Abstract
Neutrophil recruitment to site of inflammation plays a pivotal role in host defense. However, an overwhelming activation and accumulation of neutrophils in the tissue may cause tissue damage and autoimmunity due to release of cytokines, oxidants, and proteases. Neutrophil adhesion in acute inflammation is initiated by activation of αLβ2 (LFA-1), which can be induced by rolling on E-selectin (slowly) or by exposure to the chemokine CXCL1 (rapidly). Despite the clinical importance, cell-intrinsic molecular mechanisms of negative regulation of integrin adhesiveness and neutrophil recruitment are poorly understood. Mice deficient in the tyrosine phosphatase Shp1 show increased leukocyte adhesion, but interpretation of these data is limited by the severe global phenotype of these mice. Here, we used mice with global and myeloid-restricted deletion of Shp1 to study neutrophil arrest, adhesion, crawling and transendothelial migration in vitro and in vivo. Shp1 deficiency results in an increased neutrophil adhesion in vivo. However, neutrophil crawling, transmigration and chemotaxis were reduced in these mice. Mechanistically, Shp1 binds and controls PIPKIγ-activity and thereby modulates PtdIns(4,5)P2 levels and adhesion. Thus, Shp1 is involved in the deactivation of integrins and regulation of neutrophil recruitment into inflamed tissue.
Keywords: integrin deactivation, neutrophil recruitment, signaling
Introduction
Inflammatory responses require efficient recruitment of leukocytes (1). Neutrophils are the first line of cellular defense against infecting microorganisms and play a central role in innate immunity and inflammatory processes (2). They rapidly migrate to sites of inflammation and release potent oxidants, proteases, and cationic peptides, which may induce collateral tissue damage (1). Thus, uncontrolled accumulation and activity of neutrophils can lead to profound tissue damage (2).
Neutrophil recruitment occurs in a cascade-like fashion (3, 4). Selectins and chemokines activate signaling pathways that cooperate to amplify neutrophil recruitment during inflammation (5, 6). The signaling pathway, triggered by selectin engagement, forces LFA-1 in the extended conformation (7), which mediates slow leukocyte rolling and neutrophil recruitment (3). During rolling, neutrophils are exposed to chemokines, which are presented on inflamed endothelium, that cause integrin activation, converting the neutrophil from a rolling behavior to firm endothelial adhesion (3). Following arrest, neutrophils undergo actin-dependent polarization and lateral migration or crawling on endothelial cells in search for permissive sites, before final transendothelial migration out of blood vessels (6, 8). Several proteins and kinases have been demonstrated to regulate pro-adhesive signaling pathways by selectins and chemoattractants (9). Negative regulators of integrin activation have also been described. However, it is still unknown how integrin activation is turned off, which would naturally restrict inflammatory cell recruitment. Clearly, integrin deactivation is required to prevent overwhelming neutrophil recruitment that may lead tissue damage.
The protein tyrosine phosphatase Src homology 2 domain-containing protein tyrosine phosphatase1 (Shp1, encoded by the Ptpn6 gene) is expressed in all hematopoietic cells (10) and substantial effort has been devoted to study the function of this phosphatase in the immune system (11, 12). However, the detailed role of how Shp1 regulates inflammation remains unclear. The motheaten mouse has a spontaneous autosomal recessive mutation in Ptpn6 leading to inflammation and immune deficiency (13). The inflammatory phenotype caused by transfer of bone marrow cells from motheaten mice into wild type animals is abolished by using anti-CD11b antibodies (14), suggesting that Shp1 is involved in the tight regulation of β2-integrin activation.
Shp1 is involved in the regulation of multiple signaling pathways (11, 15, 16). Previous in vitro work has focused on investigating cells isolated from mice containing Ptpn6 mutations. Neutrophils from Ptpn6me/me and Ptpn6me-v/me-v mice are hyperadhesive in in vitro assays (17). Ptpn6me-v/me-v macrophages are also hyperadhesive and show an increased responsiveness to different cytokines (18–20). Alterations in immune cell function may be a consequence of indirect effects caused by the inflammatory milieu in gene-deficient mice (21). Cell-intrinsic abnormalities caused by loss of Shp1 function may also cause abnormal leukocyte recruitment. However, it is still unknown how Shp1-deficiency affects the different steps of the neutrophil recruitment cascade in vivo.
Shp1 interacts with and regulates the activity of different signaling molecules, including Vav, SFKs, and Slp76 (22–24). The phosphatidylinositol-phosphate kinase Iγ (PIPKIγ) has also been found to associate with Shp1 (25). The group of PIPKIs consists PIPKIα, β and γ isoforms which are involved in the production of phosphatidylinositol (4,5)-bisphosphate (PtdIns (4,5)P2) (26). PtdIns (4,5)P2 is subsequently converted into phosphatidylinositol (3,4,5)-trisphosphate (PtdIns (3,4,5)P3) by PI3K (27). PtdIns (4,5)P2 and PtdIns (3,4,5)P3 participate in a variety of cell functions, including chemotaxis, superoxide production, and phagocytosis (27, 28). Several splice variants of PIPKIγ are located at distinct subcellular localizations in hematopoietic cells, including a 90 kDa extended isoform which is able to bind talin-1 (29).
The present study was designed to identify how Shp1 in neutrophils regulates selectin- and chemokine-induced integrin activation and neutrophil recruitment. We demonstrate that Shp1 negatively regulates β2-integrin activation, thereby controlling different steps of the leukocyte recruitment cascade. We also show that PIPKIγ is involved in neutrophil recruitment and that the regulation of PIPKIγ and PI3K by Shp1 in neutrophils determines the production of PtdIns (4,5)P2 and Akt thus regulates chemotaxis. Thus, this study pinpoints the importance of Shp1 in neutrophils as a negative regulator of selectin- and chemokine-induced integrin activation and neutrophil recruitment.
Materials and Methods
Animals
8–12-wk-old C57Bl/6 (JANVIER), Ptpn6−/− (More precisely motheaten viable, Ptpn6me-v/me-v mice, expressing normal levels of mutated form of Shp1 with very low catalytic activity. This mutation is leading to a chronic inflammation of the skin, production of autoantibodies and lethal pneumonitis due to increased numbers of neutrophils and macrophages in the lungs after 9–12 weeks (30)), Ptpn6+/+S100a8-cre (21), Ptpn6fl/flS100a8-cre (conditional knockout causing Shp1-deficiency in neutrophils, with an efficiency of an average of 80 % Shp1-deficient neutrophils for the mice used in the described experiments) (21), and PIPKIγ90−/− (lacking the 90 kDa isoform of PIPKIγ) (29) were used throughout this study. Mice were housed in a special pathogen–free facility. The Animal Care and Use Committee of the University of Muenster (Germany) approved all animal experiments.
Due to the phenotype of Ptpn6−/− mice, we used chimeric mice derived from transfer of bone marrow cells from Ptpn6−/− into lethally irradiated wild type mice, for all in vivo experiments. Bone marrow chimeras were generated as described previously (31).
Cell lines and constructs
Stable knockdown of SHP1 in promyelocytic HL-60 cells was performed by lentiviral transduction of shRNA as described previously (32) (sequence: CCGGGGAGCATGACACAACCGAATACTCGAGTATTCGGTTGTGTCATGCTCCTTTTTG). The knockdown efficiency was confirmed by Western blot (Shp1 (C19), Santa Cruz Biotechnology, Heidelberg, Germany).
During cell culture, the Shp1 knockdown was maintained by puromycin selection.
Intravital microscopy
Mice were anesthetized using injection of ketamine hydrochloride (125 mg/kg, Sanofi Winthrop Pharmaceuticals, USA) and xylazine (12.5 mg/kg, TranquiVed, Phonix Scientific, USA) (i.p.) and the cremaster muscle was prepared for intravital imaging as previously described (6, 31, 33). Some mice were pretreated with either PBS or the Shp-1/2 inhibitor NSC87877 (0.15 mg/mouse i.p., EMD Millipore, Darmstadt, Germany) prior the TNF-α injection (34). Measurements were performed in postcapillary venules with a diameter between 20–40 µm. To determine leukocyte adhesion, 500 ng CXCL1 were injected via the carotid artery. The number of adherent cells prior and following CXCL1 injection was analyzed. In order to determine selectin mediated slow rolling, adhesion, and transmigration in vivo, mice were injected intrascrotally with TNF-α (500 ng R&D Systems, Minneapolis, MN, USA) 2 h prior the preparation of the cremaster muscle. Intravital microscopy was performed on an upright microscope (Axioskop; Zeiss, Jena, Germany) with a 40 x 0.75 NA saline immersion objective. We determined leukocyte rolling velocity and adhesion by transillumination intravital microscopy. The number of extravasated leukocytes was investigated by reflected light oblique transillumination (RLOT) microscopy as previously described (35–37). Emigrated cells were determined in an area 75 x 100 µm to each side of a vessel (representing 1.5 x 104µm2 tissue area).
Clustering of surface adhesion molecules (LFA-1) was performed as previously described (35, 36). Recorded images were analyzed off-line using ImageJ and AxioVision (Carl Zeiss) software. The microcirculation was recorded using a digital camera (Sensicam QE, Cooke, Germany). Blood flow centerline velocity was measured using a dual photodiode sensor system (Circusoft Instrumentation, Hockessin, Germany). Centerline velocities were converted to mean blood flow velocities as previously described.
Intravascular crawling assay
By using intravital microscopy, the leukocyte intravascular crawling behavior was determined as described previously (32). Briefly, the anti-Gr-1 antibody (750 ng, clone RB6–8C5), labeled with Alexa Fluor 488 (Molecular Probes, Eugene, Oregon, USA) was injected directly prior the experiment. Following preparation and exteriorization, the M. cremaster was superfused with 5 nM CXCL2 (R&D Systems, Wiesbaden-Nordenstadt, Germany) and timelapse microscopy was performed for 2 h.
In vitro chemotaxis assay
In vitro chemotaxis assay was performed as described previously (38). Following isolation, bone marrow derived murine neutrophils were seeded on fibronectin-coated (50 µg/ml) chemotaxis µ-slides (Ibidi). Within the chemotaxis slide, a CXCL1 gradient (1 ng/ml) was applied. Cell movement was recorded over a period of 30 min by using time-lapse microscopy (2 frames/min). For analysis, cells were tracked with Manual Tracking (ImageJ) and analyzed with Chemotaxis plug-in (Ibidi). We analyzed the accumulated distance, velocity and chemotaxis index of the cells (38).
In vitro transmigration assay
PMN transmigration experiments were performed as described previously (39). Briefly, bend.5 cells were activated for 16 h with 5 nM TNF-α on 6.5-mm-diameter transwell filters with 5-µm pore size. Afterwards, cells were washed with DMEM medium containing 10% FCS and 25 mM HEPES. The upper reservoir was then filled with 100 µl of supplemented DMEM medium containing 5 × 105 PMNs. After 30 min, the number of transmigrated PMNs in the lower reservoirs containing 600 µl of supplemented DMEM medium with 40 ng/ml CXCL1 was quantified using a Casy Cell Counter (Innovatis).
ICAM-1 binding-assay
Murine bone marrow derived neutrophils were isolated and suspended in HBSS containing 1 mM CaCl2 and MgCl2. Afterwards, neutrophils were left unstimulated or were stimulated with CXCL1 (100ng/ml, 3 min, 37°C) (PeproTech), in the presence of ICAM-1/Fc (20 µg/mL; R&D Systems) and allophycocyanin-conjugated anti–human IgG1 (Fc-specific; Southern Biotechnology). By using an anti-CD11b (clone M1/70, 10 µg/mL) blocking antibody, Mac-1–dependent ICAM-1 binding was prevented. Cells were fixed on ice and neutrophils were stained with FITC-conjugated anti-Ly6G (Biolegend). ICAM-1 binding was measured using flow cytometry.
Flow chamber systems
In order to investigate the rolling velocity of murine neutrophils on E- or P-selectin, we used an autoperfused flow chamber (6, 31, 33). Rectangular glass capillaries (20×200 µm) were filled either with E-selectin (2.5 µg/ml, R&D Systems, MN, USA) or P-selectin (20 µg/ml, R&D Systems) alone or in combination with ICAM-1 (2 µg/ml in combination with E-selectin, 5 µg/ml in combination with P-selectin, R&D Systems) for 2 h and then blocked for 2 h using casein (1%, Thermo Scientific). One side of the chamber was connected to a mouse carotid artery. The other side of the chamber was connected to a PE 50 tubing (Becton Dickinson, Sparks, MD, USA) and used to control the wall shear stress in the capillary. To investigate chemokine-induced adhesion in vitro (33), glass capillaries were coated with P-selectin (50 µg/ml) and ICAM-1 (15 µg/ml) or P-selectin and ICAM-1 in combination with CXCL1 (25 µg/ml).
Adhesion Flow chamber
Adhesion flow chamber experiments were carried out as described previously (7). Briefly, protein-G coated glass capillaries were coated with E-selectin (6.6 µg/ml) and IgG1 (25 µg/ml) or KIM127 (25 µg/ml) for 1 hour and blocked with Casein (Thermo Fisher Scientific, Bonn, Germany). In other experiments, capillaries were coated with P-Selectin (20 µg/ml), IL-8 (50 µg/ml, Peprotech, Rocky Hill, NJ), and IgG1 (5 µg/mL) or mAb24 (5 µg/mL, generous gift from N. Hogg) or Mac-1 (CBRM1/5, 40 µg/ml, Biologend). HL-60 cells were resuspended in human plasma with a density of 5×106/ml living cells. The flow chamber was perfused with the cell suspension for 2 min and washed with phosphate-buffered saline (1 mM MgCl2/CaCl2) for 1 min. In representative images, the number of cells per field of view was determined.
Biochemical experiments
For biochemical assays, bone-marrow derived murine neutrophils were isolated and suspended in PBS (containing 1 mM each CaCl2 and MgCl2). Subsequently the cells were either incubated under rotating conditions (65 rpm) for 10 minutes on uncoated or E-selectin (3 µg/ml) coated coverslips in multiwell plates or stimulated with CXCL1 (100ng/ml, 37°C). Cells were lysed in RIPA buffer and lysates were boiled with sample buffer (10 min, 95°C) or incubated with Sepharose A/G beads (Santa Cruz Biotechnology, Inc., Dallas, USA) anti-Syk, anti-Shp1 or anti-PIPKIγ antibody (Santa Cruz Biotechnology, Inc., Dallas, USA) for 4 h at 4°C. Beads were washed four times, and bound proteins were eluted by adding boiling sample buffer. Cell lysates and immunoprecipitates were run on 10% SDS-PAGE and immunoblotted using antibodies against phosphotyrosine (4G10; Millipore), Akt, phospho-Akt (Ser473), PLCγ2, phospho-PLCγ2 (Tyr1217) (all from Cell Signaling Technology, Danvers, USA), and Fgr, Hck, Lyn and Syk (Santa Cruz Biotechnology, Inc.). Immunoblots were developed using an ECL system (GE Healthcare). Densitometric quantification was performed using ImgaeJ software.
Statistics
Statistical analysis was performed with SPSS Statistics (version 21.0, IBM, Armonk, NY). Differences between the groups were evaluated by one-way ANOVA analysis of variance, Student-Newman-Keuls test or t-test where appropriate. Data are presented as mean ±SEM, and p < 0.05 was considered statistically significant.
Results
Shp1 is involved in different steps of the leukocyte recruitment cascade
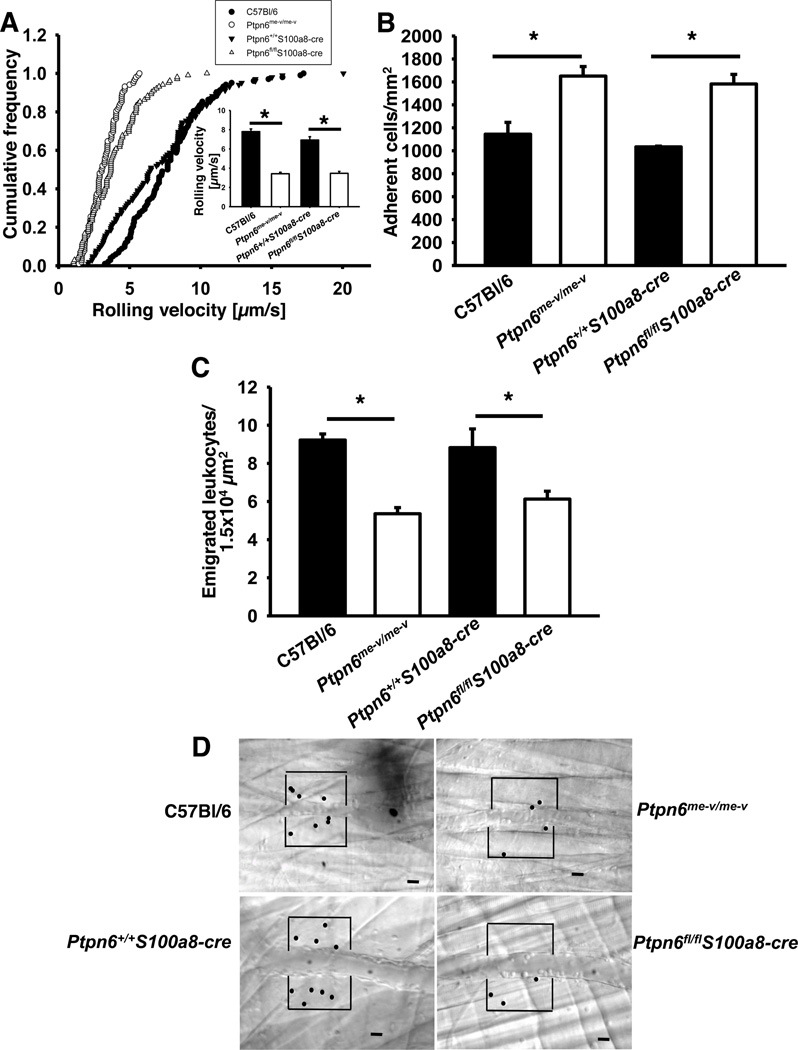
To investigate the role of Shp1 in the different steps of the recruitment cascade in the systemic circulation, we performed intravital microscopy of the cremaster muscle. Two hours after TNF-α injection, leukocyte tethering and rolling along the endothelial surface is mediated by P- and E-selectin expressed on inflamed endothelial cells of the cremaster muscle (5, 6). The rolling velocity of Shp1-deficient neutrophils (Ptpn6me-v/me-v bone marrow chimeras, hereafter referred to as Ptpn6−/−, and Ptpn6fl/flS100a8-cre) was significantly reduced compared to the rolling velocity of wild type neutrophils (C57Bl/6 and Ptpn6+/+S100a8-cre; Fig 1A). Both E-selectin and G-protein coupled receptor (GPCR) signaling mediate leukocyte intravascular adhesion in venules of the cremaster muscle after TNF-α treatment (5, 6). The number of adherent cells was significantly higher in Shp1-deficient mice (Ptpn6−/− and Ptpn6fl/flS100a8-cre) compared to wild type mice (C57Bl/6 and Ptpn6+/+S100a8-cre) (Fig 1B). In contrast, the number of emigrated cells was significantly decreased in Shp1-deficient mice compared to wild type mice (Fig 1C). Representative video micrographs of wild type mice (C57Bl/6 and Ptpn6+/+S100a8-cre) and Shp1-deficient mice (Ptpn6−/− and Ptpn6fl/flS100a8-cre) 2h after TNF-α injection are shown in Figure 1D. Microvascular parameters (vessel diameters, centerline velocities, wall shear rates) counts were similar among the groups (data not shown). These data suggest that Shp1 in neutrophils regulates different steps of the leukocyte recruitment cascade. In this model, selectin- dependent slow leukocyte rolling, chemokine-induced adhesion, and transmigration of neutrophils are affected in the absence of Shp1.
Figure 1. Shp1 is involved in different steps of the leukocyte recruitment cascade.
(A–D) Intravital microscopy of postcapillary venules in the murine cremaster muscle, 2 h after intrascrotal TNF-α injection. (A) Cumulated histogram of rolling velocities of neutrophils from wild type (•), Ptpn6−/− (○), Ptpn6+/+S100a8-cre (▼), and Ptpn6fl/flS100a8-cre (Δ) neutrophils (n=3). Inset data are means +/− SEM. (B–C) Adherent cells per square millimetre (B) and the number of extravasated cells per 1.5×104 mm2 tissue area surrounding postcapillary venules (C). (n=3). (D) Representative RLOT pictures 2 h after TNF-α application. Scale bar=20 µm. * p < 0.05. Intravital microscopy was performed on an upright microscope (Axioskop; Zeiss, Jena, Germany) with a 40 × 0.75 NA saline immersion objective.
Deficiency of Shp1 increases chemokine-induced arrest and post-adhesion strengthening
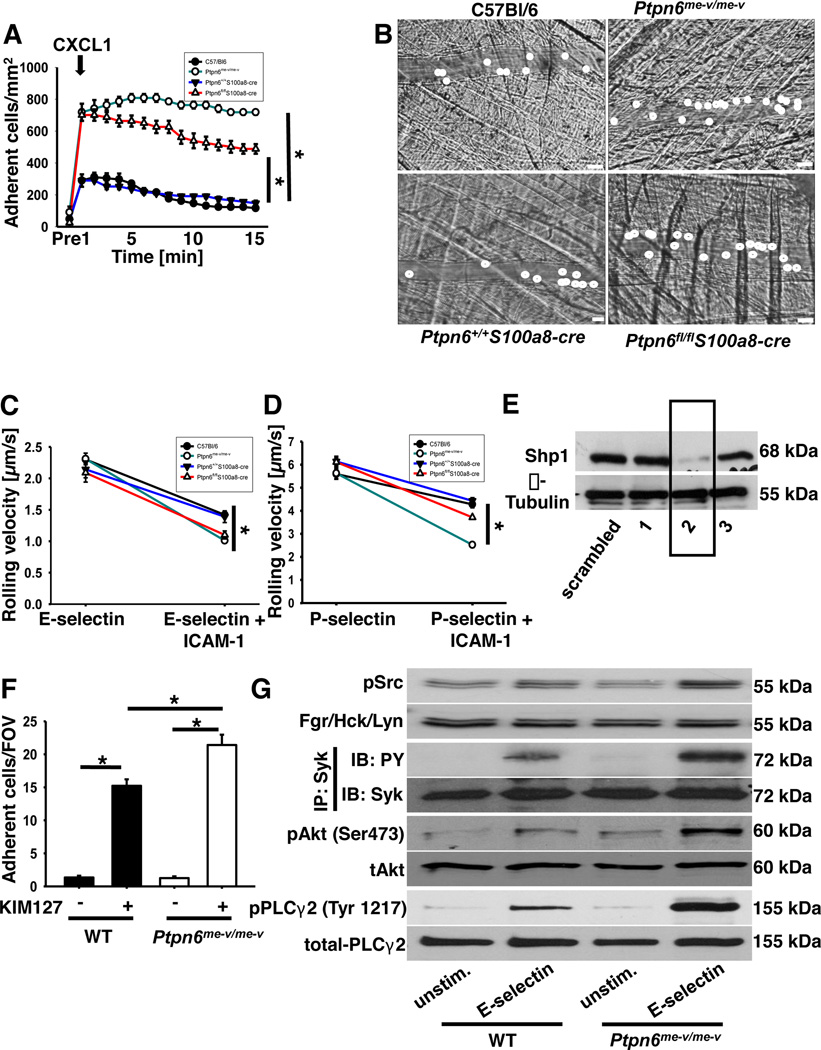
In order to dissect the role of Shp1 in these two signaling pathways leading to integrin activation, we performed chemokine-induced arrest in the cremaster muscle (33, 40). In this model, neutrophil rolling in the venules of the cremaster muscle is due to P-selectin expression on the endothelium. Injection of CXCL1 induces a shift in integrin conformation from a closed to an open conformation that subsequently induces neutrophil arrest (41). The number of adherent cells after CXCL1 injection is significantly increased in Shp1-deficient mice (Ptpn6−/− and Ptpn6fl/flS100a8-cre) compared to wild type mice (C57Bl/6 and Ptpn6+/+S100a8-cre; Fig 2A–B). While the number of adherent leukocytes in wild type (C57Bl/6 and Ptpn6+/+S100a8-cre) venules decreased over time, the number of adherent leukocytes in the venules of Shp1-deficient mice (Ptpn6−/− and Ptpn6fl/flS100a8-cre) mice remained significantly higher (Fig 2A). These data suggest that Shp1 is involved in chemokine induced-arrest, which is dependent on the activation of the β2-integrin LFA-1, and in Mac-1-dependent post-adhesion strengthening of neutrophils in the murine M.cremaster.
Figure 2. Deficiency of Shp1 influences chemokine-dependent as well as selectin-dependent pathways.
(A) Chemokine induced arrest of neutrophils in postcapillary venules of wild type (•), Ptpn6−/− (○), Ptpn6+/+S100a8-cre (▼), and Ptpn6fl/flS100a8-cre (Δ) mice before and following intravenous CXCL1 injection (500 ng, i.v.). Intravital microscopy was performed on an upright microscope (Axioskop; Zeiss, Jena, Germany) with a 40 × 0.75 NA saline immersion objective. (B) Representative images of postcapillary venules of wild type, Ptpn6−/−, Ptpn6+/+S100a8-cre, and Ptpn6fl/flS100a8-cre mice 1 min following CXCL1 injection (B). Scale bar=20 µm. (C–D) Rolling velocities on E-selectin and E-selectin plus ICAM-1 (C) or P-selectin and P-selectin plus ICAM-1 (D) of neutrophils from wild type (•), Ptpn6−/− (○), Ptpn6+/+S100a8-cre (▼) and Ptpn6fl/flS100a8-cre (Δ) mice. (E) Protein levels of Shp1 in HL-60 cells following transfection with three different shRNAs in comparison to protein level following transduction with scrambled shRNA. (F) HL-60 cells (transduced with scrambled shRNA or construct 2) were analyzed using a flow chamber adhesion assay with E-selectin and either an antibody specific for the intermediate confirmation of LFA-1 (KIM127) or an isotype control. Adherent cells per field of view were counted and means ± SEM are displayed. (G) BM-derived neutrophils were plated on uncoated (unstim) or E-selectin–coated wells for 10 min, and then lysates were prepared. Representative Western blots of either immunoprecipitations or total lysates of neutrophils of wild type and Ptpn6−/− mice showing the phosphorylation of Src kinases and the downstream molecules Syk, Akt and PLCγ2. For the analysis of Syk phosphorylation, lysates were immunoprecipitated with a Syk (n=3) antibody followed by immunoblotting with a general phosphotyrosine (4G10) antibody or total-Syk-antibody. Total lysates were immunoblotted with antibodies to phosphorylated Src-kinases (Tyr416; n=3), total Src (n=3), phosphorylated PLCγ2 (Tyr1217; n = 3), total PLCγ2 (n = 3), phosphorylated Akt (Ser472; n = 3), total Akt (n = 3). * p < 0.05.
Shp1 regulates selectin-dependent neutrophil slow rolling and selectin-mediated intracellular signaling
To further confirm the in vivo data, rolling velocity, integrin activation, and intracellular signaling were analyzed in vitro. To examine E-selectin-mediated rolling velocity, autoperfused flow chamber experiments were performed (6). In wild type neutrophils, the rolling velocity on E-selectin was 2.3 ± 0.06 µm/s and decreased to 1.42 ± 0.04 µm/s when neutrophils rolled on E-selectin and ICAM-1. This reduction of the rolling velocity is mediated by the binding of activated LFA-1 to ICAM-1 (7). Neutrophils from Shp1-deficient mice (Ptpn6−/− and Ptpn6fl/flS100a8-cre) showed the same rolling velocity on E-selectin (Ptpn6−/−: 2.31 ± 0.09 µm/s, Ptpn6fl/flS100a8-cre: 2.09 ± 0.15 µm/s), but displayed a significantly slower rolling velocity on E-selectin and ICAM-1 compared to wild type (Fig 2C). The same results were seen on P-selectin and P-selectin plus ICAM-1 (Fig 2D).
E-selectin engagement induces the activation of LFA-1, which is accompanied by formation of the extended conformation of LFA-1 (7). To directly scrutinize the role of Shp1 in selectin-mediated integrin activation, we used an immobilized reporter antibody assay to directly investigate the extended conformation of LFA-1 (antibody KIM127) (7). As reporter antibodies are only available for human cells, we stably knocked down Shp1 in the promyelocytic cell line HL-60 by transducing the cells with short hairpin RNA constructs against Shp1. Down-regulation of Shp1 was confirmed by Western blot analysis (construct 2; Fig 2E). In the immobilized reporter antibody assay, LFA-1 in the extended conformation binds to the reporter antibody (KIM127) and mediates cell adhesion. On flow chambers coated with selectin in combination with an IgG antibody, few HL-60 (WT and Ptpn6−/−) cells adhered on the surface. The immobilized reporter antibody assay revealed that the number of adherent cells per field of view was significantly increased when the expression of Shp1 was down-regulated (Fig 2F).
Selectin engagement induces the phosphorylation of different signaling molecules leading to the activation of the β2-integrin LFA-1 (42). To investigate this in Shp1 deficient cells, we stimulated bone-marrow derived neutrophils from wild type mice and Ptpn6−/− mice, then examined the phosphorylation of Src family kinases (Tyr416), Syk, Akt, as a PI3K readout, and PLCγ2 (Fig 2G). Phosphorylation of the different signaling molecules increased in wild type neutrophils following the stimulation with E-selectin. In Ptpn6−/− neutrophils the phosphorylation level of these molecules was significantly elevated after E-selectin stimulation compared to phosphorylation levels in wild type neutrophils (Fig 2G; Western blot densitometry is shown in the Supplemental Data, Fig S1A–D). All together, these data demonstrate that the regulation of the selectin-mediated intracellular signaling which is leading to integrin activation is altered in Shp1-deficient neutrophils.
Gαi-mediated integrin activation is regulated by Shp1
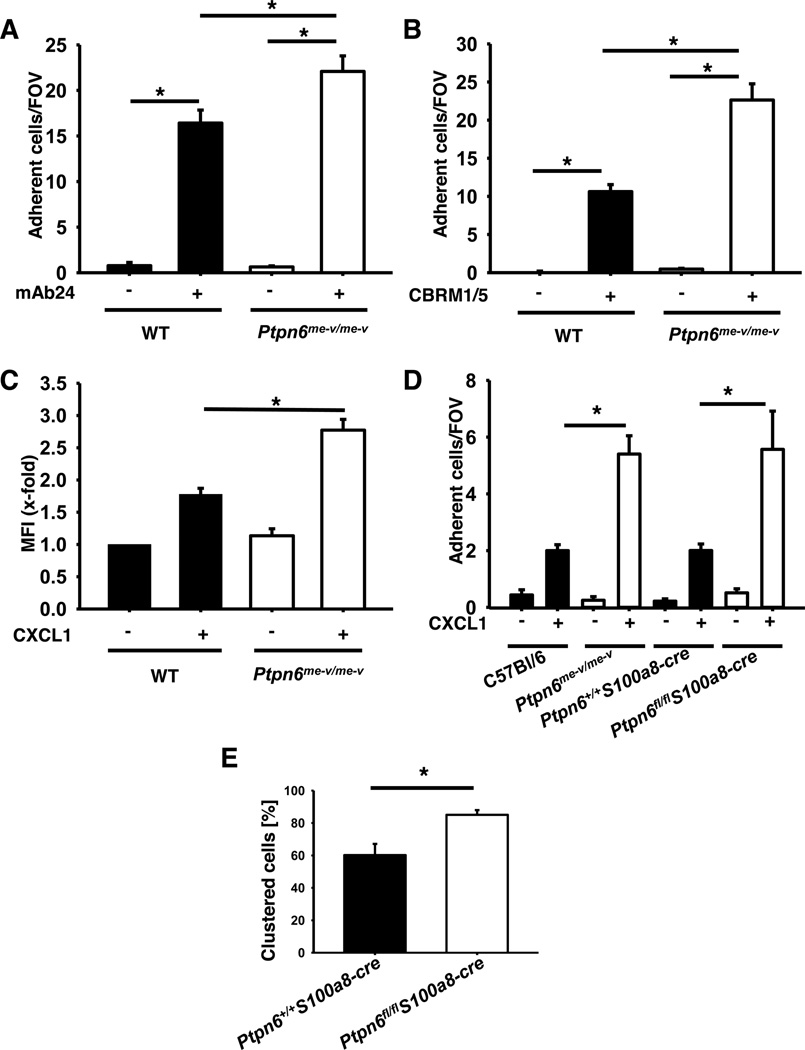
As the in vivo data suggest that Shp1 is also involved in chemokine-induced integrin activation, we investigated Gαi-signaling and integrin activation in vitro by using different assays. To directly test whether Shp1 is involved in GPCR-mediated integrin activation under flow conditions, we used the Shp1 knock-down HL-60 cell line in an immobilized reporter antibody assay (7). Here, we observed an increased number of adherent wild type cells, in the presence of CXCL1, when the chambers were coated with a reporter antibody against activated LFA-1 (mAb24, high affinity conformation, Fig 3A) or Mac-1 (CBRM1/5, Fig 3B) compared to IgG control antibody. In control chambers, Shp1-knock-down cells showed the same number of adherent cells compared to wild type cells (Fig 3A–B). However, in the presence of the reporter antibodies, the number of adherent Shp1-knock-down cells was significantly increased (Fig 3A–B). These data suggest that Shp1 in neutrophils modulates chemokine-induced LFA-1 and Mac-1 activation.
Figure 3. Gαi-signaling is regulated by Shp1.
(A–B) The number of adherent HL-60 cells (scrambled and construct 2) on a flow chamber coated with G protein, P-selectin, IL-8 and a control IgG antibody or reporter antibodies for LFA-1 (A) or Mac-1 (B) activation. (C) Adherent cells per field of view were counted and means ± SEM are displayed. ICAM-1 binding of unstimulated or CXCL1 stimulated (100 ng/ml, 3 min, 37°C) neutrophils from wild type and Ptpn6−/− mice. ICAM-1 binding was measured by FACS. (D) Number of adherent cells in wild type and Ptpn6−/− mice on capillaries coated with P-selectin or P-selectin in combination with CXCL1. Percentage of rolling neutrophils showing LFA-1 clustering in postcapillary venules of the cremaster muscle of wild type and Ptpn6−/− mice 2 h following intrascrotal TNF-α injection (E). * p < 0.05.
To investigate the adhesive state of LFA-1, an ICAM-1-binding assay was performed using Ptpn6−/− and wild-type neutrophils which were incubated with soluble murine ICAM-1 (32). By using flow cytometry, the LFA-1 mediated ICAM-1 binding of unstimulated in comparison to CXCL1 stimulated neutrophils was investigated. Wild type neutrophils showed a strong binding after CXCL1 stimulation, whereas Ptpn6−/− neutrophils after CXCL1 stimulation bound significantly more ICAM-1 compared to wild type neutrophils (Fig 3C).
In order to investigate the adhesion phenotype in vitro, adhesion flow-chamber experiments were performed. P-selectin and ICAM-1 were co-immobilized in the presence or absence of the chemokine CXCL1 and the number of adherent neutrophils was determined. While in chambers coated with P-selectin and ICAM-1 only few wild type (C57Bl/6 and Ptpn6+/+S100a8-cre) and Shp1-deficient (Ptpn6−/− and Ptpn6fl/flS100a8-cre) neutrophils adhered, the number of adherent neutrophils increased in the chambers where CXCL1 was co-immobilized with P-selectin and ICAM-1. Concurrent with the results of our in vivo experiments, the number of adherent neutrophils per field of view, was significantly increased in Shp1-deficient mice (Ptpn6−/− and Ptpn6fl/flS100a8-cre) mice compared to wild type mice (C57Bl/6 and Ptpn6+/+S100a8-cre; Fig 3D).
As LFA-1 adhesiveness is regulated by integrin affinity and avidity (clustering), we investigated LFA-1 clustering in the next step. To this purpose, a fluorescently labeled LFA-1 antibody was injected intravenously and intravital microscopy of the cremaster muscle was performed 2 h after TNF-α injection. In wild type mice (Ptpn6+/+S100a8-cre), approximately 60% of rolling neutrophils presented a LFA-1 cluster, in contrast in Ptpn6fl/flS100a8-cre mice this percentage increased to approximately 80% (Fig 3E). These data indicate that Shp1 is involved in the regulation of GPCR-mediated changes in LFA-1 avidity on neutrophils.
Elimination of Shp1 in neutrophils abolishes intravascular crawling and migration
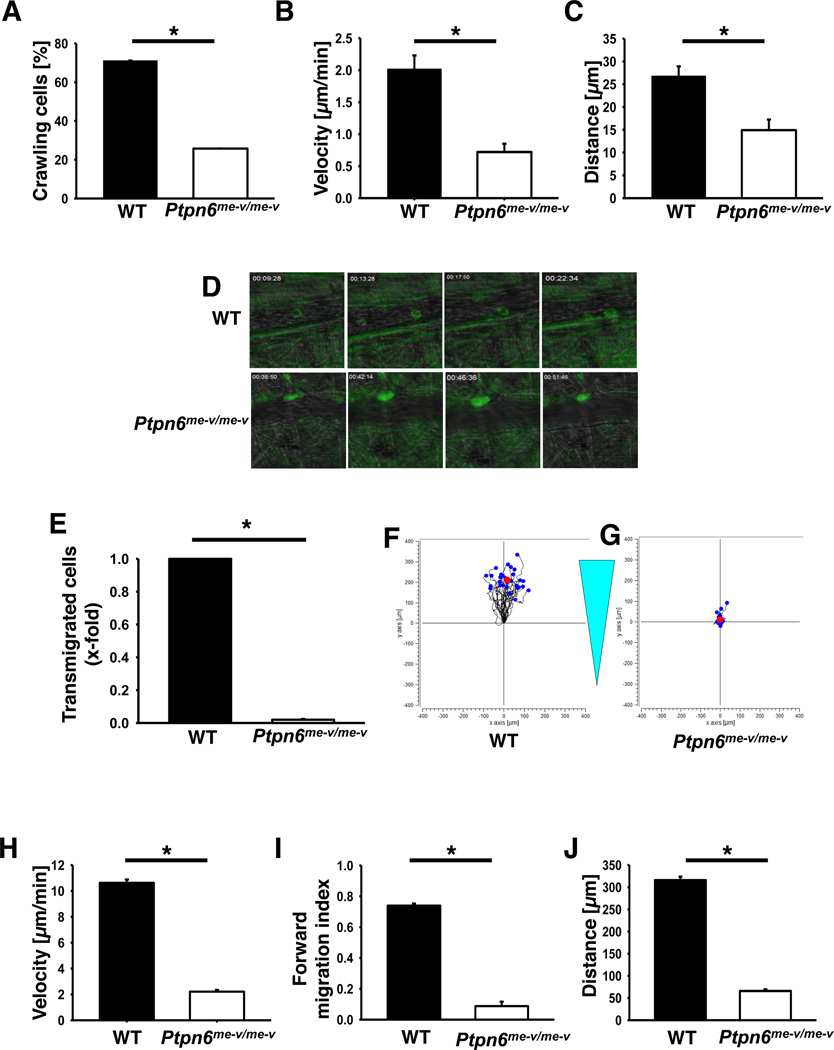
The β2-integrin Mac-1 has a critical role in intravascular crawling of leukocytes (32). To investigate the role of Shp1 in this step, we performed intravital microscopy of the cremaster muscle after stimulation with the chemokine CXCL2. During CXCL2 stimulation, leukocytes adhesion was observed in wildtype and Ptpn6−/− mice. In Ptpn6−/− mice, the percentage of adherent cells that crawled was significantly reduced compared to wild type mice (Fig 4A). Furthermore, Ptpn6−/− leukocytes that crawled, had a reduced crawling velocity (Fig 4B) and distance (Ptpn6−/−: 14.9 µm; WT 26.8 µm, Fig 4C–D) compared to wild type leukocytes. In Figure 4D, representative images of adherent neutrophils within postcapillary venules of wild type (upper row) and Ptpn6−/− mice (lower row) during CXCL2 superfusion are shown.
Figure 4. Crawling, migration and transmigration is disturbed in Ptpn6 deficient neutrophils.
Percentage of crawling cells (A), crawled distance (B), crawling velocity (C) and representative images (D) of wild type or Ptpn6−/− neutrophils in postcapillary venules of the cremaster muscle during CXCL2 superfusion (n=4). Data are presented as means +/− SEM. Intravital microscopy for the intravascular crawling documentation was performed on an upright microscope (Axioskop; Zeiss, Jena, Germany) with a 40 × 0.75 NA saline immersion objective. (E) Transmigration of wild type and Ptpn6−/− neutrophils along a CXCL1 gradient through a TNF-α stimulated bend.5 cell layer. (F–G) Representative plots of an in vitro chemotaxis assay with wild type (F) and Ptpn6−/− (G) neutrophils on fibronectin along a CXCL1 gradient. Velocity (H), forward migration index (I) and distance (J) of neutrophils in migration experiments (n=3). Data presented as mean +/− SEM. * p < 0.05.
To further examine the role of Shp1 in neutrophil migration, we investigated whether Shp1 is also involved in the transmigration step of the leukocyte adhesion cascade. To scrutinize this, we used an in vitro transmigration assay. In comparison to wild type neutrophils, almost no Ptpn6−/− neutrophils transmigrated through the bend.5 cell layer towards CXCL1 (Fig 4E).
Furthermore, we investigated whether Shp1 is involved in chemotaxis by using an in vitro assay (Fig 4F–J). Wild type neutrophils, adherent on fibronectin, were able to migrate along a CXCL1 gradient (Fig 4F). In contrast, Shp1 deficient neutrophils were unable to migrate along the chemotactic gradient and adhered at the starting point (Fig 4G). Migration velocity (Fig 4H), forward migration index (Fig 4I) and migration distance (Fig 4J) of Shp1-deficient neutrophils were significantly reduced compared to wild type neutrophils (Fig 4H–J). Together, these data indicate that Shp1 also plays a pivotal role in Mac-1-dependent processes. Shp1 is critically involved in neutrophil intravascular crawling, transmigration, and chemotaxis.
Shp1 modulates PtdIns (4,5)P2 levels in neutrophils by regulating PIPKIγ activity
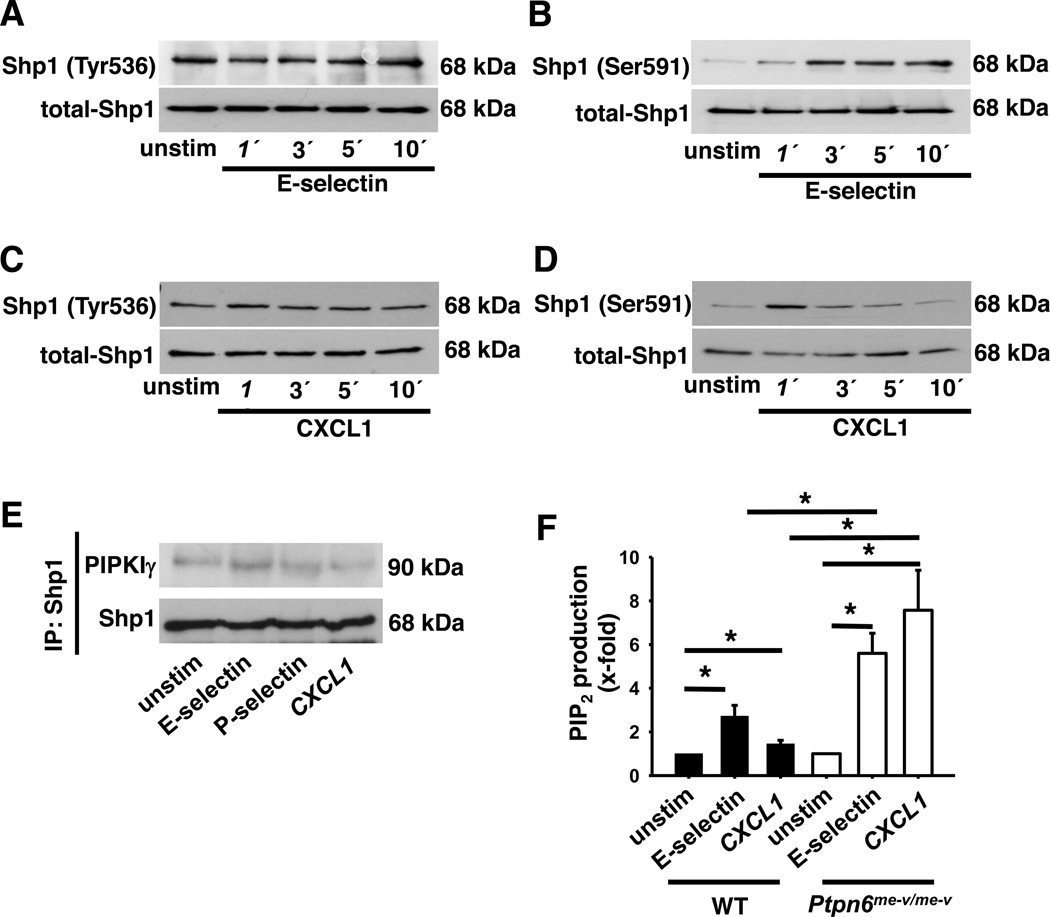
To demonstrate how Shp1 regulates signaling pathways involved in neutrophil recruitment, we investigated the activation of Shp1 after the stimulation of neutrophils with a chemokine or selectin. Phosphorylation of Tyr536 on Shp1 leads to an increased phosphatase activity, whereas phosphorylation of Ser591 reduces phosphatase activity (43). E-selectin engagement did not influence the phosphorylation of the tyrosine in position 536 (Fig 5A and Fig S1E), but increased the phosphorylation of the serine in position 591 (Fig 5B and Fig S1F). Similar to E-selectin engagement, the stimulation of neutrophils with the chemokine CXCL1 did not influence the phosphorylation of Tyr536 (Fig 5C and Fig S1G), but transiently increased the phosphorylation of Ser591 (Fig 5D and Fig S1H), suggesting that Shp1 phosphatase activity is modulated in neutrophils after stimulation with chemokines or selectins.
Figure 5. Shp-1 interacts with PIPKIγ and regulates its activity.
BM-derived neutrophils were plated on uncoated (unstim) or E-selectin–coated wells (A+B) or were stimulated with CXCL1 (C+D) for the indicated time periods. Afterwards lysates were prepared. Representative Western blots of total lysates of wild type neutrophils showing the phosphorylation of tyrosine536 (A+C) and serine591 (B+D). (E) Representative co-immunoprecipitation of unstimulated, E-selectin and CXCL1 stimulated wild type neutrophils. (F+G) PtdIns (4,5)P2 levels of unstimulated, E-selectin or CXCL1 stimulated wild type and Shp1-deficient neutrophils measured by ELISA. Data are presented as means +/− SEM.
Shp1 can interact with different molecules. Here, we investigated the interaction between PIPKIγ and Shp1. By using Co-immunoprecipitation, we demonstrated that Shp1 physically interacts with PIPKIγ in both resting cells and following stimulation with chemokines or selectins (Fig 5E). As PIPKIγ produces PtdIns (4,5)P2, we investigated the PtdIns (4,5)P2 concentration in WT and Ptpn6−/− neutrophils in both resting and stimulated cells. Under baseline conditions, wild type and Ptpn6−/− neutrophils had similar PtdIns (4,5)P2 concentrations, however following either E-selectin or CXCL1 stimulation, the levels of PtdIns (4,5)P2 were significantly elevated in Ptpn6−/− neutrophils compared to wild type cells (Fig 5F).
Shp1 influences leukocyte recruitment by regulating PIPKIγ activity
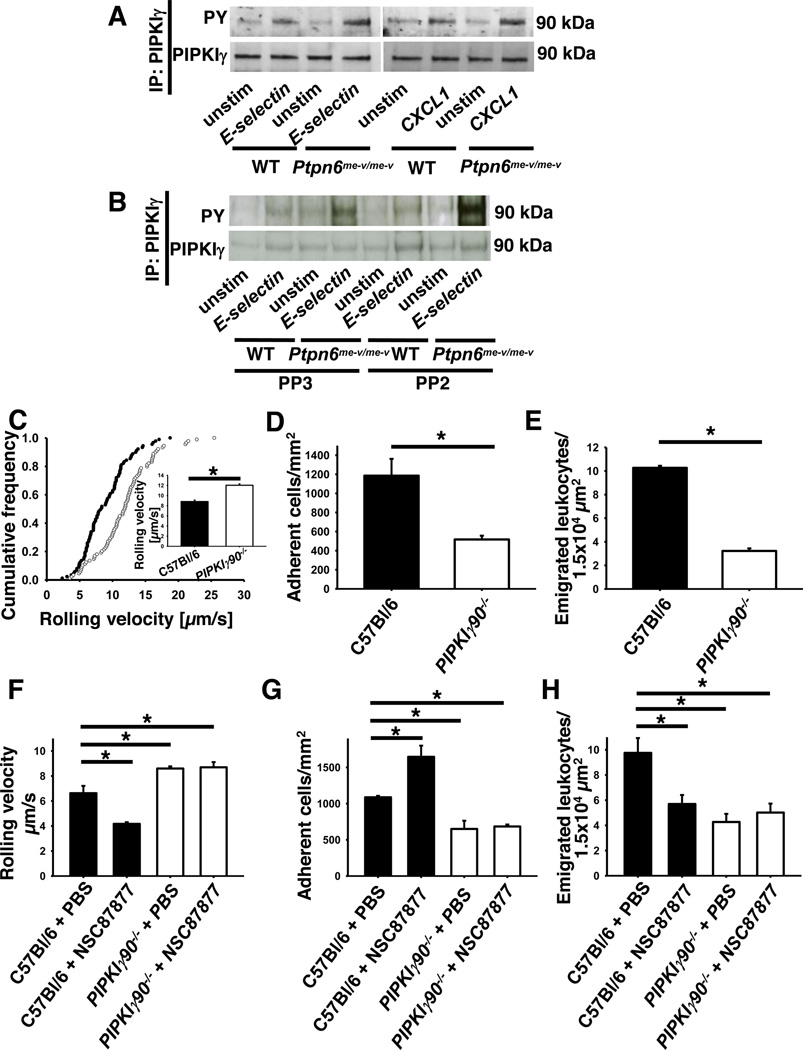
Given that activity and subcellular localization of PIPKIγ is influenced by tyrosine phosphorylation (44), we examined the phosphorylation of PIPKIγ in wild type and Ptpn6−/− neutrophils. Stimulation of wild type neutrophils with E-selectin or chemokine induced the tyrosine phosphorylation of PIPKIγ, which was significantly enhanced in Ptpn6−/− neutrophils (Fig 6A and Fig S1I). The phosphorylation of PIPKIγ is not Src family kinase dependent (Fig 6B and Fig S1J). In summary, the present results suggest that Shp1 modulates PtdIns (4,5)P2 levels by regulating PIPKIγ activity in a Src family-kinase-independent matter.
Figure 6. Increased PIPKIγ activity affects different steps of the leukocyte recruitment cascade.
Representative Western blots of immunoprecipitations of PIPKIγ in unstimulated, E-selectin and CXCL1 stimulated wild type and Ptpn6−/− neutrophils showing the phosphorylation of PIPKIγ in the absence (A+B) or presence (B) of a Src family kinase inhibitor. (C–H) Intravital microscopy of postcapillary venules in the murine cremaster muscle, 2 h after intrascrotal TNF-α injection. Neutrophil rolling velocities (C+F), number of adherent cells (D+G) and emigrated cells (E+H) from wild type (•) and PIPKIγ90−/− (○) mice either untreated (C–E)) or pretreated with the Shp-inhibitor (F–H) (n=3). Intravital microscopy was performed on an upright microscope (Axioskop; Zeiss, Jena, Germany) with a 40 x 0.75 NA saline immersion objective. Inset data are means +/− SEM. Adherent cells per square millimetre (D+G) and the number of extravasated cells per 1.5×104 mm2 tissue area surrounding postcapillary venules (E+H). (n=3). * p < 0.05.
To further investigate the role of PIPKIγ in neutrophil recruitment, we performed intravital microscopy of the murine cremaster muscle in PIPKIγ90−/− mice, which lack the 90 kDa isoform of PIPKIγ (29). Two hours after TNF-α injection, the selectin-dependent neutrophil rolling velocity in PIPKIγ90−/− mice (12.0 µm/s) was significantly increased in comparison to wild type mice (8.8 µm/s, Fig 6C). The number of adherent (Fig. 6D) and transmigrated leukocytes (Fig. 6E) in PIPKIγ90−/− mice was decreased compared to wild type mice. These data indicate an involvement of PIPKIγ in the selectin- and chemokine-triggered signaling pathways in neutrophils during the recruitment cascade.
As we demonstrated that the activity of PIPKIγ is regulated by Shp1 following selectin- and chemokine-stimulation, we investigated in the next step whether these two molecules are involved in the same signaling pathway regulating neutrophil recruitment in vivo. Therefore, we performed intravital microscopy of the murine cremaster muscle following TNF-α injection in PIPKIγ90−/− mice as well as wild type mice pretreated with a Shp-inhibitor (NSC87877) (Fig. 6F–H). As expected, the rolling velocity of inhibitor-treated wild type neutrophils was decreased in comparison to control neutrophils (Fig. 6F). While the selectin-dependent rolling velocity was increased in PIPKIγ90−/− neutrophils, treatment with the Shp-inhibitor had no effect on these cells. Similar results were seen with the number of adherent and transmigrated cells: treatment of wild type mice with the Shp-inhibitor recapitulated the Ptpn6−/− phenotype of increased neutrophil adhesion and reduced transmigration, while treatment of the PIPKIγ90−/− mice had no effect on the poor adhesion and transmigration of these neutrophils (Fig. 6G–H). All together, the data suggest that the phosphatase Shp1 is involved in the regulation neutrophil recruitment and that Shp1 and PIPKIγ are located in the same signaling pathways.
Discussion
The function of Shp1 in the regulation of integrin adhesiveness and leukocyte recruitment has not previously been appreciated. We demonstrated that stimulation of neutrophils with selectins or chemokines deactivates the activity of Shp1 leading to integrin over-activation and an altered neutrophil recruitment. Our studies were initiated by the unexpected finding that neutrophil adhesion in gene-deficient mice is enhanced, whereas the number of extravasated neutrophils is reduced. This is in line with reduced intravascular crawling, transmigration capacity and chemotaxis in Ptpn6-deficient neutrophils. For the different steps of the leukocyte recruitment cascade, a tight regulation of integrin activation and deactivation is essential. However, in the absence of Shp1, β2-integrin adhesiveness was enhanced after stimulation with chemokines or selectins explaining the observed phenotype. We demonstrated that Shp1 interacts with PIPKIγ and regulates the activity of PIPKIγ in a Src-family kinase-independent fashion. The regulation of PIPKIγ by Shp1 led to an increased production of PtdIns (4,5)P2 in Ptpn6-deficient neutrophils.
Whereas inflammatory reactions are beneficial and necessary for host defense, they need to be balanced and controlled to prevent harmful consequences and tissue destruction. Different signaling pathways exist which ensure rapid and efficient integrin activation on leukocytes. However, different endogenous mechanisms counteract and balance integrin activation, thereby limiting leukocyte recruitment and the extent of inflammation (40, 45–47). Different protein tyrosine kinases and phosphatases are required for initiating and limiting downstream signaling following cellular stimulation. In neutrophils, PIR-B has been found to be constitutively phosphorylated and associated with Shp1 (48). Cellular stimulation results in transiently diminished phosphorylation of PIR-B and reduced association of the molecule with Shp1 allowing maximization of downstream signaling events (48). Here, we show that Shp1 activity, assessed by Ser591 phosphorylation, is also transiently reduced following chemokine or selectin stimulation. The serine/threonine kinase that phosphorylates Shp1 on Ser591 is unknown but it may be regulated by other signaling molecules involved in neutrophil activation. A recently published study suggests that the GTPase Rap1b is involved in the regulation of Shp1 activity (34). Kumar et al. (34), found that stimulation of wild type neutrophils with fMLP induced Shp1 activation, whereas Shp1 activation was reduced in Rap1b−/− neutrophils, suggesting that Rap1b either directly, or more likely indirectly through affects on serine kinases, modulates Shp1 activity. As the phosphorylation state of different signaling molecules and integrin activity in unstimulated Shp1-deficient neutrophils and wild type neutrophils is similar, Shp1 is a phosphatase exclusively regulating cell function in stimulated neutrophils. Hence, in this model, Shp1 acts as a cell-intrinsic negative regulator required for turning off chemokine- and selectin-induced signaling.
To our knowledge, this is the first study showing that Shp1 interacts with and regulates the activity of PIPKIγ. It is known that Shp1 regulates the phosphorylation and activation of Src family kinases (12), however, the regulation of PIPKIγ by Shp1 following chemokine and selectin stimulation is Src-family kinase independent. PIPKIγ is involved in PtdIns (4,5)P2 production. PtdIns (4,5)P2 and PtdIns (3,4,5)P3, which is produced by PI3K isoforms, are involved in integrin activation and different steps of neutrophil recruitment (49). In line with other reports, we demonstrated increased PtdIns (4,5)P2 levels after stimulation with chemokines or selectins. Different publications demonstrated an increased interaction of PIPKIγ with talin, as well as the nature of this interaction, following phosphorylation of Tyrosine Y644 (50). Talin is required for inducing LFA-1 extension to the intermediate affinity state, which is selectin-dependent, as well as the high-affinity conformation, which is chemokine-dependent (9). The increased phosphorylation of PIPKIγ leads to an increased interaction with talin and this may explain the increased integrin activity in Shp1 deficient neutrophils. Additionally, the interaction between PIPKIγ and talin targets PIPKIγ to focal adhesions (FA) (51), leading to an increased PtdIns (4,5)P2 concentration at FAs in Shp-1 deficient neutrophils. The interaction of talin with β2-integrins in FAs is strengthened by PtdIns (4,5)P2 (52).
The increased PIPKIγ activity in Shp1-deficient neutrophils leads to increased accumulation of PtdIns (4,5)P2 which subsequently upregulate integrin affinity and avidity, explaining the hyperadhesive phenotype of Ptpn6−/− neutrophils. PtdIns (4,5)P2 accumulation on the plasma membrane, promotes the detachment of α-actinin-1 from the cytoplasmic tail of the integrin, which allows attachment of PtdIns (4,5)P2-activated talin1, leading to an increased integrin affinity and avidity. Moreover, PIPKIγ directly interacts with the head domain of talin1 (53), which is an antiparallel homodimer (54), thus leaving one head domain free to bind integrins. Interestingly, PIPKIγ has been implicated in conformer-specific regulation of LFA-1 affinity in lymphocytes. Thus it is through these mechanisms that the increased PIPKIγ activity in Shp1-deficient neutrophils leads to an increased extended and high-affinity conformation of LFA-1 triggered by selectins and chemokines, respectively.
PI3K-PtdIns (3,4,5)P3–Akt- signaling pathways are involved in selectin-mediated slow leukocyte rolling, post-adhesion strengthening and chemotaxis, as deletion of PI3K in leukocytes abrogates these functions (55–57). As Shp1-deficient neutrophils are hyperadhesive, it is tentative to speculate that PtdIns (3,4,5)P3-levels are increased in Shp1-deficient cells. This speculation is supported by the fact that Akt phosphorylation, a readout for PI3K activation, is increased and PtdIns (4,5)P2 levels in these cells are elevated. The fact that either under activation of the Akt pathway (in PI3K deficient cells) or over activation of the pathway (in Shp1 deficient cells) leads to defective leukocyte recruitment suggests that PtdIns (4,5)P2,-PI3K-PtdIns (3,4,5)P3–Akt- signaling pathways have to be tightly regulated to ensure balance of signaling to allow both the adhesion and de-adhesion steps required for leukocyte recruitment into inflamed tissue (17, 34).
Supplementary Material
Acknowledgments
We would like to thank Reinhard Fässler for sharing the PIPKIγ90−/− mice with us.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (ZA428/6-1, ZA428/9-1, and SFB1009_A5 to A.Z.), Cells-in-Motion Cluster of Excellence EXC 1003–CiM (University of Münster, Germany; to A.Z.), and in part by the US National Institutes of Health (RO1 AI65495 and RO1 AI68150 to C.A.L.).
Author contributions
A.S. designed and did most of the experiments, analyzed the results, and prepared the manuscript; H.B. did biochemistry experiments, S.V. did some flow chamber as well as intravital microscopy experiments; C.A. provided the Ptpn6+/+S100a8-cre and Ptpn6fl/flS100a8-cre mice; C.S. performed biochemistry assays; M.B. performed chemotaxis assays and C.L. and A.Z. provided overall supervision, helped design all of the experiments, and prepared the manuscript.
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews. Immunology. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews. Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nature medicine. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. The Journal of experimental medicine. 2004;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood. 2010;116:617–624. doi: 10.1182/blood-2010-01-266122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. The Journal of experimental medicine. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefort CT, Ley K. Neutrophil arrest by LFA-1 activation. Frontiers in immunology. 2012;3:157. doi: 10.3389/fimmu.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plutzky J, Neel BG, Rosenberg RD. Isolation of a src homology 2-containing tyrosine phosphatase. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annual review of immunology. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- 12.Tsui FW, Martin A, Wang J, Tsui HW. Investigations into the regulation and function of the SH2 domain-containing protein-tyrosine phosphatase, SHP-1. Immunologic research. 2006;35:127–136. doi: 10.1385/IR:35:1:127. [DOI] [PubMed] [Google Scholar]
- 13.Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. The Journal of heredity. 1975;66:250–258. doi: 10.1093/oxfordjournals.jhered.a108625. [DOI] [PubMed] [Google Scholar]
- 14.Koo GC, Rosen H, Sirotina A, Ma XD, Shultz LD. Anti-CD11b antibody prevents immunopathologic changes in viable moth-eaten bone marrow chimeric mice. Journal of immunology. 1993;151:6733–6741. [PubMed] [Google Scholar]
- 15.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends in biochemical sciences. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Seminars in immunology. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 17.Kruger J, Butler JR, Cherapanov V, Dong Q, Ginzberg H, Govindarajan A, Grinstein S, Siminovitch KA, Downey GP. Deficiency of Src homology 2-containing phosphatase 1 results in abnormalities in murine neutrophil function: studies in motheaten mice. Journal of immunology. 2000;165:5847–5859. doi: 10.4049/jimmunol.165.10.5847. [DOI] [PubMed] [Google Scholar]
- 18.Chen HE, Chang S, Trub T, Neel BG. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Molecular and cellular biology. 1996;16:3685–3697. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao H, Yang W, Berrada K, Tabrizi M, Shultz L, Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Experimental hematology. 1997;25:592–600. [PubMed] [Google Scholar]
- 20.Roach TI, Slater SE, White LS, Zhang X, Majerus PW, Brown EJ, Thomas ML. The protein tyrosine phosphatase SHP-1 regulates integrin-mediated adhesion of macrophages. Current biology : CB. 1998;8:1035–1038. doi: 10.1016/s0960-9822(07)00426-5. [DOI] [PubMed] [Google Scholar]
- 21.Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity. 2013;38:489–501. doi: 10.1016/j.immuni.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. Journal of immunology. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 25.Bairstow SF, Ling K, Anderson RA. Phosphatidylinositol phosphate kinase type Igamma directly associates with and regulates Shp-1 tyrosine phosphatase. The Journal of biological chemistry. 2005;280:23884–23891. doi: 10.1074/jbc.M500576200. [DOI] [PubMed] [Google Scholar]
- 26.Ling K, Schill NJ, Wagoner MP, Sun Y, Anderson RA. Movin’ on up: the role of PtdIns(4,5)P(2) in cell migration. Trends in cell biology. 2006;16:276–284. doi: 10.1016/j.tcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 28.Oude Weernink PA, Schmidt M, Jakobs KH. Regulation and cellular roles of phosphoinositide 5-kinases. European journal of pharmacology. 2004;500:87–99. doi: 10.1016/j.ejphar.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Wernimont SA, Legate KR, Simonson WT, Fassler R, Huttenlocher A. PIPKI gamma 90 negatively regulates LFA-1-mediated adhesion and activation in antigen-induced CD4+ T cells. Journal of immunology. 2010;185:4714–4723. doi: 10.4049/jimmunol.1001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. “Viable motheaten,” a new allele at the motheaten locus. I. Pathology. The American journal of pathology. 1984;116:179–192. [PMC free article] [PubMed] [Google Scholar]
- 31.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. The Journal of experimental medicine. 2008;205:2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herter JM, Rossaint J, Block H, Welch H, Zarbock A. Integrin activation by P-Rex1 is required for selectin-mediated slow leukocyte rolling and intravascular crawling. Blood. 2013;121:2301–2310. doi: 10.1182/blood-2012-09-457085. [DOI] [PubMed] [Google Scholar]
- 33.Zarbock A, Deem TL, Burcin TL, Ley K. Galphai2 is required for chemokine-induced neutrophil arrest. Blood. 2007;110:3773–3779. doi: 10.1182/blood-2007-06-094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Xu J, Kumar RS, Lakshmikanthan S, Kapur R, Kofron M, Chrzanowska-Wodnicka M, Filippi MD. The small GTPase Rap1b negatively regulates neutrophil chemotaxis and transcellular diapedesis by inhibiting Akt activation. The Journal of experimental medicine. 2014;211:1741–1758. doi: 10.1084/jem.20131706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Block H, Herter JM, Rossaint J, Stadtmann A, Kliche S, Lowell CA, Zarbock A. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. The Journal of experimental medicine. 2012;209:407–421. doi: 10.1084/jem.20111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stadtmann A, Brinkhaus L, Mueller H, Rossaint J, Bolomini-Vittori M, Bergmeier W, Van Aken H, Wagner DD, Laudanna C, Ley K, Zarbock A. Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur J Immunol. 2011 doi: 10.1002/eji.201041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stadtmann A, Germena G, Block H, Boras M, Rossaint J, Sundd P, Lefort C, Fisher CI, Buscher K, Gelschefarth B, Urzainqui A, Gerke V, Ley K, Zarbock A. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. The Journal of experimental medicine. 2013;210:2171–2180. doi: 10.1084/jem.20130664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ittner A, Block H, Reichel CA, Varjosalo M, Gehart H, Sumara G, Gstaiger M, Krombach F, Zarbock A, Ricci R. Regulation of PTEN activity by p38delta-PKD1 signaling in neutrophils confers inflammatory responses in the lung. The Journal of experimental medicine. 2012;209:2229–2246. doi: 10.1084/jem.20120677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. The Journal of experimental medicine. 2011;208:1721–1735. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nature medicine. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 41.Lefort CT, Rossaint J, Moser M, Petrich BG, Zarbock A, Monkley SJ, Critchley DR, Ginsberg MH, Fassler R, Ley K. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood. 2012;119:4275–4282. doi: 10.1182/blood-2011-08-373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Kruhlak MJ, Hao JJ, Shaw S. Rapid T cell receptor-mediated SHP-1 S591 phosphorylation regulates SHP-1 cellular localization and phosphatase activity. Journal of leukocyte biology. 2007;82:742–751. doi: 10.1189/jlb.1206736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong X, Wang X, Misra S, Qin J. Structural basis for the phosphorylation-regulated focal adhesion targeting of type Igamma phosphatidylinositol phosphate kinase (PIPKIgamma) by talin. Journal of molecular biology. 2006;359:47–54. doi: 10.1016/j.jmb.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 45.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, Ballantyne CM, Constant SL, Aird WC, Papayannopoulou T, Gahmberg CG, Udey MC, Vajkoczy P, Quertermous T, Dimmeler S, Weber C, Chavakis T. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, Martinello M, Constantin G, Laudanna C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009;10:185–194. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- 47.Zarbock A, Kempf T, Wollert KC, Vestweber D. Leukocyte integrin activation and deactivation: novel mechanisms of balancing inflammation. J Mol Med (Berl) 2012;90:353–359. doi: 10.1007/s00109-011-0835-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Meng F, Chu CL, Takai T, Lowell CA. The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity. 2005;22:235–246. doi: 10.1016/j.immuni.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Insall RH, Weiner OD. PIP3, PIP2, and cell movement--similar messages, different meanings? Developmental cell. 2001;1:743–747. doi: 10.1016/s1534-5807(01)00086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ling K, Doughman RL, Iyer VV, Firestone AJ, Bairstow SF, Mosher DF, Schaller MD, Anderson RA. Tyrosine phosphorylation of type Igamma phosphatidylinositol phosphate kinase by Src regulates an integrin-talin switch. The Journal of cell biology. 2003;163:1339–1349. doi: 10.1083/jcb.200310067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 52.Martel V, Racaud-Sultan C, Dupe S, Marie C, Paulhe F, Galmiche A, Block MR, Albiges-Rizo C. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. The Journal of biological chemistry. 2001;276:21217–21227. doi: 10.1074/jbc.M102373200. [DOI] [PubMed] [Google Scholar]
- 53.Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- 54.Isenberg G, Goldmann WH. Peptide-specific antibodies localize the major lipid binding sites of talin dimers to oppositely arranged N-terminal 47 kDa subdomains. FEBS letters. 1998;426:165–170. doi: 10.1016/s0014-5793(98)00336-6. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 56.Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. Journal of leukocyte biology. 2006;80:1491–1499. doi: 10.1189/jlb.0306227. [DOI] [PubMed] [Google Scholar]
- 57.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, Zarbock A. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.