronted with infants born at 25-26 weeks gestation. As for the pain and suffering of the children themselves during their time in the NICU, it is, to our mind, most reasonable for the parents to be the judge of whether, and when, NICU intervention is ever, or still, ‘worth it’.
Figure 8.
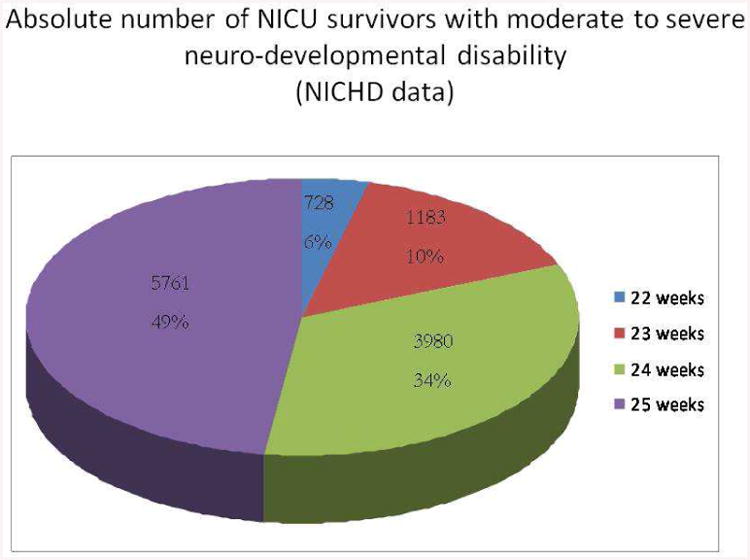
Percent of all infants born <26 weeks gestation who survive with NDI vs gestational age. Most survivors with NDI are born at higher GA, precisely because survival depends strongly on GA while the percentage of survivors who will be impaired does not.
Predicting the Outcome of Neonatal Resuscitation
When predicting outcomes of neonatal resuscitation for parents, only two things matter – timing and positive predictive value. Figure 9 presents a complex time-line illustrating this point. Four time-points are illustrated [9,10]. Many, probably most, neonatal decisions about resuscitation for infants born extremely prematurely are discussed before the infant's birth – in an antenatal consultation. In the context of the data available antenatally (GA, SGA status, antenatal steroids, twinning, and gender), the data available – either from the local institution or using the NICHD calculator [5], are used to predict either overall survival or ‘intact’ survival for a population of infants born sharing the same features. These data inform and constrain the conversations between the parents, OBs, and neonatologists regarding the appropriateness of resuscitation for their infant.
Figure 9.
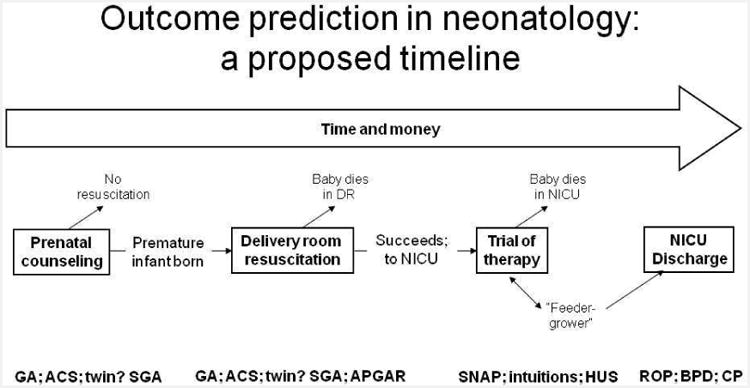
Timeline for outcome prediction for infants. Four possible opportunities for counseling are presented.
The second time point is immediately after delivery, in the minutes while the infant lies in the delivery room suite. Here, in addition to confirming GA, SGA, twinning, and gender, the neonatologist also learns about Apgar scores – how the infant ‘looks’ at birth (Apgar @ 1”) and how the infant responds to initial resuscitation (Apgar @ 5”). Although little data exist correlating Apgar scores to ultimate outcomes, either mortality or morbidity, in the ELBW population, many neonatologists appear to value this information and use it to influence the initiation, or duration, of their delivery room resuscitative efforts [11].
The relevant third time point for predicting the outcome of neonatal resuscitation is during the NICU stay. Here, to be ethically relevant (that is, to envision a clinical course distinct from continuing intervention – extubation and palliative care) we would optimally obtain data while the child is requiring mechanical ventilation. One could envision a number of possible predictive data variables – individual occurrences like sepsis or NEC or IVH, composite scores of abnormal physiology like SNAP or SNAPPE or CRIB, or something less tangible but no less real like healthcare professional intuition of impending demise. We shall examine each of these in the paragraphs that follow.
The final time point for predicting the outcome of neonatal resuscitation is at the time of NICU discharge. Here, of course, we know best about the condition of each baby – at the same time it is too late to do much about it. That is, other than attempting to optimize post-discharge early intervention, there is nothing to withdraw, and no alternative to continued support for the infant at home.
These data illustrate the reasons why antenatal counseling is so imprecise. Antenatal counseling suffers from the inevitable uncertainty associated with statistical descriptions of the outcomes of populations – as opposed to individuals – born at the border of viability (roughly 22 – 26 weeks gestation). Moreover, in our experience, parents desire prognostic information about their baby, not 100 more-or-less similar babies.
This uncertainty does not go away in the delivery room. In the delivery room, we can confirm what the infant weighs, examine for congenital anomalies, and assess responses to initial resuscitative efforts. Although neonatologists often describe making assessments in the DR about the usefulness or futility of further interventions (11), none of these assessments has been shown to predict either death or future neuro-developmental impairment in any helpful way.
Instead, we now believe that the best time to predict the outcome of neonatal resuscitation for individual infants, and to counsel their parents accordingly, occurs while the infants is in the NICU, on a ventilator. At that time, there are still choices to be made about life-sustaining treatment. The ventilator can be withdrawn. Decide too soon and the prognostic uncertainty is too great. Wait too long, e.g., until NICU discharge, and, although there may be improved prognostic accuracy (12), there is no longer any treatment to withhold or withdraw.
During the first days after NICU admission, while infants remain on mechanical ventilation, we can determine more robust predictors of outcome than those that are available in the delivery room. These predictors come in two categories – hard-data available in algorithms and ‘softer’ data available from the clinical judgments of healthcare professionals. Consider algorithms first. Algorithms award points for physiologic derangements – the more abnormal the respirations, or blood pressure, or electrolytes, the higher the score. Since patients who die are clearly more physiologically deranged than patients who live, it is perfectly reasonable to assume that, over time, serial illness severity scores will diverge – growing ever higher for patient populations who are on the path to die, and growing lower for patient populations whose physiology is improving and who will eventually survive.
As Figure 10 demonstrates, this perfectly reasonable expectation is completely wrong. Figure 10 plots the SNAPPE-II scores of two populations of infants born < 1000g at the University of Chicago, one population that will eventually die in the NICU and another population that will survive to NICU discharge [13]. Several points are apparent. First, at the time of birth, eventual non-survivors do have higher SNAPPE-II scores than survivors – as would be anticipated by the definition of SNAPPE-II. However, and more importantly for our argument, over time the differences between the SNAPPE-II scores of the non-surviving population and the scores for those infants who will survive converge – not diverge.
Figure 10.
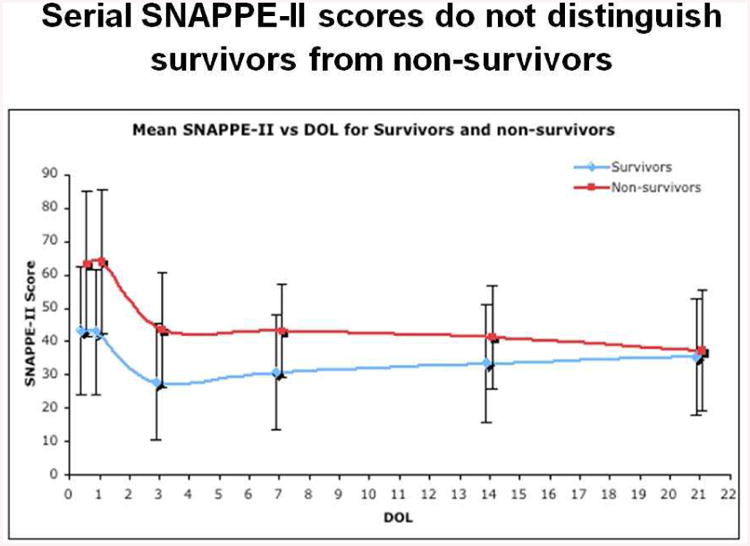
Serial SNAPPE-II scores vs Day of Life for NICU survivors and non-survivors. Over time, serial SNAPPE-II scores become less, not more, able to distinguish survivors from non-survivors.
How can this be? How do the illness severity scores of the two populations grow closer, not farther apart, over time? The answer is simple -- the sickest infants, those with the highest SNAPPE-II scores and most likely to die, do die, quickly, lowering the SNAPPE-II scores of the residual population of non-survivors. Consequently, even though the surviving infants' SNAPPE-II scores also improve, the overall distance between the two populations narrows. Serial illness severity scores will be of no help in counseling parents of infants who have been resuscitated.
What about non-algorithmic predictors such as clinical judgment? Clinical judgment is criticized as imprecise and as simply masking an implicit algorithm that could be better analyzed if it could be made explicit. Most clinicians recognize, however, that some things are hard to define, even if you “know them when you see them.” We have tested the predictive power of clinical judgment.
Our methods are as follows: For each day over the past fourteen years at the University of Chicago, we have been assessing the predictive power of the clinical judgments of healthcare professionals (RNs, NNPs, residents, fellows, attendings) who are asked one simple question each day that the child remains on mechanical ventilation – ‘do you think this infant is going to survive to be discharged, or die in the NICU’? We limit the questioning to infants who require mechanical ventilation, precisely because for those infants the parents have an ethically available option – extubation and palliative care.
Our first finding is a simple one. Sixty percent of babies are never predicted to die before discharge by any health professional in the NICU [cf. Figure 11]. Another 15% are predicted to die by only one person on one day of ventilation, 10% are predicted to die by more than one person, and 15% have at least one day of unanimous predictions of ‘die before discharge’.
Figure 11.
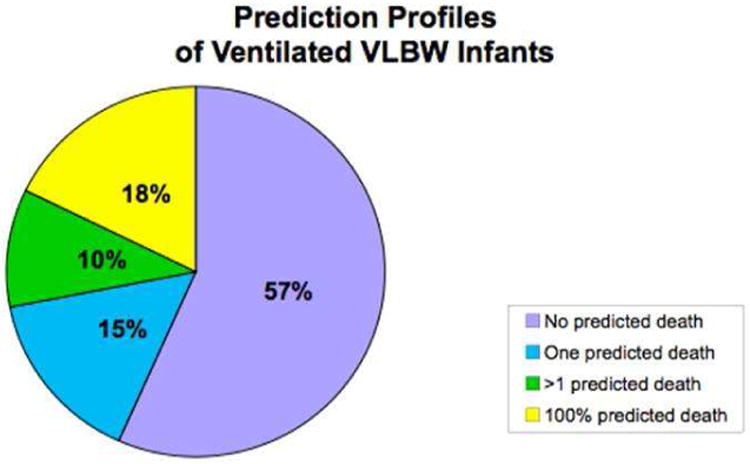
Prediction profiles for ventilated NICU infants. The majority of ventilated NICU infants are never predicted to die by any caretaker on any day.
Of the 60% of infants who were never predicted to die – not surprisingly, almost all survived to NICU discharge. However, of the 40% of infants who received at least one prediction of ‘die before discharge’ – nearly half of these infants survived as well [13]. This is not very good predictive power.
Perhaps we can do better if we attempt to predict a combined outcome – either death in the NICU or neuro-developmental impairment (NDI), defined as MDI or PDI < 70 on the Bayley II score at 24 months of corrected age. It turns out that serial algorithms (SNAPPE-II scores) are very unhelpful at distinguishing the population of infants who will either die or survive with NDI from those infants who will survive unscathed. How well do clinical judgments of ‘die before discharge’ predict the combined outcome of death or NDI?
Figure 12 presents these data – and they are some of the most illuminating in this entire chapter [10]. The figure presents the likelihood of the combined outcome of either death or NDI on the ‘y-axis’ as a function of predictive features on the x-axis. The two predictors displayed are head ultrasound (normal, Grade 1, Grade 2, Grade 3/4/PVL) and caretaker clinical judgments of ‘die before discharge’ (none, one, more than one on at least one day, or at least one day with a unanimous prediction of die). Several points are apparent from Figure 12. First, considering the grey bars (all patients in each HUS family), there is an increased risk of death or NDI as HUS worsens – this is not a surprise.
Figure 12.
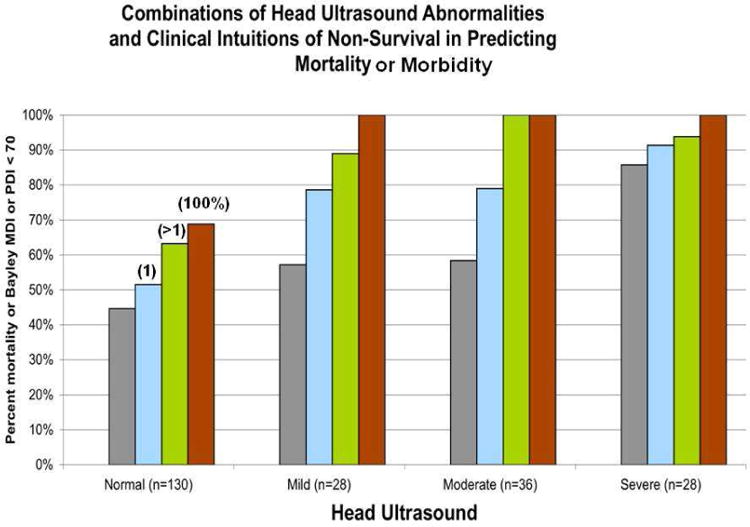
Predictive power of the combination of head ultrasound abnormalities and healthcare professional judgments that the infant will ‘die before discharge’ for the outcome of death or survival with NDI. The positive predictive value of ‘death or NDI’ exceeds 95% for infants with a corroborated prediction of ‘die’ and an abnormal HUS.
More importantly, the predictive value for the combined adverse outcome of death or NDI increases when clinical judgments (the colored bars) are added to each HUS group. That is, clinical judgments and HUS each add predictive power over using either one alone. Moreover, for infants with moderate or severe HUS abnormality who have even one day of corroborated prediction of die before discharge, the PPV of death or NDI is at least 96% -- the likelihood that these infants will be alive without NDI at two years of age is less than 4%. That, we would argue, warrants a conversation with significant ethical import.
Moreover, for each HUS group, never having had a prediction of die before discharge improves the likelihood of a normal outcome. This too is an important conversation to have.
How does the combination of HUS and clinical judgments during the NICU stay compare to using gestational age at the time of birth to predict outcomes for ELBW infants? Figure 13 reveals several important comparisons. Again the y-axis is likelihood of either death or NDI, while the X-axis now displays 177 patients as a function of both gestational age and the number of adverse ‘events’ in the NICU (either abnormal HUS, prediction of die before discharge, both, or neither). The first comparison (left-most columns) is the simplest – 23-24 week infants are more likely to have an adverse outcome (either death or NDI) than 25-27 weekers. This is not news.
Figure 13.
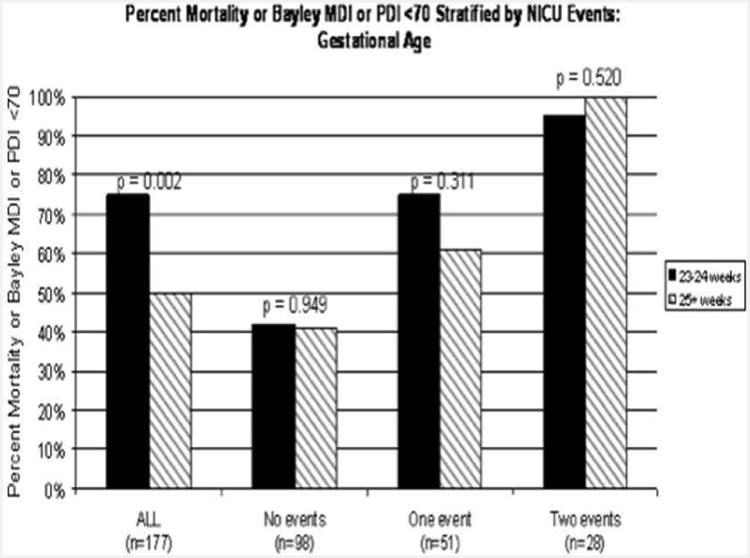
Likelihood of death or survival with NDI stratified by NICU trajectory – either abnormal head ultrasound or caretaker judgments of ‘die before discharge’. The predictive power of gestational age disappears when infants are stratified by events that occur in the NICU.
However, what is news is the comparisons of the three families of columns to the right – here, 23-24 week infants are compared with 25-27 week infants if they have had zero, one, or two ‘events’ – that is, either a prediction of ‘die before discharge’ or an abnormal HUS. Two points are clear – one simple and the other more profound. First, infants who have had two events are more likely to have an adverse outcome than infants who have had one event, who in turn are more likely to do poorly than infants who have had neither an abnormal HUS nor a prediction of die. That, again, is not surprising.
However, the second comparison is remarkable. For each grouping of patients – 0, 1, or 2 adverse events – there is no distinction comparing 23-24 weekers to 25-27 week infants. In other words, although younger gestation infants are more likely to have one of these two adverse events (the left-most columns), once you have either or both of the adverse events (abnormal HUS or prediction of ‘die’) gestational age drops out as a predictive factor. And, the PPV for infants with both abnormal HUS and a prediction of ‘die’ for the combined outcome of die or survive with NDI is > 95%, independent of gestational age.
This predictive accuracy is approaching the kind of power that parents are asking for when we counsel them about whether or not to resuscitate their infants at the time of delivery. We are now capable of offering parents two aspects of information that are unavailable at birth – 1) the individualized trajectory for their particular baby, not 100 more-or-less similar babies; and 2) the knowledge that the predictive value of either death or survival with NDI for infants with both abnormal HUS and a prediction of ‘die before discharge’ is over 95%, about as high as medical certainty is likely to ever achieve.
This comparison – between what we know before birth and what we learn in the NICU – is captured nicely in Figure 14, which displays the time course of our gaining knowledge about the likely outcomes of individual infants after their resuscitation. Here a good outcome increases along the y-axis, and again two major groups of infants are displayed – 23-24 week gestation vs 25 – 27 week gestation.
Figure 14.
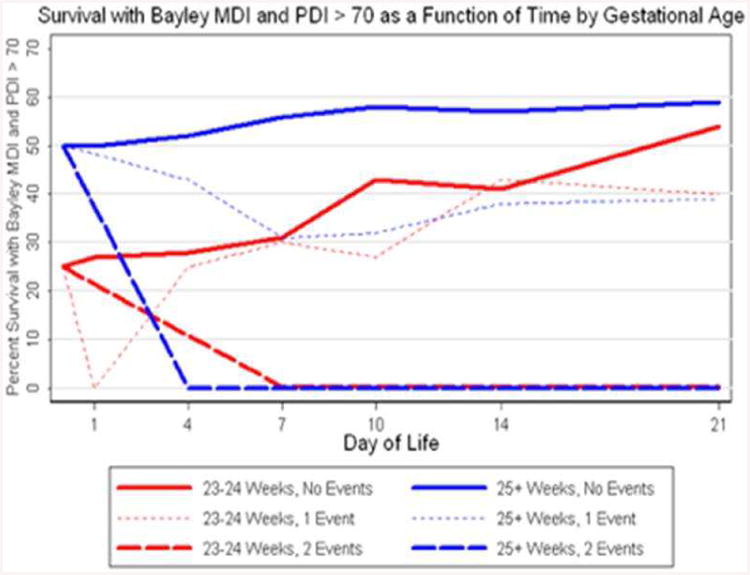
Likelihood of intact survival as a function of day of life for infants with 0, 1, or 2 ‘events’ in the NICU (either abnormal HUS or caretaker intuition of ‘die before discharge’). Less than one week is needed to clarify the likely outcome for infants born >25 wks. For infants born at 23-24 wks, three weeks are needed to clarify their likely outcome.
At birth, again, it is clear that 25-27 weekers have roughly a 50% likelihood of survival without NDI, significantly higher than the 25% likelihood for infants born at 23-24 weeks gestation. This phenomenon has been documented by many other groups. However, as Figure 14 makes clear, it does not take long for the 25+ weekers to receive their ‘hits’ – that is, within roughly a week the vast majority of 25+ week infants who will go on to die or survive with NDI will have ‘declared themselves’ – and allow us to discuss this ‘declaration’ with their parents. And again, those 25+ weekers will have the same likelihood of a bad outcome (>95%) as the 23-24 week infants who also two bad prognostic predictors.
In contrast, as Figure 14 also reveals, for infants born at 23-24 weeks gestation, it will take between 2-3 weeks for their ‘good’ clinical course to declare itself. By roughly day of life 21, the likelihood of a good outcome for a 23-24 week infant who has had neither an abnormal HUS nor a prediction of ‘die before discharge’ becomes as good as the outcome for infants born at 25-27 weeks gestation.
In both cases, for infants who develop abnormal HUS/predictions of ‘die’, and for infants who do not, we learn more about their likely outcome during their NICU stay than we could possible have predicted at the time of delivery. This powerful effect of the passage of time on our prognostic capability is, we would argue, the most important ethical contribution we can make to the process of parental decision-making for infants born at the threshold of viability.
Here is a nice analogy – betting on baseball games. Traditionally, you have to place your bet before the game starts. You can take into account whatever you wish (team records, starting pitchers, home field advantage, etc.) but you are obligated to make your decision before the first pitch. This, we argue, is analogous to decision-making before delivery, where gestational age, SGA status, antenatal steroids, twins, and gender are the factors the parents get to take into account before they decide to place their bet on resuscitation – or not.
But what if the betting rules were changed? What if you could continue to place bets while you learned information about the game while the innings passed – strictly analogous to resuscitating your baby at birth and then learning about the baby's course while ventilated in the NICU? Don't you think you'd become a better bettor as time went on? Figure 15 presents data from 411 major league baseball games, plotting the likelihood of winning eventually as a function of whether a team was leading at the end of a particular inning [14]. It's clear that at the beginning of the game, the likelihood of betting success is roughly 50-50. However, by the end of the 8th inning, the team that is leading wins 94% of the time.
Figure 15. Likelihood of eventually winning if a baseball team is leading at the end of each inning.
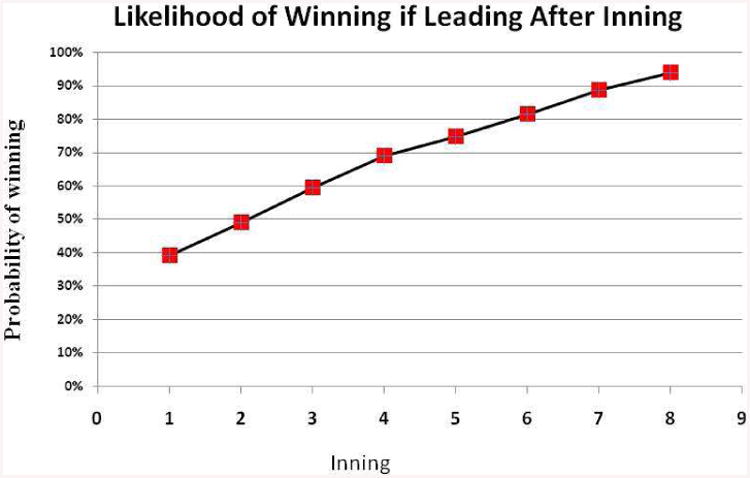
Here, then, is the claim, in its boldest framing. If we let you bet on baseball while the game was still going on, you would do a lot better than if we forced you to bet using the pre-game line. Why don't we allow the same options for the parents of extremely low gestation age newborns?
Summary
We have discussed in this chapter ethical issues surrounding the resuscitation of infants who are at great risk to die or survive with significant morbidity. We have chosen not to take a philosophic approach – that is, we have not spoken much about autonomy, beneficence, paternalism, or best-interests. Rather, we have chosen to address data. Specifically, we have introduced data regarding three separate aspects of the morality of resuscitation for these infants – money, outcomes, and prediction.
We have demonstrated that there are no credible financial arguments against NICU care (including post-NICU costs) for infants born at the border of viability – rather, the NICU is a bargain in terms of dollars devoted to infants who will survive to discharge as opposed to die in hospital. Moreover, the NICU is particularly cost-effective when compared with medical interventions in adults – either adult ICUs or other widely-accepted adult procedures.
We have noted that of the four possible outcomes after birth (comfort care, death in the NICU, survival with NDI, survival without NDI), gestational age influences some (death in the NICU) much more than others (percentage of survivors with NDI). Consequently, for parents who view ‘giving their child a chance’ by starting NICU intervention as a worthwhile option, counseling about resuscitation as a function of gestational age appears to have limited support from the data.
Finally, we have noted that prediction is possible at four stages of the resuscitation process – before birth (antenatal counseling), in the delivery room, in the NICU while the child remains on the ventilator (when there are ethical alternative to continued NICU intervention – namely extubation and palliative care), and at the time of discharge. We have presented data suggesting that antenatal and delivery room predictions are inadequately accurate, and prediction at the time of discharge is too late. Rather, we suggest that learning about individual infants from data collected during the trajectory of their own NICU experience (specifically, abnormal head ultrasound and healthcare professional intuitions that the child will ‘die before discharge’) can offer a positive predictive value of >95% for the combined outcome of death or survival with neuro-developmental impairment. This predictive value is worth talking about.
We end with a plea. We need more study of parental attitudes, both about infants who die in the NICU and infants who survive with NDI. We also need to continue to search for better predictors of infant outcome, while infants remain on ventilators and ethical alternatives exist. We owe this to our colleagues, our patients, and their parents.
Key Points.
We have introduced data regarding three separate aspects of the morality of resuscitation for these infants – money, outcomes, and predictive ability.
There are no credible financial arguments against NICU care for infants born at the border of viability.
NICU care for ELBW infants is particularly cost-effective when compared with medical interventions in adults, even when post-NICU care is included in the calculations.
For parents who view ‘giving their child a chance’ by starting NICU intervention as a worthwhile option, counseling about resuscitation as a function of gestational age appears to have limited support from the data.
Antenatal and delivery room predictions are inadequately accurate, and prediction at the time of discharge is too late. Rather, abnormal head ultrasound and healthcare professional intuitions that the child will ‘die before discharge’ may offer a positive predictive value of >95% for the combined outcome of death or survival with neuro-developmental impairment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meadow WL, Lantos JD, Reimschisel T, Mokalla M. Distributive justice across generations: epidemiology of ICU care for the very young and the very old. In: Hageman J, Freed G, editors. Ethical issues in Neonatal Intensive Care, Clinics in Perinatology. Vol. 23. 1996. pp. 597–608. [PubMed] [Google Scholar]
- 2.Buchh B, Graham N, Harris B, Sims S, Corpuz M, Lantos J, Meadow W. Neonatology has always been a bargain -- even when we weren't very good at it. ACTA Pediatrica. 2007;96:659–63. doi: 10.1111/j.1651-2227.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 3.Lantos JD, Meadow WL. Costs and end-of-life care in the NICU: lessons for the MICU? J Law Med Ethics. 2011 Summer;39(2):194–200. doi: 10.1111/j.1748-720X.2011.00588.x. [DOI] [PubMed] [Google Scholar]
- 4.Meadow WL, Hall J, Frain L, Ren Y, Pohlman A, Plesha-Troyke S, Gresens W, Lantos JD. Some are Old, Some are New, Life and death in the ICU. Seminars in Perinatology. 2003;27:471–79. doi: 10.1053/j.semperi.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Behrman RE, Butler AS, editors. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Ch 12: Societal Costs of Preterm Birth. Washington (DC): National Academies Press (US); 2007. Preterm Birth: Causes, Consequences, and Prevention. [PubMed] [Google Scholar]
- 6.Tyson J, Parikh N, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity – moving beyond gestational age. New Engl J Med. 2008;358:1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marlow N, Wolke D, Bracewell MA, Samara M. the EPICure Study Group: Neurologic and Developmental Disability at Six Years of Age after Extremely Preterm Birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. http://www.nejm.org/doi/full/10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 8.Larroque B, Ancel PY, Marchand-Martin L, Cambonie G, Fresson J, Pierrat V, Roze JC, Marpeau L, Thiriez G, Alberge C, et al. Special care and school difficulties in 8-year-old very preterm children: the Epipage cohort study. PLoS One. 2011;6(7):e21361. doi: 10.1371/journal.pone.0021361. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0021361#pone.0021361-Larroque1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meadow W, M.D. Ph.D, Lagatta J, Andrews B, Caldarelli L, Keiser A, Laporte J, Plesha-Troyke S, Subramanian M, Wong S, Hron J, Golchin N, Schreiber M. Just, in time: Ethical implications of serial predictions of mortality and morbidity for ventilated premature infants. Pediatrics. 2008;121:732–740. doi: 10.1542/peds.2006-2797. [DOI] [PubMed] [Google Scholar]
- 10.Lagatta J, Andrews B, Caldarelli L, Schreiber M, Plesha-Troyke S, Meadow W. Early Neonatal Intensive CareUnit Therapy Improves Predictive Power for the Outcomes of Ventilated Extremely Low Birth Weight Infants. J Pediatr. 2011;159(3):384–391. doi: 10.1016/j.jpeds.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Singh J, Fanaroff J, Andrews B, Caldarelli L, Lagatta J, Plesha-Troyke S, Lantos JD, Meadow W. Resuscitation in the ‘gray zone’ of viability: determining physician preferences and predicting infant outcomes. Pediatrics. 2007;120(3):519–26. doi: 10.1542/peds.2006-2966. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt Barbara, Asztalos Elizabeth V, Roberts Robin S, Robertson Charlene MT, Sauve Reginald S, Whitfield Michael F. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethac in prophylaxis in preterms. JAMA. 2003;289(9):1124–9. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 13.Meadow WL, Frain L, Ren Y, Lee G, Soneji S, Lantos J. Serial assessment of mortality in the neonatal intensive care unit by algorithm and intuition: certainty, uncertainty, and informed consent. Pediatrics. 2002;109:878–886. doi: 10.1542/peds.109.5.878. [DOI] [PubMed] [Google Scholar]
- 14.Meadow W, Meadow X, Tanz RR, Lagatta J, Lantos JD. The Power of a Trial of Therapy – Football as a Proof of Concept; ACTA Paediatrica. 2011;100(2):167–69. doi: 10.1111/j.1651-2227.2010.02113.x. [DOI] [PubMed] [Google Scholar]


