Abstract
Understanding the ecology of the gastrointestinal tract and the impact of the contents on the host mucosa is emerging as an important area for defining both wellness and susceptibility to disease. Targeted delivery of drugs to treat specific small intestinal disorders such as small bowel bacterial overgrowth and targeting molecules to interrogate or to deliver vaccines to the remote regions of the small intestine has proven difficult. There is an unmet need for methodologies to release probes/drugs to remote regions of the gastrointestinal tract in furthering our understanding of gut health and pathogenesis. In order to address this concern, we need to know how the regional delivery of a surrogate labeled test compound is handled and in turn, if delivered locally as a liquid or powder, the dynamics of its subsequent handling and metabolism. In the studies we report on in this paper, we chose 13C sodium acetate (13C-acetate), which is a stable isotope probe that once absorbed in the small intestine can be readily measured non-invasively by collection and analysis of 13CO2 in the breath. This would provide information of gastric emptying rates and an indication of the site of release and absorptive capacity. In a series of in vitro and in vivo pig experiments, we assessed the enteric-protective properties of a commercially available polymer EUDRAGIT®L100-55 on gelatin capsules and also on DRcaps®. Test results demonstrated that DRcaps®coated with EUDRAGIT®L100-55 possessed enhanced enteric-protective properties, particularly in vivo. These studies add to the body of knowledge regarding gastric emptying in pigs and also begin the process of gathering specifications for the design of a simple and cost-effective enteric-coated capsule for delivery of acid-labile macromolecules to the small intestine.
Keywords: Breath testing, Pig, Endoscopic capsule, Gastric emptying, Biomarker delivery, Gastrointestinal tract
1. Introduction
As the immune system of the gastrointestinal (GI) tract constitutes the first line of defence against mucosal pathogens (van Ginkel et al., 2000; Holmgren and Czerkinsky, 2005; Takahashi et al., 2009), the ability to deliver acid-labile compounds such as therapeutic drugs and also vaccine antigens directly to the small intestine offers opportunities for non-invasive treatments and vaccination strategies. Although a significant amount of work has been conducted using micro-encapsulated molecules, sophisticated and expensive equipment is required for these formulations (Sue et al., 1993; Shirley et al., 2001; Bueno da Costa et al., 2002; Mccarron et al., 2008). We therefore have developed a simple and inexpensive enteric-stable capsule with an ability to deliver milligram amount payloads to the small intestine of pigs. Non-invasive functional breath tests have been used previously in animal models (Anderson et al., 2002; Pelton et al., 2004; Butler, 2008; Terry et al., 2012) with more applications/examples in humans (Ishii et al., 2001; Festi et al., 2005; Tooley et al., 2006; de Lacy Costello et al., 2013; Pizzoferrato et al., 2013). In our studies, we chose 13C sodium acetate (13C-acetate) for a surrogate biomarker of “payload” in the GI tract of pigs as it is relatively inexpensive and its metabolism in the duodenum to 13CO2 can be non-invasively measured and quantified from exhaled breath, enabling determination of gastric emptying rates (Braden et al., 1995; 2004; Barbosa et al., 2005).
The use of synthetic polymer-coated capsules, which provide protective properties against the low pH of the stomach while also being rapidly degraded in neutral pH as encountered in the small intestine, was pivotal in our investigations. In our studies, we used EUDRAGIT®L100-55 (Evonik Industries, Essen, North Rhine-Westphalia, Germany) and DRcaps®(Capsugel®, Morristown, NJ, USA) capsules as they have been extensively reported and marketed to provide exceptional enteric protection while also being rapidly degraded at pH 5.5 (Khan et al., 1999; Terao et al., 2001; Jelvehgari et al., 2010; Calija et al., 2013).
We therefore have instigated in vitro testing and small-scale pig studies using EUDRAGIT®L100-55 coating of both gelatin capsules and also commercially available DRcaps®to investigate proof of concept for an inexpensive but effective capsule with non-invasive end uses in human gastro-intestinal functional studies and therapeutic drug/vaccine delivery.
2. Materials and methods
2.1. Capsule coating and in vitro studies
In our current study we first embarked on in vitro experiments to determine the basic properties that would be required to provide enteric stability of the 13C-acetate payload. Following consultation with Evonic Industries and the use of in-house technologies, we developed the following capsule coating protocol. Gelatin capsules (size 000) were filled with 250 mg of crystal violet and a stainless steel bar (6.3 mm×12 mm). Loaded capsules were then arranged in a dipping tray and suspended in a pre-prepared EUDRAGIT®L100-55 mixture (EUDRAGIT®L100-55, 9.0 g; polyethylene glycol 400, 1.4 g; Tween 80, 0.1 g; acetone, 38 ml; isopropyl alcohol, 57 ml; and water, 5 ml) for 15 s, permitting 2/3 of the capsules’ surface to be coated and then allowed to dry for 30 min. Capsules were then inverted and re-inserted into the dipping tray and the remaining 1/3 of the capsules’ surface was coated. Capsules were subsequently placed on the laboratory bench at ambient temperature and allowed to completely dry for 72 h. Specific residual solvent analysis was not performed; however, all coated capsules were free of acetone odor after 72 h of drying. In a series of experiments, we then tested the in vitro enteric protective ability of both the DRcaps®and gelatin capsules coated with EUDRAGIT®L100-55 either once, twice, thrice, or four times. Uncoated gelatin capsules or commercially available DRcaps®capsules used for controls were loaded with crystal violet and the stainless steel bar but were not EUDRAGIT®L100-55 coated. As we intended to perform an in vitro release (or dissolution) study using crystal violet dye as the test compound, a paddle system dissolution apparatus was used in accordance with pharmacopoeia standards. In these experiments, a dissolution bath (USP 23 type II Apparatus Vankel VK6010, Varian Inc., Palo Alto, CA, USA) was assembled and pre-warmed to 37 °C. A volume of 400 ml of 0.1 mol/L HCl (pH 1.0) was placed into 3×dissolution cups and rotation was set at 100 r/min. Enteric protection/capsule integrity, as measured by visual detection after release of blue color, was assessed at 60, 90, and 120 min. At the 120-min mark, 200 ml of 0.05 mol/L phosphate buffer (Na2HPO4, pH 7.5) was added to the cups, effectively adjusting the pH to 7.0, representative of porcine intestinal conditions (Evans et al., 1988) and also in accordance with the pH range (6.8–8.0) as accepted by the U.S. Food and Drug Administration Guidance for Industry (http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070237.pdf ). Integrity was again monitored until the capsule had disintegrated (n≥3 for each different capsule coating type).
2.2. In vivo pig studies
In vivo studies were conducted on a total of thirteen female Large White×Duroc pigs (age 8–12 weeks) obtained from the Roseworthy Campus, University of Adelaide (Roseworthy, Australia) and subsequently housed at the South Australian Health and Medical Research Institute Animal Facility at Gilles Plains in Adelaide, Australia. The pigs were housed singularly alongside neighboring companions in raised metal cages (2 m×2 m) with rubber matting covering the bottom surface of the enclosures. During maintenance periods, food and water were provided ad libitum. Room temperature was maintained at 20 °C with a 12-h light/12-h dark cycle.
Prior to the procedures, all animals were fasted from food overnight but allowed access to water. In all studies, a baseline breath sample was taken from the pigs 5 min prior to gavage. Pigs were briefly anaesthetized via an intramuscular injection of 20 mg/kg of ketamine (100 mg/ml) (CEVA Animal Health, Glenorie NSW, Australia) delivered through an 18 G needle. Once fully anaesthetized, a measured length of 12 mm inner diameter (ID) polypropylene tubing was then placed through the mouth and directed carefully into the stomach. To assess the liquid gastric emptying time, pigs were gavaged via tubing with 250 mg of 13C-acetate dissolved in 100 ml of water via a 30-ml syringe. To elucidate the gastric emptying time and the enteric protective capabilities of the EUDRAGIT®L100-55, pigs were gavaged with a quadruple-coated gelatin capsule containing 250 mg of powdered 13C-acetate followed by 100 ml of acidified water (pH 3.5) via a 30-ml syringe. Finally, we aimed to demonstrate both the gastric liquid emptying phase and the enteric protective properties of EUDRAGIT®L100-55 simultaneously in an individual pig. In these studies, 250 mg of powdered 13C-acetate was enclosed in a size 0 DRcaps®which had been placed into a size 00 DRcaps®(double DRcaps®). The outer DRcaps®was also further coated with EUDRAGIT®L100-55 four times (quadruple-coated). In these later studies, groups of pigs were gavaged with 250 mg of 13C-acetate dissolved in 100 ml of acidified water (pH 3.5) via a 30-ml syringe, simultaneously with the quadruple-coated, double DRcaps®. In all studies, exhaled breath samples were collected from conscious pigs using an adapted mask set up constructed from a cut down lower end of an empty intravenous bag with the outlet tube attached to a 3-way stopcock and 20-ml syringe. This mask was held over the pig’s snout while the syringe plunger was withdrawn. By closing the stopcock, the breath sample could be transferred to evacuated tubes for analysis. 13CO2 was measured using the SerCon Automated Breath 13C Isotope Ratio Mass Spectrometer (SerCon, Crewe, UK). Breath test results are shown as the change from baseline of the ratio of 13C/12C detected in the expired CO2 (Symonds et al., 2008). CO2 levels were always checked to ensure consistent sampling.
3. Results
3.1. In vitro studies
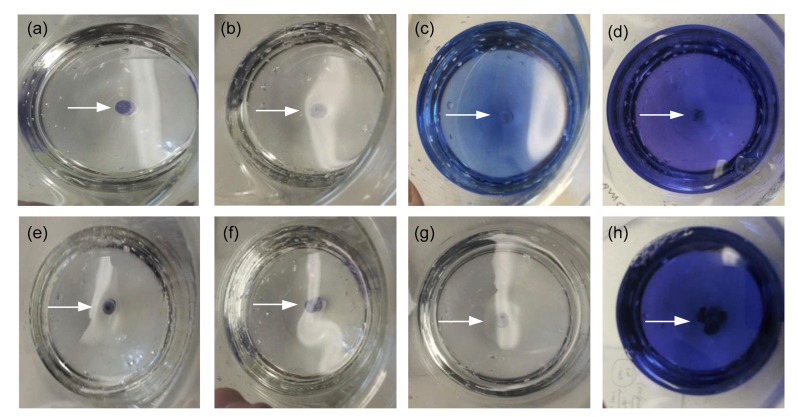
Under the low pH conditions (pH 1.0), the singly and doubly coated gelatin capsules had completely disintegrated within 30 min. The uncoated control gelatin capsules had completely disintegrated within 5 min (data not shown). Conversely, gelatin capsules triple-coated or quadruple-coated with the EUDRAGIT®L100-55 survived for 60 (Figs. 1a and 1e) and 90 min (Figs. 1b and 1f), respectively, without any visible sign of disintegration. Slight disintegration of triple-coated gelatin capsules was observed at 120 min (Fig. 1c). However, quadruple-coated gelatin capsules survived 120 min without evident dye leakage under these conditions (Fig. 1g). Subsequent exposure of the triple- and quadruple-coated (EUDRAGIT®) gelatin capsules to pH 7.0 resulted in total disintegration after 30 min (Figs. 1d and 1h). In preliminary in vitro experiments, uncoated DRcaps®surprisingly disassembled into two separate pieces within 90 min of exposure to pH 1 with disintegration occurring at the closure seal of the capsule (data not shown) and therefore was not used for initial in vivo studies.
Fig. 1.
In vitro assessment of triple- and quadruple-coated (EUDRAGIT®L100-55) gelatin capsules
Triple-coated (EUDRAGIT®L100-55) capsule integrity following incubation for 60 (a), 90 (b), and 120 min (c) at pH 1.0 and then 30 min at pH 7.0 (d). Quadruple-coated (EUDRAGIT®L100-55) capsule integrity following incubation for 60 (e), 90 (f), and 120 min (g) at pH 1.0 and then 30 min at pH 7.0 (h). All incubations were conducted at 37 °C with 100 r/min rotation. All pictures are plan views of the dissolution cups. Blue areas indicate dye content release
3.2. In vivo pig studies
The initial stage to establish the utility of the pig model of gastric emptying was to determine a dose-response curve with 13C-acetate administered in a liquid form. As expected, release of 13CO2 in the breath of the pigs was detected within 5 min of gavage and rapidly peaked within 45 min (Figs. 2a and 2b). The height of the individual peaks also demonstrated a dose-response profile of 13CO2 release in the breath (Fig. 2a: 250 mg, peak 159; 125 mg, peaks 62 and 73; 65 mg, peak 41) confirming the reproducibility of this protocol. We next compared the release of 13CO2 in the breath following dosing of pigs with powdered 13C-acetate delivered in gelatin capsules quadruple-coated with EUDRAGIT®L100-55. We observed a significant delay in the release of 13CO2 with the average 13CO2 peak observed at 110 min after the capsule administration, compared with a peak occurring at around 30 min after the 13C-acetate was administered in the liquid form indicating release of the labelled 13CO2 in the small intestine (Fig. 2b). In our final study, we investigated the combined administration of 250 mg of 13C-acetate in a liquid form simultaneously with 250 mg of powdered 13C-acetate in a double DRcaps®quadruple-coated with EUDRAGIT®L100-55. In these studies, where pigs were dosed with both liquid 13C-acetate simultaneously with EUDRAGIT®L100-55 coated DRcaps®we observed a biphasic profile of 13CO2 release. The first peak appeared at 30 min post-dosing representing the liquid gastric emptying phase. This was followed by a significantly delayed and broader peak at 180 min indicative of duodenal release of the 13C-acetate. These results demonstrated the enhanced enteric-protective properties of the EUDRAGIT®L100-55 coated DRcaps®compared with EUDRAGIT®L100-55 coated gelatin capsules.
Fig. 2.
13CO2 release in the breath of pigs following dosing with either liquid or enteric-coated capsules containing powdered 13C-acetate
(a) In the first set of small-scale pilot studies, pigs (n=1 per dose) were gavaged with 250, 125, or 65 mg of 13C-acetate dissolved in 100 ml water. (b) In the second set of pilot studies, pigs were gavaged with 250 mg of powdered 13C-acetate in a gelatin capsule quadruple-coated with EUDRAGIT®L100-55 (n=4) or with 250 mg of 13C-acetate dissolved in 100 ml acidified water simultaneously with 250 mg of powdered 13C-acetate enclosed in a double DRcaps®quadruple-coated with EUDRAGIT®L100-55 (n=2). Breath samples were collected throughout the duration of the study and assessed for the presence of 13CO2 and expressed as delta/baseline. Data are represented as mean and standard error of the mean
4. Discussion
Fundamental to our studies was the development of an in vivo assay to assess gastric emptying. In our initial studies, we observed reproducible dose-response curves for 13CO2 release in the breath of pigs and these results enabled us to effectively assess the enteric-stability of our coated capsules (Fig. 2a). Preliminary in vitro studies clearly demonstrated the utility of EUDRAGIT®L100-55 for enteric-like protection of gelatin capsules. However, this protection was less evident in the gastro-intestinal tract of a pig. We therefore tested the in vivo protective capability of EUDRAGIT®L100-55 coated gelatin capsules and also to further enhance the enteric-protective properties of our capsules, EUDRAGIT®L100-55 coated DRcaps®. In these studies, where pigs were dosed with liquid 13C-acetate simultaneously with powdered 13C-acetate in a double DRcaps®quadruple-coated with EUDRAGIT®L100-55, we observed a biphasic profile of 13CO2 release. The first peak was evident at 30 min post-dosing representing the liquid gastric emptying phase, followed by a significantly delayed and broader peak at 180–220 min, which is indicative of duodenal release of the 13C-acetate. These observations indicate a superior gastric-stability of the EUDRAGIT®L100-55 coated DRcaps®over EUDRAGIT®L100-55 coated gelatin capsules. To our knowledge, this data is the first to apply a direct comparison of the enteric-stability of EUDRAGIT®L100-55 coated capsules and DRcaps® in vitro and in vivo in a pig model. Additionally, these results add to the body of knowledge regarding gastric emptying in pigs and the utility of non-invasive breath testing when the probe is delivered directly to the small intestine.
In conclusion, this current study investigated the combined use of enteric-protective coatings, breath tests, and the collection of preliminary specifications for the design of a simple but effective capsule for use in both pigs and humans with the aim of optimizing the construction of novel, non-invasive devices and targeted therapeutics. It showed that breath tests are feasible when the probe (13C-acetate) is delivered in situ in the small intestine in powdered form in a capsule suitably coated with enteric-protective coatings. Compared with oral delivery of 13C-acetate in liquid form, significant delays in the release of 13CO2 were observed when 13C-acetate powder was delivered either in gelatin capsule or in DRcaps®both quadruple-coated with EUDRAGIT®L100-55. The breath test signals also showed different patterns when compared with 13C-acetate delivered orally in liquid form; however, the aggregate signal was similar. The DRcaps®demonstrated enhanced enteric-protective properties compared with the gelatin capsules when both were coated with EUDRAGIT®L100-55. Finally, these studies describe the effectiveness of a simple and inexpensive endoscopic capsule, which is able to deliver powdered compounds to the duodenum of pigs, signifying the potential for use for studies aimed at delivering acid-labile compounds to the lower gastro-intestinal tract. Related studies have also signified the potential for tracking the location of the capsule within the GI tract (Pham and Aziz, 2014).
Acknowledgments
We wish to thank Evonik Industries for their kind gift of the EUDRAGIT®L100-55 and also Capsugel®for their supply of the DRcaps®. We acknowledge Associate Professor Taher OMARI of the Women’s and Children’s Hospital, Adelaide, Australia for additional supportive funding. We also acknowledge Pfizer Australia, Sydney, NSW, Australia for additional funding.
Footnotes
Project supported by the Australian Research Council (ARC) Linkage Project Grant (No. LP0990847)
Compliance with ethics guidelines: Darren S. MILLER, Anne Michelle PARSONS, John BRESLAND, Paul HERDE, Duc Minh PHAM, Angel TAN, Hung-yao HSU, Clive A. PRESTIDGE, Tim KUCHEL, Rezaul BEGG, Syed Mahfuzul AZIZ, and Ross N. BUTLER declare that they have no conflict of interest.
All animal studies were approved by the SA Pathology/Central Health Network Animal Ethics Committee in accordance with the Australian code of practice for the care and use of animals for scientific purposes.
References
- 1.Anderson DL, Bartholomeusz FD, Kirkwood ID, et al. Liquid gastric emptying in the pig: effect of concentration of inhaled isoflurane. J Nucl Med. 2002;43(7):968–971. [PubMed] [Google Scholar]
- 2.Barbosa L, Vera H, Moran S, et al. Reproducibility and reliability of the 13C-acetate breath test to measure gastric emptying of liquid meal in infants. Nutrition. 2005;21(3):289–294. doi: 10.1016/j.nut.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Braden B, Adams S, Duan LP, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108(4):1048–1055. doi: 10.1016/0016-5085(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 4.Braden B, Peterknecht A, Piepho T, et al. Measuring gastric emptying of semisolids in children using the 13C-acetate breath test: a validation study. Dig Liver Dis. 2004;36(4):260–264. doi: 10.1016/j.dld.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Bueno da Costa MH, Quintilio W, Tanizaki MM, et al. Heat shock protein micro-encapsulation as a double tool for the improvement of new generation vaccines. J Liposome Res. 2002;12(1-2):29–35. doi: 10.1081/LPR-120004773. [DOI] [PubMed] [Google Scholar]
- 6.Butler RN. Non-invasive tests in animal models and humans: a new paradigm for assessing efficacy of biologics including prebiotics and probiotics. Curr Pharm Des. 2008;14(14):1341–1350. doi: 10.2174/138161208784480180. [DOI] [PubMed] [Google Scholar]
- 7.Calija B, Cekic N, Savic S, et al. pH-sensitive microparticles for oral drug delivery based on alginate/oligochitosan/Eudragit®L100-55 “sandwich” polyelectrolyte complex. Colloids Surf B Biointerfaces. 2013;110:395–402. doi: 10.1016/j.colsurfb.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 8.de Lacy Costello BP, Ledochowski M, Ratcliffe NM. The importance of methane breath testing: a review. J Breath Res. 2013;7(2):024001. doi: 10.1088/1752-7155/7/2/024001. [DOI] [PubMed] [Google Scholar]
- 9.Evans DF, Pye G, Bramley R, et al. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Festi D, Capodicasa S, Sandri L, et al. Measurement of hepatic functional mass by means of 13C-methacetin and 13C-phenylalanine breath tests in chronic liver disease: comparison with Child-Pugh score and serum bile acid levels. World J Gastroenterol. 2005;11(1):142–148. doi: 10.3748/wjg.v11.i1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(Suppl. 4):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 12.Ishii T, Furube M, Hirano S, et al. Evaluation of 13C-phenylalanine and 13C-tyrosine breath tests for the measurement of hepatocyte functional capacity in patients with liver cirrhosis. Chem Pharm Bull (Tokyo) 2001;49(12):1507–1511. doi: 10.1248/cpb.49.1507. [DOI] [PubMed] [Google Scholar]
- 13.Jelvehgari M, Zakeri-Milani P, Siahi-Shadbad MR, et al. Development of pH-sensitive insulin nanoparticles using Eudragit L100-55 and chitosan with different molecular weights. AAPS PharmSciTech. 2010;11(3):1237–1242. doi: 10.1208/s12249-010-9488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan MZ, Prebeg Z, Kurjakovic N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release. 1999;58(2):215–222. doi: 10.1016/S0168-3659(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 15.Mccarron PA, Donnelly RF, Al-Kassas R. Comparison of a novel spray congealing procedure with emulsion-based methods for the micro-encapsulation of water-soluble drugs in low melting point triglycerides. J Microencapsul. 2008;25(6):365–378. doi: 10.1080/02652040802000656. [DOI] [PubMed] [Google Scholar]
- 16.Pelton NS, Tivey DR, Howarth GS, et al. A novel breath test for the non-invasive assessment of small intestinal mucosal injury following methotrexate administration in the rat. Scand J Gastroenterol. 2004;39(10):1015–1016. doi: 10.1080/00365520410003416. [DOI] [PubMed] [Google Scholar]
- 17.Pham DM, Aziz SM. A real-time localization system for an endoscopic capsule using magnetic sensors. Sensors. 2014;14(11):20910–20929. doi: 10.3390/s141120910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pizzoferrato M, del Zompo F, Mangiola F, et al. Specific 13C functional pathways as diagnostic targets in gastroenterology breath-tests: tricks for a correct interpretation. Eur Rev Med Pharmacol Sci. 2013;17(Suppl. 2):45–50. [PubMed] [Google Scholar]
- 19.Shirley IM, Scher HB, Perrin RM, et al. Delivery of biological performance via micro-encapsulation formulation chemistry. Pest Manag Sci. 2001;57(2):129–132. doi: 10.1002/1526-4998(200102)57:2<129::AID-PS265>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Sue MS, Liu KM, Yu HS. The gastro-intestinal absorption of griseofulvin can be enhanced by encapsulation into liposomes. Kaohsiung J Med Sci. 1993;9(1):1–8. [PubMed] [Google Scholar]
- 21.Symonds EL, Tran CD, Butler RN, et al. Gastric emptying is altered with the presence of gastritis. Dig Dis Sci. 2008;53(3):636–641. doi: 10.1007/s10620-007-9928-8. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi I, Nochi T, Yuki Y, et al. New horizon of mucosal immunity and vaccines. Curr Opin Immunol. 2009;21(3):352–358. doi: 10.1016/j.coi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Terao T, Matsuda K, Shouji H. Improvement in site-specific intestinal absorption of furosemide by Eudragit L100-55. J Pharm Pharmacol. 2001;53(4):433–440. doi: 10.1211/0022357011775721. [DOI] [PubMed] [Google Scholar]
- 24.Terry R, van Wettere WH, Whittaker AL, et al. Using the noninvasive 13C-sucrose breath test to measure intestinal sucrase activity in swine. Comp Med. 2012;62(6):504–507. [PMC free article] [PubMed] [Google Scholar]
- 25.Tooley KL, Saxon BR, Webster J, et al. A novel non-invasive biomarker for assessment of small intestinal mucositis in children with cancer undergoing chemotherapy. Cancer Biol Ther. 2006;5(10):1275–1281. doi: 10.4161/cbt.5.10.3303. [DOI] [PubMed] [Google Scholar]
- 26.van Ginkel FW, Nguyen HH, Mcghee JR. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000;6(2):123–132. doi: 10.3201/eid0602.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]