Abstract
Tumor cell extravasation into the brain requires passage through the blood-brain barrier, which is a highly protected microvascular environment fortified with tight junction (TJ) proteins. TJ integrity can be regulated under physiological and pathophysiological conditions. There is evidence that exercise can modulate oxidation status within the brain microvasculature and protect against tumor cell extravasation and metastasis formation. In order to study these events, mature male mice were given access to voluntary exercise on a running wheel (exercise) or access to a locked wheel (sedentary) for five weeks. The average running distance was 9.0 ± 0.2 km/day. Highly metastatic tumor cells (murine Lewis lung carcinoma) were then infused into the brain microvasculature through the internal carotid artery. Analyses were performed at early stage (48 hours) and late stage (3 weeks) post tumor cell infusion. Immunohistochemical analysis revealed fewer isolated tumor cells extravasating into the brain at both 48 hours and 3 weeks post surgery in exercised mice. Occludin protein levels were reduced in the sedentary tumor group, but maintained in the exercised tumor group at 48 hours post tumor cell infusion. These results indicate that voluntary exercise may participate in modulating blood-brain barrier integrity thereby protecting the brain during metastatic progression.
Keywords: Exercise, metastasis, bioluminescence, blood-brain barrier, tight junction
1. Introduction
The benefits to overall fitness and well-being from regular exercise are undisputed. Additionally, the success of exercise to promote health and welfare following cancer diagnosis and treatment, e.g. diminish fatigue, support strength and mobility and aid in quality of life, is largely accepted [1,2]. While the ability of exercise to modulate the adverse ramifications of cancer therapy is well documented, the impact of physical activity on cancer progression and metastasis remains less understood. Direct effects of exercise on cancer development and progression have been more difficult to elucidate, and results from individual studies are often conflicting [3,4]. Several factors can influence the incongruous results reported in the literature including differences in methodology, tumor type, tumor location and exercise volume and strategy [3,4].
Metastatic progression and the formation of secondary tumor sites remain a challenge for advancing cancer treatment strategies [5]. Particularly in the brain, where the blood-brain barrier (BBB) selectively restricts both tumor cell trafficking and therapeutic interventions, it is imperative that regulatory mechanisms underlying that selectivity be better understood [5]. Lung and breast tumors as well as melanoma are the most common sources of brain metastases [6]. However the majority of exercise and cancer data comes from breast cancer followed by gastrointestinal and then prostate [3]. New reports in lung cancer patients suggest that exercise improves many physiological factors such as functional capacity, emotional and anxiety levels but not overall quality of life [7]; however in a 20 year study from Finland, exercise was seen to reduce lung cancer risk [8].
We chose a voluntary running model to mimic a moderately trained individual and diminish the potential effects of short, intensive workouts. Wheel exposure has been shown to impact various aspects of mouse physiology [9]. We demonstrated previously that voluntary exercise in mice altered oxidation status in the brain microvessels and modulated activation of redox-sensitive small GTPases [10]. In the current study we demonstrate that exercise regulates expression of barrier proteins in brain microvessels at early and late stages of tumor progression in an experimental model of brain metastasis formation.
2. Materials and methods
2.1 Tumor cell line
D122-luc, (Lewis lung carcinoma cells expressing luciferase, a gift from Dr. Lea Eisenbach, Weizmann Institute of Science, Rehovot, Israel) were cultured in Dulbecco’s modified Eagle’s medium GlutaMax (DMEM+Glut; Invitrogen) [11], supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen, Camarillo, CA). D122-luc cells were transfected with green fluorescent protein (GFP)–encoding vector (pBMN-GFP; Orbigen, San Diego, CA) to produce D122-luc/GFP cells. D122-Luc/GFP-positive cells were selected by culturing with 3 μg/ml puromycin followed by cell sorting. Cells were maintained in 1 μg/ml puromycin-containing media. All cells were used in the third or fourth passage for the study.
2.2 Animal housing, surgical procedures and bioluminescent imaging
Male C57BL/6 mice (Jackson Labs, Bar Harbor, ME), 12 weeks of age, were singly housed in plastic cages containing a running wheel (Coulbourn Instruments, Whitehall, PA). As used in our previous study [10], exercising mice had voluntary access to the running wheel while sedentary cages included the same running wheel in the locked position. Wheel revolutions were monitored using Clocklab software (Actimetrics, Wilmette, IL). Mice had ad libitum access to chow and water at all times. The lighting schedule was 12 hours of light followed by 12 hours of darkness, lights on at 6AM, Eastern Standard Time. Mice were euthanized at the conclusion of the study using carbon dioxide followed by decapitation. All procedures, which complied with the guidelines of the American Association for Accreditation of Animal Care (AAALAC), were approved by the University of Miami Institutional Animal Care and Use Committee.
Following five weeks of monitoring in exercise or sedentary conditions, mice were anesthetized with isoflurane, and 1.0 × 10 06 D122-luc/GFP cells were slowly infused through the internal carotid artery, as described previously [10,12]. Briefly, the common carotid artery (CCA) was isolated along with internal (ICA) and external (ECA) carotid arteries. The ECA was ligated while the CCA was temporarily closed with a vessel clip. A small incision was made in the ECA proximal to the bifurcation point of the CCA and tubing was inserted into the CCA. Tumor cells were slowly infused through the ICA. Blood flow returned once the vessel clip was removed from the CCA and the surgical site was cleaned and closed. Mice were under close observation for up to 3 weeks. Developing brain metastatic nodules were monitored weekly for up to 3 weeks using the IVIS Xenogen Bioluminescence Imager (Caliper Life Sciences, Hopkinton, MA). Prior to imaging, mice were anesthetized with isoflurane and injected i.p. with D-luciferin potassium salt (2 mg/100 μl) to induce bioluminescence of D122-luc/GFP cells. Identical instrument settings were used for all measurements under three-minute acquisition time. Brains were then dissected and sliced in 2mm sections using a brain block (Braintree Scientific, Braintree, MA) to image tumor growth within brain regions.
2.3 Immunohistochemistry
Brains used for immunohistochemistry were removed, rinsed in PBS, and placed in 10% neutral buffered formalin for fixation. The brains were paraffin embedded and cut into a series of 5-μm thick coronal sections with 150-μm intersections. Sections were mounted onto glass slides, deparaffinized, and subjected to antigen retrieval in citrate buffer (pH 6.0) boiling at 95 °C for 20 min. Endogenous peroxidase activity was reduced by treatment with 3% H2O2 in methanol for 20 min. The slides were rinsed in TBS containing 0.1% Tween-20 (TBS-T), blocked with 5% rabbit serum in TBS-T, and incubated with chicken α-GFP monoclonal antibody (1:400; Aves Lab, Tigard, OR) at 4°C overnight, followed by incubation with rabbit α-chicken IgY-HRP secondary antibody (1:400; Genway Biotech, San Diego, CA) for 90 min at room temperature. Diamino benzidine from Vector Laboratories (Burlingame, CA) was used for color developing. The sections were counterstained with hematoxylin (Sigma, St Louis, MO) and examined under light microscopy with Nikon Eclipse Ti-U and Zeiss Stemi 2000-CS instruments.
2.4 Microvessel isolation
Microvessels were isolated as previously reported [10,13]. Briefly, brains were removed and placed immediately in ice-cold PBS. Brains were homogenized using isolation buffer (102 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 15 mM HEPES, 25 mM NaHCO3, 10 mM glucose, 1 mM Na pyruvate with proteinase inhibitors). Then, 26% dextran (M.W. 75,000) in isolation buffer was added, and samples were centrifuged (5,800 xg; 4 °C) for 20 min. The microvessel containing pellets were resuspended in isolation buffer and filtered through a 120 μm mesh filter paper and re-pelleted by centrifugation (1,500 xg; 4 °C) for 10 min. Microvessel enriched fractions were smeared (~7 μl/slide) onto glass slides, heat fixed for 10 min at 98 °C, rinsed with chilled PBS, and fixed in 4% paraformaldehyde for 10 min at room temperature. Slides were rinsed with PBS and processed for immunofluorescence (described below). The remain pellets were resuspended in RIPA lysis buffer (0.5M Tris-HCl, pH 7.4, 1.5M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10mM EDTA, from Millipore) and incubated for 30 min at 4 °C homogenizing every 5 min with hand-held homogenizer from Kontes (Thomas Scientific, Swedesboro, NJ) and lysates were collected by centrifugation (14,000 xg; 4 °C) for further analysis.
2.5 Immunofluorescence
Slides containing microvessels were permeablized with 0.1% Triton X-100 for 30 min at room temperature then rinsed with PBS. Slides were blocked using 3% BSA in PBS for 1 hour at room temperature followed by overnight incubation at 4 °C with primary antibodies occludin, and zona occludens-1 (ZO-1) at concentrations 1:200 dilution in 3% BSA in PBS both obtained from Invitrogen (Camarillo, CA). Following washes with PBS, secondary antibodies, α-mouse Alexa fluor 488 and α-rabbit Alexa fluor 594 from Abcam (Cambridge, MA) were applied at 1:200 dilution in 3% BSA in PBS. Following 90 min incubation at room temperature, protected from light, the slides were washed and mounted with ProLong® Gold Antifade with dapi from Thermo Fisher Scientific (Life Technologies, Grand Island, NY) and coverslips. Images were acquired using a Nikon Eclipse Ti-U fluorescence microscope and the NES Elements software.
2.6 Western Blotting
Protein concentration from homogenized microvessels was measured using BCA™ Protein Assay Kit (Thermo Scientific, Rockford, IL). Equal amounts of protein lysates (20 μg) were loaded and separated on a 4–20% sodium dodecyl sulphate-polyacrylamide gels (SDS-PAGE), transferred using nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) and analyzed by Western Blotting. Nitrocellulose membranes were blocked with 3% bovine serum albumin (BSA) dissolved with Tris-buffered saline containing 0.1% Tween-20 (TBS-T), and incubated with the respective antibodies overnight at 4 °C. Occludin and ZO-1 antibodies were purchased from Invitrogen (Camarillo, CA). Claudin-5 antibody was purchased from Millipore (Temecula, CA). β-tubulin antibody was obtained from Sigma-Aldrich (St. Louis, MO). All antibodies were diluted with 3% BSA in TBS-T buffer at 1:1000 except for β-tubulin which was diluted 1:20,000. Secondary antibodies were purchased from LI-COR biosciences (Lincoln, NE) and incubated at room temperature for 1 hour at 1:20,000 concentrations. Following washing with TBS-T blots were imaged on the LI-COR Odyssey® CLx scanner and analyzed using Image Studio software provided with the instrument.
2.7 Statistical Analysis
Data were graphed and analyzed using GraphPad Prism software (La Jolla, CA). Tumor growth and measures of tumor metastasis were analyzed by nonparametric Mann-Whiteny test. Protein expression was quantified using two-way ANOVA, with Fisher’s LSD with significance value at p<0.05. Western blots were quantified using Image Studio and normalized to the signal for β-tubulin. The mean values for treated groups are represented relative to sedentary vehicle controls.
3. Results
3.1 Exercised and sedentary mice display variations in tumor growth in vivo
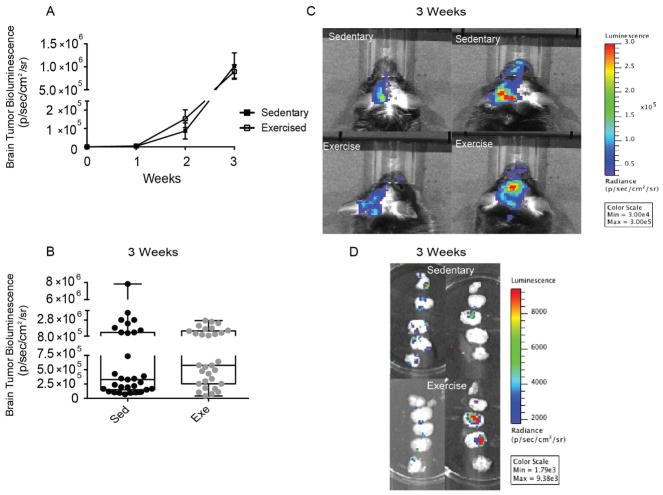
Following surgical infusion of D122-luc/GFP tumor cells and recovery, once per week mice were imaged to monitor tumor progression. Both sedentary and exercised mice began to show bioluminescent signals of tumor growth 2 weeks post-surgery. In addition, both groups exhibited similar dynamics of tumor progression (Fig. 1A). IVIS data analyzed at the 3rd week (i.e., the terminal time point) displayed variability within both groups and no significant difference in D122-luc/GFP bioluminescence (Fig. 1B). Raw IVIS data from the 3rd week showed strongest bioluminescence on the side ipsilateral to tumor cell infusion (Fig. 1C). Sliced brain sections also demonstrated tumor growth throughout the brain with strongest signal from mid-sections (Fig. 1D) in both sedentary and exercised groups, demonstrating variation within both treatments.
Figure 1. Exercised and sedentary mice display variation in tumor growth in vivo.
(A) Tumor progression over 3 weeks of observation. Sedentary mice closed squares, exercised mice open squares. Values are mean ± SEM. (B) Summary data of bioluminescent signal from D122-luc/GFP tumor cells in the brains of sedentary (black circles) and exercised (gray circles) mice at the 3rd (final) week of study. Each circle represents an individual mouse. Box and wisker plot. (C) Representative in vivo imaging system (IVIS) pictures of sedentary and exercised mice showing tumor growth at 3 weeks. (D) Representative IVIS images of sliced brains from sedentary and exercised mice showing tumor growth throughout the brain. (Bioluminescent signal measured in radiance, photons/second/square centimeter/square radian).
3.2 Immunohistochemistry of brain sections
In order to further quantify metastatic progression, brains were processed using immunohistochemistry by probing with α-GFP antibody. The majority of metastatic nodules appeared in the lateral and third ventricles within the mouse brain (Fig. 2A). Tumor cell counting and measurement of metastatic lesions was performed at 40X and 10X magnification, respectively. Tumor cells were observed in and around microvessels as well as within brain parenchyma in both sedentary and exercised groups (Fig. 2B). Solitary tumor cells or tumor cells in small groups were counted 48 hours and 3 weeks post tumor cell infusion. At both time points exercised mice show a decrease in the number of individual cells (Fig. 2C and 2D). The difference was significant within the 3 week cohort (Fig. 2D) demonstrating that exercise influenced the presence of solitary tumor cells within the brain. The number of metastatic nodules and the size of these lesions was similar between sedentary and tumor groups (Fig. 2E and 2F).
Figure 2. Immunohistochemistry of brain sections.

(A) Representative images of brain sections from sedentary and exercised mice, coronal sections 1.25X and stained for α-GFP. Arrows indicate tumor masses in the latteral and third ventricles at 3 weeks post tumor cell infusion. (B) Representative brain sections images captured at 40X and 100X (box inset), showing individual tumor cells and small clusers of tumor cells around microvessels and within brain parenchyma at 3 weeks post tumor cell infusion, indicated by black arrows. (C) Summary data for the number of individual tumor cells 48 hours post tumor cell infusion. (D) Summary data for individual tumor cells not part of metastatic nodules following 3 weeks of tumor growth. (E) Summary data for the number of metastatic nodules 3 weeks post tumor cell infusion. (F) Summary data for metastatic area (mm2) 3 weeks post tumor cell infusion. Values are mean ± SEM, *p<0.05.
3.3 Exercise impacts tight junction (TJ) protein expression in brain microvessels
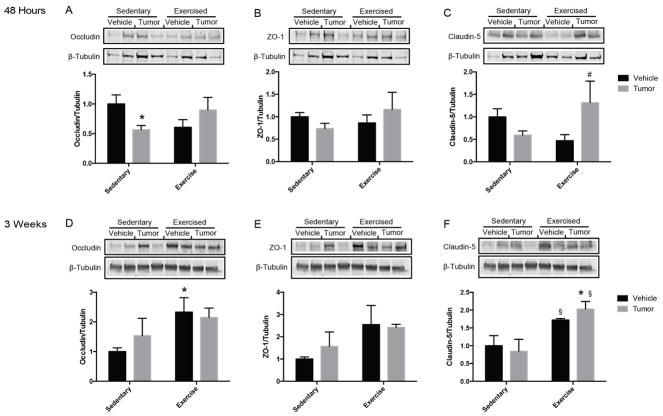
Three proteins central to TJ function were examined to determine if exercise modifies BBB integrity. At 48 hours post tumor cell infusion, levels of occludin were significantly reduced and the expression of ZO-1 and claudin-5 exhibited a tendency to be reduced in sedentary tumor mice compared to sedentary vehicle mice (Fig. 3A, 3B, and 3C). In contrast, the expression of occludin and ZO-1 were maintained at the control levels in exercised tumor mice and claudin-5 expression was significantly increased, indicating enhanced barrier regulation by exercise. At 3 weeks post surgery, there were no changes in TJ protein expression in the tumor-growing mice as compared to vehicle controls (Fig. 3D, 3E, and 3F); however, exercise alone increased the baseline expression of occludin and claudin-5 protein levels.
Figure 3. Exercise modulated tight junction protein expression in brain microvessels.
Representative western blots and quantitative summary data for tight junction protein expression. Levels of occludin (A, D), ZO-1 (B, E), and claudin-5 (C, F) were measured 48 hours (A, B, C) and 3 weeks (D, E, F) post tumor cell infusion in isolated brain microvessels and normalized to β-tubulin levels. Vehicle bars in black, tumor bars in gray. Values are mean ± SEM, *p<0.05 vs sedentary vehicle, #p<0.05 vs exercise vehicle, §p<0.05 vs sedentary tumor.
3.4 Colocalization of TJ proteins
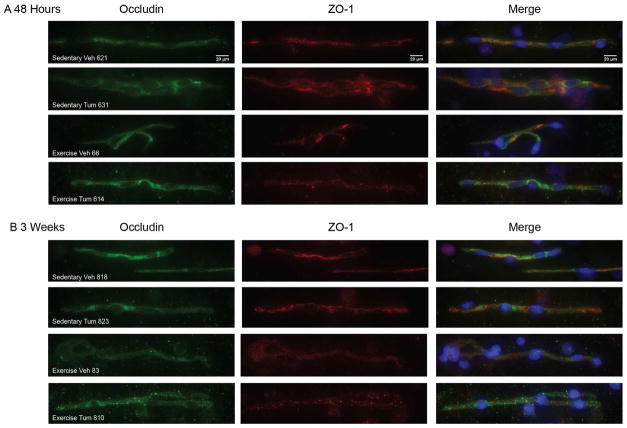
In addition to the overall expression levels, colocalization of TJ proteins is critical for a proper assemble of TJs and functions of the BBB. To visualize patterns of colocalization, isolated microvessels were prepared for immunofluorescence using antibodies against occludin and ZO-1. Patterns of occludin staining were similar among all treatment groups at both 48 hours and 3 weeks post tumor cell infusion (Fig. 4A and 4B, left panels). ZO-1 expression appeared less evenly distributed than occludin (center panels); however, the proteins overlapped in merged images demonstrating that proper assembly into TJs were present in isolated microvessels in all studied groups (Fig. 4A and 4B, right panels).
Figure 4. Colocalization of tight junction proteins.
Representative fluorescent images of isolated brain microvessels from sedentary and exercised mice at (A) 48 hours (B) 3 weeks post tumor cell infusion. Occludin (green), and ZO-1 (red) immunreactivity was evaluated using a Nikon Eclipse Ti-U fluorescence microscope and NES Elements software at 40X magnification. Merged images show occludin and ZO-1 overlap as well as nuclei (stained with DAPI, blue).
4. Discussion
Studies have shown that regular exercise and a healthy lifestyle early on in one’s life may reduce risk for certain diseases, including some types of cancer, as well as improve outcomes following diagnosis [4]. In the context of brain metastasis, it is important to point out that all blood-borne cells and therapeutics must pass through the BBB to gain access to the brain. Therefore, greater understanding of regulation and signaling events within brain endothelial cells is essential for more progressive prevention and/or treatment options. Previously, we reported that exercise can modulate redox status in microvessels comprising the BBB, suggesting a role for exercise in maintaining barrier integrity [10]. At the signaling levels, exercise modulated activity of redox-sensitive small GTPases, which participate in BBB remodeling and contribute to barrier disruption [14]. Our current study was based on the hypothesis that early lifestyle choices, such as exercise, can affect cancer progression in the brain specifically related to cerebrovascular health and BBB disruption. While all mice exposed to blood-borne tumor cells formed brain metastases, exercised mice exhibited a reduction in the number of solitary tumor cells within the brain both at the early and later stages of tumor development. At 48 hours post tumor cell infusion, expression of TJ proteins was reduced in microvessels from sedentary tumor mice but not in exercised tumor mice. Furthermore, exercise significantly unregulated occludin in vehicle-infused mice and claudin-5 in both tumor cell and vehicle-infused mice when analyzed at 3 week time point.
The BBB is comprised of endothelial cells that form a selective vascular network through use of TJ complexes [15]. Within TJ complexes there are several molecules that interact in the extracellular junctional space and others act as anchors within the endothelial cell to create a barrier that is impermeable to most blood-borne substances [15]. Occludin was the first transmembrane TJ protein identified [16]. Changes in BBB permeability partially coincide with alterations in occludin expression [17] and epithelial barrier function [18]. On the other hand, the intestinal barrier in occludin knockout mice remains operative [19]. In addition, other work has demonstrated that occludin could play a role outside of permeability, including redox regulation [19,20].
The claudin family of proteins has 24 members identified; mainly located in endothelial and epithelial cells, with claudin-5 being the most abundant type found in brain endothelium [6,15]. The selectivity of claudin-5 expression in brain endothelial cells suggests it plays a crucial role in BBB function [6]. Indeed, it has been demonstrated that claudin-5 participates in modulating permeability to ions as well as macromolecules [21,22]. Several studies indicated that increased permeability of the BBB was accompanied by decreased claudin-5 expression [6]. Interestingly, it was demonstrated that Rho kinase (ROCK) could induce phosphorylation of claudin-5 (and occludin) leading to TJ disruption and promoting monocyte migration through the BBB in a model of HIV-1 encephalitis [23,24]. ROCK is activated by RhoA GTPases [25]. These data support our previous findings that RhoA was less activated in microvessels from exercised mice, especially high runners following tumor cell infusion [10]. Coupled with our current observations that claudin-5 expression is maintained in microvessels from exercised mice while microvessels from sedentary mice showed decreased expression at 48 hours post tumor cell infusion suggests exercise could impact maintenance of TJs through the RhoA/ROCK signaling pathway.
Zona occludens (ZO) proteins are scaffolding proteins that interact with occludin and other claudin family members to organize proteins at the plasma membrane and “anchor” TJs within the cytoplasm [26]. ZO-1 overexpression results in reduced proliferation of cultured cells; thus, it is not surprising that cancer cells can promote a decrease in ZO-1 expression [27]. Indeed, it has been demonstrated that matrix metalloproteinases secreted by circulating cancer cells can increase BBB permeability by targeting TJ proteins including ZO-1 [28]. At early stages of tumor growth in the current study, microvessels from sedentary mice tended toward decreased ZO-1 expression, while exercise showed an indication towards maintained or increased TJ protein expression. This observation is consistent with the evidence that endurance swimming increased mRNA levels of ZO-1 in rat small intestine [29].
While exercise is universally accepted as beneficial to the body, in the context of pathology the picture becomes less clear. It is important to consider the impact of exercise alone as well as the type of exercise model as different intensity of activity may confer a different outcome. In the current study we used voluntary running over time to mimic a trained individual before exposure to tumor cells. Exercise impacted brain endothelial cell TJs during tumor development, suggesting that such conditioning can impact BBB integrity and may provide benefit in the early stages of metastases formation.
Highlights.
We found fewer extravasating tumor cells in the brains from exercised mice
We observed tight junction disruption in brain microvessels from tumor cell infused sedentary mice
Exercise alone altered tight junction protein expression in brain microvessels
Acknowledgments
This work was supported by the NIH/NCI grant R01 CA133257 and Alltech Nutrigenomics. The authors would also like to thank the imaging core at the University of Miami, particularly Bernard Jay Wasserlauf and Marbella Chavarria from pathology resource services.
Abbreviations
- TJ
Tight junction
- BBB
Blood-brain barrier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gretchen Wolff, Email: g.wolff11@med.miami.edu.
Sarah J. Davidson, Email: s.davidson9@umiami.edu.
Jagoda K. Wrobel, Email: JWrobel@med.miami.edu.
Michal Toborek, Email: MToborek@med.miami.edu.
References
- 1.Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: A translational perspective. Brain Behav Immun. 2013;30(Supplement):S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemanne D, Cassileth B, Gubili J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology (Williston Park) 2013;27:580–585. [PubMed] [Google Scholar]
- 5.Wilhelm I, Molnar J, Fazakas C, Hasko J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013;14:1383–1411. doi: 10.3390/ijms14011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia W, Lu R, Martin TA, Jiang WG. The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (review) Mol Med Rep. 2014;9:779–785. doi: 10.3892/mmr.2013.1875. [DOI] [PubMed] [Google Scholar]
- 7.Quist M, Adamsen L, Rorth M, Laursen JH, Christensen KB, Langer SW. The Impact of a Multidimensional Exercise Intervention on Physical and Functional Capacity, Anxiety, and Depression in Patients With Advanced-Stage Lung Cancer Undergoing Chemotherapy. Integr Cancer Ther. 2015 doi: 10.1177/1534735415572887. [DOI] [PubMed] [Google Scholar]
- 8.Pletnikoff PP, Tuomainen TP, Laukkanen JA, Kauhanen J, Rauramaa R, Ronkainen K, Kurl S. Cardiorespiratory fitness and lung cancer risk: A prospective population-based cohort study. J Sci Med Sport. 2015 doi: 10.1016/j.jsams.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Duncan MJ, Franklin KM, Peng X, Yun C, Legan SJ. Circadian rhythm disruption by a novel running wheel: roles of exercise and arousal in blockade of the luteinizing hormone surge. Physiol Behav. 2014;131:7–16. doi: 10.1016/j.physbeh.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff G, Balke JE, Andras IE, Park M, Toborek M. Exercise modulates redox-sensitive small GTPase activity in the brain microvasculature in a model of brain metastasis formation. PLoS One. 2014;9:e97033. doi: 10.1371/journal.pone.0097033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbach L, Segal S, Feldman M. MHC imbalance and metastatic spread in Lewis lung carcinoma clones. Int J Cancer. 1983;32:113–120. doi: 10.1002/ijc.2910320118. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Swartz KR, Toborek M. Vessel microport technique for applications in cerebrovascular research. J Neurosci Res. 2009;87:1718–1727. doi: 10.1002/jnr.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park M, Kim HJ, Lim B, Wylegala A, Toborek M. Methamphetamine-induced Occludin Endocytosis Is Mediated by the Arp2/3 Complex-regulated Actin Rearrangement. J Biol Chem. 2013;288:33324–33334. doi: 10.1074/jbc.M113.483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4:e8045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, Turner JR. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal. 2011;15:1195–1219. doi: 10.1089/ars.2010.3542. [DOI] [PubMed] [Google Scholar]
- 21.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kluger MS, Clark PR, Tellides G, Gerke V, Pober JS. Claudin-5 controls intercellular barriers of human dermal microvascular but not human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:489–500. doi: 10.1161/ATVBAHA.112.300893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Mariscal L, Dominguez-Calderon A, Raya-Sandino A, Ortega-Olvera JM, Vargas-Sierra O, Martinez-Revollar G. Tight junctions and the regulation of gene expression. Semin Cell Dev Biol. 2014;36:213–223. doi: 10.1016/j.semcdb.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One. 2011;6:e20599. doi: 10.1371/journal.pone.0020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teerapornpuntakit J, Dorkkam N, Wongdee K, Krishnamra N, Charoenphandhu N. Endurance swimming stimulates transepithelial calcium transport and alters the expression of genes related to calcium absorption in the intestine of rats. Am J Physiol Endocrinol Metab. 2009;296:E775–786. doi: 10.1152/ajpendo.90904.2008. [DOI] [PubMed] [Google Scholar]