Abstract
In connection with the global demand for safe human food and the production of environmentally friendly aquaculture products, acidifiers are natural organic acids and salts that have received considerable attention as animal-feed additives. The current study was designed to evaluate the effects of potassium diformate (KDF) on the growth performance and immunity of cultured Oreochromis niloticus (O. niloticus). Four iso-nitrogenous and iso-caloric rations containing graded levels of KDF, including 0% (control basal diet), 0.1%, 0.2% and 0.3%, were fed separately to four equal fish groups (30 fish/group with an initial body weight of 53.49 ± 6.15 g) for sixty days. At the end of the experimental period, the fish groups fed on 0.2% and 0.3% KDF exhibited significant improvements in their feed intake, live weight gain, specific growth rate, feed conversion ratio and protein efficiency ratio, with concomitant improvement of their apparent protein digestibility (p < 0.05). Dietary supplementation of 0.3% KDF appeared to stimulate the beneficial intestinal flora; a proliferation was observed of indigenous probionts (Eubiosis) associated with the relative activation of cellular and humeral innate immunity (phagocytic activity/index, nitroblue tetrazolium reduction test and serum/gut mucous lysozyme activity). The cumulative mortality of the fish groups fed on KDF and challenged orally with Aeromonas hydrophila was lower than that of the control group. The resistance against diseases increased with dietary KDF in a dose-dependent manner. Thus, we conclude that the use of acidifiers can be an efficient tool to achieve sustainable, economical and safe fish production.
Keywords: Acidifiers, Growth performance, Eubiosis, Gut probionts, Innate immunity, Challenge test
Introduction
The long-term administration of antibiotic growth promoters, AGPs, in aquafeeds creates an optimal environment to enable antibiotic resistance genes to multiply [1]. The treated animals become “reservoirs” for the production and distribution of antibiotic-resistant bacteria. A wide variety of natural growth promoters (NGPs), including plant extracts, prebiotics, probiotics and organic acids, have been broadly applied worldwide with reasonable success. Organic acids and their salts have been used as a potential replacement of AGPs to improve the performance and the health of livestock [2]. Formic, acetic, propionic, and citric acid are the most commonly used dietary organic acids in aquaculture. Particularly, the salts of formic acid KDF have been recently used in tropical and cold-water fish. Formic acid KDF was the first substance approved as a possible non-antibiotic growth promoter by the European Union [Commission Reg (EC) number 1334/2001] [3].
Dietary acidifiers have demonstrated effectiveness in enhancing the growth performance and the nutrient availabilities in various aquatic species. They reduce the pH of the digesta of the stomach and the foregut, which in turn stimulates the pepsin activity, improving protein digestibility and mineral absorption [4,5]. Dietary inclusion of citric acid/formic acid enhances the bioavailability of minerals, including phosphorus, magnesium, calcium and iron in rainbow trout (Oncorhynchus mykiss), sea bream (Pagrus major) and Indian carp (Labeo rohita) [5,6]. These short-chain organic acids are generally absorbed through the intestinal epithelia by passive diffusion, providing energy for renewing the intestinal epithelia and maintaining the gut health [6]. Despite the reported improvement in the nutrient availabilities of aquatic animals fed on dietary acidifiers, contradictory results have been reported on the growth promoting effects. Oral administration of potassium diformate (KDF) significantly improves the feed intake, the live weight gain, the feed conversion ratio and the protein efficiency ratio of various tilapia species [7–11]. In contrast, Petkam et al. [12] and Zhou et al. [3] reported no significant improvement in the growth performance of tilapia fed on organic acids/salt blend or KDF, respectively, at various dietary levels.
From another point of view, KDF can improve the general health status of cultured animals by its stronger antimicrobial effect towards coliform bacteria, Escherichia coli and Salmonella sp., than towards lactobacilli [3]. It was reported that the total bacteria per gram of faeces was significantly reduced in the fish fed with an organic acid blend and KDF diets [10]. Similarly, Da Saliva et al. [13] indicated that propionate, butyrate and acetate salts exhibit the highest inhibitory capacity against vibrio species in marine shrimp. These acids can penetrate through the cell wall of gram-negative bacteria and release protons into the cytoplasm. Thus, the bacteria consume a large amount of ATP to excrete protons in trying to maintain a balanced intracellular pH, resulting in the depletion of cellular energy with eventual cell death [14]. Although the scientific publications focused on the antimicrobial effects of organic acids are numerous, very few publications have tackled their effects on the indigenous beneficial flora, lactic acid bacteria (LAB), which has become a major source of concern as one of the most common probiotic bacteria used in aquafeeds [15]. To our knowledge, there have been no previous reports about the ability of acidifiers to influence the humoral and cellular non-specific immunity of cultured tilapia. As a result, the current study was planned to assess the effect of potassium diformate, KDF (Aquaform)® on the growth performance, protein digestibility, gastrointestinal pH, gut beneficial flora, innate immunity and survival of Oreochromis niloticus challenged with pathogenic Aeromonas hydrophila.
Material and methods
Experimental fish
One hundred and twenty apparently healthy O. niloticus were obtained from a private fish farm. Fish acclimated to the laboratory conditions for two weeks before being randomly divided into four groups (30 fish/treatment, three replicates/tank) representing four nutritional groups. One group served as the control, and the other three groups represented the feed additives tested. The experimental fish (mean individual initial weight of 53.49 ± 6.15 g) were fed to satiation, 2% of a total body weight two times/day (at 0800 and 0400) for 60 days and weighed biweekly to adjust the daily requirements [16]. All Institutional and National Guidelines for the care and use of fisheries were followed.
Experimental unit
The present study was conducted in the Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Cairo University. The experimental fish were stocked in 12 glass aquaria (80 cm × 30 cm × 40 cm) supplied with de-chlorinated tap water. The water was aerated continuously by using an air compressor (BOYU S 2000 Air pump, Malaysia). The photoperiod was 12 h light/12 h dark. The water temperature was maintained at (24 ± 1 °C) using a 250-Watt immersion heater with a thermostat. The water temperature and the dissolved oxygen level were recorded daily (by Metteler Toledo, model 128, s/No 1242), and the average range of dissolved oxygen was greater than 5.8 mg/l. Other water quality parameters, including pH and ammonia level, were measured every two days with a pH meter (Orion model 720A, s/No 13062) and ammonia meter (Hanna ammonia meter); the average range of the total ammonia was 0.12–0.23 mg/l, and the pH was in the range of 7.2 ± 0.5 during the experiment.
Experimental diet
Four iso-nitrogenous and iso-caloric diets were formulated from practical ingredients to satisfy the nutrient requirements of O. niloticus according to NRC [16] (Table 1). The control (basal diet) and the other diets were supplemented by 0.1%, 0.2% and 0.3% (KDF) Aquaform®, which contains 35% free formic acid, 35% formate and 30% potassium (ADDCON, NordicAS, Porsgrunn, Norway). The experimental diets were formulated to contain nearly 28% crude protein. The diets were prepared by individually weighing each component and thoroughly mixing the minerals, vitamins and additives with corn. The organic acid powder was mixed thoroughly in the stated quantities into a small amount of feed (1 kg) in a pre-mixer. Water was added until the mixture became suitable for making pellets. The wet mixture was passed through a pellet machine with a 2-mm diameter. The produced pellets were dried at room temperature and kept frozen until the beginning of the experiment. The tested diets were analysed for crude protein (CP %), ether extract (EE %), crude fibre (CF %), ash (%) and moisture %, according to the procedures described by the standard A.O.A.C. methods [17]. The nitrogen free-extract (NFE %) was calculated by the differences.
Table 1.
Ingredients and composition of basal diet.
| Ingredient | % |
|---|---|
| Fish meal (65%) | 10 |
| Soy bean meal (46%) | 35 |
| Yellow Corn | 17.29 |
| Wheat bran | 15 |
| Rice polish | 20 |
| Vitamin c | 0.01 |
| Mono calcium phosphate (23.7) | 0.2 |
| Calcium carbonate | 1.5 |
| Sodium chloride | 0.7 |
| Premixa | 0.3 |
| Chemical analysis of the diet (%) | |
| Moisture | 9.25 |
| Dry matter | 90.75 |
| Ash | 6.4 |
| Ether extract | 5.57 |
| Crude fiber | 4.8 |
| Crude protein | 28 |
| NFEb | 45.98 |
| Gross energyc (kcal/100 g) | 399.35 |
Each kg vitamin and mineral mixture premix contained Vitamin A, 4.8 million IU, D3, 0.8 million IU; E, 4 g; K, 0.8 g; B1, 0.4 g; Riboflavin, 1.6 g; B6, 0.6 g, B12, 4 mg; Pantothenic acid, 4 g; Nicotinic acid, 8 g; Folic acid, 0.4 g Biotin,20 mg, Mn, 22 g; Zn, 22 g; Fe, 12 g; Cu, 4 g; I, 0.4 g, Selenium, 0.4 g and Co, 4.8 mg.
Nitrogen free extract.
Gross energy. Based on 5.65 kcal/g protein, 9.45 kcal/g fat and 4.1 carbohydrate kcal/g [16].
Growth performance and feed utilisation
The body weight of the fish per group was recorded on an individual basis at biweekly intervals. The cumulative feed consumption per group was also recorded on a biweekly basis. The feed conversion ratio per group was calculated at biweekly intervals by taking into consideration the biweekly body weight gain and the feed consumption of the respective group. The protein efficiency ratio and the specific growth rate were also calculated [18].
Faeces collection technique
During the last three days of the experimental period, the triplicate groups of fish were fed the basal and the experimental diets mixed with an indicator (chromic oxide 5 g/kg diet). The fish were fed three meals daily between 0900 and 1600 h, and the feed was offered only as long as the fish were actively feeding, to avoid wastage. One hour after the last meal, the uneaten feed particles and faeces were removed from the system. One-third of the water in the tanks was drained to ensure that the cleaning procedure was complete. The faeces were then allowed to settle overnight. Faecal samples were collected each morning at 0800 h. The faeces were immediately collected on filter paper, dried in an oven at 60 °C and kept in airtight containers at −20 °C. The daily faecal samples from each aquarium were pooled over the three successive days until sufficient sample was available for chemical analyses [19,20].
Apparent protein digestibility (APD)
The apparent protein digestibility (APD) was calculated as follows [21]:
where D = % crude protein of diet, F = % crude protein of faeces, Di = % digestion indicator (AIA) of diet, and Fi = % digestion indicator (AIA) of faeces.
Serum analysis
Five blood samples/replicate were collected using clean syringes from the caudal vessels of fish at the termination of the experiment. The blood samples were centrifuged at 1500g for 15 min at 4 °C. The sera were used for the determination of serum transaminases, alanine aminotransferase ALT and aspartate aminotransferase AST [22], urea [23], creatinine [24] and blood urea nitrogen (BUN) [25].
Measurement of gastro-intestinal pH and total colony count of LAB
Two hours postprandial, five fish/replicate were opened, and their gastrointestinal tracts were removed. The full stomach was opened, and the respective pH was determined directly using a digital pH meter (HANNA HI 2210 benchtop pH meter supplied with HI 1131B glass body pH electrode, HI7662 temperature electrode). The intestinal tract was divided into three equal parts (upper gut, middle and lower gut). A 0.5 g sample of the content of each part (fluid and solids) was mixed with 4.5 ml of distilled water for pH measurement [10].
For the total colony count of LAB, one gram of intestinal content was homogenised with 9 ml of sterile normal saline and mixed for 1 min. Subsequently, a dilution series was prepared in sterile saline from 10−1 to 10−5. One millilitre of each dilution was transferred and mixed with 20 ml of deMan-Rogosa-Sharpe (MRS) (Conda, Spain). The plates were incubated anaerobically at 37 °C for 48–72 h [26]. The averages of triplicate plates were used to express the counts as log CFU (colony forming units) per gram of sample [27]. The isolates were examined for cellular morphology and gram staining and for catalase and oxidase activity.
Immunological measurements
Cellular innate immune response: Phagocytic assay and oxygen radicals (NBT reduction activity)
Five blood samples/replicate were collected on 100 IU/ml sodium heparin for measurement of the cellular innate immune response. Three millilitres of heparinised blood was carefully overlaid onto an equal volume of a histo-paque medium (1.077 g/ml, Sigma–Aldrich, St. Louis, MO, USA) on a polystyrene tube. The sample was centrifuged at 1500g for 20 min at 4 °C for preparation of viable leucocytes from the peripheral blood. The leukocytes at the interface were collected and washed twice with (Roswell park memorial institute medium, RPMI-1640 supplemented with 100 IU/ml penicillin and 1 mg/ml streptomycin). The cell precipitate was re-suspended in (RPMI1640 supplemented with 3% foetal calf serum, 100 IU/ml penicillin and 1 mg/ml streptomycin). The number of viable cells was detected using the trypan blue exclusion method [28] and adjusted to 4 × 107 ml−1 using the culture medium.
The phagocytic activity was adapted from the method described by Esteban et al. [29]. One millilitre of the cell suspension was placed onto a 1 ml volume of a (1 × 106 Candida albicans) suspension and incubated at 37 °C for one hour. Ten microlitres of the mixture was spread onto the clean slide and stained with Giemsa stain. Under the oil immersion lens of an Olympus CX22 bright-field biological microscope, approximately 200 phagocytic cells were counted. The phagocytic activity and index were calculated using the following equation: Percentage of phagocytosis = no. of ingesting phagocytes/total no. of phagocytes.
Phagocytic index = no. of ingested C. albicans cells/no. of ingesting phagocytes.
To measure the NBT, peripheral blood leucocytes (1 × 106 cells per well) were incubated with an equal volume of nitroblue tetrazolium 0.2% for 2 h at 28 °C. The supernatants were removed, and the cells were fixed with 100% (v/v) methanol for 5 min. Each well was washed twice with 125 ml of 70% (v/v) methanol. The fixed cells were allowed to air-dry. The reduced NBT (in the form of the blue precipitate formazan) was dissolved using 120 ml of 2 N potassium hydroxide (KOH) and 140 ml of dimethyl sulphoxide (DMSO, Sigma–Aldrich, St. Louis, MO, USA) per well. The turquoise-blue solution was measured with the enzyme-linked immunosorbent assay, Elisa reader at the wavelength 630 nm.
Lysozyme activity (serum and gut mucus)
Five serum samples/replicate were collected, and then the fish were euthanised, and the entire intestine was removed. The guts were opened and scraped carefully with a rubber spatula. The intestinal mucus samples were collected and centrifuged at 1500g. The supernatants were filtered with 0.22 μm Millipore filters before testing. The serum and the mucus lysozyme activities were measured using the turbidometric method, as previously described by Esteban et al. [29]. A twenty-five microlitres sample of serum and mucus was added to 175 μl (0.75 mg/ml Micrococcus lysodeikticus) in flat-bottomed, 96-well plates. The reduction in the absorbance at 450 nm was measured from 0 to 15 min at 25 °C in the ELISA reader. One unit of lysozyme activity was defined as a reduction in absorbance of 0.001 min−1, and the units of lysozyme activity were calculated using the hen egg white lysozyme standard curve.
Challenge test
Bacterial strain
A virulent strain of A. hydrophila was isolated from a naturally diseased cultured O. niloticus during 2010 in a private fish farm at Kafer El-shekh governorate. It was cultured in brain–heart infusion broth (Lab M, USA) at 25 °C for 24 h. The broth culture was centrifuged at 1500g/10 min. The bacterial precipitate was re-suspended in phosphate buffered saline. The bacterial concentration was adjusted to 1.5 × 106 CFU/ml using the plate counting technique [27].
Coating of feed pellets
The fish basal diet was mixed thoroughly with the saline culture (weight equal volume) to obtain 1.5 × 106 cfu/g food. The food loaded with pathogenic bacteria was coated with gelatine. This preparation was performed on the day of challenge. Based on the data for the daily requirements of the fish, the amount of bacteria in the experimental feed was 2.5 ± 0.2 × 106 cfu/fish/day [30].
Oral infection
At the end of the feeding trial, fifteen fish/groups were fasted for 24 h. They were fed on an infected diet once/day for the three successive days. Signs of disease and their mortality were monitored for 15 days post challenge. Throughout this period, the fish were fed on the basal diet to apparent satiation once/day.
Statistical analysis
The data obtained were statistically assessed by the analysis of variance (ANOVA, through the general linear model procedure of the SPSS14.0 software). The values were expressed as means ± standard error. Duncan’s multiple range tests were used to test the significance of the difference between means by considering the differences significant at p < 0.05.
Results
Growth performance and feed utilisation
The effects of the dietary supplementation of KDF on the growth performance and feed utilisation of O. niloticus are summarised in Table 2. At the end of the feeding trial, the fish groups fed on (0.2% and 0.3% KDF) showed a significant (p < 0.05) increase in the live body weight gain by (14.9% and 15.8%), respectively, and SGR by (11.6% and 12.9%), compared to the control group. In contrast, the group fed on (0.1% KDF) showed a numerical increase in the live body weight gain by (5%) versus the control group. The results of the feed utilisation in terms of FCR and PER of the fish groups supplemented with (0.2% and 0.3% KDF) showed a significant improvement in the FCR of (9.3% and 9.1%), respectively, whereas the fish group supplemented with (0.1% KDF) showed a non-significant improvement in the FCR of (3.7%). The PER was significantly (p < 0.05) increased in fish fed diets supplemented with (0.2% and 0.3% KDF) compared to those a fed diet supplemented with (0.1%KDF) and the control diet.
Table 2.
Growth performance and apparent protein digestibility of O. niloticus at the end of feeding trial.
| Items | Control | KDFA 1 g/kg | KDFA 2 g/kg | KDFA 3 g/kg |
|---|---|---|---|---|
| Initial weight (g) | 53.55 ± 6.42 | 53.50 ± 5.98 | 53.47 ± 6.32 | 53.45 ± 5.88 |
| Final weight (g) | 85.15a ± 8.56 | 86.75a ± 8.74 | 89.87b ± 9.24 | 90.16b ± 9.53 |
| Total feed intake (/fish/2M) | 72.27 | 73.06 | 75.28 | 76.09 |
| Weight gain (g) | 31.60a ± 3.12 | 33.24a ± 2.95 | 36.39b ± 3.88 | 36.70b ± 3.92 |
| SGRB | 0.77a ± 0.07 | 0.80a ± 0.07 | 0.86b ± 0.09 | 0.87b ± 0.09 |
| PERC | 1.56a ± 0.21 | 1.62a ± 0.18 | 1.72b ± 0.23 | 1.71b ± 0.21 |
| FCRD | 2.28a ± 0.31 | 2.20a ± 0.35 | 2.07b ± 0.26 | 2.07b ± 0.32 |
| APDE | 83.73a ± 8.61 | 84.12a ± 8.55 | 89.03b ± 9.12 | 89.38b ± 9.32 |
Data represented as means ± SE (n = 30). Within rows, values with different superscripts a, b, c and d indicating that their corresponding means are significantly different at (p < 0.05) according to one way ANOVA followed by Duncan test.
Body weight (BW): fish were weighted every 15 day to the nearest g.
Weight gain (WG) = average final weight (g) − average initial weight (g) {the average of WG based on the calculation of the average weight gain of the replicate/group}.
KDF Potassium di-formate, aquaform® (ADDCON, NordicAS, Porsgrunn, Norway).
Specific growth rate = (Ln. Final body weight − Ln. Initial body weight) × 100/experimental period (days).
Protein efficiency ratio = weight gain (g)/protein intake (g).
Feed conversion ratio = feed intake (g)/body weight gain (g).
Apparent protein digestibility.
Apparent protein digestibility
The APD was improved for tilapia fed on diets supplemented by (0.1%, 0.2% and 0.3% KDF) compared to the fish group fed on the control diet. A better digestibility was obtained with the group supplemented with (0.2% and 0.3% KDF), as shown in Table 2.
Biochemical serum analysis
The data in Table 3 show that a non-significant difference was found among all experimental groups, including the control group, for both the ALT and AST activity. The data for urea, creatinine and BUN showed a slight, non-significant reduction.
Table 3.
Serum biochemical parameters.
| Items | Control | 0.1% KDFa | 0.2% KDFa | 0.3% KDFa |
|---|---|---|---|---|
| AST (U/L) | 83.62 ± 9.1 | 84.63 ± 8.72 | 82.92 ± 8.3 | 81.83 ± 8.24 |
| ALT (U/L) | 20.50 ± 2.22 | 20.83 ± 2.35 | 21.12 ± 2.24 | 21.23 ± 2.48 |
| Urea (mg/dl) | 3.31 ± 0.36 | 3.17 ± 0.31 | 3.22 ± 0.32 | 3.19 ± 0.35 |
| Creatinine (mg/dl) | 0.69 ± 0.07 | 0.66 ± 0.06 | 0.67 ± 0.07 | 0.66 ± 0.07 |
| BUN (mg/dl) | 2.66 ± 0.27 | 2.63 ± 0.29 | 2.55 ± 0.23 | 2.49 ± 0.25 |
Data represented as means ± SE (n = 5/replicate). “All means are not significantly different according to one way ANOVA and p < 0.05.
KDF Potassium di-formate, aquaform® (ADDCON, NordicAS, Porsgrunn, Norway), AST aspartate amino transferase, ALT Alanine amino transferase, BUN blood urea nitrogen.
Gastro-intestinal pH and total lactic acid bacterial count
The stomach pH of the treated fish groups was lowered by the addition of KDF into the fish diet (Table 4). A dietary inclusion of (0.2% and 0.3% KDF) resulted in a significant (p < 0.05) reduction in the stomach pH compared with those of fish fed on the control diet. The pH levels decreased from 3.4 in the control group to 2.96 in the group fed on 0.3% KDF. However, no significant difference was found between the stomach pH of the control group and the group fed on 0.1% KDF. The upper gut pH showed a pH reduction by increasing the dose of the salt of the organic acid. A significant reduction of 0.45 in the pH level in the group treated with 0.3% KDF and of 0.23 in the group treated with 0.2% KDF compared with control group was observed. The results recorded a numerical reduction in the pH of other gut portions in all treated groups, but these were not significantly lower than those of the control group. There was a significant increase in the LAB count isolated from the gut of the treated groups fed on 0.3% KDF compared with those for the other treated groups. The LAB count varied from (23 ± 0.2 × 102 cfu/g) in the control group to (24 ± 0.3 × 103 cfu/g) in the groups fed on 0.3% KDF (Table 4). The isolated bacteria were gram positive cocci and bacilli, which were non-motile and oxidase- and catalase-negative.
Table 4.
Gastro-intestinal pH and total LAB count at the end of feeding trial.
| Items | Control | 0.1% KDFa | 0.2% KDFa | 0.3% KDFa |
|---|---|---|---|---|
| Stomach pH | 3.43a ± 0.35 | 3.29a ± 0.27 | 3.05b ± 0.33 | 2.96b ± 0.29 |
| Intestinal tract | ||||
| Upper | 6.88a ± 0.73 | 6.81a ± 0.65 | 6.65b ± 0.66 | 6.43c ± 0.74 |
| Middle | 6.66a ± 0.62 | 6.66a ± 0.65 | 6.63a ± 0.62 | 6.61a ± 0.62 |
| Lower | 7.34a ± 0.77 | 7.33a ± 0.82 | 7.23a ± 0.72 | 7.12a ± 0.75 |
| Total LAB count (g) | 23 × 102a ± 0.25 | 34 × 102a ± 0.45 | 35 × 102a ± 0.43 | 24 × 103b ± 0.31 |
Data represented as means ± SE (n = 5/replicate). Within rows, values with different superscripts indicating that their corresponding Means are significantly different at (p < 0.05) according to one way ANOVA followed by Duncan test.
KDF Potassium di-formate, aquaform® (ADDCON, NordicAS, Porsgrunn, Norway).
Immunological findings
Cellular and humeral innate parameters
All of the fish groups fed on KDF showed a significant increase (p < 0.05) in the innate immunological parameters versus those of the control group. The statistical analysis strongly favoured the 0.2–0.3% KDF-treated groups. The findings of the cellular innate immunity exhibited a significant increase (p < 0.05) in phagocytic activity (82.13%) and index (1.8) in the fish group fed on 0.3% KDF compared with the control groups, which had (52.16%) phagocytic activity and (1.49) phagocytic index (Fig. 1). The NBT reduction activity showed a similar pattern. The fish group fed on 0.3% KDF recorded the highest optical density, 1.75 versus 0.819 in the control group. The highest lysozyme activity both in the fish serum and the intestinal mucus was recorded in the 0.3% KDF group, compared with the results for the control group (Table 5).
Fig. 1.
Phagocytic cells of 0.3% KDF fish group engulfed more the one Candida albicans (Giemsa stain 1000×).
Table 5.
Immunological findings of fish groups at the end of experimental period.
| Items | Control | 0.1% KDFa | 0.2%KDFa | 0.3%KDFa |
|---|---|---|---|---|
| Phagocytic assay | ||||
| Activity (%) | 52.16a ± 6.10 | 66.2b ± 7.15 | 79.00b ± 7.50 | 82.13c ± 8.20 |
| Index | 1.49a ± 0.14 | 1.74b ± 0.13 | 1.76b ± 0.13 | 1.80b ± 0.25 |
| NBT (O.D. at 630 nm) | 0.819a ± 0.11 | 1.06ab ± 0.08 | 1.22b ± 0.15 | 1.75c ± 0.03 |
| Lysozyme activity | ||||
| Serum (μg/ml) | 233.1a ± 24.2 | 251.5ab ± 26.5 | 277.7bc ± 28.03 | 306c ± 34.43 |
| Intestinal mucus | 104.4a ± 14.7 | 119.1ab ± 14.7 | 144.9bc ± 11.03 | 177.98c ± 18.7 |
Data represented as means ± SE (n = 5/replicate). Within rows, values with different superscripts indicating that their corresponding Means are significantly different at (p < 0.05) according to one way ANOVA followed by Duncan test.
NBT nitro blue tetrazolium.
KDF Potassium di-formate, aquaform® (ADDCON, NordicAS, Porsgrunn, Norway).
Challenge test
The mean cumulative mortality of the experimental fish groups 15 days post challenge with A. hydrophila is illustrated in Fig. 2. Tilapia fed on the control diet showed the highest mortality rate (40%) compared with the potassium-diformate-supplemented groups, which showed a reduction in the mortality rate from 13% in the groups treated with 0.1% and 0.2% to 7% in the group fed on 0.3% KDF.
Fig. 2.
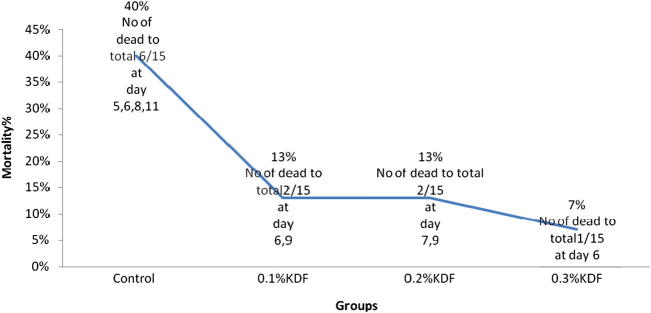
Mortality percentage post challenge with A. hydrophila orally.
Discussion
There is currently a great interest in the commercial use of organic acids/salts in aquafeeds, both to enhance the growth performance and to control disease [1]. Dietary supplementation of 0.2% and 0.3% potassium diformate significantly improve the growth performance and protein digestibility of O. niloticus. Similarly, Ramli et al. [8] indicated significant improvements in the growth and feed-utilisation efficiency of hybrid tilapia (Oreochromis sp.) fed a casein-based diet containing potassium diformate (KDF). Lim et al. [9] also observed that graded levels of dietary KDF up to 10 g/kg tended to improve the weight gain and feed efficiency in O. niloticus. Furthermore, red hybrid tilapia fed diets supplemented with 2 g/kg KDF showed a tendency towards increased body weight gain, feed utilisation and nutrient digestibility [10]. Cuvin-Aralar et al. [11] reported better growth and FCR in juvenile Nile tilapia given diets supplemented with 0.3% KDF compared to the control diets. However, our results are not in accordance with that obtained by Zhou et al. [3] and Petkam et al. [12]. Various factors, such as species and the physiological age of the experimental fish, the type and level of organic acids, the diet composition and the culture conditions may all influence the manifestation of the potential growth-promoting effects of dietary organic acids in aquaculture [10].
To date, the mode of action of organic acid compounds has been speculated in fish. The reduction of the stomach and the upper gut pH in KDF-supplemented fishes may be the primary reason for improving the growth performance and protein digestibility. The lower gastric pH associated with a higher pepsin activity contributes to improve the protein digestibility and nitrogen retention [7]. This obviously appeared in the results of the apparent protein digestibility, which increased by 6.75% in the 0.2% and 0.3% KDF-treated groups more than the other two groups (p < 0.05). Ng et al. [10] reported that dietary KDF at 2 g/kg decreased the diet pH and reduced the digesta pH of the stomach and gut of red hybrid tilapia. This KDF-supplemented diet markedly decreased the total bacterial counts in faeces. Because the low molecular weight lipophilic organic acids can diffuse across the cell membrane of gram-negative bacteria, acidification of their metabolism can lead to bacterial cell death. This may be the second reason for improving the growth performance.
Lowering of the gut pH with dietary KDF has an eubiotic effect on the allochthonous, beneficial lactic acid bacteria. This was significantly detected in the LAB count of the fish group fed 0.3% KDF. The LAB count was elevated from 23 × 102 CFU/g in the control group to 24 × 103 CFU/g. Lactic acid bacteria are able to grow at a relatively low pH, which means that they are more resistant to organic acids/salts than gram-negative bacteria [13]. These indigenous probiotic bacteria have the ability to colonise the intestinal surface and form a barrier, serving as the first defence to limit direct attachment or interaction of fish pathogenic bacteria to the gut mucosa [15]. It was reported that dietary KDF stimulates the colonisation of certain gut bacteria and inhibits the growth of others in hybrid tilapia [3]. It improved the relative richness of certain intestinal allochthonous bacteria, such as Mycobacterium sp. Partial MHSD12-like, Mycobacterium peregrinum-like, Pseudomonas sp. HMPB4-like and six uncultured bacterium like species. However, alpha Proteobacterium IMCC1702-like, Rhodococcus sp. P14-like, and three uncultured bacterium-like species were depressed in the gut. Similarly, Owen et al. [31] reported the tendency for a relative increase in the proportion of gram-positive bacteria of Clarias gariepinus treated with sodium butyrate. The eubiotic effect of KDF on the proliferation of indigenous probionts may be the third reason for improving the growth performance because this gram-positive bacterium plays a vital role in fermentation of certain non-digestible carbohydrates and increases the availability of nutrients [15].
The result of ALT and AST means that fish could tolerate the addition of 0.1%, 0.2% and 0.3% KDF without any deleterious effects on the liver and kidney functions. These results are in full agreement with those of El-Kerdawy [32]. In contrast, Abdel-Azeem et al. [33] showed that the level of AST was reduced, although ALT was not significantly affected. The findings for urea, creatinine and BUN coincide with those of Sturkie, [34] who revealed that the dietary addition of an organic acid slightly reduced the serum concentration of uric acid. This result could result from the better utilisation of proteins and amino acid digestibility because urea is the major end product of protein metabolism.
Not much is known about the use of acidifiers as immunostimulants in cultured fish. KDF was able to modify microbial communities in tilapia guts, which in turn may account for its ability to initiate an immune response. It has been reported that the quantity and quality of immune cells in gut mucosa depend on the continuous stimulation provided by indigenous intestinal flora [35]. Inclusion of KDF in the fish diet has a significant impact on the cellular and humoral non-specific immunity of O. niloticus. This was obviously recorded in the results of the phagocytic activity, the nitro-blue tetrazolium reduction test and the lysozyme activity of the serum and the intestinal mucus. Balcázar et al. [35] observed a correlation between the colonisation ability of indigenous LAB and the non-specific humoral response, such as an alternative, complementary pathway activity and lysozyme activity in brown trout. This could explain the indirect activation of the non-specific immunity of treated fish groups.
The cumulative mortality after 15 day post challenge with A. hydrophila in the diet was reduced in the fish fed on the 0.3% KDF-supplemented diet, followed by the other two supplements (Fig. 2). Inversely, no significant effects were detected in the mortality of the Nile tilapia fed a diet supplemented with a different level of KDF after 14 days post challenge with Streptococcus iniae [3], despite the fact that KDF was reported to be effective against Vibrio anguillarum [8]. An explanation for this may be that, as gram-positive bacteria, S. iniae have high intracellular potassium concentrations, which provide a counteracting effect for the acid anions of the dissociated organic acids [36]. Conversely, it can acidify the cytoplasm of gram-negative bacteria, such as A. hydrophila and V. anguillarum, resulting in eventual cell death. The antimicrobial effects of organic acids have been augmented with increased LAB densities and their antimicrobial products in the fish gut. The colonisation of LAB inhibits the attachment and invasion of the pathogenic bacteria, following the competitive exclusion theory of these probiotic bacteria against pathogens.
Conclusions
The results indicate the promising potential of acidifiers in fish diets and provide evidence to encourage aquafeed manufacturers to consider using such additives. The dietary inclusion of KDF not only enhances the growth performance and the apparent protein digestibility of O. niloticus, but it also has an eubiotic effect on the proliferation of indigenous LAB, which plays a prominent role in activation of the immune response against diseases.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
The authors are thankful to Dr. Mohamed Marzouk, Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Cairo University, Egypt, for his valuable recommendations throughout the work and his careful revision of the manuscript. Additionally, we are thankful to Dr. Azza Kamal, Department of Biochemistry, Animal Health Research Institute, Dokki, for helping in the serum analysis.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Lückstädt C. Use of organic acids as feed additives – sustainable aquaculture production the non-antibiotic way. Int Aquafeed. 2006;9:21–26. [Google Scholar]
- 2.Lim C., Lückstädt C., Klesius P.H. Review: use of organic acids, salts in fish diets. Global Aquacult Advocat. 2010;5:45–46. [Google Scholar]
- 3.Zhou Z., Liu Y., He S., Shi P., Gao X., Yao B., Ringo E. Effects of dietary potassium diformate (KDF) on growth diformate (KDF) on growth performance, feed conversion and intestinal bacterial community of hybrid tilapia (Oreochromis niloticus x O. aureus) Aquaculture. 2009;291:89–94. [Google Scholar]
- 4.Lückstädt C. Effect of organic acid containing additives in worldwide aquaculture – sustainable production the non-antibiotic way. In: Lückstädt C., editor. Acidifiers in animal nutrition – a guide for feed preservation and acidification to promote animal performance. Nottingham University Press; Nottingham: 2007. pp. 71–79. [Google Scholar]
- 5.Jun-sheng L., Jian-lin L., Ting-ting W. Ontogeny of protease, amylase and lipase in the alimentary tract of hybrid Juvenile tilapia (Oreochromis niloticus X Oreochromis aureus) Fish Physiol Biotechnol. 2006;32:295–303. [Google Scholar]
- 6.Vielma J., Lall S. Dietary formic acid enhances apparent digestiblity of minerals in rainbow trout, Oncorhynchus mykiss (Walbaum) Aquac Nutr. 1997;3:265–268. [Google Scholar]
- 7.Liebert F, Mohamed K, Lückstädt C. Effects of diformates on growth and feed utilization of all male Nile Tilapia fingerlings (Oreochromis niloticus) reared in tank culture. In: XIV International symposium on fish nutrition and feeding, Qingdao, China, Book of Abstracts; 2010. p. 190.
- 8.Ramli N, Heindl U, Sunanto S. Effect of potassium-diformate on growth performance of tilapia challenged with Vibrio anguillarum. Bali, Indonésia: World Aquaculture Society; 2005. p. 9–13.
- 9.Lim C, Klesius P, Luckstadat C. Effects of dietary levels of potassium diformate on growth, feed utilization and resistance to Streptococcus iniae of Nile tilapia, Oreochromis niloticus. In: Proceeding of the fourteenth international symposium on fish nutrition and feeding, Qingdao, China; 2010. p. 170.
- 10.Ng W.K., Koh C.B., Sudesh K., Siti-Zahrah A. Effects of dietary organic acids on growth, nutrient digestibility and gut microflora of red hybrid tilapia, Oreochromis sp., and subsequent survival during a challenge test with Streptococcus agalactiae. Aquac Res. 2009;40:1490–1500. [Google Scholar]
- 11.Cuvin-Aralar M, Kühlmann KJ, Schroeder K, Lückstädt C. Effect of potassium diformate (KDF) on growth performance of male Nile tilapia (Oreochromis niloticus). In: XIV international symposium on fish nutrition and feeding, Qingdao, China, Book of Abstracts; 2010. p. 187.
- 12.Petkam R, Luckstadt C, Nittayachit P, Sadao S, Encarnacao P. Evaluation of a dietary organic acid blend on tilapia Oreochromis niloticus growth performance. Busan, Korea: World Aquaculture; 2008 [Abstract].
- 13.Da Saliva B.C., Vieira F.N., Mourino J.P., Ferrira G.S., Seiffert W.Q. Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture. 2013;384–387:107–110. [Google Scholar]
- 14.Defoirdt T., Boon N., Sorgeloos P., Verstraete W., Bossier P. Short-chain fatty acids and poly-β-hydroxyalkanoates: (new) biocontrol agents for a sustainable animal production. Biotechnol Adv. 2009;27:680–685. doi: 10.1016/j.biotechadv.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Denev S., Staykov Y., Moutafchieva R., Beev G. Rev. Microbial ecology of gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture. Int Aquat Res. 2009;1:1–29. [Google Scholar]
- 16.NRC (National Research Council). Nutrient requirements of fish National academy of science. Washington, DC; 1993. 141pp.
- 17.AOAC. In: Cunnil PA, editor. Official methods of analysis of the association official analytical chemists, vol. 1, 16th ed. Arlington, USA: AOAC International; 1995.
- 18.Abu-Elala N., Marzouk M., Moustafa M. Use of different Saccharomyces cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. Int J Vetrinary Sci Med. 2013;1:21–29. [Google Scholar]
- 19.Bureau D.P., Harris A.M., Cho C.Y. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss) Aquaculture. 1999;180:354–358. [Google Scholar]
- 20.Zhou Qi-Cun, Tan Bei-Ping, Mai Kang-Sen, Liu Yong-Jain. Apparent digestibility of selected feed ingredients for juvenile Cobia Rachycentron canadum. Aquaculture. 2004;241:441–451. [Google Scholar]
- 21.Furukawa A., Tsukahara H. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull Jpn Soc Sci Fish. 1966;32(6):502–506. [Google Scholar]
- 22.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;1:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 23.Patton C.J., Crouch S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal Chem. 1977;49:464–469. [Google Scholar]
- 24.Fabiny D.L., Eriinghausen G. Automated reaction-rate method for determination of serum creatinine with the centrifichem. Clinc Chem. 1971;8:696–700. [PubMed] [Google Scholar]
- 25.Fawcett J.K., Scott J.E. Colorimetric determination of blood urea nitrogen. J Clin Path. 1960;13:156. [Google Scholar]
- 26.Ghiasi F. Predominant lactic acid bacteria isolated from the intestines of silver carp in low water temperature. Afr J Biotechnol. 2011;10:12717–12721. [Google Scholar]
- 27.Buller NB. Bacteria from fish and other aquatic animals: a practical identification manual. CABI pub.; 2004. ISBN: 0-85199-738-4.
- 28.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol 2001; 21: A.3B.1–2. [DOI] [PubMed]
- 29.Esteban M.A., Cuesta A., Ortuño J., Meseguer J. Immunomodulatory effects of dietary intake of chitin on gilthead sea bream (Sparus aurata L.) innate immune system. Fish Shellfish Immunol. 2001;11:303–315. doi: 10.1006/fsim.2000.0315. [DOI] [PubMed] [Google Scholar]
- 30.Kwon S.R., Lee E.H., Nam Y.K., Kim S.K., Kim K.H. Efficacy of oral immunization with Edwardsiella tarda ghosts against edwardsiellosis in Olive flounder (Paralichthys olivaceus) Aquaculture. 2007;269:84–88. [Google Scholar]
- 31.Owen MAG, Waines P, Bradley G, Davies S. The effect of dietary supplementation of sodium butyrate on the growth and micro£ora of Clarias gariepinus (Burchell 1822). In: XII International symposium on fish nutrition and feeding, Biarritz, France; 2006. p. 149 [Abstract].
- 32.El-Kerdawy D.M.A. Acidified feed for growing rabbits. Egypt J Rabbit Sci. 1996;6:143–156. [Google Scholar]
- 33.Abdel-Azeem F., El-Hommosany Y.M., Nematallah G.M. Effect of citric acid in diets with different starch and fiber levels on productive performance and some physiological traits of growing rabbits. Egypt J Rabbit Sci. 2000;10:121–145. [Google Scholar]
- 34.Sturkie P.D. 4th ed. Springer-Verlag Inc.; New Work, NY: 1986. Avian physiology. [Google Scholar]
- 35.Balcázar J., De Blas I., Ruiz-Zarzuela I., Vendrell D., Gironés O., Muzquiz J. Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss) FEMS Immunol Medical Microbiol. 2007;51:185–193. doi: 10.1111/j.1574-695X.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 36.Russell J.B., Diez-Gonzales F. The effects of fermentation acids on bacterial growth. Adv Microb Physiol. 1998;39:205–234. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]




