Abstract
Alexandria Governorate contracted an international company in the field of municipal solid waste management for the collection, transport and disposal of municipal solid waste. Construction and operation of the sanitary landfill sites were also included in the contract for the safe final disposal of solid waste. To evaluate the environmental impacts associated with solid waste landfilling, leachate and groundwater quality near the landfills were analyzed. The results of physico-chemical analyses of leachate confirmed that its characteristics were highly variable with severe contamination of organics, salts and heavy metals. The BOD5/COD ratio (0.69) indicated that the leachate was biodegradable and un-stabilized. It was also found that groundwater in the vicinity of the landfills did not have severe contamination, although certain parameters exceeded the WHO and EPA limits. These parameters included conductivity, total dissolved solids, chlorides, sulfates, Mn and Fe. The results suggested the need for adjusting factors enhancing anaerobic biodegradation that lead to leachate stabilization in addition to continuous monitoring of the groundwater and leachate treatment processes.
Keywords: Environmental impacts, Groundwater pollution, Heavy metals, Leachate, Solid waste disposal
Introduction
The social and environmental impacts imposed by municipal solid waste (MSW) received attention in recent decades [1]. Consequently, several policies, strategies, plans and methods have been developed in the field of MSW management. These include waste reduction and waste recovery for reuse, recycling, composting and incineration for energy generation in addition to landfilling of final rejects [2]. Landfills and/or open dumpsites were the common practice for MSW disposal all over the world [3]. Currently, sanitary landfill represents a viable and the most commonly used method for solid waste disposal all over the world because it may achieve the reclamation of derelict land [4]. Also, properly designed and operated sanitary landfills eliminated some adverse environmental impacts that result from other solid waste final disposal alternatives such as burning in open-air burning sites and open-pit dumping. However, other impacts may arise from gas and leachate formation if not well controlled. These impacts include fires and explosions, vegetation damage, unpleasant odors, landfill settlement, groundwater pollution, air pollution and global warming [1]. In developing countries, landfills have been largely unsuccessful because the landfill sites have a very limited time frame of usage [2]. It is also receiving MSW, commercial and industrial wastes which may contain hazardous substances and can increase the health risks emanating from the leachate and gases [4].
In 1999, 23% of the collected solid waste from Alexandria, Egypt, was recovered for compost production. The remaining 77% was open dumped in an uncontrolled manner on both the banks of Maryout Lake and three open dump sites, causing detrimental effects [5]. Nowadays, sanitary landfilling became the main disposal method where 78% of the generated solid waste is transferred to sanitary landfill and the remaining 22% is recovered for compost production [6].
Over 20–30 years MSW in closed landfill cells is converting into gases, liquid and inert solids. Landfill leachate is one of the main sources of groundwater and surface water pollution if it is not properly collected and treated and safely disposed as it may percolate through soil reaching water aquifers [7]. Therefore, the current study focuses on the characteristics of leachate generated from landfill sites in Alexandria, Egypt and its impacts on the groundwater quality.
Background information
Waste and leachate quantities
In 2010, Alexandria region had a population of 4.42 million and a total area of 2679 km2 [8]. It produces 2700 tons of solid waste every day which may increase to 3400 tons/day during summer. Municipal waste, mainly derived from households sector, also includes some institutional, commercial and industrial sources which represent around 1600 tons/day [6].
All the generated solid wastes (2700 tons) are collected daily and transported to 3 transfer stations: Oum Zgheiou, Moharam Bey, and Montazah. They serve three districts west, middle and east of Alexandria. Biodegradable organic waste that represents around 600 tons of the daily MSW generation is transferred to 3 compost plants (Montazah, Abis 1 and Abis 2); 150,000 tons/year of compost is produced and sold to farmers as a fertilizer or soil conditioner contributing to the development of agricultural activities. The remaining wastes are transported to Borg El-Arab Landfill site during winter and El-Hammam landfill site during summer [6,9]. The quantity of leachate produced in Borg El-Arab and El-Hammam landfills is about 6000 m3/month for each one [10].
Landfill sites description
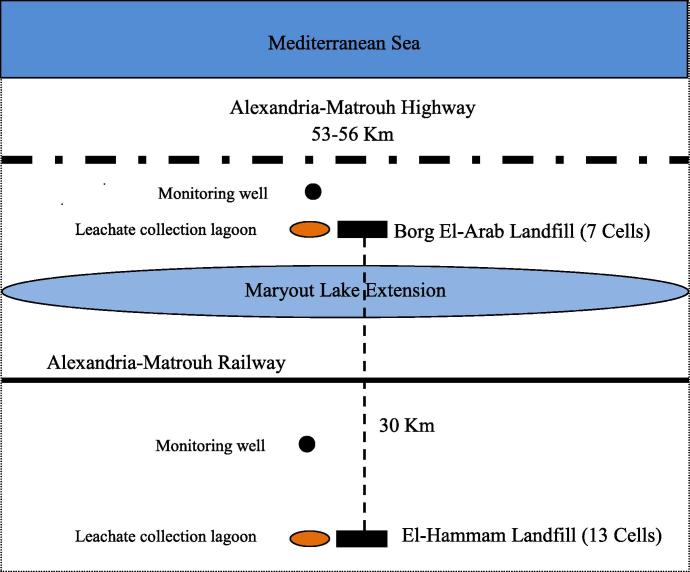
Borg El-Arab landfill site locates parallel to the Mediterranean sea shoreline and also parallel to the Northern Coast Road “Alexandria-Matrouh Road”. It distances around 850 m south the Mediterranean sea coast shoreline and 250 m south the Northern Coast Road “Alexandria-Matrouh Road”. The eastern border of the site is at the sign of km 53 and the western border is at the sign 56 km on the Northern Coast Road “Alexandria-Matrouh Road”. El-Hammam landfill site locates around 30 km south of Borg El-Arab Landfill site (Fig. 1).
Fig. 1.
Lay-out of the study area in Borg El-Arab and El-Hammam Landfills sites.
Borg El-Arab site occupies an area of 0.75 km2 (3 km length, 250 m width, and 9–25 m depth) [11]. The total area of El-Hammam landfill site is 1.19 km2 (1700 m length, 700 m width, and 11.5 m depth) [10]. Borg El-Arab site includes 7 landfill cells while El-Hammam landfill site includes 13 landfill cells [5,10]. Each cell is large enough for one to two years of MSW generated by Alexandria governorate. The cell capacity is around 1.5 million tons and the waste generation is around 1 million ton/year [5].
Landfilling is performed by trench method. Daily, the delivered solid waste is weighed at the landfill site, dumped into the cell, compacted and covered with soil layer to minimize fire risk, reduce landfill odors, and reduce windblown garbage. Covering the waste with soil consumes a significant volume of cell capacity. Also, these soil layers decrease the velocity of leachate movement within the cell and hence may cause localized leachate trapping within the cell. Therefore, soil covering layer is removed, leaving a small depth of sand on top of the existing waste. The new waste is then placed above this layer of soil. The waste covering and de-covering activities take place every day till the cell is totally filled [5,10].
The landfill cells is lined with 2 polyethylene layers and compacted clay layer to prevent or to minimize the leachate percolation to the groundwater through decreasing the permeability coefficient to 1 × 10−7 cm/s. The collected leachate is pumped out of the collection trench and directed to the leachate treatment lagoons. The leachate is treated in the lagoons by evaporation using mechanical aerators and heat. The purpose of the mechanical aerator is to enhance the evaporation process and decomposition of the organic content of the leachate [5,10].
Gases resulted from solid wastes biodegradation are burned and the produced heat is used for drying the lagoons leachate [5,10]. The methane produced due to waste anaerobic decomposition from landfill is collected and combusted through flares reducing the greenhouse gas emissions into the atmosphere. Both landfill sites are equipped with an extensive landfill gas capture system, a biogas pumping station, and 3 enclosed high efficiency flares [12].
Material and methods
Sampling and analysis
Leachate samples were collected and analyzed to assess their characteristics and stability. Groundwater samples were collected from two monitoring wells, one at each site, which are drilled around the landfills sites in order to monitor the closer aquifer extent of contamination. Sampling was conducted every two months over one year giving a total of six leachate samples and 12 groundwater samples. From each site, three leachate samples and six groundwater samples were collected. In each site, leachate samples were collected during season specific for landfill operation. However, groundwater samples were collected bimonthly from each site.
All the samples were collected, preserved, and analyzed according to the Standard Methods for the Examination of Water and Wastewater [13]. In landfills, leachate pollutant measurements included organic contaminants [measured as Biochemical Oxygen Demand (BOD) or Chemical Oxygen Demand (COD)], ammonia, nitrates, total nitrogen, suspended solids, heavy metals and soluble inorganic salts [7]. Eight heavy metals [nickel (Ni), lead (Pb), copper (Cu), manganese (Mn), chromium (Cr), cadmium (Cd), zinc (Zn), and iron (Fe)] were chosen because of their availability in landfill leachates [3]. Heavy metals were determined using Atomic Absorption Spectrophotometer Schimadzu model AA-6650 flame system [13].
Statistical analysis
The data collected were tabulated and analyzed using Statistical Package for Social Sciences (SPSS) version 11.0 software package [14]. They were presented in the form of range, arithmetic mean, standard deviation and 95% confidence intervals. In order to determine the factors which had higher detection rate and larger impact, the correlation between the heavy metals content in leachate samples was analyzed. Statistical differences between the means of leachate and groundwater samples were compared using t-test at p-value ⩽ 0.05 [14].
Results and discussion
Leachate characterization and biodegradability
Physical and chemical characteristics of leachate
The results of physical and chemical analyses of the leachate samples are presented in Table 1. It is evident from this table that pH ranged from 7.0 to 7.8 which is suitable for methanogenic bacteria. Similar results were obtained by Tränkler et al. [15] who found that leachate samples had a slightly high pH and remained in the range of 7.0–8.0 during the operations which indicates the short acidic phase and early methanogenic phase. On the other hand, Bahaa-eldin et al. [16] found that the average value of pH was 6.7 for the municipal landfill leachate in Malaysia indicating the young leachate and the waste degradation was at its late stage of acidic phase.
Table 1.
Physical and chemical analyses of leachate samples collected from sanitary landfills in Alexandria, Egypt.
| Parameters | Unit | Leachate samples |
||
|---|---|---|---|---|
| n = 6 | Min | Max | ||
| PH | – | 7.0 | 7.8 | – |
| Conductivity | μS/cm | 35,260 | 42,857 | 40921 ± 861 |
| Total dissolved solids | mg/l | 24,954 | 30,482 | 27452 ± 605 |
| Chlorides | mg/l | 9500 | 16,250 | 11387 ± 119 |
| Total suspended solids | mg/l | 3278 | 14,464 | 12985 ± 137 |
| Chemical oxygen demand | mg/l | 12,850 | 16,350 | 15629 ± 206 |
| Biochemical oxygen demand | mg/l | 9620 | 11,700 | 10824 ± 95 |
| Total nitrogen | mg/l | 382 | 954 | 583 ± 76 |
| Ammonia-N | mg/l | 190 | 410 | 321 ± 68 |
| Nitrate-N | mg/l | 0.36 | 2.9 | 1.4 ± 0.2 |
| Sulfates | mg/l | 298 | 720 | 596 ± 87 |
| Phosphates | mg/l | 0.29 | 0.52 | 0.37 ± 0.04 |
Hassan and Ramadan [5] evaluated landfill leachate characteristics and found the mean values of conductivity and total dissolved solids were 41,637 μS/cm and 30,083 mg/l, respectively. This finding confirmed the results of the present study where the range of conductivity extended from 35,260 to 42,857 μS/cm with a mean value of 40,921 μS/cm and the mean value of dissolved inorganic solids was 27,452 mg/l. Lower results were obtained by Bahaa-eldin et al. [16] who found that the conductivity of the leachate from the landfill in Malaysia was 31.68 μS/cm. Although, Olivero-Verbel et al. [17] and Chofqi et al. [18] showed that leachates collected from landfill in Colombia and Morocco had high conductivity of 22,000 μS/cm and 26,000 μS/cm respectively, these values were lower than those found in the present study.
In the present study, chlorides widely ranged from 9500 to 16,250 mg/l with a mean value of 11,387 mg/l. Lower chloride values (2050; 5680 and 7000 mg/l) than those of the present study were observed by Bahaa-eldin et al. [16], Chofqi et al. [18] and Monje-Ramirez and Orta de Velásquez [19], respectively.
In the current study, BOD ranged between 9620 and 11,700 mg/l with a mean value of 10,824 mg/l and COD values ranged between 12,850 and 16,350 mg/l with an average of 15,629 mg/l. Ratio of BOD5/COD (0.69) indicated that the leachate had high biodegradability through anaerobic phase. Chofqi et al. [18] studied the leachate originating from the El Jadida municipal landfill in Morocco and found that the leachate had the mean values of COD and BOD5 of 1000 mg/l and 60 mg/l, respectively. The ratio BOD5 to COD was 0.06. This indicates that the leachate was stabilized and the landfill was in the methanic phase of anaerobic degradation. Lower results were recorded in another study in Colombia landfill where the maximum leachate COD value was 4480 mg/l [17]. The results of the current study were in contradiction with Monje-Ramirez and Orta de Velásquez [19] who found that leachates obtained from the Bordo Poniente, Mexico sanitary landfill were well-stabilized (BOD5/COD < 0.01); on the average, they had a COD of 5000 mg/l, and a BOD5 of 20 mg/l. Although, higher mean values of BOD and COD (28,833 and 45,240 mg/l; respectively) than those of the present study were reported by Hassan and Ramadan [5], the ratio BOD5 to COD of their study was 0.63 which is similar to the current study results. Chen [20] studied the effects of landfill age and rainfall on landfill leachate in Taiwan, the results showed that BOD and COD concentrations (296 and 3340 mg/l, respectively) were below the values of the present study and indicated that the leachate had reached the mature stage.
Young leachates are more polluted than the mature ones where BOD5 may reach up to 81,000 mg/l for young and 4200 mg/l for mature samples [7]. BOD5/COD ratio in young landfill, where biological activity corresponds to the acid phase of anaerobic degradation, reaches values of 0.85 [18]. Old landfills produce stabilized leachate with relatively low COD and low biodegradability (BOD5:COD ratio < 0.1) [7].
In the present study, the variation in different parameters values may be attributed to the fluctuations in waste type and characteristics, the absence of waste shredding before disposal, compaction of the waste which retards degradation, and landfilling meteorological conditions such as temperature and pressure.
Observed ammonia concentrations ranged from 190 to 410 mg/l with a mean value of 321 mg/l. At this concentration the methanogenic is only slightly inhibited by ammonia, but at higher values of pH and temperature, such that the equilibrium shift NH4 to NH3, the latter that is more toxic can cause inhibition of the methanogenic archaea. Higher mean values of ammonia concentrations (600 mg/l) than those reported in the present study were obtained by Hassan and Ramadan [5].
In the present study, it is expected that the mean values of total Kjeldahl nitrogen (583 mg/l) and phosphates (0.37 mg/l) decrease during the stabilization process as found by Hassan and Ramadan [5] (mean values of 973 mg/l for total nitrogen and 0.33 mg/l for total phosphate). This may be attributed to the compaction of the wastes in the landfill. In mature leachate ammonia-NH3/total Kjeldahl nitrogen ratio is usually greater than 70%. In the leachate under study, ammonia-NH3 represents 55% of total nitrogen and ammonification was not yet complete then nitrates or nitrites have not been produced.
In Morocco, Chofqi et al. [18] collected leachate samples from El Jadida landfill and the mean results showed that the leachate had high concentrations of nitrates and sulfates (290 mg/l and 1150 mg/l, respectively). High nitrate values indicate that the environment was oxidized, thus the sulfate reduction not occurred, so sulfate concentrations were higher than those of the present study where sulfates and nitrates had mean concentrations of 596 mg/l and 1.4 mg/l, respectively. In our study, sulfate may be resulted from the decomposition of proteins. In addition, the leachate organic matter has not been fully biodegraded yet and sulfur has not been released; therefore the sulfate concentrations were lower than those found by Chofqi et al. [18]. On the other hand, the results of the current study agreed with Hassan and Ramadan [5] who found that nitrates and sulfates values of landfill leachate had low mean concentrations with a mean value of 1.0 mg/l and 535 mg/l, respectively. Hassan and Ramadan [5] revealed that although landfills are considered anaerobic environments, oxygen input can occur from heterogeneous mixture of wastes and rainwater. Oxidizing conditions in the landfill may cause volatilization and nitrification reactions. Volatilization leaves enriched free ammonia-NH3 while nitrification converts ammonia to nitrate, consequently lead to increase in nitrate concentrations. However, the more prevalent reducing conditions in the landfill may cause reduction of nitrate to ammonia or to N2, which results in a decrease in nitrate values and an increase in ammonia concentrations. This finding is not consistent with our results.
Heavy metals concentrations in landfill leachate
Table 2 shows heavy metals concentrations of leachate samples collected from sanitary landfills in Alexandria, Egypt. It is clear from this table that leachate content of heavy metals can show significant variation where Cr had low concentration ranging from 0.029 to 0.094 mg/l while Zn and Mn had high mean values of 0.749 mg/l and 0.839 mg/l, respectively. However, Pb shows a lower mean value of 0.019 mg/l. High concentrations of Zn can be attributed to disposal of large quantities of industrial wastes within landfills. Although Rapti-Caputo and Vaccaro [21] recorded that the chemical composition of the landfill leachate in Italy with an important content of heavy metals can exhibit considerable temporal variation, their results are in contradiction with the findings of the current study where Cr showed continuous increase with concentrations varying between 0.13 and 0.36 mg/l. Differently, Zn had more or less stable concentrations equal to 0.10–0.50 mg/l. In contrast, the Pb content of the leachate presented a continuous decrease from 1.0 to 0.05 mg/l. Similar results were obtained by Hassan and Ramadan [5] who found the mean values of Zn and Mn were 0.724 and 0.730 mg/l, respectively. Higher results were obtained by Olivero-Verbel et al. [17] who studied composition and toxicity of leachates from a MSW landfill in Colombia and found that the Ni concentrations ranged between 0.173 and 0.359 mg/l. However, Cu and Mn concentrations were <0.025–0.053 mg/l and <0.030–0.165 mg/l, are lower than those recorded by the present study. In addition, Pb had mean a value of <0.10 mg/l and Cd concentrations ranged from 0.039 to 0.295 mg/l. On the other hand, Fe concentrations (0.426–11.49 mg/l) with a mean value of 6.314 mg/l reported in the current study were lower (23 mg/l) than those recorded by Chofqi et al. [18].
Table 2.
Heavy metals concentrations of leachate samples collected from sanitary landfills in Alexandria, Egypt.
| Heavy metals | Unit | Leachate samples |
||
|---|---|---|---|---|
| n = 6 | Min | Max | ||
| Nickel | mg/l | 0.037 | 0.167 | 0.096 ± 0.015 |
| Lead | mg/l | 0.008 | 0.025 | 0.019 ± 0.004 |
| Copper | mg/l | 0.016 | 0.172 | 0.074 ± 0.026 |
| Manganese | mg/l | 0.260 | 1.39 | 0.839 ± 0.165 |
| Chromium | mg/l | 0.029 | 0.094 | 0.062 ± 0.044 |
| Cadmium | mg/l | 0.002 | 0.261 | 0.094 ± 0.026 |
| Zinc | mg/l | 0.342 | 0.974 | 0.749 ± 0.235 |
| Iron | mg/l | 0.426 | 11.49 | 6.314 ± 1.827 |
The Pearson correlation matrix for all heavy metal content of leachate samples collected from sanitary landfills in Alexandria, Egypt is displayed in Table 3. The results indicate a significant correlation among each of Zn, Mn and Fe at the level of p ⩽ 0.05.
Table 3.
Pearson correlation coefficients among heavy metals concentrations of leachate samples collected from sanitary landfills in Alexandria, Egypt.
| Heavy metals | Nickel | Lead | Copper | Manganese | Chromium | Cadmium | Zinc | Iron |
|---|---|---|---|---|---|---|---|---|
| n = 6 | r (p-Value) | r (p-Value) | ||||||
| Nickel | ||||||||
| Lead | −.132 | |||||||
| Copper | .941 | .185 | ||||||
| (.001⁎) | ||||||||
| Manganese | −.021 | −.260 | −.041 | |||||
| Chromium | .670 | .541 | .730 | −.219 | ||||
| Cadmium | .962 | .134 | .601 | −.403 | .083 | |||
| (.021⁎) | ||||||||
| Zinc | −.217 | −.209 | −.086 | .985 | −.351 | −.253 | ||
| (.000⁎) | ||||||||
| Iron | −.316 | −.382 | −.205 | .362 | −.594 | −.690 | .397 |
Correlation is significant at p ⩽ 0.05.
Groundwater contamination
Physical and chemical characteristics of monitored well water
The results of physical, chemical and heavy metals analyses of well water samples collected from sanitary landfill in Alexandria, Egypt are given in Tables 4 and 5. The results in these tables show that the water quality at the wells near the landfill is significantly different at p ⩽ 0.05 from the recommended groundwater quality indicating that the landfill leachate most likely influenced.
Table 4.
Physical and chemical analyses of monitoring well samples at Alexandria’s solid waste sanitary landfills, Egypt.
| Parameters | Unit | Well 1 |
Well 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 12 | Min | Max | p-Value | Min | Max | p-Value | |||
| PH | – | 7.4 | 8.8 | – | – | 7.12 | 8.1 | – | – |
| Conductivity | μS/cm | 4200 | 21,500 | 12745 ± 120 | .000⁎ | 3720 | 16,800 | 10354 ± 76 | .000⁎ |
| Total dissolved solids | mg/l | 3263 | 16,276 | 9895 ± 93 | .000⁎ | 2855 | 14,781 | 8721 ± 58 | .000⁎ |
| Chlorides | mg/l | 2240 | 11,750 | 6890 ± 45 | .000⁎ | 1030 | 8200 | 4685 ± 30 | .000⁎ |
| Total suspended solids | mg/l | 682 | 1591 | 1197 ± 29 | .002⁎ | 180 | 1348 | 867 ± 16 | .000⁎ |
| Chemical oxygen demand | mg/l | 78 | 190 | 80 ± 5 | .000⁎ | 45 | 120 | 68 ± 2 | .000⁎ |
| Biochemical oxygen demand | mg/l | 36 | 95 | 60 ± 1 | .000⁎ | 16 | 78 | 45 ± 1 | .000⁎ |
| Total nitrogen | mg/l | 1.0 | 1.7 | 1.3 ± 0.1 | .007⁎ | 0.9 | 1.4 | 1.10 ± 0.09 | .001⁎ |
| Ammonia-N | mg/l | 1.2 | 5.1 | 1.7 ± 0.9 | .000⁎ | 0.14 | 0.55 | 0.28 ± 0.06 | .006⁎ |
| Nitrate-N | mg/l | 0.22 | 0.36 | 0.25 ± 0.07 | .043 | 0.05 | 0.24 | 0.13 ± 0.02 | .075 |
| Sulfates | mg/l | 647 | 1100 | 784 ± 42 | .004⁎ | 240 | 900 | 543 ± 31 | .439 |
| Phosphates | mg/l | 0.08 | 0.15 | 0.10 ± 0.06 | .423 | 0.02 | 0.09 | 0.05 ± 0.01 | .124 |
| Oil and grease | mg/l | ND† | ND† | ND† | – | ND† | ND† | ND† | – |
95% CI = 1.96.
Significant at p ⩽ 0.05.
ND: Not Detected; the detection level was 0.06 mg/l.
Table 5.
Heavy metals concentrations of monitoring well samples at Alexandria’s solid waste sanitary landfills, Egypt.
| Heavy metals | Unit | Well 1 |
Well 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 12 | Min | Max | p-Value | Min | Max | p-Value | |||
| Nickel | mg/l | 0.01 | 0.152 | 0.057 ± 0.020 | .424 | 0.007 | 0.147 | 0.029 ± 0.013 | .052 |
| Lead | mg/l | 0.004 | 0.009 | 0.005 ± 0.001 | .746 | 0.002 | 0.005 | 0.0010 ± 0.0001 | .083 |
| Copper | mg/l | 0.004 | 0.067 | 0.026 ± 0.014 | .829 | 0.001 | 0.026 | 0.013 ± 0.007 | .024 |
| Manganese | mg/l | 0.182 | 0.673 | 0.357 ± 0.210 | .000⁎ | 0.039 | 0.439 | 0.257 ± 0.190 | .000⁎ |
| Chromium | mg/l | 0.006 | 0.158 | 0.039 ± 0.015 | .515 | ND† | 0.058 | 0.028 ± 0.008 | .069 |
| Cadmium | mg/l | 0.001 | 0.051 | 0.011 ± 0.006 | .107 | ND† | 0.027 | 0.005 ± 0.001 | .000⁎ |
| Zinc | mg/l | 0.001 | 0.343 | 0.148 ± 0.032 | .281 | 0.001 | 0.153 | 0.043 ± 0.021 | .000⁎ |
| Iron | mg/l | 0.044 | 5.90 | 1.23 ± 0.74 | .352 | 0.014 | 3.53 | 0.456 ± 0.300 | .000⁎ |
95% CI = 1.96.
Significant at p ⩽ 0.05.
ND: Not Detected; the detection level was 0.01 ppm.
In the present study, conductivity of the two investigated monitored wells recorded high values with means of 10,354 and 12,745 μS/cm and a maximum value of 21,500 μS/cm monitored in one of them. Total dissolved solid values ranged from 2855 to 16,276 mg/l. Improperly lined landfills may lead to increased total dissolved solids concentrations in groundwater. High mean values of chloride content (4685 and 6890 mg/l) and sulfates concentrations (543 and 784 mg/l) are also observed for the two monitoring wells. Such contents of chloride and sulfates are much higher than the acceptable upper limits for drinking purposes as suggested by WHO [22] (250 mg/l for chloride and 500 mg/l for sulfates). This may be attributed to contamination of the studied wells from landfills leachates, industrial effluents or sea water intrusion. In agreement, Bahaa-eldin et al. [16] investigated the effect of municipal landfill leachate on groundwater quality in Malaysia. Their results showed that the elevated concentration of chloride (355.48 mg/l), nitrate (10.40 mg/l), nitrite (14.59 mg/l), ammonia (11.61 mg/l), iron (0.97 mg/l), and lead (0.32 mg/l) indicates that the groundwater quality was extremely affected by the migrated leachate from the landfill site. However, groundwater contains little or no organic matter where the mean BOD and COD concentrations of the two monitoring wells ranged between 45–60 mg/l and 68–80 mg/l, respectively. This indicates that there is no organic contamination from the leachate to the groundwater surrounding the site. This has also been found by Hassan and Ramadan [5] who assessed the impacts of the same sanitary landfill leachate on the groundwater and found that no organic contamination of piezometer wells around the active cells of landfill.
In the present study, all heavy metals mean concentrations of the two monitoring wells showed low values as shown in Table 5 and were below the allowable limits for drinking described by EPA [23] except Mn (0.257–0.357 mg/l) and Fe (0.456–1.23 mg/l) which far exceeded the limits (0.05 mg/l for Mn and 0.3 mg/l for Fe). Water Stewardship Information Series [24] stated that Mn and Fe may be present in samples as a naturally occurring constituent of groundwater from weathering of Fe and Mn bearing minerals and rocks. Industrial effluent, acid-mine drainage and sewage may also contribute Fe and Mn to local groundwater.
Reyes-López et al. [25] assessed the groundwater contamination by landfill/open dump site in México. The results showed that the monitoring wells had higher average conductivity (15,400 μS/cm) and COD (172.5 mg/l) than those of the present study. However, domestic wells were characterized by lower average conductivity (4200 μS/cm) and COD (31.4 mg/l). High conductivity and COD values may be due to the presence of landfill leachate in wells located near the site and organic strength produced by it. Low BOD values compared with measured COD confirms that groundwater samples contain large amounts of non-biodegradable organic matter. This finding is not consistent with our results.
Rapti-Caputo and Vaccaro [21] studied the chemical composition of an unconfined aquifer system in Italy and the influence of the landfill leachate on it. They found that the pH values of groundwater samples were between 7.16 and 7.9. Chlorides values ranged from 10.15 to 467.5 mg/l. Nitrates and sulfates concentrations were extended from 1.9 to 166 mg/l and from 23 to 1128 mg/l, respectively.
Chofqi et al. [18] evaluated groundwater wells pollution located near El Jadida landfill in Morocco and found that conductivity had lower values (4500–8000 μS/cm) than those of the present study. Mean chlorides and sulfates values were 1620 and 1000 mg/l, respectively. The concentration can exceed 2500 mg/l for chloride and 1000 mg/l for sulfates in the landfill owing to the infiltration of highly salt loaded leachate and it constituted a salinity plume near the landfill. For wells located far from the landfill, high salinity records are related to seawater intrusion [5]. Also, high metallic concentrations (15–25 μg/l in Cd and 60–100 μg/l in Cr) are detected in these wells [18].
New Jersey Department of Health and Senior Services (NJDHSS) studied 20 wells pollution located adjacent to the Dover Township Municipal Landfill (DTML) in 1997 and found that 90% of these wells contained lead (1.5–27.4 μg/l) higher than those of the current study. Lead may be present as a naturally occurring constituent of groundwater or as the result of corrosion of well materials and plumbing. In 1999, 10 monitoring wells on site at DTML were investigated and are consistent with our results where 30% of these wells showed Cd in excess of the drinking water Maximum Contaminant Level (MCL) (5 μg/l) [23,26] and low levels (less than the Action Level of 15 μg/l) [23] of Pb (up to 2.0 μg/l). In 2000, 11 on- and off-site monitoring wells were evaluated by NJDHSS and showed that 18% of these wells had Cd in excess of the drinking water MCL (5 μg/l) [23] and 72.7% of them had low levels of Pb (up to 8.0 μg/l) below the Action Level (15 μg/l) [23].
In Sri Lanka, impact of landfill site on well water was assessed and it is contrary to the present study where the well water is unacceptably acidic and COD level ranged from 20 to 100 mg/l. This COD values may be explained by the leachate maturing process. Also, higher Cd levels (25–38 μg/l) exceeding the permissible limit of 5 μg/l given by EPA [23] than those of the current study were detected. The high Cd content resulted from co-disposal of industrial waste with MSW. The BOD level (1.0–4.0 mg/l) was low indicating that the well water at that time has not been contaminated with fresh leachate [27].
Conclusion and recommendations
The main environmental concern in this study is the effect of landfills leachate on the groundwater quality. Based on the findings from this study, the Alexandria landfills, operational since 2001, is in the initial stabilization process and the leachate had high biodegradability through anaerobic phase (BOD5/COD = 0.69). Although leachate was characterized by high contents of organic and inorganic chemicals as well as the toxic nature arising from heavy metals concentrations, the groundwater through monitoring wells around the active cells did not has severe contamination, whereas certain parameters exceeded the WHO and EPA standards. These parameters included conductivity, total dissolved solids, chlorides, sulfates, Mn and Fe. This may be the result of proper lining of landfill cells and leachate ponds. Combating impacts such as organic load from leachate may require MSW undergo one week of bulk composting prior to landfilling. Also, shredding of MSW is recommended to increase the rate of biological degradation. The results support the need for continuous monitoring of the groundwater.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
The authors gratefully acknowledge Mohamed H. Ramadan, Professor of Environmental Chemistry and Biology, Environmental Health Department, High Institute of Public Health, Alexandria University, Egypt for his cordial revise, precious guidance and generous assistance; Samia G. Saad, Professor of Environmental Chemistry and Biology, Environmental Health Department, High Institute of Public Health, Alexandria University, Egypt for her constructive suggestion, valuable guidance and constant encouragement; Veolia Environmental Services in Alexandria Governorate for their assistance and support during the course of this research.
Footnotes
Peer review under responsibility of Cairo University.

References
- 1.Calvo F., Moreno B., Zamorano M., Szanto M. Environmental diagnosis methodology for municipal waste landfills. Waste Manage. 2005;25:768–779. doi: 10.1016/j.wasman.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Narayana T. Municipal solid waste management in India: from waste disposal to recovery of resources. Waste Manage. 2009;29:1163–1166. doi: 10.1016/j.wasman.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Nagendran R., Selvam A., Joseph K., Chiemchaisri C. Phytoremediation and rehabilitation of municipal solid waste landfills and dumpsites: a brief review. Waste Manage. 2006;26:1357–1369. doi: 10.1016/j.wasman.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Erses A.S., Fazal M.A., Onaya T.T., Craig W.H. Determination of solid waste sorption capacity for selected heavy metals in landfills. J Hazard Mater. 2005;B121:223–232. doi: 10.1016/j.jhazmat.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Hassan A.H., Ramadan M.H. Assessment of sanitary landfill leachate characterizations and its impacts on groundwater at Alexandria. J Egypt Public Health Assoc. 2005;80:27–49. [PubMed] [Google Scholar]
- 6.Veolia Environmental Services. Renewable energy – energy production from solid and household waste. Symposium meeting Room C. Egypt, Alexandria: Bibliotheca Alexandrina conference centre; 30 July 2011.
- 7.Bashir M.J.K., Isa M.H., Kutty S.R.M., Awang Z.B., Abdul Aziz H., Mohajeri S. Landfill leachate treatment by electrochemical oxidation. Waste Manage. 2009;29:2534–2541. doi: 10.1016/j.wasman.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Wikipedia. The free encyclopedia. Egypt Governorates. <http://ar.wikipedia.org/wiki/%D9%85%D8%AD%D8%A7%D9%81%D8%B8%D8%A7%D8%AA_%D9%85%D8%B5%D8%B1> [updated 29.11.11; cited 26.11.11].
- 9.Veolia Environment Organization. Waste management system in Alexandria, Egypt. An innovative and sustainable public-private partnership to improve the living environment in a large, developing city. Egypt, Alexandria: VE, Information Services Section. <http://www.unhabitat.org/downloads/docs/032-Waste%20Management%20System%20in%20Alexandria%20(Egypt).doc>; 2006 [updated 12.01.13; cited 10.01.13].
- 10.Integral Consult. Environmental impact assessment: Al-Hammam landfill project. <http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2006/02/21/000011823_20060221153642/Rendered/INDEX/E12870v10EIA0A1aft0Revised020010005.txt>; 2005 [updated 20.10.12; cited 11.10.12].
- 11.UNFCCC (United Nations Framework Convention on Climate Change). Onyx Alexandria landfill gas capture and flaring project. Clean development mechanism project design document. Version 4. Reference no. 0508. Egypt, Alexandria: Borg El Arab and El Hammam Landfills. <http://wbcarbonfinance.org/docs/Egypt_Onyx_PDD.pdf>; 2006.
- 12.Veolia Environmental Services. Monitoring report. Onyx Alexandria landfill gas capture and flaring project. Version 1, VES. Egypt, Alexandria. <http://cdm.unfccc.int/filestorage/Y/X/S/YXSB0UQTOE2G714ALZ3WP6FHNKMR5V/Monitoring%20Report.pdf?t=WUp8bHdodTM0fDBwuXQy2ZSnx1TTc8o0Y-ln>; 2011.
- 13.Eaton A.D., Franson M.A.H., American Water Works Association, Water Environment Federation . 21st ed. American Public Health Association; Washington: 2005. Standard method for the examination of water and wastewater. [Google Scholar]
- 14.Van Gerald, Lloyd D., Patrick J., Thomas L. 2nd ed. John Wiley & Sons, Inc.; United States of America: 2004. Biostatistics: a methodology for the health sciences. [Google Scholar]
- 15.Tränkler J., Visvanathan C., Kuruparan P., Tubtimthai O. Influence of tropical seasonal variations on landfill leachate characteristics—results from lysimeter studies. Waste Manage. 2005;25:1013–1020. doi: 10.1016/j.wasman.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Bahaa-eldin E.A.R., Yusoff I., Samsudin A.R., Yaacob W.Z.W., Rafek A.G.M. Deterioration of groundwater quality in the vicinity of an active open-tipping site in West Malaysia. Hydrogeol J. 2010;18:997–1006. [Google Scholar]
- 17.Olivero-Verbel J., Padilla-Bottet C., De la Rosa O. Relationships between physicochemical parameters and the toxicity of leachates from a municipal solid waste landfill. Ecotoxicol Environ Saf. 2008;70:294–299. doi: 10.1016/j.ecoenv.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Chofqi A., Younsi A., Lhadi E., Mania J., Mudry J., Veron A. Environmental impact of an urban landfill on a coastal aquifer (El Jadida, Morocco) J Afr Earth Sci. 2004;39:509–516. [Google Scholar]
- 19.Monje-Ramirez I., Orta de Velásquez M.T. Removal and transformation of recalcitrant organic matter from stabilized saline landfill leachates by coagulation–ozonation coupling processes. Water Res. 2004;38:2359–2367. doi: 10.1016/j.watres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Chen P.H. Assessment of leachates from sanitary landfills: impact of age, rainfall, and treatment. Environ Int. 1996;22:225–237. [Google Scholar]
- 21.Rapti-Caputo D., Vaccaro C. Geochemical evidences of landfill leachate in groundwater. Eng Geol. 2006;85:111–121. [Google Scholar]
- 22.WHO. WHO’s drinking water standards 1993: WHO’s guidelines for drinking-water quality. Geneva: Lenntech Water Treatment and Air Purification Holding B.V.; 1993.
- 23.EPA. Drinking water quality standards. Waterford, Wisconsin 53185: Edstrom Industries; 2003.
- 24.Water Stewardship Information Series. Iron & manganese in groundwater. British Columbia: The British Columbia Ground Water Association. <http://www.env.gov.bc.ca/wsd/plan_protect_sustain/groundwater/library/ground_fact_sheets/pdfs/fe_mg(020715)_fin2.pdf>; 2007.
- 25.Reyes-López J.A., Ramírez-Hernández J., Lázaro-Mancilla O., Carreón-Diazconti C., Garrido M.M.L. Assessment of groundwater contamination by landfill leachate: a case in México. Waste Manage. 2008;28:S33–S39. doi: 10.1016/j.wasman.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 26.The New Jersey Department of Health and Senior Services, Hazardous Site Health Evaluation Program, Consumer and Environmental Health Service, Division of Epidemiology, Environmental and Occupational Health. Public Health Assessment: Dover Township Municipal Landfill. CERCLIS, Number: NJD980771570, Silverton Private Well Contamination Investigation, CERCLIS Number: NJD981877780, New Jersey: Dover Township, Ocean County; 2001.
- 27.Bandara N.J.G.J., Hettiaratchi J.P.A. Environmental impacts with waste disposal practices in a suburban municipality in Sri Lanka. Int J Environ Waste Manage. 2010;6(1/2):107–116. [Google Scholar]