Abstract
With increasing age, naïve CD4 T cells acquire intrinsic defects that compromise their ability to respond and differentiate. Type I IFNs, pervasive constituents of the environment in which adaptive immune responses occur, are known to regulate T cell differentiation and survival. Activated naïve CD4 T cells from older individuals have reduced responses to type I IFN, a defect that develops during activation and is not observed in quiescent naïve CD4 T cells. Naïve CD4 T cells from young adults upregulate the expression of STAT1 and STAT5 after activation, lowering their threshold to respond to type I IFN stimulation. The heightened STAT signaling is critical to maintain the expression of CD69 that regulates lymphocyte egress and the ability to produce IL-2 and to survive. Although activation of T cells from older adults also induces transcription of STAT1 and STAT5, failure to exclude SHP1 to the signaling complex blunts their type I IFN response. In summary, our data show that type I IFN signaling thresholds in naïve CD4 T cells after activation are dynamically regulated to respond environmental cues for clonal expansion and memory cell differentiation. Naïve CD4 T cells from older adults have a defect in this threshold calibration. Restoring their ability to respond to type I IFN emerges as a promising target to restore T cell responses and improve the induction of T cell memory.
Introduction
With advancing age, the immune system loses competence to generate adaptive immune responses (1–5). Mortality and morbidity from infections increases; more than 90% of all influenza-related deaths in the United States occur in older adults (6). In particular, older individuals are more prone to develop complications from newly arising infectious organisms such as West Nile fever or Severe Acute Respiratory Syndrome (7–12). Vaccinations are powerful interventions that have been extremely successful to change the natural history of infections in the young and, therefore, should be ideal tools to promote healthy aging. Considerable efforts have gone into annual influenza vaccinations; however, the induction of protective immunity elicited by influenza as well as other vaccines remains inadequate in older adults (13–16). Despite increasing compliance with vaccine recommendations, the annual influenza epidemics remain a medical challenge (17).
A better understanding of the age-associated defects in adaptive immunity holds the promise to design age-targeted interventions to improve vaccine responses (3, 18). Given the dramatic decline in thymic T cell production in humans, older individuals have been suspected to lack a sufficiently diverse T cell receptor (TCR) repertoire to respond to the universe of foreign antigens which would make it difficult to improve vaccine responses (19, 20). While initial studies in the mouse and in humans supported this concept (21–23), more recent studies have led to the conclusion that T cell generation and homeostasis is quite different in humans and mice (24). Moreover, recent estimates of human TCR richness by next-generation sequencing have shown an unexpected complexity of the human repertoire even in older individuals that makes frank holes in the repertoire an improbable explanation for the age-associated T cell defect (19, 25). T cell homeostatic proliferation appears to be efficient to maintain not only T cell numbers but also TCR diversity, in particular for human CD4 T cells that are important for vaccine-induced antibody responses (26).
Alternatively to contraction in TCR diversity, increasing dysfunctionality of naïve T cells could explain defective vaccine responses (27–30). Age-associated defects could occur at the level of initial T cell activation, the level of subsequent clonal expansion into differentiated effector cells and finally the ability to survive as long-lived memory cell. Gene expression studies comparing naïve CD4 T cells from young and older individuals after stimulation with the superantigen toxic shock syndrome toxin (TSST) and myeloid dendritic cells (DC) suggested that the ability to respond to strong stimuli was preserved (31). As well, aged human CD4 naïve T cells were able to be activated by novel antigens and produce IL-2 when stimulated with rabies virus or Etr proteins from tick-borne encephalitis virus (32). However, suboptimal stimulation uncovered a heightened TCR activation threshold due to overexpression of the dual specific phosphatase 6. The associated initial blunting of ERK phosphorylation may compromise the response to low affinity antigens (33).
The current study was designed to examine pathways that are operational several days after initial antigen encounter when naïve CD4 T cells differentiate and develop into effector and memory T cells; and to identify defects in these pathways that may contribute to suboptimal vaccine responses in older adults. In initial gene expression arrays on day 3 after activation with DC/TSST, we observed a signature of type I IFN-inducible genes in young naïve CD4 T cell responses that were reduced in older individuals. Here, we show that CD4 T cell priming is associated with sensitization of STAT1 and STAT5 responses to IFN-α. This increased sensitivity of activated CD4 T cells is attenuated in older adults. Since type I IFN responses regulate T cell survival and migration, the signaling defect in activated CD4 T cells may contribute to the impaired adaptive immune responses.
Materials and Methods
Study population and cells
Peripheral blood mononuclear cells (PBMC) were obtained from eighty-seven 20–35 and eighty-six 65–85 year-old adults. Individuals with acute diseases, current or previous history of immune-mediated diseases or cancer except limited basal cell carcinoma or chronic diseases not controlled on oral medications were excluded. The study was approved by the Stanford Institutional Review Board. CD4 naïve T cells were purified from PBMC by negative isolation of total CD4 T cells using CD4+ T Cell Isolation Kit II (Miltenyi Biotec) followed by depleting of CD45RO+ cells. In some experiments, T cells were isolated from peripheral blood with Human T Cell Enrichment Cocktail (STEMCELL Technologies Inc.). To generate DC, CD14+ monocytes were positively selected by anti-CD14 magnetic microbeads (Miltenyi Biotec Inc.) and then cultured with 800 U/ml−1 granulocyte-macrophage-CSF and 1000 U/ml−1 IL-4 (R&D Systems) for six days. Dendritic cells (DCs) were matured with 1100 U/ml−1 TNF-α (R&D Systems) and 1 μg/ml−1 PGE2 (Sigma) for 24 h. For DC–T cell cocultures, 25×103 naïve CD4+ cells were stimulated with 0.5×103 of DC and 0.1 μg/ml−1 TSST-1 (Toxin Technology) for four days. Cell and TSST concentrations were chosen to minimize alloreactive responses and keep stimulation conditions suboptimal. Alternatively, purified naïve CD4 T cells were stimulated with Dynabeads human T-expander CD3/CD28 (Life Technologies) for three days.
Transcript quantification by qPCR
Total RNA was isolated using Trizol (Invitrogen Life Technologies) and cDNA was synthesized using AMV reverse transcriptase (Roche Applied Science) and random primers. Transcripts of target genes were quantified by SYBR qPCR. The primers used for qPCR were: IFIT1: 5′-GCAGAACGGCTGCCTAATTT-3′ and 5′-TCAGGCATTTCATCGTCATC-3′; OAS3:5′-GTCAAACCCAAGCCACAAGT-3′ and 5′-CTCCTTCCACAACCCCTGTA-3′; STAT1: 5′-ACCTTGCAGAACAGAGAACAC-3′ and 5′-GGCATTCTGGGTAAGTTCAGT-3′; STAT5A: 5′-GCAGCCAGACCTATTCCTCCT-3′ and 5′-TCTCTTAGGAGGGCAAAGCT-3′; STAT5B: 5′-CGACCTCTCCATCTTCAGCT-3′ and 5′-CACAAACACATACTCGCACT-3′; PTPN6: 5′-TCAAGGTCATGTGCGAGGGT-3′ and 5′-CCTCGTGGCATAGTACGGCTG-3′; SOCS1: 5′-TTTTCGCCCTTAGCGTGAAGA-3′ and 5′-GAGGCAGTCGAAGCTCTCG-3′; SOCS3: 5′-GGCCACTCTTCAGCATCTCTGT-3′ and 5′GCATCGTACTGGTCCAGGAACT-3′; PIAS1: 5′-AACCCACCAGTCTAGCATC-3′ and 5′-GGAAGTGATCTTCTTGTGGAC-3′; PIASy: 5′-AGCTGTGCAAGGCACTGGTCA-3′ and 5′-GCTTCTTCTCGTTCATCTGCAG-3′; 18s rRNA: 5′-GTTGAACCCCATTCGTGATG-3′ and 5′-GCCTCACTAAACCATCCAA-3′. Results were compared to qPCR of serial dilutions of gene-specific standards to determine transcript numbers and were normalized to 1×106 18s rRNA transcripts.
Flow cytometry
Unstimulated or activated CD4 naïve T cells (stimulated with anti-CD3/CD28 Dynabeads for three days) were stimulated with 10,000 U/ml−1 IFN-α (PBL Assay Science) at 37°C. Cells were fixed in BD Cytofix™ fixation buffer; permeabilized with BD Perm Buffer III and stained with anti-CD4-PerCP, anti-phospho-STAT1 (pY701)-FITC, or anti-phospho-STAT5 (pY694)-APC. For intracellular staining of IL-2, activated CD4 naïve T cells were restimulated with Dynabeads human T-expander CD3/CD28 in the presence of brefeldin A for 6 h. Cells were stained with anti-CD4-PerCP, fixed with BD Cytofix/Cytoperm buffer and then stained with anti-IL-2-FITC. Apoptosis was assayed using BD Annexin V: PE Apoptosis Detection Kit I following procedures suggested by the manufacturer. All flow antibodies and buffers were from BD Biosciences. IFN-α/β receptors (IFNAR) surface staining was performed using anti-IFNAR1-PE or –IFNAR2-PE (PBL Assay Science). Cytometry data were collected on an LSRII flow cytometer and analyzed with FACS Diva or FlowJo software.
Immunoprecipitation and Western blotting
Unstimulated and activated CD4 naïve T cells were lysed in lysis buffer containing proteinase and phosphatase inhibitor cocktails (Cell Signaling Technology, Inc.). Lysates were electrophoresed on a 9% SDS-polyacrylamide gel and then transferred to a PVDF membrane (Bio-Rad Laboratories). The blots were blocked and probed with anti-phospho-STAT1 (pY701), anti-STAT1, anti-phospho-STAT5 (pY694), anti-STAT5, anti-SOCS1, anti-SOCS3 Ab (all from Cell Signaling Technology, Inc.), and anti-SHP-1 Ab (EMD Millipore). For immunoprecipitation, lysates were precleared with protein G agarose beads (Santa Cruz Biotechnology, Inc.) at 4°C for 1 h followed by incubation with anti-human IFNAR1 Ab (PBL Assay Science) at 4°C overnight. The immune complexes were captured by protein G agarose beads and then subjected to blotting assays using the antibodies described above.
Quantitative colocalization analysis
CD4 naïve T cells were activated with anti-CD3/CD28 mAb for three days followed by 10,000 U/mL−1 IFN-α-stimulation for 5 min. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.05% Triton X-100 and incubated with rabbit-anti-SHP-1 and mouse-anti-human IFNAR 1 Abs at 4°C overnight. After washing, cells were incubated with Alexa Fluor 488-labeled goat anti-rabbit and Alexa Fluor 594-labeled goat anti-mouse antibodies (Life Technologies) at room temperature for 1 h. Cells were then subjected to confocal microscopy. Images were captured using a LSM710 microscope system and analyzed with ZEN 2010 software (Carl Zeiss).
Statistical analysis
Comparisons were performed by two-way ANOVA or by t-test, where appropriate, using SigmaStat 3.0 software.
RESULTS
Age-associated defect in type I IFN responses of naïve CD4 T cells
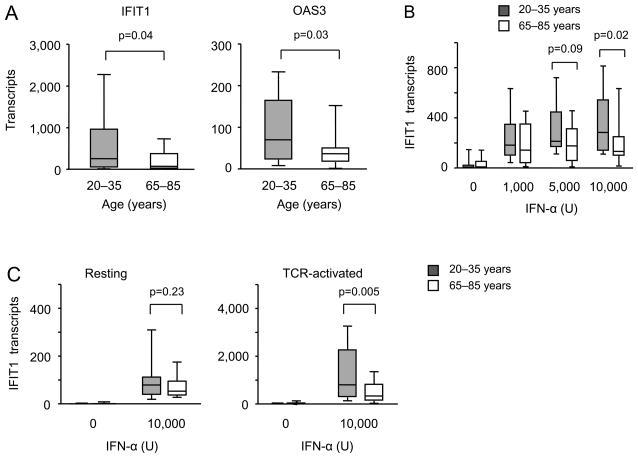
In comparing gene expression in CD4 T cells from young and elderly individuals after stimulation with the superantigen TSST and DC from a young individual, we identified several signatures of differentially expressed genes on day 3 after stimulation (31, 34). As previously reported, gene ontology enrichment analysis using the David Bioinformatics Resource tool (35, 36) showed enrichment for GO terms associated with zinc binding and transportation in genes overexpressed in old activated naïve CD4 T cells. Conversely, probes with reduced expression in old CD4 T cells were significantly enriched for genes that are controlled by an interferon response element (ISRE) (p<10−17). Among others, these genes included classical type I IFN-inducible genes such as IFIT1, Mx-1 and various OAS variants (data not shown). To confirm this observation, we stimulated purified naïve CD4 T cells from eighteen 20 to 35 year-old and eighteen 65 to 85 year-old individuals with TSST and monocyte-derived DC for three days and assessed the transcription of the ISRE regulated genes IFIT1 and OAS3 (Figure 1A). Transcripts of both genes were significantly higher in the activated T cells from young individuals.
Figure 1. Expression of ISRE-responsive genes in activated CD4 naïve T cells declines with age.
IFIT1 and OAS3 expressions were quantified by qPCR. Transcript numbers expressed relative to 1×106 18s RNA transcripts from 20–35 (shaded boxes) and 65–85 (open boxes) year-old adults are shown as box plots with medians, 25th and 75th percentiles as boxes and 10th and 90th percentile as whiskers. (A) Purified naïve CD4+ T cells from young (n=18) and older (n=18) adults were stimulated with the superantigen TSST and mDCs. IFIT1 (left panel) and OAS3 (right panel) transcripts were quantified on day 3 of culture. (B) CD4 naïve T cells were stimulated with Dynabeads human T-expander CD3/CD28 in the presence of indicated concentrations of IFN-α for three days; IFIT1 expression was quantified using qPCR. Data are from twelve young and twelve older adults. (C) Unstimulated (left panel, n=10) and activated naïve CD4 T cells (right panel, Dynabeads human T-expander CD3/CD28 stimulation for three days, n=13) from young and older adults were stimulated with 10,000 U mL−1 IFN-α. Cells were harvested after 8 h and IFIT1 transcripts were quantified.
To determine whether the defective transcription of ISRE-regulated genes was caused by a lower production of type I IFN in the culture or by reduced sensitivity of the elderly CD4 T cells to the type I IFN stimulus, we stimulated purified naïve CD4 T cells in the absence of DC by cross-linking CD3 and CD28 in the presence of increasing concentrations of IFN-α. Results from 12 young and 12 elderly adults shown Figure 1B demonstrated a reduced responsiveness in the older adults which was particularly evident at the higher concentrations of type I IFN. Of note, T cells cultured in the presence of type I IFN were less responsive to type I IFN restimulation irrespective of age, suggesting the induction of a negative feedback loop under these conditions. To determine whether the age-associated defect already existed in fresh naïve CD4 T cells or whether it was acquired after T cell activation, we compared IFIT inducibility in nonstimulated and day 3-activated naïve CD4 T cells. No significant difference between young and old adults was seen when IFN responses of fresh ex vivo naïve CD4 T cells were compared (Figure 1C, left panel). Inducibility of IFIT1 expression increased with cell activation and culture, but significantly more so for naïve CD4 T cells from young adults (Figure 1C, right panel) suggesting that old CD4 T cells fail to gain type I IFN responsiveness with T cell activation and differentiation.
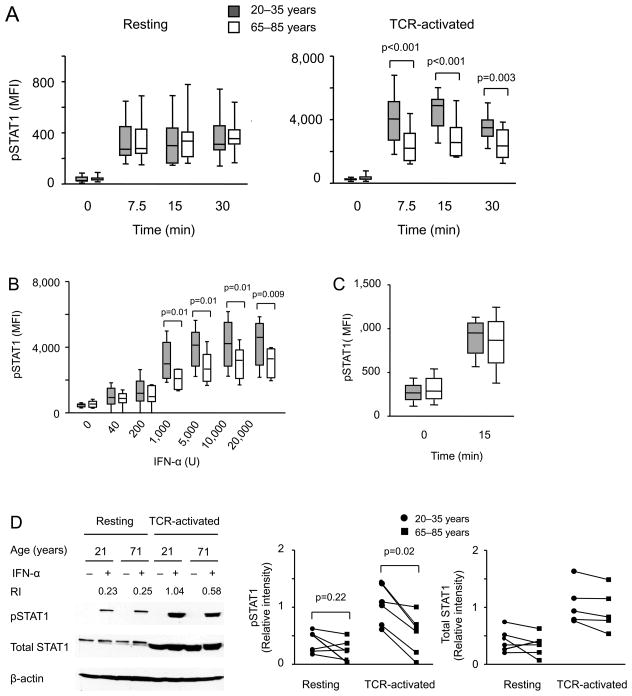
Type I IFNs transmit their signal through STAT1/2 to activate ISRE-dependent genes. To determine whether JAK-STAT activation is intact in elderly T cells, we quantified STAT1 phosphorylation by PhosFlow in fifteen 20 to 35 and fifteen 65 to 85 year-old individuals after stimulation with 10,000 U/ml IFN-α. No difference was seen when naïve unstimulated CD4 T cells were compared (Figure 2A, left panel). Compared to unstimulated CD4 naïve T cells, activated cells respond to type I IFN with more vigorous STAT1 phosphorylation. Naïve CD4 T cells activated by anti-CD3/CD28 stimulation for 72 h from older individuals were clearly inferior to phosphorylate STAT1 (Figure 2A, right panel). Stimulation with increasing doses of IFN-α showed that STAT1 phosphorylation plateaued at concentrations of 5,000 U/ml irrespective of age. An age-associated reduction in STAT phosphorylation was seen starting at concentrations of 1,000 U/ml and could not be overcome by increasing the dose (Figure 2B). The defect appeared to be specific for type I IFNs since STAT1 phosphorylation after type II IFN was intact (Figure 2C). Western blotting confirmed the age-associated reduction in STAT1 responses to IFN-α stimulation in TCR-activated, but not quiescent naïve CD4 T cells (Figure 2D). Interestingly, expression of STAT1 was massively increased in day 3-activated T cells which may explain their increased responsiveness to IFN-α. However, this increase was not reduced with age.
Figure 2. STAT1 phosphorylation after type I IFN stimulation of activated naïve CD4 T cells declines with age.
(A) Resting (left panel, sixteen young and sixteen older individuals) and day 3 anti-CD3/CD28-activated (right panel, fifteen young and fifteen older) CD4 naïve T cells were treated with 10,000 U/mL−1 IFN-α at 37°C. STAT1 phosphorylation was measured by flow cytometry at the indicated time points. (B) Anti-CD3/CD28-activated CD4 naïve T cells from eight young and eight older individuals were stimulated with increasing doses of IFN-α at 37°C for 15 min; STAT1 phosphorylation was measured. (C) Anti-CD3/CD28-activated CD4 naïve T cells from five young and five older individuals were treated with 10,000 U/mL−1 IFN-γ at 37°C for 15 min. STAT1 phosphorylation was determined. (D) Resting and anti-CD3/CD28-activated CD4 naïve T cells from young and older individuals were left untreated or treated with 10,000 U/mL−1 IFN-α at 37°C for 10 min; phosphorylated STAT1 and total STAT1 levels were determined by Western blotting. Representative blots (left) and summary data (right) from six young (aged 20–35 years, circles) and six old (aged 65–85 years, squares) adults are shown. Samples from a young and an older individual that were cultured and analyzed by Western blotting in parallel are connected by a line. RI = relative intensity of pSTAT1 compared to β-actin.
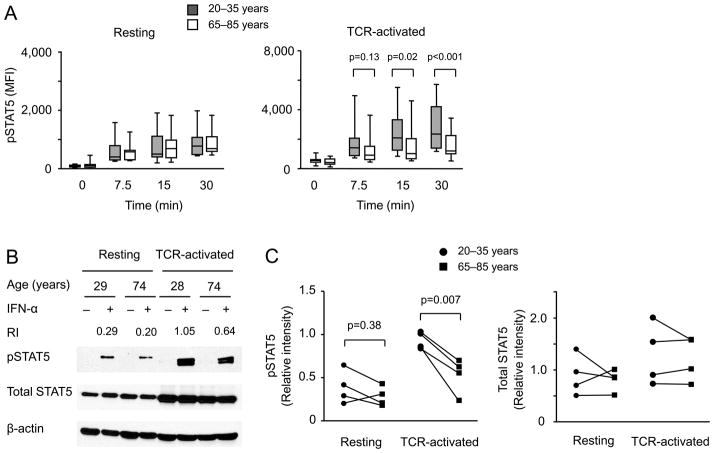
Although the classical type I IFN signaling pathway entails STAT1/2 activation, the type I IFN receptor is promiscuous and also activates other STAT pathways (37). STAT5 phosphorylation showed a similar pattern as STAT1, i.e., the STAT5 response to type I IFN stimulation was increased in activated CD4 T cells and this increase was more pronounced in the young than the old (Figure 3).
Figure 3. STAT5 phosphorylation after type I IFN stimulation of activated naïve CD4 T cells declines with age.
(A, B) Phosphorylated STAT5 and total STAT5 were determined by flow cytometry (A) in resting (sixteen young and sixteen older) and anti-CD3/CD28-activated (fifteen young and fifteen older) CD4 naïve T cells and by Western blotting (B) as described in Figure 2A and D. (C). Summary data from Western blots are from four young (circles) and four older individuals (squares). Again paired samples are indicated by a line.
Mechanisms of age-associated impaired type I IFN receptor signaling in activated naïve T cells
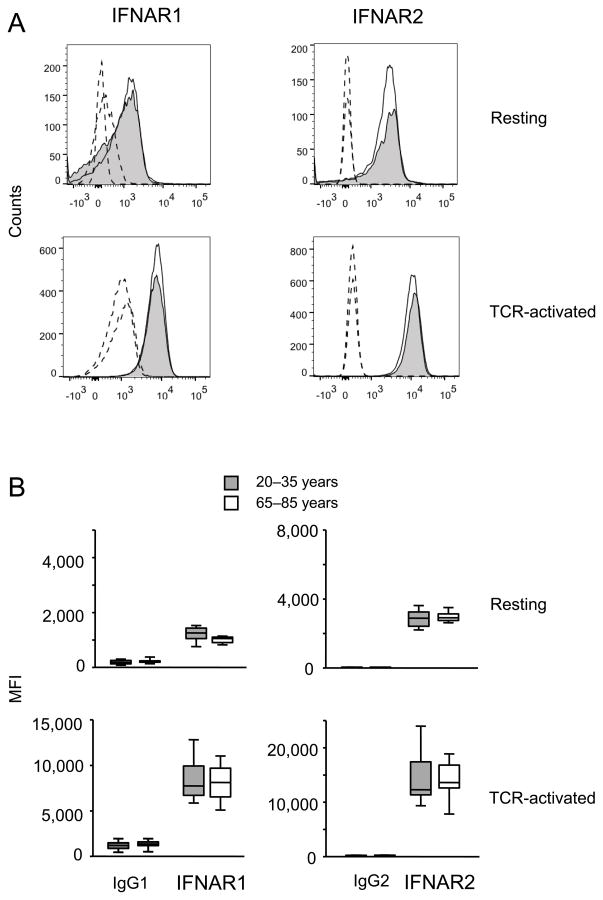
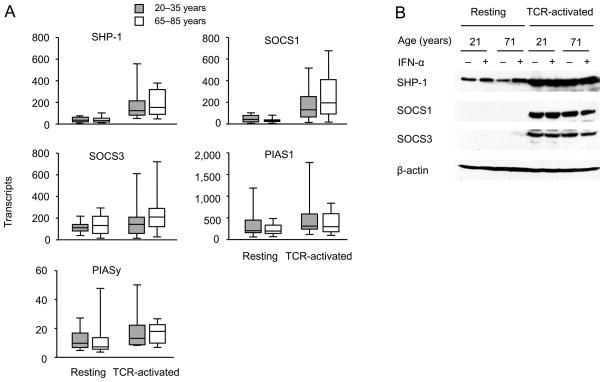
Our analysis so far has shown that activation of CD4 T cells renders them more sensitive to type I IFN stimulation, a process that is attenuated with age. Western blot analysis showed increased expression of STAT1 and STAT5 with activation that did not appear to account for the age-associated difference in responsiveness. To confirm the Western blot data in a larger population of young and older adults, we compared STAT1 and STAT5a and b transcripts by qPCR. All three STAT molecules were higher expressed in activated T cells, however irrespective of age (Figure 4). Both chains of IFNAR were expressed on naïve CD4 T cells. Mean fluorescence intensities increased after activation, however, irrespective of age (Figure 5). Similarly, we did not find a difference in transcription of the negative STAT regulators SHP-1, SOCS1 and SOCS3, PIAS1 and PIASy, when activated naïve CD4 T cells from young and older individuals were compared (Figure 6A). Western blot studies corroborated these results (Figure 6B).
Figure 4. Age does not inhibit activation-induced increase in STAT1 and STAT5 expression.
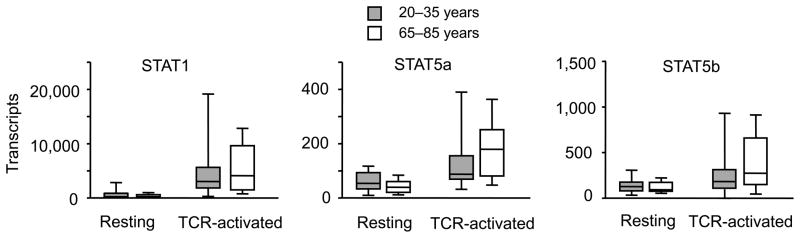
STAT1 and STAT5 transcription were quantified in resting and anti-CD3/CD28-activated CD4 naïve T cells from young (shaded boxes, n= 18) and older adults (open boxes, n=16) by qPCR. Transcript numbers are expressed relative to 1 × 106 18s RNA transcripts.
Figure 5. Expression of IFNAR is independent of age.
Expression of IFNAR1 and R2 in resting and anti-CD3/CD28-activated CD4 naïve T cells was analyzed by flow cytometry. (A) Representative data from a 31 year-old (open histogram) and a 72 year-old (shaded histogram) individual are shown. Dashed lines: IgG control; solid lines: IFN-R. (B) Summary data from ten young and ten older individuals are shown.
Figure 6. Expression of negative regulators of STAT signaling is independent of age.
(A) SHP-1, SOCS1, SOCS3, PIAS1 and PIASy transcription in resting and anti-CD3/CD28-activated CD4 naïve T cells from young (shaded boxes, n=18) and older adults (open boxes, n=16) were quantified by qPCR. (B) Western blots from resting and anti-CD3/CD28-activated CD4 naïve T cells from young and older individuals were prepared as described in Figure 2D and probed for SHP-1, SOCS1 and SOCS3. Blots shown are representative of four.
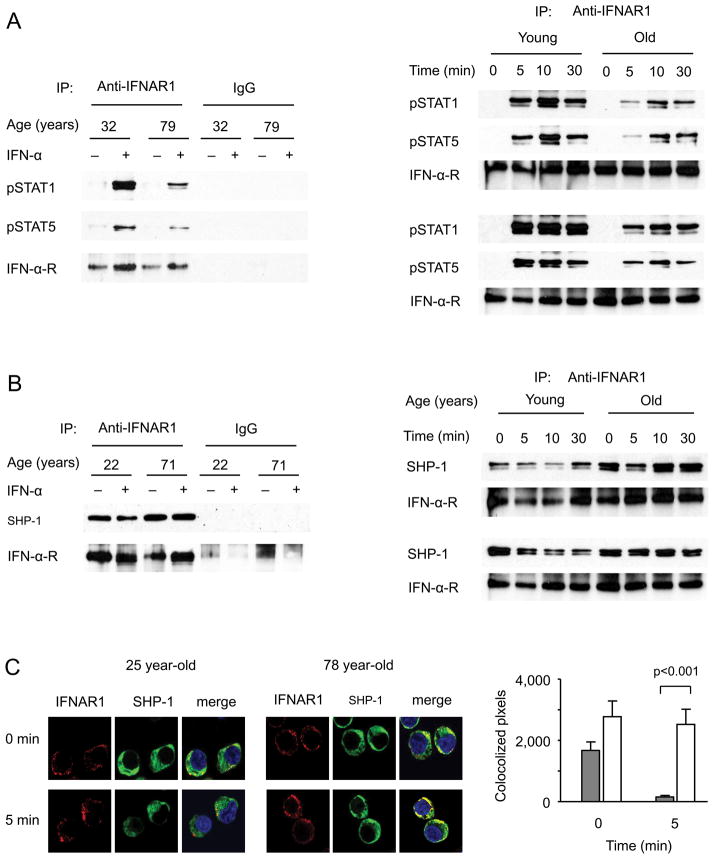
To examine the type I IFN receptor signaling complex, we precipitated the complex from naïve CD4 T cells three days after activation and analyzed the precipitate by Western blotting. Precipitation was more efficient after type I IFN stimulation which was largely independent of age as can be seen from the staining for the IFNAR1 (Figure 7). Phosphorylated STAT1 and STAT5 were markedly reduced in the precipitates of older CD4 T cells (Figure 7A). We consistently found increased concentrations of SHP-1 in the precipitates from older T cells (Figure 7B) while the cytoplasmic concentrations were not increased. A careful kinetic analysis of the SHP-1 in the receptor complex showed that in young adults SHP-1 was present before stimulation and was excluded from the complex upon IFN-α stimulation to slowly return after 30 min or later (Figure 7B, right panel). In contrast, CD4 T cells from older adults already exhibit more SHP-1 in the complex before stimulation. More importantly, they were not able to exclude SHP-1 from the signaling complex upon stimulation. SOCS1 did not appear to be enriched in the precipitates from activated older CD4 T cells; however, the concentrations of SOCS1 in the precipitate were too low to be confidently quantified with the currently available commercial antibodies (data not shown).
Figure 7. SHP-1 exclusion from the type I IFN receptor signaling complex decreases with age.
(A, B). CD4 naïve T cells from young and older adults activated with anti-CD3/CD28 mAb for three days were stimulated with 10,000 U/mL−1 IFN-α for 10 min (left panels) or indicated times (right panels). IFN receptor complexes were immunoprecipitated and examined by Western blotting. (A) Western blots showing reduced pSTAT1 and pSTAT5 in older adults are representative of three experiments (left panel). In two additional pairs of a young and old individual, the time courses of phosphorylated STAT1 in the precipitate is shown. (B) Western blots show failure to exclude SHP-1 from the signaling complex after stimulation in older adults. The left panel shows results at before and 10 min after IFN-α stimulation and is representative of three experiments. A more detailed time course is shown in the right panel. (C) Colocalization of IFNAR1 and SHP-1 on cell membrane was examined by confocal microscopy. Representative images (left) and mean±SEM colocalized pixels of 50 cells (right) from a 25 year-old and a 78 year-old individual are shown. Data are representative of three experiments.
To confirm an age-related difference in SHP-1 recruitment to the signaling complex, we used quantitative colocalization analysis by confocal fluorescence microscopy. CD3/CD28-activated T cells cultured for three days in vitro were stained with antibodies to SHP-1 and IFNAR1. T cells from older adults exhibit higher colocalization, which, in contrast to young adults, did not significantly decline with IFN-α stimulation (Figure 7C).
Functional consequences of impaired type I IFN receptor signaling
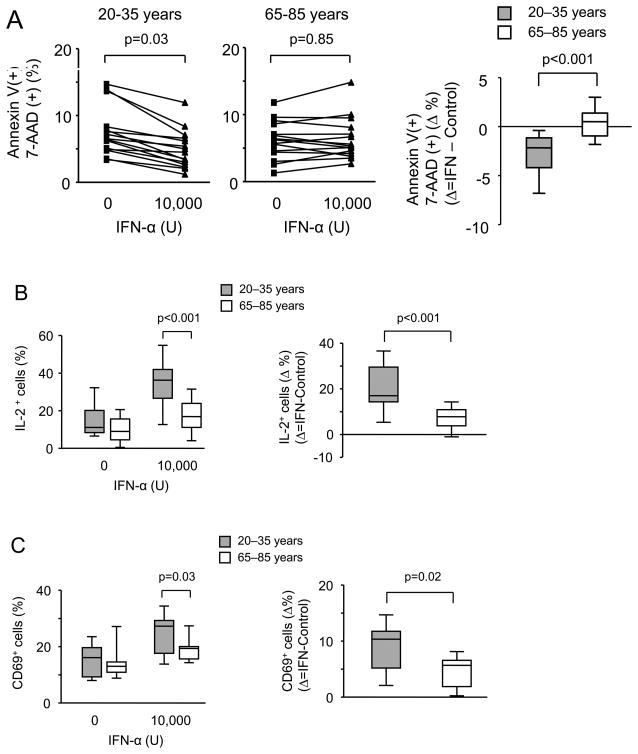
Previous studies have reported an important function of type I IFN responses in T cell survival (38, 39). To explore functional consequences of the increased sensitivity of activated T cells from young individuals to type I IFN stimulation, naïve CD4 T cells were stimulated with CD3/CD28 cross-linking for 72 h followed by three day culture with 10,000 U IFN-α before apoptosis rates in proliferating T cells were determined. IFN-α was mildly protective for activated T cells from young individuals, a similar effect was not seen for T cells from individuals older than 65 years (Figure 8A). We explored several pathways that could protect cells from undergoing apoptosis. We did not find a differential induction in anti-apoptotic molecules such as Bcl-XL. However, we found a striking difference in the ability to produce IL-2. On day 3 after stimulation, naïve CD4 T cells have largely lost the ability to produce IL-2 upon restimulation, more so in old than in young adults although this trend did not reach significance. IFN-α could restore this defect in CD4 T cells from young individuals while it was significantly less effective with old T cells (Figure 8B).
Figure 8. Functional consequences of impaired type IFN signaling in older adults.
CD4 naïve T cells were stimulated with anti-CD3/CD28 Ab. IFN-α (10,000 U/mL−1) was added on day 3. Representative original cytometry data for all experiments summarized below are shown in Suppl. Figure 1. (A) Frequencies of Annexin V and 7-AAD double positive cells were determined on day 6 in cultures from fifteen young and fifteen older adults (left panel). Right panel shows Δ frequencies in cultures with and without added IFN-α. (B) On day 4, cells were restimulated with anti-CD3/CD28 in the presence of Brefeldin A and analyzed for IL-2 production by flow cytometry. Frequencies of IL-2–producing CD4 T cells from fifteen young (shaded boxes) and fifteen older adults (open boxes) are shown in the left panel. Right panel shows Δ frequencies in cultures with and without added IFN-α. (C) Type I IFN induction of CD69 in activated CD4 naïve T cells from nine young (shaded boxes) and nine older adults (open boxes) is shown in the left panel. Right panel shows Δ frequencies in cultures with and without added IFN-α.
Type I IFN has been reported to inhibit lymphocyte egress from lymphoid tissue after activation, mostly by inducing or sustaining CD69 expression (40). To determine the influence of age on the type I IFN response, day 3-activated naïve CD4 T cells were stimulated with IFN-α for 24 h and CD69 induction was assessed (Figure 8C). Activated CD4 naïve T cells from young individuals responded significantly better to IFN-α with CD69 expression suggesting that elderly CD4 T cells are less retained in secondary lymphoid organs after antigen-induced activation.
DISCUSSION
Successful vaccine responses require the activation of antigen-specific T cells by antigenic peptides presented by DC and the subsequent clonal expansion of these T cells and their differentiation into effector cells. While most of these effector cells undergo clonal contraction, few cells survive as long-lived memory cells constituting the quintessential objective of vaccination. This process is impaired in older individuals (16, 18). Defects in DC activation and early T cell signaling contribute to this failure and can be in part compensated by adjuvant-mediated DC activation or increasing the antigen dose (41); however, these interventions have so far not been sufficient to overcome the impaired vaccine responses (42–45). The current studies were built on the hypothesis that age affects the ability of naïve T cells to expand and differentiate into effector and memory cells. We found that activated naïve CD4 T cells have a blunted response to type I IFN stimulation that impairs IL-2 production and cell survival and may, therefore, have a negative impact on the ability to establish T cell memory.
Type I IFN are key components of the innate immune response rapidly triggered by bacterial and viral infections. They, therefore, form a physiologic environment for the evolving adaptive T cell response, in part, since plasmacytoid DCs are the major producers of IFN-α. In animal models, they act as an adjuvant enhancing the magnitude of primary and secondary T cell responses (46–50). Studies in mice that lacked the type I IFN receptor selectively on T cells have allowed characterizing the IFN effects in detail. CD8 responses are highly dependent on type I IFN-induced signals and CD8 memory formation in the setting of LCMV infection in the absence of type I IFN signaling is greatly diminished (51). The role of type I IFN in CD4 T cell responses has been less studied in murine models. However, antibody production after immunization is no longer upregulated by type I IFN if T cells lack the IFN receptor, supporting the notion that T helper cell activity is dependent on type I IFN stimulation (52).
The effects of type I IFN on T cells are dependent on the timing of the IFN signal in relation to TCR stimulation. Human CD4 naïve T cells preincubated with IFN-α show decreased proliferative activities upon TCR triggering, but IFN-α does not inhibit expansion of preactivated T cells (53). Type I IFN curtails phosphorylation of MEK1 and ERK1/2 upon stimulation leading to diminished IL-2 production and IL-2Rα expression in IFN-α pretreated CD4 T cells (54). In contrast, IFN-α upregulates expression of proliferation-promoting genes including IL-2Rα, c-myc, and pim-1 in activated human T cells (55). As one possible explanation for the different outcomes, type I IFN signaling pathways have been proposed to change with T cell activation.
Type I IFN predominantly signals through the JAK-STAT pathway. Receptor occupancy leads to the juxtaposition of TYK2 on IFNAR1 and JAK1 on IFNAR2. The subsequent phosphorylation events recruits and phosphorylates STAT1, 3 and 5, and dependent on the cell type, also STAT4 and 6 (56–58). The classical pathway signals through STAT1/2 heterodimers that in association with IRF9 bind to ISRE eliciting a predominantly anti-proliferative effect. In mice, T cells after activation switch from the anti-proliferative STAT1 signal to STAT4 phosphorylation that is required for IFN-γ induction, while STAT3 and STAT5 provide anti-apoptotic signals (59–61). Such a switch was not observed in our studies with human naïve CD4 T cells. On the contrary, TCR stimulation induced the expression of STAT1 and STAT5 disproportionately to SOCS1 and sensitized activated T cells to respond to type I IFN stimulation with increased STAT1 and STAT5 phosphorylation while STAT3 and STAT4 did not change. Gene expression arrays of activated T cells after type I IFN stimulation demonstrated the expression of ISRE responsive genes indicative of the STAT1/2 pathway; quantification of selected ISRE-responsive genes such as IFIT1 by qPCR in activated vs unstimulated T cells confirmed this increased responsiveness.
The activation-induced sensitization of the STAT1 and STAT5 pathways to type I IFN stimuli was subdued in elderly naïve CD4 T cells. While type I IFN-induced STAT1 and STAT5 phosphorylation in unstimulated CD4 T cells was not influenced by age, naïve CD4 T cells from older individuals did not exhibit an increased sensitivity several days after activation to the same extent that was typical for CD4 T cell from young adults. Our data are consistent with the notion that activation of young and less so elderly T cells is associated with a sensitization to type I IFN signaling through the classical pathways without that switches in pathway usage contribute to the observed differences.
Type I IFN signaling is controlled at several levels. Receptor expression did not change with age. T cell activation induced transcription of STAT1 and STAT5 which may explain the increased IFN responsiveness; however, no age-associated difference was seen. Various phosphatases can dephosphorylate JAK kinases (62–65). SOCSs inhibit STAT activation proximally through several mechanisms (66–69). We found SOCS1 and 3 to be induced by activation, but not to higher degree in T cells from older individuals. PIAS proteins function as more distal STAT inactivators (70, 71). Again, no age-dependent difference in PIAS transcription was seen. Immunoprecipitation experiments identified age-associated difference in the presence of SHP-1 in the signaling complex. SHP-1 has been shown to be an important regulator of type I IFN signaling, presumably by dephosphorylating JAK1 and not TYK2 (62). SHP-1, generally binding with its SH2 domain to phosphotyrosines in cell surface receptors, can directly bind to JAK1 (65). Consistent with observations by David et al in mouse macrophages (62), we found SHP-1 to be present in the IFN receptor complex of activated CD4 T cells before IFN-α stimulation; upon IFN binding it was rapidly excluded. A SHP-1–mediated dephosphorylating activity on JAK kinases would explain that both STAT1 and STAT5 phosphorylation were reduced in activated naïve CD4 T cells from older individuals. The cause for the increased receptor recruitment of SHP-1 remains to be elucidated.
Type I IFN stimulation of activated T cells has a plethora of functional consequences that are important for effector cell differentiation and memory formation (72). We did not observe increased anti-proliferative effects of STAT1/2 signaling in the young as one might have suspected from the increased STAT1 signaling. Type I IFN are known to function as a survival factor for activated T cells which may be important to counteract clonal downsizing and favor memory cell survival (38). Conversely, type I IFN may also deliver pro-apoptotic signals, possibly through the induction of FasL or TRAIL (73–76). In our system, we demonstrated an anti-apoptotic effect of type I IFN on activated T cells in vitro that was reduced in older adults, consistent with impaired IFN signaling. Perhaps more importantly, we find that type I IFN stimulation enables activated CD4 T cells to produce IL-2 upon restimulation. Again, this effect was seen more in young than in older CD4 T cells. Increased IL-2 production would not only contribute to the survival of activated CD4 T cells themselves but also support the clonal expansion of antigen-specific CD8 T cells. Finally, type I IFNs are important to retain activated T cells in lymphoid tissues by upregulating and sustaining CD69 expression that inhibits sphingosine 1-phosphate receptor-1 function (40). Indeed, type I IFN was less able to induce CD69 expression in activated naïve CD4 T cells from older than young adults. Since sustained CD69 expression will delay lymphocyte egress from lymphoid tissue and thereby promote T cell differentiation and lymphoid follicle formation, this defect could greatly contribute to the impaired adaptive immunity in older individuals.
T cell responsiveness to type I IFN, commonly produced in viral and bacterial infections, is known to be pivotal for the generation of adaptive immune responses. Our findings identify a defect in the IFN signaling pathway that may contribute to reduced T cell responses. The increased threshold to IFN stimulation of activated naïve CD4 T cells renders older individuals more resistant to the activity of this cytokine. Increased type I IFN stimulation may be able to overcome this defect, but needs to be carefully timed. Based on our data, type I IFN responses are needed in T cells several days after the initial antigenic stimulation, while type I IFN in the early stages of T cell activation will induce the activation of negative feedback loops and be likely counterproductive.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health grants U19 AI090019, R01 AG015043, U19 AI057266 and R01 AI108891 (JJG), and R01 AI044142, R01 AR042527 and P01 HL058000 (CMW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no conflict of interest.
References
- 1.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–189. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 3.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikolich-Zugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T Cell Maintenance and Function in Human Aging. J Immunol. 2015;194:4073–4080. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Nagata N, Iwata N, Hasegawa H, Fukushi S, Harashima A, Sato Y, Saijo M, Taguchi F, Morikawa S, Sata T. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am J Pathol. 2008;172:1625–1637. doi: 10.2353/ajpath.2008.071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206:2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jean CM, Honarmand S, Louie JK, Glaser CA. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg Infect Dis. 2007;13:1918–1920. doi: 10.3201/eid1312.061265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 14.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–3069. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Boraschi D, Aguado MT, Dutel C, Goronzy J, Louis J, Grubeck-Loebenstein B, Rappuoli R, Del Giudice G. The gracefully aging immune system. Sci Transl Med. 2013;5:185ps188. doi: 10.1126/scitranslmed.3005624. [DOI] [PubMed] [Google Scholar]
- 16.Duraisingham SS, Rouphael N, Cavanagh MM, Nakaya HI, Goronzy JJ, Pulendran B. Systems biology of vaccination in the elderly. Curr Top Microbiol Immunol. 2013;363:117–142. doi: 10.1007/82_2012_250. [DOI] [PubMed] [Google Scholar]
- 17.Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, Rappuoli R. Influenza vaccine immunology. Immunol Rev. 2011;239:167–177. doi: 10.1111/j.1600-065X.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 18.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi Q, Zhang DW, Weyand CM, Goronzy JJ. Mechanisms shaping the naive T cell repertoire in the elderly - thymic involution or peripheral homeostatic proliferation? Exp Gerontol. 2014;54:71–74. doi: 10.1016/j.exger.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 21.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 22.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Lewinsohn DA, Nikolich-Zugich J. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee J-Y, Olshen RA, Weyand CM, Goronzy JJ. Diversity and clonal selection in the human T cell repertoire. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1409155111. Epub August 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson PL, Yates AJ, Goronzy JJ, Antia R. Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc Natl Acad Sci U S A. 2012;109:21432–21437. doi: 10.1073/pnas.1209283110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes L, Lefebvre JS. Age-related Deficiencies in Antigen-Specific CD4 T cell Responses: Lessons from Mouse Models. Aging Dis. 2011;2:374–381. [PMC free article] [PubMed] [Google Scholar]
- 28.Haynes L, Swain SL. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin Immunol. 2012;24:350–355. doi: 10.1016/j.smim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Brahmakshatriya V, Swain SL. CD4 T cell defects in the aged: causes, consequences and strategies to circumvent. Exp Gerontol. 2014;54:67–70. doi: 10.1016/j.exger.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Sharpless NE. Tumor suppressor mechanisms in immune aging. Curr opin immunol. 2009;21:431–439. doi: 10.1016/j.coi.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee WW, Cui D, Czesnikiewicz-Guzik M, Vencio RZ, Shmulevich I, Aderem A, Weyand CM, Goronzy JJ. Age-dependent signature of metallothionein expression in primary CD4 T cell responses is due to sustained zinc signaling. Rejuvenation Res. 2008;11:1001–1011. doi: 10.1089/rej.2008.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez I, Marx F, Gould EA, Grubeck-Loebenstein B. T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. Exp Gerontol. 2004;39:597–605. doi: 10.1016/j.exger.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu M, Li G, Lee WW, Yuan M, Cui D, Weyand CM, Goronzy JJ. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A. 2012;109:E879–888. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 36.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8:907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 38.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lombardi G, Dunne PJ, Scheel-Toellner D, Sanyal T, Pilling D, Taams LS, Life P, Lord JM, Salmon M, Akbar AN. Type 1 IFN maintains the survival of anergic CD4+ T cells. J Immunol. 2000;165:3782–3789. doi: 10.4049/jimmunol.165.7.3782. [DOI] [PubMed] [Google Scholar]
- 40.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 41.Oviedo-Orta E, Li CK, Rappuoli R. Perspectives on vaccine development for the elderly. Curr Opin Immunol. 2013;25:529–534. doi: 10.1016/j.coi.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, Golding H. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2:15ra15. doi: 10.1126/scitranslmed.3000624. [DOI] [PubMed] [Google Scholar]
- 43.Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, Brauer V, Banzhoff A, Rappuoli R, Del Giudice G, Castellino F. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A. 2009;106:3877–3882. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, Sigurdardottir B, Hoeper A, Graham IL, Edelman R, He F, Nino D, Capellan J, Ruben FL. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–7663. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 46.Belardelli F, Gresser I. The neglected role of type I interferon in the T-cell response: implications for its clinical use. Immunol Today. 1996;17:369–372. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 47.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 49.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 50.Bracci L, La Sorsa V, Belardelli F, Proietti E. Type I interferons as vaccine adjuvants against infectious diseases and cancer. Expert Rev Vaccines. 2008;7:373–381. doi: 10.1586/14760584.7.3.373. [DOI] [PubMed] [Google Scholar]
- 51.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 53.Dondi E, Rogge L, Lutfalla G, Uze G, Pellegrini S. Down-modulation of responses to type I IFN upon T cell activation. J Immunol. 2003;170:749–756. doi: 10.4049/jimmunol.170.2.749. [DOI] [PubMed] [Google Scholar]
- 54.Zella D, Romerio F, Curreli S, Secchiero P, Cicala C, Zagury D, Gallo RC. IFN-alpha 2b reduces IL-2 production and IL-2 receptor function in primary CD4+ T cells. J Immunol. 2000;164:2296–2302. doi: 10.4049/jimmunol.164.5.2296. [DOI] [PubMed] [Google Scholar]
- 55.Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, Julkunen I. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood. 1999;93:1980–1991. [PubMed] [Google Scholar]
- 56.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rauch I, Muller M, Decker T. The regulation of inflammation by interferons and their STATs. JAKSTAT. 2013;2:e23820. doi: 10.4161/jkst.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 60.Tanabe Y, Nishibori T, Su L, Arduini RM, Baker DP, David M. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol. 2005;174:609–613. doi: 10.4049/jimmunol.174.2.609. [DOI] [PubMed] [Google Scholar]
- 61.Kallal LE, Biron CA. Changing partners at the dance: Variations in STAT concentrations for shaping cytokine function and immune responses to viral infections. JAKSTAT. 2013;2:e23504. doi: 10.4161/jkst.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.David M, Chen HE, Goelz S, Larner AC, Neel BG. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995;15:7050–7058. doi: 10.1128/mcb.15.12.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You M, Yu DH, Feng GS. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol. 1999;19:2416–2424. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du Z, Shen Y, Yang W, Mecklenbrauker I, Neel BG, Ivashkiv LB. Inhibition of IFN-alpha signaling by a PKC- and protein tyrosine phosphatase SHP-2-dependent pathway. Proc Natl Acad Sci U S A. 2005;102:10267–10272. doi: 10.1073/pnas.0408854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279:821–824. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 67.Zimmerer JM, Lesinski GB, Kondadasula SV, Karpa VI, Lehman A, Raychaudhury A, Becknell B, Carson WE., 3rd IFN-alpha-induced signal transduction, gene expression, and antitumor activity of immune effector cells are negatively regulated by suppressor of cytokine signaling proteins. J Immunol. 2007;178:4832–4845. doi: 10.4049/jimmunol.178.8.4832. [DOI] [PubMed] [Google Scholar]
- 68.Dimitriou ID, Clemenza L, Scotter AJ, Chen G, Guerra FM, Rottapel R. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol Rev. 2008;224:265–283. doi: 10.1111/j.1600-065X.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 69.Piganis RA, De Weerd NA, Gould JA, Schindler CW, Mansell A, Nicholson SE, Hertzog PJ. Suppressor of cytokine signaling (SOCS) 1 inhibits type I interferon (IFN) signaling via the interferon alpha receptor (IFNAR1)-associated tyrosine kinase Tyk2. J Biol Chem. 2011;286:33811–33818. doi: 10.1074/jbc.M111.270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ungureanu D, Vanhatupa S, Kotaja N, Yang J, Aittomaki S, Janne OA, Palvimo JJ, Silvennoinen O. PIAS proteins promote SUMO-1 conjugation to STAT1. Blood. 2003;102:3311–3313. doi: 10.1182/blood-2002-12-3816. [DOI] [PubMed] [Google Scholar]
- 71.Liu B, Mink S, Wong KA, Stein N, Getman C, Dempsey PW, Wu H, Shuai K. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat Immunol. 2004;5:891–898. doi: 10.1038/ni1104. [DOI] [PubMed] [Google Scholar]
- 72.Tough DF. Modulation of T-cell function by type I interferon. Immunol Cell Biol. 2012;90:492–497. doi: 10.1038/icb.2012.7. [DOI] [PubMed] [Google Scholar]
- 73.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oshima K, Yanase N, Ibukiyama C, Yamashina A, Kayagaki N, Yagita H, Mizuguchi J. Involvement of TRAIL/TRAIL-R interaction in IFN-alpha-induced apoptosis of Daudi B lymphoma cells. Cytokine. 2001;14:193–201. doi: 10.1006/cyto.2001.0873. [DOI] [PubMed] [Google Scholar]
- 75.Takada E, Shimo K, Hata K, Abiake M, Mukai Y, Moriyama M, Heasley L, Mizuguchi J. Interferon-beta-induced activation of c-Jun NH2-terminal kinase mediates apoptosis through up-regulation of CD95 in CH31 B lymphoma cells. Exp Cell Res. 2005;304:518–530. doi: 10.1016/j.yexcr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 76.Fraietta JA, Mueller YM, Yang G, Boesteanu AC, Gracias DT, Do DH, Hope JL, Kathuria N, McGettigan SE, Lewis MG, Giavedoni LD, Jacobson JM, Katsikis PD. Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection. PLoS Pathog. 2013;9:e1003658. doi: 10.1371/journal.ppat.1003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.