Abstract
Background
Dementia costs are critical for influencing healthcare policy, but limited longitudinal information exists. We examined longitudinal informal care costs of dementia in a population-based sample.
Methods
Data from the Cache County Study included dementia onset, duration and severity assessed by the Mini Mental State Examination (MMSE), Clinical Dementia Rating Scale (CDR) and Neuropsychiatric Inventory (NPI). Informal cost of daily care (COC) was estimated based on median Utah wages. Mixed models estimated the relationship between severity and longitudinal COC in separate models for MMSE and CDR.
Results
287 subjects [53% female, mean (sd) age was 82.3(5.9) years] participated. Overall COC increased by 18%/year. COC was 6% lower per MMSE-point increase and compared with very mild dementia, COC increased over 2-fold for mild, 5-fold for moderate and 6-fold for severe dementia on the CDR.
Conclusions
Greater dementia severity predicted higher costs. Disease management strategies addressing dementia progression may curb costs.
1. Introduction
Among older adults, dementia prevalence is high and quickly rising. Without significant advances in prevention and treatment options, worldwide estimates of 35.6 million today will reach 115 million by the year 2050(1). Alzheimer’s disease (AD), the most common type of dementia in late life, afflicts 5.2 million Americans; this number is projected to reach 13.8 million in 2050(2).
Regardless of etiology, most conditions causing dementia are progressive, with continuous cognitive and functional decline, and often accompanied by unwanted neuropsychiatric symptoms (NPS). As a result, dementia is one of the most costly conditions for older adults, placing significant burden on patients, their family members, healthcare systems and society. In the U.S., aggregate direct healthcare costs including short- and long-term institutional stays for dementia patients are estimated by the Alzheimer’s Association at $203 billion in 2013 and projected to increase to $1.2 trillion by 2050(2). Despite major differences between the individual cost components of dementia and cardiovascular disease (disease onset age and related factor of loss of workforce productivity, and institutionalization) for very rough comparison purposes, the estimated cost of dementia is on the order of that of cardiovascular disease in 2009 and 2010 [excluding nursing home costs of those with cardiovascular disease (3, 4)].
Direct costs associated with dementia care include physician and nursing services, home care, institutional care and informal caregiving costs. With the exception of the latter, all of these costs must be purchased. Informal caregiving costs can be valued in terms of replacement cost or forgone wages and accounts for a substantial portion of the total costs of dementia care (e.g., 11). The total costs of dementia care have been shown to increase as the condition progresses from mild to more severe stages(5).
Prior studies that have estimated the component costs of dementia care and their predictors have predominantly used information from administrative databases such as Medicare databases. While a benefit of such studies is the large sample size, the sources have significant limitations such as lower sensitivity of disease ascertainment, which can lead to inaccurate estimates of individual costs(2,6). Further, many of these studies are cross-sectional in nature, and do not capture individual-level longitudinal costs. Informal caregiver costs that are generally not paid by Medicare are also excluded. Two large studies have examined longitudinal costs of care relying on information from patient surveys: The Predictors Study in the United States(5,7,8) and a French study(9). Notable strengths of The Predictors Study included follow-up time of 7 years and longitudinal estimates of unpaid caregiver costs. The French study had a large sample size and examined the association of costs with worsening functional impairment. However, The Predictors Study drew dementia patients from academic clinical centers while the French study drew dementia patients from a randomized clinical trial. Neither study’s population is representative of typical community-dwelling dementia patients and their caregivers, for example, the greater likelihood of individuals from clinic samples to be married and of higher socioeconomic status than persons drawn from the general community(10). Recently Hurd et al.(11) estimated that the yearly cost of care attributable to dementia ranged from $41,689 to $56,689 (in 2010 dollars) depending on the method used to estimate informal caregiving costs. Informal care costs accounted for 31% to 49% of the total costs of dementia care. The study sample was drawn from the Health and Retirement Study (HRS), using individual, direct clinical assessment to identify a subsample with dementia and imputation to identify the final large sample with probable dementia (N = 10,903). As noted by the authors, the estimated total costs of dementia care were lower than those of the Alzheimer’s Association(2) likely due to differences in study populations, dementia severity, and correction for costs of care attributable to comorbid conditions. While the Hurd sample is likely to be representative of a typical community-dwelling population with dementia, costs of dementia care or time devoted to dementia care vary with dementia severity(8,12,13) or the presence of NPS(14). These factors were not examined in the HRS.
The Cache County Dementia Progression Study (DPS), is comprised of community dwelling participants, where all dementia cases were clinically diagnosed(15) and followed for as many as 7 years, with rich information including caregiver hours, dementia progression, and NPS(16). These data enable an estimation of the effect of disease severity and NPS on longitudinal informal dementia care costs in the community. With projected rising dementia prevalence and associated costs of informal dementia care, quantifying longitudinal informal care costs is critical for influencing health care policy and resource planning. While there are cross-sectional estimates of these costs across disease severity, there is scant longitudinal information using U.S. data. Our objective was to determine the effect of dementia severity on the longitudinal costs of informal dementia care in this population-based sample of persons with dementia.
2. Methods
This study used extant data from the prospective, population-based study of dementia, the DPS, which observed persons with dementia and their caregivers semiannually for up to 7.8 years (mean=1.6; sd=1.9; median=1.3). Cases of dementia were identified from the Cache County Study on Memory in Aging in four triennial waves of dementia screening and ascertainment, described previously(15,17). The eligible participants, permanent residents of Cache County, Utah, aged 65 years or older in 1995 (Wave 1) and found to be without dementia, were rescreened and assessed in subsequent waves: 1998–1999, 2002–2004, and 2005–2007. All cases of dementia were identified from a detailed clinical examination that included a physical and neurological examination, neuropsychological testing, and clinical information provided by a knowledgeable informant. Subsequently, participants with suspected dementia were asked to complete brain imaging and standard laboratory tests for dementia, and a physician exam to aid in the differential diagnosis of dementia. A panel of experts in neurology, geropsychiatry, neuropsychology or cognitive neuroscience assigned a consensus diagnosis for each case. Persons were classified with dementia if they met DSM-III-R criteria(18). AD was assigned following National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria(19) and vascular dementia, the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria(20). Age of dementia onset was assigned as the age at which the individual unambiguously met DSM-III-R criteria for dementia. Incident cases of dementia were followed by the Cache County Study after 18-months and subsequently with their caregivers in the DPS. Only incident, non-institutionalized participants were included in the analyses.
2.1 Procedures of the DPS
Starting in 2002, the DPS invited surviving Cache County Study participants with dementia and their caregivers to join a longitudinal study to examine factors that influence the rate of progression of cognitive, functional and NPS in dementia(16). Subjects completed a core battery of neuropsychological tests at semi-annual visits with supplemental measures at alternating (annual) visits. The core battery included the Mini-Mental State Exam (MMSE) and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological test battery(21). A caregiver was interviewed about the participant’s current functional abilities using the Dementia Severity Rating Scale (DSRS)(22), and NPS using the UCLA 12-item Neuropsychiatric Inventory (NPI)(23). At each odd-numbered visit, the caregiver also completed an interview about the amount of time the participant received assistance in daily tasks and other sources of support including community services. The former survey formed the basis for estimating caregiver costs (see Measures). Following each visit, the assessment team, in consultation with a study neuropsychologist or geropsychiatrist, completed the Clinical Dementia Rating (CDR)(24) based on all available clinical data. Ratings of 0(normal), 0.5(questionable), 1(mild), 2(moderate), 3(severe), 4(profound) and 5(terminal) were assigned to each of the following domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. A global CDR was determined, following a standard algorithm and a sum of boxes (CDR-SB) score was also calculated by summing scores across domains(24). The range of values for the CDR-SB was 0–30. Research staff also assigned a rating of overall health using the General Medical Health Rating based on a standard physical exam (review of systems, blood pressure), review of medical history and medications, and frailty(25). The range of values for the GMHR are: 1(poor), 2(fair), 3(good), and 4(excellent).
2.2 Measures
2.2.1 Outcome variable: Informal costs of care
As noted above, at alternating visits, caregivers completed a caregiver activity survey about how much time was spent over the past 24 hours by all caregivers providing supportive care for the participant in areas such as answering questions, leaving reminders, using transportation, dressing, assisting with meals and eating, assisting with grooming, and supervising the participant’s activities. Values for each activity were summed to a maximum of 16 hours, following previous studies(5,26).
To estimate the cost of informal care in 2012 dollars, we used the Utahn median hourly wage as reported in the U.S. Bureau of Labor’s Occupational Employment Statistics(27) for the year of the visit (spanning from 2002–2011), adjusting for inflation using a derived Medical Consumer Price Index (MCPI) multiplier based on the “medical care services” values from the annual average Urban Consumer Price Index (CPI-U). The following example illustrates conversion of 2002 Utah median hourly wage into 2012 dollars. The 2002 MCPI multiplier was calculated [(2012 CPI-U)/(2002 CPI-U)] and then multiplied by the 2002 Utahn median hourly wage of $12.23, yielding the 2012 equivalent wage of $18.39.
In a similar fashion, we estimated the equivalent national costs using this sample, by obtaining the median hourly wage for U.S. citizens(27). The Utah estimates of informal costs were used in inferential statistical models whereas the national estimates were provided for descriptive purposes only. To describe informal care costs at various levels of dementia severity, we used the global CDR (0.5=very mild, 1=mild, 2=moderate, 3–5=severe) at the last visit of each participant.
2.2.2 Predictor variables
To examine factors associated with informal costs of dementia care, we used the MMSE(28) as a cognitive indicator of dementia severity, and the CDR-SB as a functional indicator at each visit. The MMSE is a well-known and validated cognitive test used to gauge level of dementia severity(29). Its items assess orientation, working and episodic memory, language and praxis. As described previously, sensory and motor adjustments were made for items confounded by physical impairment for up to 10% of the total score(16). Total scores on the MMSE range from 0–30. We chose the CDR-SB over the global CDR due to the increased range of values of the former, and lower variability in the latter as most participants were in the mild range at the beginning of the observation period. Due to significant correlations between the MMSE and CDR(16), we conducted informal cost models separately using either measure as a time-varying predictor.
We also examined whether NPS at each visit predicted the cost of informal care, using the total NPI score. The NPI assesses delusions, hallucinations, depression, anxiety, irritability, agitation/aggression, disinhibition, euphoria, aberrant motor behavior, apathy, sleep and appetite. Each domain receives a score from 0 (not present) to a maximum of 12, which represents the product of frequency and intensity ratings to yield a domain severity score. The scores across all 12 domains are summed to yield a total NPI score that ranges from 0–144. NPI was used as a time-varying predictor of informal care costs.
Other covariates examined in inferential models included age of dementia onset, type of dementia (Alzheimer’s, vascular, other), overall general health rating (GMHR), gender, and education. Only significant (p <= 0.05) terms were retained in the adjusted models.
2.3 Analytic approach
We provided descriptive statistics of costs by levels of dementia severity, testing for mean differences using analysis of variance. To examine longitudinal informal costs of care, we modeled these costs at each visit as the outcome and time, modeled from dementia onset, as a predictor variable using linear mixed effects models with time and intercept as random effects. These models afford the flexibility of accommodating unequally spaced visits across participants and incomplete visits or dropouts, where subjects contribute data up to the point of nonparticipation. We also used a log link function to the gamma distribution to accommodate the positively skewed distribution of cost data. To test whether each predictor significantly affected the average informal cost of care across time, each predictor was entered individually and retained if −2 Log Likelihood chi-square test (-2LL) comparing a model with and without the term was significant at p<0.05. Significance of each predictor on the rate of change in informal costs, was tested with -2LL comparing a model with and without the predictor*time interaction, retaining the interaction if p<0.05. All statistical analyses were run using SPSS version 21 software.
3. Results
Overall, 287 members of the DPS cohort met the study inclusion criteria, and contributed a total of 611 assessments. Females comprised 53.3% of the sample. Mean(SD) age at dementia onset was 82.3(5.9) years. A majority (99%) of the cohort was white, 62% were diagnosed with solely Alzheimer’s disease. Table 1 provides demographic information and levels of dementia severity at start of study observation. Overall, females were in better health, were slightly more functionally impaired (CDR scale), had longer dementia duration and exhibited more NPS compared to males. Median(range) and mean(SD) costs of informal dementia care at the start of study observation were $9.73($0–$294.19)/day and $40.07($75.41)/day in 2012 dollars respectively.
Table 1.
Dementia Progression Study Cohort Characteristics at Start of Study Observation
| Characteristic | Total | Female | Male | p-value | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | % | N | % | N | % | ||
| Sample Size | 287 | 100.0 | 153 | 53.3 | 134 | 46.7 | |
| Age at dementia onset, years | |||||||
| Median (range) | 83 (68, 104) | 83 (68, 96) | 82 (69, 104) | 0.934 | |||
| Mean (sd) | 82.33 (5.89) | 82.35 (5.69) | 82.29 (6.15) | ||||
| 65–74 | 30 | 10.5 | 17 | 11.1 | 13 | 9.7 | 0.811 |
| 75–84 | 155 | 54.0 | 80 | 52.3 | 75 | 56.0 | |
| ≥85 | 102 | 35.5 | 56 | 36.6 | 46 | 34.3 | |
| Education | |||||||
| Less than high school | 43 | 15.0 | 18 | 11.8 | 25 | 18.7 | <0.001 |
| High school | 103 | 35.9 | 73 | 47.7 | 30 | 22.4 | |
| At least some college | 141 | 49.1 | 62 | 40.5 | 79 | 59.0 | |
| Dementia Type | |||||||
| Alzheimer’s disease | 178 | 62.0 | 103 | 67.3 | 75 | 56.0 | 0.120 |
| Vascular dementia w/o AD | 38 | 13.2 | 16 | 10.5 | 22 | 16.4 | |
| Other dementia | 71 | 24.7 | 34 | 22.2 | 37 | 27.6 | |
| General Medical Health Rating | |||||||
| Median (range) | 3 (0, 4) | 3 (0, 4) | 3 (0, 4) | 0.026 | |||
| Mean (sd) | 2.94 (0.56) | 3.01 (0.55) | 2.87 (.56) | ||||
| 2=fair/poor | 53 | 18.5 | 22 | 14.4 | 31 | 23.1 | 0.118 |
| 3=good | 196 | 68.3 | 106 | 69.3 | 90 | 67.2 | |
| 4= excellent | 37 | 12.9 | 24 | 15.7 | 13 | 9.7 | 0.365 |
| missing | 1 | 0.3 | 1 | 0.7 | 0 | 0 | |
| Died during study (2002–2011) | 219 | 76.3 | 115 | 75.2 | 104 | 77.6 | |
| Dementia duration in years at Start of Study Observation | |||||||
| Median | 3.16 | 3.69 | 2.60 | <0.001 | |||
| Mean (sd) | 3.54 (1.94) | 4.01 (2.12) | 3.01 (1.56) | ||||
| Follow up time in years† | |||||||
| Median, among those with follow-up | 2.34 | 2.37 | 2.31 | 0.980 | |||
| Mean (sd), among those with follow-up | 2.77 (1.63) | 2.77 (1.64) | 2.77 (1.64) | ||||
| 0 follow up (baseline only) | 131 | 45.6 | 81 | 52.9 | 50 | 37.3 | 0.070 |
| Number of assessments per person | |||||||
| Median | 2 | 1 | 2 | 0.004 | |||
| Mean (sd) | 2.13 (1.40) | 1.90 (1.22) | 2.39 (1.53) | ||||
| Mini Mental State Examination (range 0 – 30)) | |||||||
| Median | 22.36 | 22.00 | 22.76 | 0.051 | |||
| Mean (sd) | 20.71 (6.43) | 20.00 (7.03) | 21.50 (5.60) | ||||
| Clinical Dementia Rating Scale (range 0–4) | |||||||
| Median | 1 | 1 | 1 | <0.001 | |||
| Mean (sd) | 1.19 (0.72) | 1.33 (0.75) | 1.02 (0.66) | ||||
| Neuropsychiatric Inventory (range 0–48) | |||||||
| Median | 8 | 12 | 6 | 0.011 | |||
| Mean (sd) | 11.58 (11.05) | 13.12 (10.79) | 9.81 (11.13) | ||||
| Hours giving care (max of 16hr/day) at Start of Study Observation | |||||||
| Median | 0.58 | 0.92 | 0.50 | 0.318 | |||
| Mean (sd) | 2.30 (4.27) | 2.53 (4.49) | 2.03 (4.01) | ||||
| Informal costs of dementia care ($/day) at Start of Study Observation | |||||||
| Median | 9.73 | 15.18 | 8.58 | 0.328 | |||
| Mean (sd) | 40.07 (75.41) | 44.15 (79.08) | 35.41 (70.99) | ||||
Follow-up from first assessment to last assessment
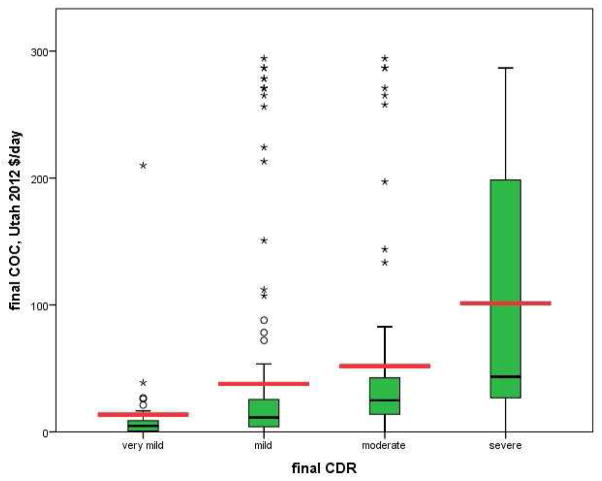
The relationship between average daily costs of informal care and dementia severity measured at each participant’s final assessment indicates higher informal costs with more severe dementia (see Table 2 and Figure 1). Results of bivariable analyses yielded the following mean(SD) daily informal costs: very mild dementia [(CDR=0–0.5); (n=32); $13.63($37.63)]; mild dementia [(CDR=1); (n=145); $37.84($72.57)]; moderate dementia [(CDR=2); (n=71); $51.78($76.13)]; and severe dementia [(CDR≥3); (n=35); $101.23($104.13)]. Similar results were obtained for overall U.S. wages.
Table 2.
Average Daily Costs of Informal Dementia Care among DPS cohort by Dementia Severity (2012 Dollars)
|
FINAL Observations N=283† |
Very Mild CDR = 0 or 0.5 | Mild CDR = 1 | Moderate CDR=2 | Severe CDR =3 or 4 | ||
|---|---|---|---|---|---|---|
|
|
||||||
| N = 32 | N = 145 | N = 71 | N = 35 | p-value | ||
| Utah | Mean (SD) | 13.63 (37.63) | 37.84 (72.57) | 51.78 (76.13) | 101.23 (104.13) | <0.001 |
| 75th Percentile | 8.89 | 26.13 | 42.95 | 257.68 | ||
| Median | 4.71 | 11.27 | 24.85 | 43.48 | ||
| 25th Percentile | 0.34 | 4.03 | 13.90 | 25.50 | ||
|
| ||||||
| USA | Mean (SD) | 14.80 (40.17) | 39.40 (75.37) | 52.43 (76.56) | 107.30 (113.48) | <0.001 |
| 75th Percentile | 9.67 | 28.32 | 46.46 | 278.75 | ||
| Median | 5.07 | 12.23 | 26.72 | 42.18 | ||
| 25th Percentile | 0.36 | 4.36 | 14.52 | 27.52 | ||
Of the 287 subjects, four were missing CDR at their final assessment and thus excluded from this table.
Figure 1. Average Daily Costs of informal care increase with severity of dementia.
The distribution of average daily costs of care by CDR score at final visit are depicted below in 2012 dollars (corrected using MCPI for average Utahn wages) to show the unadjusted effect of dementia severity on average daily costs of care. The heavy black lines within each “box” and the heavy red lines represent median and mean daily costs of care respectively. Lower and upper box edges represent the 25th and 75th percentiles respectively, while circles and asterisks represent outlier (1.5 * interquartile range) and far outlier (3.0*interquartile range) values respectively. While daily cost of care data were non-normally distributed (highly skewed); all means were significantly different from one another. N = 283†
† Of the 287 subjects, four were missing CDR at their final assessment and thus excluded from this figure.
Costs of informal dementia care over time
The daily costs of informal dementia care over time in this sample increased 18% per year from the onset of dementia, irrespective of any covariates [expβ=1.18; (95% CI 1.120–1.239)].
3.1 Costs of dementia care with time-varying MMSE
Results of the multivariate linear mixed effects models on the association of dementia severity over time on daily costs are presented in Table 3 and Figure Panel 2 (n=579 assessments). In the adjusted MMSE model, daily informal costs increased approximately 8% [expβ=1.084; (95% CI 1.027–1.143)] per year and less severe dementia was associated with lower daily informal costs, with 6% [expβ=0.94; (95% CI 0.922–0.960)] lower informal costs for a one-point increase in score (Figure 2). NPS increased daily informal costs by 2% [expβ=1.019; (95% CI 1.006 – 1.032)] for each point increase in NPI score. Additionally, lower informal care costs were associated with not having completed high school [expβ=0.497; (95% CI 0.339–0.729)], vascular dementia (compared to all other dementias) [expβ=0.519; (95% CI 0.370–0.729)], and better health [expβ=0.652; (95% CI 0.441–0.964)].
Table 3.
Association of dementia severity with informal costs of dementia care since dementia onset
| Mini Mental State Exam (MMSE) Model | Clinical Dementia Rating Scale Model | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N = 279 (580)† | N = 284 (603)† | |||||||
|
| ||||||||
| Variable | Unadjusted‡ | Adjusted‡ | Unadjusted‡ | Adjusted‡ | ||||
|
| ||||||||
| expβ | 95% CI | expβ | 95% CI | expβ | 95% CI | expβ | 95% CI | |
| Time in years | 1.070 | 1.015 1.127 | 1.084 | 1.027 1.143 | 1.051 | 0.985 1.120 | 1.050 | 0.987 1.116 |
| Dementia Severity | ||||||||
| MMSE (Time-varying) | 0.932 | 0.913 0.951 | 0.940 | 0.922 0.960 | ||||
| CDR (Time-varying) | ||||||||
| Categorical, vs. Very mild | ||||||||
| Mild | 2.958 | 1.764 4.961 | 2.690 | 1.725 4.194 | ||||
| Moderate | 5.466 | 3.397 8.797 | 4.998 | 3.231 7.730 | ||||
| Severe | 7.288 | 4.252 12.489 | 6.318 | 3.733 10.692 | ||||
| Less than HS education (vs. completed HS/GED) | 0.497 | 0.339 0.729 | ** | |||||
| Any vascular dementia (vs. no vascular dementia) | 0.519 | 0.370 0.729 | 0.480 | 0.355 0.650 | ||||
| NPI | 1.019 | 1.006 1.032 | ||||||
| Good/Excellent GMHR (vs. poor/fair) | 0.652 | 0.441 0.964 | 0.734 | 0.532 1.012 | ||||
Models adjusted for: age at dementia onset, gender, apoe4, general medical health rating (GMHR), education, type of dementia, and neuropsychiatric inventory (NPI); non-significant covariates were removed from the model, often to help models converge.
The potential sample for each analysis was N = 287 subjects (with 611 observations), however some observations were unable to be included in the analysis due to missing variable(s).
Multivariable mixed models using GEE with gamma distribution and log link.
Model did not converge with inclusion of this covariate.
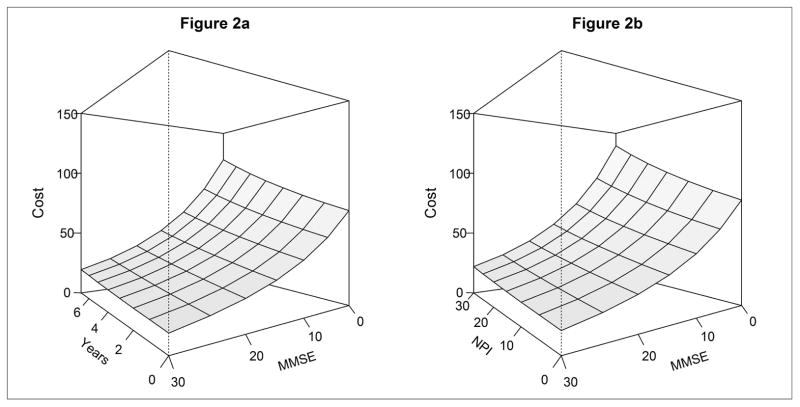
Figure 2. a–2b. Change in the cost of informal dementia care by MMSE and NPI scores over time.
Figure panels 2 displays the informal dementia care costs in the fully adjusted, time-varying MMSE model for an individual without vascular dementia, at least a high school education, and in good/excellent health. Panel 2a displays the change in informal care costs by time and MMSE score holding NPI score constant at 10 (median of all time points). For illustration, the corresponding informal care costs associated with an MMSE of 20 with dementia duration of 4 years = $28/day; the informal care costs associated with an MMSE of 10 with dementia duration of 7 years = $66/day. Panel 2b displays the change in informal care costs by MMSE and NPI holding time constant at 3.7 years (median of all time points). For illustration, the corresponding informal care costs at an MMSE of 20 with NPI score of 10 = $27/day; the informal care costs associated with an MMSE score of 10 and NPI score of 30 = $74/day.
3.2 Cost of informal dementia care with time-varying CDR
In the adjusted CDR model, daily informal costs increased approximately 5% [expβ=1.050; (95% CI 0.987–1.116)] per year and by dementia severity, which was modeled categorically. Compared to very mild (CDR score 0–0.5) there was a 2.7-fold, 5.0-fold and 6.3-fold higher daily informal costs for mild [CDR score=1; expβ=2.69; (95% CI 1.725–4.194)], moderate [CDR score=2; expβ=4.998; (95% CI 3.231–7.730)] and severe [CDR score≥3; expβ=6.318; (95% CI 3.733–10.692)] dementia, respectively (see Table 3 and Figure 3). Vascular dementia and better health were associated with lower informal costs by approximately 52% [expβ=0.480; (95% CI 0.355–0.650)] and 27% [expβ=0.734; (95% CI 0.532–1.012)], respectively. Note that a model including education in the presence of the other significant covariates did not converge.
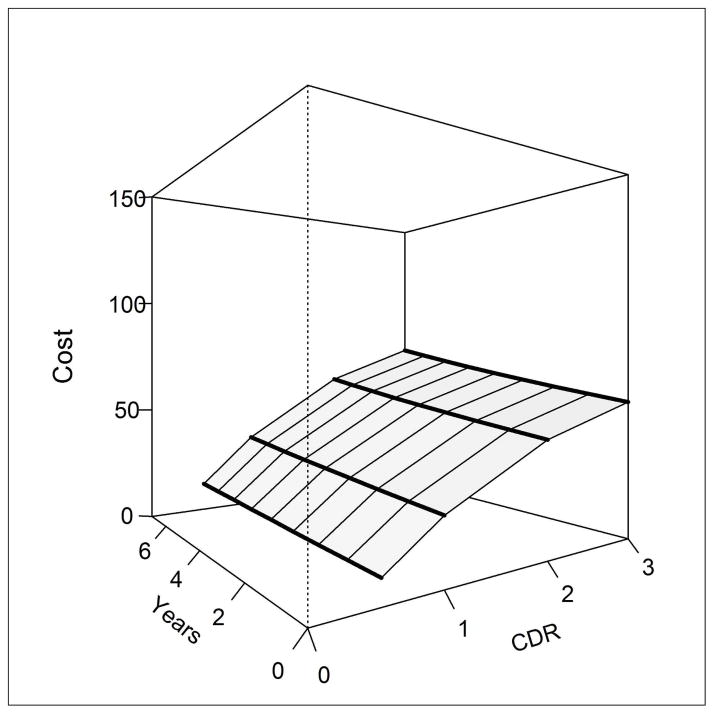
Figure 3. Change in the cost of informal dementia care by CDR over time.
Figure 3 displays the informal dementia care costs in the fully adjusted time-varying CDR model for an individual without vascular dementia and in good/excellent health. For illustration, the corresponding informal care costs associated with a CDR of 1 (mild) with dementia duration of 4 years = $11/day; the informal care costs associated with a CDR of 3 (severe) with dementia duration of 7 years = $62/day. CDR values are: 0.5 (very mild), 1 (mild), 2 (moderate), 3 (severe).
4. Discussion
Using the population-based community cohort with dementia from the Cache County Study, we estimated that from the onset of dementia, the costs of informal dementia care increased by approximately 18% per year. The increasing daily cost of informal dementia care was associated with worsening dementia severity. When holding dementia severity constant, we found an average 5–8% annual increase in daily informal costs. Our finding of lower educational status predicting lower daily informal costs may be confounded with socioeconomic status such that Medicaid-funded services may have offset informal costs for this subsample.
In models controlling for cognitive impairment, we found more severe NPS to be associated with higher daily informal care costs over time. Previous work in this and other samples suggest that the course of NPS (unlike those of cognitive and functional abilities) show marked fluctuation and variability over the course of dementia(16). Our results raise the potential that successful treatment or management of NPS may result in a reduction in the costs of informal dementia care. In future work examination of specific symptom types (e.g., psychotic or affective symptoms) and their association with the cost of informal dementia care may be informative.
Our overall findings are of the same magnitude when compared with those of other studies(5,7–9,11,31). In the Predictors Study of a community-dwelling dementia cohort drawn from university Alzheimer’s clinic patients(5), Zhu et al. found that costs of dementia increase as disease severity progresses from mild to more severe disease(5). Specifically, baseline total annual direct costs of care (medical and non-medical) per person were approximately $9239 (2004 dollars) per year at milder stages of disease and after four years of follow-up these annual costs increased to approximately $19,925 per patient per year, representing approximately a 20% annual increase in these costs(5). In examining measures of functional impairment and patient dependency, Zhu et. al. found that a one-point increase in the Blessed Dementia Rating Scale (BDRS) was associated with a 7.7% increase total direct costs(32). Further, Zhu et al. found that increases in BDRS and Dependence Scale both resulted in increases in costs of dementia care but differentially increased medical, nonmedical costs as well as caregiver time(32).
In a French community-dwelling dementia clinical trial cohort drawn from 50 French memory clinics with two years of follow-up, Rapp et al. found that cognitive (MMSE) and functional(9,19,33) decline as well as NPS were associated with increased overall costs of dementia care. Informal costs of care were found to significantly increase with worse cognition and function.
In the nationally representative HRS, the average annual monetary cost per person attributable to dementia was either $41,689 or $56,290 using a forgone wage model or an equivalent cost of paid care model for informal care costs respectively(11). Relevant to the current project, adjusted annual informal care costs were estimated at $13,188 (forgone wage model) or $27,789 (equivalent cost of paid care model) in 2010 dollars. These findings do not consider severity of dementia. In comparison, we reported that the median daily informal costs in Cache County range from $4.71 per day (2012 dollars) for very mild dementia to $43.48 per day for severe dementia; this translates to annual costs per person of approximately $1,719–$15,870 respectively, generally in line with Hurd’s findings(11). Similarly, our mixed model results predict similar increases to that of the Predictors study(5). Notwithstanding the difficulties in comparing our results to those of the French study, we also found that greater cognitive and functional impairments along with the presence of NPS (when controlling for MMSE) contribute to higher informal costs(9).
Study limitations include the homogeneity of the population and small sample size. As well, informal cost data were derived from caregiver hours that were obtained using an activity survey, where the primary caregiver is interviewed and as such is subject to recall bias. Additionally, we do not report on nursing home/institutional care (28 dementia participants were excluded from the analyses due to nursing home residence). Furthermore, caregiver hours and informal care costs from those in our cohort who resided in assisted living facilities are likely to be underestimated.
Study strengths include the uniqueness of the longitudinal population-based community-dwelling cohort with dementia with high participation rates and frequent follow-ups (see Tschanz et al., 2011; 16). This detailed data allowed a longitudinal examination of caregiving hours, a feature that is important to establishing “real cost” estimates for informal care in community-dwelling populations. Further, the caregiver survey included the estimation of caregiver time for multiple caregivers. Finally, our study quantified changes in informal costs associated with changes in scores on commonly used clinical scales; this can allow for assessment/quantification of the value or impact of disease management strategies and intervention by reduction in changes or deterioration on these measures.
In conclusion, our main finding is that daily costs of informal dementia care increase substantially with disease progression over time, with greater dementia severity predicting higher costs in a population-based community-dwelling cohort with dementia. Identifying and targeting interventions to slow progression in community-dwellers with dementia is fundamentally important to decreasing annual costs of dementia care as both informal caregiving and institutionalization are becoming universally acknowledged to be two of the costliest components of caring for those with dementia. Likewise, as NPS are modifiable and responsive to pharmacological and non-pharmacological interventions, continued efforts to reduce these symptoms may reduce the overall costs of care(34,35).
Research in Context
Systematic Review: In our literature review, we sought studies of informal, longitudinal costs of dementia care with measures of disease severity conducted among community-dwellers with dementia. The few longitudinal studies identified involved medical-center or clinical trial cohorts, however lacked information on informal caregiving costs or disease severity. Hurd’s recent study (NEJM 2013) had informal care costs among community dwellers with dementia, but was cross-sectional.
Interpretation
In our population-based community-dwelling cohort with dementia, we demonstrated that daily informal costs of dementia care increase substantially overtime with greater dementia severity and behavioral symptoms predicting higher costs. Targeting interventions to slow progression in dementia is important to decreasing annual costs of dementia care.
Future Directions
Extending our findings to larger, racially diverse, urban community-dwelling dementia cohorts will provide important results concerning generalizability of these findings and help to prioritize appropriate disease management strategies.
Acknowledgments
This study was supported by NIH grants R01AG21136 and R01AG11380. We are indebted to Ronald Munger, MPH, Ph.D., Kathleen Piercy, Ph.D., and the participants and caregivers of the Cache County Studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer’s Disease International. World Alzheimer Report 2009 Executive Summary. 2009 [Google Scholar]
- 2.Alzheimer’s Association. 2013 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia. 2013;9(2) doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation Heart failure. 2013;6(3):606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu CW, Scarmeas N, Torgan R, Albert M, Brandt J, Blacker D, et al. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology. 2006;67(6):998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DH, Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–15. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu CW, Scarmeas N, Torgan R, Albert M, Brandt J, Blacker D, et al. Clinical characteristics and longitudinal changes of informal cost of Alzheimer’s disease in the community. J Am Geriatr Soc. 2006;54(10):1596–602. doi: 10.1111/j.1532-5415.2006.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu CW, Leibman C, McLaughlin T, Scarmeas N, Albert M, Brandt J, et al. The effects of patient function and dependence on costs of care in Alzheimer’s disease. J Am Geriatr Soc. 2008;56(8):1497–503. doi: 10.1111/j.1532-5415.2008.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapp T, Andrieu S, Molinier L, Grand A, Cantet C, Mullins CD, et al. Exploring the relationship between Alzheimer’s disease severity and longitudinal costs. Value Health. 2012;15(3):412–9. doi: 10.1016/j.jval.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Kokmen E, Ozsarfati Y, Beard CM, O’Brien PC, Rocca WA. Impact of referral bias on clinical and epidemiological studies of Alzheimer’s disease. J Clin Epidemiol. 1996;49(1):79–83. doi: 10.1016/0895-4356(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 11.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–34. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leicht H, Heinrich S, Heider D, Bachmann C, Bickel H, van den Bussche H, et al. Net costs of dementia by disease stage. Acta Psychiatr Scand. 2011;124(5):384–95. doi: 10.1111/j.1600-0447.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 13.Andersen CK, Lauridsen J, Andersen K, Kragh-Sorensen P. Cost of dementia: impact of disease progression estimated in longitudinal data. Scand J Public Health. 2003;31:119–25. doi: 10.1080/14034940210134059. [DOI] [PubMed] [Google Scholar]
- 14.Lacey LA, Niecko T, Leibman C, Liu E, Grundman M. Association between Illness Progression Measures and Total Cost in Alzheimer’s Disease. J Nutr Health Aging. 2013;17(9):745–50. doi: 10.1007/s12603-013-0368-1. [DOI] [PubMed] [Google Scholar]
- 15.Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53(2):321–31. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 16.Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19(6):532–42. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology. 2002;58(2):209–18. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 18.Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; 1987. Revised. [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Roman GC, Tatemichi T, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 21.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–14. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 22.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10(1):31–9. [PubMed] [Google Scholar]
- 23.Cummings JL. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):10S–6S. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 25.Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren Am, et al. The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47(7):487–91. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 26.Penrod JD, Kane RL, Finch MD, Kane RA. Effects of post hospital Medicare home health and informal care on patient functional status. Health Service Research. 1998;33:513–29. [PMC free article] [PubMed] [Google Scholar]
- 27.Download Occupational Employment and Wage Estimates. [cited 2013 February 23]. Available from: http://www.bls.gov/oes/oes_dl.htm.
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15(3):346–53. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 30.Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090–7. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 31.Zhu CW, Sano M. Economic considerations in the management of Alzheimer’s disease. Clin Interv Aging. 2006;1(2):143–54. doi: 10.2147/ciia.2006.1.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu CW, Leibman C, McLaughlin T, Zbrozek AS, Scarmeas N, Albert M, et al. Patient dependence and longitudinal changes in costs of care in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;26(5):416–23. doi: 10.1159/000164797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 34.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 35.Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Advances in Psychiatric Treatment. 2004;10:171–7. [Google Scholar]