Highlights
-
•
Pazopanib, in addition to dasatinib and statins, activates the Hippo pathway.
-
•
Pazopanib induces the proteasomal degradation of YAP/TAZ.
-
•
YAP/TAZ inhibitors reduce viability of YAP/TAZ-dependent breast cancer cells.
-
•
YAP/TAZ inhibitors sensitize cancer cells to anti-cancer drugs.
Abbreviations: YAP, yes-associated protein; TAZ, transcriptional coactivator with PDZ-binding motif; TEAD, TEA domain family member; GPCR, G-protein coupled receptor; FDA, food and drug administration; NF2, neurofibromin 2; HMG-CoA, hydroxymethylglutaryl-coenzyme A; CTGF, connective tissue growth factor
Keywords: YAP, TAZ, Statins, Dasatinib, Pazopanib, Combination therapy
Abstract
YAP and TAZ oncoproteins confer malignancy and drug resistance to various cancer types. We screened for small molecules that inhibit the nuclear localization of YAP/TAZ. Dasatinib, statins and pazopanib inhibited the nuclear localization and target gene expression of YAP and TAZ. All three drugs induced phosphorylation of YAP and TAZ, and pazopanib induced proteasomal degradation of YAP/TAZ. The sensitivities to these drugs are correlated with dependence on YAP/TAZ in breast cancer cell lines. Combinations of these compounds with each other or with other anti-cancer drugs efficiently reduced cell proliferation of YAP/TAZ-dependent breast cancer cells. These results suggest that these drugs can be therapeutics and chemosensitizers for YAP/TAZ-dependent breast cancers.
1. Introduction
YAP and TAZ are transcriptional co-activators involved in tissue growth and stem cell maintenance in normal tissue through binding to TEADs. They are regulated through phosphorylation by the Hippo pathway, leading to inhibition of the nuclear translocation and the proteasomal degradation [1]. The Hippo pathway is activated by cellular density [2–4], soluble factors via GPCR [5,6], and actin cytoskeleton organization [7,8]. YAP and TAZ activations are also implicated in the tumorigenesis and malignancy of various cancers including breast [9], colon [10], lung [11], liver [12], and mesothelioma [13]. TAZ is associated with the maintenance of breast cancer stem cells and drug resistance [14]. Therefore, YAP and TAZ play causative roles in carcinogenesis and cancer progression, and inactivation of YAP and TAZ by small molecules is a promising strategy for therapeutics of various cancers with their activation [15].
In drug discovery, one of the successful strategies is the exploitation of established drugs that have already been approved for treatment of other cancers or non-cancerous diseases (i.e., drug repositioning, drug repurposing, or indication switch). The major advantage of this approach is that the pharmacokinetic, pharmacodynamics and toxicity profiles of these drugs are well known, making their rapid shifts to clinical trials possible [16,17].
In this study, we screened for small molecules which inactivate YAP and TAZ from drugs with known targets for the drug repositioning against breast cancer. We found that dasatinib, statins, and pazopanib inhibited their nuclear localization and TEAD-dependent transcription, and induced YAP/TAZ phosphorylation. Pazopanib induced proteasomal degradation of YAP and TAZ. Furthermore, we explored the possibility of chemotherapy with them for breast cancer, and found that the sensitivities to these compounds are correlated with the dependence on YAP and TAZ in these cell lines. Combination of the YAP/TAZ inhibitors or of those with anti-cancer agents efficiently suppressed the breast cancer cell growth. Our findings thus opened the window for the application of dasatinib, statins, and pazopanib, clinically used drugs, for breast cancers with activation of YAP and TAZ.
2. Materials and methods
2.1. Cell culture and treatments
MDA-MB-231, MDA-MB-453, HBC-4, HBC-5, MCF-7, BSY-1, ZR-75-1, and SKBR-3 breast cancer cell lines were maintained in RPMI-1640 containing 10% FBS and penicillin/streptomycin. HEK293 was maintained in Dulbecco’s modified Eagle medium containing 10% FBS and penicillin/streptomycin. Dasatinib was purchased from JS Research Chemicals Trading Co. Fluvastatin, doxorubicin, and paclitaxel were purchased from Wako Pure Chemicals. Geranylgeranyl diphosphate (GGPP), farnesyl diphosphate (FPP), GGTI-286, and FTI-276 were purchased from Sigma. MG-132 was purchased from Merck Mllipore, and pazopanib from ChemieTek.
2.2. Antibodies
For immunofluorescence, 1/200 rabbit anti-YAP antibody (H-125, Santa Cruz), 1/400 rabbit anti-YAP/TAZ antibody (D24E4, Cell Signaling), 1/200 phalloidin-AlexaFluor 594 (Life Technologies), and 1/1000 anti-rabbit IgG-AlexaFluor 488 conjugate (Life Technologies) were used. For immunoblot, 1/3000 rabbit anti-YAP antibody (H-125, Santa Cruz), 1/3000 rabbit anti-TAZ antibody (#2149, Cell Signaling), 1/3000 rabbit anti-YAP/TAZ antibody (D24E4, Cell Signaling), 1/10,000 mouse anti-GAPDH antibody (6C5, Millipore), 1/5000 anti-mouse IgG-HRP (GE Healthcare), and 1/5000 anti-rabbit IgG-HRP (GE Healthcare), were used. Antibodies for Western blot were diluted in Can Get Signal reagents (Toyobo). Western blot using standard SDS–PAGE gel or gels containing Phos-tag-acrylamide (SuperSep Phos-tag, Wako) was performed as previously described [18].
2.3. Screening of the inhibitors inhibiting nuclear localization of YAP
MDA-MB-231 cells (10,000–15,000 cells) were inoculated in a μclear imaging plate (Corning) and 24 h later, 10 μM chemicals in a SCADS inhibitor kit (provided by the Screening Committee of Anticancer Drugs, Japan) was added and incubated for 6 h. Cells were fixed with 4% paraformaldehyde and immunostained with anti-YAP antibody as described below.
2.4. Immunofluorescence and imaging
Paraformaldehyde-fixed cells were permeabilized with 0.3% TritonX-100 in PBS and blocked with 3% FBS in PBST for 30 min. They were then incubated with anti-YAP antibody at 4 °C overnight and washed with PBS three times. Cells were incubated with anti-rabbit IgG-Alexa Fluor 488 for 1 h at room temperature, and washed with PBS three times. For confocal microscopy, cells were mounted in Prolong Gold reagent containing 10 μg/ml Hoechst 33342 (Life Technologies). When appropriate, cells were stained with phalloidin-Alexa Fluor 594 (Life Technologies) prior to mounting. Images were obtained with an FV1000-D confocal microscope equipped with a 40× objective lens using FV10-ASW software (Olympus). For screening of small molecules, PBS containing 10 μg/ml Hoechst 33342 was added and images were obtained by IN Cell Analyzer 2000 (GE Healthcare) using a 40× objective lens.
2.5. Reporter assay
8xGTIIC-luciferase was obtained from Addgene (Addgene #34615). MDA-MB-231 cells were transfected with 1.4 μg of 8xGTIIC-luciferase plasmid and 50 ng of pRL-CMV (Promega) by using lipofectamine 2000 (Life Technologies) or Viafect transfection reagent (Promega) as previously described [7]. After 24 h, cells were treated with chemicals for 18 h. Luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions.
2.6. Quantitative real time PCR
Total RNA was prepared with ISOGEN (Nippon Gene) and cDNA was synthesized with ReverTra Ace qPCR RT master mix with gDNA remover (TOYOBO). Real time PCR was performed with QuantiTect SYBR Green PCR Master Mix (Qiagen) using the Eco Real Time PCR system (Illumina). The sequences of PCR primers for CTGF and GAPDH were as follows; CTGF-F: AGGAGTGGGTGTGTGACGA, CTGF-R: CCAGGCAGTTGGCTCTAATC, GAPDH-F: AGCCACATCGCTCAGACAC, GAPDH-R: GCCCAATACGACCAAATCC.
2.7. RNAi
siRNA transfection was performed using Lipofectamine RNAiMAX (Life Technologies) according to the manufacturer’s instructions. The siRNA sequence is listed as followed; siYAP: GACAUCUUCUGGUCAGAGAUU, siTAZ: ACGUUGACUUAGGAACUUUUU [14].
2.8. MTT assay
MTT assay was performed as previously described [18]. 3000–10,000 cells suspended in RPMI-1640 containing 1% FBS were seeded on 96 well plates. Fifteen μl of medium containing drugs was added, and cells were incubated for 4 days.
2.9. Colony formation assay
MDA-MB-231 or MCF-7 cells (1000–2000 cells per well) were seeded on 24 well plates and treated with inhibitors for 10 days. Cells were fixed with 4% formaldehyde and stained with 0.5% crystal violet.
3. Results
3.1. Dasatinib, fluvastatin, and pazopanib inhibit the function of YAP/TAZ transcriptional co-activator
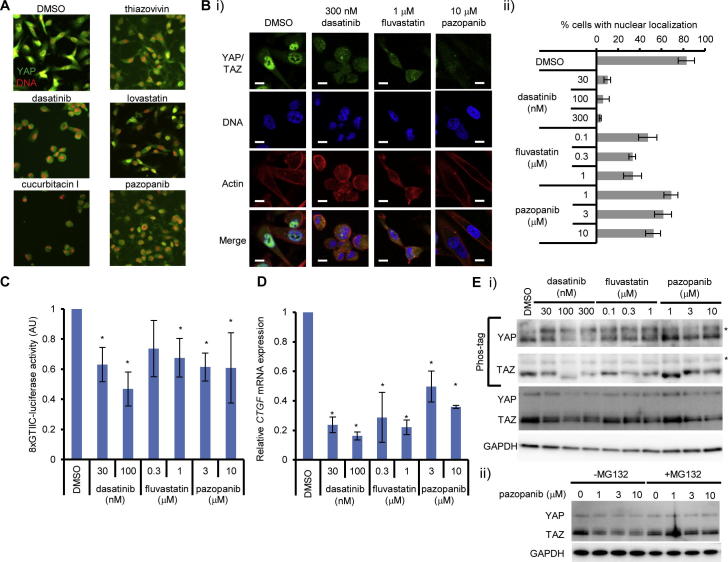
To identify drugs targeting YAP and TAZ, we performed image-based screening for small molecules which inhibit their nuclear localization using MDA-MB-231 breast cancer cell line. MDA-MB-231 harbors homozygous mutation in NF2, which positively regulates the Hippo pathway; therefore, YAP and TAZ are constitutively activated in this cell line [19]. We screened about 400 chemicals with known targets from SCADS inhibitor kit (provided by the Screening Committee of Anticancer Drugs, Japan) which consists of classical anti-cancer agents, kinase inhibitors, metabolic pathway inhibitors, and signaling pathway inhibitors, including FDA-approved drugs [20]. MDA-MB-231 cells were treated with chemicals and nuclear localization of YAP was evaluated by immunofluorescence. Chemicals which induced nuclear exclusion of YAP were treated as positive. We found that thiazovivin, dasatinib, lovastatin, cucurbitacin I, and pazopanib inhibited the nuclear localization of YAP (Fig. 1A). Among them, dasatinib, statins, and pazopanib are approved as clinically used drugs, and we therefore analyzed them further. We found that dasatinib, fluvastatin, and pazopanib inhibited nuclear localization of YAP and TAZ in the nanomolar to micromolar range, although the effect of pazopanib on the nuclear localization of YAP/TAZ was relatively weak compared to the other two drugs (Fig. 1B). They also inhibited the YAP/TAZ-TEAD-dependent reporter activity (Fig. 1C). We found that they also reduced the transcripts of CTGF, whose transcription is dependent on YAP/TAZ (Fig. 1D). Dasatinib and fluvastatin are known to change actin dynamics, and this leads to phosphorylation of YAP/TAZ [19]. Dasatinib inhibits SRC and affects YAP nuclear localization [21]. Statins inhibit HMG-CoA reductase and lead to impaired geranylgeranylation of RHOA, resulting in the inactivation of YAP/TAZ as reported recently [22,23]. We reproduced the geranylgeranylation-dependent inactivation of YAP/TAZ by fluvastatin, as the simultaneous addition of geranylgeranyl diphosphate (GGPP), but not farnesyl diphosphate (FPP), with fluvastatin canceled the effect of fluvastatin (Supplementary Fig. S1). We also found that the direct inhibition of protein geranylgeranylation recapitulated phosphorylation of YAP (Supplementary Fig. S1). These results confirmed the RHOA-dependent inactivation of YAP/TAZ by statins [22,23].
Fig. 1.
Dasatinib, fluvastatin, and pazopanib inhibit the function of YAP/TAZ transcriptional co-activator. (A) Identification of small molecule agents which inhibit the nuclear localization of YAP. MDA-MB-231 cells were treated with a chemical library for 8 h. YAP was immunostained. Red: DNA, green: YAP. Each agent was used at 10 μM. (B) Dasatinib, fluvastatin, and pazopanib inhibited the nuclear localization of YAP and TAZ. MDA-MB-231 cells were treated with inhibitors for 8 h and YAP and TAZ were visualized by immunofluorescence. (i) Representative images of immunofluorescence. Bar represents 10 μm. (ii) Cells with nuclear localization of YAP and TAZ were counted. At least 75 cells were counted for each sample. Data represents mean and standard deviation from three independent experiments. (C) Inhibition of TEAD-dependent promoter activity by dasatinib, fluvastatin, and pazopanib. MDA-MB-231 cells were transfected with 8xGTIIC-luciferase TEAD reporter plasmid with pRL-CMV control plasmid, and treated with inhibitors overnight. Relative luciferase activity (8xGTIIC-luciferase/Renilla luciferase) was measured. Data represents mean and standard deviation from at least three independent experiments. *P < 0.02. (D) Inhibition of endogenous target genes of YAP and TAZ by dasatinib, fluvastatin, and pazopanib. MDA-MB-231 cells were treated with inhibitor for 18 h and total RNA was prepared. CTGF mRNA was quantified by quantitative RT-PCR. Data represents mean and standard deviation from at least three independent experiments. *P < 0.02. (E) Dasatinib, fluvastatin, and pazopanib activate the Hippo pathway. (i) Induction of phosphorylation of YAP and TAZ by dasatinib, fluvastatin and pazopanib. MDA-MB-231 cells were treated with inhibitors for 8 h. Whole cell extract was analyzed by SDS–polyacrylamide gel containing Phos-tag acrylamide. Asterisks show phosphorylated YAP and TAZ. Data is representative of at least three independent experiments. (ii) Degradation of YAP and TAZ by pazopanib is proteasome-dependent. MDA-MB-231 cells were treated with pazopanib for 8 h in the presence or absence of 10 μM MG132. Whole cell extract was analyzed by Western blot. Data is representative of at least three independent experiments.
Pazopanib inhibits VEGFR and PDGFR signaling. VEGF signaling induces activation of RHOA in cervical cancer cells as well as vascular endothelial cells [24,25]. Inhibition of VEGF signaling is known to impair the activation of SRC and FAK, leading to impaired activation of RHOA. PDGF signaling also induced activation of RHOA [26]. MDA-MB-231 is known to express VEGFR2, a VEGFR family member [27] and PDGFR [28]. We hypothesized that the phosphorylation of YAP/TAZ accounted for the inhibition of YAP/TAZ nuclear localization by pazopanib. Therefore, we examined whether pazopanib induced the phosphorylation of YAP and TAZ, and found the increase in the ratio of phosphorylated YAP and TAZ by this drug by pazopanib similar to that by dasatinib and fluvastatin (Fig. 1E i), suggesting that pazopanib also induces YAP/TAZ phosphorylation. We further noted that treatment of cells with pazopanib reduced the total amount of YAP/TAZ in the cell. This reduction was canceled by a proteasome inhibitor, MG-132, (Fig. 1E ii) suggesting that pazopanib facilitates degradation of YAP/TAZ by the ubiquitin–proteasome system as previously described [29].
3.2. YAP/TAZ-dependent breast cancer cell lines are sensitive to dasatinib, fluvastatin, and pazopanib
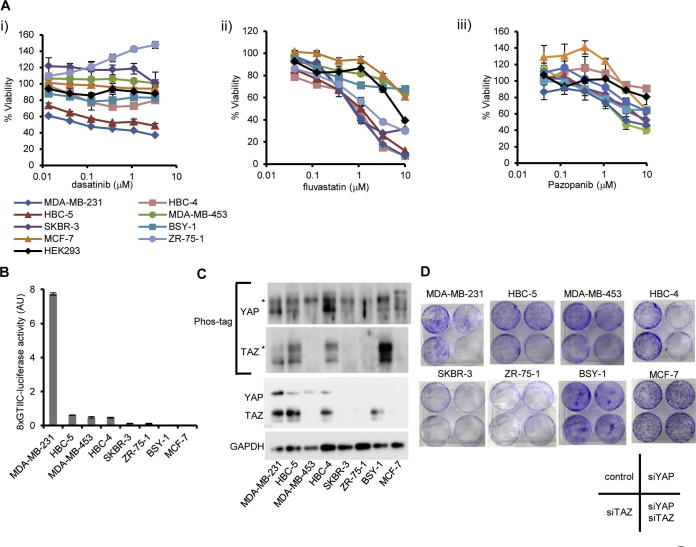
YAP and TAZ activation is frequently observed in various cancers and is associated with resistance to anti-cancer agents, invasiveness, stemness, and poor prognosis [9,14,30–32]. We hypothesized that dasatinib, statins, and pazopanib are effective for YAP/TAZ-dependent breast cancer cells and therefore addressed whether they suppressed the breast cancer cell growth. The response to these drugs was different among cell lines, and we found that MDA-MB-231 was most sensitive to these inhibitors. On the other hand, some cell lines (MCF-7, for example), and HEK293 (human embryonic kidney cell), were resistant to them (Fig. 2A). Examining the level of YAP/TAZ-dependent transcription in these cell lines, we found that MDA-MB-231 showed the highest YAP/TAZ-dependent transcriptional activity among eight breast cancer cell lines (Fig. 2B), which is consistent with the NF2 mutation in this cell line [19]. MDA-MB-231 expressed higher YAP and TAZ, and phosphorylation of YAP and TAZ was lower than that in other cell lines (Fig. 2C). We hypothesized that the activation and dependence of YAP and TAZ is one determinant of the effectiveness of these YAP/TAZ inhibitors and examined the colony formation assay using cells treated with YAP and TAZ siRNA. Colony formation was dramatically reduced when both YAP and TAZ were depleted in MDA-MB-231 (Fig. 2D). Therefore, cell growth of MDA-MB-231 is YAP/TAZ-dependent. On the other hand, some cell lines which are resistant to the agents, including MCF-7, are also resistant to silencing of YAP and TAZ by siRNA (Fig. 2D), suggesting MCF-7 is a YAP/TAZ-independent cell line. In summary, we concluded that there was a correlation between the dependence of YAP/TAZ in breast cancers and the sensitivities to dasatinib, fluvastatin, and pazopanib.
Fig. 2.
YAP/TAZ-dependent breast cancer cell lines are sensitive to dasatinib, fluvastatin, and pazopanib. (A) Sensitivity of breast cancer cell lines to (i) dasatinib, (ii) fluvastatin, and (iii) pazopanib. Cells were treated with drugs for 4 days. Viability was measured by MTT assay. Data represents mean and standard deviation from triplicate. Data is representative of at least three independent experiments. HEK293 (black line) was used as normal cell control. (B) Activation states of YAP/TAZ-dependent transcription in breast cancer cell lines. Cells were transfected with 8xGTIIC-luciferase and pRL-CMV vector. Promoter activity was measured 2 days after transfection. Data represents mean and standard deviation from triplicate. Data is representative of at least two independent experiments. (C) Expression of YAP and TAZ in breast cancer cell lines. Asterisks show phosphorylated YAP and TAZ. (D) Dependence of cell growth on YAP and TAZ. Cells were transfected with siRNA for YAP and TAZ and were diluted in 6 well plates. Colony formation was measured by crystal violet. Data is representative of at least two independent experiments.
3.3. Combination of dasatinib, fluvastatin and pazopanib efficiently reduced the viability of MDA-MB-231
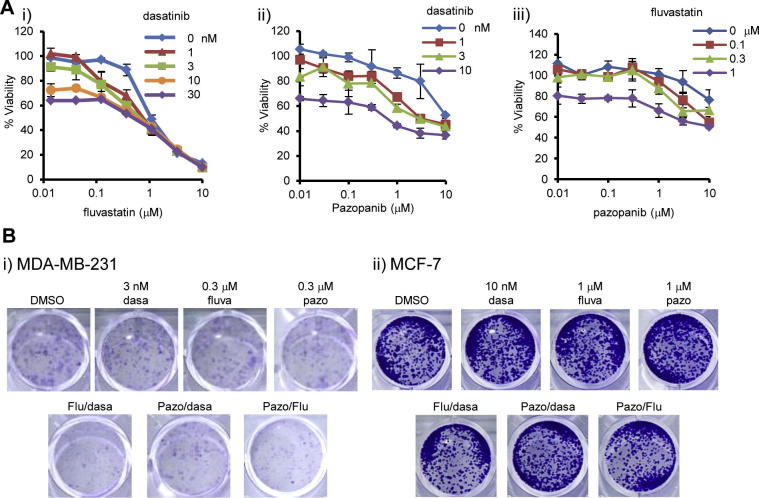
Although dasatinib, fluvastatin, and pazopanib can inactivate YAP and TAZ in breast cancers, their doses were relatively high compared to clinically relevant ones. Higher blood concentration of these drugs can be a risk factor for adverse effects. Reduced concentrations of them to cancer cells are relevant in terms of drug safety. Their use in combination is a feasible approach for this. We first combined the dasatinib, fluvastatin, and pazopanib for MDA-MB-231 cell line, and found that their combinations efficiently reduced viability of the cells (Fig. 3A). Although emergence of the colonies was not largely affected, the colony sizes were efficiently reduced by the combination of these inhibitors (Fig. 3B). Then again, single agents or their combinations did not reduce colony formation of MCF-7, YAP/TAZ-independent cell line (Fig. 3B). These results suggested that combinations of these agents are one of the efficient strategies against YAP/TAZ-dependent cell lines.
Fig. 3.
Combination of dasatinib, fluvastatin and pazopanib efficiently reduced the viability of MDA-MB-231. (A) Combination therapy using dasatinib, fluvastatin, and pazopanib for MDA-MB-231. (i) Fluvastatin in combination with dasatinib. (ii) Pazopanib with dasatinib. (iii) Pazopanib with fluvastatin. Cells were treated with inhibitors for 4 days and viability was measured by MTT assay. Data represents mean and standard deviation of triplicate. Data is a representative of at least three independent experiments. (B) Colony formation reduced by the combination of dasatinib, fluvastatin, and pazopanib in MDA-MB-231. (i) MDA-MB-231 and (ii) MCF-7 cells were treated with the combination of inhibitors (dasa: dasatinib, fluva: fluvastatin, pazo: pazopanib) for 7 or 10 days. Colony formation was determined by staining with crystal violet. Data is representative of three independent experiments.
3.4. Dasatinib, fluvastatin, and pazopanib sensitize YAP/TAZ-dependent cancer cells to doxorubicin and paclitaxel
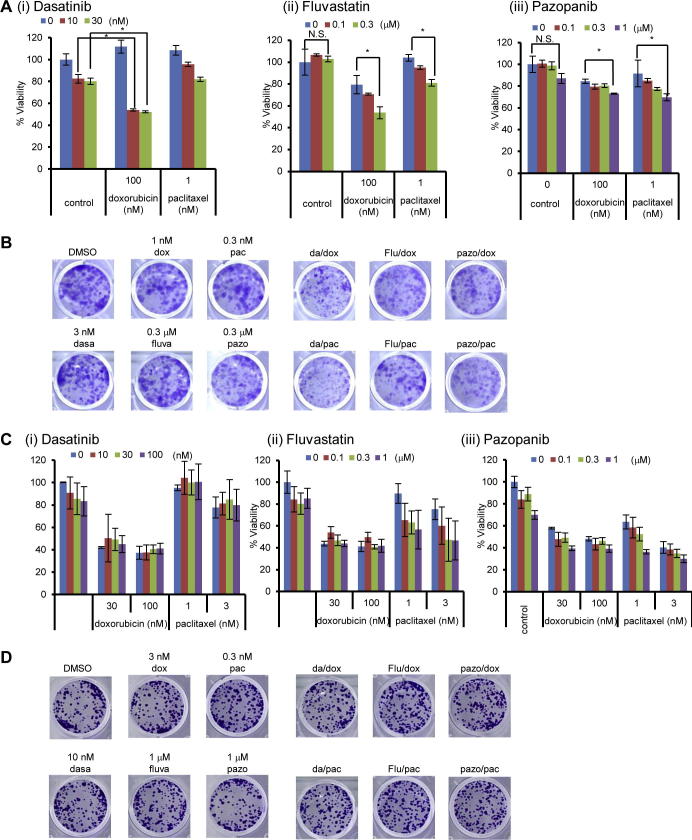
TAZ confers the resistance to paclitaxel and doxorubicin, and its depletion leads to sensitization of cells to these anti-cancer drugs in breast cancer cells [9,32]. Therefore, we hypothesized that dasatinib, fluvastatin, and pazopanib can act as chemosensitizers for YAP/TAZ-dependent breast cancer cells. MDA-MB-231 was then treated with paclitaxel or doxorubicin together with dasatinib, fluvastatin, or pazopanib. We found that some combination of YAP/TAZ inhibitors with these anti-cancer agents synergistically reduced cell viability (Fig. 4A). Colony formation was also significantly reduced by the combination therapies (Fig. 4B).
Fig. 4.
Dasatinib, fluvastatin, and pazopanib sensitize YAP/TAZ-dependent cancer cells to doxorubicin and paclitaxel. (A) Combination therapy with doxorubicin and paclitaxel with (i) dasatinib, (ii) fluvastatin, or (iii) pazopanib. Cells were treated with dasatinib, fluvastatin, or pazopanib in combination with doxorubicin or paclitaxel for 4 days. Viability was measured by MTT assay. Data represents mean and standard deviation from triplicate, and data is representative of at least three independent experiments. *P < 0.02. N.S.: not significant. (B) Colony formation of MDA-MB-231 cells treated with dasatinib, fluvastatin, and pazopanib in combination with doxorubicin or paclitaxel. MDA-MB-231 cells were treated with the combination of inhibitors (dasa: dasatinib, fluva: fluvastatin, pazo: pazopanib, dox: doxorubicin, pac: paclitaxel) for 10 days. Colony formation was determined by staining with crystal violet. Data is representative of two independent experiments. (C) Effect of (i) dasatinib, (ii) fluvastatin, and (iii) pazopanib as chemosensitizers in MCF-7, YAP/TAZ-independent cell line. Viability was measured by MTT assay. Data represents mean and standard deviation from triplicate, and data is representative of at least two independent experiments. (D) Colony formation of MCF-7 cells treated with dasatinib, fluvastatin, and pazopanib in combination with doxorubicin or paclitaxel. MCF-7 cells were treated with the combination of inhibitors (dasa: dasatinib, fluva: fluvastatin, pazo: pazopanib, dox: doxorubicin, pac: paclitaxel) for 7 days. Colony formation was determined by staining with crystal violet. Data is representative of three independent experiments.
Chemosensitizing effects were not consistently observed when MCF-7, whose cell growth is YAP/TAZ-independent, was treated with these combinations (Fig. 4C). Colony formation of this cell line was also not affected by the combination of agents (Fig. 4D). These results suggested that dasatinib, fluvastatin, and pazopanib can be chemosensitizers for YAP/TAZ-dependent breast cancer cells.
4. Discussion
YAP and TAZ are emerging targets for breast cancer treatment. In this study we identified the clinically used drugs, dasatinib, statins, and pazopanib as small molecule agents which inhibit the nuclear localization of YAP/TAZ transcriptional co-activators. These agents are effective for YAP/TAZ-dependent breast cancer; their combination increased the efficacy. Furthermore, they can also act as chemosensitizers of doxorubicin and paclitaxel. We proposed them as candidate chemotherapeutic agents for YAP/TAZ-dependent breast cancers.
Thiazovivin, cucurbitacin I, dasatinib, fluvastatin, and pazopanib inhibit nuclear localization of YAP/TAZ, and activate the Hippo pathway. Thiazovivin is a RHO-kinase inhibitor and modulates actin cytoskeletal dynamics. Inhibition of RHO-kinase is known to inhibit nuclear localization of YAP/TAZ [19], indicating the validity of our screening. Cucurbitacin I is a plant poison and indirectly affects actin dynamics [33]. Changes in the cytoskeletal dynamics can activate YAP/TAZ as previously reported [7]. Among them, we focused on three FDA-approved drugs. Dasatinib, a SRC inhibitor, changes actin dynamics, leading to decrease in nuclear localization of YAP and TAZ [21]. Recent papers have shown that statins inhibit nuclear localization of YAP/TAZ via reduction of geranylgeranylation of RHO family GTPases, followed by phosphorylation [22,23]. In addition to them, we found that pazopanib, a multikinase inhibitor, inhibited the nuclear localization of YAP/TAZ, leading to reduced transcription. Pazopanib also induced phosphorylation of YAP, similar to that by dasatinib or fluvastatin (Fig. 1E). Pazopanib is known as a multikinase inhibitor, and mainly targets VEGFR and PDGFR. Previous studies revealed that VEGFR2 and PDGFR are expressed in MDA-MB-231 [27,34–36], and that their signalings are involved in migration and cell growth [34,37]. In vascular endothelial cells, VEGFR signaling affects actin filament dynamics through the RHO/ROCK pathway in vascular endothelial cells [38]. Thus, inhibition of VEGFR2 in MDA-MB-231 may influence cytoskeletal rearrangements, resulting in Hippo pathway activation. Furthermore, pazopanib induced the proteasome-dependent degradation of YAP and TAZ, which was not prominent in the cells treated with dasatinib and fluvastatin (Fig. 1E). Pazopanib more strongly induced proteasomal degradation than dasatinib or fluvastatin. Although further study is required to know the precise mechanisms of proteasomal degradation of YAP/TAZ by pazopanib, it might exemplify a unique class of Hippo pathway activators.
We found that the efficacy of dasatinib, fluvastatin, and pazopanib is correlated with the dependency on YAP/TAZ in breast cancer cell lines. MDA-MB-231 exhibited higher YAP/TAZ-dependent transcriptional activity and its cell growth was YAP/TAZ-dependent (Fig. 2). This cell line was sensitive to these agents, whereas the cell line with low dependence on YAP/TAZ, MCF-7, for example, was resistant to them (Fig. 2E). We further showed that the combination of the identified compounds was effective for MDA-MB-231 (Fig. 3). YAP/TAZ expression is associated with poor prognosis in many cancers, including breast cancer [9,11,39]. YAP/TAZ is also associated with cancer stem cell traits in breast cancer cells [14]; therefore, these agents might be effective in YAP/TAZ-dependent cancers. We also found that dasatinib, fluvastatin, and pazopanib sensitize MDA-MB-231 to doxorubicin and paclitaxel. YAP/TAZ is associated with drug resistance to classical chemotherapeutics [9,32,40]. Combination therapies efficiently reduced the viability of MDA-MB-231 (Fig. 4). Drug resistance is a bottleneck for anti-cancer therapy, and reversing drug resistance to susceptibility is an attractive strategy. The agents identified in this study are effective against YAP/TAZ-dependent cell line (Fig. 2). In recent molecular targeted approaches in cancer therapeutics, the stratification of patients with biomarkers is important. Expression or activation status of YAP/TAZ might be a predictor for these drugs, and such stratification might be important for anti-YAP/TAZ therapy. Our findings can offer an alternative regimen for the treatment of breast cancer using clinically approved drugs.
Acknowledgements
We thank Professor Stefano Piccolo for 8xGTIIC-luciferase plasmid, and the Screening Committee of Anticancer Drugs supported by a Grant-in-Aid for Scientific Research on Innovative Areas, Scientific Support Programs for Cancer Research, from The Ministry of Education, Culture, Sports, Science and Technology, Japan for providing the SCADS inhibitor kits. We also thank Arowu R Tanaka for discussion, and Sayaka Osaka, Keisuke Abe, Shiori Kato, Miki Numahata, Namiko Abe, Satomi Murai, Chie Ishikawa and Emi Takahashi for technical assistance. This work was supported by MEXT/JSPS KAKENHI Grant Number 26830113, and in part by a Grant-in-Aid for the Strategic Medical Science Research Center from MEXT Japan 2010–2014.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.fob.2015.06.007.
Supplementary data
Impaired protein geranylgeranylation is involved in fluvastatin-induced inactivation of YAP/TAZ. (A) Inhibition of the nuclear localization of YAP and TAZ by fluvastatin was reversed by GGPP, but not by farnesyl diphosphate (FPP). MDA-MB-231 cells were treated with fluvastatin in the presence or absence of GGPP or FPP for 8 h, then were fixed and YAP and TAZ were visualized by immunofluorescence. (i) Representative images of immunofluorescence. Bar represents 10 μm. (ii) Cells with nuclear localization of YAP and TAZ were counted; at least 89 cells were counted for each sample. Data represents mean and standard deviation of three independent experiments. (B) Restoration of endogenous target genes of YAP and TAZ by GGPP, but not by FPP in fluvastatin-treated cells. MDA-MB-231 cells were treated with fluvastatin, and GGPP or FPP for 18 h. CTGF mRNA expression was quantified by quantitative RT-PCR. Data is representative of at least three independent experiments. (C) Phosphorylation of YAP by fluvastatin is canceled by GGPP, but not by FPP. MDA-MB-231 cells were treated with fluvastatin, and GGPP or FPP for 8 h. Whole cell extract was analyzed by SDS–polyacrylamide gel containing Phos-tag acrylamide. Arrow indicates phosphorylated YAP. Data is representative of at least two independent experiments. (D) Direct inhibition of protein geranylgeranylation, but not farnesylation, also inhibited the nuclear localization of YAP and TAZ. MDA-MB-231 cells were treated with 10 μM GGTI-286 or FTI-276 for 8 h and YAP and TAZ were visualized by immunofluorescence. (i) Representative images of immunofluorescence. Bar represents 10 μm. (ii) Cells with nuclear localization of YAP and TAZ were counted; at least 64 cells were counted for each sample. Data represents mean and standard deviation from three independent experiments. (E) Inhibition of endogenous target genes of YAP and TAZ by the inhibition of protein geranylgeranylation. MDA-MB-231 cells were treated with inhibitor for 18 h. CTGF mRNA expression was quantified by quantitative RT-PCR. Data is representative of at least three independent experiments. (F) Induction of phosphorylation of YAP and TAZ by the inhibition of protein geranylgeranylation. MDA-MB-231 cells were treated with inhibitors for 8 h. Whole cell extract was analyzed by SDS–polyacrylamide gel containing Phos-tag acrylamide. Arrow indicates phosphorylated YAP. Data is representative of at least two independent experiments.
References
- 1.Mo J.S., Park H.W., Guan K.L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15(6):642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ota M., Sasaki H. Mammalian tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135(24):4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- 4.Kim N.G., Koh E., Chen X., Gumbiner B.M. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. USA. 2011;108(29):11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu F.X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller E., Yang J., DeRan M., Wu C., Su A.I., Bonamy G.M. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 2012;19(8):955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Wada K., Itoga K., Okano T., Yonemura S., Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138(18):3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 8.Zhao B., Li L., Wang L., Wang C.Y., Yu J., Guan K.L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26(1):54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartucci M., Dattilo R., Moriconi C., Pagliuca A., Mottolese M., Federici G. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2014 doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Shi S., Guo Z., Zhang X., Han S., Yang A. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013;8(6):e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Noguchi S., Saito A., Horie M., Mikami Y., Suzuki H.I., Morishita Y. An integrative analysis of the tumorigenic role of TAZ in human non-small cell lung cancer. Clin. Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3328. [DOI] [PubMed] [Google Scholar]
- 12.Moeini A., Cornella H., Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer. 2012;1(2):83–93. doi: 10.1159/000342405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami H., Mizuno T., Taniguchi T., Fujii M., Ishiguro F., Fukui T. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71(3):873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 14.Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Liu-Chittenden Y., Huang B., Shim J.S., Chen Q., Lee S.J., Anders R.A. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duenas-Gonzalez A., Garcia-Lopez P., Herrera L.A., Medina-Franco J.L., Gonzalez-Fierro A., Candelaria M. The prince and the pauper. A tale of anticancer targeted agents. Mol. Cancer. 2008;7:82. doi: 10.1186/1476-4598-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y.Y., Jones S.J. Drug repositioning for personalized medicine. Genome Med. 2012;4(3):27. doi: 10.1186/gm326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oku Y., Tareyanagi C., Takaya S., Osaka S., Ujiie H., Yoshida K. Multimodal effects of small molecule ROCK and LIMK inhibitors on mitosis, and their implication as anti-leukemia agents. PLoS One. 2014;9(3):e92402. doi: 10.1371/journal.pone.0092402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 20.Kawada M., Inoue H., Masuda T., Ikeda D. Insulin-like growth factor I secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res. 2006;66(8):4419–4425. doi: 10.1158/0008-5472.CAN-05-4239. [DOI] [PubMed] [Google Scholar]
- 21.Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15(6):637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Wu Y., Wang H., Zhang Y., Mei L., Fang X. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl. Acad. Sci. USA. 2013;111(1):E89–E98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorrentino G., Ruggeri N., Specchia V., Cordenonsi M., Mano M., Dupont S. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 2014;16(4):357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 24.Bryan B.A., Dennstedt E., Mitchell D.C., Walshe T.E., Noma K., Loureiro R. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24(9):3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He M., Cheng Y., Li W., Liu Q., Liu J., Huang J. Vascular endothelial growth factor C promotes cervical cancer metastasis via up-regulation and activation of RhoA/ROCK-2/moesin cascade. BMC Cancer. 2010;10:170. doi: 10.1186/1471-2407-10-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pertz O., Hodgson L., Klemke R.L., Hahn K.M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440(7087):1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 27.Timoshenko A.V., Rastogi S., Lala P.K. Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br. J. Cancer. 2007;97(8):1090–1098. doi: 10.1038/sj.bjc.6603993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jechlinger M., Sommer A., Moriggl R., Seither P., Kraut N., Capodiecci P. Autocrine PDGFR signaling promotes mammary cancer metastasis. J. Clin. Investig. 2006;116(6):1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B., Li L., Tumaneng K., Wang C.Y., Guan K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24(1):72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q., Zhang N., Gray R.S., Li H., Ewald A.J., Zahnow C.A. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28(5):432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen D., Sun Y., Wei Y., Zhang P., Rezaeian A.H., Teruya-Feldstein J. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat. Med. 2012;18(10):1511–1517. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai D., Ho K.C., Hao Y., Yang X. Taxol resistance in breast cancer cells is mediated by the Hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 33.Knecht D.A., LaFleur R.A., Kahsai A.W., Argueta C.E., Beshir A.B., Fenteany G. Cucurbitacin I inhibits cell motility by indirectly interfering with actin dynamics. PLoS One. 2010;5(11):e14039. doi: 10.1371/journal.pone.0014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price D.J., Miralem T., Jiang S., Steinberg R., Avraham H. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 2001;12(3):129–135. [PubMed] [Google Scholar]
- 35.Yu Y.C., Yang P.M., Chuah Q.Y., Huang Y.H., Peng C.W., Lee Y.J. Radiation-induced senescence in securin-deficient cancer cells promotes cell invasion involving the IL-6/STAT3 and PDGF-BB/PDGFR pathways. Sci. Rep. 2013;3:1675. doi: 10.1038/srep01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachelder R.E., Wendt M.A., Mercurio A.M. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62(24):7203–7206. [PubMed] [Google Scholar]
- 37.Yoshiji H., Gomez D.E., Shibuya M., Thorgeirsson U.P. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res. 1996;56(9):2013–2016. [PubMed] [Google Scholar]
- 38.Bryan B.A., Dennstedt E., Mitchell D.C., Walshe T.E., Noma K., Loureiro R. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24(9):3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuen H.F., McCrudden C.M., Huang Y.H., Tham J.M., Zhang X., Zeng Q. TAZ expression as a prognostic indicator in colorectal cancer. PLoS One. 2013;8(1):e54211. doi: 10.1371/journal.pone.0054211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touil Y., Igoudjil W., Corvaisier M., Dessein A.F., Vandomme J., Monte D. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin. Cancer Res. 2014;20(4):837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Impaired protein geranylgeranylation is involved in fluvastatin-induced inactivation of YAP/TAZ. (A) Inhibition of the nuclear localization of YAP and TAZ by fluvastatin was reversed by GGPP, but not by farnesyl diphosphate (FPP). MDA-MB-231 cells were treated with fluvastatin in the presence or absence of GGPP or FPP for 8 h, then were fixed and YAP and TAZ were visualized by immunofluorescence. (i) Representative images of immunofluorescence. Bar represents 10 μm. (ii) Cells with nuclear localization of YAP and TAZ were counted; at least 89 cells were counted for each sample. Data represents mean and standard deviation of three independent experiments. (B) Restoration of endogenous target genes of YAP and TAZ by GGPP, but not by FPP in fluvastatin-treated cells. MDA-MB-231 cells were treated with fluvastatin, and GGPP or FPP for 18 h. CTGF mRNA expression was quantified by quantitative RT-PCR. Data is representative of at least three independent experiments. (C) Phosphorylation of YAP by fluvastatin is canceled by GGPP, but not by FPP. MDA-MB-231 cells were treated with fluvastatin, and GGPP or FPP for 8 h. Whole cell extract was analyzed by SDS–polyacrylamide gel containing Phos-tag acrylamide. Arrow indicates phosphorylated YAP. Data is representative of at least two independent experiments. (D) Direct inhibition of protein geranylgeranylation, but not farnesylation, also inhibited the nuclear localization of YAP and TAZ. MDA-MB-231 cells were treated with 10 μM GGTI-286 or FTI-276 for 8 h and YAP and TAZ were visualized by immunofluorescence. (i) Representative images of immunofluorescence. Bar represents 10 μm. (ii) Cells with nuclear localization of YAP and TAZ were counted; at least 64 cells were counted for each sample. Data represents mean and standard deviation from three independent experiments. (E) Inhibition of endogenous target genes of YAP and TAZ by the inhibition of protein geranylgeranylation. MDA-MB-231 cells were treated with inhibitor for 18 h. CTGF mRNA expression was quantified by quantitative RT-PCR. Data is representative of at least three independent experiments. (F) Induction of phosphorylation of YAP and TAZ by the inhibition of protein geranylgeranylation. MDA-MB-231 cells were treated with inhibitors for 8 h. Whole cell extract was analyzed by SDS–polyacrylamide gel containing Phos-tag acrylamide. Arrow indicates phosphorylated YAP. Data is representative of at least two independent experiments.