Abstract
Introduction
Assessment of oral motor behavior in a mouse is challenging due to the lack of currently available techniques that are non-invasive and allow long-term assessment in a home cage environment. The purpose of this study was to evaluate incising behavior using mouse chow attached to a three-dimensional force transducer that was mounted on the existing home cage. In addition, a persistent hyperalgesia condition was introduced to evaluate the sensitivity of the technique to identify incising behavioral changes.
Methods
Incising activity of CD-1 male and female mice (n=48) was evaluated over a 24 hour recording session during four baseline and six longitudinal hyperalgesia assessment sessions using custom written software. A pre-clinical persistent pain model was used to induce hyperalgesia in the masseter muscle by repetitive acidic saline injections. Sex and age differences were evaluated for multiple incising variables during both light and dark cycles during baseline and hyperalgesia conditions.
Results
Significant sex differences were found for multiple incising variables but not for age. Discrete incising frequencies were identified in the range of 4.6-10.4 Hz and were reproducibly found in both female and male mice. A significant shift to lower incising frequencies was observed after repetitive acidic saline injections compared to neutral saline injections. This shift to lower frequencies of incising returned to baseline levels after approximately four weeks but was statistically longer in female compared to male mice. Significant differences were also found for chow intake (reduced) and weight change during the hyperalgesia condition. No significant differences were found for total number of incisions or number of incising episodes per day or incising force.
Conclusions
The findings from this study support the use of recording three dimensional incising forces as a sensitive measure of incising behavior. This novel technique allowed the identification of specific incising variables that were differentially affected in female and male mice during a persistent hyperalgesia. The data were collected in the home cage environment with minimal bias such as experimenter interaction. Similar to other dental pain studies, mice were able to maintain normal incising activity levels per day (total incisions, total number of incising episodes) even in the presence of hyperalgesia.
Keywords: central pattern generator, mastication, incising, 3D force transducer, pain
1.0 Introduction
Oral motor assessment in a rodent model such as mice can provide insight into the effects of genetic disorders, anxiety/stress or medications on the central pattern generator for mastication and feeding behavior. Although food and water intake measures over time can provide some indication of deficiencies or alterations in feeding, these measures do not differentiate the impact of reduced satiation (reduced drive), changes in oral motor control (reduced masticatory ability) and reduced overall activity.
Assessment of oral motor behaviors in mice during incising and mastication has been limited by the invasiveness and associated stress of the different recording techniques, the short duration of the recordings and the unique environment outside of the traditional home cage. Multiple techniques have been reported such as mandibular strain gauge recordings [1], electromyographic (EMG) monitoring [2-4] and/or jaw tracking using a magnetic field sensor mounted on the head [5-7]. Many of these techniques are invasive and require surgical implantation of electrodes and tethering the mouse to recording equipment or have telemetry instrumentation implanted. Most recordings from these techniques have been short in duration, lasting from a few minutes to a few hours while eating different foodstuffs such as grains or chow [3, 4].
Evaluation of behavior has advanced in recent years using automated computer assessment of video recordings to identify behavior and tracking of activity in the home cage environment. The advantages of these approaches compared to the traditional assessments of behavior during normal and experimentally altered conditions have been discussed by Spruijt et al. and are significant [8]. Evaluation of incising or mastication is especially problematic since small changes in jaw position or bite force cannot be evaluated without special equipment to detect these behavioral changes to an experimental condition. There is a need to develop techniques that have adequate sensitivity to detect changes in oral function but do not require a surgical manipulation or require tethering of the animal to the recording equipment.
The purpose of this study was to evaluate a new technique that is non-invasive, does not require training, can be used in a home cage environment to minimize stress, and assesses incising force in three dimensions over an extended period such as 24 hrs. Repetitive acidic saline injections were used to create persistent masticatory muscle hyperalgesia and alter incising activity to test the sensitivity of this technique over a 35 day period in male and female mice.
2.0 Materials and Methods
2.1 Animals
Equal numbers of male and female CD-1 mice (total n = 48) from the same distribution source (Charles Rivers) were housed in one animal room with four mice of the same sex housed in the same home cage. Each mouse was individually assessed in an identical home cage located in a sound attenuation chamber (ENV-022V, Med Associates, Inc.) in the same animal room, so minimal relocation of animals was required for the behavioral assessment. All mice were housed and assessed with ad libitum access to water and food and were maintained in a normal 12 hr light/dark cycle at an average temperature of 25°C. Each sound attenuation chamber was ventilated and equipped with the same 12 hr light/dark cycle schedule to maintain continuity with the animal room. The animal care and experimental procedures were in compliance and approved by the University of Florida Institutional Animal Care and Use Committee.
2.2 Data Acquisition
Audio, video and incising force recordings were acquired during a 24 hr period from four cages simultaneously. Video recordings (B&W) were made for the entire 24 hr period using a small analog camera (HRC-20P with 6 mm lens, SCS, Enterprises, Inc.) connected to a DVR to assess on-going behavior such as general activity, posture and grooming activities and to validate incising behavior. Three dimensional incising forces were assessed using a multi-axis force transducer (NANO17-E, ATI Industrial Automation) attached to a custom food holder with three pieces of standard chow (Harlan Laboratories). The force transducer-mounted chow was positioned in the standard chow tray and the mouse accessed the chow through the wire top (Fig. 1). Episodes of incising forces/sound were recorded using custom-written software (LabView 2013, National Instruments, Inc.). Analog forces were recorded using an A/D converter (PCIe-6343 X Series Multifunction DAQ, National Instruments, Inc.) at 500 Hz and 16 bit resolution while sound was recorded at 51.2 kHz/channel and with a 24 bit resolution (Model 9234, National Instruments, Inc.) using an industrial microphone (Model 378B02, 1/2″ microphone, bandwidth 3.15 Hz - 20kHz, PCB Piezotronics, Inc.). Force/sound recordings were initiated when the 3-D force resultant (instantaneously calculated from the X, Y and Z forces) exceeded a set threshold (2.4 N) and ended after a pre-determined period of no incising (4 sec). Post-processing of the acquired signals was accomplished using custom-written software (LabView 2013, National Instruments, Inc.) to remove baseline bias and digitally filter (bandwidth 0-31.25 Hz) the individual force axis recordings (X, Y and Z forces), calculate the resultant and automatically identify peak force amplitude and peak time during each episode of incising. Incising episodes with five or fewer incising force peaks were removed from the analysis. Each incising episode was analyzed to determine the instantaneous incising frequency between two force peaks. For each of these pair of peaks, the second peak was evaluated for peak force, and top 10% incising forces. Incising episode duration, total number of incising episodes, total number of incisions, weight of chow consumed and body weight were also assessed for each 24 hour recording.
Fig. 1.

Position and orientation of multi-axis force transducer mounted on the home cage with mouse chow attached to the transducer. White arrows represent X and Y axes and black arrow represents the Z axis. Positive is represented by the direction of the arrowhead.
2.3 Pain Model Description
A persistent mild to moderate jaw closing muscle hyperalgesia was induced by two 20 μl injections of sterile acidic saline (pH = 4.0) into the left masseter muscle separated by five days. Repetitive injections within 3-7 day window have been shown to elicit hyperalgesia in muscle such as the lateral gastrocnemius that lasts for over four weeks [9]. A control group consisted of two 20 μl injections of sterile neutral saline (pH = 7.0) into the left masseter muscle separated by five days. The injection was made into the center of the muscle as assessed from the anterior/posterior and dorsal/ventral borders of the masseter and the needle was inserted until lightly touching the body of the mandible and then retracted approximately 0.5 mm. Mice were anesthetized using an intraperitoneal injection of xylazine (15 mg/kg) and ketamine (75 mg/kg) prior to the acid/neutral saline injections.
2.4 Incising Recordings
Four baseline recordings and seven post-injection recordings (1 day after the first injection and 3, 7, 14, 21, 28 and 35 days after the second injection) were made to assess incising behavior. Mouse weight was monitored at each recording to determine any adverse effects on the mice. During this study, no mouse was ever found to be in overt distress such as weight loss > 10%, ruffled fur, persistent face grooming on the side of injection or lack of activity. The animals were weighed, transferred to the home cage for recording and were placed in individual sound attenuation chambers by the same male experimenter (CGW).
2.5 Statistical Analyses
Incising variables were analyzed for differences between sex and age. When data satisfied the requirement for parametric analyses, a mixed model Analysis of Variance was calculated and, when significant, post-hoc testing was conducted using the Fisher Least Significant Difference (LSD) test. Data not satisfying the requirements of parametric analyses were tested using appropriate non-parametric analyses. A probability level of less than 0.05 was used for all statistical testing. Three age groups of male and female mice were evaluated to determine the effect of age on incising: 3 months (n = 12 per sex for the baseline/hyperalgesia condition; 4 per sex for the control condition), 6 months (n = 4 per sex for the baseline/hyperalgesia condition) and 9 months (n = 4 per sex for the baseline/hyperalgesia condition). Incising activity over 24 hrs was normalized to total activity for each day and then averaged for baseline recordings days 2-4 and hyperalgesia recordings at 7, 14 and 21 days after the second injection. Baseline 1 was found to be variable and not representative of the actual baseline recordings and was excluded from the analysis. Days 7, 14 and 21 were chosen as days that represented hyperalgesia. Total number of incisions and incising episodes for each recording day after the introduction of jaw muscle hyperalgesia (3-35 days post-injection, D3-D35) was normalized to the mean of the total number of incisions and incising episodes during the baseline recordings and differences between sex and over time were evaluated. Correlation coefficients were converted to z-scores using Fisher's z-transformation (arctanh(r)) to normalize the coefficients prior to statistical testing and means and standard deviations were converted back to correlation coefficients using the inverse calculation (tanh(z)).
3.0 Results
3.1 Chow Intake During Baseline and Hyperalgesia Conditions
A decrease in the amount of chow eaten per day is one indicator of the presence of allodynia or hyperalgesia. Twelve males and twelve females, age 3 months, were evaluated for three baseline measures of chow intake and this mean baseline served as a standard for normalizing the amount of chow consumed during the days after the repetitive acidic saline injections. Not all mice responded to the repetitive acidic saline injections. It was observed that 75% of the mice (either male or female) had a statistically significant decrease in chow intake over a period of 28 days for females and 14 days for males (Fig. 2) except for day 7 when no significant differences among the groups were detected. These mice were considered to be responders to the acidic saline injection. The remaining 25% of the male and female mice with acidic saline injections had no difference in the amount of chow consumed compared to mice that had neutral saline injections (p > 0.52 for females; p > 0.44 for males) and these mice were considered non-responders to the acidic saline injections.
Fig. 2.

Amount of mouse chow consumed is expressed as a percent of mean baseline for mice that received repetitive acidic saline injections (acid and acid non-responders (NR)) and mice that received repetitive neutral saline injections. No statistically significant differences were found between mice that received neutral saline injections and the acidic saline non-responders.
3.2 Mouse Weight Changes During Baseline and Hyperalgesia Conditions
As an independent measure of the effect of acidic saline and neutral saline injections, mouse weight was monitored during baseline and hyperalgesia conditions. These mice were approximately 3 months old and were growing during the period of data collection so a least squares analysis of the baseline weights allowed the calculation of expected weights during the post-injection measurements (Fig. 3 A-B). No statistically significant effect on body weight was observed during the control (neutral saline) condition or for the non-responders to acidic saline injections identified from their chow intake (Fig. 3 C-D). However, a significant decrease in expected weight was observed in both female and male mice responding to the acidic saline injections (same mice that experienced a significant decrease in chow consumed per day) (Fig.3 C-D). The decrease started on day 14 after the repetitive acidic saline injections for the females and continued for the duration of weight monitoring (42 days). The males experienced a significant decrease on day 28 which stabilized on days 35 and 42. These findings of weight loss were consistent with the decreased chow intake. Further analyses included only the mice considered responding to the repetitive acidic saline injections.
Fig. 3.

Typical weight change in a female (A) and male (B) mouse during baseline and after acidic saline injections into the left masseter muscle. Note that the increase in the weight during the baseline and post-hyperalgesia recordings was almost linear (dotted lines) while there was relatively no change in weight during hyperalgesia. Percent change in mouse weight (mean ± st. err.) was calculated from projected weights from a least-square calculation using the baseline measures (C and D). Both females and males stopped increasing weight after the repetitive acidic saline injections and had different durations of weight change during the monitoring period.
3.3 Incising Behavior Over 24 Hours
Male and female incising activity during the 24 hr recording periods was found to vary over time in a similar pattern with minimal activity between the daytime hours of 0900-1300 and peak activity between the nighttime hours of 2200-0000. Incising activity then gradually reduced after midnight but had a small reproducible burst of activity at 0600-0700 (the onset of the light cycle). A mixed model ANOVA evaluating the effect of independent variables of sex (M or F), condition (baseline or hyperalgesia) and time (24 hourly time points) on the dependent variable (% incising activity) found a significant interaction among all three independent variables (F = 82.2, p < 0.000). Incising activity was found to be significantly different at specific time points between average baseline recordings (M vs F), between average hyperalgesia recordings (M vs F), and between baseline and hyperalgesia conditions for each sex and over time (Fig. 4).
Fig. 4.

Plots of average normalized incising activity over 24 hours for male and female mice during baseline (average of recordings of baselines B2-B4) and hyperalgesia conditions (average of recordings on days 7, 14 and 21 after the repetitive saline injections). The vertical axis represents the percentage of the total incising acitivty over the 24 hour recording period. Light and dark cycles are depicted by a horizontal line below the time scale.
Incising activity during baseline measurements in both male and female mice started increasing in the afternoon and continued until peak activity at 2200 (10 PM) for males and midnight (0000) for females. Incising activity during hyperalgesia was not different in females compared to their baseline except for at three time points (0200, 0800, 1500). Male mice had significantly higher incising activity during hyperalgesia at one time point in the morning (0100) and significantly lower incising activity during the afternoon/evening (1400, 2000-2100) compared to their baseline levels. Most of the incising activity during hyperalgesia occurred nocturnally between 1800 and 0600 (6PM -6AM) (mean ± st. error = 71% ± 0.02% vs 68% ± 0.03, baseline vs hyperalgesia, female, p > 0.10; 77% ± 0.02% vs 75% ± 0.03, baseline vs hyperalgesia, male, p > 0.10). Cumulative incising activity per 24 hours of recording and averaged over the baseline (B2-B4) or the hyperalgesia condition (D7, D14, D21) was not found to be significantly different between condition (baseline or hyperalgesia) or age or sex (ANOVA for repeated measures, p > 0.10). Total number of incising episodes was normalized to the average baseline and was statistically tested for differences between sex and over time (during hyperalgesia) and no significant differences were found (ANOVA for repeated measures, p > 0.10).
3.4 Incising Force Resultants
Raw incising forces recorded in the X, Y and Z directions were digitally filtered and the resultant was calculated. Peaks were automatically identified in the resultant waveform and the amplitude and timing were stored on the hard drive for each peak during each episode of incising (Fig. 5). The mean of the top 10% of the peak forces was calculated to determine the average incising force for each sex during each condition, baseline (B2-B4) and hyperalgesia (D7, D14, D21). An ANOVA for repeated measures was calculated to test the effect of sex and condition and was not found to be statistically significant for main effects or interactions of these variables.
Fig. 5.
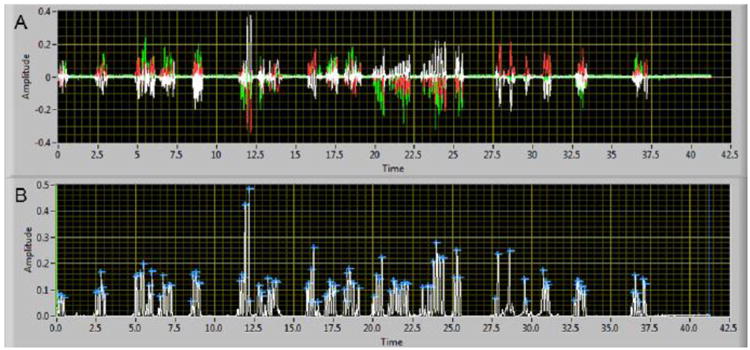
An example (Female-334, D7) of the multiaxis force recordings (A) and the calculated resultant with peak forces automatically identified (B). Each force axis (X, white; Y, red, Z, green) is superimposed (A) and the incising force peaks are easily observed. The calculated resultant (B) was analyzed using a software algorithm to automatically identify peaks (blue cross) in the waveform that had an amplitude that was greater than 2.4 N.
3.5 Incising Frequencies
Five discrete incising frequencies were consistently identified in all mice (mean ± st. error): 4.6 ± 0.01 Hz, 5.3 ± 0.01 Hz, 6.2 ± 0.01 Hz, 7.6 ± 0.02 Hz and 10.4 ± 0.03 Hz. Although previous studies have reported a range of incising frequencies with a single peak frequency, this is the first observation of separate and discrete frequencies that can be observed during incising. No statistically significant sex or condition (baseline or pain) main effect, or interaction was identified but the main effect of peak frequency time was statistically different and post-hoc testing found that each frequency was statistically different from all others (three way ANOVA, main effect time, F = 28392, p < 0.0001; LSD test, p < 0.001). Typical examples of the normalized amplitude frequency plots over time for each sex (normalized to the number of incisions for the recording day) are shown in Fig. 6 and demonstrate the reproducibility of the preferred incising frequencies.
Fig. 6.

Two typical examples of the distribution of incising frequencies for baseline and pain conditions for one female (A) and male (B) mouse. Note the reproducible distribution of frequencies with peaks identified at 4.6, 5.3, 6.2, 7.6 and 10.4 Hz. The amplitude of the distributions was normalized to the total number of incising peak frequencies.
The normalized percentage of peak frequency for each recording day was found to vary according to the condition (baseline or hyperalgesia), particularly for the middle frequency amplitude peaks (5.3, 6.2 and 7.6 Hz). A typical example of the variation in normalized amplitude for a female and male mouse for baselines 2-4 and D3-35 recordings is shown in surface plots (Fig. 7). It can be observed that the peak frequency at 5.3 Hz is more frequent after repetitive acidic saline injections compared to baseline while the peak frequency at 7.6 Hz is less frequent compared to the baseline recordings. The peak frequencies at 4.6 Hz and 10.4 Hz were low in occurrence in this example and were not included in the surface plot. Similar responses during hyperalgesia over time were observed for female and male mice but the males had a shorter duration response (note the male 7.6 Hz response returns to baseline levels at day 28 while the female response has not returned to baseline levels).
Fig. 7.
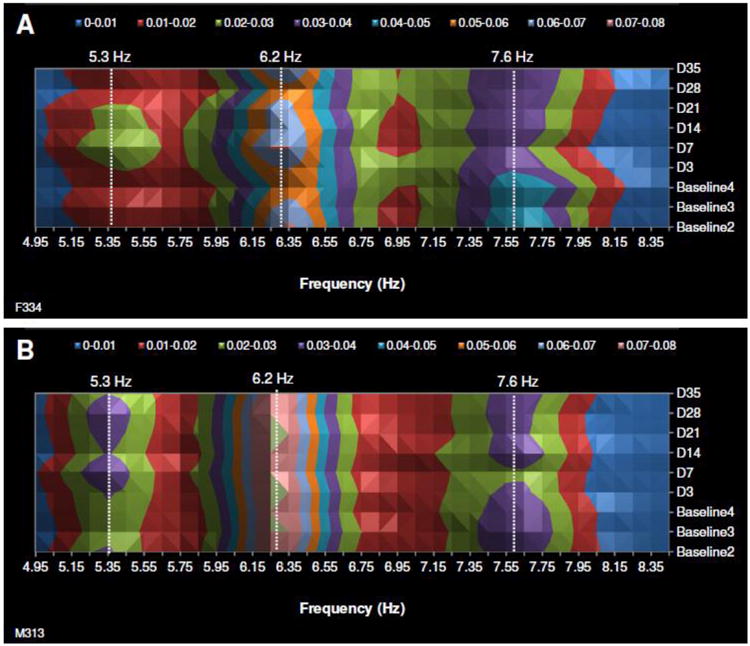
Two typical examples of surface plots that depict the change in incising frequency occurrence (%) during the hyperalgesia condition (Days 3-35) compared to the baseline condition (Baseline 2-4, Baseline 1 was excluded from all statistical comparisons). The three major incising frequencies are shown and similar responses can be observed in both female (top panel) and male (bottom panel) incising frequency changes over time. The color scale at the top of each graph represents normalized percent.
3.6 Frequency Differences When Incising Chow vs Biting Bars
Evaluation of mouse incising forces and the corresponding video found that mice had a low amplitude incising activity that involved biting the wire enclosure of the cage top, although only a few (n = 3) had a sufficient number of these type of events to be analyzed. The occurrence of biting the cage top events was more frequent in the baseline recordings compared to recordings during the hyperalgesia condition. After selection of this low amplitude, rhythmic force and analyzing the distribution of incising frequencies, it was found that a different distribution of frequencies was associated with biting the wires compared to incising the chow (Fig. 8). A small, but detectable, proportional shift to a lower median frequency (10.4 to 7.6 Hz) was observed in the low amplitude incising activity of biting the cage top during the hyperalgesia condition (day 7) compared to the baseline condition. This was consistent with the shift to lower incising frequencies (7.6 and 6.2 Hz to 5.3 Hz) after repetitive acidic saline injections compared to baseline for the chow incising (Fig. 8).
Fig. 8.

Normalized amplitude of incising frequencies identified for incising chow and incising on the cage bars during baseline and hyperalgesia conditions. Note the shift in frequency distribution to a slower rhythm during the hyperalgesia condition (D7) compared to the baseline condition (B3).
3.7 Association Between Incising Frequencies
Discrete incising frequencies were found for all mice but the percentage of incising for each recording day at each frequency was found to vary. To examine potential associations among incising frequencies, mean correlation coefficients were calculated for each frequency pair for each sex during control and hyperalgesia conditions (Fig. 9) and were found to range from -0.90 to 0.67. Correlation coefficients were not found to be significantly different when considering comparisons between sex and condition and among different ages (p > 0.10). The frequency pair with the highest correlation coefficients (>-0.85) for both male and female mice was the pair 5.3 Hz vs 7.6 Hz and was a negative relationship. Generally, there appeared a negative correlation (when greater than -0.40) between non-adjacent frequencies with a difference of greater than 2 Hz between frequencies.
Fig. 9.

Mean (± st. error) of correlation coefficients for each pair of frequencies identified during incising. No significant differences were found between female (A) and male (B) correlation coefficients.
When considering the relationship among the three most frequent frequencies (see Fig. 6 for an example of the relative occurrences of 5.3, 6.2 and 7.6 Hz), the negative relationship between frequencies 5.3 and 7.6 was the most strongly correlated. This highly correlated, inverse relationship between the number of incising events at 5.3 Hz and 7.6 Hz could be expressed as a ratio (7.6 Hz/5.3 Hz) and could be used as an indicator of transitions from higher to lower frequencies of incising observed after repetitive acidic saline injections compared to neutral saline injections (see Fig. 7 for an example). This ratio was calculated for the 24 hour recordings made for baseline and hyperalgesic conditions for each sex (Fig. 10). The baseline recording ratios were averaged and the hyperalgesia condition ratios were normalized to the average baseline for each animal. A decrease in the ratio represented a decrease in the number of incising events at 7.6 Hz and an increase in the number of incising events at 5.3 Hz and, therefore, slower incising. Significant differences were found for these ratios between the control and the hyperalgesia conditions for both female and male mice (Fig. 10). However, a more robust response can be observed in the female mice compared to the males that lasted at least 35 days while a shorter duration hyperalgesia response (<14 days) was found in the male mice. Older and younger mice were evaluated for differences in response to hyperalgesia using this ratio and no significant differences were found (Fig. 10).
Fig. 10.

A ratio of the number of incising events at 7.6 Hz and 5.3 Hz was calculated for each recording day for each condition, sex and age and the ratios were normalized to the average of the baseline ratios. A decrease in the ratio observed during the hyperalgesia condition represented a higher number of 5.3 Hz incising events compared to the number of incisions at 7.6 Hz (or a transition to a lower overall frequency of incising). The control condition was a repetitive injection of neutral saline (pH = 7.0). No data were acquired for the male controls at the time point one day post first injection (1d-post 1st inj) due to a computer malfunction.
4.0 Discussion
4.1 Discrete Incising Frequencies
There are a number of unique findings from this study. For example, this study is the first to record and quantify incising activity characteristics over a 24 hour period in the home cage environment. During this full day of recording, it was found that incising occurs over a range of frequencies for chow that is a constant size and hardness. There have been previous reports of incising, licking and chewing frequencies in a rodent model that range from 4-11 Hz with incising (8-11 Hz) [5, 10] being greater than licking (4-7.5 Hz) [5]. Licking frequency has been shown to vary according to the level of vigilance [11]. Chewing frequency (4-6 Hz) [5, 12, 13] has been reported to be the slowest frequency compared to incising and licking frequencies and is dependent on the size and hardness of the food. The chewing frequency has been reported to be as high as 11 Hz while eating a soft diet such as pudding [12]. In this study, discrete incising frequencies were observed over a range from 4-10 Hz, occurred predominantly at night and were observed in both female and male mice in the age range that was examined (3 months to 9 months). These discrete frequencies (4.6, 5.3, 6.2, 7.6, 10.4 Hz) were not harmonically-related with differences of 0.7, 0.9, 1.4 and 3.1 Hz and were not related to the methodological techniques used to record the incising activity. These discrete incising frequencies may be an example of quasiperiodic oscillations (torus) that have been observed in rhythmic movements [14]. It has been suggested, based on theoretical work, that quasiperiodic oscillations can enhance the flow of information and neural network performance when compared to single frequency periodic oscillations [15, 16]. Interestingly, in this study, pairs of frequencies were found to be inversely correlated with correlation coefficients ranging from -0.4 to -0.9 but the highest correlations were frequency pairs that were not adjacent to each other and were separated by at least 1.6 Hz. These inverse correlations may reflect bistability associated with reciprocally inhibitory neuronal networks [17]. Discrete chewing frequencies suggestive of bistability have been observed from EMG recordings of natural chewing activity in humans although they were not specifically identified in the publication (see fig. 1A in Po et al. 2011 [18]).
4.2 Incising Sex Differences
4.2.1 Full Day Recordings During Baseline and Hyperalgesic Conditions
The 24 hour recordings allowed an examination of diurnal and nocturnal incising activity during male and female feeding behavior and uncovered discrete sex differences that have not been reported previously for CD-1 mice. However, the pattern of feeding over 24 hours is very similar to behavior reported for a mixed strain (129sv/C57BL6J/FBVN) [19]. In the baseline condition, discrete differences in peak incising times were found between males (2200 hrs or 10 PM) and females (0 hrs or midnight). Both males and females had less than 14% of their incising activity between 0900 and 1500 (9 AM - 3 PM) and started a gradual rise in activity after 1600 (4 PM) and this is consistent with the nocturnal activity of rodents. No differences in the timing of peak incising were found between males and females during hyperalgesia with both peaking at 2200 hrs (10 PM). After the acidic saline injections, the male mice had a decrease in incising activity during the first half of the dark cycle and an increase in the latter part of the dark cycle while female incising activity was relatively similar to their baseline condition except for the early light cycle phase. The shift to a lower frequency of incising could be related to the hyperalgesia but may also be related to stress. Experimentally-induced stress has been shown to elicit differential effects on rats and was dependent on the strain and sex [20]. Male rats were found to be more sensitive to stress compared to females and stress caused significantly less feeding and more effect on body weight than females. However, in a study of male mice, no stress effects could be determined using three different pre-clinical pain models involving the hindlimb [21]. In another study of tooth pain in both male and female mice, no differences were found for feeding behavior between sex or after the introduction of a dental pulp injury [22]. In our study of jaw muscle hyperalgesia, no differences were observed for the total number of incisions or the number of episodes of incising. These findings may be interpreted as a lack of stress associated with this pre-clinical model of mild to moderate muscle hyperalgesia or may reflect the lack of the expression of stress-induced changes in a mouse. Since the mouse is a prey animal, there would be advantages to not show vulnerabilities such as pain and stress [21].
4.2.2 Effect of Hyperalgesia on Incising Frequency
Hyperalgesia in the orofacial region from deep regions such as jaw muscles, temporomandibular joint and bone cause a segmental response with an inhibitory effect on agonist muscles during maximal contraction and a small excitatory effect on antagonist muscles that are normally quiescent with a net result of slowing of movement around a joint [23] and a lowering of the frequency of chewing [24]. The resulting hyperalgesia from repetitive injections of acidic saline into the masseter muscle was shown in this study to differentially affect females over males and resulted in slowing the frequency of incising over a period of 3-4 weeks after the second injection. This model parallels the experience of human jaw muscle pain where females are affected much more often than males. Most animal studies have used acute preparations to demonstrate the effect of hyperalgesia on oral behavior as predicted by the Pain Adaptation Model [23] while this study has demonstrated a prolonged effect of hyperalgesia on incising frequency. The acid injection model was originally reported by Sluka et al. examining the effects of repetitive injections of acidic saline into the lateral gastrocnemius muscle of a rat [25]. In her model, bilateral allodynia was produced from unilateral repetitive acidic injections and lasted over four weeks. No evidence of an inflammatory effect was observed in the lateral gastrocnemius muscle after the repetitive acidic saline injections. We have used the acidic saline injections into the masseter muscle of a mouse as a pre-clinical pain model for mild-moderate masticatory muscle hyperalgesia and observed bilateral neuroplastic changes in the trigeminal ganglia that have a similar time course as the hyperalgesia reported for the lateral gastrocnemius [26]. The differential effect of a more robust response from females over males was also consistent with the neuroplastic changes that we observed in the trigeminal ganglia [26]. We also did not observe an inflammatory effect consistent with a muscle fiber injury after the repetitive acidic saline injections. In this study, we have used the ratio of two incising frequencies (7.6 Hz and 5.3 Hz) that had a high inverse correlation to monitor the shift to lower incising frequency with pain and the return to a higher frequency after the reduction of pain over time. The mouse model cannot be easily evaluated for allodynia or hyperalgesia in the facial region due to movement of the head to avoid the calibrated filaments. Also, the inability to have the mouse stay immobile to perform the test is another factor that limits reflex assessment. Therefore, the identification of a non-invasive technique such as recording incising forces in three dimensions in a home cage environment can provide objective information regarding pain and is an advancement in orofacial pain assessment.
4.2.3 Effect of Hyperalgesia on the Top 10% of Biting Forces
No significant differences were found between the baseline and hyperalgesia conditions or between sex for the top 10% of biting forces. This finding might seem opposite to the Pain Adaptation Model that describes a lower maximal voluntary contraction of agonist muscles during pain [23]. The chow that was used for this study was the standard mouse chow (Purina) and most likely did not require the mouse to use their maximal biting force to incise into the chow. Future studies that utilize a range of hardness of mouse chow would be necessary to address the impact of pain on maximum voluntary contraction using the three-dimensional force recordings.
4.2.4 Effect of Hyperalgesia on Mouse Weight
Significantly different effects on weight gain between female and male mice were identified in response to persistent hyperalgesia after acidic saline injection into the jaw muscle. After repetitive acidic saline injections, female mice ceased to have the significant weight gain observed during baseline recordings; no significant weight gain was observed for 42 days post acidic saline injection. The lack of weight gain is consistent with the reduced chow intake that was also found in this study. In comparison, male mice also ceased to have weight gain after initiation of hyperalgesia but this effect only lasted 21 days. In addition, the percent weight difference (expected minus actual) was twice as great in female mice as male mice. Although the female mice may have slowed their growth while males continue to grow as adults and this may account for the sex differences, the growth rate after recovery from hyperalgesia was similar to the growth rate during the baseline recordings. Therefore, it is unlikely that effect on weight was due to growth differences and is more likely due to the impact of the acidic saline injections.
Weight loss has been commonly associated with an injury involving the masticatory apparatus. In this study, chow weight intake decreased during the first three days after the final acidic saline injection and then returned back to baseline levels where it remained. However, mice that did not receive acidic saline injections (and the non-responders) steadily increased their chow intake over the same period of time. This finding is similar to a study by Gibbs et al. that evaluated the effect of a dental pulp injury on feeding [22]. Mouse body weight, however, did not increase during the time of study after the dental pulp injury. In our study of jaw muscle hyperalgesia, mouse body weight did not increase over four weeks while control mice had a significant increase in body weight. Both groups of mice were not different in their total number of incisions or total incising episodes. These similar outcomes from models of tooth or muscle pain suggest that necessary adjustments to incising/feeding behavior were made to accommodate hyperalgesia conditions and to allow approximately normal food intake. Further assessment of the incising force records may allow some insight into the types of accommodations that were involved.
We found that 25% of the female and male mice did not respond to repetitive acidic saline injections based on their chow intake and body weight gain. This may be related to the small volume of acid that was injected and that the first and second injections were not in the same location in the masseter muscle. It has been shown that the acid is buffered rapidly in the masseter muscle and if both injection locations were not in close proximity, it would be similar to providing single injections into the muscle and no long term hyperalgesia would be elicited [25]. A larger volume of saline into the muscle for each injection might allow a larger overlap of exposure to the acidic saline and might reduce the number of non-responders. Since the percentage of non-responders was the same in females and males, this interpretation would be favored over a physiological difference that did not allow the development of the persistent hyperalgesia condition.
4.3 Non-Invasive Assessment of Incising as a Measure of Masticatory Muscle Hyperalgesia
Hyperalgesia assessment in rodent orofacial pain models has been performed using various techniques such as mechanical stimulation with Semmes-Weinstein monofilaments [27, 28], dowel gnawing [29], thermal assessment [30, 31], facial expression [32], air puff stimulation [28], bite force assessment [33], face grooming patterns after formalin injection [34, 35] and anesthetic-induced hindpaw motor behavior [36]. These techniques required anesthetizing the animal [36], confinement or restraint of the animal [28, 29, 33], shaving the face of the rodent [30, 31], had a short duration of hyperalgesia [34, 35] or required human intervention [27, 32]. Human intervention, particularly human males, has been shown to have the potential to introduce experimenter bias [37].
These techniques can be performed in rats but have limited application to evaluation of hyperalgesia in mice. Mice have much higher activity levels than rats and cannot be handled as easily making mechanical assessment using von Frey filaments (or Semmes-Weinstein filaments) a greater challenge to obtain reproducible results. New orofacial hyperalgesia assessments are needed to evaluate the mouse in the home cage environment that do not require an experimenter to be present to make the assessment. Also, monitoring both dark and light cycles allows the assessment of the effects of persistent pain on circadian rhythm and sleep. Our evaluation of incising using a three-dimensional force transducer in the home cage environment over a 24 hour period has provided data on the incising frequency and force during baseline and hyperalgesia conditions and has allowed the detection of sex differences among some of these parameters. The potential bias of experimenter interaction has been minimized using this approach and the recording apparatus has been adapted to the current mouse cage so that mouse adaptation is maximized. Combining the incising force data with general behavior assessment could provide a more complete profile of orofacial pain assessment in the mouse orofacial pain model.
Highlights.
A multiaxis force transducer was used to assess incising in a mouse model
Sex differences for several incising parameters were identified
Incising was composed of five discrete frequencies, not a continuous distribution
Female incising was affected more than male incising in a pre-clinical model of pain
This technique minimizes experimenter bias and is conducted in the mouse home cage
Acknowledgments
This work was supported by the National Institute of Dental and Craniofacial Research/National Institutes of Health (DE021849).
Footnotes
Conflict of Interest: The authors have no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross CF, Dharia R, Herring SW, Hylander WL, Liu ZJ, Rafferty KL, et al. Modulation of mandibular loading and bite force in mammals during mastication. J Exp Biol. 2007;210:1046–63. doi: 10.1242/jeb.02733. [DOI] [PubMed] [Google Scholar]
- 2.Okayasu I, Yamada Y, Maeda T, Yoshida N, Koga Y, Oi K. The involvement of brain-derived neurotrophic factor in the pattern generator of mastication. Brain Res. 2004;1016:40–7. doi: 10.1016/j.brainres.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Weijs WA, Dantuma R. Functional anatomy of the masticatory apparatus in the rabbit. Neth J Zool. 1981;31:99–147. [Google Scholar]
- 4.Herring SW, Grimm AF, Grimm BR. Functional heterogeneity in a multipinnate muscle. Am J Anat. 1979;154:563–76. doi: 10.1002/aja.1001540410. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Masuda Y, Fujimoto Y, Matsuya T, Yamamura K, Yamada Y, et al. Electrophysiological analysis of rhythmic jaw movements in the freely moving mouse. Physiol Behav. 2002;75:377–85. doi: 10.1016/s0031-9384(01)00662-x. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Masuda Y, Kishino M, Ishida T, Maeda N, Morimoto T. Characteristics of mastication in the anodontic mouse. J Dent Res. 2002;81:594–7. doi: 10.1177/154405910208100903. [DOI] [PubMed] [Google Scholar]
- 7.Koga Y, Yoshida N, Kobayashi K, Ichiro O, Yamada Y. Development of a three-dimensional jaw-tracking system implanted in the freely moving mouse. Med Eng Phys. 2001;23:201–6. doi: 10.1016/s1350-4533(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 8.Spruijt BM, Peters SM, de Heer RC, Pothuizen HH, van der Harst JE. Reproducibility and relevance of future behavioral sciences should benefit from a cross fertilization of past recommendations and today's technology: “Back to the future”. J Neurosci Methods. 2014;234:2–12. doi: 10.1016/j.jneumeth.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid Injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther. 2002;302:1146–50. doi: 10.1124/jpet.102.033167. [DOI] [PubMed] [Google Scholar]
- 10.Druzinsky RE. Incisal biting in the mountain beaver (Aplodontia rufa) and woodchuck (Marmota monax) J Morphol. 1995;226:79–101. doi: 10.1002/jmor.1052260106. [DOI] [PubMed] [Google Scholar]
- 11.Vajnerova O, Bielavska E, Jiruska P, Brozek G. Level of vigilance influences licking frequency in rats. Physiol Res. 2003;52:243–9. [PubMed] [Google Scholar]
- 12.Thomas NR, Peyton SC. Relationship of masseter electromyographic activity to mandible position in the freely-moving rat. Arch Oral Biol. 1983;28:1043–6. doi: 10.1016/0003-9969(83)90060-2. [DOI] [PubMed] [Google Scholar]
- 13.Thomas NR, Peyton SC. An electromyographic study of mastication in the freely-moving rat. Arch Oral Biol. 1983;28:939–45. doi: 10.1016/0003-9969(83)90090-0. [DOI] [PubMed] [Google Scholar]
- 14.Bondarenko VE, Cymbalyuk GS, Patel G, Deweerth SP, Calabrese RL. Bifurcation of synchronous oscillations into torus in a system of two reciprocally inhibitory silicon neurons: experimental observation and modeling. Chaos. 2004;14:995–1003. doi: 10.1063/1.1795471. [DOI] [PubMed] [Google Scholar]
- 15.Borisyuk RM, Borisyuk GN. Information coding on the basis of synchronization of neuronal activity. Biosystems. 1997;40:3–10. doi: 10.1016/0303-2647(96)01624-3. [DOI] [PubMed] [Google Scholar]
- 16.Hoppensteadt FC, Izhikevich EM. Oscillatory neurocomputers with dynamic connectivity. Physical Review Letters. 1999;82:2983–6. [Google Scholar]
- 17.Mamiya A, Nadim F. Dynamic interaction of oscillatory neurons coupled with reciprocally inhibitory synapses acts to stabilize the rhythm period. J Neurosci. 2004;24:5140–50. doi: 10.1523/JNEUROSCI.0482-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Po JM, Kieser JA, Gallo LM, Tesenyi AJ, Herbison P, Farella M. Time-frequency analysis of chewing activity in the natural environment. J Dent Res. 2011;90:1206–10. doi: 10.1177/0022034511416669. [DOI] [PubMed] [Google Scholar]
- 19.Mathew D, Zhou P, Pywell CM, van der Veen DR, Shao J, Xi Y, et al. Ablation of the ID2 gene results in altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. PLoS One. 2013;8:e73064. doi: 10.1371/journal.pone.0073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faraday MM. Rat sex and strain differences in responses to stress. Physiol Behav. 2002;75:507–22. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- 21.Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152:990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbs JL, Urban R, Basbaum AI. Paradoxical surrogate markers of dental injury-induced pain in the mouse. Pain. 2013;154:1358–67. doi: 10.1016/j.pain.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: A discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–94. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz G, Lund JP. Modification of rhythmical jaw movements by noxious pressure applied to the periosteum of the zygoma in decerebrate rabbits. Pain. 1995;63:153–61. doi: 10.1016/0304-3959(95)00028-Q. [DOI] [PubMed] [Google Scholar]
- 25.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Widmer CG, Morris-Wiman J. Bilateral trigeminal ganglia neuroplastic changes after unilateral masseter acidic exposure. J Dent Res. 2005;84(Spec Iss A):1521. [Google Scholar]
- 27.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–6. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 28.Krzyzanowska A, Pittolo S, Cabrerizo M, Sanchez-Lopez J, Krishnasamy S, Venero C, et al. Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. J Neurosci Methods. 2011;201:46–54. doi: 10.1016/j.jneumeth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Dolan JC, Lam DK, Achdjian SH, Schmidt BL. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods. 2010;187:207–15. doi: 10.1016/j.jneumeth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–95. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Cha M, Kohan KJ, Zuo X, Ling JX, Gu JG. Assessment of chronic trigeminal neuropathic pain by the orofacial operant test in rats. Behav Brain Res. 2012;234:82–90. doi: 10.1016/j.bbr.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao L, Long H, Zhang L, Chen H, Zhou Y, Ye N, et al. Evaluation of pain in rats through facial expression following experimental tooth movement. Eur J Oral Sci. 2014;122:121–4. doi: 10.1111/eos.12110. [DOI] [PubMed] [Google Scholar]
- 33.Khan J, Benoliel R, Herzberg U, Mannes AJ, Caudle RM, Young A, et al. Bite force and pattern measurements for dental pain assessment in the rat. Neurosci Lett. 2008;447:175–8. doi: 10.1016/j.neulet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan L, Yuan H, Duan L, Cao R, Gao B, Shen J, et al. Blocking the glial function suppresses subcutaneous formalin-induced nociceptive behavior in the rat. Neurosci Res. 2007;57:112–9. doi: 10.1016/j.neures.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Vos BP, Hans G, Adriaensen H. Behavioral assessment of facial pain in rats: face grooming patterns after painful and non-painful sensory disturbances in the territory of the rat's infraorbital nerve. Pain. 1998;76:173–8. doi: 10.1016/s0304-3959(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 36.Ro JY, Capra NF. Assessing mechanical sensitivity of masseter muscle in lightly anesthetized rats: a model for craniofacial muscle hyperalgesia. Neurosci Res. 2006;56:119–23. doi: 10.1016/j.neures.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–32. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]


