Abstract
Objectives
To determine the effect of hydration media on ex vivo corneal elasticity.
Methods
Experiments were conducted on forty porcine eyes retrieved from an abattoir (10 eyes each for PBS, BSS, Optisol, 15% Dextran). The epithelium was removed and the cornea was excised with an intact scleral rim and placed in 20% Dextran overnight to restore its physiological thickness. For each hydration media, corneas were evenly divided into two groups: one with an intact scleral rim and the other without. Corneas were mounted onto a custom chamber and immersed in a hydration medium for elasticity testing. While in each medium, corneal elasticity measurements were performed for 2 hours: at 5-minute intervals for the first 30 minutes and then 15-minute intervals for the remaining 90 minutes. Elasticity testing was performed using nanoindentation with spherical indenters and Young’s modulus was calculated using the Hertz model. Thickness measurements were taken before and after elasticity testing.
Results
The percentage change in corneal thickness and elasticity was calculated for each hydration media group. BSS, PBS, and Optisol showed an increase in thickness and Young’s moduli for corneas with and without an intact scleral rim. 15% Dextran exhibited a dehydrating effect on corneal thickness and provided stable maintenance of corneal elasticity for both groups.
Conclusions
Hydration media affects the stability of corneal thickness and elasticity measurements over time. 15% Dextran was most effective in maintaining corneal hydration and elasticity, followed by Optisol.
Keywords: Atomic Force Microscopy, cornea, mechanical properties, hydration, nanoindentantion
1. Introduction
Corneal biomechanics is an important parameter in the development and improvement of ophthalmic technologies related to the cornea. Establishing a correlation between corneal structure and function, corneal biomechanics not only functions as a quantitative measure of the progression of various corneal pathologies but also serves as an objective measure of the effectiveness of diagnostic and therapeutic methods designed to combat corneal diseases. Measurement of corneal biomechanical response is of much importance because of its influence on present and future corneal-related diagnostic and therapeutic technologies.
Several characterization techniques have been designed to increase understanding of corneal biomechanical response. New techniques such as the Ocular Response Analyzer (ORA) 1–3 and shear wave imaging 4–6 have been developed to measure corneal mechanical properties in vivo. However, direct measurement of standard mechanical property parameters of the corneal tissue, such as Young’s modulus of elasticity, has only been conducted in the ex vivo experimental setting. Ex-vivo biomechanical testing methods currently applied to measure corneas include tensile stretching (or strip extensiometry) 7–20, bulge/inflation testing 18, 21–27, nanoindentation testing (atomic force microscopy, AFM) 28–35, indentation testing 36, shear testing 4, 37, and acoustic radiation force elastic microscopy 38. Despite the existence of a variety of characterization methods available, the published values derived from such techniques lack reproducibility, evidenced by the large range of reported corneal Young’s modulus of elasticity values in literature (0.57kPa – 41MPa) 7–18, 21–36, 39. Although the varied nature of the characterization methods may contribute to the sizable range of published elasticity values, one should not assume that this is the sole reason. Other factors that should be taken into consideration include differences in sample preparation, corneal hydration, age, and postmortem time. The focus of this paper is the importance of corneal hydration.
For ex-vivo corneal characterization studies, a true reflection of the in-situ biomechanical response is heavily influenced by the hydration of the post-mortem cornea 40. For this reason, researchers use various media to maintain corneal hydration during mechanical testing; such media include saline solutions like PBS or HBSS 9, 23, 28, 29, 33, Dextran solutions 30–32, 40–42, oils 14, 36, as well as commercial ophthalmic solutions 22, 24. Although numerous studies have been conducted to investigate the effect of different media on corneal thickness and swelling changes over time 40–46, only a few studies looking into the impact of corneal hydration solutions on corneal biomechanical response have been published 27, 47. The purpose of this study is to expand upon these previous studies by using different hydration media and investigating how the corneal limbus affects biomechanical measurements by using corneal samples with and without the intact scleral rim present at sub-micron scale of observation. The elasticity of the cornea over time was measured using atomic force microscopy (AFM).
2. Materials and Methods
Experiments were conducted on 40 porcine eyes (10 eyes for each hydration medium; <3 days postmortem). The eyes were retrieved from an abattoir, placed in a bag filled with saline, and shipped to the laboratory overnight. Upon arrival in the laboratory, the corneal epithelium was removed using a cotton-tipped applicator. The porcine cornea was then excised with a generous scleral rim and placed in 20% Dextran, anterior stroma down, to restore corneal thickness to physiological levels40, 43, 44. The intact corneas remained in 20% Dextran for 24 hours at room temperature. Pachymetry measurements were taken after 24 hours to ensure the equilibrium and restoration of the corneal thickness to physiological levels 48.
With the physiological thickness restored, one group of porcine corneas (5 corneas each for each hydration medium) was further excised within the limbus (all sclera removed), and the other group (5 corneas each for hydration medium) was left with the intact scleral rim around the cornea. The porcine cornea was then mounted onto a custom corneal holder33 and positioned so that the central region of the corneal sample was oriented directly under the AFM cantilever. The corneal holder was then filled with a hydration medium, either PBS (D1283, Sigma Aldrich, St. Louis, MO), HBSS (04-315Q, Lonza, Walkersville, MD), 15% Dextran (15 grams of dextran in 100mL of PBS; D8821, avg. molecular weight: 64,000–76,000 g/mol, Sigma Aldrich, St. Louis, MO), or Optisol (Optisol-GS, 50006-OPT, Bausch and Lomb, Rochester, NY), prior to corneal elasticity testing. While maintained in each medium, corneal elasticity measurements were performed for 2 hours; measurements were conducted at 5-minute intervals for the first 30 minutes and then at 15-minute intervals for the remaining 90 minutes.
Elasticity characterization testing was conducted using a custom-built atomic force microscopy (AFM) system. The details of the AFM system and testing protocol have been previously described 31, 32, 49. Tipless AFM cantilevers (nominal spring constant: 4.5 N/m, NSC12 series, Mikromasch, San Jose, CA) were modified with glass microspheres (59–74μm diameter, 15926-100, Polysciences Inc) and calibrated with a reference force calibration cantilever (nominal spring constant: 10–30 N/m, CLFC-NOBO, Bruker, Camarillo, CA) to measure its spring constant. Regulated by a piezoelectric mechanism (60μm maximal expansion, P-841.40, Physik Instrumente, Germany), the spherical-tipped cantilevers were lowered onto the corneal samples at an approach speed of 15μm/s and then retracted at that same speed, once the maximal indentation force of 1000V (<20nN) was applied. Recordings of the cantilever’s deflection from the photodiode’s voltage output and the cantilever’s indentation from the piezoelectric displacement were then used to derive the sample’s force-indentation curves (after the cantilever deflection on a hard surface is factored out and the measured spring constant of the cantilever is integrated). A custom curve-fitting MATLAB program is used to analyze to the force-indentation curves with the Hertz model for spherical indenters 50:
where F is the measured force (in Newtons), E is Young’s modulus (in Pascals), ν is Poisson’s ratio (ν=0.49 for the cornea 51–53), R is the radius of the spherical indenter (in meters), and D is the measured indentation (in meters). Experiments were performed at room temperature. Corneal thickness measurements were taken with an ultrasonic pachymeter (DGH 55 Pachmate, DGH Technology Inc., Exton, PA) before and after elasticity testing.
3. Results
Thickness
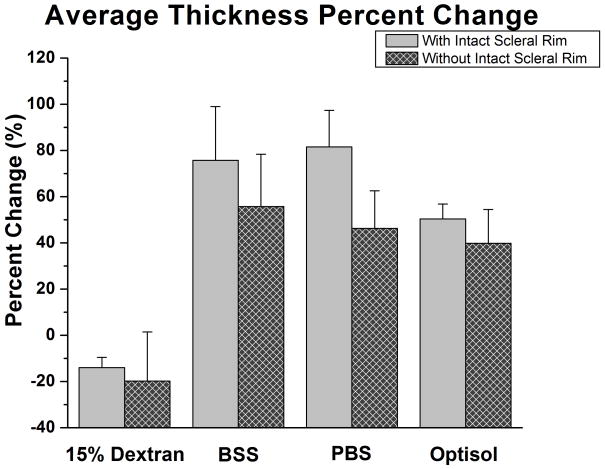
The average central corneal thickness for all the eyes at the start of the experiments was 562 ± 72μm (range: 436–684μm; Table 1). The percentage change of the corneal thickness (change in thickness relative to initial thickness) was calculated for each sample. For the intact scleral rim, the percent change in thickness was: −14.0 ± 4.5% for 15% Dextran, 75.7 ± 23.3% for BSS, 81.6 ± 15.8% for PBS, and 50.4 ± 6.5% for Optisol (Figure 1). For the group where corneas were excised within the scleral rim, the percentage change in thickness was: −19.8 ± 21.3% for 15% Dextran, 55.8 ± 22.6% for BSS, 46.3 ± 16.3% for PBS, and 39.9 ± 14.6% for Optisol (Figure 1). A paired Student’s t-test was performed to compare the initial and final corneal thickness measurements for each hydration media group. Such tests resulted in significance for all hydration media with the corneal scleral rim left intact (p<0.01); however only BSS, PBS, and Optisol were statistically significant when the scleral rim was removed (15% Dextran: p=0.095; BSS, PBS, and Optisol: p<0.01).
Table 1.
Corneal Thickness Measurements performed before (at the initial time) and after (at 2 hours) the AFM mechanical testing in the different hydration media.
| Corneal Thickness (μm) [Average ± Standard Deviation] | ||||
|---|---|---|---|---|
| With Intact Sclera | Without Intact Sclera | |||
| Hydration Media | At Initial Time | At 2 Hours | At Initial Time | At 2 Hours |
| 15% Dextran | 492.4 ± 21.4 | 423.2 ± 23.7 | 611.6 ± 85.4 | 476.0 ± 55.0 |
| PBS | 500.3 ± 41.2 | 886.2 ± 35.5 | 596.2 ± 48.1 | 870.2 ± 94.7 |
| BSS | 502.4 ± 41.8 | 881.9 ± 126.3 | 611.6 ± 64.4 | 943.2 ± 85.6 |
| Optisol | 610.4 ± 17.4 | 918.6 ± 58.4 | 687.0 ± 16.0 | 850.6 ± 109.5 |
Figure 1.
Bar Graph of the Average Corneal Thickness Percentage Change. A bar graph comparing the thickness percentage changes after 120 minutes in different hydration media. Samples with the intact sclera had a greater change in thickness than samples without the intact sclera.
Corneal Elasticity
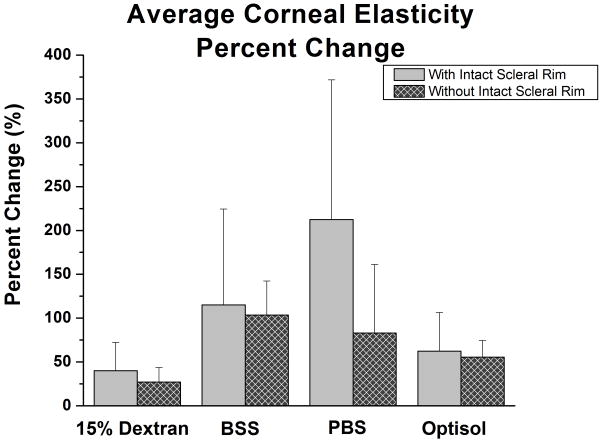
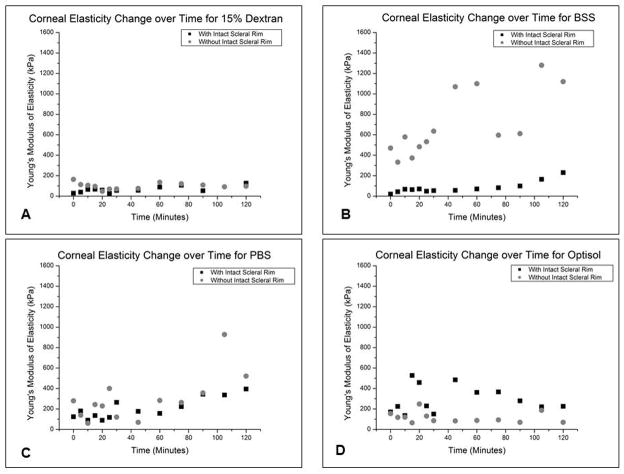
The percentage change of the effective Young’s modulus (change in Young’s modulus relative to initial Young’s modulus) was calculated for each corneal sample. For the corneas excised with the scleral rim left intact, the average corneal elasticity percentage change was: 40.1 ± 32.2% for 15% Dextran, 115.1 ± 109.3% for BSS, 212.4 ± 159.3% for PBS, and 62.4 ± 44.1% for Optisol (Figure 2; Table 2). While for the corneas excised within the scleral rim perimeter, the average corneal elasticity percentage change was: 27.1 ± 16.5% for 15% Dextran, 103.4 ± 39.0% for BSS, 83.1 ± 78.1% for PBS, and 55.6 ± 18.9 % for Optisol (Figure 2; Table 2). 15% Dextran and Optisol exhibited elasticity profiles that fluctuated around a median line, while BSS and PBS exhibited linearly increasing Young’s modulus profiles over time for both corneal samples with and without intact scleral rim (Figure 3). A paired Student’s t-test was performed to compare the initial and final corneal elasticity measurements for each hydration media group. No statistical significance existed for all hydration media with and without the intact scleral rim (p>0.01 for all hydration media). The average AFM indentation depth applied onto all corneas during elasticity testing was 4.1 ± 1.4 μm.
Figure 2.
Bar Graph of the Average Corneal Elasticity Percentage Change. A bar graph comparing the corneal Young’s modulus percentage changes after 120 minutes in different hydration media. Samples with the intact sclera had a greater change in Young’s modulus than samples without the intact sclera.
Table 2.
The measured corneal Young’s modulus of elasticity performed at the initial time and final time point of 2 hours for each hydration media.
| Young’s Modulus of Elasticity (kPa) [Average ± Standard Deviation] | ||||
|---|---|---|---|---|
| With Intact Sclera | Without Intact Sclera | |||
| Hydration Media | At Initial Time | At 2 Hours | At Initial Time | At 2 Hours |
| 15% Dextran | 117.1 ± 85.4 | 131.7 ± 99.3 | 228.6 ± 340.7 | 153.4 ± 195.3 |
| PBS | 130.8 ± 74.4 | 299.1 ± 260.4 | 438.4 ± 277.5 | 619.1 ± 427.5 |
| BSS | 86.4 ± 59.1 | 102.6 ± 91.6 | 541.1 ± 280.4 | 769.2 ± 577.3 |
| Optisol | 151.3 ± 116.9 | 145.6 ± 75.8 | 292.9 ± 149.3 | 199.7 ± 215.9 |
Figure 3.
Scatter Plots of the Corneal Elasticity Profile during AFM Testing for each Hydration Medium. For one representative sample per hydration media, Young’s modulus is plotted as a function of time. Corneal samples with and without intact scleral rim are included in each graph: A.) 15% Dextran, B.) BSS, C.) PBS, and D.) Optisol.
4. Discussion
Published values of ex-vivo corneal mechanical properties vary greatly. Factors contributing to such variation include differences in characterization techniques (which reveal the anisotropic nature of the cornea), post-mortem time, age23, 53, 55–59, and sample preparation. However, in this study, the factor of sample preparation was investigated. Corneal sample preparation, which encompasses manipulation required to prepare the sample for biomechanical testing, varies depending on the biomechanical characterization method used. For example, tensile stretching requires the cornea to be excised into thin strips, while bulge testing and AFM allow measurements to be performed on an intact cornea. With the existence of many variables attributing to the high variation of published ex-vivo corneal mechanical property values, the need for experimental standardization arises. For this reason, this study was undertaken to further our understanding of the influence of cornea hydration on mechanical property measurements ex vivo. The cases of keeping the scleral rim intact with cornea (which is used for AFM, inflation testing, shear testing, and acoustic radiation force elastic microscopy), by excising the cornea with a scleral rim, and having the scleral rim removed from the cornea (which is common for tensile stretching) were also investigated to determine how the presence of the corneal scleral rim affected these biomechanical measurements.
Post-mortem corneas, whether human corneas from the eye bank or animal globes like porcine eyes from an abattoir, arrive in an edematous state with thicknesses above their respective physiological range. Performing mechanical characterization testing on such edematous corneas would yield biomechanical results influenced by high water content, thus not reflecting an accurate measure of in-situ corneal responses. Therefore, the restoration of the corneal thickness to the normal physiological thickness range before characterization testing is imperative. Some researchers explicitly make mention of measuring the corneal thickness before characterization testing 20, 22–24, 30–32, 37, 38 but only a few take measures to address corneal thickness restoration before characterization testing 30–32, 38. Based on the results of this present study, measures should also be taken to address the corneal hydration (and thickness), through the use of hydration media, during measurements as they affect corneal biomechanical properties.
In this study, corneal elasticity profiles, obtained through AFM indentation testing, were mapped over the span of 2 hours in the hydration solutions of 15% dextran, PBS, BSS, and Optisol (Figure 3). In addition, corneal thickness was performed before and after mechanical testing. Mechanical testing and thickness measurements were conducted on two corneal samples groups: corneas with the scleral rim left intact and corneas excised within the scleral rim perimeter. The elasticity profiles of BSS and PBS respectively showed a steady increase in Young’s modulus (Figure 3B an 3C), while the profiles of 15% Dextran and Optisol fluctuated around a median (Figure 3A and 3D). For both corneal samples where the scleral rim was left intact and removed, 15% Dextran was most effective in maintaining corneal thickness and yielded the least change in corneal elasticity over the two hour experimental span. Although producing the minimum change in corneal thickness out of the hydration media, it is important to note that the 15% Dextran caused corneal dehydration. This same dehydrating effect has been observed previously by Hamaoui et al (2003)43, Duffey et al (1989)45 and Terry et al (1994)42, where they noted initial corneal thinning followed by thickness stability. Perhaps a lower concentration of Dextran would be necessary to minimize the observed corneal shrinkage. In fact, 8% Dextran was included in the Kling and Marcos (2013)28 study and showed a lesser dehydrating effect on the corneal thickness than that seen for the 15% Dextran concentration used in this study. Further investigation using the 8% Dextran concentration should be conducted. After 15% Dextran, Optisol followed in its effectiveness to maintain corneal hydration. Similar to this study, corneal thickening in Optisol was also observed in the study of Bourne et al (2001)46, while the study of Jablonski-Stiemke and Edelhauser (1998)47 revealed that the mean percent water content within the cornea increased significantly in Optisol, without the corneal epithelium present. BSS and PBS were not effective at all and resulted in extreme corneal swelling. A previous study by Hatami-Marbini and Rahimi (2014)48 also noted an increase in corneal thickness for PBS and BSS (4.9% increase for PBS and 4.2% increase for BSS). However, since their thickness measurements were performed after only 10 minutes in the hydration media, the percent increases were much smaller than those seen in the current study. The studies of Terry et al (1994) and Duffey et al (1989) also showed the rehydrating effect of BSS on corneal thickness as up to a 22% and 14.7% increase in corneal thickness was respectively observed when cornea samples were subjected to BSS drops on the anterior corneal surface for one hour.
Young’s modulus of elasticity increased for all samples, indicating an increase in stiffness. The greater the level of corneal swelling, the larger the percentage change in corneal Young’s modulus. This was most apparent for the samples placed in BSS and PBS, which had the highest percentage changes in both corneal thickness and elasticity compared 15% Dextran and Optisol. Previous studies of Kling & Marcos (2013)28 and Hatami-Marbini & Rahimi (2014)48 also investigated the effect of different corneal media solutions on corneal biomechanical responses using inflating testing and uniaxial tensile testing, respectively. The Kling and Marcos (2013)28 study investigated the change in hysteresis of corneas (with the scleral rim intact) during different intraocular pressure levels with the corneal samples in 20% dextran, 8% dextran, 0.125% riboflavin-20% dextran, or Optisol-GS. The slope of the curves as IOP increases can be related to the corneal stiffness. Kling and Marcos found that corneas in Dextran solutions were less stiff than those in Optisol, which mirrors the results of the current study (more swollen samples were stiffer)28. The Hatami-Marbini and Rahimi (2014)48 study performed tensile stretching experiments on corneal strips in 12% NaCl, 0.9% NaCl, PBS, ophthalmic balanced saline solution (OBSS), and mineral oil. The trend found in the Hatami-Marbini and Rahimi (2014)48 study showed that increasing corneal thickness yielded a decrease in corneal tangent modulus, which contradicts the trends found within this study. Such discrepancy in the qualitative results of this current study and that of Hatami-Marbini and Rahimi may stem from the difference in characterization technique. When the cornea swells due to the hydration media, the hydration solution occupies the interfibrillar space. At the high levels of corneal swelling observed in this current study, it may be possible that there is so much fluid in the corneal tissue that it causes the collagen fiber interconnectivity to be overextended. Since AFM indentation is a compressive technique and the indentations upon the cornea were performed instantaneously, a stiffening effect was observed because the tautness of the collagen fibers did not allow the hydration solution to displace easily within the collagenous network. The results of this study may imply that the elastic property of the cornea may not structurally correspond to the collagen fibers within the corneal ultrastructure only, but may also be influenced by the amount of extracellular matrix between the collagenous networks as well. Therefore, it is imperative to make sure that the corneal samples subjected to biomechanical characterization are within the physiological thickness range before testing and such thickness can be maintained during testing, to yield biomechanical responses close to physiological form.
Corneal samples excised within the scleral rim consistently yielded lower percentage changes in thickness and elasticity, independent of the hydration media used. The observation of such phenomenon may stem from an increase in peripheral diffusion. Since the corneal sample no longer has a circumferential impermeable boundary that restricts fluid flow, hydration media is able to flow in both the axial and the transverse directions. Therefore, corneal samples without the intact scleral rim are more susceptible to both the inflow and outflow of hydration media, and the thickness changes were observed as less dramatic than those observed with the intact scleral rim present. Since samples without the intact scleral rim are consistently thinner than samples with the limbus in the same hydration media, there is a corresponding increase in Young’s modulus, as described in the previous paragraph. For hydration media BSS and PBS, this degree of increase in Young’s modulus is shown to be greater than of 15% Dextran and Optisol. Tissue swelling causes an overextension of the collagen fibers. When the corneal sample with the intact scleral rim was indented upon, the corneal fluid content was unable to be displaced circumferentially, resulting in a stiffening effect.
In summary, this study confirms that corneal hydration media does have an effect on measured ex-vivo corneal elasticity and thickness over time. Measures should be performed to carefully select a corneal hydration medium that effectively maintains corneal hydration during corneal biomechanical testing. For the both cases of corneal samples having the intact scleral rim and corneal samples excised within the scleral rim, 15% Dextran exhibited the most effectiveness in stabilizing corneal thickness and maintaining corneal elasticity over the span of 2 hours.
Acknowledgments
Grant support United Negro College Fund (UNCF)-MERCK Science Research Dissertation Fellowship (JD); National Institute of Health (NIH) National Research Service Award Individual Predoctoral Fellowship (1F31EY021714-01, JD).
Footnotes
The authors do not have any proprietary or financial interest in any of the devices presented.
Presented in part at the Association for Research in Vision and Ophthalmology 2013 meeting.
References
- 1.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refr Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Vinciguerra P, Albe E, Mahmoud AM, et al. Intra- and Postoperative Variation in Ocular Response Analyzer Parameters in Keratoconic Eyes After Corneal Cross-linking. J Refract Surg. 2010;26:669–676. doi: 10.3928/1081597X-20100331-01. [DOI] [PubMed] [Google Scholar]
- 3.Glass DH, Roberts CJ, Litsky AS, et al. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Invest Ophth Vis Sci. 2008;49:3919–3926. doi: 10.1167/iovs.07-1321. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen T-M, Aubry J-F, Touboul D, et al. Monitoring of Cornea Elastic Properties Changes during UV-A/Riboflavin-Induced Corneal Collagen Cross-Linking using Supersonic Shear Wave Imaging: A Pilot Study. Invest Ophth Vis Sci. 2012;53:5948–5954. doi: 10.1167/iovs.11-9142. [DOI] [PubMed] [Google Scholar]
- 5.Touboul D, Gennisson JL, Nguyen TM, et al. Supersonic Shear Wave Elastography for the In Vivo Evaluation of Transepithelial Corneal Collagen Cross-Linking. Invest Ophth Vis Sci. 2014;55:1976–1984. doi: 10.1167/iovs.13-13445. [DOI] [PubMed] [Google Scholar]
- 6.Roy AS, Rocha KM, Randleman JB, et al. Inverse computational analysis of in vivo corneal elastic modulus change after collagen crosslinking for keratoconus. Exp Eye Res. 2013;113:92–104. doi: 10.1016/j.exer.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 8.Hoeltzel DA, Altman P, Buzard K, et al. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J Biomech Eng. 1992;114:202–215. doi: 10.1115/1.2891373. [DOI] [PubMed] [Google Scholar]
- 9.Jayasuriya AC, Ghosh S, Scheinbeim JI, et al. A study of piezoelectric and mechanical anisotropies of the human cornea. Biosens Bioelectron. 2003;18:381–387. doi: 10.1016/s0956-5663(02)00144-6. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y, Yang J, Huang K, et al. A comparison of biomechanical properties between human and porcine cornea. J Biomech. 2001;34:533–537. doi: 10.1016/s0021-9290(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 11.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refr Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 12.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refr Surg. 2009;35:540–546. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Lanchares E, del Buey MA, Cristobal JA, et al. Biomechanical property analysis after corneal collagen cross-linking in relation to ultraviolet A irradiation time. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2011;249:1223–1227. doi: 10.1007/s00417-011-1674-0. [DOI] [PubMed] [Google Scholar]
- 14.Nash IS, Greene PR, Foster CS. Comparison of mechanical properties of keratoconus and normal corneas. Exp Eye Res. 1982;35:413–424. doi: 10.1016/0014-4835(82)90040-9. [DOI] [PubMed] [Google Scholar]
- 15.Kohlhaas M, Spoerl E, Schilde T, et al. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refr Surg. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 16.Seiler T, Matallana M, Sendler S, et al. Does Bowman’s layer determine the biomechanical properties of the cornea? Refractive & Corneal Surgery. 1992;8:139–142. [PubMed] [Google Scholar]
- 17.Hammer A, Richoz O, Mosquera SA, et al. Corneal Biomechanical Properties at Different Corneal Cross-Linking (CXL) Irradiances. Invest Ophth Vis Sci. 2014;55:2881–2884. doi: 10.1167/iovs.13-13748. [DOI] [PubMed] [Google Scholar]
- 18.Kling S, Remon L, Pérez-Escudero A, et al. Corneal Biomechanical Changes after Collagen Cross-Linking from Porcine Eye Inflation Experiments. Invest Ophth Vis Sci. 2010;51:3961–3968. doi: 10.1167/iovs.09-4536. [DOI] [PubMed] [Google Scholar]
- 19.Scarcelli G, Kling S, Quijano E, et al. Brillouin Microscopy of Collagen Crosslinking: Noncontact Depth-Dependent Analysis of Corneal Elastic Modulus. Invest Ophth Vis Sci. 2013;54:1418–1425. doi: 10.1167/iovs.12-11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wernli J, Schumacher S, Spoerl E, et al. The Efficacy of Corneal Cross-Linking Shows a Sudden Decrease with Very High Intensity UV Light and Short Treatment Time. Invest Ophth Vis Sci. 2013;54:1176–1180. doi: 10.1167/iovs.12-11409. [DOI] [PubMed] [Google Scholar]
- 21.Hjortdal JO. Regional elastic performance of the human cornea. J Biomech. 1996;29:931–942. doi: 10.1016/0021-9290(95)00152-2. [DOI] [PubMed] [Google Scholar]
- 22.Elsheikh A, Wang DF, Brown M, et al. Assessment of corneal biomechanical properties and their variation with age. Curr Eye Res. 2007;32:11–19. doi: 10.1080/02713680601077145. [DOI] [PubMed] [Google Scholar]
- 23.Elsheikh A, Alhasso D, Rama P. Biomechanical properties of human and porcine corneas. Exp Eye Res. 2008;86:783–790. doi: 10.1016/j.exer.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Elsheikh A, Geraghty B, Rama P, et al. Characterization of age- related variation in corneal biomechanical properties. J Roy Soc Interface. 2010;7:1475–1485. doi: 10.1098/rsif.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjortdal JO, Ehlers N. Effect of excimer laser keratectomy on the mechanical performance of the human cornea. Acta Ophthalmol Scand. 1995;73:18–24. doi: 10.1111/j.1600-0420.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 26.Jue B, Maurice DM. The mechanical properties of the rabbit and human cornea. J Biomech. 1986;19:847–853. doi: 10.1016/0021-9290(86)90135-1. [DOI] [PubMed] [Google Scholar]
- 27.Kling S, Marcos S. Effect of Hydration State and Storage Media on Corneal Biomechanical Response From In Vitro Inflation Tests. J Refract Surg. 2013;29:490–497. doi: 10.3928/1081597X-20130617-08. [DOI] [PubMed] [Google Scholar]
- 28.Last JA, Liliensiek SJ, Nealey PF, et al. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. Journal of Structural Biology. 2009;167:19–24. doi: 10.1016/j.jsb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Last JA, Thomasy SM, Croasdale CR, et al. Compliance profile of the human cornea as measured by atomic force microscopy. Micron. 2012;43:1293–1298. doi: 10.1016/j.micron.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombardo M, Lombardo G, Carbone G, et al. Biomechanics of the Anterior Human Corneal Tissue Investigated with Atomic Force Microscopy. Invest Ophth Vis Sci. 2012;53:1050–1057. doi: 10.1167/iovs.11-8720. [DOI] [PubMed] [Google Scholar]
- 31.Dias J, Diakonis VF, Kankariya VP, et al. Anterior and posterior corneal stroma elasticity after corneal collagen crosslinking treatment. Exp Eye Res. 2013;116:58–62. doi: 10.1016/j.exer.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias JM, Ziebarth NM. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp Eye Res. 2013;115:41–46. doi: 10.1016/j.exer.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomasy SM, Raghunathan VK, Winkler M, et al. Elastic modulus and collagen organization of the rabbit cornea: Epithelium to endothelium. Acta Biomater. 2014;10:785–791. doi: 10.1016/j.actbio.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifert J, Hammer CM, Rheinlaender J, et al. Distribution of Young’s Modulus in Porcine Corneas after Riboflavin/UVA-Induced Collagen Cross-Linking as Measured by Atomic Force Microscopy. Plos One. 2014;9:e88186. doi: 10.1371/journal.pone.0088186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu KS, Li SY, Lo ACY, et al. Micro-scale Stiffness Change of Cornea Tissues Suffered from Elevated Intraocular Pressure Investigated by Nanoindentation. Soft Materials. 2011;11:244–253. [Google Scholar]
- 36.Winkler M, Chai D, Kriling S, et al. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest Ophth Vis Sci. 2011;52:8818–8827. doi: 10.1167/iovs.11-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sondergaard AP, Ivarsen A, Hjortdal J. Corneal Resistance to Shear Force After UVA-Riboflavin Cross-Linking. Invest Ophth Vis Sci. 2013;54:5059–5069. doi: 10.1167/iovs.12-10710. [DOI] [PubMed] [Google Scholar]
- 38.Mikula E, Hollman K, Chai D, et al. Measurement of corneal elasticity with an acoustic radiation force elasticity microscope. Ultrasound in medicine & biology. 2014;40:1671–1679. doi: 10.1016/j.ultrasmedbio.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kling S, Ginis H, Marcos S. Corneal Biomechanical Properties from Two-Dimensional Corneal Flap Extensiometry: Application to UV-Riboflavin Cross-Linking. Invest Ophth Vis Sci. 2012;53:5010–5015. doi: 10.1167/iovs.12-9583. [DOI] [PubMed] [Google Scholar]
- 40.Borja D, Manns F, Lamar P, et al. Preparation and Hydration Control of Corneal Tissue Strips for Experimental Use. Cornea. 2004:23. doi: 10.1097/00003226-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Terry MA, Ousley PJ, Zjhra ML. Hydration Changes in Cadaver Eyes Prepared for Practice and Experimental Surgery. Arch Ophthalmol. 1994;112:538–543. doi: 10.1001/archopht.1994.01090160118031. [DOI] [PubMed] [Google Scholar]
- 42.Hamaoui M, Tahi H, Chapon P, et al. Corneal preparation of eye bank eyes for experimental surgery. Cornea. 2001;20:317–320. doi: 10.1097/00003226-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Swinger CA, Kornmehl EW. Dehydration of post-mortem eyes for practice and experimental surgery. Ophthalmic surgery. 1985;16:182–183. [PubMed] [Google Scholar]
- 44.Duffey RJ, Tchah H, Lindstrom RL. Human cadaver corneal thinning for experimental refractive surgery. Refractive & corneal surgery. 1989;5:41–42. [PubMed] [Google Scholar]
- 45.Bourne WM, Nelson LR, Maguire LJ, et al. Comparison of Chen Medium and Optisol-GS for human corneal preservation at 4 degrees C: results of transplantation. Cornea. 2001;20:683–686. doi: 10.1097/00003226-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Jablonski-Stiemke MM, Edelhauser HF. Storage of human corneas in dextran and chondroitin sulfate–based corneal storage medium: Changes in stromal free sodium. Arch Ophthalmol. 1998;116:627–632. doi: 10.1001/archopht.116.5.627. [DOI] [PubMed] [Google Scholar]
- 47.Hatami-Marbini H, Rahimi A. Effects of bathing solution on tensile properties of the cornea. Exp Eye Res. 2014;120:103–108. doi: 10.1016/j.exer.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Faber C, Scherfig E, Prause JU, et al. Corneal thickness in pigs measured by ultrasound pachymetry in vivo. Scand J Lab Anim Sci. 2008;35:39–43. [Google Scholar]
- 49.Ziebarth NM, Arrieta E, Feuer WJ, et al. Primate lens capsule elasticity assessed using Atomic Force Microscopy. Exp Eye Res. 2011;92:490–494. doi: 10.1016/j.exer.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hertz H. Uber die beruhrung fester elastischer korper (on the contact of elastic solids) J Reine Angew Math. 1881:92. [Google Scholar]
- 51.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. Journal of Cataract & Refractive Surgery. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 52.Cartwright NEK, Tyrer JR, Marshall J. Age-Related Differences in the Elasticity of the Human Cornea. Invest Ophth Vis Sci. 2011;52:4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez DC, Niazy AM, Kurtz RM, et al. Finite element analysis applied to cornea reshaping. J Biomed Opt. 2005:10. doi: 10.1117/1.2136149. [DOI] [PubMed] [Google Scholar]