Abstract
Regulatory T cells (Treg), a subset of CD4+ T cells, dramatically accumulate with age in humans and mice and contribute to age-related immune suppression. Recently, we showed that a majority of accumulating Treg in aged mice expressed low levels of CD25 and their accrual is associated with declining levels of IL-2 in aged mice. Here, we further investigated the origin of CD25lo Treg in aged mice. First, aged Treg had high expression of neuropilin-1 and Helios and had a broad Vβ repertoire. Next, we analyzed the gene expression profile of Treg, naïve, and memory T cells in aged mice. We found that the gene expression profile of aged CD25lo Treg were more related to young CD25lo Treg than to either naïve or memory T cells. Further, the gene expression profile of aged Treg was consistent with recently described “effector” Treg. Additional analysis revealed that nearly all Treg in aged mice were of an effector phenotype (CD44hiCD62Llo) and could be further characterized by high levels of ICOS and CD69. ICOS contributed to Treg maintenance in aged mice, as in vivo antibody blockade of ICOSL led to a loss of effector Treg, and this loss was rescued in Bim-deficient mice. Further, serum levels of IL-6 increased with age and contributed to elevated expression of ICOS on aged Treg. Finally, Treg accrual was significantly blunted in aged IL-6-deficient mice. Together, our data show a role for IL-6 in promoting effector Treg accrual with age likely through maintenance of ICOS expression.
Introduction
The immune system undergoes significant, progressive changes with age that contribute to a dramatic decline in the efficacy of immune responses in the elderly, leading to increased incidences of infections, cancers, and decreased vaccine efficacy (1, 2). This suppressed immune phenotype observed in the elderly has been termed ‘immunosenescence’, and is driven by defects in both the innate and adaptive immune systems (3, 4). Within the adaptive immune system, T cells exhibit intrinsic defects in T cell receptor (TCR) signaling, which reduces their ability to proliferate in response to antigen stimulation (5–8). T cells also exhibit defects at the population level, as aged mice have reduced naïve T cells due to thymic involution and a constrained repertoire due to clonal expansion of memory T cells (9–13). Finally, we and others have shown that FoxP3+ regulatory T cells (Treg), a subset of CD4+ T cells, significantly accumulate with age and also contribute to age-related immunosenescence (14–18).
Several factors contribute to Treg homeostasis, including production in the thymus, survival and conversion in the periphery. IL-2 has been described as a major Treg survival factor, as Treg are decreased significantly in IL-2-deficient mice (19, 20). In additional to IL-2, other common γ chain cytokines, such as IL-15, contribute redundantly to Treg survival, as CD122 or CD132 deficient mice have a greater loss of Treg compared to IL-2 deficient mice (19, 21–23). Nonetheless, it is clear that such cytokine signaling promotes Treg homeostasis by antagonizing the pro-apoptotic activity of Bim (24, 25). However, IL-2 levels decrease with age, favoring the accrual of Treg that have dramatically reduced levels of Bim and are less dependent on IL-2 for survival (25). Further, combined neutralization of IL-2/15 in vivo led to significant, but not complete reduction of Treg in aged mice (25), suggesting other factors contribute to Treg accrual and homeostasis with age.
In addition to thymic production, Treg can also be derived from peripheral conversion of naïve CD4+ T cells via multiple mechanisms (26). Although these converted Treg normally predominate in the gut tissues, they can populate secondary lymphoid organs sufficient to control autoimmunity under conditions where thymic production is absent (27). Using one in vitro model of Treg conversion, we have shown that, if anything, Treg conversion is reduced in aged mice (28). The lack of distinguishing markers has hampered the ex vivo identification of peripherally converted Treg, until recent gene expression profiles have identified neuropilin-1 (Nrp-1) and Helios as markers of thymically-derived Treg (29–31). However, it remains unclear whether the in vivo accumulation of Treg in aged mice reflects an expanded peripheral Treg pool or a persisting thymic Treg pool.
Other cytokine-independent mechanisms can also contribute to Treg maintenance, as co-stimulatory receptors CD28 and inducible co-stimulator (ICOS) have been shown to affect Treg homeostasis (32, 33). Recent work has defined two subsets of Treg that differ in their homeostatic requirements: “central” Treg (CD44lo CD62Lhi) which appear to be more dependent on IL-2 signaling, while “effector” Treg (CD44hi CD62Llo) appear to be more dependent on ICOS signaling for their maintenance (34). With age, it is unclear if the accumulating Bimlo Treg population that is less dependent on IL-2 is reflective of an increase in the “effector” Treg subset.
Aging is also associated with altered systemic cytokine production, and while some cytokines such as IL-2 decline (25), others such as IL-6 increase with age (35). Increased inflammatory cytokines are reflective of an overall increase in inflammation that occurs with age, which has been termed “inflammaging” (36). It is unclear how this increased inflammatory environment may affect Treg homeostasis with age. However, in young mice LPS has been shown to promote ICOS expression and expansion of “effector” Treg (34). Increased IL-6 may promote Treg maintenance as IL-6 has been shown to promote the survival of naïve T cells, and decrease Bim expression within activated T cells (37, 38). Further, one study using IL-6 transgenic mice showed that increasing the levels of IL-6 in vivo can enhance the numbers of Treg (39). Conversely, IL-6 has been shown to inhibit Treg differentiation in vitro by promoting Th17 lineage commitment along with TGFβ signaling (40, 41). As Th17 cells are also increased with age (42, 43), the role of IL-6 in promoting Treg accrual remains unclear.
In this study, we further characterized Treg in aged mice and determined the role of IL-6 and ICOS in their homeostasis. We found that Treg in aged mice have a predominately effector phenotype and that ICOS is critical for their maintenance, likely by inhibiting Bim-mediated death. In addition, we found that IL-6 contributed to Treg accrual in aged mice and promoted expression of ICOS on Treg. Thus, while IL-6 clearly promotes inflammation, our data suggest a novel role of IL-6 to counterbalance this inflammation by elevating Treg.
Materials and Methods
Mice and antibody treatments
Young C57BL/6 mice were purchased from Taconic Farms (Germantown, NY, USA) or were received from the National Institutes of Aging (NIA) colony located at Charles River Laboratories (Wilmington, MA, USA). Old C57BL/6 mice were aged in house or were received from the NIA colony. FoxP3-IRES-DTR-GFP knock-in C57BL/6 mice (44) and FoxP3-IRES-YFP/Cre mice (45) were a generous gift from Dr. A. Rudensky and were aged in-house. Bim-deficient [Bim knockout (KO)] mice were a kind gift from Drs. P. Bouillet and A. Strasser, and have been backcrossed to C57BL/6 mice 20 generations (Walter and Eliza Hall Institute, Melbourne, Australia). Bimf/f mice on the C57BL/6 background were generated in collaboration with Dr. P. Bouillet, and were then crossed to FoxP3-IRES-YFP/Cre mice, as previously described (25). IL-6-deficient (IL6KO) mice on the C57BL/6 background were originally purchased from The Jackson Laboratory (Bar Harbor, ME) (B6.129S2-Il6tm1Kopf/J) and maintained and aged in house. Mice were housed under specific pathogen free conditions in the Division of Veterinary Services at Cincinnati Children’s Hospital Research Foundation. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC).
Young (2–4 months) and old (18 months) C57BL/6 mice were injected i.p. with 7.5mg/kg anti-ICOSL (HK5.3, BioXcell, West Lebanon, NH.) or with rat IgG2A isotype control (2A3, BioXcell), on days 0, 3, 6, 9 and sacrificed on day 12.
Flow cytometry
Spleen, peripheral lymph nodes (inguinal, axillary, and brachial) and mesenteric lymph nodes were harvested and crushed through 100 μm filters (BD Falcon) to generate single-cell suspensions. 1×106 cells were surface stained with a combination of the following antibodies: anti-CD4, CD44, CD62L, ICOS, CCR7, CD69, CD25, Nrp-1 (all from eBioscience, San Diego, CA), Vβ1-17 (BD Biosciences, San Diego, CA). Cells were intracellularly stained for Bim (Cell Signaling Technology, Danvers, MA), Bcl-2 (generated in-house), Ki67 (eBioscience), Helios (eBioscience), and FoxP3 (eBioscience) using the eBioscience FoxP3 staining kit and protocol. For surface staining of CCR7, cells were incubated at 37C for 1 hour prior to adding the anti-CCR7 antibody. Data were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed using FACSDiva software (BD Biosciences). Histogram overlays were generated using FlowJo software (FLOWJO, LLC, Ashland, OR); the smoothing effect was applied to the histograms, and the y-axis is representing the data normalized to the mode.
IVCCA and ELISAs
IL-6 and TNF-α in vivo cytokine capture assay (IVCCA) was performed as previously described (46–48). Briefly, young (2–4 months) and old (18 months) C57BL/6 mice were injected i.v. with 10ug of biotinylated anti-IL-6 (MP5-32C11, eBioscience) and anti-TNFα (TN3, eBioscience) capture antibodies, mice were bled 24 hours later and serum was collected. A luminescent ELISA was performed using anti-IL6 (MP5-20F3, eBioscience) or anti-TNF-α (G281-2626, BD Biosciences) as the coating antibody. Serum IL-1β was measured via Multiplex Assay using Luminex (Millipore, Billerica, MA) according to the manufacturer’s instructions. Serum endotoxin (LPS) was determined using the QCL-1000 Limulus Ameboycte Lysate (LAL) endpoint assay (Lonza, Allendale, NJ), as previously described (48).
Next-Generation sequencing
Spleen cells from young (3–5 months, n=3, pooled) and old (>18 months, n=3, pooled) FoxP3-IRES-DTR-GFP mice were enriched for CD4+ T cells using the negative selection MACS CD4+ T cell Isolation kit II (Miltenyi Biotec, San Diego, CA). Cells were stained with anti-CD4, CD44, CD62L, and CD25 antibodies and the following populations were sorted by a FACSAria (BD Biosciences): CD4+ FoxP3GFP+ CD25lo (CD25lo Treg), CD4+ FoxP3GFP+ CD25hi (CD25hi Treg), CD4+ FoxP3GFP− CD44lo CD62Lhi (naïve CD4+), CD4+ FoxP3GFP− CD44hi CD62Llo (memory CD4+). >85% purity was obtained (data not shown). RNA was isolated from the sorted cells using an RNeasy Minikit (Qiagen, Valencia, CA), and amplified with the Ovation RNA-Seq System (NuGEN Technologies, San Carlos, CA). The cDNA library was generated using Illumina NGS library preparation, and sequenced on the Illumina HiSeq 2000 with single-end 100-bp reads (Illumina, San Diego, CA) in the Cincinnati Children’s Hospital RNA sequencing core.
RNA-seq analysis was performed entirely in GeneSpring NGS software (Agilent Technologies, Santa Clara, CA). Sequences were aligned to the mouse reference genome (mm9) with annotations produced by the Ensembl project. Aligned gene read counts were quantified and used to compute Reads per Kilobase per Million (RPKM) for each transcript within each sample. RPKMs were normalized using the DESeq algorithm, with read counts thresholded to 1, and the baseline was set to the median of all samples. The data was further filtered, requiring each transcript to have ≥10 reads in at least one of the eight samples (n=13940 genes).
We identified differentially expressed genes with a fold change test, using a cutoff of 2.0. Additionally, gene lists were built using rank ordering, selecting the top and bottom 500 genes expressed, based on cell type and age. In order to identify sample clustering based on top and bottom expressed genes based on the age effect, we performed a principal component analysis on the top and bottom 500 genes of old and young Tregs (n=4144 genes). Four principal components generated were adequate to account for 80% of variability, and samples were clustered based on principal component scores calculated from expression values.
The RNA-sequencing data has been deposited to NCBI Sequence Read Archive (http://trace.ncbi.nlm.nih.gov/Traces/sra/), accession number SRP058464.
Results
Aged Treg express high levels of neuropilin-1 and Helios and have broad TCR Vβ usage
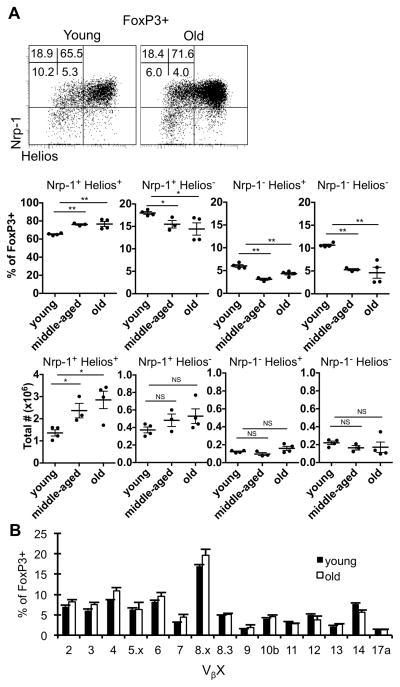
While our and others previous data showed that Treg accumulate with age (14–18, 25, 28), the origin of these cells remained unclear. One possibility was that the accrued Treg represent peripherally derived or converted Treg. To test this, we examined their expression of markers that have been reported to distinguish thymus-derived from peripheral-derived Treg, Nrp-1 and Helios (29–31). Notably, Treg from aged mice expressed high levels of both Nrp-1 and Helios (Fig. 1A), suggesting a thymic origin for these cells. Another possibility was that they were oligoclonally-expanded cells, similar to CD8+ T cells, perhaps in response to endogenous superantigen (49, 50). However, flow cytometric analysis of their TCR Vβ chains showed that aged Treg have a similar TCR Vβ usage compared to Treg from young mice (Fig. 1B). Thus, Treg in aged mice represent a relatively diverse pool of cells expressing markers denoting a thymic origin.
Figure 1.
Aged Treg are enriched for Nrp-1+ and Helios+ cells, and maintain a similar TCR Vβ usage. Splenocytes from young (3 months), middle-aged (12 months), and old (18 months) wild-type mice were stained for CD4, FoxP3, CD44, CD62L, Nrp-1, and Helios and analyzed by flow cytometry (n=4 mice/group). A, Representative dot plots show the expression of Nrp-1 and Helios in total CD4+ FoxP3+ cells. Numbers are the frequency of CD4+ FoxP3+ cells. Scatter plots show the frequency and total number of CD4+ FoxP3+ Treg that are Nrp-1+ Helios+, Nrp-1+ Helios−, Nrp-l− Helios+, or Nrp-1− Helios−. B, Splenocytes from young (3 months, n=4), and old (22 months, n=4) mice were stained for CD4, FoxP3, and Vβ2-17a and analyzed by flow cytometry. Data shows the average frequency of CD4+ FoxP3+ cells that are Vβx+ (±SE). The p values represent the difference between young and middle-aged or old mice (**p ≤ 0.01, Student’s t test). Data is representative of at least three independent experiments.
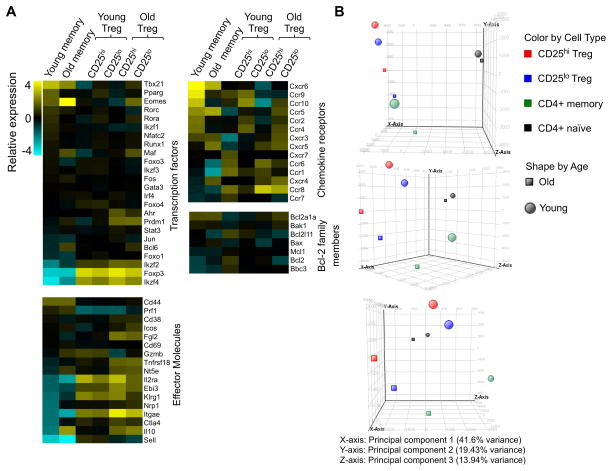
Gene expression profiling reveals an “effector” Treg phenotype in aged mice
Our prior data showed that a substantial fraction of Treg that accumulate with age express low levels of CD25 (25). To further characterize these cells, we sort-purified CD25lo and CD25hi Treg (as well as naïve and memory CD4+ T cells) from young and old FoxP3-GFP reporter mice (44), and subjected the isolated RNA from these cells to high-throughput sequencing. The CD25lo Treg, both from young and old mice, had a gene expression profile different from memory CD4+ cells and expressed genes associated with Treg [i.e. Foxp3, Ctla4, Tnfrsf18 (GITR), Il10, Itgae (CD103), Fig. 2A]. To independently determine the relationships between these populations, we performed a principle component analysis (PCA). This analysis showed a tighter clustering of CD25lo with CD25hi Treg than with naïve or memory CD4+ T cells (Fig. 2B). Thus, PCA analysis shows that Treg from aged mice are more like Treg from young mice than they are to old memory cells. However, both old CD25lo and CD25hi Treg cluster closer together than to young CD25lo and CD25hi Treg (Fig. 2B). In terms of their gene expression, both CD25lo and CD25hi Treg from aged mice had enhanced expression of genes associated with recently described “effector” Treg [Il10, Icos, Prdm1 (Blimp-1), Ebi3, Ccr6] (Fig. 2A) (34, 51, 52).
Figure 2. Aged Treg display the transcriptional signature of “effector” Treg.
Splenocytes from young (3–5months) and old (>18months) FoxP3-IRES-GFP reporter mice were sorted for CD25hi Treg (CD4+ FoxP3GFP+ CD25hi), CD25lo Treg (CD4+ FoxP3GFP+ CD25lo), memory CD4+ T cells (CD4+ FoxP3GFP− CD44hi CD62Llo), and naïve CD4+ T cells (CD4+ FoxP3GFP− CD44lo CD62Lhi), and the isolated RNA was sent for NextGeneration RNA sequencing. A, Heatmaps show the relative gene expression. B, Principle component analysis is shown.
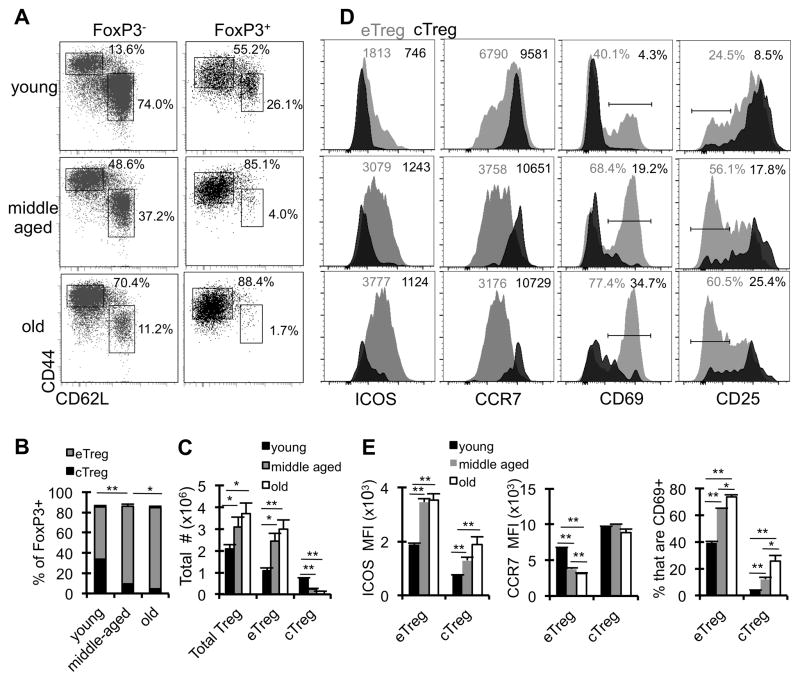
Effector Treg preferentially accumulate with age
Prior work showed that high expression of CD44 and low expression of CD62L marked an “effector” Treg population (34). Further, these effector Treg were less dependent on IL-2 (34), similar to what we previously reported for CD25lo Treg (25). So we longitudinally characterized the “effector” (CD44hi CD62Llo) vs “central” (CD44lo CD62Lhi) phenotype of Treg in young (3 months), middle-aged (12 months), and old (>18 months) mice. Strikingly, by middle age, most Treg had acquired an effector phenotype (>85%), which increased only slightly in old mice (Fig. 3A, B). The increase in effector Treg frequency with age occurs mainly within the lymphoid tissues and not the non-lymphoid tissues, which are comprised predominately of effector Treg even in young mice (Supp. Fig. 3A–B). Further, the overall increase in numbers of Treg in aged mice was largely due to the accrual of “effector” Treg (Fig. 3C).
Figure 3. Treg that accumulate with age have an effector Treg phenotype.
Splenocytes from young (3months, n=4), middle-aged (12 months, n=4), and old (≥18months, n=4) wild-type mice were stained with antibodies against CD4, CD44, CD62L, ICOS, CCR7, CD69, CD25, and FoxP3, and analyzed by flow cytometry. A, The dot plots show representative frequencies of CD4+ FoxP3− cells that are memory (CD44hi CD62Llo) and naïve (CD44lo CD62Lhi) and the frequencies of CD4+ FoxP3+ cells that are effector Treg (CD44hi CD62Llo) and central Treg (CD44lo CD62Lhi). B, Data shows the frequency of FoxP3+ that are effector Treg (eTreg, gray) or central Treg (cTreg, black) (±SE). The statistics are comparing the effector Treg populations. C, Data shows the total number of cells that are FoxP3+ (total Treg), eTreg, and cTreg in young (black), middle-aged (gray), and old (white) mice (±SE). D, The representative histograms show the expression of ICOS, CCR7, CD69, and CD25 on eTreg (gray) and cTreg (black). The numbers are the MFI (ICOS and CCR7) or the frequency of eTreg and cTreg (CD69 and CD25). E, The bar graphs show the average MFI of ICOS and CCR7 expression on eTreg and cTreg in young (black), middle-aged (gray), and old (white) mice, as well as the frequency of cells that are CD69+ (±SE). *p ≤ 0.05, **p ≤ 0.01 (Student’s t test). Data are representative of at least three independent experiments.
To further characterize effector Treg in aged mice, we assessed their expression of ICOS, CD69, CCR7, and CD25, all markers described to be differentially expressed between effector and central Treg (34), and identified by our RNAseq analysis as changing in aged Treg. As expected, both ICOS and CD69 are increased in effector Treg with age, and this is evident already by middle age (Fig. 3D, E). The progressive increase in ICOS expression with age is not Treg-specific, as CD4+ FoxP3− T cells (both memory and naïve) also have an increase in ICOS expression, however the fold-increase is less compared to Treg (1.4 fold in CD4+ memory vs 1.75 fold in effector Treg; Supp. Fig. 1). Further, effector Treg express lower levels of CCR7 and CD25 compared to central Treg, and the expression of these markers further decreases with age (Fig. 3D, E). Together, these data show that the accumulating Treg population has reduced heterogeneity and phenotypically become more effector-like with age.
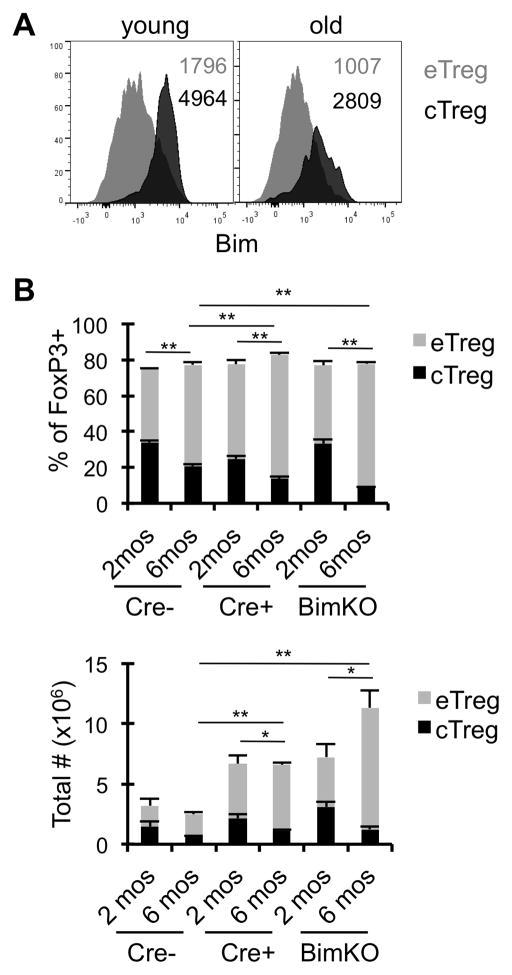
Deletion of Bim promotes effector Treg accrual
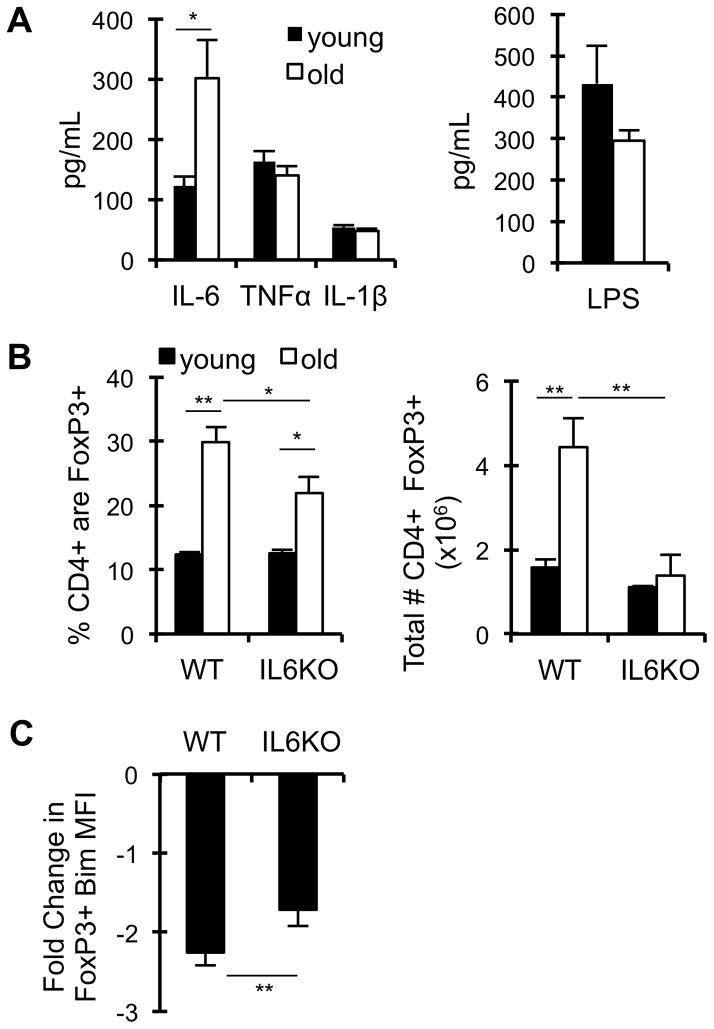
We have previously shown that Bim is a critical negative regulator of Treg homeostasis (25, 28). Intracellular flow cytometric analysis showed that central Treg have higher expression of Bim relative to effector Treg (Fig. 4A). Given that central Treg maintain higher expression of Bim, even in aged mice, it is possible that the high levels of Bim within central Treg may drive their decline via apoptosis. To test this, we examined Treg subsets in aged mice with Treg-specific deletion (FoxP3-Cre Bimf/f) or germline deletion of Bim (BimKO). The deletion of Bim did not rescue the loss of central Treg with age (Fig. 4B), even though Bim was effectively deleted in both eTreg and cTreg (Supp. Fig. 2). In fact, the accrual of effector Treg was accelerated in mice with either a germline or Treg-specific loss of Bim. Combined, these data show that effector, but not central, Treg accrual with age is limited by Bim-mediated death.
Figure 4. Deletion of pro-apoptotic Bim promotes effector Treg accrual.
Splenocytes from young (2–4 months, n=4) and old (≥18months, n=4) mice were stained with antibodies against CD4, CD44, CD62L, Bim, and FoxP3, and analyzed by flow cytometry. A, Representative histograms show the expression of Bim in eTreg (gray) and cTreg (black) from young and old wild-type mice, and numbers are the MFI. B, Splenocytes from FoxP3-Cre− Bimf/f, FoxP3-Cre+ Bimf/f, and BimKO mice that were 2 months or 6 months old (n=4 mice/group) were stained for CD4, CD44, CD62L, and FoxP3. Data shows the frequency of CD4+ FoxP3+ cells that are eTreg (gray) or cTreg (black), or the total number of cells (±SE). *p ≤ 0.05, **p ≤ 0.01 (Student’s t test). The statistics are comparing the effector Treg populations. Data are representative of at least two independent experiments.
Aged IL6KO mice have reduced Treg
Previous work showed that inflammatory stimuli such as LPS altered expression of ICOS and CCR7 on Treg, resulting in a more “effector” Treg phenotype (34). As LPS is known to drive inflammatory cytokine expression, we first determined the levels of inflammatory cytokines in aged mice. Similar to prior reports, we found that aged mice have a 3-fold increase in serum IL-6; however, no change in the inflammatory cytokines TNFα and IL-1β or in serum LPS was observed (Fig. 5A). To assess whether the accumulation of IL-6 with age contributes to Treg accrual, IL-6KO mice were aged to ≥18 months. As expected, young IL-6KO mice had no difference in Treg frequencies or numbers compared to WT mice (Fig. 5B). However, aged IL-6KO mice had significantly reduced frequencies and numbers of Treg (Fig. 5B). This reduction was mainly within the lymphoid tissues (spleen, pLN, mLN), and not within some non-lymphoid tissues (liver, IEL) (Supp. Fig. 3B). Given the role of Bim in limiting Treg accrual, we assessed the effect of IL-6 on Bim expression in Treg with age. We found that the diminution of Bim expression with age is impeded in IL-6 deficient mice (Fig. 5C). Importantly, aged IL-6KO mice had similar frequencies of effector Treg compared to WT mice, and the effector Treg in aged IL-6KO mice had higher Bim expression (data not shown). Together, these data show that IL-6 represses Bim and promotes Treg accrual with age.
Figure 5. IL-6 represses Bim and promotes Treg accrual with age.
A, Young (4 months, black, n=6) and old (18 months, white, n=6) wild-type mice were intravenously injected with biotinylated α-IL-6 and α-TNFα capturing antibodies, serum was collected 24 hours later, and IL-6 and TNFα levels were measured by ELISA. Serum IL-1β levels were measured by Multiplex. Serum LPS was measured by LAL assay. Data shows the average serum IL-6, TNFα, IL-1β, and LPS (±SE). B–C, Splenocytes from young (3 months, n=5–8/group) and old (≥18 months, n=5–8/group) wild-type (WT) and IL-6KO mice were stained with antibodies against CD4, CD44, CD62L, Bim, and FoxP3, and analyzed by flow cytometry. B, Data shows the frequency of CD4+ that are FoxP3+, and the total number of cells that are CD4+ FoxP3+ (±SE). C, Data shows the fold-decrease in Bim expression in total CD4+ FoxP3+ cells with age (±SE). Data is representative of two independent experiments. *p ≤ 0.05, **p ≤ 0.01 (Student’s t test).
IL-6 promotes ICOS expression on Treg
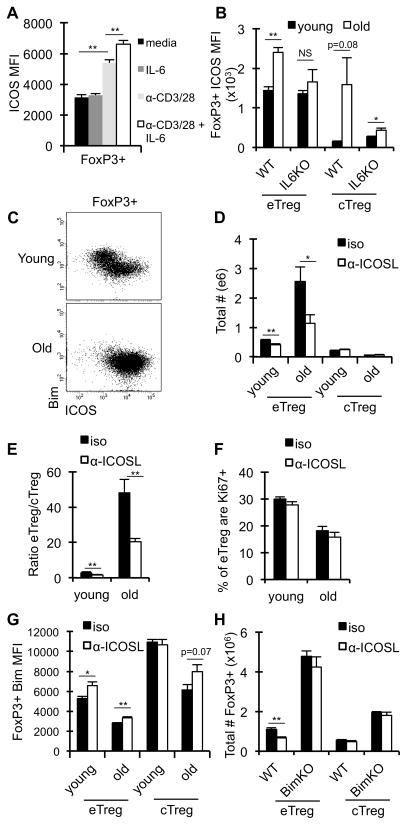
T cell receptor signaling promotes the expression of ICOS (53), however it is unclear if cytokine signaling can induce ICOS expression. Given the increase in both serum IL-6 and Treg ICOS expression with age, we asked whether IL-6 promotes ICOS expression on Treg. While IL-6 alone does not have a significant effect on ICOS expression ex vivo, IL-6 did have an additive effect in combination with TCR signaling (Fig. 6A). Further, the absence of IL-6 resulted in decreased ICOS expression on aged effector and central Treg in vivo (Fig. 6B). Thus, IL-6 enhances TCR-driven ICOS expression on Treg and contributes to the increase in ICOS expression with age.
Figure 6. ICOS contributes to effector Treg maintenance in aged mice.
A–H, Splenocytes from young (2–4 months) and old (≥18months) mice were stained with antibodies against CD4, CD44, CD62L, Bim, ICOS and FoxP3, and analyzed by flow cytometry. A, Splenocytes from young wild-type (WT) mice (2 months, n=4) were cultured for 24 hours with IL-6 (5 ng/ml), anti-CD3/CD28 (3μg/ml), or both. Data shows the average ICOS MFI on total CD4+ FoxP3+ cells (±SE). B, Data show the ICOS MFI on effector Treg and central Treg isolated from the spleens of young (n=5–8/group) and old (n=5–8/group) WT and IL-6KO mice (±SE). C, Representative dots plots show the expression levels of Bim against ICOS on total CD4+ FoxP3+ cells from WT mice. D–G, Young (n=5) and old (n=5) WT mice were treated with isotype control (black) or α-ICOSL blocking antibody (white) for 12 days and splenocytes were analyzed by flow cytometry. Data show the total number of CD4+ FoxP3+ cells that are eTreg or cTreg in young and old mice (D), the ratio of eTreg to cTreg in young and old mice (E), the frequency of eTreg that are Ki67+ (F), and the average Bim MFI in effector Treg and central Treg (±SE) (G). H, Young (3 months, n=4–5 mice/group) WT and BimKO mice were treated with isotype control (black) or α-ICOSL blocking antibody (white) for 12 days. Data show the total number of CD4+ FoxP3+ that are eTreg or cTreg (±SE). *p ≤ 0.05, **p ≤ 0.01 (Student’s t test).
ICOS promotes effector Treg maintenance in old mice
Given that ICOS/ICOSL interactions are critical for effector Treg homeostasis in young mice (32, 34), we examined the role of ICOS in effector Treg homeostasis in old mice. Notably, ICOS signals predominately through a PI3K/Akt/FOXO pathway, which is known to affect Bim expression in T cells (54, 55). In Treg, high expression of ICOS correlates with lower expression of Bim, in both young and old Treg (Fig. 6C). To test whether ICOS/ICOSL interactions are critical for Treg maintenance in old mice and whether such interactions affect the expression of Bim, we neutralized ICOSL in young and old mice. Neutralization of ICOSL resulted in a significant decrease in the number of effector Treg in young and old mice (Fig. 6D), while the numbers of central Treg were not affected regardless of age. The fold loss of effector Treg was greater in old mice compared to young mice (2.3-fold vs 1.4-fold, respectively), resulting in a substantially skewed effector/central Treg ratio in aged mice treated with α-ICOSL (Fig. 6E). Importantly, the effects of α-ICOSL on Treg homeostasis were not due to decreased proliferation, as the frequency of Ki-67+ cells were not changed after α-ICOSL treatment (Fig. 6F). Further, the numbers of dendritic cells were not changed after α-ICOSL treatment (data not shown). Instead, α-ICOSL treatment led to a slight, albeit statistically significant, increase in expression of Bim in the effector Treg (Fig. 6G). Given the critical role for Bim in mediating apoptosis of Treg, it is possible that further increases in Bim due to ICOSL blockade results in cell death, making it difficult to detect the potential magnitude of Bim induction. To test whether the loss of effector Treg was driven by Bim-mediated death, BimKO mice were treated with α-ICOSL. Importantly, the loss of effector Treg was rescued in the absence of Bim (Fig. 6H). Together, these data show that aged effector Treg are more dependent on ICOS for survival and that ICOS enhances Treg survival by antagonizing Bim.
Discussion
Our and others data show that regulatory T cells accumulate with age and contribute to suppressed immune responses (14–18, 28). Further, there is a growing appreciation that the Treg population is heterogeneous, comprised of subsets that have differences in transcriptional regulation, tissue localization, and functionality (34, 52, 56, 57). To date, it is unclear what subsets contribute to Treg accrual with age. We previously reported that a population of CD25lo Treg preferentially accrual with age (25), and here we show that these cells resemble the recently defined “effector” Treg (FoxP3+ CD44hi CD62Llo), both at the transcriptional and protein levels. The effector Treg that accumulate with age have increased expression of ICOS and CD69. ICOS promotes the maintenance of aged effector Treg, likely by reducing Bim-mediated death. Further, we show that the inflammatory environment in aged mice, namely IL-6, is required to maintain optimal ICOS expression and Treg accrual. This study elucidates a novel pathway of Treg accrual and maintenance with age, mediated by an IL-6-ICOS-Bim axis.
There are two sources of Treg that may contribute to the accumulated Treg pool in aged hosts, thymically-derived Treg and Treg that are converted in the periphery from conventional CD4+ T cells. One study has suggested that Treg accrual with age is absent when peripheral induction of Treg is impaired (58). However, this study only looked at Treg frequencies at 8–12 months of age, a time point where we only see modestly increased Treg frequencies, and the reported frequencies of Treg in their wild-type mice were substantially elevated compared to what we historically see (58). Here, we used Nrp-1 and Helios expression to differentiate between thymic and peripheral-derived Treg, and showed that aged Treg are enriched for Nrp-1+ Helios+ cells, suggesting aged Treg are of thymic origin. While the specificity of these molecules as markers for thymic Treg remains controversial, it is clear that peripheral-derived Treg in the gut are negative for expression of Nrp-1 and Helios (29, 59–61). Given the reduction in Treg in the thymus with age (28), the accrual of thymus-derived Treg with age is likely due to increased survival. Indeed, we showed that Bim deficient mice have accelerated Treg accrual that begins after cells have left the recent thymic emigrant compartment (28), and importantly these cells remain Nrp-1+ Helios+ (data not shown). Thus, within the secondary lymphoid organs, the Treg that accumulate appear to be largely thymus-derived.
We and others have defined Treg subsets as CD25lo and CD25hi (16, 25), however with age these populations become more homogeneous at the protein and transcript level, and thus CD25 is likely not the best marker to differentiate aged Treg subsets. Instead, we assessed Treg subsets as “effector” and “central” Treg, as recently described (34), and showed that it is the effector Treg that accumulate with age. Further, the effector Treg have increased expression of ICOS with age and are partially dependent on ICOS for their maintenance. Mechanistically, TCR signaling promotes ICOS expression (34). Further, LPS-induced inflammation promoted increased ICOS expression (34). However, how ICOS is controlled in aged Treg is unclear. Our data show that the pro-inflammatory cytokine IL-6 can enhance ICOS expression in the context of TCR signaling, however whether IL-6 promotes ICOS expression on aged Treg through direct or indirect mechanisms remains unclear. IL-6 can directly signal in Treg, and young and old Treg express similar levels of IL-6 receptor (data not shown), although IL-6-induced STAT3 phosphorylation is slightly impaired in old Treg compared to young Treg (43, 53). ICOS expression is also upregulated downstream of TCR/CD28 via NFATc2 and ERK signaling (53). IL-6 can induce ERK activation (62), and thus may enhance TCR/CD28-driven ICOS expression on Treg through ERK. We cannot exclude indirect pathway(s) by which IL-6 promotes ICOS expression on Treg, as IL-6 can affect DC and macrophage maturation (63, 64). Indeed, DC can promote Treg homeostasis (65). Thus, elevated IL-6 may promote enhanced antigen-presentation and CD80/CD86 expression, prolonging TCR/CD28 signaling. Future work will determine if Treg or other non-Treg cells need to express IL-6Rα in order to promote Treg accrual.
ICOS signaling promotes effector Treg homeostasis (34), however the mechanisms still remain unclear. Smigiel et al. showed that blocking ICOSL selected against Bcl-2lo Treg without affecting proliferation, suggesting that the loss in Treg was due to cell death (34). Mechanistically, ICOS signaling may promote survival by inhibiting Bim-mediated death through activation of the PI3K/Akt pathway (54), which is known to limit Bim expression through modulating FOXO3a transcription factor activation (66, 67). Indeed, Bim is a critical negative regulator of T cell survival and homeostasis, and we have shown that the levels of Bcl-2 determine the levels of Bim a T cell can tolerate (68). Consistently, we show that blocking ICOSL results in increased Bim expression, and the absence of Bim rescues the loss of effector Treg. Thus with age, enhanced ICOS expression on effector Treg likely limits Bim expression via activation of the PI3K/Akt/FOXO pathway, promoting effector Treg survival.
The central Treg population decreases with age, and this loss may be driven by multiple non-mutually exclusive mechanisms. First, Treg production declines with age due to thymic involution (12, 28). The majority of Treg in the thymus are central Treg (34), thus thymic production is likely a major source of this population. Second, central Treg can become effector Treg, a process driven by TCR signaling (34). Indeed, we found that transfer of CD25hi Treg (likely mostly central Treg) converted to CD25lo Treg (likely mostly effector Treg) after adoptive transfer (25). Further, both effector and central Treg in old mice have an increased frequency of CD69+ cells, a marker of recent T cell activation, supporting a model of central Treg activation and conversion. Lastly, we showed that serum IL-2 levels declined with age (25), which may select against central Treg as these cells are more dependent on IL-2 for maintenance (34). IL-2 promotes Treg survival by combating Bim-mediated death (24, 25). However, deletion of Bim did not rescue the loss of central Treg with age. These data argue against increased Bim-mediated cell death as driving the loss of central Treg with age, however we cannot exclude the role for another pro-apoptotic, such as Puma. Alternatively, it is possible that the potential Treg conversion of central Treg to effector Treg is dominant to the death process.
Our data showing that IL-6 promotes Treg accrual is seemingly contradictory to the literature showing that IL-6, along with TGFβ, inhibits Treg while promoting Th17 differentiation (40, 41, 69). This role of IL-6 was established with in vitro cultures using undifferentiated naïve CD4+ T cells (40, 41). In vivo, models of limiting IL-6 promote increased Treg in the context of inflammation (70–72), supporting an inhibitory role of IL-6 on peripheral Treg induction. However, the effect of IL-6 signaling on thymically-derived Treg is less clear. A recent study has shown that IL-6 can induce a “reprogramming” of peripheral Treg through downregulating the transcription factor Eos (73). Eoslo Treg maintained normal FoxP3 expression but exhibited both pro- and anti-inflammatory properties, depending on the tissue localization of the Treg and the inflammatory environment (73). Aged Treg do not exhibit decreased Eos expression (data not shown), thus elevated IL-6 with age is not likely driving Treg “reprogramming”. Additionally, increased in vivo IL-6 levels can promote increased Treg numbers, as IL-6 transgenic mice have elevated thymus-derived Treg numbers and these Treg are functional, while induction of peripheral-derived Treg was inhibited (39). Thus, the role of IL-6 on Treg differentiation and homeostasis is multifactorial and likely cell context dependent.
Recently, the PTEN-mTORC2 axis has been implicated in regulating Treg homeostasis and functionality (74, 75). Interestingly, PTEN deficient Treg have an effector Treg phenotype similar to aged Treg, with elevated CD44, ICOS and CD69 expression (75). At the transcriptional level aged Treg do not have decreased PTEN expression (data not shown), however it is unclear whether or not PTEN signaling changes with age and contributes to effector Treg homeostasis. Understanding effector Treg homeostasis is of broader relevance, as humans have a population of ICOS+ IL-10+ Treg cells that resemble effector Treg in mice (76). Thus, future studies investigating the heterogeneity within the effector Treg subset with age is of clinical relevance, as they may further elucidate Treg homeostasis and functionality with age, and may provide potential therapeutic targets for manipulating Treg numbers and function in the elderly.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Institute of Health Grant AG033057 (to D.A.H. and C.A.C.).
The authors would like to thank the members of the Hildeman, Chougnet, and Divanovic labs for their helpful input and discussion. The authors thank Dr. Monica Cappelletti and Daniel Giles for performing the IL-6 ELISA and LAL assay.
References
- 1.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nature immunology. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murasko DM, Jiang J. Response of aged mice to primary virus infections. Immunological reviews. 2005;205:285–296. doi: 10.1111/j.0105-2896.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 3.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature immunology. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Current opinion in immunology. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. Journal of immunology. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 6.Miller RA. Effect of aging on T lymphocyte activation. Vaccine. 2000;18:1654–1660. doi: 10.1016/s0264-410x(99)00502-2. [DOI] [PubMed] [Google Scholar]
- 7.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunological reviews. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 8.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. Journal of immunology. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 9.Clambey ET, van Dyk LF, Kappler JW, Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunological reviews. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. Journal of immunology. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang Q, Wagner WM, Wikby A, Walter S, Aubert G, Dodi AI, Travers P, Pawelec G. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. Journal of clinical immunology. 2003;23:247–257. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- 12.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Molecular immunology. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 14.Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, Salmon M, Taams LS, Krueger J, Greenwood J, Klein N, Rustin MH, Akbar AN. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. The Journal of experimental medicine. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. Journal of immunology. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. Journal of immunology. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. Journal of immunology. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 18.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. The Journal of clinical investigation. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 20.Malek TR. The biology of interleukin-2. Annual review of immunology. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 21.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. Journal of immunology. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. Journal of immunology. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 23.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. Journal of immunology. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. Journal of immunology. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raynor J, Sholl A, Plas DR, Bouillet P, Chougnet CA, Hildeman DA. IL-15 Fosters Age-Driven Regulatory T Cell Accrual in the Face of Declining IL-2 Levels. Frontiers in immunology. 2013;4:161. doi: 10.3389/fimmu.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Frontiers in immunology. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, Crozat K, Sovath S, Moresco EM, Theofilopoulos AN, Beutler B, Hoebe K. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS biology. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, Plas DR, Hildeman DA. A major role for Bim in regulatory T cell homeostasis. Journal of immunology. 2011;186:156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. The Journal of experimental medicine. 2012;209:1723–1742. S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. The Journal of experimental medicine. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of immunology. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. Journal of immunology. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 33.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. Journal of immunology. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 34.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. The Journal of experimental medicine. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mysliwska J, Bryl E, Foerster J, Mysliwski A. Increase of interleukin 6 and decrease of interleukin 2 production during the ageing process are influenced by the health status. Mech Ageing Dev. 1998;100:313–328. doi: 10.1016/s0047-6374(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 36.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 37.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. Journal of immunology. 1997;158:5791–5796. [PubMed] [Google Scholar]
- 38.Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, Teuscher C, Haynes L, Rincon M. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. The Journal of experimental medicine. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, Ripley B, Serada S, Nishikawa T, Kimura A, Nomura S, Kishimoto T, Naka T. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. Journal of immunology. 2011;186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 40.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang X, Yang Z, Zhang R, Arnaboldi P, Lu G, Li Q, Wang W, Zhang B, Cui M, Zhang H, Liang-Chen J, Qin L, Zheng F, Huang B, Xiong H. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cellular immunology. 2011;266:208–217. doi: 10.1016/j.cellimm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun L, V, Hurez J, Thibodeaux SR, Kious MJ, Liu A, Lin P, Murthy K, Pandeswara S, Shin T, Curiel TJ. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging cell. 2012;11:509–519. doi: 10.1111/j.1474-9726.2012.00812.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 45.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 6. Chapter 6. 2003. p. 28. [DOI] [PubMed] [Google Scholar]
- 47.Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse. International immunology. 1999;11:1811–1818. doi: 10.1093/intimm/11.11.1811. [DOI] [PubMed] [Google Scholar]
- 48.Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, Sheridan R, Xanthakos SA, Steinbrecher KA, Sartor RB, Kohli R, Karp CL, Divanovic S. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830–1839. doi: 10.1002/hep.26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Punkosdy GA, Blain M, Glass DD, Lozano MM, O’Mara L, Dudley JP, Ahmed R, Shevach EM. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3677–3682. doi: 10.1073/pnas.1100213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. The Journal of clinical investigation. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature immunology. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 53.Tan AH, Wong SC, Lam KP. Regulation of mouse inducible costimulator (ICOS) expression by Fyn-NFATc2 and ERK signaling in T cells. The Journal of biological chemistry. 2006;281:28666–28678. doi: 10.1074/jbc.M604081200. [DOI] [PubMed] [Google Scholar]
- 54.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature reviews Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 56.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nature reviews Immunology. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 57.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. The Journal of experimental medicine. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 60.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CWW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CSS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annual review of immunology. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 62.Heinrich P, Behrmann I, Haan S, Hermanns H, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature immunology. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 64.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. Journal of immunology. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 65.Darrasse-Jèze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao K-hH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. The Journal of experimental medicine. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 67.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. The Journal of biological chemistry. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 68.Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, Hildeman DA. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. Journal of immunology. 2011;186:5729–5737. doi: 10.4049/jimmunol.1100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XHH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, Drobyski WR. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el B, Lehr HA, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. The Journal of clinical investigation. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, Shi H, Munn DH. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity. 2013;38:998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nature immunology. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nature immunology. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.