Abstract
Cold storage (at 4 °C) offers a compromise between the benefits and disadvantages of cooling. It allows storage of organs or cells for later use that would otherwise quickly succumb to warm ischemia, but comprises cold ischemia that, when not controlled properly, can result in severe damage as well by both similar and unique mechanisms. We hypothesized that polyethylene glycol (PEG) 35 kDa would ameliaorate these injury pathways and improve cold primary hepatocyte preservation. We show that reduction of the storage temperature to below zero by means of supercooling, or subzero non-freezing, together with PEG supplementation increases the viable storage time of primary rat hepatocytes in University of Wisconsin (UW) solution from 1 day to 4 days. We find that the addition of 5% PEG 35 kDa to the storage medium prevents cold-induced lipid peroxidation and maintains hepatocyte viability and functionality during storage. These results suggest that PEG supplementation in combination with supercooling may enable a more optimized cell and organ preservation.
Keywords: hepatocytes, preservation, supercooling, University of Wisconsin solution, lipid peroxidation, polyethylene glycol
Introduction
The plasma membrane plays a key role in cell viability, as the protective barrier between cells and their environment. A change in temperature alters the physical properties of cellular membranes [15], and could be detrimental to cell survival [17]. Protection of the cell membrane is therefore one of the main concerns when devising cold preservation protocols. Typically, cells are preserved in University of Wisconsin (UW) solution at 0–4 °C – conditions similar to whole-organ preservation – when storing for a limited period of time. Better viability and functionality of primary hepatocytes after storage could be achieved by a customized preservation technique, based on supercooling. Recent work from our laboratory is based on this premise, lowering the conventional storage temperature of 4 °C (hypothermic storage) to −4.4 °C, without the formation of ice. In doing so, we were able to increase the viable storage time of rat hepatocytes and whole livers [1,18].
Recent work from our laboratory has shown that membrane damage is evident in primary hepatocytes after cold storage or supercooling [18]. Moreover, we found that only livers that were stored in UW supplemented with 5% polyethlylene glycol 35 kDa (PEG) yielded successful orthotopic transplantation after 96 hours of supercooling preservation [1]. PEG has shown multiple benefits in cell and organ preservation, including antioxidant capacity, preventing edema, membrane stabilization and, in the context of subzero preservation, freeze-protection [2,13,14]. In the present study, we compared supercooling storage of primary hepatocytes at −4.4 °C to the current standard of cold storage at 4 °C for extending preservation times, specifically investigating the protective effect of PEG on supercooled hepatocytes and the potential role of lipid peroxidation.
Materials and Methods
Hepatocyte Isolation
Primary rat hepatocytes obtained from the Cell Resource Core at Massachusetts General Hospital (MGH) were used for all experiments. The animals were maintained in accordance with National Research Council guidelines, and the experimental protocols were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital. Briefly, primary hepatocytes were isolated from 2–3 month old female Lewis rats (Charles River, MA), as described previously [6] by the MGH Cell Resource Core. Routinely, 200–300 million cells were isolated with viability between 93–98% as determined by trypan blue exclusion. Cells were kept on ice for a maximum of 5 minutes which was logistically necessary to initiate the processing for plating or storage, in Dulbecco’s Modified Eagle Medium (DMEM, Gibco), supplemented with 20 ng/mL EGF (Sigma), 7.5 ug/mL hydrocortisone (Upjohn Co), 7 ug/mL glucagon, 0.5 unit/mL insulin, 200 units/mL penicillin, 200 ug/mL streptomycin and 10% FBS (Gibco).
Experimental groups
Primary hepatocytes were treated in five groups; two supercooling groups, in which cells were preserved at −4.4 °C, and two groups stored at 4 °C. At both temperatures, one group was preserved in UW solution and one in UW solution supplemented with PEG. A control group was cultured fresh without storage.
Cold storage and supercooling
Directly after isolation, fresh cells were plated for culture on a 12-well plate as described below (control), or prepared for storage. For storage, cells were centrifuged at 300g for 5 min at 4 °C and gently resuspended in UW solution (CoStorSol, Preservation Solution Inc.) or in UW solution supplemented with 5% PEG 35 kDa (Sigma) at 2 million cells/mL. The suspensions were aliquotted into pre-cooled 1.5 mL cryovials (Nalgene), 1 mL/vial, and transferred for storage at either 4 °C (cold storage), or −4.4 °C (supercooling). The supercooling temperature was based on an optimal viability found in previous experiments [18]. Cold stored samples were stored in a temperature-controlled room for 1, 4 or 6 days, Supercooled samples were stored in a portable controlled-rate freezer (Engel MHD-13, Engel-USA Inc.) for matching durations. Please note that in the case of supercooling, the actual freezer setting was 24°F (−4.4 °C). To minimize temperature variations, the device was placed in the same cold room as the cold controlled samples. In our observations the temperature remained within a range of about ±2°F, where the thermostat cut in and cut out were observed. Usta et al have tested different temperatures for hepatocyte supercooling in this setting & environment [18] and have shown that lower temperatures (−7 °C and −10 °C) leads to significantly increased likelihood of freezing.
After storage, cells were spun down at 300g for 5 min at 4 °C and the supernatant was collected for lactate dehydrogenase (LDH) analysis. The cells were washed once, resuspended in culture medium and then were allowed to recover at 37 °C while being gently rotated. We found previously that determining the cell viability directly upon taking them out of storage overestimates the number of viable cells, therefore viability was determined after allowing the cells to recover for 1 h at 37 °C. LDH quantification was performed after 1 h of recovery as well. The cells were spun down after, washed once, resuspended in 1 mL culture medium, and the viability was determined by trypan blue exclusion. 0.4 M live cells were plated per well in a 12-well plate as described below. Cells were examined daily and imaged with a Zeiss Axiovert 200 M Microscope using phase contrast microscopy. Experiments were performed on three different hepatocyte isolation batches and in triplicate for each isolation.
Hepatocyte culture
Hepatocytes were cultured after storage or fresh (controls) in collagen sandwich gels at 37 °C and exposed to 10% CO2, 90% O2. Collagen (type I) extracted from Lewis rat-tail tendons was used for culture (Cell Resource Core, MGH). The first collagen layer (1 part 10x DMEM pH 7.4, 9 parts collagen solution at 1.11 mg/mL) was allowed to gel at 37 °C in a 12-well culture dish (200 μL/well). Following storage, cells were left to recover in suspension for 1 h at 37 °C before plating. 0.4 million viable hepatocytes in culture medium were seeded on this layer and allowed to attach at 37 °C for 4 h, after which the media was refreshed. At 24 h (in the case of fresh cells) or 48 h after seeding (in the case of stored cells), the media was removed, the cells were washed with PBS once and were then overlaid with a second layer of collagen, which was allowed to gel for 1 h at 37 °C. After gelling, 500 μL culture medium was added to each well. The medium was changed daily for 12 days and was sampled and stored at −20 °C for further analysis.
Measurement of albumin, LDH and lipid peroxidation
Albumin secreted in the medium was analyzed by ELISA, as described previously [5], using an anti-rat albumin polyclonal antibody (Organon Teknika Corp).
Membrane integrity was assessed by measuring the amount of cytoplasmic LDH released into the medium, using a cytotoxicity detection kit for LDH (Sigma) following the manufacturer’s protocol. LDH levels are presented as a percentage of LDH in fresh cells, which was obtained by sonication of freshly isolated cells followed by LDH analysis, reflecting the total LDH concentration.
Cold-induced cell injury has been associated with the formation of reactive oxygen species [10]. To further elucidate the nature of cellular injury that hepatocytes sustain during storage, we used thiobarbituric acid-reactive substances (TBARS) to analyze the cells for the presence of lipid peroxidation. During lipid peroxidation, a polar oxygen moiety (hydroperoxy group) is introduced into the hydrophobic tails of unsaturated fatty acids. The end product of lipid peroxidation is malondialdehyde (MDA) [11]. In the TBARS assay, thiobarbituric acid reacts with MDA to yield a measurable product, detected using an assay (Zeptometrix), following the manufacturer’s protocol. For each sample, 8 million cells were used that were sonicated in 500 μL PBS over ice. 100 μL of the homogenate was used in the assay.
Statistical analysis
To analyze the differences in cellular viability and cellular metabolite output (Albumin, LDH, TBARS) over time using different temperatures and the addition of PEG to the storage medium, 2-way repeated measures analysis of variance (ANOVA) with bonferroni post-testing was used. A p value of p<0.05 was applied to determine statistical significance.
Results
Supercooling with UW PEG reduces cell injury after extended preservation
To assess membrane damage under the various storage conditions and preservation parameters used in this study, LDH leakage was measured in the storage solution right after storage (cold) as well as after the 1 h recovery phase at 37 °C of the same samples. Figure 1 shows LDH release as a percentage of the total amount of LDH in fresh cells. Regardless of the storage medium, temperature or storage time, LDH release is more significant after the recovery phase at 37 °C for an hour than after storage. With the exception of the cells stored for 6 days at 4 °C, which have significantly higher LDH release during storage, LDH release is 3.5 – 11.2 % for both cold storage and supercooling in UW solution immediately after cold storage (Fig. 1A). The samples supplemented with PEG have a lower LDH release during storage in all groups (p<0.01), indicating that the addition of PEG suppresses LDH leakage. LDH release is significantly higher for cells stored for 6 days at 4 °C, regardless of the storage medium (31% for UW and 29% for UW PEG stored cells), indicating that cells cannot withstand 6 days of storage at 4 °C in either storage medium and reinforcing the benefit of supercooled preservation. The effect of PEG is mitigated during rewarming, although a significant effect is still noted after prolonged storage. In addition, this experiment shows that damage due to supercooling or cold storage becomes more apparent after bringing the cells back to their physiological temperature (Fig. 1B).
Figure 1. Lactate dehydrogenase (LDH) leakage.
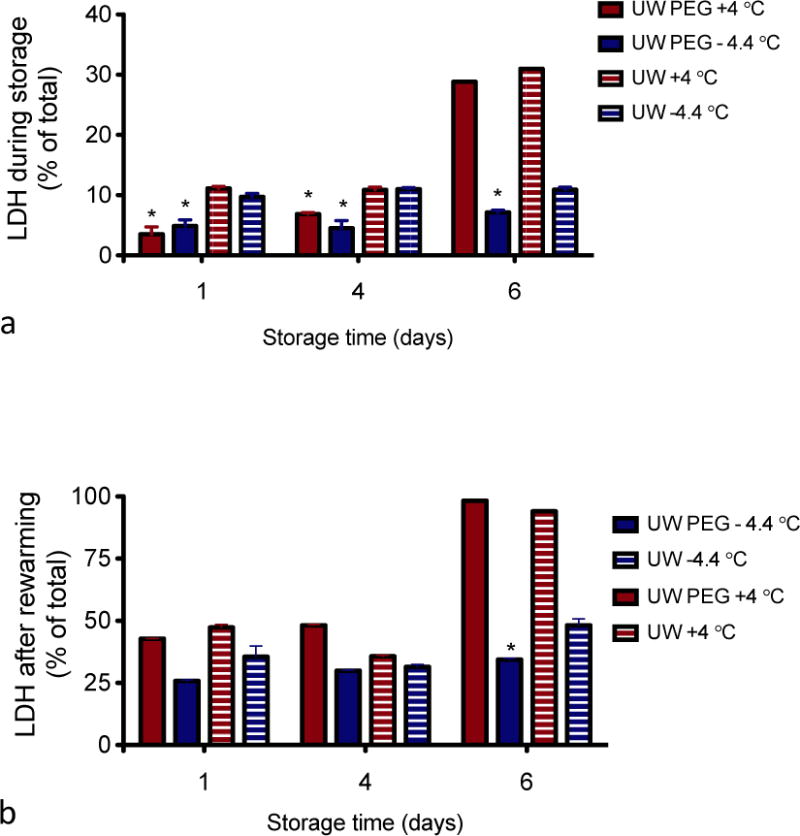
LDH concentration was determined at the end of storage (a) and following 1 h of recovery at 37 °C (b). LDH release is normalized to total cellular LDH content of freshly isolated cells determined by sonication * = significantly lower than without PEG.
Lowering of the storage temperature, and supplementation with PEG improves cell viability after extended preservation
Figure 2 shows cell viability as a function of the preservation time (1, 4 and 6 days) and temperature (4 °C and −4.4 °C). The viability is represented as a percentage of the viability of freshly isolated cells. We found that the majority of primary hepatocytes were able to endure storage for 24 hours, with insignificant differences between storage groups. However, when the storage time is extended, both the storage temperature and medium become important; at 4 days, the viability is significantly higher when cells are supercooled compared to cold storage (p<0.01). Additionally, when UW solution is supplemented with PEG cell survival improved at supercooled temperature (p<0.001). These differences were even more pronounced when cells are stored for 6 days (p<0.001).
Figure 2. Cell viability after storage.
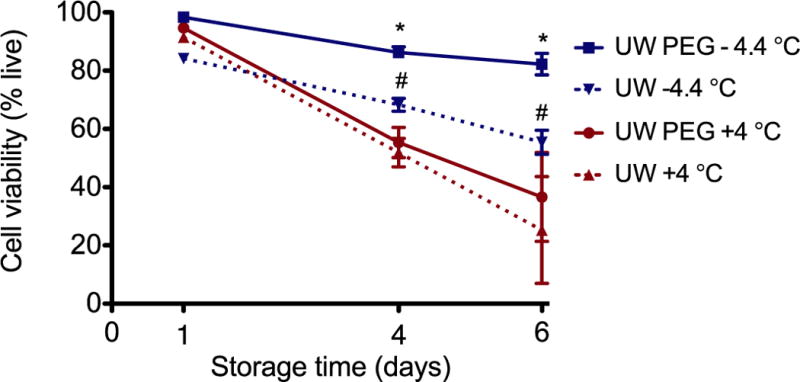
The viability was determined following an hour of recovery at 37 °C and is represented as a percentage of the viability of freshly isolated cells. (* = significantly higher than UW −4.4 °C and higher than with UW PEG at 4 °C. # = significantly higher than UW 4 °C, without PEG)
Albumin secretion and morphology are best preserved by supercooling and is contingent on supplementation with PEG
Figure 3A shows there are no significant differences in albumin secretion between the various storage conditions when cells are stored for 1 day, and secretion is higher than fresh cells that have been injured during isolation (collagenase treatment and perfusion digestion shear stress [6]). Increasing duration of storage to 4 days, storage modality influences synthetic capacity, with a better-preserved albumin production at −4.4 °C in UW PEG, which is consistent with cell viability results (Fig. 3B). In this group albumin secretion is similar to that of control cells. In all other groups, the albumin levels drop below those measured in fresh control cells. After 6 days of storage at 4 °C cells were no longer viable. However, cells supercooled for 6 days in UW PEG survived and displayed albumin production similar to fresh control cells (Fig. 3C), showing the importance of PEG supplementation (P<0.05). In contrast, supercooled cells in UW solution without PEG supplementation did not show any albumin production.
Figure 3. Albumin production in culture following storage.
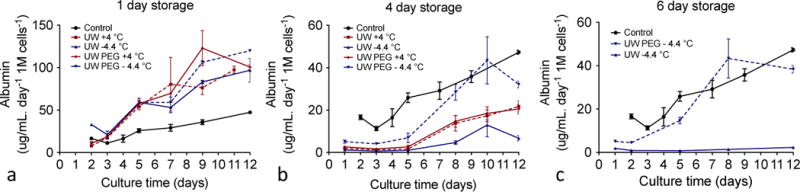
Cells were plated after 1, 4 and 6 days of storage (a, b, c, respectively) and albumin production was quantified for each group. After 6 days, cells stored at 4 °C were not longer viable and there were no albumin results.
Figure 4 shows phase-contrast microscopy of hepatocytes that were cultured for 7 days after a 4-day storage period at −4.4 °C. Whereas the cells that were stored in UW are mostly rounded (middle panel), the cells stored in UW PEG display the trabecular structure (right panel) comparable to fresh cells (left panel).
Figure 4. Morphology of hepatocytes in plate culture.
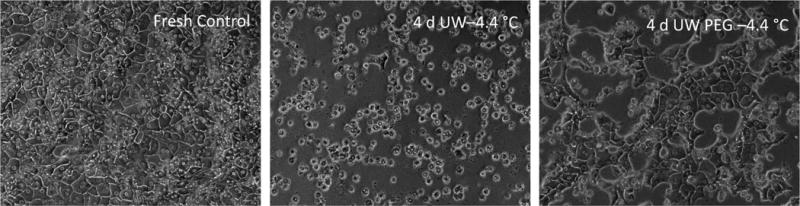
Fresh hepatocyte morphology (a), compared to hepatocytes after 4 days of preservation at −4.4 °C without (b) and with PEG supplementation (c).
Supplementing UW solution with PEG during storage reduces lipid peroxidation in hepatocytes
Similar to the LDH experiments described above, we measured MDA formation right after cold storage or supercooling, and after the 1 h recovery at 37 °C. As shown in Figure 5A, right after storage, all cells stored in UW solution only (at either 4 °C or −4.4 °C) display a 1.5- to 2.5-fold increase in lipid peroxidation when compared to fresh cells. The amount of lipid peroxidation in the samples supplemented with PEG is significantly lower than just UW solution at both temperatures (p<0.001). In addition, similar to our observations with LDH, lipid peroxidation already occurs after 1 day of storage. Lipid peroxidation subsequently decreases during the 1 h recovery at 37 °C in the cells stored in UW solution, whereas it remains approximately the same at low levels in the cells stored in UW solution supplemented with PEG, regardless of storage temperature (Fig. 5B).
Figure 5. Lipid peroxidation (thiobarbituric acid reactive substances).
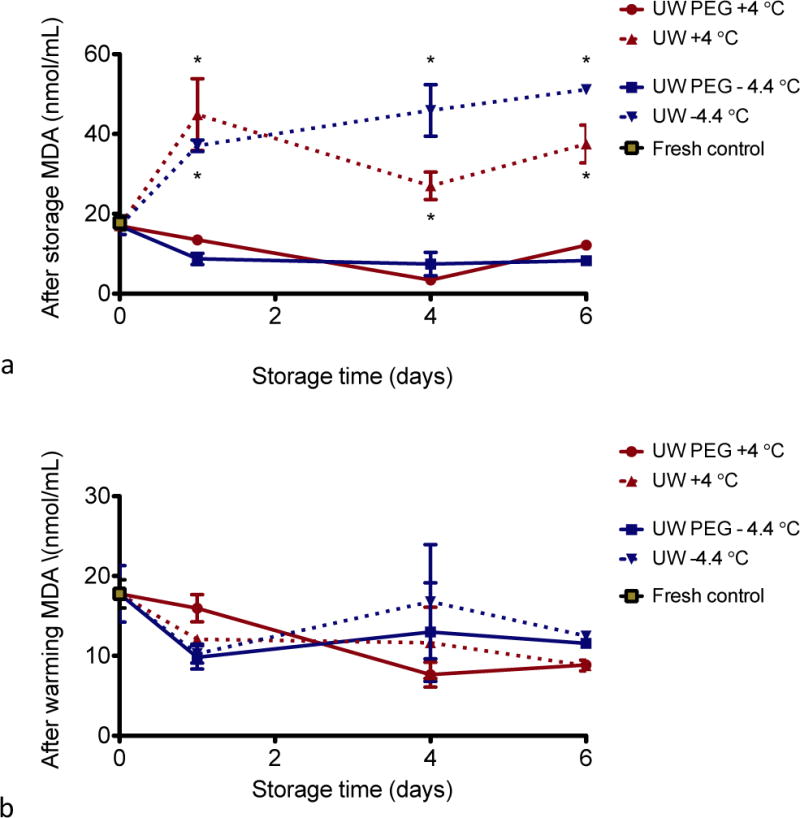
TBARS assay was performed at the end of storage (a) and following 1 h of recovery at 37 °C (b). * = significantly higher than without PEG.
Discussion
The development of the UW solution for cold storage in the late 1980s by Folkert Belzer and James Southard greatly increased the quality and storage time of preserved organs and has since been used as a preservation solution for various organs, tissues and cells [1]. In UW solution, whole-liver preservation times of up to 12 hours are well tolerated. Even so, a further extension of viable liver storage to several days is very desirable, as it would allow transport of organs between transplant centers, more rigorous tissues matches, and thus more life-saving transplant surgeries. As such, our laboratory has recently developed a novel method to produce transplantable rat livers after 3 and 4 days of storage, which involves supercooling of the liver to −6 °C in UW solution supplemented with PEG [1]. Moreover, extending the viable preservation of single cells would greatly enhance cell transplantation, bio-artificial liver devices and the logistical planning of these procedures. To understand the effects of supercooling, in the current study we looked at its effects on the cellular level. In this paper we have shown that in order to successfully increase storage time of primary hepatocytes, the storage temperature needs to be decreased, rendering a non-frozen, supercooled state, and supplementation of the UW solution with PEG is required for preserved viability and function. Lipid peroxidation is significantly reduced, and is suggested to play a role in injury during cold storage and rewarming.
When storing primary hepatocytes for only 24 hours, our experiments demonstrate that the viability does not differ significantly between standard storage conditions at 4 °C (cold storage) or at our decreased temperature of −4.4 °C (supercooling) in either UW or UW supplemented with PEG. Interestingly however, LDH release as well as lipid peroxidation after 1 day of storage is comparable to after 4 days of storage, indicating that cold-induced damage already occurs during short-term storage. It becomes apparent, since the albumin secretion and cellular morphology were found not to be inferior to fresh cells, that the damage sustained after 24 hours is quickly reversible and/or does not impair cellular machinery significantly. Extending the storage time beyond 1 day causes more extensive damage that is evidently not reversible when the cells are stored in UW at 4 °C, as shown morphologically, and by albumin secretion and in viability. In fact, only cells stored at −4.4 °C in UW supplemented with PEG were able to regain a functionality that is comparable to fresh cells after 6 days of storage. This indicates that both the effects of lower storage temperature and PEG supplementation of the storage medium are prerequisites for long-term viable preservation without loss of function.
In prior work we demonstrate that supercooling at −4.4°C better preserves ATP stores over storage at +4°C [18]. The work here and the work of others suggest that there are multiple additional mechanisms involved in hypothermic cell survival and killing [12]. Notably, studies in bacteria, plants, worms, flies and human cells have shown that the cell membrane is a primary site of chilling or cold-shock injury, which is manifested by damage to intracellular organelles and the leakage of ions and other solutes across cell membranes [15]. One of the main consequences of cold ischemia is excessive production of reactive oxygen species (ROS), which can result in lipid peroxidation [4]. In this context, it has been shown that the anti-oxidant additive in UW solution (glutathione) is not sufficient to preserve cells beyond 24 hours as it has a half-life of 1 day [9]. Our results show that the cells sustain membrane damage as a result of cold as well as supercooling storage, and that this was significantly reduced by the addition of PEG to the UW solution. PEG has been well noted to benefit in the context of cell and organ preservation, and most likely one of it’s effects is to shield the lipids from free radicals. PEG was noted to suppress the diffusion of free radicals, thereby playing the role of an indirect antioxidant to inhibit lipid peroxidation [3]. Low molecular weight PEG (5kDa) is reported to accumulate in hepatocytes [13]. MWs of 400–20,000 were noted to activate JNK signaling which may be either protective or harmful [7] as well as interact with membrane glycerophospholipids [8], and the 35KDa PEG was noted to both protect against necrosis and enable membrane stabilization[8] in renal cells. The exact mechanisms remain to be elucidated, but our results demonstrate clear reduction in intracellular MDA levels as a direct result of 35kDa PEG. Also to this point, our experiments show that upon removal of PEG during the rewarming phase (since it is toxic at 37 °C), lipid peroxidation increases slightly but noticably. Screening for other additives to alleviate and/or prevent membrane damage and oxidative stress in particular for use in rewarming is likely to further optimize preservation.
In conclusion, UW solution is sufficient to preserve cells for short periods of time at 4 °C, but in order to obtain viable and functional cells after 4 days of storage, the temperature needs to be reduced to −4.4 °C, and the UW solution needs to be supplemented with 5% PEG 35 kDa, which prevents peroxidation of lipids and maintains hepatocellular function and viability. Improved cell survival after supercooling preservation will facilitate various cell-based treatments, organ transplantation and the day-to-day logistics of in vitro studies.
Acknowledgments
Funding from the US National Institutes of Health (R01EB008678, R01DK096075, R01DK084053), and the Shriners Hospitals for Children is gratefully acknowledged.
Abbreviations
- UW
University of Wisconsin
- PEG
polyethylene glycol
- MGH
Massachusetts General Hospital
- DMEM
Dulbecco’s Modified Eagle Medium
- LDH
lactate dehydrogenase
- TBARS
thiobarbituric acid-reactive substances
- MDA
malondialdehyde
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors of this manuscript have conflicts of interest to disclose: Authors are inventors on patent application WO/2011/002926 (KU and MLY); WO/2011/35223(TAB, OBU, MLY, KU), and MGH Disclosure 22743 (BGB, KU). KU has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. Dr Uygun’s interests are managed by the MGH and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Berendsen TA, Bruinsma BG, Puts CF, Saeidi N, Usta OB, Uygun BE, Izamis ML, Toner M, Yarmush ML, Uygun K. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat Med. 2014;20:790–793. doi: 10.1038/nm.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatnagar BS, Martin SM, Teagarden DL, Shalaev EY, Suryanarayanan R. Investigation of PEG crystallization in frozen PEG-sucrose-water solutions: II. Characterization of the equilibrium behavior during freeze-thawing. J Pharm Sci. 2010;99:4510–4524. doi: 10.1002/jps.22182. [DOI] [PubMed] [Google Scholar]
- 3.Cheng CY, Wang JY, Kausik R, Lee KY, Han S. Nature of interactions between PEO-PPO-PEO triblock copolymers and lipid membranes: (II) role of hydration dynamics revealed by dynamic nuclear polarization. Biomacromolecules. 2012;13:2624–2633. doi: 10.1021/bm300848c. [DOI] [PubMed] [Google Scholar]
- 4.Crockett EL. The cold but not hard fats in ectotherms: consequences of lipid restructuring on susceptibility of biological membranes to peroxidation, a review. J Comp Physiol B. 2008;178:795–809. doi: 10.1007/s00360-008-0275-7. [DOI] [PubMed] [Google Scholar]
- 5.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 6.Dunn JC, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992;116:1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutheil D, Underhaug Gjerde A, Petit-Paris I, Mauco G, Holmsen H. Polyethylene glycols interact with membrane glycerophospholipids: is this part of their mechanism for hypothermic graft protection? Chem Biol. 2009;2:39–49. doi: 10.1007/s12154-009-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutheil D, Rioja-Pastor I, Tallineau C, Goujon JM, Hauet T, Mauco G, Petit-Paris I. Protective effect of PEG 35,000 Da on renal cells: paradoxical activation of JNK signaling pathway during cold storage. Am J Transplant. 2006;6:1529–40. doi: 10.1111/j.1600-6143.2006.01343.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans PJ, Tredger JM, Dunne JB, Halliwell B. Catalytic metal ions and the loss of reduced glutathione from University of Wisconsin preservation solution. Transplantation. 1996;62:1046–1049. doi: 10.1097/00007890-199610270-00002. [DOI] [PubMed] [Google Scholar]
- 10.Guillouzo A, Rialland L, Fautrel A, Guyomard C. Survival and function of isolated hepatocytes after cryopreservation. Chem Biol Interact. 1999;121:7–16. doi: 10.1016/s0009-2797(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez LA, Granger DN. Role of antioxidants in organ preservation and transplantation. Critical care medicine. 1988;16:543–549. doi: 10.1097/00003246-198805000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Kruuv J, Glofcheski DJ, Lepock JR. Evidence for two modes of hypothermia damage in five cell lines. Cryobiology. 1995;32:182–190. doi: 10.1006/cryo.1995.1017. [DOI] [PubMed] [Google Scholar]
- 13.Mack JE, Kerr JA, Vreugdenhil PK, Belzer FO, Southard JH. Effect of polyethylene glycol on lipid peroxidation in cold-stored rat hepatocytes. Cryobiology. 1991;28:1–7. doi: 10.1016/0011-2240(91)90002-6. [DOI] [PubMed] [Google Scholar]
- 14.Oltean M, Joshi M, Björkman E, Oltean S, Casselbrant A, Herlenius G, Olausson M. Intraluminal polyethylene glycol stabilizes tight junctions and improves intestinal preservation in the rat. Am J Transplant. 2012;12:2044–2051. doi: 10.1111/j.1600-6143.2012.04067.x. [DOI] [PubMed] [Google Scholar]
- 15.Quinn PJ. Principles of membrane stability and phase behavior under extreme conditions. J Bioenerg Biomembr. 1989;21:3–19. doi: 10.1007/BF00762209. [DOI] [PubMed] [Google Scholar]
- 16.Steinhardt RA. The mechanisms of cell membrane repair: A tutorial guide to key experiments. Ann N Y Acad Sci. 2005;1066:152–165. doi: 10.1196/annals.1363.017. [DOI] [PubMed] [Google Scholar]
- 17.Uemura M, Steponkus PL. A Contrast of the Plasma Membrane Lipid Composition of Oat and Rye Leaves in Relation to Freezing Tolerance. Plant Physiol. 1994;104:479–496. doi: 10.1104/pp.104.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usta OB, He X, Kim Y, Ozer S, Bruinsma BG, Lee J, Demir E, Berendsen TA, Puts CF, Izamis ML, Uygun K, Uygun BE, Yarmush ML. Supercooling as a Viable Non-Freezing Cell Preservation Method of Rat Hepatocytes. PLoS ONE. 2013;8:e69334. doi: 10.1371/journal.pone.0069334. [DOI] [PMC free article] [PubMed] [Google Scholar]


