Abstract
Number processing deficits are frequently seen in children prenatally exposed to alcohol. Although the parietal lobe, which is known to mediate several key aspects of number processing, has been shown to be structurally impaired in fetal alcohol spectrum disorders (FASD), effects on functional activity in this region during number processing have not previously been investigated. This fMRI study of 49 children examined differences in activation associated with prenatal alcohol exposure in five key parietal regions involved in number processing, using tasks involving simple addition and magnitude comparison. Despite generally similar behavioral performance, in both tasks greater prenatal alcohol exposure was related to less activation in an anterior section of the right horizontal intraparietal sulcus known to mediate mental representation and manipulation of quantity. Children with fetal alcohol syndrome and partial fetal alcohol syndrome appeared to compensate for this deficit by increased activation of the angular gyrus during the magnitude comparison task.
Keywords: fMRI, Fetal alcohol spectrum disorders, Fetal alcohol syndrome, Prenatal alcohol exposure, Number processing, Magnitude comparison, Parietal
Abbreviations: AA, absolute alcohol; ADHD, attention-deficit/hyperactivity disorder; ANOVA, analysis of variance; ARND, alcohol-related neurodevelopmental disorder; DD, developmental dyscalculia; EA, exact addition; EA_CTL, control block in the exact addition task; FAS, fetal alcohol syndrome; FASD, fetal alcohol spectrum disorders; HE, heavily exposed; IPS, intraparietal sulcus; LSD, least-squares difference; PFAS, partial fetal alcohol syndrome; PJ, proximity judgment; PJ_CTL, control block in the proximity judgment task; PSPL, posterior superior parietal lobule; ROI, region of interest; TS, Turner syndrome; UCT, University of Cape Town; VBM, voxel-based morphometry; WISC-III, Wechsler Intelligence Scale for Children, Third Edition
Highlights
-
•
We use fMRI to examine the effects of prenatal alcohol on key number processing parietal regions.
-
•
The children performed simple exact addition and magnitude comparison.
-
•
Behavioral performance in the scanner was similar between groups in both tasks.
-
•
Greater prenatal alcohol related to less activation in the right intraparietal sulcus.
-
•
FAS/PFAS group compensate by using angular gyrus more during magnitude comparison.
1. Introduction
Prenatal alcohol exposure causes impairment in brain structure and function, leading to cognitive and behavioral deficits that range in severity (Archibald et al., 2001; Sowell et al., 2001; Riley and McGee, 2005; Astley et al., 2009). Fetal alcohol syndrome (FAS), the most severe of the fetal alcohol spectrum disorders (FASD), is characterized by distinctive craniofacial dysmorphology (short palpebral fissures, thin vermilion, flat philtrum), small head circumference and pre- and/or postnatal growth retardation (Hoyme et al., 2005). The craniofacial dysmorphology is also seen in partial FAS (PFAS), together with either small head circumference, retarded growth, or neurobehavioral deficits. Heavily exposed (HE) individuals lacking the distinctive dysmorphology are diagnosed with alcohol-related neurodevelopmental disorder (ARND) if they exhibit cognitive and/or behavioral impairment (Stratton et al., 1996).
Prenatal alcohol exposure is associated with a broad range of cognitive deficits, including low IQ (Streissguth et al., 1990; Jacobson et al., 2004), poor attention and executive function (Kodituwakku et al., 1995; Coles et al., 1997; Mattson et al., 1999; Burden et al., 2005a), and slower cognitive processing speed (Streissguth et al., 1990; Jacobson et al., 1993; Jacobson et al., 1994; Coles et al., 2002). Among the cognitive deficits seen in relation to prenatal alcohol exposure, arithmetic is especially sensitive, and mathematical deficits are seen even after controling for IQ (Coles et al., 1991; Streissguth et al., 1990; Streissguth et al., 1994; Chiodo et al., 2004; Jacobson et al., 2004; Burden et al., 2005b), and impaired numerosity is already seen in infants with FAS (S. Jacobson et al., 2011a). When academic achievement tests are administered to exposed individuals, arithmetic is consistently more impaired than reading or spelling (Streissguth et al., 1991; Goldschmidt et al., 1996; Kerns et al., 1997; Howell et al., 2006).
Although the parietal lobe has been known to be involved in number processing since the beginning of the 20th century (Henschen, 1919), fMRI has provided a more extensive understanding of the neuroanatomy of this domain of processing. Based on brain lesion and neuroimaging findings, Dehaene and associates (Dehaene, 1992; Dehaene and Cohen, 1996) have proposed a triple-code model of number processing that incorporates the three different systems of representation that may be used in number processing tasks: the quantity system, the verbal system and the visual system. In addition, they have posited a core quantity system—a nonverbal abstract representation of numerical quantity, which has been localized bilaterally in the anterior portion of the horizontal segments of the intraparietal sulcus (IPS). This area is hypothesized to support number processing irrespective of the notation used; that is, whether represented symbolically as Arabic numbers or sequences of words or analogically by numbers of dots. The verbal processing of numbers is posited to be based on the left angular gyrus (close to the language areas), while the bilateral posterior superior parietal lobules (PSPLs) are hypothesized to be involved in spatial and non-spatial attentional processes contributing to the visual processing of numbers. Rivera et al. (2005) found age-related increases in activation of the anterior IPS during number processing between school age and early adulthood, which they attributed to the development of increasing functional specialization of this region as symbolic number processing becomes increasingly automatic. Results from a meta-analysis of studies comparing number processing in children vs. adults generally support Dehaene's model but suggest that the localization of parietal activations is more notation-specific in children and that right IPS activations in non-symbolic magnitude comparison are slightly more anterior than those observed in adults (Kaufmann et al., 2011).
Data from behavioral studies that we have conducted in two different cohorts suggest that a specific deficit in the ability to represent and manipulate quantity may play a critical role in the poor arithmetic performance seen in FASD (J. Jacobson et al., 2011). A 224-item, 7-subtest, computer-based number processing test that we developed in collaboration with S. Dehaene, was administered to 262 adolescents from the Detroit Longitudinal Prenatal Alcohol Exposure Cohort. A factor analysis of the seven subtests yielded two factors, one reflecting exact and approximate calculation (“Calculation”). The other factor, on which number comparison and proximity judgment loaded most strongly, reflected the ability to represent and manipulate quantity (“magnitude comparison”), corresponding to Dehaene's core quantity system. In a path analytic model, the relation of prenatal alcohol exposure to calculation was fully mediated by its effects on magnitude comparison, suggesting that magnitude comparison is a core deficit involved in the poor arithmetic performance seen in these children. These findings were subsequently confirmed in a sample of school-age children in Cape Town, South Africa (S. Jacobson et al., 2011a). In the Detroit cohort, attention-deficit/hyperactivity disorder (ADHD) was related to poorer performance on all seven number processing subtests, but, by contrast to the pattern seen in the alcohol-exposed children, the associations were markedly stronger with calculation than magnitude comparison. Moreover, IQ significantly mediated the effect of ADHD on calculation, suggesting that the effects of ADHD on aspects of calculation not specific to the representation of number, such as attention and executive function, mediate the poorer number processing seen in that disorder.
This behavioral evidence linking prenatal alcohol exposure to impairment in the core quantity system is consistent with evidence from MRI studies reporting alcohol-related structural impairment in the parietal region, including disproportionate size reductions in the parietal lobe (Archibald et al., 2001; Chen et al., 2012). A high resolution structural MRI, surface-based image analysis indicated that the brains of alcohol-exposed individuals were narrower in the inferior parietal and perisylvian regions (Sowell et al., 2002), and voxel-based morphometry (VBM) analysis revealed gray matter abnormalities that were most prominent in the left perisylvian cortices of the parietal and temporal lobes (Sowell et al., 2001). In addition, significant cortical thickness excesses were observed in children with FASD in large areas of bilateral temporal, bilateral inferior parietal and right frontal regions (Sowell et al., 2008).
In the first fMRI study of number processing in FASD, adults with and without prenatal alcohol exposure were administered a task involving subtraction from 11 of a series of numbers that appeared on the screen (Santhanam et al., 2009). Exposed individuals with alcohol-related dysmorphology exhibited poorer task performance and lower activation in regions known to be associated with arithmetic processing, including the right inferior and left superior parietal regions and medial frontal gyrus, compared with controls. However, it was not clear whether the reduced activation in the dysmorphic participants reflected a specific deficit in fronto-parietal function or completion of fewer problems due to the difficulty of the task. Using simpler proximity judgment and single-digit addition problems, we found that children activate the same fronto-parietal regions activated in number processing by adults (Meintjes et al., 2010a). Although children with FAS and PFAS performed as well as controls on the simple tasks administered in the scanner, they activated a markedly more diffuse parietal region extending into the angular gyrus, precuneus and posterior cingulate and for, exact addition, also the postcentral gyrus (Meintjes et al., 2010b). However, the FAS/PFAS and control groups did not differ significantly in the degree of activation in the anterior portion of the IPS and other regions linked by Dehaene and associates to number processing, possibly due to the lack of statistical power in the small sample on which that whole brain voxel-wise analysis was conducted.
In this study, we examine the effects of both FASD diagnosis and continuous measures of prenatal alcohol exposure on brain activation in the five parietal structures found by Dehaene and associates to mediate number processing in a larger sample that includes not only children with FAS/PFAS and nonexposed controls, but also nonsyndromal heavily-exposed (HE) children (Suttie et al., 2013). We have found that continuous measures of prenatal alcohol exposure based on a maternal report obtained during pregnancy are often more sensitive in detecting effects of prenatal alcohol exposure than diagnoses based on dysmorphic features in studies using diverse neuroimaging techniques, including tensor-based morphometry (Meintjes et al., 2014) and magnetic resonance spectroscopy (du Plessis et al., 2014). Based on the behavioral findings from our Detroit and Cape Town studies, our central hypothesis was that prenatal alcohol exposure would be associated with reduced activation of the anterior IPS, the region believed to mediate abstract representation of numerical quantity.
2. Methods
2.1. Participants
Participants were 65 right-handed, 8- to 12-year-old children from the Cape Coloured (mixed ancestry) community in Cape Town, South Africa, of whom 40 had been heavily exposed to alcohol prenatally and 25 were controls in the same age range (S. Jacobson et al., 2011b). The Cape Coloured community is composed primarily of descendants of white European settlers, Malaysian slaves, Khoi-San aboriginals, and black African ancestors. The incidence of FASD in this population is exceptionally high due to poor socioeconomic circumstances and historical practices of compensating farm laborers with wine, which have contributed to a tradition of heavy recreational weekend binge drinking (May et al., 2007). Thirty-seven children were the older siblings of participants in our Cape Town Longitudinal Cohort (Jacobson et al., 2008). The others were identified by screening all of the 8- to 12-year-old children from an elementary school in a rural section of Cape Town, where there is a very high incidence of alcohol abuse among local farm workers (Meintjes et al., 2010b).
2.2. Procedure
Our research nurse and staff driver transported the mother and child from their home to our child development laboratory at the Faculty of Health Sciences campus of the University of Cape Town (UCT) for a 3-hour neuropsychological assessment and to Groote Schuur Hospital for a neuroimaging assessment, which was administered on the following day. All examiners were blind with regard to maternal alcohol history and the child's FASD diagnostic status, except in the most severe cases where it was obvious. Written informed consent was obtained from each mother; written assent, from each child. Approval for human research was obtained from the Wayne State University Human Investigation Committee and the UCT Faculty of Health Sciences Human Research Ethics Committee.
Each mother was interviewed in her primary language, Afrikaans or English, regarding her alcohol consumption during pregnancy, using a timeline follow-back approach (Sokol et al., 1985; Jacobson et al., 2002). Volume was recorded for each type of beverage consumed each day, converted to absolute alcohol (AA) using multipliers proposed by Bowman et al. (1975), and averaged to provide summary measures of alcohol consumption during pregnancy. Two groups of women were recruited: (1) heavy drinkers, who consumed at least 14 standard drinks per week (1.0 oz AA/day) on average or engaged in binge drinking (5 or more drinks/occasion) and (2) controls whose mothers abstained or drank only minimally during pregnancy—92.0% (all but two) of the control mothers abstained; one drank 2 drinks on 11 occasions; the other, 2 drinks on 1 occasion. Data from the alcohol consumption interview were tabulated to provide three continuous measures of drinking during pregnancy: average oz AA consumed per day, AA/drinking day (dose/occasion) and frequency (days/week). Number of cigarettes smoked on a daily basis was also recorded, as was the use of illicit drugs (yes/no). Mothers were also interviewed regarding their education. Mothers and children were given breakfast, lunch, and a snack during the morning at each laboratory visit. The mother received a small monetary compensation for each visit and photograph of her child, and the child was given a small gift.
In September 2005, we organized a clinic in which each child was independently examined for growth and FAS dysmorphology by two expert U.S.-based FAS dysmorphologists (H.E. Hoyme and L.K. Robinson) using the Hoyme et al. (2005) protocol; a subset of children who could not attend the clinic was examined by a Cape Town-based dysmorphologist (N. Khaole) (Jacobson et al., 2008). There was substantial agreement among the examiners on the assessment of all dysmorphic features, including the three principal fetal alcohol-related features—philtrum and vermilion (which were measured on the Astley and Clarren (2001) rating scales) and palpebral fissure length (median r = 0.78). FAS and PFAS diagnoses were determined at a case conference by the dysmorphologists (HEH and LKR), SWJ, JLJ, and CDM. Nine children met the Hoyme et al. criteria for full FAS: at least two of the principal dysmorphic features, small head circumference (bottom 10th percentile), and low weight or short stature (bottom 10th percentile). Nine met the criteria for PFAS; that is, two features, confirmed maternal alcohol consumption during pregnancy, and at least one of the following: small head circumference, low weight, short stature, or low IQ (<70).
The FAS and PFAS groups were combined for the analyses, but the HE children were treated as a separate group. The decision not to combine the FAS/PFAS and HE groups was based on our findings in Diwadkar et al. (2013) that for some aspects of cognition, distinctly different brain activation patterns are seen in these two groups. Analyses were repeated with all alcohol exposed children combined into one group.
2.3. Neuropsychological assessment
Each child was administered the computer-based number processing test described above, which included two 16-item subtests, one of which assessed Exact Addition (EA); the other, Proximity Judgment (PJ) (Kopera-Frye et al., 1996; Jacobson et al., 2003). In EA, a series of problems involving single and/or double digits are displayed on the screen, and the child enters the solution on the computer keypad. In PJ, a double-digit number is displayed on the left side of the screen, and the child presses a button to indicate which of the two double-digit numbers shown on the right side is numerically closer to it. Each child was also assessed on 7 of the 10 subtests from the Wechsler Intelligence Scale for Children, Third Edition (WISC-III)—Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion—and Matrix Reasoning from the WISC-IV. The IQ subtests were selected to represent the four dimensions of the WISC-III: Verbal Comprehension (Similarities), Perceptual Organization (Block Design, Picture Completion, Matrix Reasoning), Freedom from Distractibility (Digit Span, Arithmetic), and Processing Speed (Coding, Symbol Search). Only Similarities were administered in the verbal domain because the other verbal subtests appeared to be less valid in this cross-cultural context. IQ was estimated from these subtests using Sattler's (1992) formula for computing Short Form IQ; validity coefficients for Short Form IQ based on 5 or more subtests consistently exceed r = 0.90. Handedness was assessed with the Annett (1970) Behavioral Handedness Inventory.
2.4. Neuroimaging assessment
2.4.1. Magnetic resonance imaging protocol
All scans were acquired using a 1.5 T Magnetom Symphony MRI Scanner (Siemens Medical Systems, Erlangen, Germany). High-resolution anatomical images were acquired in the sagittal plane using a three-dimensional inversion recovery gradient echo sequence (72 slices, TR = 1900 ms, TE = 3.93 ms, TI = 1100 ms, slice thickness 2 mm, 250 mm field of view, resolution 1.4 × 1.0 × 2 mm3). During the fMRI protocol, 154 functional volumes sensitive to blood oxygen level dependent contrast were acquired with a T2*-weighted gradient echo, echo planar imaging sequence (TR = 2000 ms, TE = 50 ms, 20 interleaved slices, 5 mm thick, gap 1 mm, 230 mm field of view, resolution 3.6 × 3.6 × 5 mm3).
2.4.2. Functional MRI experimental tasks
Simplified versions of the EA and PJ subtests from the computer-based number processing assessment described above were administered. The tasks were simplified to make it easier for the children in the exposed groups to achieve acceptable scores for task accuracy in the scanner. EA was simplified in that the child selected the correct answer from two choices displayed on the screen and the sum was never greater than 12. PJ was simplified by including fewer difficult problems (i.e., problems with response choices that were only 1–2 units apart). EA was selected because it is the easiest of the calculation subtests; PJ was selected to represent magnitude comparison because the range of the neurobehavioral scores for Number Comparison is truncated. Each child practiced these tasks initially in a mock scanner built for this study, which was important in reducing anxiety and facilitating completion of the MRI scans. The experimental tasks were programmed using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) and were presented using a data projector and a rear projection screen mounted at the foot of the patient bed. The child held a Lumitouch response system (Photon Control Inc., Burnaby, Canada) in his/her right hand and responded using the right index and middle finger. The child was able to talk to the examiner using an intercom that is built into the scanner and could stop the scan at any time by squeezing a ball held in his/her left hand.
The EA and PJ tasks were administered using a self-paced block design, in which the child completed as many problems as possible during each 40 s block. In the active blocks, a fixation square was displayed for 500 ms prior to each trial (Fig. 1A). In the EA blocks, two numbers were displayed one above the other for 1000 ms, after which two possible solutions appeared horizontally below the two numbers. The display remained on the screen until either the child made a selection or the 40 s time limit for the block expired. Sums were selected randomly from a list in which the solution was never greater than 12 and from which tie problems (e.g., 2 + 2) and sums involving unity had been excluded. The child selected the correct answer from the two choices displayed below by pressing the button on the same side as the correct answer. The control blocks in the EA task (EA_CTL) followed the same format but with two identical Greek symbols displayed vertically initially for 1000 ms (Fig. 1B). Two different Greek symbols were then displayed horizontally below the vertical symbols and the child selected the one that was identical to the initial two by pressing the button on the side of that symbol. Each block was repeated three times in the following order: EA, EA_CTL, rest, EA, EA_CTL, rest, EA_CTL, EA and rest. In the rest blocks, the fixation square was displayed for 20 s, resulting in a total task duration of 5 min. The PJ task followed the same format, the only difference being that a single number (the “target”) was now displayed for 1000 ms, and the child was instructed to select from two numbers displayed horizontally below it, the one numerically closer to the target. Problems were selected randomly from a list of 1-digit and 2-digit numbers. In the control blocks for the PJ task, the target consisted of a single Greek symbol, followed by two Greek symbols displayed horizontally below it. The child pressed the button on the same side as the symbol that was identical to the target. Each block was repeated three times in the order PJ, PJ control (PJ_CTL), rest, PJ, PJ_CTL, rest, PJ_CTL, PJ and rest, with the fixation square displayed for 20 s during the rest blocks, for a total task duration of 5 min.
Fig. 1.
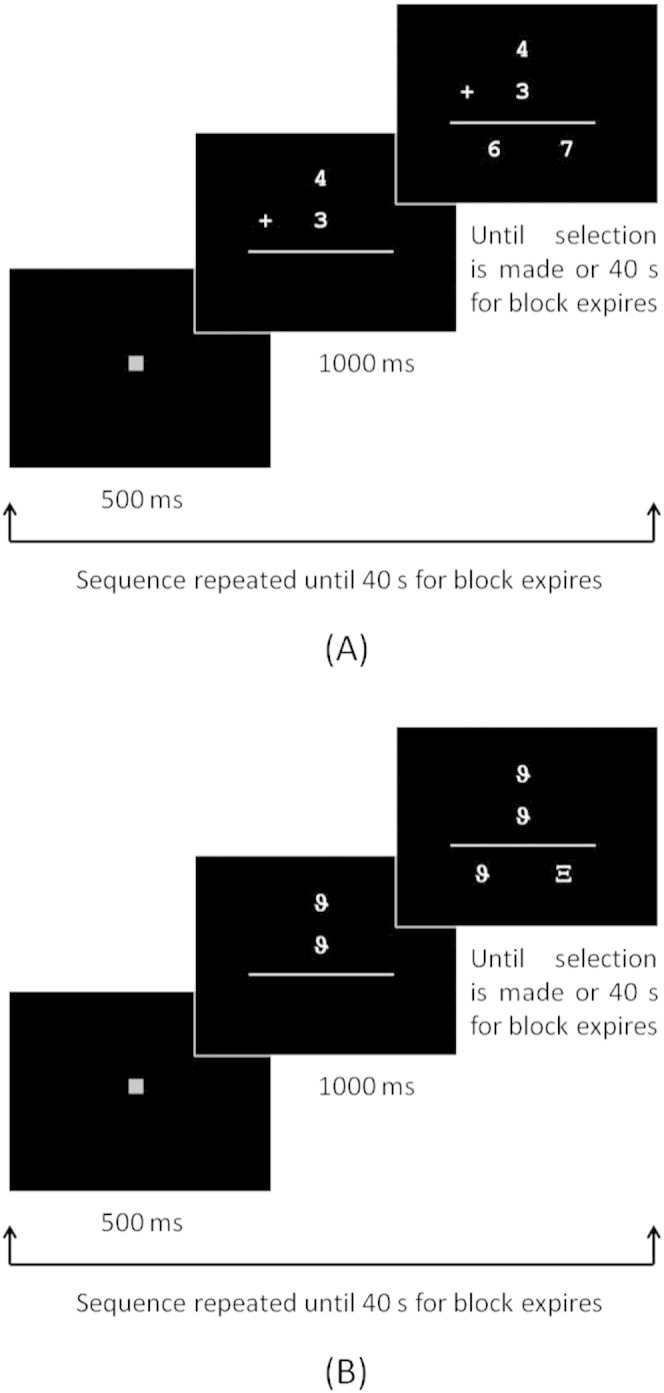
Schematic of task design showing the format and timing for blocks of (A) exact addition trials and (B) control trials. The same format and timing were used for the proximity judgment task.
2.4.3. Behavioral performance
Responses for the number processing tasks administered in the scanner were recorded on a computer. Number of trials attempted, number of trials correct, and accuracy (% correct) were computed for each task. Functional data were excluded for children with poor task performance (<66% correct) to ensure that all those included in the analysis had been mentally engaged in the task. No imaging data were obtained for 6 children (4 FAS/PFAS, 1 HE, 1 control). For PJ, data of 9 children (3 FAS/PFAS, 3 HE, 3 controls) were excluded from the neuroimaging analyses due to poor performance, and another control child fell asleep during the task. For EA, one HE child was not able to perform the task, and data from 19 children (6 FAS/PFAS, 7 HE, 6 controls) were excluded due to poor performance.
2.4.4. fMRI analysis
All fMRI analyses were performed using Brain Voyager QX (Brain Innovation, Maastricht, the Netherlands). Four dummy images were acquired in each run that were excluded from all analyses. Images were motion corrected relative to the first volume of the functional run with trilinear/sinc interpolation. Images were corrected for different slice acquisition times and linear trends, spatially smoothed using a Gaussian filter (FWHM 4 mm), and temporally smoothed with a high pass filter of 2 cycles/point. All data exceeding the movement criterion of a 3 mm displacement or 3.0° rotation within a functional run were rejected. In addition to the previously noted exclusions for poor performance, PJ data for 2 control children and EA data for 1 control child were discarded due to excessive motion. Each child's functional data sets were co-registered to his/her high-resolution anatomical MRI, rotated into the AC–PC plane and normalized to Talairach space using a linear transform calculated on the anatomical images. The 3.6 × 3.6 × 5 mm3 fMRI voxels were interpolated during Talairach normalization to 3 × 3 × 3 mm3.
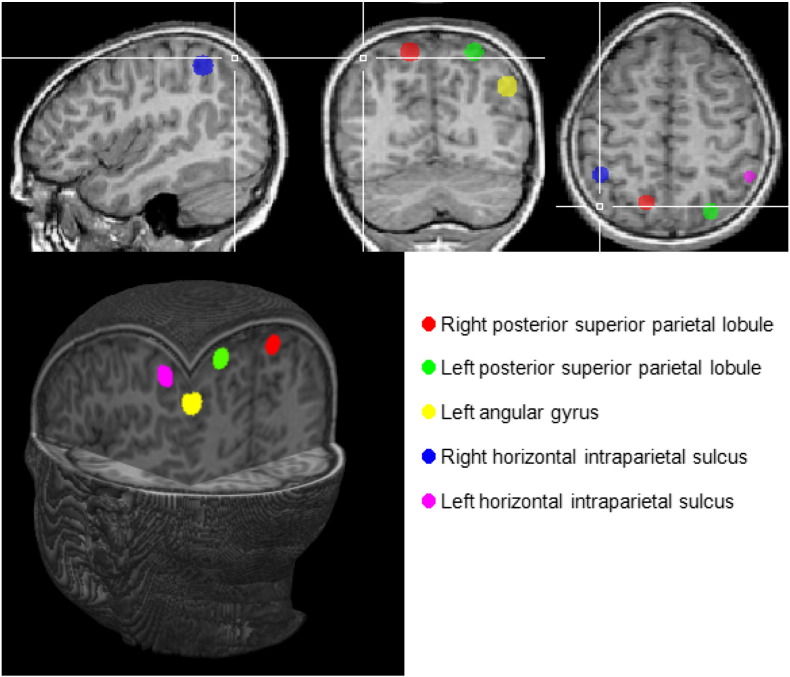
A priori regions of interest (ROIs) were defined for each of the five parietal regions identified in Dehaene et al.'s (2003) meta-analysis, namely bilateral anterior horizontal intraparietal sulcus (IPS), bilateral PSPL and left angular gyrus. Each ROI consisted of a sphere, with a radius of 6 mm, centered on the coordinates derived from the meta-analysis. These regions are illustrated in Fig. 2. Separate subject analyses were performed on the average signal in each ROI using the general linear model with predictors based on the known experimental blocks convolved by the standard hemodynamic function. The six motion correction parameters were z-transformed and then added as predictors of no interest. The beta values generated by this analysis, which reflect the mean percent signal change for each condition for each subject, were used to calculate percent signal change during the numeric task compared to the control task. One outlier with percent signal change values >3 SD beyond the mean for the right and left PSPL and left IPS regions was excluded from analyses of those regions on the PJ task, and one outlier for the left PSPL and right IPS was excluded from analyses of those regions on EA.
Fig. 2.
Regions identified in Dehaene's meta-analysis that were used as regions of interest in this study.
2.4.5. Statistical analyses
All variables were examined for normality of distribution. AA/day and AA/occasion were positively skewed and were log transformed (log X + 1). The following variables with outliers greater than 3 SD beyond the mean were transformed by recoding all outlying values to one point beyond the next most extreme observed value: parity (n = 1), mother's grade (n = 1), lead exposure (n = 1), number of correct EA trials inside the scanner (n = 1), and PJ accuracy inside the scanner (n = 2).
Seven control variables were assessed for consideration as potential confounders of the relation of prenatal alcohol exposure to number processing: three demographic characteristics (parity, and mother's age at delivery and years of education), two child characteristics (child gender and age at assessment), and two neurotoxic exposures (maternal smoking during pregnancy and postnatal lead exposure) that are known to impact on the child's academic performance. Lead exposure, which was based on a venous blood sample obtained from the child, was included because lead levels in this population are within the range in which subtle but meaningful effects on cognitive function have been consistently reported (e.g., Lanphear et al., 2000; Chiodo et al., 2004). Each control variable that was weakly related to a given outcome measure (at p < 0.10) was considered a potential confounder of the effect of each exposure measure on the outcome in question.
The outcome measures were accuracy (% correct) inside and outside the scanner, number of trials attempted and number completed inside the scanner, and percent signal change relative to the control task in each of the ROIs for each of the tasks. The relation of diagnostic group (FAS/PFAS; heavily-exposed (HE) nonsyndromal; control) to each of the outcome measures was examined using analyses of variance (ANOVA). Post-hoc comparisons were computed using the least-squares difference (LSD) approach. Each ANOVA was then rerun as an analysis of covariance including as covariates each of the control variables related to the outcome in question at p < 0.10 to adjust for potential confounding. The relation of two continuous measures of prenatal alcohol exposure—AA/day and AA/drinking day—to each of the outcome measures was examined using Pearson correlation analysis. Multiple regression analyses were then run relating each of the continuous exposure measures to each of the outcomes controling for potential confounders.
Pearson correlation was used to examine the relation of percent signal change in each of the ROIs to behavioral performance inside and outside the scanner.
3. Results
3.1. Sample characteristics
Sample characteristics for the 65 children recruited are summarized in Table 1. The mothers of children with FAS and PFAS reported having consumed an average of 13.2 standard drinks of alcohol per drinking occasion during pregnancy; the mothers of the HE group, 11.8 standard drinks. The groups were generally similar in terms of the control variables, except that the mothers of the HE group smoked more during pregnancy than the mothers of the control or FAS/PFAS children and that on average, the control children's primary caregivers were more educated than either of the other groups. There was very little drug use, with only one mother of an HE child reporting use of marijuana during pregnancy. The FAS/PFAS and HE groups scored more poorly on the WISC IQ test than the control children; and the FAS/PFAS, more poorly than the HE group. On the WISC subtests, the alcohol-exposed children performed more poorly than the controls in both the number processing (i.e., Arithmetic) and verbal (i.e., Similarities) domains. The low IQ scores of all of the children in the sample reflect the highly disadvantaged circumstances and poor quality of education available in this community.
Table 1.
Sample characteristics (N = 65).
| FAS/PFAS (n = 18) |
Heavy exposed (n = 22) |
Control (n = 25) |
F or χ2 | |
|---|---|---|---|---|
| Prenatal alcohol exposure | ||||
| Absolute alcohol/day (oz)a | 2.8 (2.1) | 2.1 (1.9) | 0.0 (0.0) | 19.90*** |
| Absolute alcohol/occasion (oz)a | 6.5 (3.4) | 5.9 (5.4) | 0.1 (0.3) | 21.98*** |
| Frequency (days/week)a | 2.9 (1.2) | 2.6 (1.8) | 0.0 (0.1) | 41.29*** |
| Potential confounders | ||||
| Maternal | ||||
| Parity | 2.6 (1.2) | 2.0 (1.0) | 2.1 (1.4) | 1.29 |
| Years of educationb | 6.7 (2.5) | 6.9 (2.4) | 8.5 (2.0) | 4.27* |
| Smoking during pregnancy (cigs/day)c | 6.6 (5.1) | 12.1 (9.0) | 5.6 (7.1) | 4.99** |
| Mother's age at delivery | 26.3 (5.9) | 24.5 (5.5) | 24.0 (5.8) | 0.92 |
| Child | ||||
| Sex (% male) | 33.3 | 45.5 | 48.0 | 1.00 |
| Age at assessment | 10.1 (1.1) | 10.6 (1.3) | 10.2 (1.2) | 1.09 |
| Blood lead concentration (µg/dL)d | 7.0 (3.6) | 5.2 (1.9) | 6.7 (2.6) | 2.56† |
| IQ scores | ||||
| WISC Estimated Full Scale IQ scoree | 59.8 (10.5) | 67.9 (10.2) | 76.0 (11.0) | 12.25*** |
| Freedom from Distractibilityf | 74.4 (16.5) | 80.6 (10.4) | 87.4 (12.4) | 5.20** |
| Arithmeticg | 4.4 (2.5) | 5.9 (2.6) | 6.9 (2.6) | 4.96** |
| Digit Spanh | 6.3 (3.7) | 7.0 (1.7) | 8.3 (2.9) | 2.80† |
| Codingi | 4.6 (2.3) | 6.5 (2.1) | 6.8 (2.5) | 4.31* |
| Matrix Reasoningj | 3.8 (2.1) | 4.6 (2.4) | 5.4 (2.3) | 2.38 |
| Block Designk | 3.6 (2.1) | 5.5 (2.6) | 6.4 (3.3) | 5.24** |
| Symbol Searchl | 4.0 (2.2) | 4.7 (2.5) | 6.8 (2.9) | 6.86** |
| Similaritiesm | 2.3 (2.0) | 3.7 (2.0) | 5.4 (2.4) | 11.48*** |
| Picture Completionn | 4.7 (3.3) | 5.2 (3.0) | 6.1 (2.9) | 1.07 |
Means (SD).
†p < 0.10; *p < 0.05; **p < 0.01; ***p < 0.001.
Cont < FAS/PFAS, HE, both ps < 0.001.
Cont > FAS/PFAS, p = 0.012; cont > HE, p = 0.020.
HE > cont, p = 0.004; HE > FAS/PFAS, p = 0.022.
FAS/PFAS > HE, p = 0.042; cont > HE, p = 0.077.
Cont > FAS/PFAS, p = 0.001; cont > HE, p = 0.012; HE > FAS/PFAS, p = 0.019.
Cont > FAS/PFAS, p = 0.002; cont > HE, p = 0.082.
Cont > FAS/PFAS, p = 0.003; HE > FAS/PFAS, p = 0.067.
Cont > FAS/PFAS, p = 0.026.
Missing for 4 FAS/PFAS, 1 HE, and 4 controls. cont > FAS/PFAS, p = 0.008; HE > FAS/PFAS, p = 0.017.
Missing for 2 FAS/PFAS, 1 HE, and 3 controls.
Missing for 1 control. cont > FAS/PFAS, p = 0.002; HE > FAS/PFAS, p = 0.039.
Cont > FAS/PFAS, p = 0.001; cont > HE, p = 0.009.
Cont > FAS/PFAS, p < 0.001; cont > HE, p = 0.006; HE > FAS/PFAS, p = 0.051.
Missing for 1 control.
Fifty-nine children were scanned: 14 with FAS or PFAS, 21 HE nonsyndromal and 24 controls. After exclusions due to excessive motion and/or poor accuracy, 49 children with usable data for at least one task remained (47 for PJ; 38 for EA). The children with usable scanner data did not differ from those in the initial sample in terms of alcohol exposure or diagnostic group (all ps > 0.15). The excluded children were 1.1 years younger on average than those included (t(63) = 3.56, p = 0.001), and their estimated IQ scores were lower (t(63) = 2.73, p = 0.008), but they did not differ from the children whose data were included in maternal education, parity, smoking during pregnancy, and age at delivery, or child sex and blood lead concentrations (all ps > 0.10).
3.2. Neuropsychological assessments
Behavioral performance on the number processing tasks is summarized by group and in relation to prenatal alcohol exposure in Tables 2 and 3, respectively. All the children in the sample are included in the analyses of the data collected outside the scanner, but behavioral performance inside the scanner is shown only for those whose neuroimaging data were included in the data analysis. Outside the scanner the FAS/PFAS group performed more poorly than the other two groups on PJ and more poorly than the controls on EA (Table 2). Both continuous measures of prenatal alcohol exposure were related to poorer PJ performance outside the scanner, effects that remained significant after controling for potential confounders (Table 3).
Table 2.
Comparison of behavioral performance on proximity judgment and exact addition by diagnostic group.
| FAS/PFAS | HE | Control | F | Fa | |
|---|---|---|---|---|---|
| Proximity judgment | |||||
| Outside scanner | |||||
| N | 18 | 22 | 25 | ||
| Age | 10.5 (1.0) | 10.6 (1.3) | 10.2 (1.2) | 1.09 | 1.09 |
| Accuracy (% correct)b,c,d | 58.0 (29.8) | 85.8 (14.3) | 88.0 (11.8) | 14.94**f | 12.75** |
| Inside scanner (simplified version) | |||||
| N | 11 | 18 | 18 | ||
| Age | 10.5 (1.0) | 10.9 (1.2) | 10.5 (1.2) | 0.66 | 0.66 |
| Trials attemptedb | 33.9 (5.6) | 38.9 (8.2) | 39.3 (6.1) | 2.42†,g | 2.35 |
| Trials correct b | 29.1 (4.4) | 32.9 (7.6) | 35.2 (5.2) | 3.40*,h | 4.56* |
| Accuracy (% correct)b,e | 86.3 (8.6) | 84.3 (9.1) | 90.1 (9.9) | 1.73 | 2.17 |
| Exact addition | |||||
| Outside scanner | |||||
| N | 18 | 22 | 25 | ||
| Age | 10.5 (1.0) | 10.6 (1.3) | 10.2 (1.2) | 1.09 | 1.09 |
| Accuracy (% correct)b,c | 66.3 (33.8) | 81.8 (29.2) | 87.8 (19.5) | 3.30*,i | 2.63† |
| Inside scanner (simplified version) | |||||
| N | 8 | 13 | 17 | ||
| Age | 10.6 (1.0) | 11.2 (1.2) | 10.5 (1.1) | 1.57 | 1.57 |
| Trials attempted | 28.8 (7.7) | 34.5 (7.3) | 30.4 (8.7) | 1.56 | 1.56 |
| Trials correct b | 24.4 (7.4) | 30.5 (7.6) | 27.1 (9.0) | 1.47 | 0.98 |
| Accuracy (% correct)b,e | 84.7 (12.1) | 88.2 (9.8) | 88.3 (7.4) | 0.45 | 0.56 |
Values are means (standard deviation).
† p < 0.10; * p < 0.05; ** p < 0.001.
Adjusted for potential confounders.
Controlled for child's age.
Controlled for mother's age.
Controlled for lead exposure.
Controlled for mother's smoking.
Cont, HE > FAS/PFAS, both ps < 0.001.
Cont > FAS/PFAS, p = 0.047; HE > FAS/PFAS, p = 0.062.
Cont > FAS/PFAS, p = 0.012.
Cont > FAS/PFAS, p = 0.014; HE > FAS/PFAS, p = 0.079.
Table 3.
Relation of degree of prenatal alcohol exposure to behavioral performance on proximity judgment and exact addition.
| N | Absolute alcohol per day r |
Absolute alcohol per day β |
Absolute alcohol per occasion r |
Absolute alcohol per occasion β |
|
|---|---|---|---|---|---|
| Proximity judgment | |||||
| Outside scanner | |||||
| Accuracy (% correct)a,b,c | 65 | −0.39**** | −0.36*** | −0.33** | −0.32*** |
| Inside scanner (simplified version) | |||||
| Trials attempteda | 47 | −0.13 | −0.12 | −0.15 | −0.16 |
| Trials correcta | 47 | −0.30* | −0.29* | −0.30* | −0.31* |
| Accuracy (% correct)a,d | 47 | −0.32* | −0.35* | −0.29* | −0.31† |
| Exact addition | |||||
| Outside scanner | |||||
| Accuracy (% correct)a,b | 65 | −0.22† | −0.18 | −0.17 | −0.15 |
| Inside scanner (simplified version) | |||||
| Trials attempted | 38 | 0.16 | 0.16 | 0.10 | 0.10 |
| Trials correcta | 38 | 0.05 | 0.05 | 0.03 | −0.01 |
| Accuracy (% correct)a,d | 38 | −0.21 | −0.37† | −0.15 | −0.26 |
† p < 0.10; * p < 0.05; ** p < 0.01; *** p < 0.005; **** p < 0.001.
Controlled for child's age.
Controlled for mother's age.
Controlled for lead exposure.
Controlled for mother's smoking.
By contrast, no group differences in performance accuracy were seen when simplified versions of the tasks were administered inside the scanner (Table 2). The only differences between the groups on either task was that in PJ the FAS/PFAS group attempted fewer trials, reflecting a slower rate of completing the PJ problems, and got fewer trials correct than those of the control group. AA/day and AA/occasion were inversely correlated with the number of PJ trials completed correctly and accuracy inside the scanner (Table 3).
3.3. Neuroimaging assessments
The diagnostic groups showed few differences in terms of activation of the five parietal ROIs. On the PJ task, the groups differed only in the left angular gyrus (F(2,44) = 6.93, p = 0.002, means ± standard deviation (Ms ± SD) = 0.17 ± 0.11, −0.02 ± 0.17 and 0.00 ± 0.11 for the FAS/PFAS, HE and control groups, respectively), with more activation in the FAS/PFAS group than in either the HE (p = 0.001) or control groups (p = 0.003; see Fig. 3). On the EA task, none of the ROIs showed significant group differences, but the effect on the right IPS fell just short of significance (F(2,34) = 2.96, p = 0.065), with the controls showing more activation than the HE group (p = 0.020) (Ms ± SD = 0.03 ± 0.10, −0.03 ± 0.15, and 0.07 ± 0.08 for the FAS/PFAS, HE and control groups, respectively). When combining the FAS/PFAS and HE groups to compare activation in exposed children to unexposed controls, controls showed more activation relative to exposed children in the right IPS during EA (t(35) = 2.06, p = 0.47; Ms ± SD = 0.00 ± 0.13 and 0.07 ± 0.08 for the exposed and control groups, respectively).
Fig. 3.
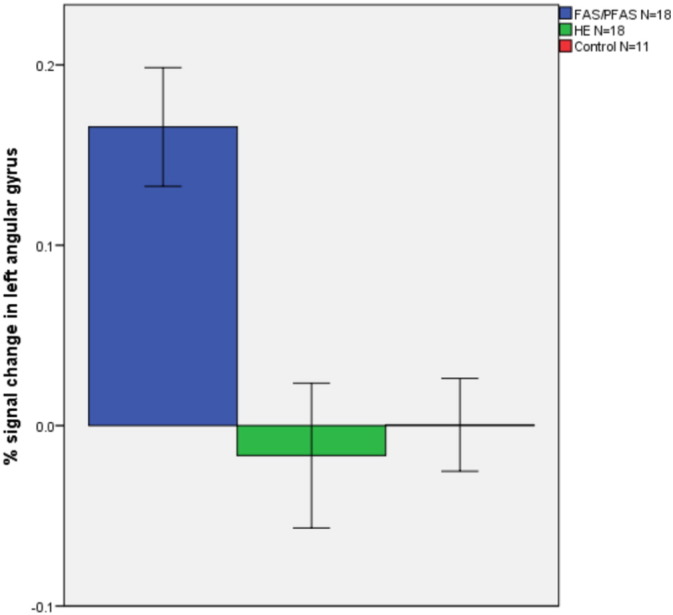
Percent signal change during proximity judgment compared to the control task in the left angular gyrus in each of the diagnostic groups (N = 47). Values are means ± standard error.
By contrast, increasing AA/day and AA/drinking day were both related to lower percent signal change relative to the control task in the right IPS on both the PJ and EA tasks (Table 4 and Fig. 4), effects that remained significant after controling for potential confounders. Since the children in the FAS/PFAS group performed fewer trials during PJ than the HE and control children, we also examined whether the effects of alcohol on activation during PJ remained significant after controling for the number of trials attempted. All differences and associations remained essentially unchanged (βs relating AA/day and AA/occasion to activation of right IPS were −0.35 and −0.36, respectively; both ps < 0.02). In addition, after controling for the confounding influences of maternal smoking during pregnancy and age at delivery, greater AA/day was related to reduced activation in the left PSPL during EA, suggesting that these control variables were suppressors. The relation of both continuous measures of prenatal alcohol exposure to reduced percent signal change in the left angular gyrus on the EA task fell short of statistical significance (Table 4).
Table 4.
Relation of extent of prenatal alcohol exposure to the percent signal change compared to a control task in the a priori number processing regions of interest during (a) proximity judgment and (b) exact addition.
| N | Talairach coordinates | Absolute alcohol per day r |
Absolute alcohol per day β |
Absolute alcohol per occasion r |
Absolute alcohol per occasion β |
|
|---|---|---|---|---|---|---|
| (a) Proximity judgment | ||||||
| R posterior superior parietal lobule | 46 | 15, –63, 56 | 0.06 | 0.06 | 0.12 | 0.12 |
| L posterior superior parietal lobulea | 46 | −22, −68, 56 | −0.08 | −0.02 | −0.13 | −0.06 |
| L angular gyrus | 47 | −41, −66, 36 | 0.17 | 0.17 | 0.18 | 0.18 |
| R horizontal intraparietal sulcus | 47 | 41, −47, 48 | −0.36* | −0.36* | −0.37** | −0.37** |
| L horizontal intraparietal sulcus | 46 | −44, −48, 47 | −0.13 | −0.13 | −0.16 | −0.16 |
| (b) Exact addition | ||||||
| R posterior superior parietal lobuleb | 38 | 15, −63, 56 | 0.25 | 0.02 | 0.21 | 0.01 |
| L posterior superior parietal lobuleb,c | 37 | −22, −68, 56 | −0.12 | −0.36* | −0.12 | −0.32† |
| L angular gyrus | 38 | −41, −66, 36 | −0.29† | −0.29† | −0.31† | −0.31† |
| R horizontal intraparietal sulcusc,d | 37 | 41, −47, 48 | −0.48*** | −0.49*** | −0.43** | −0.46*** |
| L horizontal intraparietal sulcus | 38 | −44, −48, 47 | −0.05 | −0.05 | 0.04 | 0.04 |
p < 0.10.
p < 0.05,
p < 0.01.
p < 0.005.
Controlled for mother's grade.
Controlled for maternal smoking.
Controlled for mother's age.
Controlled for parity.
Fig. 4.
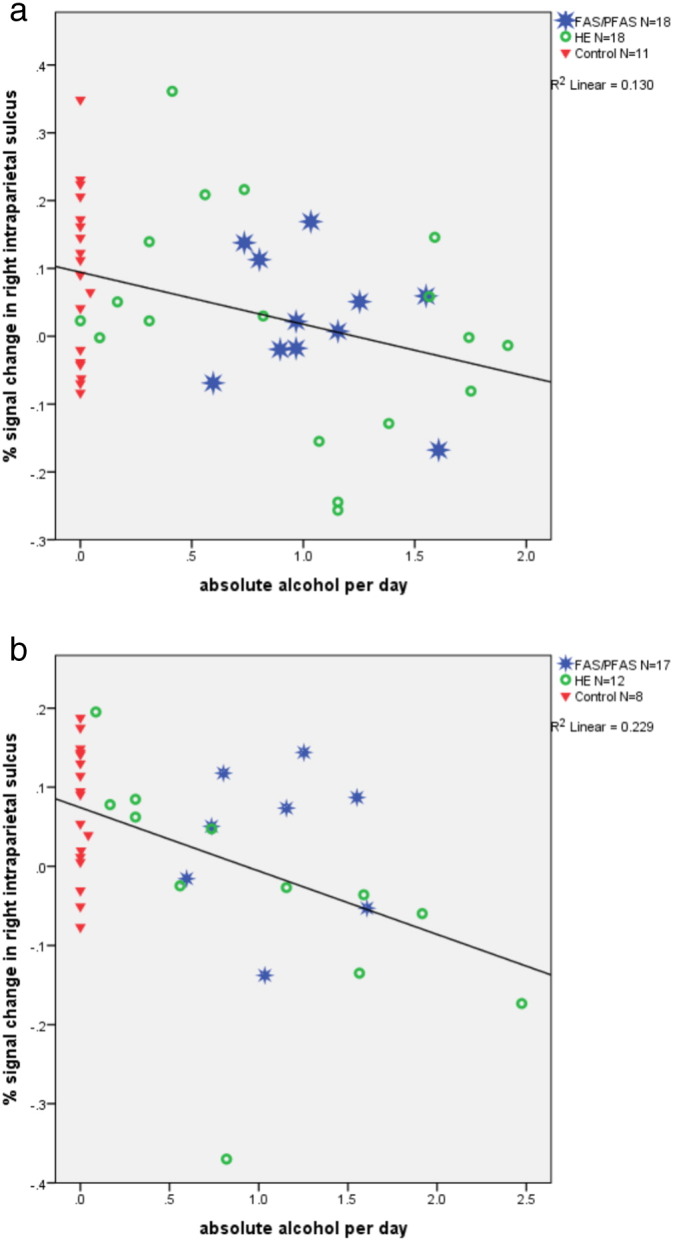
Relation of absolute alcohol/day to percent signal change in the right horizontal intraparietal sulcus for PJ (a) and EA (b), respectively.
Greater percent signal change in the right IPS was related to better EA performance outside the scanner (r = 0.35, p = 0.033). For PJ, greater percent signal change in the right PSPL was associated with poorer accuracy outside the scanner, r = −0.31, p = 0.037, and greater signal change in the left PSPL was associated with completion of fewer problems correctly inside the scanner, r = −0.35, p = 0.016. As can be seen in Fig. 5, the inverse relation between the left PSPL activation and the number of correct PJ trials was strongest in the FAS/PFAS group (r = −0.76, p = 0.007, for FAS/PFAS, compared with r = −0.40, p = 0.113, for HE and r = −0.31, p = 0.214 for controls).
Fig. 5.
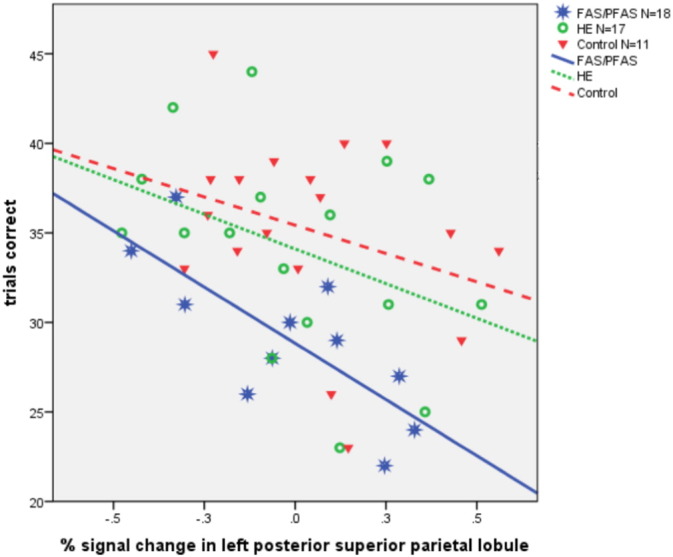
Relation of percent signal change in the left posterior superior parietal lobule during proximity judgment to the number of sums answered correctly inside the scanner (FAS/PFAS: R2 = 0.57; HE: R2 = 0.16; control: R2 = 0.10).
4. Discussion
This study examined the relation of FASD diagnosis and continuous measures of prenatal alcohol exposure to activation of the five parietal regions identified by Dehaene et al. (2003) as most critical for number processing, using tasks involving simple addition and number comparison. Despite generally similar behavioral performance between diagnostic groups on the simplified tasks administered inside the scanner, the effects of prenatal exposure were observed in altered patterns of brain activation. Greater prenatal alcohol exposure was related to less activation in the right IPS during both the PJ and EA tasks and to less left PSPL activation during EA. In addition, the FAS/PFAS group activated the left angular gyrus more than the other groups in the PJ task.
When more challenging versions of the number processing tasks were administered outside the scanner, FASD diagnosis was associated with poorer performance on both EA and PJ, and degree of prenatal alcohol exposure was associated with poorer performance on PJ. While neither measure of alcohol exposure was related to performance on the simpler EA task administered in the scanner, the children with FAS or PFAS performed the PJ task more slowly than the other groups in the scanner, and the continuous measures of exposure were associated with less accurate in-scanner PJ performance. These findings suggest that these heavily exposed children found the magnitude comparison processing required for PJ more challenging than simple addition problems that could be solved by rote memory. These findings are also consistent with the higher sensitivity of magnitude comparison to prenatal alcohol exposure seen in our Detroit longitudinal cohort (J. Jacobson et al., 2011).
This study is the first to demonstrate a direct effect of prenatal alcohol exposure on activation of the IPS during number processing. The bilateral IPS have been repeatedly linked to nonverbal representation of quantity (Dehaene et al., 2003). This region is activated when numbers are read even when no arithmetic manipulation is required (Eger et al., 2003), but activation is greater when comparing numbers (Chochon et al., 1999) or performing calculations (Burbaud et al., 1999; Chochon et al., 1999; Pesenti et al., 2000) and often larger on the right (Rosselli and Ardila, 1989; Dehaene and Cohen, 1996; Langdon and Warrington, 1997; Chochon et al., 1999; Pinel et al., 2001). This area is more active when manipulating large numbers (Kiefer and Dehaene, 1997; Stanescu-Cosson et al., 2000), performing more complex arithmetic manipulations (e.g., with 3 rather than 2 operands; Rivera et al., 2002), or comparing numbers separated by a small numerical distance (Dehaene and Cohen, 1996; Pinel et al., 2001). It is well-established that numbers closer together (e.g., 2 and 3) are more difficult (i.e., take longer) to compare than numbers further apart (e.g., 2 and 9) (Moyer and Landauer, 1967). Thus, the observed effect of prenatal alcohol exposure on poorer recruitment of the IPS provides evidence of a fetal alcohol-related deficit in mental representation and manipulation of quantity, which is consistent with the behavioral evidence from our Detroit study suggesting a specific effect of prenatal alcohol on magnitude comparison (J. Jacobson et al., 2011).
Although our continuous measure of prenatal alcohol exposure showed a dose-dependent decrease in activation of the right IPS during both tasks, diagnostic group differences were only seen in this region when all alcohol exposed children were combined into one group and compared to unexposed controls, a result that seems to be driven by the reduced activation in this region during EA in the HE children specifically. This finding is consistent with our previous study, in which children with FAS or PFAS from this cohort were compared with healthy controls using a whole brain, voxelwise approach (Meintjes et al., 2010b). Although only the control children showed significant activation of the IPS in that study, the between-group difference was not significant. The only other previous study to examine number processing in relation to fetal alcohol exposure also found an alcohol exposure-dependent response in a right inferior parietal region that included the IPS, with controls showing the most activity, during a subtraction task (Santhanam et al., 2009). The finding that our continuous measures of prenatal alcohol exposure were more sensitive than diagnosis in detecting effects on brain function is consistent with our findings in several other neuroimaging studies (De Guio et al., 2014; du Plessis et al., 2014; Meintjes et al., 2014).
The poorer activation of the right IPS seen in the alcohol-exposed children in this study during number processing is also seen in children with developmental dyscalculia (DD) (Kucian et al., 2006; Price et al., 2007; Kaufmann et al., 2009a; Kaufmann et al., 2009b; Rubinsten and Henik, 2009; Mussolin et al., 2010; Ashkenazi et al., 2012) and poor arithmetical fluency (De Smedt et al., 2011). DD is a specific learning disability believed to be genetic in origin, which is characterized by impairment in the processing of numerical and arithmetical information in individuals with normal intelligence. In DD, activations of the bilateral IPS also fail to exhibit the increased response to differences in numerical distance seen in normal control children (Mussolin et al., 2010). A voxel-based morphometry study found less gray matter density in the left IPS in low birthweight children with DD, compared with healthy controls (Isaacs et al., 2001). Impaired recruitment of the IPS during tasks involving number processing has also been found in Turner syndrome (TS), a genetic disorder involving a chromosomal defect, in which math is an area where deficits are commonly noted (Molko et al., 2003). Similarly, in a study of children with Fragile X syndrome, another form of mental retardation, Rivera et al. (2002) found that, although the right IPS was activated by healthy controls during 3-operand arithmetic problems, it was not activated by Fragile X patients presented with those problems.
In the PJ task, we found greater activation in the left angular gyrus in the FAS/PFAS group than either the HE or control groups. The angular gyrus is adjacent to the perisylvian language processing network and is associated with the verbal processing of numbers (Dehaene et al., 2003). In typically developing children, it is activated more during addition and multiplication than during subtraction, presumably because addition and multiplication facts are more likely to be retrieved from long-term memory (Stanescu-Cosson et al., 2000; Simon et al., 2002; Delazer et al., 2005). The increased activation of the angular gyrus suggests that children with FAS and PFAS may rely on verbal recitation of the numbers and/or verbally mediated subtraction operations to solve the PJ problems, instead of the type of nonverbal quantity processing that has been shown to be mediated by the right anterior IPS (Chochon et al., 1999). Verbal mediation may provide a compensatory strategy for these children, whose ability to activate the IPS appears to be impaired. This finding is consistent with results from our previous whole brain voxelwise analysis, in which there was a significant group difference in the same region of the left angular gyrus [−42,−65,36], with greater activation in the FAS/PFAS group than the controls (Meintjes et al., 2010b). We added the HE group to the analyses in the present study and found that, although this group was exposed at similar levels to the FAS/PFAS group, the HE children did not appear to rely on the angular gyrus to perform the PJ task. By contrast, on the EA task, in which verbal recall of number facts is presumed to be the most efficient strategy, our data suggest less activation of the angular gyrus by the more heavily exposed children (Table 4).
Lower levels of activation in the right IPS was related to poorer EA performance outside the scanner. By contrast, Price et al. (2013) reported greater activation in the right IPS during single digit arithmetic calculation in adolescents with lower math scores. The authors note that their findings suggest that, while IPS-mediated quantity processing mechanisms appear to play an important role in the development of elementary arithmetic skills, individuals who continue to rely on them in adolescence may achieve poorer mathematical competence than their peers who do not. A large body of behavioral research has shown that children typically undergo a process of development in arithmetic skill whereby simple calculations are initially computed through procedural strategies, but then gradually come to be solved by memory retrieval (Geary et al., 1991; Ashcraft, 1982). Development beyond quantity-based calculation strategies may be essential for the development of more advanced mathematical competence.
In the PJ task, greater activation of the right PSPL was associated with worse performance outside the scanner, and greater activation of the left PSPL was associated with fewer trials correct inside the scanner. The PSPL, which is activated during counting (Piazza et al., 2002) and a variety of visual–spatial tasks, is believed to support the engagement of attention during number processing (Pinel et al., 2001; Dehaene et al., 2003). The greater activation of these regions by children with less optimal PJ performance suggests engagement of increased attention to compensate for their poorer facility in magnitude comparison. As shown in Fig. 5, the inverse relation between the left PSPL activation and PJ performance was strongest in the FAS/PFAS group, which appeared to find the PJ task particularly challenging. By contrast, in the EA task prenatal alcohol exposure was related to less activation of the left PSPL, a region that may facilitate attentional engagement required for simple addition at this age but may no longer be needed by children who have mastered simple proximity judgment.
Since working memory and attention, which are also mediated, in part, by the parietal lobes (e.g., Ravizza et al., 2004; Diwadkar et al., 2013), contribute substantially to number processing performance, impairments in these domains may play a role in the number processing deficits observed in FASD. Moreover, two studies have reported reduced activation in working memory tasks in individuals with FASD compared to controls in posterior parietal regions close to the PSPL ROI used in our study (Astley et al., 2009; Gautam et al., 2014). The specific section of the IPS found to mediate the effects of prenatal alcohol exposure on number processing in this study has, however, not been implicated in working memory or attention per se.
One limitation of this study was that the maternal report of drinking during pregnancy was obtained retrospectively several years after the child's birth. Nevertheless, the validity of these reports is supported by our findings that they are predictive of neuroimaging and neurobehavioral outcomes (Meintjes et al., 2010b; J. Jacobson et al., 2011; S. Jacobson et al., 2011a). Predictive validity for childhood IQ was r = −0.36 and −0.40, for AA/day and AA/occasion, respectively, both ps < 0.001. A second limitation was the relatively small size of the FAS/PFAS and HE groups and the greater variability in performance accuracy outside the scanner in the FAS/PFAS group. Not surprisingly, the children who were excluded due to poor performance were younger and had lower IQ scores. Presumably, performance on this test will improve with age, and we assume that the fetal alcohol-related patterns of brain activation seen in this study will also be manifest once these younger children acquire sufficient math skills to perform these tasks. With regard to IQ, although proportionately more low IQ children were unable to complete the neuroimaging tasks, the sample on whom the neuroimaging data were obtained did include a substantial number of children with low IQs (<70). Thus, the results can be generalized to all but the most severely retarded children.
Because the children were socioeconomically and educationally deprived, we cannot determine the degree to which the results would hold for children from an educationally less deprived background. Moreover, due to the poor educational background of the children, we had to use very easy problems to ensure that they would be able to perform them. If more difficult problems could have been used, a more pronounced group difference might have been seen. The need to use easy problems also precluded the use of a potentially more powerful parametric design to examine the effect of increasing difficulty on the children's brain activations. The age range of the children—7.9 to 13.4 years—was relatively large, particularly in light of the changes in number processing strategy that may emerge during this period. A smaller age range might have yielded stronger associations. Nevertheless, it is interesting, that, although all the behavioral performance measures were related to age, suggesting an age-dependent effect of education, none of the brain activations were even weakly related to age but instead showed associations with maternal factors (age, education, smoking, and parity). We did not control for multiple comparisons, due to the fact that we examined only a limited number of regions rather than the whole brain. Region-of-interest analyses are advantageous in that multiple comparisons are much less of an issue and SNR is increased by averaging across the voxels in a region.
5. Conclusions
This study demonstrates poor recruitment of the right IPS during number processing tasks by children with heavy prenatal alcohol exposure, confirming our previous report based on behavioral data of a fetal alcohol-related deficit in mental representation and manipulation of quantity (J. Jacobson et al., 2011). Our neuroimaging data also indicated increased activation of the angular gyrus in the FAS/PFAS group during the proximity judgment task, suggesting that these children may use a verbal mediation strategy to compensate for impairment in magnitude comparison. By contrast, prenatal alcohol exposure was related to lower levels of activation of the angular gyrus during the exact addition task, for which verbal mediation to retrieve addition facts is presumed to be an efficient strategy. Although activation of the left PSPL, which is believed to support attentional engagement during number processing, was increased in children with less optimal PJ performance, particularly in the FAS/PFAS group, prenatal alcohol was associated with lower levels of activation of this region during the EA task, suggesting less capacity to devote attentional resources to the verbal retrieval of addition facts required for simple addition at this age. In summary, these data demonstrate that prenatal alcohol exposure alters activation of each of the elements of the parietal network known to be critical for number processing, providing additional evidence for a specific fetal alcohol-related deficit in the core quantity system for representation and manipulation of quantity identified by Dehaene et al. (2003). These findings suggest that remediation focused on the core quantity concepts might be particularly effective in children with FASD who exhibit difficulty with mathematical processing.
Acknowledgments
This research was funded by a Fogarty International Research Collaboration Award from the National Institutes of Health (R03 TW007030), a Focus Area grant (FA2005040800024) from the National Research Foundation of South Africa, the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa, the Medical Research Council of South Africa, a Children's Bridge grant from the Office of the President of Wayne State University (WSU), and the Joseph Young, Sr., Fund from the State of Michigan. Data analysis was funded, in part, by R01 AA06781 from the National Institute on Alcohol Abuse and Alcoholism. The dysmorphology assessments were conducted in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders U01-AA014790 and U24AA014815. We thank H.E. Hoyme, L.K. Robinson, and N. Khaole, who performed the dysmorphology examination of the children, and Stanislas Dehaene, for his consultation on the development of the number processing assessment tasks. We also thank M. September, N. Dodge, and our UCT and WSU research staff, for their work on this project, and the staff at the Cape Universities Brain Imaging Centre for their contributions to the acquisition of the neuroimaging data. We thank the mothers and children who have participated in our Cape Town research study.
Contributor Information
E.M. Meintjes, Email: ernesta.meintjes@gmail.com.
J.L. Jacobson, Email: joseph.jacobson@wayne.edu.
References
- Annett M. A classification of hand preference by association analysis. Br. J. Psychol. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Archibald S.L., Fennema-Notestine C., Gamst A., Riley E.P., Mattson S.N., Jernigan T.J. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev. Med. Child Neurol. 2001;43(3):148–154. [PubMed] [Google Scholar]
- Ashcraft M.H. The development of mental arithmetic: A chronometric approach. Dev. Rev. 1982;2(3):213–236. [Google Scholar]
- Ashkenazi S., Rosenberg-Lee M., Tenison C., Menon V. Weak task-related modulation and stimulus representations during arithmetic problem solving in children with developmental dyscalculia. Dev. Cogn. Neurosci. 2012;2:S152–S166. doi: 10.1016/j.dcn.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley S.J., Aylward E.H., Olson H.C., Kerns K., Brooks A., Coggins T.E., Davies J., Dorn S., Gendler B., Jirikowic T., Kraegal P., Maravilla K., Richards T. Functional magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. J. Neurodev. Disord. 2009;1(1):61–80. doi: 10.1007/s11689-009-9004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley S.J., Clarren S.K. Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol Alcohol. 2001;36(2):147–159. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Bowman R.S., Stein L.I., Newton J.R. Measurement and Interpretation of Drinking Behavior. J. Stud. Alcohol Drugs. 1975;36(09):1154. doi: 10.15288/jsa.1975.36.1154. [DOI] [PubMed] [Google Scholar]
- Burbaud P., Camus O., Guehl D., Bioulac B., Caillé J.M., Allard M. A functional magnetic resonance imaging study of mental subtraction in human subjects. Neurosci. Lett. 1999;273(3):195–199. doi: 10.1016/s0304-3940(99)00641-2. [DOI] [PubMed] [Google Scholar]
- Burden M.J., Jacobson S.W., Sokol R.J., Jacobson J.L. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol. Clin. Exp. Res. 2005;29(3):443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Burden M.J., Jacobson S.W., Jacobson J.L. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol. Clin. Exp. Res. 2005;29(8):1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Chen X., Coles C.D., Lynch M.E., Hu X. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum. Brain Mapp. 2012;33(7):1663–1676. doi: 10.1002/hbm.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo L.M., Jacobson S.W., Jacobson J.L. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol. Teratol. 2004;26(3):359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chochon F., Cohen L., Van De Moortele P.F., Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. J. Cogn. Neurosci. 1999;11(6):617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- Coles C.D., Brown R.T., Smith I.E., Platzman K.A., Erickson S., Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol. Teratol. 1991;13(4):357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Coles C.D., Platzman K.A., Raskind‐Hood C.L., Brown R.T., Falek A., Smith I.E. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol. Clin. Exp. Res. 1997;21(1):150–161. [PubMed] [Google Scholar]
- Coles C.D., Platzman K.A., Lynch M.E., Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol. Clin. Exp. Res. 2002;26(2):263–271. [PubMed] [Google Scholar]
- De Guio F., Mangin J.F., Riviere D., Perrot M., Molteno C.D., Jacobson S.W., Meintjes E.M., Jacobson J.L. A study of cortical morphology in children with fetal alcohol spectrum disorders. Hum. Brain Mapp. 2014;35(5):2285–2296. doi: 10.1002/hbm.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt B., Holloway I.D., Ansari D. Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. Neuroimage. 2011;57(3):771–781. doi: 10.1016/j.neuroimage.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Varieties of numerical abilities. Cognition. 1992;44(1):1–42. doi: 10.1016/0010-0277(92)90049-n. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. Towards an anatomical and functional model of number processing. Math. Cogn. 1996;1:83–120. [Google Scholar]
- Dehaene S., Piazza M., Pinel P., Cohen L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003;20(3):487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Delazer M., Ischebeck A., Domahs F., Zamarian L., Koppelstaetter F., Siedentopf C.M., Kaufmann L., Benke T., Felber S. Learning by strategies and learning by drill—Evidence from an fMRI study. Neuroimage. 2005;25(3):838–849. doi: 10.1016/j.neuroimage.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Diwadkar V.A., Meintjes E.M., Goradia D., Dodge N.C., Warton C., Molteno C.D., Jacobson S.W., Jacobson J.L. Differences in cortico-striatal-cerebellar activations during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum. Brain Mapp. 2013;34(8):1931–1945. doi: 10.1002/hbm.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis L., Jacobson J.L., Jacobson S.W., Hess A.T., van der Kouwe A.J.W., Avison M.J., Molteno C.D., Stanton M.E., Stanley J.A., Peterson B.S., Meintjes E.M. An in vivo 1H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with feta alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2014;38(5):1330–1338. doi: 10.1111/acer.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E., Sterzer P., Russ M.O., Giraud A.L., Kleinschmidt A. A supramodal number representation in human intraparietal cortex. Neuron. 2003;37(4):719–726. doi: 10.1016/s0896-6273(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Gautam P., Nuñez S.C., Narr K.L., Mattson S.N., May P.A., Adnams C.M., Riley E.P., Jones K.L., Kan E.C., Sowell E.R. Developmental trajectories for visuo-spatial attention are altered by prenatal alcohol exposure: A longitudinal FMRI study. Cereb. Cortex. 2014;162 doi: 10.1093/cercor/bhu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary D.C., Brown S.C., Samaranayake V. Cognitive addition: A short longitudinal study of strategy choice and speed-of-processing differences in normal and mathematically disabled children. Dev. Psychol. 1991;27(5):787–797. [Google Scholar]
- Goldschmidt L., Richardson G.A., Stoffer D.S., Geva D., Day N.L. Prenatal alcohol exposure and academic achievement at age six: a nonlinear fit. Alcohol. Clin. Exp. Res. 1996;20(4):763–770. doi: 10.1111/j.1530-0277.1996.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Henschen S.E. Über sprach-, musik-und rechenmechanismen und ihre lokalisationen im großhirn. Z. f. d. g. Neur. u. Psych. 1919;52(1):273–298. [Google Scholar]
- Howell K.K., Lynch M.E., Platzman K.A., Smith G.H., Coles C.D. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J. Pediatr. Psychol. 2006;31(1):116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Hoyme H.E., May P.A., Kalberg W.O., Kodituwakku P., Gossage J.P., Trujillo P.M., Buckley D.G., Miller J.H., Aragon A.S., Khaole N., Viljoen D.L., Jones K.L., Robinson L.K. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs E.B., Edmonds C.J., Lucas A., Gadian D.G. Calculation difficulties in children of very low birthweight: A neural correlate. Brain. 2001;124(9):1701–1707. doi: 10.1093/brain/124.9.1701. [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Jacobson J.L., Sokol R.J., Martier S.S., Ager J.W. Prenatal alcohol exposure and infant information processing ability. Child Dev. 1993;64(6):1706–1721. [PubMed] [Google Scholar]
- Jacobson S.W., Jacobson J.L., Sokol R.J. Effects of fetal alcohol exposure on infant reaction time. Alcohol. Clin. Exp. Res. 1994;18(5):1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Chiodo L.M., Sokol R.J., Jacobson J.L. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Dodge N., Dehaene S., Chiodo L.M., Sokol R.J., Jacobson J.L. Evidence for a specific effect of prenatal alcohol exposure on ‘number sense. Alcohol. Clin. Exp. Res. 2003;27:A121. [Google Scholar]
- Jacobson S.W., Jacobson J.L., Sokol R.J., Chiodo L.M., Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5‐year intellectual function. Alcohol. Clin. Exp. Res. 2004;28(11):1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Stanton M.E., Molteno C.D., Burden M.J., Fuller D.S., Hoyme H.E., Robinson L.K., Khaole N., Jacobson J.L. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol. Clin. Exp. Res. 2008;32(2):365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson J.L., Dodge N.C., Burden M.J., Klorman R., Jacobson S.W. Number processing in adolescents with prenatal alcohol exposure and ADHD: differences in the neurobehavioral phenotype. Alcohol. Clin. Exp. Res. 2011;35(3):431–442. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.W., Jacobson J.L., Stanton M.E., Meintjes E.M., Molteno C.D. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychol. Rev. 2011;21(2):148–166. doi: 10.1007/s11065-011-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.W., Stanton M.E., Dodge N.C., Pienaar M., Fuller D.S., Molteno C.D., Meintjes E.M., Hoyme H.E., Robinson L.K., Khaole N., Jacobson J.L. Impaired delay and trace eyeblink conditioning in school‐age children with fetal alcohol syndrome. Alcohol. Clin. Exp. Res. 2011;35(2):250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann L., Vogel S.E., Starke M., Kremser C., Schocke M. Numerical and non-numerical ordinality processing in children with and without developmental dyscalculia: Evidence from fMRI. Cogn. Dev. 2009;24(4):486–494. [Google Scholar]
- Kaufmann L., Vogel S.E., Starke M., Kremser C., Schocke M., Wood G. Developmental dyscalculia: compensatory mechanisms in left intraparietal regions in response to nonsymbolic magnitudes. Behav. Brain Funct. 2009;5(1):35. doi: 10.1186/1744-9081-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann L., Wood G., Rubinsten O., Henik A. Meta-analyses of developmental fMRI studies investigating typical and atypical trajectories of number processing and calculation. Dev. Neuropsychol. 2011;36(6):763–787. doi: 10.1080/87565641.2010.549884. [DOI] [PubMed] [Google Scholar]
- Kerns K.A., Don A., Mateer C.A., Streissguth A.P. Cognitive deficits in nonretarded adults with fetal alcohol syndrome. J. Learn. Disabil. 1997;30(6):685–693. doi: 10.1177/002221949703000612. [DOI] [PubMed] [Google Scholar]
- Kiefer M., Dehaene S. The time course of parietal activation in single-digit multiplication: Evidence from event-related potentials. Math. Cogn. 1997;3(1):1–30. [Google Scholar]
- Kodituwakku P.W., Handmaker N.S., Cutler S.K., Weathersby E.K., Handmaker S.D. Specific impairments in self‐regulation in children exposed to alcohol prenatally. Alcohol. Clin. Exp. Res. 1995;19(6):1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kopera-Frye K., Dehaene S., Streissguth A.P. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34(12):1187–1196. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Kucian K., Loenneker T., Dietrich T., Dosch M., Martin E., Von Aster M. Impaired neural networks for approximate calculation in dyscalculic children: a functional MRI study. Behav. Brain Funct. 2006;2(31):1–17. doi: 10.1186/1744-9081-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon D.W., Warrington E.K. The abstraction of numerical relations: a role for the right hemisphere in arithmetic? J. Int. Neuropsychol. Soc. 1997;3(3):260–268. [PubMed] [Google Scholar]
- Lanphear B.P., Dietrich K., Auinger P., Cox C. Cognitive deficits associated with blood lead concentrations < 10 microg/dL in US children and adolescents. Public Health Rep. 2000;115(6):521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Goodman A.M., Caine C., Delis D.C., Riley E.P. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 1999;23(11):1808–1815. [PubMed] [Google Scholar]
- May P.A., Gossage J.P., Marais A.S., Adnams C.M., Hoyme H.E., Jones K.L., Robinson L.K., Khaole N.C.O., Snell C., Kalberg W.O., Hendricks L., Brooke L., Stellavato C., Viljoen D.L. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88(2):259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes E.M., Jacobson S.W., Molteno C.D., Gatenby J.C., Warton C., Cannistraci C.J., Gore J.C., Jacobson J.L. An fMRI study of magnitude comparison and exact addition in children. Magn. Reson. Imaging. 2010;28(3):351–362. doi: 10.1016/j.mri.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes E.M., Jacobson J.L., Molteno C.D., Gatenby J.C., Warton C., Cannistraci C.J., Hoyme H.E., Robinson L.K., Khaole N., Gore J.C., Jacobson S.W. An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol. Clin. Exp. Res. 2010;34(8):1450–1464. doi: 10.1111/j.1530-0277.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- Meintjes E.M., Narr K.L., van der Kouwe A.J.W., Molteno C.D., Pirnia T., Gutman B., Woods R.P., Thompson P.M., Jacobson J.L., Jacobson S.W. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. NeuroImage Clin. 2014;5:152–160. doi: 10.1016/j.nicl.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molko N., Cachia A., Rivière D., Mangin J.F., Bruandet M., Le Bihan D., Cohen L., Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40(4):847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- Moyer R.S., Landauer T.K. Time required for judgements of numerical inequality. Nature. 1967;215:1519–1520. doi: 10.1038/2151519a0. [DOI] [PubMed] [Google Scholar]
- Mussolin C., De Volder A., Grandin C., Schlögel X., Nassogne M.C., Noël M.P. Neural correlates of symbolic number comparison in developmental dyscalculia. J. Cogn. Neurosci. 2010;22(5):860–874. doi: 10.1162/jocn.2009.21237. [DOI] [PubMed] [Google Scholar]
- Pesenti M., Thioux M., Seron X., Volder A.D. Neuroanatomical substrates of Arabic number processing, numerical comparison, and simple addition: A PET study. J. Cogn. Neurosci. 2000;12(3):461–479. doi: 10.1162/089892900562273. [DOI] [PubMed] [Google Scholar]
- Piazza M., Mechelli A., Butterworth B., Price C.J. Are subitizing and counting implemented as separate or functionally overlapping processes? Neuroimage. 2002;15(2):435–446. doi: 10.1006/nimg.2001.0980. [DOI] [PubMed] [Google Scholar]
- Pinel P., Dehaene S., Riviere D., LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage. 2001;14(5):1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Price G.R., Holloway I., Räsänen P., Vesterinen M., Ansari D. Impaired parietal magnitude processing in developmental dyscalculia. Curr. Biol. 2007;17(24):R1042–R1043. doi: 10.1016/j.cub.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Price G.R., Mazzocco M.M.M., Ansari D. Why mental arithmetic counts: Brain activation during single digit arithmetic predicts high school math scores. J. Neurosci. 2013;33(1):156–163. doi: 10.1523/JNEUROSCI.2936-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza S.M., Delgado M.R., Chein J.M., Becker J.T., Fiez J.A. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Riley E.P., McGee C.L. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp. Biol. Med. 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rivera S.M., Menon V., White C.D., Glaser B., Reiss A.L. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Hum. Brain Mapp. 2002;16(4):206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S.M., Reiss A.L., Eckert M.A., Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb. Cortex. 2005;15(11):1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Rosselli M., Ardila A. Calculation deficits in patients with right and left hemisphere damage. Neuropsychologia. 1989;27(5):607–617. doi: 10.1016/0028-3932(89)90107-3. [DOI] [PubMed] [Google Scholar]
- Rubinsten O., Henik A. Developmental dyscalculia: Heterogeneity might not mean different mechanisms. Trends Cogn. Sci. 2009;13(2):92–99. doi: 10.1016/j.tics.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Santhanam P., Li Z., Hu X., Lynch M.E., Coles C.D. Effects of prenatal alcohol exposure on brain activation during an arithmetic task: an fMRI study. Alcohol. Clin. Exp. Res. 2009;33(11):1901–1908. doi: 10.1111/j.1530-0277.2009.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler J.M. Jerome M. Sattler, Inc.; San Deigo: 1992. Assessment of children 3rd edition. [Google Scholar]
- Simon O., Mangin J.F., Cohen L., Le Bihan D., Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33(3):475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Sokol R.J., Martier S., Ernhart C. Identification of alcohol abuse in the prenatal clinic. In: Chang N.C., Chao H.M., editors. NIAAA Research Monograph 17: Early Identification of alcohol abuse. DHHS Publication, U.S. Department of Health and Human Resources; Washington, DC: 1985. pp. 85–128. [Google Scholar]
- Sowell E.R., Thompson P.M., Mattson S.N., Tessner K.D., Jernigan T.L., Riley E.P., Toga A.W. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12(3):515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Mattson S.N., Tessner K.D., Jernigan T.L., Riley E.P., Toga A.W. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb. Cortex. 2002;12(8):856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Mattson S.N., Kan E., Thompson P.M., Riley E.P., Toga A.W. Abnormal cortical thickness and brain–behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb. Cortex. 2008;18(1):136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu-Cosson R., Pinel P., Van de Moortele P.F., Le Bihan D., Cohen L., Dehaene S. Cerebral bases of calculation processes: Impact of number size on the cerebral circuits for exact and approximate calculation. Brain. 2000;123(11):2240–2255. doi: 10.1093/brain/123.11.2240. [DOI] [PubMed] [Google Scholar]
- Stratton K.R., Howe C.J., Battaglia F.C. National Academies; 1996. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. [Google Scholar]
- Streissguth A.P., Barr H.M., Sampson P.D. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol. Clin. Exp. Res. 1990;14(5):662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth A.P., Aase J.M., Clarren S.K., Randels S.P., LaDue R.A., Smith D.F. Fetal alcohol syndrome in adolescents and adults. J. Am. Med. Assoc. 1991;265(15):1961–1967. [PubMed] [Google Scholar]
- Streissguth A.P., Barr H.M., Olson H.C., Sampson P.D., Bookstein F.L., Burgess D.M. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: Adolescent data from a population‐based prospective study. Alcohol. Clin. Exp. Res. 1994;18(2):248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Suttie M., Foroud T., Wetherill L., Jacobson J.L., Molteno C.D., Meintjes E.M., Hoyme H.E., Khaole N., Robinson L.K., Riley E.P., Jacobson S.W., Hammond P. Facial dysmorphism across the fetal alcohol spectrum. Pediatrics. 2013;131(3):e779–e788. doi: 10.1542/peds.2012-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]