Abstract
Rationale and Objectives
We retrospectively determined if signal enhancement ratio (SER), a quantitative measure of contrast kinetics using volumetric parameters, could reduce the number of biopsy recommendations without decreasing the number of cancers detected when applied to suspicious lesions seen on breast magnetic resonance imaging (MRI).
Materials and Methods
A retrospective review of Breast Imaging Reporting and Data System (BIRADS) 4 or 5 lesions seen on breast MRI in 2008 that were clinically and mammographically occult yielded a final sample size of 73 lesions in 65 patients. Images were processed with in-house software. Parameters used to predict benignity/malignancy included SER total tumor volume (lesion volume above a 70% initial enhancement level), SER partial tumor volume (volume with “washout” and “plateau” kinetics), SER washout tumor volume, peak SER, and peak percent enhancement. Thresholds were determined to retrospectively discriminate benign from malignant histopathology. Clinical impact was assessed through the reduction in the number of biopsies recommended (by eliminating benign lesions discriminated by SER).
Results
Based on the original radiologist interpretations, 73 occult lesions were called suspicious and biopsied with a predictive value of biopsies (PPV3) of 18/73 (25%). SER parameters were found to be significantly associated with histopathology (P < .05). Biopsy recommendations could be reduced using SER parameters of SER partial tumor volume (73 to 40), SER total tumor volume (73 to 45), and peak percent enhancement (73 to 55) without removing true positives.
Conclusion
The adjunctive use of SER parameters may reduce the number of recommended biopsies without reducing the number of cancers detected.
Breast magnetic resonance imaging (MRI) is the most sensitive method for detecting and diagnosing breast cancer. MRI is able to identify new malignancies occult to mammography or clinical breast examination (1,2) and is used widely in clinical practice (3). However, because of its variable specificity, breast MRI has been controversial for causing a high proportion of benign biopsies and changes in surgical management (4,5). Improving diagnostic accuracy of histopathology by imaging alone would strongly impact the clinical management of these lesions. Increasing the specificity of MRI is a particular unmet need and could be resolved through better discrimination of which lesions are benign and therefore don’t require biopsy.
Signal enhancement ratio (SER), a quantitative method for characterizing neoangiogenesis in breast cancer, is a semiautomated, reproducible, computational analysis of MRI images acquired in regular clinical practice (6). It measures change in contrast signal intensity over three time points and acts as a surrogate marker for contrast kinetics. The overall analysis monotonically approximates the redistribution rate constant (kep) in a two-compartment pharmacokinetic model when postcontrast time points are sampled appropriately (7,8). Variations of the SER technique have been incorporated in computer-aided detection breast MRI software. The SER technique has been applied in a variety of implementations, including curve classification, most-suspicious pixel cluster, or enhancing lesion volume (9–14). Although these investigations have looked at the ability of SER to improve diagnostic accuracy, few have specifically reviewed the impact of volumetric parameters.
The objective of this retrospective study was to investigate the ability of volume-based SER to reduce the number of biopsy recommendations without decreasing positive predictive value of biopsies (PPV3) in suspicious occult lesions seen on breast MRI.
MATERIALS AND METHODS
Study Subjects and Lesions
This study was approved by our institutional review board and is compliant with the Health Insurance Portability and Accountability Act. A retrospective review was performed of patients who received breast MRI at our institution between January 1, 2008, and December 31, 2008, looking for the keywords “BIRADS 4,” “BIRADS 5,” “suspicious,” and “biopsy.” Over this period, each breast MRI study was prospectively interpreted by one of seven Mammography Quality Standards Act-certified attending radiologists who specialize in breast imaging. Studies were reported prospectively as part of routine clinical care and in accordance with the American College of Radiology publication of the Breast Imaging Reporting and Data System (BIRADS) Atlas (15). Suspicious lesions were included if they were occult to prior mammographic and clinical breast examination (within the past 12 months), as determined by comparison with prior mammographic and clinical notes. Lesions were biopsied either through targeted ultrasound or MRI guidance. Lesions that did not go on to biopsy were excluded from SER analysis and not included in this study. Diagnoses of lesions as benign or malignant (Table 1) were based on the biopsy pathology report from the electronic medical record. In addition for benign biopsies, subsequent imaging and clinical records were reviewed to ensure that patients did not develop cancer within 1 year of the benign breast biopsy. It is our standard practice to prospectively ascertain radiologic-pathologic concordance for all biopsies and to consult with pathology directly as necessary.
TABLE 1.
Histopathology of 73 Biopsied Breast Lesions
| Histopathology | Malignant (n = 18) |
Benign (n = 55) |
|---|---|---|
| Malignant | ||
| Ductal carcinoma in situ (DCIS) | 3 | |
| Invasive ductal (DCIS) | 12 | |
| Invasive lobular | 1 | |
| Invasive periductal stromal | 1 | |
| Invasive papillary | 1 | |
| Benign | ||
| Fibroadenoma | 13 | |
| Normal breast tissue | 12 | |
| Fibrocystic | 7 | |
| Stromal fibrosis | 5 | |
| Lymph node | 5 | |
| Usual ductal hyperplasia | 3 | |
| Apocrine metaplasia | 3 | |
| Pseudoangiomatous stromal hyperplasia | 3 | |
| Atypical lobular hyperplasia | 1 | |
| Intraductal papilloma | 1 | |
| Lobular carcinoma in situ | 1 | |
| Cysts | 1 |
MRI Acquisition
Bilateral axial breast MRI studies were performed on a 1.5 Tesla imager (Signa; GE Medical Systems, Milwaukee, WI). A three-dimensional fast gradient-recalled-echo imaging sequence was performed to produce high-spatial-resolution, fat-suppressed images with full coverage of both breasts. Bilateral axial studies were imaged with the following parameters: repetition time/echo time = 9/4.4 ms, flip angle 10°, number of excitations (NEX)= 1, matrix: 512 × 320, field of view 29–36 cm, slice thickness 2 mm. The contrast agent, gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany), was administered intravenously at a dose of 0.1 mmol/kg of body weight. Injections were performed with an MRI-compatible remote-controlled power injector (Spectris; Medrad, Indianola, PA) at a rate of 1.2 mL/sec. Contrast material injection was followed by a 10-mL saline flush administered at the same flow rate. Acquisition times varied between 210–225 seconds. Three time points were acquired: precontrast (S0), early postcontrast (S1), and late postcontrast (S2), where S represents the corresponding signal intensity for each time point. The central phase-encoding lines of each data set were acquired halfway through the acquisition, yielding effective sample times following contrast injection of 105–112 seconds for S1 and 315–322 seconds for S2. Studies were evaluated visually for motion artifact.
Image Analysis
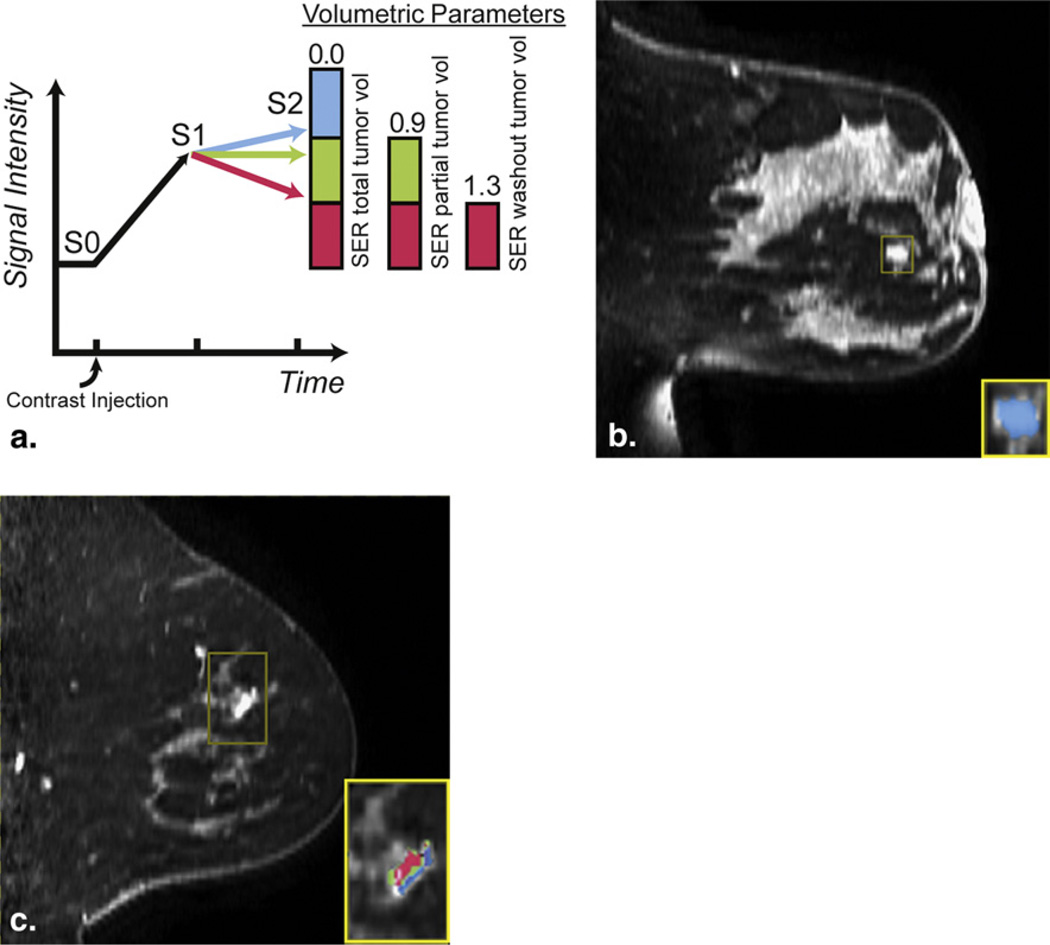
Raw image data were imported and region-of-interest box volumes were manually drawn around lesions by nonradiologist researchers (V.A.A., R.C.-Y.C) blinded to the histopathologic outcome. Lesions were identified based on the associated image numbers given in the prospective radiology report. SER values, defined as (S1-S0)/(S2-S0), were calculated on a per-voxel basis and color-coded maps were generated within the region-of-interest box (Fig 1) using in-house software programmed in IDL (ITT Visual Information Solutions, Boulder, CO). SER and percent enhancement (PE) parameters were defined a priori using criteria set by the American College of Radiology Imaging Network 6657 protocol, a multicenter trial of breast MRI (16). The ACRIN 6657 criteria are based on receiver operating characteristic (ROC) optimization in a retrospective group of 180 patients with histopathologic correlation. PE was defined as 100*(S1-S0)/(S0). An initial enhancement filter was chosen empirically and applied such that all voxels having PE <70% were assigned an SER value of 0. Voxels with SER values >1.3 were considered to have “washout” kinetics and colored red. Voxelswith SER values between 0.9–1.3 were considered to have “plateau” kinetics and colored green. Voxels with SER values <0.9 but >0 were considered to have “persistent” kinetics and colored blue. When present, nontumor elements that enhanced within the region-of-interest box (eg, blood vessels, skin border, implant edge) were manually omitted from calculation of overall SER parameters.
Figure 1.
(a) Diagram of signal enhancement ratio (SER) color map with corresponding ranges for SER parameters. SER total tumor volume (SER ≥ 0) is a combination of all kinetic curve morphologies. SER partial tumor volume (SER ≥ 0.9) combines both plateau and washout kinetic curve morphologies. SER washout tumor volume (SER > 1.3) corresponds only to washout kinetic curve morphology. (b,c) Sagittal magnetic resonance imaging (reformatted from bilateral axial scans) with inset SER voxel maps for a (b) benign lesion and (c) malignant lesion. SER is defined as (S1-S0)/(S2-S0), where S0 is the signal intensity precontrast, S1 is the signal intensity at early postcontrast (115–122 seconds), and S2 is the signal intensity at late postcontrast (315–322 seconds).
Variables used as predictors of benignity or malignancy were SER total tumor volume, SER partial tumor volume, SER washout tumor volume, peak SER, and peak PE. SER total tumor volume was defined as total volume of enhancing voxels above the initial enhancement level of 70% that have “washout,” “plateau,” or “persistent” kinetics (voxels with SER ≥ 0; colored red, green, or blue). SER partial tumor volume was defined as the total volume of enhancing voxels with “washout” or “plateau” kinetics (voxels with SER ≥ 0.9; colored either red or green). Washout SER volume was defined as the total volume of voxels with “washout” kinetics only (voxels with SER > 1.3; colored red only). SER volume parameters represent raw whole lesion volume and were not normalized to size measurements, as lesion size is associated with histopathology. Peak SER was defined as the highest mean SER of 9 or more interconnected voxels within a 3 × 3 × 3-voxel cube. Peak PE, defined as highest mean initial PE of 9 or more interconnected voxels within a 3 × 3 × 3-voxel cube, was measured to compare to peak SER. Lesions with less than 9 interconnected voxels were excluded only from analysis of peak PE and peak SER. As a comparison, lesion diameter (longest distance in axial plane) and enhancement type (mass, nonmass, and focus) were recorded based off the first postcontrast image.
Statistical Analysis
Data were statistically analyzed using Matlab 2007a (The Mathworks Inc, Natick, MA). Distribution of data was first assessed for normality using Lillefors test. Normally distributed data were analyzed with Student t-test; non-normally distributed data were analyzed using Wilcoxon rank-sum test. Thresholds were defined as SER values at which a parameter was 100% sensitive. Thresholds were determined only in parameters that were statistically significant for predicting pathologic outcome.
Clinical impact was retrospectively assessed through changes in biopsy recommendations using the 100% sensitivity threshold (beyond which all lesions are benign). Lesions retrospectively predicted to be benign would not have to be biopsied, decreasing the overall biopsies recommended. Lesions predicted to be malignant would still have to be biopsied and therefore would not change the biopsy recommendations, because important information is derived from the biopsy samples that affect treatment, such as hormone status. Finally, a comparison was made to the original radiologists’ MRI interpretations. The original radiologists’ interpretations were performed prospectively as part of routine clinical care and incorporated both BIRADS morphologic descriptors and a qualitative assessment of contrast kinetics.
RESULTS
A total of 577 consecutive MRI examinations were found by the initial search, of which 136 women were evaluated and received a BIRADS 4 or 5 assessments. Of these, 71 women were excluded resulting in a final sample size of 65 women with 73 lesions. Table 1 describes the characteristics of the lesions included in analysis. Patients were excluded for not receiving a biopsy (from mastectomy in 54 patients or doctor/patient refusal in 6 patients), ambiguity in which a finding was biopsied in retrospect (4 patients), transfer of care to an outside institution (4 patients), or loss to follow-up (3 patients). No studies were excluded for motion artifact. Among the 73 lesions included in the study, 18 malignant lesions (25%) were identified: 3 ductal carcinoma in situ (DCIS), 12 invasive ductal only or mixed with DCIS, 1 invasive lobular, 1 invasive papillary, and 1 periductal stromal invasive tumor. No lesions initially identified as benign were found to be malignant at 1 year. One patient was found to have lobular carcinoma in-situ at initial biopsy, but because radiologists felt the pathology was discordant with spiculated morphology by imaging, the patient went on to excisional biopsy and was again found to have lobular carcinoma in-situ. One patient with atypical lobular hyperplasia was followed with mammography and clinical breast exam but had no clinical signs of recurrence at 1 year. Further details about lesion histopathology are detailed in Table 1. Overall, there was no significant difference in mean diameter between malignant or benign lesions (P = .4 by t-test) or enhancement type (P = .12 by Fisher’s exact test).
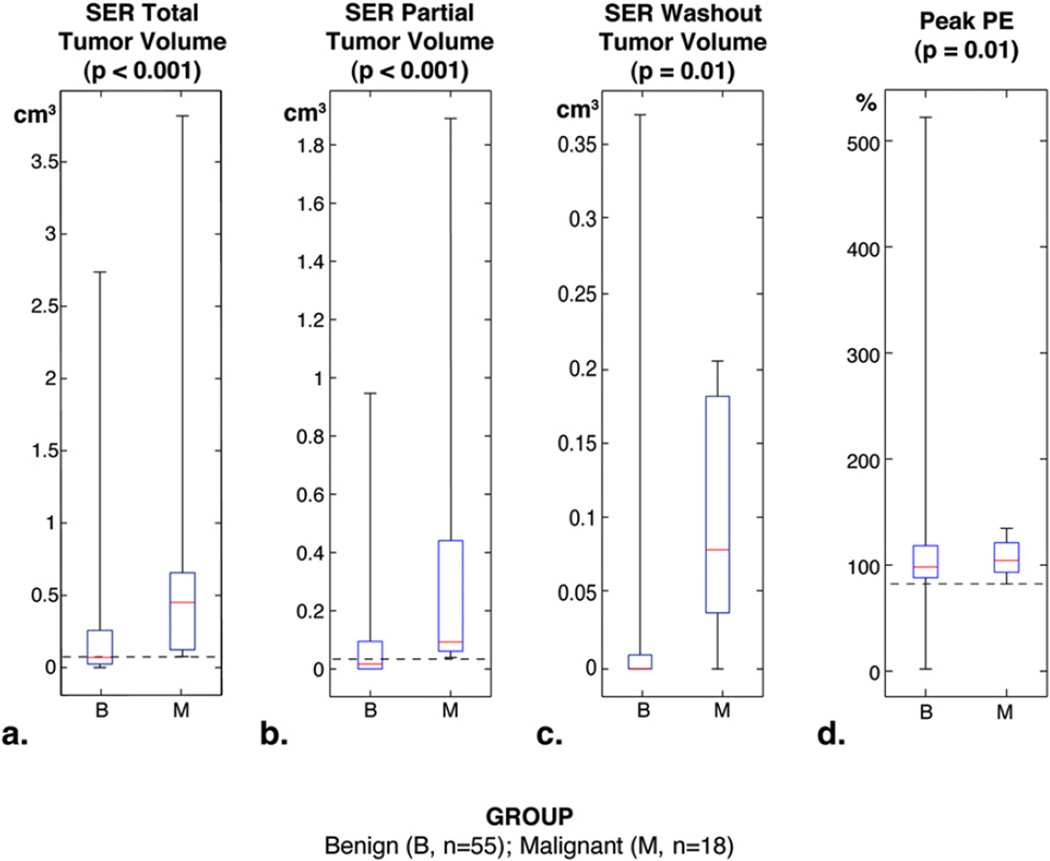
SER parameters were non-normally distributed and analyzed using Wilcoxon rank sum. Results are presented as medians for malignant and benign lesions with corresponding P values; a complete statistical summary is presented in Table 2. SER total tumor volume was significantly different between malignant versus benign lesions (0.45 cm3 vs. 0.07 cm3, P < .001), as well as SER partial tumor volume (0.09 cm3 vs. 0.02 cm3, P < .001), SER washout tumor volume (0.01 cm3 vs. 0.0003 cm3, P = .01), and peak PE (103% vs. 91%, P = .01). Peak SER was not significantly different between malignant versus benign lesions (1.40 vs. 1.21, P = .10).
TABLE 2.
Comparison of Traditional MRI Parameters and Signal Enhancement Ratio (SER) Values between Malignant and Benign Lesions
| Parameter | Malignant (n = 18) | Benign (n = 55) | P Value |
|---|---|---|---|
| Mean lesion diameter, cm. (SD) | 1.3 (0.7) | 1.2 (0.8) | .40 |
| Enhancement type | .12 | ||
| Mass | 15 | 30 | |
| Nonmass | 1 | 10 | |
| Focus | 2 | 15 | |
| SER total tumor volume, cm3 | 0.45 (0.08–3.80) | 0.07 (0–2.72) | <.001 |
| SER partial tumor volume, cm3 | 0.09 (0.04–1.89) | 0.02 (0–0.98) | <.001 |
| SER washout tumor volume, cm3 | 0.01 (0–0.21) | 0.0003 (0–0.38) | .01 |
| Peak SER* | 1.40 (0.99–2.92) | 1.21 (0.42–3) | .10 |
| Peak PE | 103% (82–138) | 91% (2–522) | .01 |
Data were non-normally distributed and statistically compared nonparametrically using Wilcoxon rank-sum test. Results are expressed as median (minimum-maximum).
For peak SER, 8 benign cases were excluded because of <9 voxel interconnectivity.
Using empirically derived thresholds for predicting benign lesions, the changes in biopsy recommendations and PPV3 were retrospectively determined (Table 3). Thresholds for which MRI had 100% sensitivity (Fig 2, dashed horizontal lines) were identified for SER total tumor volume (0.08 cm3), SER partial tumor volume (0.04 cm3), and peak PE (103%). Although SER washout tumor volume was significantly different, it was not able to predict benign lesions with 100% sensitivity. By the original radiologists’ MRI interpretations, 73 lesions were recommended for biopsy with a PPV3 of 18/73 (25%). Applying thresholds, SER total tumor volume discriminated 28 benign lesions, which decreased biopsy recommendations to 45 lesions. SER partial tumor volume discriminated 33 benign lesions, which decreased biopsy recommendations to 40 lesions. Peak PE correctly identified 18 benign lesions, which decreased biopsy recommendations to 55 lesions. Across all SER parameters in which thresholds were determined, all 18 malignancies would still be recommended for biopsy after SER analysis, thus preserving the number of malignancies detected but increasing the PPV3 as the number of false positives (denominator) decreased.
TABLE 3.
Clinical Impact of MRI Signal Enhancement Ratio (SER) Parameters
| Method | Biopsies Recommended |
PPV3 (Biopsy Yield of Malignancy) |
|---|---|---|
| Initial MRI interpretation | 73 | 25% |
| Total tumor volume | 45 | 40% |
| SER partial tumor volume | 40 | 45% |
| SER washout tumor volume | 73 | 25% |
| Peak PE | 55 | 33% |
MRI, magnetic resonance imaging; PE, percent enhancement; SER, signal enhancement ratio.
Biopsies recommended are based on the number of total biopsies recommended by original radiologist’s MRI interpretation minus lesions retrospectively predicted to be benign using empirically derived thresholds at which a parameter performs with 100% sensitivity.
Initial MRI interpretation is defined as the original radiologists’ interpretations performed prospectively as part of routine clinical care and incorporates both Breast Imaging Reporting and Data System morphologic descriptors and a qualitative assessment of contrast kinetics.
Figure 2.
Boxplots of signal enhancement ratio (SER) parameters comparing benign (B) and malignant (M) breast MRI lesions. Boxes represent the 25th to 75th quartile, with a median dividing line. Whiskers correspond to the minimum and maximum of data. P values were determined using Wilcoxon rank-sum test. Dashed lines represent the thresholds for SER parameters with 100% sensitivity (beyond which all lesions are benign). Thresholds were not assessed for peak SER enhancement because of nonsignificant association with pathologic outcome.
DISCUSSION
In this retrospective study, we found that the application of volume-based SER parameters as an adjunct to routine clinical interpretation may reduce the number of recommended biopsies without decreasing PPV3. This study adds to the body of evidence that contrast kinetic markers can be used to improve diagnostic specificity of breast MRI.
SER parameters at higher values were significantly associated with malignancy (Table 2). Using thresholds for 100% sensitivity, the parameter “SER partial tumor volume,” which is a composite approximation of lesions exhibiting either washout or plateau kinetics, performed best. Lesions with SER partial tumor volumes below 0.04 cm3 were all benign. Based on this approach, SER partial tumor volume correctly discriminated 45% of all biopsied lesions as benign. The parameter “SER total tumor volume,” which is a composite approximation of all three kinetic types, performed nearly as well and correctly discriminated 40% of benign lesions without overlap. SER washout tumor volume was also significantly associated with malignancy, but a threshold at 100% sensitivity could not be created because of overlap. These findings suggest that certain SER parameters could adjunctively improve the specificity of MRI by reducing false positives without sacrificing true positives. Furthermore, standard measurements of lesion diameter and lesion type did not predict benignity or malignancy in this study. This implies that SER parameters can provide useful diagnostic information to radiologists beyond standard BIRADS criteria, by combining both whole lesion volume and contrast kinetics into a single quantitative measure.
Our results also show that 85% of lesions correctly identified as benign, and therefore potentially excluded from biopsy recommendation, had <70% initial enhancement at the first post-contrast sequence. This suggests that the initial enhancement level, rather than kinetic curve morphologies, contributes most to predicting benign pathology. These findings are consistent with prior studies using qualitative (17,18) or quantitative (11,12,19–22) techniques that show lesions with initial enhancement above a certain threshold improves specificity of breast MRI. In retrospective studies of a three time-point model using the commercial product CADStream (11,19), lesions that did not meet an initial enhancement of 50%–100% were all benign, potentially excluding 25%–50% of all benign lesions without excluding malignant lesions. However, in other studies examining whole breast volume (20) or the most suspicious pixels (12) (similar to peak PE or peak SER), initial enhancement between 50% and 100% increased specificity at the expense of sensitivity.
There are several limitations of this study. First, this was a retrospective study with potential for selection bias. To minimize the potential for selection bias, we collected patients consecutively based on information available in our radiology electronic medical record. We purposely excluded suspicious breast lesions that were previously known or suspected by other modalities (9,23,24), because these lesions may artificially improve the diagnostic performance of MRI, hence introducing a different type of bias. Second, our relatively small sample size prevented analysis by pathologic subtype or lesion type. A larger sample size would improve confidence intervals to establish more precise thresholds. Third, our three time-point criteria may also differ from other studies. We established our criteria based on the American College of Radiology Imaging Network 6657 trial (16) and used postcontrast sampling times of approximately 105–112 seconds for S1 and 315–322 seconds for S2, initial enhancement level of 70%, and where persistent enhancement was defined as 10% above plateau and washout enhancement was defined as 30% below plateau. The results presented in this study may change with different threshold choices. Thresholds depend on multiple factors, particularly scan time and injection rate, and therefore need to be optimized relative to a specific MRI protocol (25).We chose 70% initial enhancement empirically based on past experience for our study protocol.
In conclusion, the adjunctive use of SER volume parameters retrospectively reduced the number of recommended biopsies without decreasing the number of cancers detected. These results suggest that adjunctive use of volume-based parameters may improve the specificity of breast MRI without affecting sensitivity. However, our results need to be validated prospectively to establish true clinical efficacy.
ACKNOWLEDGMENTS
This research was supported by NIH R01 CA 69587, NIH TL1 RR024129, Grant #IRG-97-150-10 from the American Cancer Society, and a grant from the University of California Cancer Research Coordinating Committee. We thank Nancy Hills for her biostatistical input, Christopher Jovais for compiling the patient list, and Eoin Galvin for his editing assistance.
REFERENCES
- 1.Kuhl CK. Current status of breast MRimaging. Part 2. Clinical applications. Radiology. 2007;244:672–691. doi: 10.1148/radiol.2443051661. [DOI] [PubMed] [Google Scholar]
- 2.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 3.Bassett LW, Dhaliwal SG, Eradat J, et al. National trends and practices in breast MRI. AJR Am J Roentgenol. 2008;191:332–339. doi: 10.2214/AJR.07.3207. [DOI] [PubMed] [Google Scholar]
- 4.Houssami N, Hayes D. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA: Cancer J Clin. 2009;59:290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl C, Kuhn W, Braun M, et al. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007;16(Suppl 2):S34–S44. doi: 10.1016/j.breast.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 7.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magnet Res Imaging JMRI. 1997;7:91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 8.Li KL, Henry RG, Wilmes LJ, et al. Kinetic assessment of breast tumors using high spatial resolution signal enhancement ratio (SER) imaging. Magnet Res Med. 2007;58:572–581. doi: 10.1002/mrm.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esserman L, Hylton N, George T, et al. Contrast-enhanced magnetic resonance imaging to assess tumor histopathology and angiogenesis in breast carcinoma. Breast J. 1999;5:13–21. doi: 10.1046/j.1524-4741.1999.005001013.x. [DOI] [PubMed] [Google Scholar]
- 10.Hylton NM. Vascularity assessment of breast lesions with gadolinium-enhanced MR imaging. Magn Reson Imaging Clin N Am. 1999;7:411–420. x. [PubMed] [Google Scholar]
- 11.Lehman CD, Peacock S, DeMartini WB, et al. A new automated software system to evaluate breast MR examinations: improved specificity without decreased sensitivity. AJR Am J Roentgenol. 2006;187:51–56. doi: 10.2214/AJR.05.0269. [DOI] [PubMed] [Google Scholar]
- 12.Wang LC, DeMartini WB, Partridge SC, et al. MRI-detected suspicious breast lesions: predictive values of kinetic features measured by computer-aided evaluation. AJR Am J Roentgenol. 2009;193:826–831. doi: 10.2214/AJR.08.1335. [DOI] [PubMed] [Google Scholar]
- 13.Eby PR, Partridge SC, White SW, et al. Metabolic and vascular features of dynamic contrast-enhanced breast magnetic resonance imaging and (15) O-water positron emission tomography blood flow in breast cancer. Acad Radiol. 2008;15:1246–1254. doi: 10.1016/j.acra.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karahaliou A, Vassiou K, Arikidis NS, et al. Assessing heterogeneity of lesion enhancement kinetics in dynamic contrast-enhanced MRI for breast cancer diagnosis. Br J Radiol. 2010;83:296–309. doi: 10.1259/bjr/50743919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR BI-RADS-Mammography. 4th ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 16.ACRIN. Protocol 6657. American College of Radiology Imaging Network (ACRIN) Available online at: www.acrin.org/6657_protocol.aspx. [Google Scholar]
- 17.Moon M, Cornfeld D, Weinreb J. Dynamic contrast-enhanced breast MR imaging. Magn Reson Imaging Clin N Am. 2009;17:351–362. doi: 10.1016/j.mric.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Szabo BK, Aspelin P, Wiberg MK, et al. Dynamic MR imaging of the breast. Analysis of kinetic and morphologic diagnostic criteria. Acta Radiol. 2003;44:379–386. doi: 10.1080/j.1600-0455.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams TC, DeMartini WB, Partridge SC, et al. Breast MR imaging: computer-aided evaluation program for discriminating benign from malignant lesions. Radiology. 2007;244:94–103. doi: 10.1148/radiol.2441060634. [DOI] [PubMed] [Google Scholar]
- 20.Baltzer PA, Renz DM, Kullnig PE, et al. Application of computer-aided diagnosis (CAD) in MR-mammography (MRM): do we really need whole lesion time curve distribution analysis? Acad Radiol. 2009;16:435–442. doi: 10.1016/j.acra.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Levman JE, Causer P, Warner E, et al. Effect of the enhancement threshold on the computer-aided detection of breast cancer using MRI. Acad Radiol. 2009;16:1064–1069. doi: 10.1016/j.acra.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paldino MJ, Barboriak DP. Fundamentals of quantitative dynamic contrast-enhanced MR imaging. Magn Reson Imaging Clin N Am. 2009;17:277–289. doi: 10.1016/j.mric.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Kelcz F, Furman-Haran E, Grobgeld D, et al. Clinical testing of high-spatial-resolution parametric contrast-enhanced MR imaging of the breast. AJR Am J Roentgenol. 2002;179:1485–1492. doi: 10.2214/ajr.179.6.1791485. [DOI] [PubMed] [Google Scholar]
- 24.Hauth EA, Stockamp C, Maderwald S, et al. Evaluation of the three-time-point method for diagnosis of breast lesions in contrast-enhanced MR mammography. Clin Imaging. 2006;30:160–165. doi: 10.1016/j.clinimag.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Jansen SA, Shimauchi A, Zak L, et al. Kinetic curves of malignant lesions are not consistent across MRI systems: need for improved standardization of breast dynamic contrast-enhanced MRI acquisition. AJR Am J Roentgenol. 2009;193:832–839. doi: 10.2214/AJR.08.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]