Abstract
Non-live vaccine platforms that induce potent cellular immune responses in mucosal tissue would have broad application for vaccines against infectious diseases and tumors. Induction of cellular immunity could be optimized by targeted activation of multiple innate and co-stimulatory signaling pathways, such as CD40 or toll-like receptors (TLRs). In this study, we evaluated immune activation and elicitation of T cell responses in non-human primates (NHPs) after immunization with peptide antigens adjuvanted with an agonistic αCD40Ab, with or without the TLR3 ligand poly IC:LC. We found that intravenous administration of the αCD40Ab induced rapid and transient innate activation characterized by IL-12 production and upregulated co-stimulatory and lymph node homing molecules on dendritic cells. Using fluorescently-labeled Abs for in vivo tracking, the αCD40Ab bound to all leucocytes, except T cells, and disseminated to multiple organs. CD4+ and CD8+ T cell responses were significantly enhanced when the αCD40Ab was co-administered with poly IC:LC compared to either adjuvant given alone and were almost exclusively compartmentalized to the lung. Notably, antigen-specific T cells in the bronchoalveolar lavage were sustained at ~5–10%. These data indicate that systemic administration of αCD40Ab may be particularly advantageous for vaccines and/or therapies requiring T cell immunity in the lung.
Keywords: adjuvant, CD40, dendritic cell, rhesus macaque, lung
Introduction
Most vaccines currently available primarily elicit antibody (Ab)-based protection. In contrast, vaccines for infections such as HIV-1/AIDS, tuberculosis, malaria, as well as therapeutic cancer vaccines will likely require potent cellular immune responses, with or without humoral responses. Immune stimulatory vaccine adjuvants provide a powerful approach to modulate the type of adaptive response a vaccine elicits by utilizing early innate immune activation. Adjuvants can stimulate vaccine responses by targeting antigen presenting cells (APCs) through multiple pathways, including engaging toll-like receptors (TLRs) or other activating cell surface receptors, such as CD40. CD40 is a major regulatory receptor in the TNF-R family widely expressed on APCs, including dendritic cells (DCs), monocytes, and B cells. CD40Ligand (CD40L; CD154) is expressed on activated T cells and CD40 ligation on DCs increases co-stimulatory molecule expression required for stimulation of T cell responses (1, 2). The importance of this pathway is highlighted by the fact that antigen presentation in the absence of CD40 can lead to immune tolerance (3). By contrast, CD40 activation promotes production of IL-12 and the co-stimulatory function of APCs, which enhances both CD4+/Th1 and CD8+ T cell immunity (4). In mouse models, CD40 stimulation can be delivered to DCs without T cell engagement by administering agonistic anti-CD40 antibodies (αCD40Abs), soluble CD40L or an adenovirus vector expressing CD40L, all of which have been shown to enhance CD8+ T cell responses (1, 5, 6).
In addition to targeting CD40, TLR ligands are effective activators of innate immunity and critical for optimizing T cell responses. In our previous work, we demonstrated that combining CD40 activation with TLR ligands synergistically enhanced cellular immunity in mice, with increased CD4+ and CD8+ T cell responses >10-fold compared to either adjuvant alone (7–9). T cell responses with this vaccine regimen were shown to have anti-tumor effects in mice, as assessed by tumor reduction (10–12). Poly IC:LC for a combinatorial TLR ligand is particularly promising as it is already tested in clinical cancer immunotherapy and can target multiple innate pathways: TLR3 in DCs and MDA-5 in stromal cells, inducing production of IL-12 and type I IFN, respectively. Mouse models have shown extensive evidence for potential clinical use of CD40 Abs in combination with TLR ligands as a versatile vaccine adjuvant to induce robust adaptive immunity.
The partially agonistic αCD40Ab clone SGN-40 or huS2C6, dacetuzumab, was tested in patients with chronic lymphocytic leukemia and non-Hodgkin’s lymphoma and showed it was well-tolerated, although none of the patients achieved an objective response (13, 14). Further, the agonistic αCD40Ab, clone CP-870,893, was tested in patients with advanced solid tumors and showed that a single dose can confer antitumor activity (15–17) but improved dosing intervals for optimal immune pharmacodynamics and clinical effect are required. These results highlight the need for an improved understanding of the mechanisms by which this class of Abs mediate their effects in vivo to facilitate the use of αCD40Ab as an adjuvant for inducing T cell immunity in humans.
Therefore in this study, we investigated the adjuvanticity of a novel human agonistic αCD40Ab (clone 341G2) together with poly IC:LC in non-human primates (NHPs). NHPs provide a more predictable model than mice for how immunomodulation can be achieved in humans based on their greater similarity in immune cell subsets, TLR distribution amongst APCs with humans and their outbred nature. Moreover, the ability to obtain multiple tissues from NHPs facilitates an extensive characterization of the innate and adaptive immune responses mediated by human αCD40Abs, not possible in clinical trials, which are aspects that may be critical for protection against infection or tumors.
Materials and Methods
Sample material
Approval for this animal study was granted by the Animal Care and Use Committees of the Vaccine Research Center, National Institutes of Health (NIH). Indian rhesus macaques were housed at Bioqual and handled according to the standards of the American Association for the Accreditation of Laboratory Animal Care. Human PBMCs were obtained from individuals participating in the NIH research apheresis program. Signed informed consent was obtained in accordance with the Declaration of Helsinki and approved by the relevant Institutional Review Board.
Human CD40 Ab screening
A variety of human αCD40Ab clones, including well known and novel sequences, were screened for their ability to induce DC activation and B cell proliferation in both human and rhesus macaque PBMCs in vitro (Figure S1A–C). The highest cell activation was found by the clone 341G2, which was designed based on the sequence developed by Kyowa Hakko Kirin Co., Ltd., Tokyo JP (18). The clone was therefore chosen to investigate potential synergy of CD40 and TLR signaling in vivo.
Immunizations
All doses used for immunizations were based on prior dose-response in vivo experiments and previously published ranges (14, 19, 20). For innate activity, rhesus macaques received intravenous administration of 1mg/kg αCD40Ab (clone 341G2 IgG2), 1mg poly IC:LC (Oncovir, Washington, DC) or the combination of the two. For Ab tracking studies, αCD40Ab or isotype control Ab (human IgG2 DNP) was first conjugated to Alexa680 according to manufacturer’s protocol (Molecular Probes, Carlsbad, CA, USA). The conjugated Ab was then treated with Triton X-114 to remove residual endotoxin and was validated at <0.1 endotoxin units with an Endpoint Chromogenic LAL Assay (Lonza, Basel, Switzerland), as has been performed for prior studies (21, 22). The Env peptides (Biomatik, Wilmington, DE) were resuspended to 50mg/ml in 30% DMSO prior to immunization. 1.5mg/kg Ax680 conjugated Ab was mixed with 1mg poly IC:LC immediately prior to immunization. The formulation was delivered i.v. and was immediately followed with 1mg/kg Env peptides delivered i.v. for immunogenicity studies, animals were immunized with 1.5mg/kg αCD40Ab, 1mg poly IC:LC and/or 4–8mg/kg Env peptide pool (as indicated in Figure S2A and 4A), all delivered i.v. as previously described. Control animals received intramuscular rAd5 HIV-1 Gag (1x1010 PU). Complete blood counts and liver function tests were performed 48 hours after the immunization (Idexx, Westbrook, ME) (Figure S1D). Animals were first boosted with 1mg poly IC:LC and 1mg/kg Env peptides or rAd5 HIV-1 Gag (1×1010 PU) and where indicated received a second boost of 1.5mg/kg αCD40Ab and 1mg/kg Env peptides. It should be noted that endogenous Abs against the administered αCD40Ab were not detected until after the second immunization with αCD40Ab (data not shown).
Rhesus tissue and blood sample processing
Blood PBMCs were isolated using a density-gradient with Ficoll-Paque (GE Healthcare, Fairfield, CT) according to standard procedures. All tissue samples were collected at euthanasia and processed to a single cell suspension following standard protocol. Briefly; LNs, spleen, and liver samples were manually disrupted and filtered through a 70um cell strainer. Liver samples were further purified with Ficoll-Paque. Lung and gut tissues were digested with collagenase treatment for 30 minutes and mechanically disrupted using the gentle MACS Dissociator (Miltenyi, Auburn, CA). PBMCs and single cell suspensions were washed and maintained in complete media (R10; RPMI 1640/10% FCS/100 U penicillin/0.1 mg streptomycin; Sigma-Aldrich, St. Louis, MO), or frozen in 90% heat-inactivated FBS and 10% DMSO (Sigma-Aldrich, St. Louis, MO) and stored in liquid nitrogen. Experiments were performed on both fresh and frozen cells.
Cytokine secretion analysis
Rhesus serum samples were evaluated for IL-12 p40/p70 and IFNγ levels using NHP ELISA kits (Invitrogen). All assays were performed according to manufacturer’s protocol.
Phenotypic analysis
For innate studies and Ab dissemination studies, 6–24 hours following immunization, 5×106 cells were stained with LIVE/DEAD Fixable Aqua Dead Cell kit according to manufacturers protocol (Invitrogen) and blocked with FcR-blocking reagent (Miltenyi). Samples were then surfaced stained with a panel of fluorescently labeled Abs (Supplemental Table I) to determine cell distribution and maturation.
Antigen recall assay
For assessment of Ag-specific cytokine production, PBMCs or single cell suspensions for tissues were restimulated in vitro. 1.5×106 cells were cultured in 200ul of R10 per stimulation in a 96 well plate. Samples were stimulated as previously described (23) with 2ug/ml of 9–13mer overlapping HIV-1 Env peptides (matched to immunization) in the presence of 10µg/ml brefeldin A (Sigma, St Louis, MO) overnight. Samples were stained the following morning to evaluate IFNγ, IL-2, TNF production. Cells were first stained with LIVE/DEAD Fixable AquaBlue viability dye then surface stained and intracellularly stained (Supplemental Table I).
Multiparameter flow cytometry
Samples were resuspended in 1% paraformaldehyde before acquisition using a modified LSRII flow cytometer (BD Biosciences). Results were analyzed using FlowJo version 9.7.5, Pestle version 1.7 and Spice version 5.3. Background cytokine staining was subtracted, as defined by staining in samples incubated without peptide. Where indicated, numbers of specific cell subsets are normalized to 1×105 viable cells (percent of gated cell subset out of AquaBlue low cells multiplied by 105).
CD40 stimulation
Human MDDCs were prepared as previously described (24) and cultured overnight at 1x106 cells/ml in R10 with 5ug/ml αCD40Ab clones, 5ug/ml isotype control Ab (human IgG2 DNP) or 5ug/ml poly I:C (Invitrogen). Supernatants were saved for cytokine analysis and cells were stained for maturation markers CD70 and CD80.
Results
Dynamics and activation of leucocytes after αCD40Ab administration
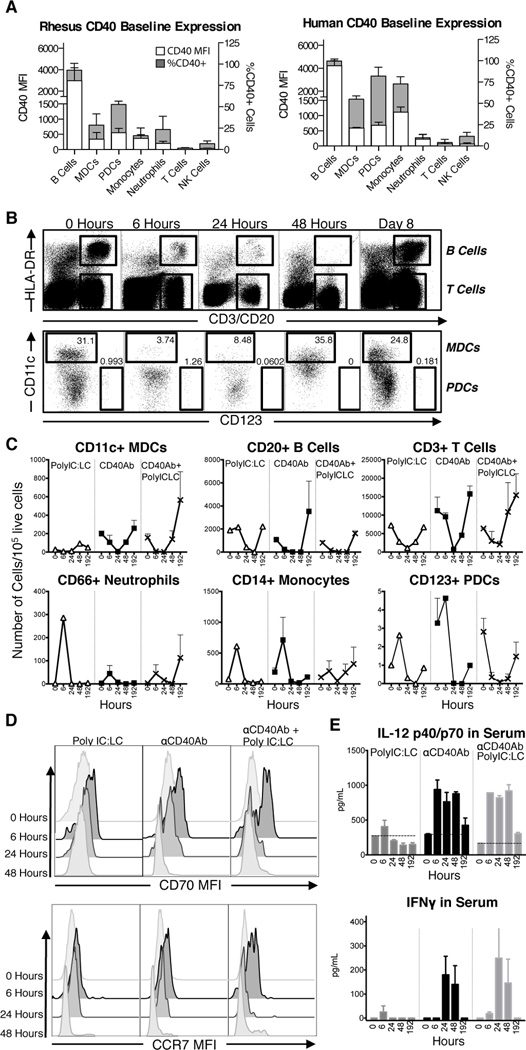
We first determined the level of CD40 expression on different leucocyte populations in blood of rhesus macaques. As expected, B cells showed the highest expression of CD40 compared to the other cell populations (Figure 1A). DC subsets, monocytes, and neutrophils expressed moderate levels of CD40, while NK cells and T cells did not have detectable CD40 expression. To highlight the similarity with humans, CD40 expression was assessed in the same cell populations in human PBMCs and showed a similar hierarchy of expression (Figure 1A). We thereafter determined the early innate activity of an αCD40Ab in vivo by evaluating how its administration affected mobilization of circulating cell populations and their activation. Human αCD40Ab clones were first screened in vitro for cell activation to select an agonistic clone suitable for in vivo investigation (Figure S1A–C). In addition to αCD40Ab, the adjuvant poly IC:LC was also used in these studies based on its potency for inducing T cell immunity.
Figure 1. In vivo innate activity of agonistic CD40 Ab.
(A) Baseline levels of CD40 expression were determined for rhesus macaque and human PBMC subsets by CD40 MFI with FMO levels subtracted (white bars) and percent of CD40+ cells (grey bars), n=3. Refer to Figure 2A for cell subset gating. (B–C) Animals were split into 3 groups (n=2/group) and immunized i.v. with αCD40Ab (1mg/kg), poly IC:LC (1mg) or the combination. Circulating PBMCs were monitored for innate activity by flow cytometry. (B) Representative flow cytometry plots from an animal receiving αCD40Ab showing rapid and transient decline of blood leucocytes, including B cells, T cells and DCs. (C) Time course (0–48 hours and day 8) for each cell type as number of cells per 105 viable cells (Mean±SEM, n=2). (D) Histograms show expression of differentiation makers CD70 and CCR7 on MDCs remaining in the blood at time points 0, 6, 24 and 48 hours. Maturation peaks at 6 hours post administration. (E) Bar graphs show serum cytokines IL-12p40/p70 and IFNγ after administration (Mean±SEM, n=2)
Animals received either intravenous (i.v.) αCD40Ab alone, αCD40Ab in combination with poly IC:LC, or poly IC:LC alone. Blood leucocytes were monitored at 6, 24, 48 hrs and 8 days (192 hrs) for cell frequencies and phenotypes using multiparameter flow cytometry. Following immunization, leucocytes quickly mobilized from the blood in all three groups (Figure 1B). There was a rapid decline of DCs, B cells, and T cells, but all cells returned to baseline levels by day 8 in all groups (Figure 1C). Monocytes and neutrophils and in some cases plasmacytoid DCs (PDCs) appeared to first increase at 6 hours post administration, before declining and returning to baseline levels (Figure 1C). As there was a transient decline of all cell populations regardless of adjuvant group, this is unlikely dependent on Ab interaction with the CD40 receptor on the cells, and may instead be an effect of systemic immune stimulation.
The DCs remaining in circulation, particularly at 6 hours post-administration, exhibited phenotypic differentiation associated with upregulated CD70 and CCR7 expression. CD11c+ myeloid DCs (MDCs) maturation was most pronounced in the αCD40Ab/poly IC:LC group (Figure 1D). We have previously shown that CD70, a co-stimulatory receptor on APCs that binds to CD27 on T cells (8, 25–27), was required for enhancement of CD4+ and CD8+ T cell responses in mice following immunization with αCD40Ab in combination with TLR agonists (8, 9, 28). In our NHP model, administration of αCD40Ab alone also induced high levels of IL-12p40/p70 in the serum at 6–48 hrs, which returned back to baseline by day 8 (Figure 1E). There was minimal IL-12 induced by poly IC:LC alone and there was no notable synergy of αCD40Ab and poly IC:LC. IFNγ was also detected in the serum, peaking at 24 hours post administration of αCD40Ab, suggesting that early IL-12 may augment subsequent IFNγ responses (Figure 1E). Finally, consistent with the in vivo observations, both human and NHP DCs produced IL-12 when exposed to αCD40Ab in vitro and upregulated CD70 and other co-stimulatory molecules such as CD80 on DCs (Figure S1A–B). In vitro exposure also induced proliferation of B cells (Figure S1C). Altogether, these data demonstrate that αCD40Ab alone induces robust innate immune activation in terms of cell mobilization, differentiation and Th1-type cytokine production.
Systemic detection of αCD40Ab after i.v. administration
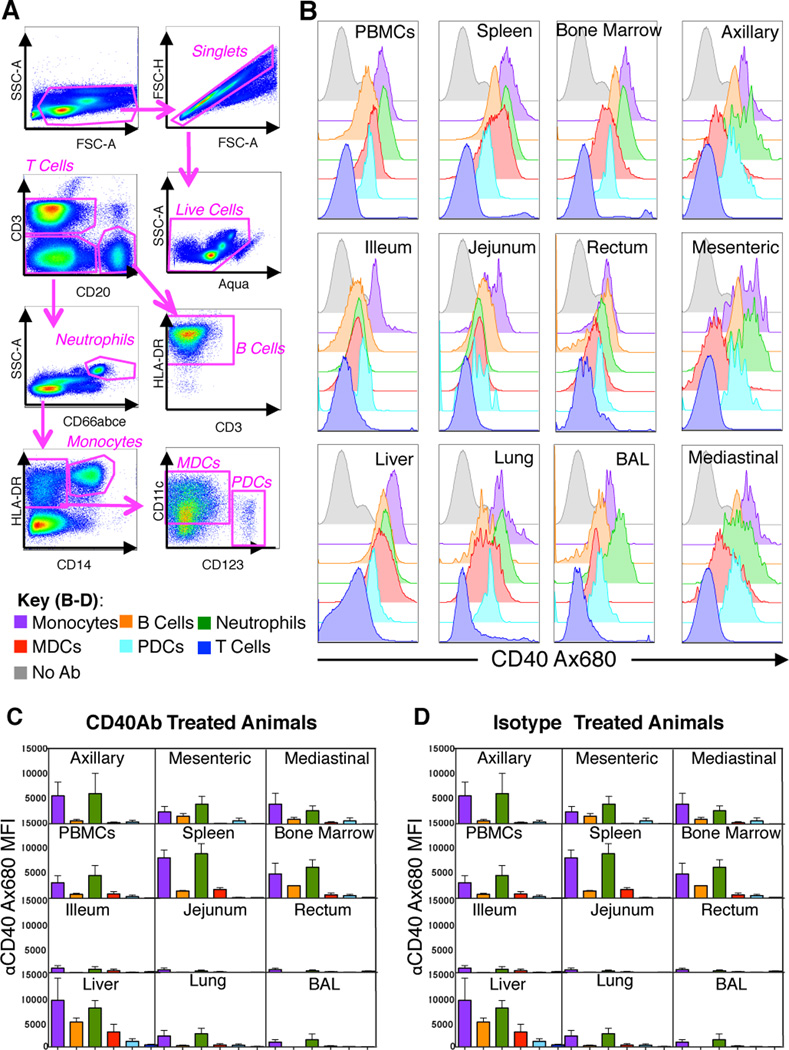
To investigate cell and organ specific targeting of the αCD40Ab following i.v. administration, the αCD40Ab was labeled with the fluorescent dye Alexa680 to track its dissemination in vivo. To mimic the conditions used in the synergistic adjuvant model reported in mice, the labeled Ab was delivered with poly IC:LC and antigen (synthetic long peptides; SLPs) (10, 12). Using flow cytometric analysis, we assessed the median fluorescence intensity (MFI) of Alexa680 at necropsy 24 hrs after vaccine administration (Figure 2A). Labeled αCD40Ab was readily detected in multiple organs and on several cell subsets at levels above background, defined by animals that received unlabeled αCD40Ab (Figure 2B). Thus, αCD40Ab distributed systemically after i.v. administration and did not target a specific organ. The highest Ab signal above background was detected in the spleen and liver (Figure 2C), consistent with the typical pharmacodynamics of i.v. delivery and the large number of immune cells, which express CD40, in the spleen. αCD40Ab binding and uptake was evident in B cells, DCs, monocytes and neutrophils (Figure 2B–C). Although neutrophils have modest expression of CD40 at steady state, they exhibited the highest binding of αCD40Ab, which suggests they may also bind Ab via Fc receptors. In contrast, T cells express neither CD40 nor Fc receptors and did not appreciably bind αCD40Ab. We performed similar experiments tracking the dissemination of an Alexa680 labeled IgG2 isotype control Ab. Several cell subsets and organs showed detectable levels of isotype Ab, although at much lower levels than the αCD40Ab. This suggests that CD40-specific interactions are responsible for the majority of αCD40Ab binding in vivo (Figure 2D).
Figure 2. In vivo dissemination of αCD40Ab after i.v. administration.
The αCD40Ab or IgG isotype control Ab were conjugated to Alexa680 and administered i.v. with poly IC:LC and a pool of HIV-1 Env peptides to rhesus macaques to determine Ab dissemination. Blood and tissues were harvested at 24 hours and evaluated by flow cytometry for alexa680 signal on a variety of cell subsets. (A) Gating scheme used to analyze cell specific Alexa680 signal. (B) Representative plots of Alexa680 signal from different cell populations, as indicated in the key, from one αCD40Ab treated animal compared to PBMCs from an unexposed animal (grey). Bar graphs show (C) αCD40Ab Alexa680 MFI on different cell subsets organized by tissue (Mean±SEM, n=3) and (D) Isotype Ab Alexa680 MFI on different cell subsets organized by tissue (Mean±SEM, n=2)
In vivo cell targeting and dendritic cell maturation after αCD40Ab administration
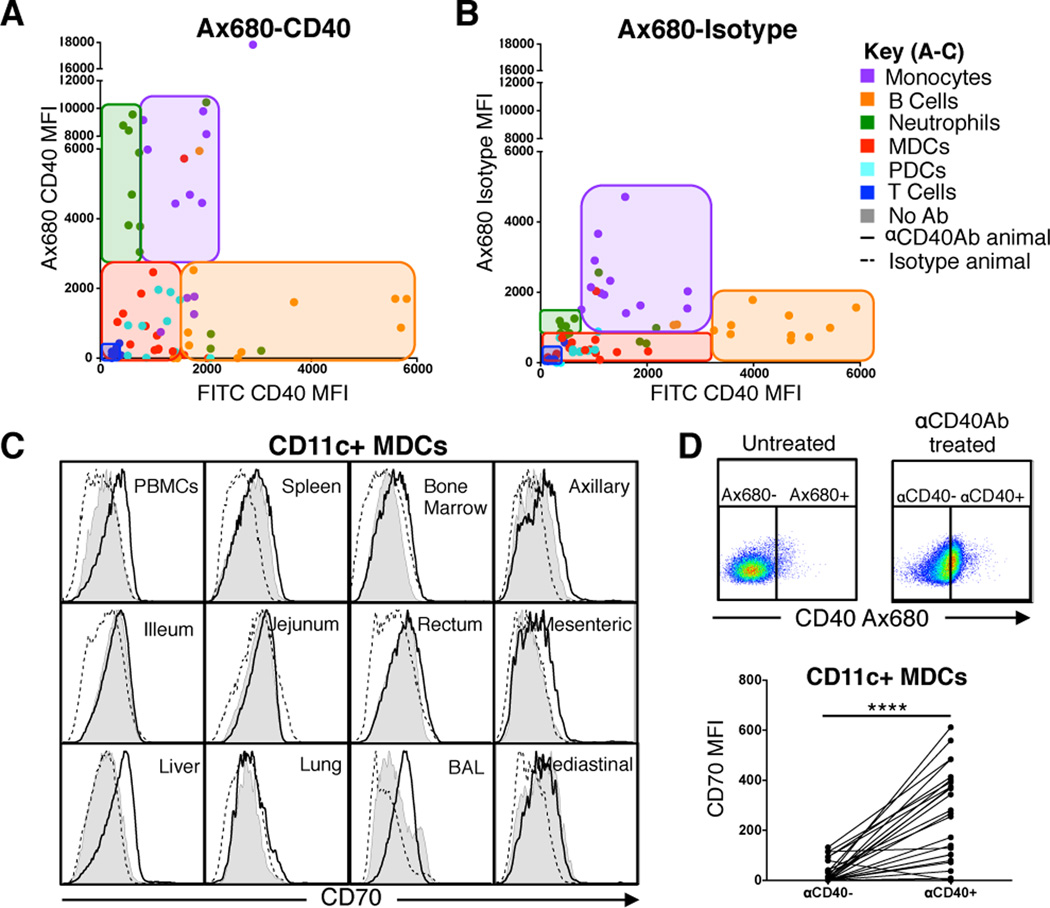
As described above (Figure 1A), the level of CD40 expression between cell types in circulation differs substantially, with B cells having the highest CD40 expression. Consistent with this observation, we found that tissue-resident B cells also expressed higher CD40 levels compared to other tissue-matched leucocytes. Despite B cells showing the highest expression of CD40, the Alexa680-labeled αCD40Ab did not preferentially target them. To demonstrate this, we correlated the MFI of CD40 expression, using the 5C3 clone as a staining CD40 Ab, with the cell matched MFI of Alexa680-labeled αCD40Ab (Figure 3A). In general, the leucocytes displayed the same hierarchy of CD40 expression regardless of the tissue they were derived from, but CD40 expression did not consistently correlate with αCD40Ab binding. The Alexa680-labeled αCD40Ab showed the highest signal on neutrophils and monocytes, despite their moderate to low CD40 expression. The same analysis using the labeled isotype control Ab showed a striking reduction in binding to neutrophils and a modest reduction in binding to monocytes and B cells (Figure 3B). Thus, expression level of CD40 on cells or in tissues did not dictate their level of binding/uptake of the Ab, but it should be noted that cells without CD40 expression, Fc receptors or endocytic activity, such as T cells, did not show any evidence of αCD40Ab binding.
Figure 3. αCD40Ab targeting is not dependent on CD40 surface expression but correlates with DC maturation.
(A–B) Cell suspensions from tissues of αCD40Ab or isotype Ab immunized animals were stained for CD40 surface expression to correlate the level of CD40 expression (CD40 FITC) to the level of delivered Ab signal (bound or internalized, Alexa680) for each cell subset. Data points are colored based on cell subset for all tissues according to key. Cell subsets were found to group together in the plot regardless of tissue location and are outlined with a tinted box (C) Representative plots of CD11c+ MDCs from indicated tissues from animals that were immunized with αCD40Ab (black line), isotype control (dashed line) or not treated (filled grey) were stained for CD70 expression 24 hours after administration. (D) CD11c+ MDCs from different tissues were gated on Alexa680 CD40 positive and negative cells, based off of signal from an untreated animal. MDCs exposed to CD40 compared to tissue and animal matched αCD40Ab- MDCs had higher expression of CD70 (normalized to tissue type from untreated animal). Statistical differences tested using paired t test ****p ≤ 0.0001.
In this analysis, we also evaluated phenotypic maturation of DCs in multiple tissues. Again we focused on upregulation of CD70 due to its importance in T cell activation with αCD40Ab. The expression of CD70 on CD11c+ MDCs was compared between animals that had received αCD40Ab, the isotype control Ab or untreated animals (Figure 3C). A modest upregulation of CD70 was found on MDCs in several tissues from the animals receiving αCD40Ab, but a striking increase in CD70 expression was found on MDCs in PBMCs, the liver and BAL. When evaluating only MDCs that showed associated αCD40Ab (Alexa680+), we found that they had significantly higher CD70 expression than the tissue matched Alexa680-negative MDCs (p>0.0001) (Figure 3D). Thus, although baseline levels of CD40 expression did not dictate the level of Ab binding, direct interaction with αCD40Ab led to maturation and upregulation of CD70.
Synergy between αCD40Ab and poly IC:LC as adjuvants for T cell responses
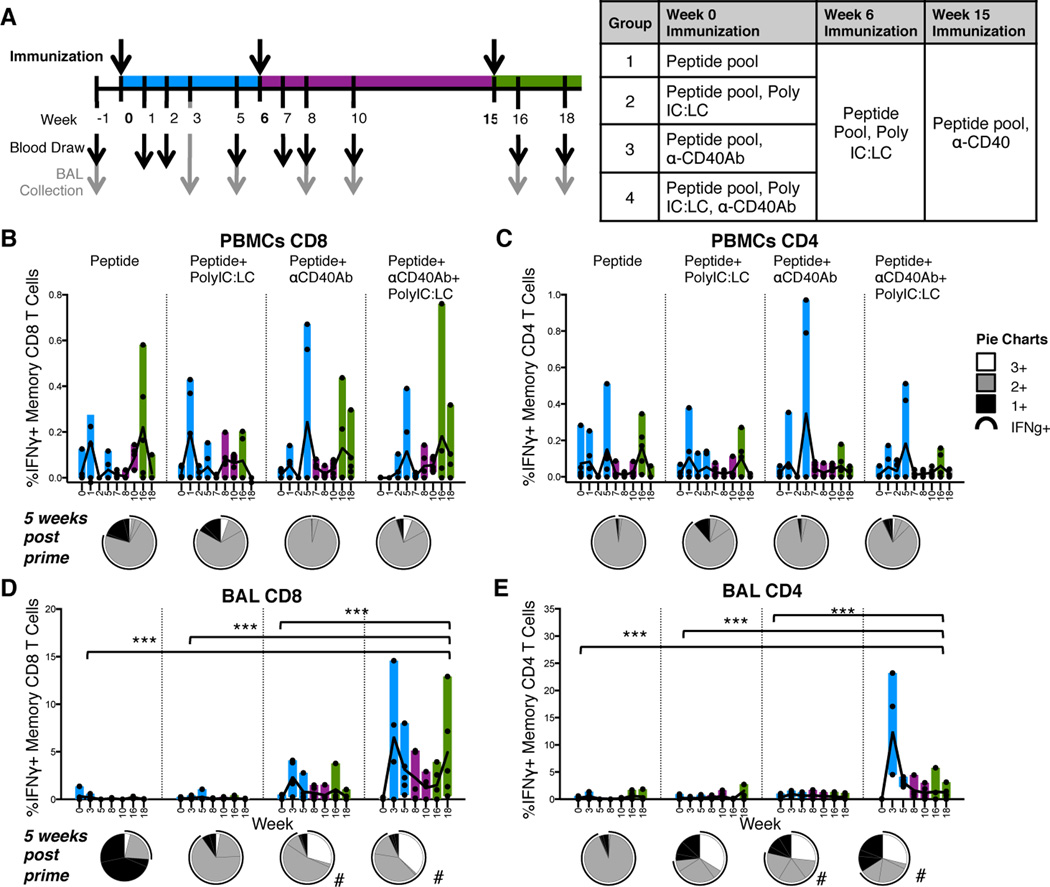
We next assessed the adjuvanticity of αCD40Ab in terms of its ability to enhance antigen-specific T cell responses. This adjuvant strategy may be most relevant for therapeutic cancer vaccines but due to the lack of cancer antigens optimized for this model, we immunized animals with a pool of six 9–13mer SLPs derived from HIV-1 envelope glycoprotein (Env) (29). These peptides are well-characterized and immunogenic in rhesus macaques (33, 34), which enabled us to focus on the adjuvant component of the immunization. The peptides should require some degree of processing by APCs for presentation on class I MHC for induction of CD8+ T cell responses (29–32) but could conceivably be loaded directly into class II MHC for induction of CD4+ T cell immunity. Throughout the study, the magnitude and quality of antigen-specific memory CD8+ and CD4+ T cell responses were assessed using multi-parameter flow cytometry and intracellular cytokine staining (ICS). Memory T cells were identified by exclusion of CCR7+ CD45RA+ naïve cells (35, 36).
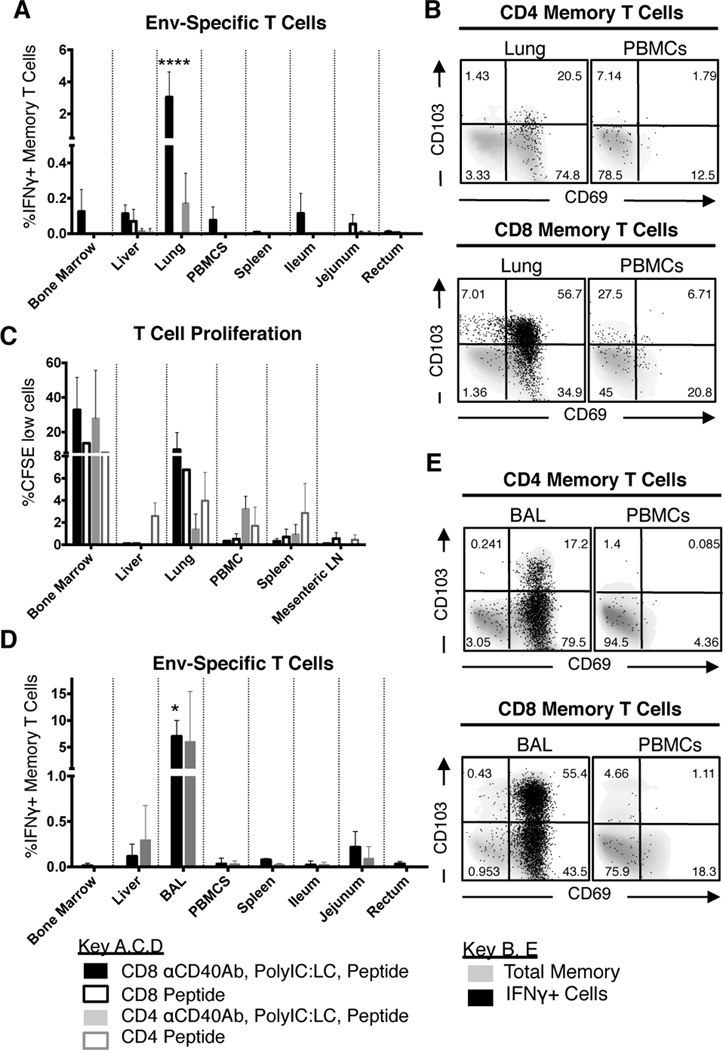
After first confirming the immunogenicity of the SLPs adjuvanted with αCD40Ab/poly IC:LC in a pilot dose study (Figure S2A–E), the individual contribution of each adjuvant component compared to the combination was assessed. For this study, rhesus macaques received the pool of Env peptides given alone or combined with either poly IC:LC, αCD40Ab or both adjuvants (Figure 4A). Given that our pilot study suggested that the high dose peptide pool promoted CD8+ responses and low dose promoted CD4+ responses, animals were primed with an intermediate dose of 4mg/kg of peptides in an effort to balance CD4+ and CD8+ T cell responses. The majority of animals regardless of group showed small but detectable Env-specific CD4+ and CD8+ T cell responses in the blood as early as 1 week after the prime (Figure 4B–C blue bars). T cell responses in PBMCs were modest and there was no significant difference between the groups (Figure 4B–C). In contrast, T cell responses in BAL were robust and groups that received αCD40Ab exhibited significantly higher Env-specific T cell responses in BAL after prime compared to the other groups, especially when co-delivered with polyIC:LC (p<0.0001 for all groups) (Figure 4D–E). High CD8+ T cell responses were found in the animals receiving αCD40Ab with or without poly IC:LC, while CD4+ T cell responses were only detected when both adjuvants were combined.
Figure 4. Development of antigen specific T cells in mucosal tissue is dependent on αCD40Ab.
Each component of the αCD40Ab/poly IC:LC immunization was tested to see individual contributions to the development of Env specific T cell responses. (A) Experimental design, rhesus macaques were divided into 4 groups (n=5/group) and immunized i.v. with one of the four indicated combinations of Env peptide pool (4mg/kg), poly IC:LC (1mg) and αCD40Ab (1.5mg/kg). At week 6 animals received a boost of the Env peptide pool (1mg/kg) with poly IC:LC (1mg) and at week 15 with the peptide pool (1mg/kg) and αCD40Ab (1.5mg/kg). Blood and BAL samples collected as indicated in (A) and used in an ICS-based assay for T cell responses. (B–E) PBMC and BAL samples were stimulated overnight with the immunizing peptide pool to recall total memory (Live/CD3+, CD45RA+ and CCR7+ naïve cells gated out) Env Specific CD8 T cells (B and D) or CD4 T cells (C and E) as determined by IFNγ production. Pie charts show the week 5 post prime proportion of total memory CD4/CD8 T cells producing IFNγ, IL-2, and TNF (white), 2+ cells producing any two of IFNγ, IL-2, and TNF (grey), and 1+ cells producing IFNγ, IL-2, or TNF alone (black). The black arc represents cells that produce IFNγ. Statistical differences between the groups (all time points combined) are indicated in bar graphs, *p ≤ 0.05 and **p ≤ 0.005 using two-way ANOVA. Statistical differences for pie graphs are represented as #p ≤ 0.05 compared with peptide alone.
In addition to the magnitude of T cell response, high proportions of multifunctional antigen-specific T cells, i.e. the ability to produce multiple cytokines at a single cell level (IFNγ, IL-2 and TNF), correlates with protection against infection (37). When multifunctionality was assessed in our study, groups that had received αCD40Ab had higher proportions of CD8+ T cells that produced more than one cytokine (Figure 4B–E, pie charts; white or grey portions). Differences in multifunctionality were most pronounced in CD8+ T cells in the BAL, where a high proportion produced all three cytokines (Figure 4D, pie charts; white portions), but only in groups that received αCD40Ab. Taken together, these data illustrate that αCD40Ab acts as an adjuvant to enhance both the magnitude and quality of antigen-specific CD8+ T cell responses.
Given the qualitative and quantitative differences in antigen-specific CD8+ T cell responses induced by αCD40Ab during priming, we assessed whether such responses would be differentially boosted. All animals were boosted at week 6 with a common boost of poly IC:LC and Env peptides, thereby controlling for the effects of the different formulations on priming only. Surprisingly, we found that this formulation provided a minimal boost of T cell responses (Figure 4B–E, purple bars), but groups primed with αCD40Ab maintained higher proportions of multifunctional T cells after boosting (Figure S3). At 15 weeks after priming and 9 weeks after the poly IC:LC boost, we administered a second boost using a formulation containing peptides with αCD40Ab. Groups that had received αCD40Ab during priming exhibited a boosting of CD8+ T cell responses in BAL (Figure 4B–E green bars). The CD8+ T cell responses were boosted much more efficiently than the CD4+ T cell responses. After the αCD40Ab boost, the proportion of multifunctional T cells in the BAL of all groups increased as compared to before the boost, although groups primed with αCD40Ab again possessed the highest proportions of such cells (Figure S3). Of note, groups that had not received αCD40Ab in the prime but received αCD40Ab in the second boost exhibited a noticeable boost of T cell responses in PBMCs but not in the BAL. This suggests that after antigen-specific T cell responses had been primed, the inclusion of αCD40Ab in the boost was not able to reprogram T cells to become BAL-resident.
αCD40Ab induces effector T cell responses predominately in the lung
Considering the widespread dissemination of αCD40Ab and the high levels of antigen-specific T cells in the BAL, we examined T cell responses in a variety of additional tissues. The two animals with the highest level of antigen-specific T cells in the BAL were euthanized 4 weeks after the third boost (αCD40Ab and peptides). As a comparison, tissues from two animals primed with peptide alone were also evaluated. Remarkably, high frequencies of antigen-specific T cells were exclusively detected in the lung (Figure 5A). The responding T cells were primarily CD8+ and were only found in animals primed with αCD40Ab, poly IC:LC and peptides, but not in animals primed with peptide alone. Most of the responding cells in the lung expressed the activation marker CD69 and about half of them were CD103+, indicating that they were tissue-resident T cells (Figure 5B).
Figure 5. Immunization with αCD40Ab elicits tissue resident T cells compartmentalized to the lung.
(A) Tissues from animals primed with αCD40Ab, poly IC:LC and Env peptide pool (filled boxes) and animals primed with peptide only (open boxes) were evaluated for Env specific T cells for weeks after the final boost (see Figure 4) as assessed by IFNγ production (Mean±SEM, n=2/group). Tissue sample T cells additionally had NKg2a+ and TCRγδ+ gated out. (B) Env specific T cells in the lung were predominantly tissue resident T cells, based on their CD103 and CD69 expression. (C) CFSE labeled cell suspensions from tissues that were harvested from the same animals were cultured for 5 days with immunizing peptides and Env specific proliferation was analyzed. Mean±SEM, n=2/group). (D) Additional animals (see Figure S2) that were primed with αCD40Ab, poly IC:LC and Env peptide pool that had previously demonstrated high levels of T cell responses were boosted with αCD40Ab, poly IC:LC and Env peptide pool. Tissues were evaluated three weeks later for Env specific T cell responses as assessed by IFNγ production (Mean±SEM, n=3). (E) Env specific T cell responses that were highest in the lung were predominantly tissue resident T cells, based on their CD103 and CD69 expression. Statistical differences compared to all other tissues are indicated in bar graphs, *p ≤ 0.05 and ****p ≤ 0.0001 using two-way ANOVA.
We also assessed proliferative capacity by restimulating cells from tissues with the Env peptides in vitro for 5 days, and were able to detect responding Env-specific CD4+ and CD8+ T cells in all animals, including those primed with peptide alone (Figure 5C). Proliferating T cells were found in the spleen, PBMCs, lung and liver, but the most robust proliferation was observed in cells derived from the bone marrow and the lung, especially cells from animals primed with αCD40Ab and poly IC:LC. This illustrates the synergistic effect of targeting CD40 and TLR3/MDA-5 simultaneously and suggests that while immunization induced effector CD4+ and CD8+ Env-specific T cells in the BAL, it also established an expandable pool of memory cells in a variety of tissues.
Due to the small number of animals examined for the T cell distribution and phenotype, an additional three animals from the dose pilot study were analyzed. These animals were primed with high dose Env peptides, αCD40Ab and poly IC:LC, then boosted once with poly IC:LC and peptides. The three animals received and additional boost with αCD40Ab and peptides three weeks before euthanasia. Again there was a striking increase in the frequency of IFNγ producing Env-specific T cells, but only in BAL (Figure 5D). We were unable to assess the lung responses in these animals, as the lung samples had low viability after in vitro restimulation, potentially reflecting ongoing T cell activation. Responding T cells in the BAL had a similar phenotype to those found in the lung tissue above, expressing high levels of CD103 (Figure 5E). Altogether, these data demonstrate that detectable antigen-specific memory T cells can be identified from a variety of tissues after expansion, but antigen-specific effector T cells are preferentially resident in the lung and BAL.
Discussion
In the presented work, the immune mechanisms leading to T cell responses in vivo after administering an agonistic αCD40Ab in an NHP model were assessed. We show that αCD40Ab rapidly spreads to multiple organs after i.v. administration. This is accompanied by mobilization of circulating leucocytes, induction of high levels of serum IL-12, phenotypic maturation of DCs, and development of robust T cell responses, particularly compartmentalized in the lung when αCD40Ab was systemically co-delivered with poly IC:LC.
An NHP model provides several advantages for performing comprehensive mechanistic and immunological evaluation in multiple tissues compared to mouse models, which can have different tissue specific distribution of innate signaling pathways. Moreover, using a human Ab provides useful data for translating these findings to human trials. In addition to clear differences between the mouse and human immune systems, recent studies have also evaluated the role of αCD40Ab crosslinking and Fc receptor engagement and found that differences in mouse Ab isotypes that mediate differential engagement of activating and inhibitory Fcγ receptors did not translate to human Abs (39, 40). When studying the commonly used murine agonistic αCD40Ab, clone FGK45, it was found that specific Fc receptor engagement was necessary for optimal activity. In contrast, the human agonistic Ab clone CP-870,893 did not show the same dependence on Fc receptor engagement (39, 40). CP-870,893 and the αCD40Ab used in this study are both of the IgG2 isotype, which typically is less likely to bind Fc receptors or activate complement as compared to the IgG1 isotype. Despite this, the Abs clearly induced a robust innate immune activity, further highlighting that human αCD40Abs can act independently of Fc receptors. However, altering the Fc portion of the Ab to improve affinity to activating Fc receptors could still potentially further enhance the function (41). It is therefore important to evaluate human Ab clones in vivo in order to fully understand the mechanisms behind Ab targeting in vaccination.
Despite some promising data on CD40 monoclonal Ab therapy, there is also evidence of negligible or negative clinical effect (13, 15, 17, 38). However, the clinical trials only evaluate αCD40Ab as a single agent, neglecting its potential as an adjuvant. In a phase I clinical trial, patients treated with clone CP-870,893 showed a strikingly similar innate profile to our animals, and the Ab was well tolerated overall (15–17, 42). In both the clinical trial and our study there was a brief cytokine release, activation of circulating cells and a transient decline returning to baseline at day 8. In the present study, the data suggest that leucocytes were mobilized to tissues rather than being depleted. For example, whole blood counts correlated with cell counts from flow cytometry and there were no differences in viability staining following administration (data not shown). In addition, DCs remaining in the blood had upregulated CCR7 expression, which directs them to LNs. Cell mobilization was also not unique to αCD40Ab administration, as poly IC:LC alone induced a similar decline and cell types such as T cells, which do not express CD40, were also affected. The redistribution of cells may therefore be a consequence of systemic delivery of immune-stimulatory agents (43–45). The similarities between innate profiles of humans and NHPs treated with agonistic αCD40Ab clones demonstrate the clinical relevance of the model.
While the blood offers some insight into the innate activation induced by αCD40Ab administration, the ability to track the Ab and examine multiple tissues after administration offered a unique opportunity to further characterize immune responses, not possible in clinical trials. We found that multiple cell types bound the αCD40Ab, but the binding and internalization did not correlate with the level of CD40 expression on the cells. However, it is clear that some level of CD40 specific interaction is necessary for αCD40Ab targeting considering the IgG isotype control Ab showed a lower Alexa680 signal. Interestingly, there may also be a role for indirect CD40 interaction, as demonstrated by the unexpected finding that in vivo binding of isotype Ab was greatly reduced in cell subsets such as neutrophils, which express relatively low levels of CD40. However, neutrophils have the ability to upregulate costimulatory molecules, including CD40, as a result of immune activation (46–48). On this note, the isotype Ab was also delivered with poly IC:LC, which leads to systemic immune activation including neutrophil mobilization (Figure 1C). However, DC differentiation and in particular IL-12 production were much more pronounced with αCD40Ab administration as compared to poly IC:LC alone. In tissues we found that CD70 upregulation was directly induced by αCD40Ab binding, and the highest levels were seen in BAL and liver, potentially reflecting a better environment for priming. The αCD40Ab was therefore superior and unique in directly stimulating DCs and inducing the strong Th1-type stimulatory immune milieu, likely leading to the adjuvant effect found in this study. Even though we did not find any evidence of obvious synergy between αCD40Ab and poly IC:LC with regards to innate immune activity, the combination of the two adjuvants clearly led to improved adaptive responses compared to either adjuvant alone.
Our immunization strategy induced remarkably high frequencies of sustainable antigen-specific T cells (5–10%) in the BAL, despite modest responses in the periphery. This suggests that there was compartmentalized priming and development of T cells responses in the lung. Prior mouse studies evaluating αCD40Ab as a vaccine adjuvant have also shown high levels of antigen-specific T cells in the lung (49). However, most mouse studies only report on the responses in peripheral blood and spleen, which appear to be readily detectable, while such responses were modest in our NHP study. It is possible that the responses in mouse are more efficiently disseminated and/or that the doses of model antigens such as OVA, rather than HIV antigens, are not comparable between the animal models.
The lung-compartmentalized responses could be a result of the i.v. route of delivery and/or are due to inherent characteristics of the αCD40Ab. We found that systemic administration of the αCD40Ab efficiently targeted lung DCs, which could lead to preferential generation of lung resident T cells. Lung resident DCs in mice were shown to imprint T cell homing to the lung through induction of CCR4 expression on the T cells (50). Although we were not able to evaluate CCR4 expression on T cells in rhesus tissues, the responding T cells appeared to be largely CD103+, indicating that they were tissue-resident memory cells (51). We also found that APCs sorted from the lung enhanced T cell proliferation and induced CD103 expression when pretreated with the αCD40Ab in vitro (data not shown). As mentioned above, the high DC maturation detected in the lung may indicate a superior environment for DC presentation and priming of T cell responses after αCD40Ab administration. Mouse data also shows that while T cells imprinted by lung DCs preferentially home to the lung, they are more flexible in their peripheral distribution than T cells imprinted by skin or gut DCs (50). This may explain our observations that antigen-specific effector T cells were preferentially in the lung, but could also be found systemically after expansion for five days in a proliferation assay. These results indicate that the immunization targeted and activated lung DCs due to the route of delivery, but does not rule out the possibility of intrinsic signaling of the αCD40Ab to imprint lung homing T cells. On this note, in a pilot study when we administered antigen and poly IC:LC subcutaneously and αCD40Ab i.v., the T cell responses were still modest in peripheral blood and higher in BAL suggesting that priming restricted to the skin draining LNs in the presence of systemic CD40 Ab still induces some degree of compartmentalized lung responses (Figure S2F–I).
Future studies are needed to elucidate whether αCD40Ab would be particularly potent for vaccine formulations to pathogens and tumors specific to the lung and warrants investigation of other therapeutic Abs e.g. anti-PD1 and anti-CD20 also delivered i.v. It will also be important to test the functionality of these T cells with tumor antigens and investigate the potential of targeting other organs with this Ab with alternative routes of delivery. While it is clear that successful next-generation vaccines to both infectious diseases and cancer will require potent and durable T cell responses, a much better understanding of how strong vaccine responses can be elicited and maintained is essential. These studies demonstrate the ability of an agonistic αCD40Ab to induce high levels of DC targeting and activation, presumably leading to substantial lung-specific CD8+ T cell responses. Altogether, such data contribute to the understanding of how T cell responses can be tailored via innate immune stimulation to be more efficacious and for improving the design of future vaccine formulations.
Supplementary Material
Acknowledgements
The authors thank John-Paul Todd, Vaccine Research Center, NIH and the animal care personnel at Bioqual. Oncovir, Inc for providing PolyIC:LC and Linnea Haeggblom for technical assistance.
Footnotes
KL is supported by grants from Vetenskapsrådet (521-2012-3377) and the Swedish Governmental Agency for Innovation Systems (Vinnova) (2010-00999). RAK and RAS are supported by intramural funds as US NIH investigators. This work was in part supported by SBIR # Phase 2 - 5R44AI080030 granted to ImmuRx Inc.
References
- 1.Hanyu K, Iida T, Shiba H, Ohashi T, Eto Y, Yanaga K. Immunogene therapy by adenovirus vector expressing CD40 ligand for metastatic liver cancer in rats. Anticancer research. 2008;28:2785–2789. [PubMed] [Google Scholar]
- 2.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 3.Buhlmann JE, Foy TM, Aruffo A, Crassi KM, Ledbetter JA, Green WR, Xu JC, Shultz LD, Roopesian D, Flavell RA, et al. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995;2:645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 4.Diehl L, Den Boer AT, van der Voort EI, Melief CJ, Offringa R, Toes RE. The role of CD40 in peripheral T cell tolerance and immunity. Journal of molecular medicine. 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- 5.Davis ID, Chen Q, Morris L, Quirk J, Stanley M, Tavarnesi ML, Parente P, Cavicchiolo T, Hopkins W, Jackson H, Dimopoulos N, Tai TY, MacGregor D, Browning J, Svobodova S, Caron D, Maraskovsky E, Old LJ, Chen W, Cebon J. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. Journal of immunotherapy. 2006;29:499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 6.Gladue RP, Paradis T, Cole SH, Donovan C, Nelson R, Alpert R, Gardner J, Natoli E, Elliott E, Shepard R, Bedian V. The CD40 agonist antibody CP-870,893 enhances dendritic cell and B-cell activity and promotes anti-tumor efficacy in SCID-hu mice. Cancer immunology, immunotherapy : CII. 2011;60:1009–1017. doi: 10.1007/s00262-011-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. The Journal of experimental medicine. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McWilliams JA, Sanchez PJ, Haluszczak C, Gapin L, Kedl RM. Multiple innate signaling pathways cooperate with CD40 to induce potent, CD70-dependent cellular immunity. Vaccine. 2010;28:1468–1476. doi: 10.1016/j.vaccine.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. Journal of immunology. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 10.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer immunology, immunotherapy : CII. 2012;61:1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Sowell RT, Schluns KS, Davis RE, Hwu P, Overwijk WW. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nature medicine. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer research. 2008;68:9892–9899. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, Harrop K, Whiting N, Drachman JG. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 14.Furman RR, Forero-Torres A, Shustov A, Drachman JG. A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2010;51:228–235. doi: 10.3109/10428190903440946. [DOI] [PubMed] [Google Scholar]
- 15.Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O’Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vonderheide RH, Burg JM, Mick R, Trosko JA, Li D, Shaik MN, Tolcher AW, Hamid O. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013;2:e23033. doi: 10.4161/onci.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O’Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi N, Miura T, Kitagawa Y, Matsushima A. Anti-cd40 antibody mutants. Google Patents. 2014 [Google Scholar]
- 19.Byrd JC, Kipps TJ, Flinn IW, Cooper M, Odenike O, Bendiske J, Rediske J, Bilic S, Dey J, Baeck J, O’Brien S. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leukemia & lymphoma. 2012;53:2136–2142. doi: 10.3109/10428194.2012.681655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park H, Adamson L, Ha T, Mullen K, Hagen SI, Nogueron A, Sylwester AW, Axthelm MK, Legasse A, Piatak M, Jr, Lifson JD, McElrath JM, Picker LJ, Seder RA. Polyinosinic-polycytidylic acid is the most effective TLR adjuvant for SIV Gag protein-induced T cell responses in nonhuman primates. Journal of immunology. 2013;190:4103–4115. doi: 10.4049/jimmunol.1202958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. The Journal of experimental medicine. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn KM, Da Costa A, Yamamoto A, Berry D, Lindsay RW, Darrah PA, Wang L, Cheng C, Kong WP, Gall JG, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gomez CE, Esteban M, Wyatt LS, Moss B, Morgan C, Roederer M, Bailer RT, Nabel GJ, Koup RA, Seder RA. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. Journal of immunology. 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lore K, Sonnerborg A, Spetz AL, Andersson U, Andersson J. Immunocytochemical detection of cytokines and chemokines in Langerhans cells and in vitro derived dendritic cells. Journal of immunological methods. 1998;214:97–111. doi: 10.1016/s0022-1759(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 25.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. Journal of immunology. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 26.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Dong H, Franklin NA, Roberts DJ, Yagita H, Glennie MJ, Bullock TN. CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help. Journal of immunology. 2012;188:3829–3838. doi: 10.4049/jimmunol.1103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurche JS, Haluszczak C, McWilliams JA, Sanchez PJ, Kedl RM. Type I IFN-dependent T cell activation is mediated by IFN-dependent dendritic cell OX40 ligand expression and is independent of T cell IFNR expression. J Immunol. 2012;188:585–593. doi: 10.4049/jimmunol.1102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nehete PN, Nehete BP, Hill L, Manuri PR, Baladandayuthapani V, Feng L, Simmons J, Sastry KJ. Selective induction of cell-mediated immunity and protection of rhesus macaques from chronic SHIV(KU2) infection by prophylactic vaccination with a conserved HIV-1 envelope peptide-cocktail. Virology. 2008;370:130–141. doi: 10.1016/j.virol.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nehete PN, Nehete BP, Manuri P, Hill L, Palmer JL, Sastry KJ. Protection by dendritic cells-based HIV synthetic peptide cocktail vaccine: preclinical studies in the SHIV-rhesus model. Vaccine. 2005;23:2154–2159. doi: 10.1016/j.vaccine.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 31.Koopman G, Beenhakker N, Nieuwenhuis I, Doxiadis G, Mooij P, Drijfhout JW, Koestler J, Hanke T, Fagrouch Z, Verschoor EJ, Bontrop RE, Wagner R, Bogers WM, Melief CJ. DNA/long peptide vaccination against conserved regions of SIV induces partial protection against SIVmac251 challenge. Aids. 2013 doi: 10.1097/QAD.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 32.Rosario M, Bridgeman A, Quakkelaar ED, Quigley MF, Hill BJ, Knudsen ML, Ammendola V, Ljungberg K, Borthwick N, Im EJ, McMichael AJ, Drijfhout JW, Greenaway HY, Venturi V, Douek DC, Colloca S, Liljestrom P, Nicosia A, Price DA, Melief CJ, Hanke T. Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. European journal of immunology. 2010;40:1973–1984. doi: 10.1002/eji.201040344. [DOI] [PubMed] [Google Scholar]
- 33.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. The New England journal of medicine. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 34.van Poelgeest MI, Welters MJ, van Esch EM, Stynenbosch LF, Kerpershoek G, van Persijn van Meerten EL, van den Hende M, Lowik MJ, Berends-van der Meer DM, Fathers LM, Valentijn AR, Oostendorp J, Fleuren GJ, Melief CJ, Kenter GG, van der Burg SH. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. Journal of translational medicine. 2013;11:88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 36.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews. Immunology. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 38.Ruter J, Antonia SJ, Burris HA, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer biology & therapy. 2010;10:983–993. doi: 10.4161/cbt.10.10.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richman LP, Vonderheide RH. Role of crosslinking for agonistic CD40 monoclonal antibodies as immune therapy of cancer. Cancer immunology research. 2014:2. doi: 10.1158/2326-6066.CIR-13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter EL, Mick R, Ruter J, Vonderheide RH. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. Journal of translational medicine. 2009;7:93. doi: 10.1186/1479-5876-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Januszkiewicz A, Lore K, Essen P, Andersson B, McNurlan MA, Garlick PJ, Ringden O, Andersson J, Wernerman J. Response of in vivo protein synthesis in T lymphocytes and leucocytes to an endotoxin challenge in healthy volunteers. Clin Exp Immunol. 2002;130:263–270. doi: 10.1046/j.1365-2249.2002.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 45.Gunzer M, Riemann H, Basoglu Y, Hillmer A, Weishaupt C, Balkow S, Benninghoff B, Ernst B, Steinert M, Scholzen T, Sunderkotter C, Grabbe S. Systemic administration of a TLR7 ligand leads to transient immune incompetence due to peripheral-blood leukocyte depletion. Blood. 2005;106:2424–2432. doi: 10.1182/blood-2005-01-0342. [DOI] [PubMed] [Google Scholar]
- 46.Fanger NA, Liu C, Guyre PM, Wardwell K, O’Neil J, Guo TL, Christian TP, Mudzinski SP, Gosselin EJ. Activation of human T cells by major histocompatability complex class II expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89:4128–4135. [PubMed] [Google Scholar]
- 47.Reinisch W, Tillinger W, Lichtenberger C, Gangl A, Willheim M, Scheiner O, Steger G. In vivo induction of HLA-DR on human neutrophils in patients treated with interferon-gamma. Blood. 1996;87:3068. [PubMed] [Google Scholar]
- 48.Beauvillain C, Delneste Y, Scotet M, Peres A, Gascan H, Guermonprez P, Barnaba V, Jeannin P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Stokes KL, Currier MG, Sakamoto K, Lukacs NW, Celis E, Moore ML. Vaccine-elicited CD8+ T cells protect against respiratory syncytial virus strain A2-line19F–induced pathogenesis in BALB/c mice. Journal of virology. 2012;86:13016–13024. doi: 10.1128/JVI.01770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikhak Z, Strassner JP, Luster AD. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. The Journal of experimental medicine. 2013;210:1855–1869. doi: 10.1084/jem.20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nature reviews. Immunology. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.