Graphical abstract
Keywords: Plague, Egypt, Re-emergence, Yersinia pestis
Abstract
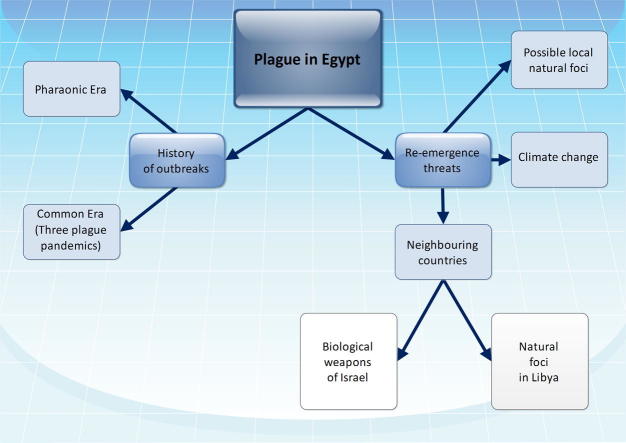
Plague is a zoonotic disease with a high mortality rate in humans. Unfortunately, it is still endemic in some parts of the world. Also, natural foci of the disease are still found in some countries. Thus, there may be a risk of global plague re-emergence. This work reviews plague biology, history of major outbreaks, and threats of disease re-emergence in Egypt. Based on the suspected presence of potential natural foci in the country, the global climate change, and the threat posed by some neighbouring countries disease re-emergence in Egypt should not be excluded. The country is in need for implementation of some preventive measures.
Introduction
Plague is a deadly infectious disease which has been responsible for a number of high-mortality epidemics throughout human history. Unfortunately, the disease is still endemic in some parts of the world. Plague natural foci are found in the tropical and sub-tropical latitudes and the warmer parts of the temperate latitudes around the globe, between the parallels 55° North and 40° South. Interestingly, known disease natural foci are found on all continents except Australia [1]. However, the continent suffered many plague outbreaks originating from shipping and eventually disappeared. Most probably the disease did not success to colonise Australia due to its failure to become established in a suitable enzootic host [2]. Worldwide, humans may be at risk of plague re-emergence. Due to the high public health significance of plague, the present work aims at reviewing the disease biology, history of outbreaks, and threats of disease re-emergence in Egypt.
Biology
Etiologic agent
In 1894, during an epidemic of plague in Hong Kong, a French-Swiss bacteriologist Alexandre Yersin discovered the causative agent which is a Gram-negative rod-shaped enterobacterium. The pathogen is a facultative anaerobe that can infect humans and other animals. Yersin named it Pasteurella pestis in honour of the Pasteur Institute where he worked. In 1967, the organism was moved to a new genus and renamed Yersinia pestis in honour of Yersin [3]. Yersinia pestis has gained attention as a possible biological warfare agent [4]. It is one of the first examples of biological warfare in history, when in 1347 plague victims were catapulted by the Mongols over the city walls of Caffa, currently known as Feodosiya which is located in Ukraine [5]. In 1940, during the World War II, a Japanese airplane released rice and wheat mixed with rat fleas infected with Y. pestis over Chushien in Chekiang Province of China. A second plane load was released three weeks later. These actions led to a local epidemic that killed 121 persons [6]. During the 1950s and 1960s, the United States and the former Soviet Union biological weapons programs developed methods to directly aerosolise particles containing Y. pestis. Soviet scientists manufactured large quantities and allegedly engineered multidrug-resistant strains of the pathogen [7]. It was estimated that 50 kg of Y. pestis released as an aerosol over a city of five million could result in 150,000 cases of pneumonic plague, with 80,000–100,000 requiring hospitalisation and 36,000 deaths [8]. Yersinia pestis has all the qualities you would look for in a potential biological weapon: a high fatality rate, no vaccine and possible air-borne transmission [7]. Antimicrobial resistance in Y. pestis is rare, but constitutes a significant international public health and biodefense threat. In 1995, the first multidrug resistant isolate of Y. pestis was identified. This strain was resistant to all first-line antibiotics as well as to the principal alternative drugs for treatment and prophylaxis [9]. The multidrug-resistant plasmid was highly transferable in vitro to other strains of Y. pestis, where it was stable. Most probably this type of replicon can also be transferred among strains of Y. pestis in their natural environment and, therefore, that resistance may spread locally in this species [9,10].
Life cycle
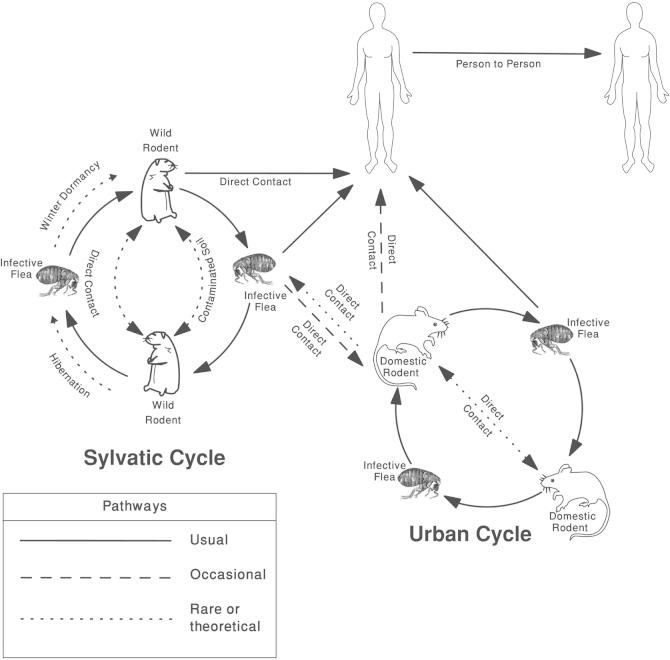
Yersinia pestis has the ability to cause disease in fleas, rodents and humans (Fig. 1). The primary carriers of the pathogen are the Oriental rat flea, Xenopsylla cheopis, and infected rodents. Xenopsylla cheopis is thought to have originated in Egypt and during the 19th century spread to all parts of the world as parasite of rats infesting ships’ cargos [11]. It was reported that fleas from other mammals have a role in human plague outbreaks [12]. Both male and female fleas feed on blood and can transmit the infection. Once internalized by the flea, Y. pestis will continue to reproduce and sticking together until a large plug is formed in the midgut, causing gastrointestinal functions to cease and the flea to starve. Although the flea cannot quell its hunger, it bites a host and continues to feed. Consequently, the flea vomits blood tainted with the bacteria back into the bite wound. The bacterium then infects a new victim, and the flea eventually dies from starvation. There are two cycles for transmission of the disease. The first is the Sylvatic Cycle, or pre-human cycle, which occurs in wild rodents and fleas. This cycle continues until either all the wild rodents are dead, or the fleas find a new food source, usually domestic rats. Once a domestic rat gets bitten, or another domestic animal, the Urban Cycle starts. If a flea that carries Y. pestis happens to bite a human then that human is infected [13]. In rare occasions, infected rodents can transmit the infection to humans through contact. Human to human transmission of the disease is possible by any of the following means: droplet contact by coughing or sneezing on another person; direct physical contact by touching an infected person. Indirect contact is usually by touching contaminated soil. Airborne transmission takes place if the causative agent can remain in the air for long periods [1]. Handling and consumption of raw or insufficiently cooked meat of infected animals like camels and goats were confirmed as means of transmission of plague to humans [14–17]. Also, the human body louse, Pediculus humanus corporis, has been proved as an efficient vector for transmission of Y. pestis [18].
Fig. 1.
Classical pathways of plague transmission (modified after Chamberlain, 2004) [19].
Pathogenicity and clinical picture
There are three main clinical varieties of human infection that commonly occur worldwide which are bubonic, septicemic, and pneumonic forms. However, rarely pharyngeal, meningeal, cellulocutaneous, pestis minor, asymptomatic, and abortive forms were reported [1,14,20–22].
Bubonic plague is the classic most common in humans. When an infected flea bites a human and contaminates the wound with regurgitated blood, the plague carrying bacteria are passed into the tissue. In human body, the bacteria can enter the lymphatic system, which drains interstitial fluid. In lymph nodes, Y. pestis replicates and stimulates severe haemorrhagic inflammation that causes the nodes to expand to the size of an egg. The expansion of lymph nodes is the cause of the characteristic “buboes” associated with the disease. Lymph nodes expansions typically arise in the groin, neck and armpits. The disease becomes evident 2–6 days after infection, and the patients typically experience a sudden onset of illness characterized by headache, shaking chills, fever, malaise and pain in the affected regional lymph nodes. Gangrene may develop in the extremities, lending it the name “Black Death” [1].
Septicemic plague occurs when Y. pestis is found in the blood. This form may be either primary or secondary to bubonic plague. The disease is contracted primarily through the bite of an infected rodent or flea, but like bubonic plague can very rarely be contracted through an opening in the skin or by cough from another infected human. After infection, the bacteria multiply in the blood, causing bacteraemia and severe sepsis. It may lead to metastatic infection of other organs or systems. Complications include plague pneumonia, plague meningitis, plague endophthalmitis, hepatic or splenic abscesses, or generalized lymphadenopathy. Early treatment with antibiotics reduces the mortality rate to 4–15% [23]. If not supported, the patient often die on the same day, symptoms first appear [1,24,25].
Pneumonic plague may be either primary or secondary. The primary variant is transmitted from human to human through the air and Y. pestis is then brought directly into the lungs. The secondary form is a complication of either the bubonic or septicemic forms. Primary pneumonic plague is the most serious form of infection. Symptoms, on top of those found in the other two forms, include a severe cough, coughing with blood (haemoptysis), chest pains, confusion, cyanosis, shock and eventual death. The incubation period is usually between two and four days, but can be as little as a few hours. Without diagnosis and treatment, the infection can be fatal in one to six days. It is 100% lethal if not treated [1,26].
Major outbreaks
Historically, it was suggested that the disease had been present since time immemorial in the areas within or near the Central Asiatic plateau which was considered as the original home of the infection [27]. Phylogenetic studies confirmed that the plague originated in Yunnan province in Southwest China [28]. Plague existed in Egypt and the Nile Valley since the Pharaonic era [29]. There have been three major human outbreaks of which are Justinian’s plague (6th and 7th centuries), the Black Death (14th and 18th centuries), and the third plague pandemic (1855–1959) [30].
The Plague of Justinian was a pandemic that afflicted the Byzantine Empire. It has been claimed as one of the greatest plagues in history. The Byzantine historian Procopius first reported the epidemic in 541 A.D. from the port of Pelusium (near Suez in Egypt). The outbreak in Constantinople was thought to have been carried to the city by infected rats (and fleas) on grain boats arriving from Egypt. This epidemic was nearly worldwide in scope, striking central and south Asia, North Africa and Arabia; and Europe all the way to Denmark and Ireland. Throughout the Mediterranean basin, until about 750 A.D., the epidemic returned in each generation. About 40% of the population of Constantinople died from the plague. It was suggested also that half of Europe’s population was wiped out before the plague disappeared in the 8th century [30].
The Black Death originated in or near China and spread by way of the Silk Road or by ship. It may have reduced world population from an estimated 450 million to between 350 and 375 million in 1400. The pandemic struck various countries in the Middle East and lead to serious depopulation and permanent change in both economic and social structures. As it spread to Western Europe, the disease entered the region from Southern Russia also. In 1347, the plague reached Alexandria in Egypt, probably through the port’s trade with Constantinople, and ports on the Black Sea. During the same year the disease travelled eastward to Gaza, and north along the eastern coast to cities in Lebanon, Syria and Palestine, including Ashkelon, Acre, Jerusalem, Sidon, Damascus, Homs, and Aleppo. During 1348–49, the disease reached Antioch. The city’s residents fled to the north, most of them dying during the journey, but the infection spread to the people of Asia Minor. The disease struck Mecca in 1349. During the same year, records showed that the city of Mawsil suffered a massive epidemic, and the city of Baghdad experienced a second round of the disease. In 1351, Yemen experienced an outbreak of the plague [30].
The third Pandemic spread to all inhabited continents, and ultimately killed more than 12 million people in India and China alone. The initial outbreak was in Yunnan Province (China) in the 1850s [30,31]. According to the World Health Organization, the pandemic was considered active until 1959, when worldwide casualties dropped to 200 per year. This pandemic may have been from two different sources. The first was primarily bubonic and was carried around the world through ocean-going trade, through transporting infected persons, rats, and cargoes harbouring fleas. The second, more virulent strain was primarily pneumonic in character with a strong person-to-person contagion. This strain was largely confined to Asia, in particular Manchuria and Mongolia. In Egypt, the third pandemic first established itself in the main ports: in Alexandria in 1899 and in Port Said in 1900. It was then dispersed inland with increasing speed and intensity, reaching its maximum during the period 1908–1912. Unlike previous epidemics, the prevalence was much greater in Upper Egypt than in Lower Egypt; thus, 6192 cases had been recorded in Upper Egypt by 1912 as against 2141 in Lower Egypt. The epidemic then fluctuated widely, although generally lessening, until 1925 when it began to show signs of abating. It disappeared from the ports (from Suez in 1930, from Port Said in 1932, and from Alexandria in 1936) and in 1937 from the northern part of Upper Egypt (the provinces of Giza, Fayoum, Minya, and Bani Sweif). In 1939, Lower Egypt finally became free of plague. In 1941, a control scheme was introduced for rats in river and canal craft to prevent the disease inland spread from the ports. So, none was reported inland from 1941 to 1945 despite an outbreak during that time in the Suez Canal Zone (Port Said, Suez and Ismailia). During the Alexandria epidemic of 1946–1947, the introduction of DDT and other control measures resulted in a sharp decrease in flea indices [32]. Recently, DDT resistance was reported in the Egyptian flea populations of Pulex irritans (the human flea) and X. cheopis [11].The epidemiological situation of recent epidemics of the disease in Egypt is not available in the literature. Mollaret (1995) mentioned the presence of many plague epidemics which have been negated or dissimulated in order to avoid isolation and quarantine. Among these epidemics were one in Egypt in 1984 [33].
Threats of disease re-emergence in Egypt
In 1999, Tikhomirov reported that natural foci of plague are known to exist in broad areas of Africa which may include Egypt [34]. This may be supported by the recent reports of the presence of natural foci in North Africa in Libya and Algeria [35–38]. The presence of natural foci of plague plays a serious role in re-emergence of the disease. Yersinia pestis bacteria may have been maintained circulating at low levels in the rodent populations without diagnosis of any human cases [39]. Also, Y. pestis can survive in the soil under laboratory conditions, possibly providing the opportunity for rodents to be infected and promoting re-emergence of the disease [40,41]. The new settlements and urbanization may help in re-emergence of plague from potential natural foci, if present. Surveillance, especially in the western part of Egypt, should be maintained to monitor the presence of potential natural foci, and spread of plague that might occur because of environment influences.
Climate change including global warming is an emerging global issue which is expected to significantly affect many countries of the world including Egypt [42–45]. An array of serious threats is apparent to develop in Egypt with the climate change, including the increase in water stress, the rise in sea level, and the rapidly increasing gap between the limited water availability and the escalating demand for water in the country [46]. Worldwide, climate has long been incriminated to be a key factor in the alternation between quiescent and active periods of plague [47]. Climate affects all components of the plague cycle (host, vector, and pathogen) in various ways and over a wide range of scales (from micro-individual flea life cycle-to macro-a plague area composed of several disjoint foci) [47]. It was suggested that seasonal variations in temperature and humidity were responsible for the seasonal patterns of human plague incidence in India [48]. Also, it was shown that human plague outbreaks in several African countries were less frequent when the weather was too hot (>27 °C) or cold (<15 °C) [49]. In Vietnam, studies showed an increased plague incidence during the hot, dry season, when it followed a period of high seasonal rainfall [50,51]. Unfortunately, the effect of global climate change especially global warming on re-emergence of a next round of plague pandemic could not be excluded.
Libya, a neighbouring country to the west, experienced several plague outbreaks during the period from 1913 to 1920, the largest of which resulted in 1449 deaths in Benghazi in 1917. Other epidemics of lower amplitude occurred in 1972, 1976, and 1977 [52]. The cases diagnosed during such epidemics were from different parts of the country scattered over a vast area. This is an evidence that an extensive epizootic of plague had taken place. Reports of plague in 1976 in places where sick camels and goats existed alerted to the role of these animals in the epidemiology of the disease in some areas [16,52]. More cases of bubonic plague were diagnosed in 1984 in two locations 25 km from Tobruk where plague foci had been noted between 1976 and 1977 [38]. After an apparent absence for 25 years, plague cases recurred in 2009 near Tobruk. An even more recent plague epidemic was reported in 2011 in the city of Tobruk [35]. The city is very close to the border with Egypt, thus the danger of plague transmission from its natural foci in Libya to Egypt should be highlighted.
Israel, a neighbouring country to the east, refused to sign the 1972 Biological and Toxin Weapons Convention and maintains a policy of ambiguity regarding government-sponsored biological weapons research and development. Speculation about the Israeli biological warfare program ranging from the mundane to the fantastic [53]. The Office of Technology Assessment in the U.S. Congress listed Israel as a country “generally reported as having [an] undeclared offensive biological warfare [program]” [54]. Speculation about Israel’s biological weapons program focuses on Israel Institute for Biological Research (IIBR), a highly classified defence research centre operated and funded by the Ministry of Defence’s Division of Research. IIBR’s mission statement and broad scientific mandate exemplify the ambiguity of dual use. A database search on Scopus® of IIBR’s publications reveals research on several select agents and toxins, with special focus on anthrax bacterium and Y. pestis. Moreover, several other research institutions, including Hebrew University of Jerusalem, Tel Aviv University, and Technion also publish select agent research [55]. Even in the presence of the peace agreements between some Arab countries and Israel, research and development of biological weapons pose a threat to the neighbouring countries including Egypt.
Conclusions
Plague is a zoonotic disease which continues to pose a major threat to humanity. Plague re-emergence in Egypt should not be excluded especially in view of the presence of suspected potential natural foci, the global climate change, and the threat posed by some neighbouring countries. Some preventive measures should be implemented, including surveillance of suspected natural foci, rodent and insect eradication campaigns, public health education, immunization, and genetic analysis of Y. pestis strains whenever found.
Conflict of interest
The author has declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biography

Wael M. Lotfy received his PhD from the Alexandria University, Egypt in 2001. He was promoted as Professor, University of Alexandria, Egypt in 2011. He is a recipient of Alexandria University Award for Encouraging of Scientific Research (2006), Alexandria University Award for Encouraging of Scientific Publications (four times) and has been listed in ‘Marquis Who’s Who in the World’ since 2009, ISBN: 9780837911410. He underwent nine months of training in Rome on Molecular and Classical Parasitology and has travelled five times in scientific missions as a visiting scholar in Biology Department, University of New Mexico, USA during the period 2004–2010. He has published 23 papers in reputed journals and three books. His name has been recorded in the international reviewer panels of many journals. He has organised several workshops and has been involved in various projects. He is a member of the Egyptian Parasitologists United (EPU), the Egyptian Society of Parasitology, the Egyptian Association of advancement of Medical Basic Sciences (EAMBS), the Eastern Mediterranean Health Genomics and Biotechnology Network (EMHGBN) and Member of the German Egyptian network of young scientists (GENYS).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.WHO . World Health Organization; Geneva, Switzerland: 1999. Plague manual: epidemiology, distribution, surveillance and control. [PubMed] [Google Scholar]
- 2.Perry R.D., Fetherston J.D. Yersinia pestis: etiologic agent of plague. Clin Microbiol Rev. 1997;10(1):35–66. doi: 10.1128/cmr.10.1.35. [PubMed PMID: 8993858; PubMed Central PMCID: PMC172914] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard-Jones N. Was Shibasaburo Kitasato the co-discoverer of the plague bacillus? Perspect Biol Med. 1973;16(2):292–307. doi: 10.1353/pbm.1973.0034. [DOI] [PubMed] [Google Scholar]
- 4.Chandler D., Landrigan I. 2nd ed. Radio and Television News Directors Foundation; Washington, DC: 2004. Bioterrorism: a journalist’s guide to covering bioterrorism. [Google Scholar]
- 5.Wheelis M. Biological warfare at the 1346 siege of Caffa. Emerg Infect Dis. 2002;8(9):971–975. doi: 10.3201/eid0809.010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drisdelle R. University of California Press; Berkeley, CA: 2010. Parasites: tales of humanity’s most unwelcome guests. [Google Scholar]
- 7.Inglesby T.V., Dennis D.T., Henderson D.A., Bartlett J.G., Ascher M.S., Eitzen E. Plague as a biological weapon: medical and public health management. Working group on civilian biodefense. JAMA. 2000;283(17):2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 8.WHO . World Health Organization; Geneva, Switzerland: 1970. Health aspects of chemical and biological weapons. [Google Scholar]
- 9.Galimand M., Guiyoule A., Gerbaud G., Rasoamanana B., Chanteau S., Carniel E. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N Engl J Med. 1997;337(10):677–680. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- 10.Dennis D.T., Hughes J.M. Multidrug resistance in plague. N Engl J Med. 1997;337(10):702–704. doi: 10.1056/NEJM199709043371010. [DOI] [PubMed] [Google Scholar]
- 11.Gratz N. Rodent reservoirs and flea vectors of natural foci of plague. In: WHO, editor. Plague manual: epidemiology, distribution, surveillance and control. World Health Organization; Geneva, Switzerland: 1999. pp. 63–96. [Google Scholar]
- 12.von Reyn C.F., Weber N.S., Tempest B., Barnes A.M., Poland J.D., Boyce J.M. Epidemiologic and clinical features of an outbreak of bubonic plague in New Mexico. J Infect Dis. 1977;136(4):489–494. doi: 10.1093/infdis/136.4.489. [DOI] [PubMed] [Google Scholar]
- 13.Keeling M.J., Gilligan C.A. Metapopulation dynamics of bubonic plague. Nature. 2000;407:903–906. doi: 10.1038/35038073. [DOI] [PubMed] [Google Scholar]
- 14.Arbaji A., Kharabsheh S., Al-Azab S., Al-Kayed M., Amr Z.S., Abu Baker M. A 12-case outbreak of pharyngeal plague following the consumption of camel meat, in north-eastern Jordan. Ann Trop Med Parasitol. 2005;99(8):789–793. doi: 10.1179/136485905X65161. [DOI] [PubMed] [Google Scholar]
- 15.Bin Saeed A.A., Al-Hamdan N.A., Fontaine R.E. Plague from eating raw camel liver. Emerg Infect Dis. 2005;11(9):1456–1457. doi: 10.3201/eid1109.050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie A.B., Chen T.H., Elberg S.S. Plague in camels and goats: their role in human epidemics. J Infect Dis. 1980;141(6):724–726. doi: 10.1093/infdis/141.6.724. [DOI] [PubMed] [Google Scholar]
- 17.Leslie T., Whitehouse C.A., Yingst S., Baldwin C., Kakar F., Mofleh J. Outbreak of gastroenteritis caused by Yersinia pestis in Afghanistan. Epidemiol Infect. 2011;139(5):728–735. doi: 10.1017/S0950268810001792. [DOI] [PubMed] [Google Scholar]
- 18.Ayyadurai S., Sebbane F., Raoult D., Drancourt M. Body lice, Yersinia pestis orientalis, and black death. Emerg Infect Dis. 2010;16(5):892–893. doi: 10.3201/eid1605.091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain N. Transmission cycles of plague http://www.microbelibrary.org/library/resources/2823-transmission-cycles-of-plague. Am Soc Microbiol. 2004 [updated 20 August 2010; cited 2013 17 May] [Google Scholar]
- 20.Hovette P., Burgel P.R., Camara P., Sane M., Auregan G., Klotz F. Pulmonic plague. Rev Pneumol Clin. 1998;54(6):373–376. [PubMed] [Google Scholar]
- 21.Marshall J.D., Jr., Quy D.V., Gibson F.L. Asymptomatic pharyngeal plague infection in Vietnam. Am J Trop Med Hyg. 1967;16(2):175–177. doi: 10.4269/ajtmh.1967.16.175. [DOI] [PubMed] [Google Scholar]
- 22.Meyer K.F., Connor C.L., Smyth F.S., Eddie B. Chronic relapsing latent meningeal plague. Arch Intern Med (Chic) 1937;59(6):967–980. [Google Scholar]
- 23.Meyer K.F. Modern therapy of plague. J Am Med Assoc. 1950;144(12):982–985. doi: 10.1001/jama.1950.02920120006003. [DOI] [PubMed] [Google Scholar]
- 24.Butler T. Plague and other yersinia infections. In: Greenough W.B. III., Merigan T.C., editors. Current topics in infectious disease. Plenum Press; New York, NY: 1983. pp. 71–92. [Google Scholar]
- 25.Pollitzer R. World Health Organization; Geneva, Switzerland: 1954. Plague (Monograph series) [Google Scholar]
- 26.Hoffman S.L. Plague in the United States: the “black death” is still alive. Ann Emerg Med. 1980;9(6):319–322. doi: 10.1016/s0196-0644(80)80068-0. [DOI] [PubMed] [Google Scholar]
- 27.Lien-Teh W., Chun I.W.H., Pollitzer R., Wu C.Y. National Quarantine Service; Shanghai, China: 1936. Plague: a manual for medical and public health workers. [Google Scholar]
- 28.Morelli G., Song Y., Mazzoni C.J., Eppinger M., Roumagnac P., Wagner D.M. Phylogenetic diversity and historical patterns of pandemic spread of Yersinia pestis. Nat Genet. 2010;42(12):1140–1143. doi: 10.1038/ng.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panagiotakopulu E. Pharaonic Egypt and the origins of plague. J Biogeogr. 2004;31:269–275. [Google Scholar]
- 30.Wade N. Europe’s plagues came from China. Study Finds. 2010;13 [Google Scholar]
- 31.Cohn S.K., Jr. Arnold Publishers; London, UK: 2003. The black death transformed: disease and culture in early renaissance europe. [PubMed] [Google Scholar]
- 32.Hussein A.G. Changes in the epidemiology of plague in Egypt, 1899–1951. Bull World Health Organ. 1955;13:27–48. [PMC free article] [PubMed] [Google Scholar]
- 33.Mollaret H.H. Concealing and denying the plague. Hist Sci Med. 1995;29(4):343–345. [PubMed] [Google Scholar]
- 34.Tikhomirov E. Epidemiology and distribution of plague. In: WHO, editor. Plague manual: epidemiology, distribution, surveillance and control. World Health Organization; Geneva, Switzerland: 1999. pp. 11–41. [Google Scholar]
- 35.Cabanel N., Leclercq A., Chenal-Francisque V., Annajar B., Rajerison M., Bekkhoucha S. Plague outbreak in Libya, 2009, unrelated to plague in Algeria. Emerg Infect Dis. 2013;19(2):230–236. doi: 10.3201/eid1902.121031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bitam I., Ayyadurai S., Kernif T., Chetta M., Boulaghman N., Raoult D. New rural focus of plague, Algeria. Emerg Infect Dis. 2010;16(10):1639–1640. doi: 10.3201/eid1610.091854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertherat E., Bekhoucha S., Chougrani S., Razik F., Duchemin J.B., Houti L. Plague reappearance in Algeria after 50 years, 2003. Emerg Infect Dis. 2007;13(10):1459–1462. doi: 10.3201/eid1310.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anonymous. Human plague in 1984. Wkly Epidemiol Rec. 1985;39:297–298. [Google Scholar]
- 39.Duplantier J.M., Duchemin J.B., Chanteau S., Carniel E. From the recent lessons of the Malagasy foci towards a global understanding of the factors involved in plague reemergence. Vet Res. 2005;36(3):437–453. doi: 10.1051/vetres:2005007. [DOI] [PubMed] [Google Scholar]
- 40.Eisen R.J., Petersen J.M., Higgins C.L., Wong D., Levy C.E., Mead P.S. Persistence of Yersinia pestis in soil under natural conditions. Emerg Infect Dis. 2008;14(6):941–943. doi: 10.3201/eid1406.080029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayyadurai S., Houhamdi L., Lepidi H., Nappez C., Raoult D., Drancourt M. Long-term persistence of virulent Yersinia pestis in soil. Microbiology. 2008;154(Pt 9):2865–2871. doi: 10.1099/mic.0.2007/016154-0. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy J.J., Canziani O.F., Leary N.A., Dokken D.J., White K.S. Cambridge University Press; Cambridge, UK: 2001. Climate change 2001: impacts, adaptation and vulnerability. Contribution of working group II to the third assessment report of the intergovernmental panel of climate change (IPCC) [Google Scholar]
- 43.IPCC . Cambridge University Press; Cambridge, UK: 1998. The regional impacts of climate change: an assessment of vulnerability. A special report of the intergovernmental panel on climate change working group II. [Google Scholar]
- 44.Houghton J.T., Jenkins G.J., Ephraums J.J. Cambridge University Press; Cambridge, UK: 1990. Scientific assessment of climate change. Report of working group I of the intergovernmental panel of climate change (IPCC) [Google Scholar]
- 45.IPCC . Cambridge University Press; Cambridge: 2000. Special report on emission scenarios. [Google Scholar]
- 46.Lotfy WM. Climate change and epidemiology of human parasitosis in Egypt. J Adv Res. 2014;5(6):607–13. [DOI] [PMC free article] [PubMed]
- 47.Ben-Ari T., Neerinckx S., Gage K.L., Kreppel K., Laudisoit A., Leirs H. Plague and climate: scales matter. PLoS Pathog. 2011;7(9):e1002160. doi: 10.1371/journal.ppat.1002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers L. The yearly variations in plague in India in relation to climate: forecasting epidemics. Proc R Soc Lond B Biol Sci. 1928;103:42–72. [Google Scholar]
- 49.Davis D.H. Plague in Africa from 1935 to 1949; a survey of wild rodents in African territories. Bull World Health Organ. 1953;5:665–700. [PMC free article] [PubMed] [Google Scholar]
- 50.Cavanaugh D.C., Marshall J.D. The influence of climate on the seasonal prevalence of plague in the Republic of Vietnam. J Wildl Dis. 1972;8:85–94. doi: 10.7589/0090-3558-8.1.85. [DOI] [PubMed] [Google Scholar]
- 51.Cavanaugh D.C., Dangerfield H.G., Hunter D.H., Joy R.J., Marshall J.D., Jr., Quy D.V. Some observations on the current plague outbreak in the Republic of Vietnam. Am J Public Health Nations Health. 1968;58:742–752. doi: 10.2105/ajph.58.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Misonne X. A natural focus of plague in Libya. Ann Soc Belg Med Trop. 1977;57(3):163–168. [PubMed] [Google Scholar]
- 53.Cohen A. Israel and chemical/biological weapons: history, deterrence, and arms control. Nonproliferation Rev. 2001;8(3):27–53. [Google Scholar]
- 54.Government Printing Office; Washington, DC: 1993. U.S. Congress-Office of Technology Assessment. Proliferation of weapons of mass destruction: assessing the risk. Report No. OTA-ISC-559. p. 65. [Google Scholar]
- 55.Monterey institute of international studies. country profiles: Israel http://www.nti.org/country-profiles/israel/biological. Nucl Threat Initiative. 2011 [cited 2013 10 July] [Google Scholar]