Abstract
Background
Energy metabolism is altered in patients with amyotrophic lateral sclerosis (ALS) but the role of diabetes is largely unknown.
Methods
We conducted a population-based case-control study of 5,108 ALS cases and 25,540 individually matched population controls during 1991–2010. Information on ALS and preexisting diabetes was retrieved from the Swedish Patient Register to explore the association of ALS with diabetes overall and with insulin-dependent or non-insulin dependent diabetes specifically. We also studied variation of the association by diabetes duration and age.
Results
In total, 224 ALS cases (4.39%) and 1,437 controls (5.63%) had diabetes before the index date, leading to an overall inverse association between diabetes and ALS risk (OR=0.79, 95%CI=0.68–0.91). The association was strong for non-insulin-dependent diabetes (OR=0.66, 95%CI=0.53–0.81) but not for insulin-dependent diabetes (OR=0.83, 95%CI=0.60–1.15) and varied as a function of diabetes duration, with the strongest association observed around six years after first ascertainment of diabetes. The association was age-specific; the inverse association was noted only among individuals aged 70 or older. In contrast, for younger individuals (<50 years), preexisting insulin-dependent diabetes was associated with a higher ALS risk (OR=5.38, 95%CI=1.87–15.51).
Conclusions
Our study suggests that there is an association between diabetes and ALS, and highlights the importance of taking into account age, insulin dependence and diabetes duration. Future studies should explore whether the association is independent of BMI.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disease. In Sweden the age-standardized incidence rate has increased from 2.32 per 100,000 person-years in 1991–1993 to 2.98 per 100,000 person-years in 2003–2005 [1], but the etiology of ALS remains largely unknown. Probable risk factors include exposure to heavy metals, pesticides, and smoking [2]. Known genetic variants are found in only a minor proportion of ALS cases without clear family history (90–95% of the total) and in two-thirds of familial cases [3].
Other diseases may share risk factors or genetic predisposition with ALS and, additionally, the pathological changes associated with specific diseases may alter future risk of ALS. Interestingly, a beneficial cardiovascular risk profile is associated with both ALS and frontotemporal dementia [4, 5]. Furthermore, a connection between metabolic disorders and ALS is supported by the fact that ALS patients are hypermetabolic and have impaired glucose tolerance [6, 7]. One study found that premorbid type 2 diabetes was associated with a 4-year delayed onset of ALS. However, two recent studies reported null associations with premorbid diabetes, except for a higher ALS risk associated with insulin-dependent diabetes before age 30 [8–10]. A literature review concluded that the role of diabetes in ALS remains unclear [11].
To address this issue, we investigated whether preexisting diabetes affects ALS risk in the Swedish population.
METHODS
Study design
We performed a nested case-control study using the Swedish Population and Housing Census conducted by Statistics Sweden in 1990. All residents who were born in Sweden and had never emigrated abroad nor been diagnosed with ALS before 1991 were selected for the study. Using the Swedish Patient, Causes of Death, and Migration Registers, we followed the study population from January 1st, 1991 to ALS diagnosis, death, emigration or December 31st, 2010, whichever came first. Cross-linkages were performed using the Swedish National Registration Numbers [12].
The Regional Ethical Review Vetting Board in Stockholm, Sweden approved the study.
ALS cases
We identified individuals with a first ALS diagnosis during follow-up through the Swedish Patient Register. Since 1964/1965, the Swedish National Board of Health and Welfare has compiled data on individual hospital discharges. This Inpatient Register achieved national coverage in 1987. The Outpatient Register contains information on visits to hospital-based specialist care from 2001 onward. Each record in the Swedish Patient Register (for Inpatient and Outpatient Registers collectively) includes discharge diagnoses coded by the Swedish Revisions of the International Classification of Diseases (ICD) codes (ICD-7 before 1969, ICD-8 1969–1986, ICD-9 1987–1996, and ICD-10 since 1997). ALS patients were defined as individuals with a main diagnosis of ALS (ICD-9 335.2 or ICD-10 G12.2). Hospital discharge data on ALS diagnosis are believed to be of high sensitivity and specificity [13].
Controls
Using incidence density sampling, we randomly selected five controls per case individually matched on year of birth, sex and area of residence (Northern, Central, or Southern Sweden). Individuals who were missing information on area of residence were excluded from the study. We defined the index date for cases as their date of diagnosis, and for controls as the date of diagnosis of their matched cases. To be eligible, the controls had to be alive and free of ALS on the index date.
Exposure
We assessed history of diabetes from 1987 to the index date through the Swedish Patient Register. Since 1987 this Register has had nationwide coverage for all hospital discharges and since 2001 it has also covered >80% hospital-based specialist care visits. We identified diagnoses of diabetes using the codes ICD-9 250 and ICD-10 E10-E11. In a sensitivity analysis, we also used information on use of antidiabetic medications extracted from the Swedish Prescribed Drug Register in order to include additional diabetes patients. This was done for the subsample with index dates between 2006 and 2010 since the Prescribed Drug Register contains information on all dispensed prescriptions of the Swedish population from July 1st, 2005 onward. All drugs are coded according to the Anatomical Therapeutic Chemical Classification System (ATC codes). Antidiabetic drugs are coded A10A or A10B.
We classified diabetes onset as early if the age at first ascertainment was ≤30 years or as late otherwise. Diabetes duration was defined as the time interval between the first available diabetes record in the Patient Register and the index date. In the main analyses, these two variables were calculated using information available after 1987. Since restricting ascertainment to 1987 onward might underestimate the duration and overestimate the age at diabetes onset, in a sensitivity analysis we further identified diabetes patients from records during the years 1964–1986 when the Inpatient Register was available but not yet nationwide (ICD-7 260, ICD-8 250).
We categorized patients as insulin-dependent if their ICD-10 code was E10 and as non-insulin-dependent if it was E11. In a sensitivity analysis, we also assigned insulin-dependent status to patients who had ever been prescribed insulin (ATC code A10A), and non-insulin-dependent status to those who were prescribed other drugs (ATC code A10B). The analyses on insulin dependence excluded individuals with unknown insulin-dependence status (i.e., absence of ICD-10 codes or with both ICD-10 E10 and E11).
Because diabetes without complications was recorded with specific ICD codes (ICD-9 250.0, ICD-10 E10.9 and E11.9), we defined cases of diabetes with complications as those with at least one diagnosis of diabetes with complications before the index date.
Covariates
The Swedish Population and Housing Census in 1990 provided information on socioeconomic status (Blue collar, White collar, Self-employed/Farmers, Other). The Swedish Education Register was established in 1985 by Statistics Sweden and annually collects information on the highest level of formal education attained for each Swedish resident. Education achieved before the index date was categorized as “≤9 years”, “9–12 years” and “University or doctoral studies”.
Statistical analysis
Analyses were performed using Stata ver. 12.1 (StataCorp, Texas, USA). We fitted conditional logistic regression models to estimate the odds ratios (ORs) for the association of ALS with diabetes, with 95% confidence intervals (CIs). We adjusted estimates for matching variables only or additionally for education and socioeconomic status. We repeated control sampling 100 times and plotted the observed distribution of the ORs. We performed analyses stratified by age at index date (<50, 50–59, 60–69, 70–79, 80+ years), sex, and calendar period of index date (1991–2000 versus 2001–2010). We tested multiplicative interaction between diabetes and age at index date by adding a continuous interaction term to the models. Departure from additivity was assessed by computing the Relative Excess Risk due to Interaction (RERI). Given that pre-clinical ALS symptoms might lead to intensified diagnostic evaluation, we repeated the analysis disregarding diabetes diagnosed during the 3-year time window before index date.
To study the impact of insulin-dependence on the association between diabetes and ALS, the subjects were classified as “No diabetes”, “Insulin-dependent diabetes” or “Non-insulin-dependent diabetes”. To estimate the effect of diabetes duration on ALS risk, we employed dummy variables (0–2, 2–4, 4–6, 6–8, 8–10, 10+ years) and cubic regression splines with 5 knots (percentiles: 5, 27.5, 50, 72.5, 95).
RESULTS
Among the 7,673,847 Swedish residents followed from 1991 to 2010, we identified 5,108 newly diagnosed cases of ALS and selected 25,540 matched controls.
Main analyses
Descriptive statistics (Table 1) showed that ALS cases were more educated (Chi squared test p=0.02) and more likely to be self-employed or white collar (p=0.02) than controls of the same age, sex and area of residence.
Table 1.
Characteristics of the amyotrophic lateral sclerosis (ALS) patients and their matched controls.
| ALS cases
|
Controls
|
|||||
|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | |
| N | 2,872 | 2,236 | 5,108 | 14,360 | 11,180 | 25,540 |
| Mean age at the index date (SD), years | 67.37 (12.18) | 69.36 (12.18) | 68.24 (12.22) | 67.36 (12.18) | 69.37 (12.18) | 68.24 (12.22) |
| Diabetes mellitus at the index date, n (%) | 144 (5.0) | 80 (3.6) | 224 (4.4) | 899 (6.3) | 538 (4.8) | 1,437 (5.6) |
| Area of residence, n (%) | ||||||
| Northern Sweden | 707 (24.6) | 519 (23.2) | 1,226 (24.0) | 3,535 (24.6) | 2,595 (23.2) | 6,130 (24.0) |
| Central Sweden | 1,513 (52.7) | 1,201 (53.7) | 2,714 (53.1) | 7,565 (52.7) | 6,005 (53.7) | 13,570 (53.1) |
| Southern Sweden | 652 (22.7) | 516 (23.1) | 1,168 (22.9) | 3,260 (22.7) | 2,580 (23.1) | 5,840 (22.9) |
| Highest achieved education, n (%) | ||||||
| ≤9 years | 1,243 (44.3) | 1,050 (48.4) | 2,293 (46.1) | 6,395 (45.8) | 5,539 (51.3) | 11,934 (48.2) |
| >9 and ≤12 years | 1,037 (37.0) | 773 (35.6) | 1,810 (36.4) | 5,033 (36.0) | 3,526 (32.7) | 8,559 (34.5) |
| University or doctoral studies | 523 (18.7) | 346 (16.0) | 869 (17.5) | 2,550 (18.2) | 1,730 (16.0) | 4,280 (17.3) |
| Missing | 69 | 67 | 136 | 382 | 385 | 767 |
| Socioeconomic status, n (%) | ||||||
| Blue collar | 992 (34.5) | 741 (33.1) | 1,733 (33.9) | 5,178 (36.1) | 3,721 (33.3) | 8,899 (34.8) |
| White collar | 1,115 (38.8) | 760 (34.0) | 1,875 (36.7) | 5,385 (37.5) | 3,584 (32.1) | 8,969 (35.1) |
| Self-employed/Farmer | 337 (11.7) | 312 (14.0) | 649 (12.7) | 1,598 (11.1) | 1,475 (13.2) | 3,073 (12.0) |
| Other | 428 (14.9) | 423 (18.9) | 851 (16.7) | 2,199 (15.3) | 2,400 (21.5) | 4,599 (18.0) |
In total, 5.63% of controls and 4.39% of cases had diabetes before the index date. Diabetes was inversely associated with ALS; the result was similar after further adjustment for education and socioeconomic status (Table 2). After repeating control sampling 100 times, the median multivariable OR was 0.80 (Figure 1). The association between diabetes and ALS varied with age at the index date (test for linear trend p<0.01, RERI=0.67); compared to the controls, ALS cases diagnosed before the age of 50 years were more likely and cases diagnosed after age 70 were less likely to have preexisting diabetes (Table 2). After stratifying by sex or calendar period of ALS diagnosis the associations did not vary greatly (Table 2).
Table 2.
Adjusted Odds Ratios (ORs) and their corresponding 95% confidence intervals (CIs) for the association of ALS with diabetes mellitus (DM).
| Variables | No. of cases with DM | No. of controls with DM | ORa (95%CI) | ORb (95%CI) |
|---|---|---|---|---|
| Overall | 224 | 1,437 | 0.77 (0.66–0.89) | 0.79 (0.68–0.91) |
| Age at the index date (years) | ||||
| <50 | 10 | 18 | 2.90 (1.31–6.44) | 3.15 (1.40–7.08) |
| 50–59 | 21 | 88 | 1.20 (0.74–1.94) | 1.20 (0.74–1.95) |
| 60–69 | 58 | 303 | 0.96 (0.72–1.27) | 0.95 (0.71–1.27) |
| 70–79 | 93 | 641 | 0.71 (0.57–0.89) | 0.71 (0.57–0.89) |
| 80 and above | 42 | 387 | 0.52 (0.37–0.72) | 0.56 (0.40–0.78) |
| Sex | ||||
| Male | 144 | 899 | 0.79 (0.66–0.95) | 0.80 (0.67–0.96) |
| Female | 80 | 538 | 0.73 (0.57–0.93) | 0.77 (0.60–0.98) |
| Calendar period | ||||
| 1991–2000 | 56 | 380 | 0.73 (0.55–0.97) | 0.77 (0.58–1.04) |
| 2001–2010 | 168 | 1,057 | 0.78 (0.66–0.92) | 0.79 (0.67–0.93) |
OR adjusted for age, sex and area of residence (matching factors).
OR adjusted for age, sex, area of residence, education and socioeconomic status; 136 cases and 767 controls with missing information on education and socioeconomic status were excluded in this analysis.
Figure 1.
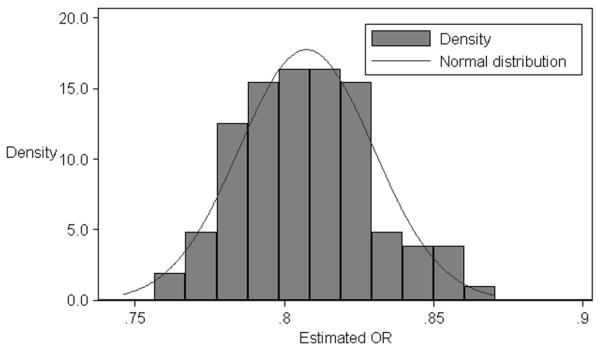
Observed distribution of the adjusted odds ratio (OR) of ALS for diabetes.
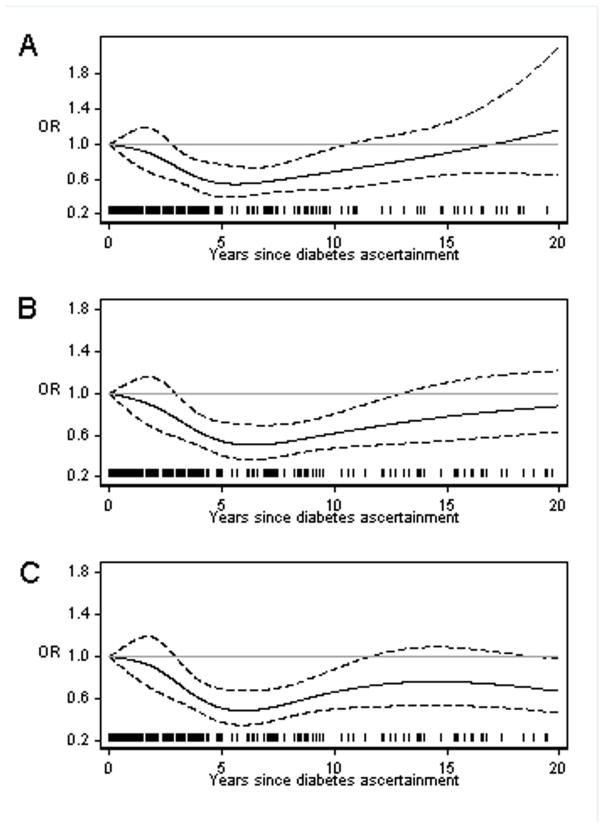
Only three cases and five controls had diabetes before age 30. Nevertheless, we observed suggestive evidence for a positive association with early onset diabetes (OR=12.05, 95%CI=2.34–62.21). Analysis by diabetes duration revealed a non-linear trend (Figure 2A). The OR first decreased with increasing duration, to a nadir (0.54) around six years after diabetes ascertainment, and then became null beyond 10 years.
Figure 2.
Temporal relationship between diabetes duration and ALS. (A) Main analysis where diabetes was ascertained from 1987 onward. (B) Sensitivity analyses where diabetes was ascertained from 1964 onward. (C) Sensitivity analyses where diabetes was ascertained from 1964 onward and excluding the individuals with diabetes before age 30.
Among the study participants 302 had insulin-dependent diabetes and 889 had non-insulin-dependent diabetes. Diabetes patients with unknown insulin dependence (77 cases and 393 controls) were on average older and less educated than those whose status was known (data not shown). The association with ALS was stronger for non-insulin-dependent diabetes (OR=0.65, 95%CI=0.52–0.79) than for insulin-dependent diabetes (OR=0.81, 95%CI=0.58–1.12). Results were similar after further adjustment for education and socioeconomic status (Table 3). Both insulin-dependent and non-insulin dependent diabetes were associated with decreased risk at older ages, but the former was associated with increased risk at younger ages (Table 3).
Table 3.
Adjusted Odds Ratios (ORs) and their corresponding 95% confidence intervals (CIs) for the association of ALS with insulin-dependent and non-insulin-dependent diabetes mellitus.
| No diabetes | Insulin-dependent diabetes | Non-insulin-dependent diabetes | |
|---|---|---|---|
| ALS cases, n (%) | 4,863 (97.1) | 43 (0.9) | 104 (2.1) |
| Controls, n (%) | 24,059 (95.8) | 259 (1.0) | 785 (3.1) |
| ORa (95%CI) | 1.00 (Ref) | 0.83 (0.60–1.15) | 0.66 (0.53–0.81) |
| ORa by Age at the index date (years) | |||
| <50 | 1.00 (Ref) | 5.38 (1.87–15.51) | 2.12 (0.37–12.10) |
| 50–59 | 1.00 (Ref) | 1.16 (0.54–2.51) | 0.77 (0.32–1.83) |
| 60–69 | 1.00 (Ref) | 1.19 (0.65–2.19) | 0.74 (0.49–1.12) |
| 70–79 | 1.00 (Ref) | 0.45 (0.25–0.82) | 0.67 (0.49–0.91) |
| 80 and above | 1.00 (Ref) | 0.46 (0.14–1.51) | 0.51 (0.33–0.80) |
OR adjusted for sex, age, area of residence, education and socioeconomic status; 136 cases and 767 controls with missing information on education and socioeconomic status were excluded in this analysis.
In total, 1,076 subjects had diabetes with complication. We observed similar inverse associations with ALS for diabetes without any complication (OR=0.79, 95%CI=0.62–1.01) and for diabetes with complications (OR=0.78, 95%CI=0.65–0.94).
Sensitivity analyses
Using the Prescribed Drug Register and restricting the analyses to participants with index dates during the period 2006–2010 (n=9,678), 10.38% of controls and 7.56% of cases had diabetes. The inverse association between diabetes and ALS persisted (OR=0.70, 95%CI=0.57–0.86); again the association was observed for non-insulin-dependent diabetes (OR=0.72, 95%CI=0.55–0.94) but not for insulin-dependent diabetes (OR=1.36, 95%CI=0.78–2.37).
Considering diabetes ascertained after 1964, we noted an increase in diabetes duration compared to duration ascertained after 1987 (median increase: 5 years). A U-shaped relationship was still seen for diabetes duration and ALS risk (Figure 2B). In total, 35 subjects had diabetes ascertained at age≤30 years (13 cases and 22 controls); the OR for early onset diabetes was 3.25 (95%CI=1.61–6.53), and for late onset diabetes 0.74 (95%CI=0.63–0.85). Duration of diabetes was longer for early onset compared to late onset diabetes (t-test of the equality of means p<0.01). When considering only late onset diabetes, ALS risk decreased with increasing diabetes duration, with a plateau beyond six years after diabetes ascertainment (Figure 2C).
Finally, we confirmed our findings when considering only diabetes diagnosed 3 or more years before the index date (OR for overall diabetes 0.66, 95%CI=0.54–0.80).
DISCUSSION
We found an inverse association between premorbid diabetes and ALS that was mainly noted for non-insulin-dependent diabetes and ALS at age 70 and above. Conversely, for insulin-dependent diabetes, we found a positive association before the age of 50 years. The group of insulin-dependent diabetics in our study likely included both type 1 diabetes and type 2 diabetes treated with insulin. Since the life expectancy for type 1 diabetes is estimated as 70 years of age [14], we speculate that the inverse association between insulin-dependent diabetes and ALS after age 70 may be largely due to insulin-dependent type 2 diabetes. Furthermore, diabetes with onset at age 30 or earlier, mainly insulin-dependent diabetes, appeared to be a risk factor for ALS, both in our main analysis and in our sensitivity analysis. Worth noting is that the latter provided a more meaningful estimate for the effect of early-onset diabetes because it relies on a better ascertainment of age at diabetes onset. Our results were consistent with an English study that found a positive association of ALS with early onset diabetes (<30 years) (relative risk 3.94) [9]. However, given the small numbers, our findings must be interpreted with care.
The biological mechanisms linking diabetes to ALS are as yet unclear. In an animal study, TDP-43, a protein encoded by the gene TARDBP which is mutated in ~4% of familial ALS cases, was shown to be a regulator of glucose and energy metabolism [15]. Specifically, overexpression of progranulin, an adipokine that mediates high-fat-induced insulin resistance, rescued mutant TDP-43 induced axonopathy in zebra fish ALS models [16,17]. Differences in associations of ALS with non-insulin-dependent type 2 and insulin-dependent type 1 diabetes may be related to differences in the pathophysiology of the two conditions. The former is characterized by insulin resistance, while the latter is an autoimmune disease destroying the beta-cells in the pancreas. Obesity, insulin resistance, or a reduction of insulin/insulin-like growth factor 1 (IGF1) signalling could all result in both type 2 diabetes and protection from neurodegeneration [18–20]. Furthermore, the two types of diabetes have both shared and independent genetic susceptibility [21].
Another potential explanation may be beneficial effects on ALS of treatments for non-insulin-dependent diabetes, such as metformin or pioglitazone. Metformin treatment in SOD1G93A transgenic mice produced small increases in motor neuron survival although it failed to significantly affect disease onset, progression or survival [22]. For pioglitazone, encouraging results have been reported in SOD1 mice, though it showed no beneficial effects on the survival of ALS patients as an add-on therapy to Riluzole [23].
The strength of the association between diabetes and ALS varied with time since first ascertainment of diabetes. Since the ascertainment date may have been years after diabetes onset and first diagnosis, the analysis by diabetes duration should be interpreted cautiously. The U-shaped association may have been a consequence of mixing insulin-dependent and non-insulin-dependent diabetes. In fact, when excluding subjects with diabetes onset at age 30 or earlier (mainly insulin-dependent diabetes), a consistently decreased ALS risk was noted. Such a pattern suggests a long induction period for diabetes to lower ALS risk, possibly because long-standing diabetes involves more profound pathophysiological changes; one possibility is greater dyslipidemia, a condition that is associated with longer survival in ALS patients [24]. This hypothesis may also explain why the decreased risk was observed only at age 70 and above.
The strengths of our study lie in the nationwide study design, the prospectively collected data, the complete follow-up, and the large sample size.
The main limitation of our study is the potential misclassification of diabetes status. The Inpatient Register data are known to have better positive predictive value than sensitivity for diabetes [25]. Indeed, the prevalence of diabetes in our data increased substantially when antidiabetic medications were used as additional source for diabetes ascertainment. The cases of diabetes undetected by the Patient Register are likely to be milder forms; thus it would be speculative to generalize our study findings to all diabetes patients. Nevertheless, since the information was prospectively and independently collected using the Patient Register, the misclassification is expected to be non-differential.
Another limitation in our data is the lack of information on BMI. BMI may be associated with both diabetes, especially non-insulin-dependent diabetes, and ALS. Elevated BMI may also increase diabetes risk and partly explain the association between diabetes and ALS. In conclusion, our study provided evidence for an inverse association between diabetes and ALS. We emphasize the importance of taking into account age, insulin dependence and diabetes duration. Future studies should explore whether the association is independent of BMI.
Acknowledgments
This study was funded by the Swedish Research Council, the Swedish Society for Medical Research, the Swedish Research Council for Health, Working life and Welfare, the Karolinska Institutet Partial Finance for PhD Students, and the Intramural Research Program of the NIH (Z01 ES 049005).
Footnotes
Conflicts of interest: None.
References
- 1.Fang F, Valdimarsdottir U, Bellocco R, et al. Amyotrophic lateral sclerosis in Sweden, 1991–2005. Archives of neurology. 2009;66(4):515–9. doi: 10.1001/archneurol.2009.13. [DOI] [PubMed] [Google Scholar]
- 2.Trojsi F, Monsurro MR, Tedeschi G. Exposure to environmental toxicants and pathogenesis of amyotrophic lateral sclerosis: state of the art and research perspectives. International journal of molecular sciences. 2013;14(8):15286–311. doi: 10.3390/ijms140815286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner MR, Hardiman O, Benatar M, et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet neurology. 2013;12(3):310–22. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korner S, Kollewe K, Ilsemann J, et al. Prevalence and prognostic impact of comorbidities in amyotrophic lateral sclerosis. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2013;20(4):647–54. doi: 10.1111/ene.12015. [DOI] [PubMed] [Google Scholar]
- 5.Kalkonde YV, Jawaid A, Qureshi SU, et al. Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2012;8(3):204–10. doi: 10.1016/j.jalz.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Desport JC, Preux PM, Magy L, et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. The American journal of clinical nutrition. 2001;74(3):328–34. doi: 10.1093/ajcn/74.3.328. [DOI] [PubMed] [Google Scholar]
- 7.Pradat PF, Bruneteau G, Gordon PH, et al. Impaired glucose tolerance in patients with amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis: official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2010;11(1–2):166–71. doi: 10.3109/17482960902822960. [DOI] [PubMed] [Google Scholar]
- 8.Jawaid A, Salamone AR, Strutt AM, et al. ALS disease onset may occur later in patients with pre-morbid diabetes mellitus. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2010;17(5):733–9. doi: 10.1111/j.1468-1331.2009.02923.x. [DOI] [PubMed] [Google Scholar]
- 9.Turner MR, Goldacre R, Ramagopalan S, et al. Autoimmune disease preceding amyotrophic lateral sclerosis: An epidemiologic study. Neurology. 2013 doi: 10.1212/WNL.0b013e3182a6cc13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seelen M, van Doormaal PT, Visser AE, et al. Prior medical conditions and the risk of amyotrophic lateral sclerosis. Journal of neurology. 2014 doi: 10.1007/s00415-014-7445-1. [DOI] [PubMed] [Google Scholar]
- 11.Lekoubou A, Matsha TE, Sobngwi E, et al. Effects of diabetes mellitus on amyotrophic lateral sclerosis: a systematic review. BMC research notes. 2014;7:171. doi: 10.1186/1756-0500-7-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. European journal of epidemiology. 2009;24(11):659–67. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisa FE, Verriello L, Deroma L, et al. The accuracy of discharge diagnosis coding for Amyotrophic Lateral Sclerosis in a large teaching hospital. European journal of epidemiology. 2009;24(10):635–40. doi: 10.1007/s10654-009-9376-1. [DOI] [PubMed] [Google Scholar]
- 14.Miller RG, Secrest AM, Sharma RK, et al. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61(11):2987–92. doi: 10.2337/db11-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stallings NR, Puttaparthi K, Dowling KJ, et al. TDP-43, an ALS linked protein, regulates fat deposition and glucose homeostasis. PloS one. 2013;8(8):e71793. doi: 10.1371/journal.pone.0071793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird AS, Van Hoecke A, De Muynck L, et al. Progranulin is neurotrophic in vivo and protects against a mutant TDP-43 induced axonopathy. PloS one. 2010;5(10):e13368. doi: 10.1371/journal.pone.0013368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu H, Deng H, Hu Z. Plasma progranulin concentrations are increased in patients with type 2 diabetes and obesity and correlated with insulin resistance. Mediators of inflammation. 2013;2013:360190. doi: 10.1155/2013/360190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly EJ, Wang H, Weisskopf MG, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14(3):205–11. doi: 10.3109/21678421.2012.735240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nature reviews Neuroscience. 2008;9(10):759–67. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj SM, Howson JM, Walker NM, et al. No association of multiple type 2 diabetes loci with type 1 diabetes. Diabetologia. 2009;52(10):2109–16. doi: 10.1007/s00125-009-1391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneb HM, Sharp PS, Rahmani-Kondori N, et al. Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1(G93A) mouse model of ALS and is harmful in female mice. PloS one. 2011;6(9):e24189. doi: 10.1371/journal.pone.0024189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupuis L, Dengler R, Heneka MT, et al. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PloS one. 2012;7(6):e37885. doi: 10.1371/journal.pone.0037885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupuis L, Corcia P, Fergani A, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70(13):1004–9. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC public health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]