Abstract
Acetylcholine receptors (AChRs) are required for body movement in parasitic nematodes and are targets of “classical” anthelmintic drugs such as levamisole and pyrantel and of newer drugs such as tribendimidine and derquantel. While neurotransmission explains the effects of these drugs on nematode movement, their effects on parasite reproduction are unexplained. The levamisole AChR type (L-AChRs) in Caenorhabditis elegans is comprised of five subunits: Cel-UNC-29, Cel-UNC-38, Cel-UNC-63, Cel-LEV-1 and Cel-LEV-8. The genome of the filarial parasite Brugia malayi contains nine AChRs subunits including orthologues of Cel-unc-29, Cel-unc-38, and Cel-unc-63. We performed in situ hybridization with RNA probes to localize the expression of five AChR genes (Bm1_35890-Bma-unc-29, Bm1_20330-Bma-unc-38, Bm1_38195-Bma-unc-63, Bm1_48815-Bma-acr-26 and Bm1_40515-Bma-acr-12) in B. malayi adult worms. Four of these genes had similar expression patterns with signals in body muscle, developing embryos, spermatogonia, uterine wall adjacent to stretched microfilariae, wall of Vas deferens, and lateral cord. Three L-AChR subunit genes (Bma-unc-29, Bma-unc-38 and Bma-unc-63) were expressed in body muscle, which is a known target of levamisole. Bma-acr-12 was co-expressed with these levamisole subunit genes in muscle, and this suggests that its protein product may form receptors with other alpha subunits. Bma-acr-26 was expressed in male muscle but not in female muscle. Strong expression signals of these genes in early embryos and gametes in uterus and testis suggest that AChRs may have a role in nervous system development of embryogenesis and spermatogenesis. This would be consistent with embryotoxic effects of drugs that target these receptors in filarial worms. Our data show that the expression of these receptor genes is tightly regulated with regard to localization in adult worms and developmental stage in embryos and gametes. These results may help to explain the broad effects of drugs that target AChRs in filarial worms.
Keywords: Brugia malayi, Acetylcholine receptors, In situ hybridization, Anthelmintic, Reproduction, Filarioideae
Graphical abstract
Highlights
-
•
Expression patterns of Brugia malayi AChR subunit genes studied by in situ hybridization.
-
•
All genes highly expressed in developing embryos and sperm precursors.
-
•
Highly expressed in the walls of uterus and Vas deferens with mature offspring.
-
•
Four of five genes expressed in body muscle of adult worms.
-
•
Expression patterns shed new light on the action of anthelmintics in filarial parasites.
1. Introduction
Parasitic nematodes commonly infect humans in the developing world, and they are responsible for an enormous burden of disease (Brooker et al., 2010). A variety of anthelmintic drugs are used to treat parasitic nematode infections. Many of these compounds act on ion channels such as acetylcholine receptors (AChRs) that occur at nematode neuromuscular junctions, in pharyngeal muscle, and in nervous tissue (Jones and Sattelle, 2004; Martin et al., 2004; Sattelle et al., 2009; Wolstenholme, 2011). This includes older drugs such as levamisole and pyrantel and recently introduced drugs such as monepantal (Kaminsky et al., 2008), derquantel (Little et al., 2011) and tribendimidine. The newer compounds are currently used in veterinary medicine, but they may be used to treat humans in the future (Xiao et al., 2005; Kaminsky et al., 2008).
Recent studies have suggested that anthelmintic drug resistance is common in some areas due to wide use of these drugs (Kaplan, 2004; Wolstenholme et al., 2004; Osei-Atweneboana et al., 2011) Circumventing drug resistance will require new anthelmintic, and it is likely that AChRs will continue to be attractive targets. Molecular characterization of nematode AChRs and improved understanding of their roles in nematode biology may facilitate development of new drugs in this class.
ACh receptors are protein complexes that are comprised of multiple subunit proteins, and each species has several different receptors. AChR proteins have been most intensively studied in Caenorhabditis elegans and in Ascaris suum. Parasitic nematodes may have fewer AChR genes than the model organism C. elegans based on limited publications (Williamson et al., 2007). C. elegans has at least 30 genes that encode AChR subunits, and these have been divided into five groups based on sequence homology (DEG-3, ACR-16, ACR-8, UNC-38 and UNC-29) (Jones et al., 2007). These subunits include examples of both α-subunits (e.g., UNC-38) and non-α-subunits (e.g., UNC-29). For example, four AChRs have been identified in C. elegans, and these include a neuronal receptor, DEG-3/DES-2 (Treinin et al., 1998), a pharyngeal muscle receptor (EAT-2), which requires a small transmembrane protein EAT-18 for normal pharyngeal pumping (McKay et al., 2004), and two body neuromuscular AChRs. One of these is sensitive to levamisole (a so-called “L-type receptor” or L-AChR) and the other is sensitive to nicotine (an N-type AChR) (Richmond and Jorgensen, 1999). Comprehensive studies of levamisole in C. elegans have improved understanding of the molecular mechanisms associated with L-AChR signaling. Based on expression in Xenopus oocytes and recordings from body wall muscle, each L-type AChR in C. elegans is composed of five subunits which include three α-subunits (UNC-38, UNC-63, and LEV-8), and two non-α-subunits, UNC-29 and LEV-1 (Boulin et al., 2008). Molecular cloning and extensive bioinformatic searches in parasitic nematode genomes have identified homologs of unc-29, unc-38 and unc-63 in all parasitic nematodes (Williamson et al., 2007; Fauvin et al., 2010; Neveu et al., 2010). Orthologs of the lev-1 gene have only been identified in Clade V nematode species (including C. elegans and Trichostrongylus species) but not in members of Clades I–IV (Martin et al., 2012).
In vitro studies have shown that the subunit composition and pharmacology of neuromuscular AChRs in parasite nematodes are quite different from those in C. elegans (Williamson et al., 2009; Boulin et al., 2011). While five subunits are required for all AChRs in C. elegans, only two (unc-38 and unc-29) are required for the levamisole-sensitive receptor in A. suum (Williamson et al., 2007), and three (UNC-29, UNC-38 and UNC-63) or four (UNC-29, UNC-38, UNC-63 and ACR-8) are required in Haemonchus contortus (Boulin et al., 2011). In C. elegans, ACR-16 is a homomeric AChR, and the only essential subunit in levamisole-resistant receptors (Touroutine et al., 2005). Orthologs of acr-16 have not been identified in other parasitic nematodes except for A. suum (Holden-Dye et al., 2013). The subunits of L-AChRs are functionally diverse. Different ratios of the two subunits (UNC-38 and UNC-29) from A. suum expressed in Xenopus oocytes formed two distinct L-AChR subtypes that exhibited different anthelmintic sensitivity (Williamson et al., 2009).
Sequence and bioinformatics analysis identified orthologs of Cel-unc-29, Cel-unc-38, and Cel-unc-63 in the Brugia malayi genome (Ghedin et al., 2007; Williamson et al., 2007); these subunit proteins are known to be present in AChRs at the nematode neuromuscular junction in C. elegans (Fleming et al., 1997; Richmond and Jorgensen, 1999; Culetto et al., 2004). Electrophysiological studies have shown that AChRs are present in body muscle of A. suum and Oesophagostomum dentatum (Martin, 1982, 1985; Robertson and Martin, 1993; Evans and Martin, 1996; Robertson et al., 1999; Qian et al., 2006), and several studies successfully reconstituted the receptors from the nematode parasites A. suum, H. contortus and O. dentatum in Xenopus oocytes (Williamson et al., 2009; Boulin et al., 2011; Bennett et al., 2012; Buxton et al., 2014). However, there are few molecular studies in filarial nematodes. Electrophysiological recordings have shown that AChRs are present in Acanthocheilonema viteae and B. malayi body muscle (Rohrer et al., 1988; Christ et al., 1990, 1994; Robertson et al., 2011; Robertson et al., 2013).
Drugs that act on AChRs in intestinal nematodes selectively open ligand-gated acetylcholine receptors in body muscle to produce depolarization, entry of sodium or calcium, contraction and spastic paralysis. These drugs paralyze nematodes and render them unable to actively maintain their position in the gut (Robertson et al., 2002; Martin and Robertson, 2007; Puttachary et al., 2010). Drugs such as levamisole that target AChRs also have potent activity against microfilariae (Mf) of filarial nematodes (B. malayi, Litomosoides carinii, Wuchereria bancrofti and Onchocerca volvulus) (Zaman and Lal, 1973; O'Holohan and Zaman, 1974; Mak and Zaman, 1980; Awadzi et al., 1982). This activity is presumably also related to effects of the drugs on parasite motility. However, levamisole is less effective for killing adult filarial worms (Mak and Zaman, 1980), and its mode of action in these parasites is poorly understood. We followed up the bioinformatics analysis of AChR subunit genes with studies to localize expression of genes that encode AChRs in B. malayi, to improve understanding of the biological roles of AChRs in filarial worms and their potential as targets for chemotherapy.
2. Materials and methods
2.1. Parasite material and slide preparation
Adult B. malayi worms were isolated from infected jirds and separated carefully by gender as previously described (Li et al., 2004). Live worms were washed twice using phosphate buffered saline (PBS) and immediately fixed in 4% formalin buffer. Fixed worms were embedded in paraffin in the Histology Core Laboratory at Washington University School of Medicine. The embedded worms were cut into 5 μm sections, using a microtome. Sections were floated onto Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA, USA) and placed on a warming block in at 65 °C for 20 min to bond the tissue to the glass. Slides were then stored at room temperature for future use.
2.2. Selection of target genes and primer design
Detailed functional studies of AChRs genes have not been performed for filarial worms. However, the genome of the filarial nematode B. malayi has been reported to contain nine genes that encode AChRs (Ghedin et al., 2007; Williamson et al., 2007). Gene-specific RNA probes were designed with consensus cDNA sequences for five B. malayi putative AChRs subunit genes retrieved from the NCBI website (http://www.ncbi.nlm.nih.gov/) either by accession number (XM_00xxxxxxx) or Pub_Locus (Bm1_xxxxx). The accession numbers were XM_001898590 (Bm1_35890; Bma-unc-29), XP_001901226 (Bm1_48815; Bma-acr-26), XP_001899560.1 (Bm1_40515; Bma-ace-12), XM_001895485 (Bm1_20330; Bma-unc-38) and XM_001899057 (Bm1_38195; Bma-unc-63). Primers for in situ listed in Table 1 were designed using PrimerQuest software (http://idtdna.com/primerquest/home), and these primers were produced by Integrated DNA Technology Inc. (Coralville, IA USA).
Table 1.
Primers for in situ hybridization.
| B. malayi gene | C. elegans homologuea | In situ forward | In situ reverse |
|---|---|---|---|
| Bm1_35890 (Bma-unc-29) | Cel-unc-29 | ACATCCAATTACCGCCGCATTTCC | ACCGCAGGTACCACCAACTGTAAT |
| Bm1_48815 (Bma-acr-26) | N/A | TTTACGATGGCACAGTCTTCTGGC | TCTGGTATGGGACGGTCAAATGGA |
| Bm1_20330 (Bma-unc-38) | Cel-unc-38 | CTGGGAACCACCTGCTATTT | CACCACTTCGTGACGGTAAA |
| Bm1_38195 (Bma-unc-63) | Cel-unc-63 | ACTTCCGATCTCCCTCTACTC | GGCCCGTAGTATGATGAGAATG |
| Bm1_40515 (Bma-acr-12) | Cel- acr-12 | TGCGGAACTAGAAGGTCAGCTACA | AGATTTGTAAATGGCTGGCGGCTG |
Nomenclature for C. elegans gene referred from Beech RN et al., 2010. Nematode parasite genes: what's in a name? Trends Parasitol 26, 334–340.
2.3. RNA probe construction and in situ hybridization
RNA probes were prepared as described previously (Li et al., 2014). Briefly, target gene sequences were amplified by PCR using B. malayi adult cDNA template as previously reported (Li et al., 2004). Amplified fragments of the selected genes were cloned into a dual promoter PCRII vector (K2060-0, Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol, and insertion of the fragments was confirmed by sequencing. Biotinylated anti-sense and sense probes were prepared by transcription from the template plasmid using MEGAscript T7 and Sp6 in vitro transcription kits (Ambion, Grand Island, NY, USA) with biotinylated NTPs (Roche Diagnostics, Indianapolis, IN, USA). The biotinylated RNA probes were purified and concentrated by ethanol precipitation, dissolved in DEPC-water, and stored at −20 °C for later use.
Paraffin sections were deparaffinized and digested as previously described (Li et al., 2014). Briefly, the sections were hybridized at 60 °C or 42 °C (depending on the probe being used) overnight in a humid chamber with 1 μg/mL of RNA probe in hybridization buffer. An in situ hybridization detection system kit (K0601, Dako, Carpinteria, CA, USA) was used for stringency wash and detection. The sections were washed and incubated for 40 min with biotinylated RNA with streptavidin-AP conjugate at room temperature. Slides were viewed using an Olympus-BX40 microscope (Olympus, Tokyo, Japan) and photographed with an Infinity2 digital microscope camera using Infinity Capture software (Lumenera, Ottawa, Ontario, Canada). The signal intensity of each object was scored as strong, moderate or weak according to the intensity of staining to provide a semi-quantitative assessment of gene expression. The stage of the embryos was defined as previously reported (Jiang et al., 2008; Li et al., 2014). Briefly, the embryonic stages were classified as follows: prelarvae (forms ranging from unfertilized eggs to morulae); developing embryos (forms ranging from morulae with a first invagination to elongated embryos with the two extremities in contact), pretzel stages (forms ranging from embryos with overlapping extremities to microfilariae coiled within the egg membrane), and stretched microfilariae (Lok et al., 1988). The distal uterus contains mostly prelarval stages, the middle portion mainly contains developing embryos, and the proximal uterus contains pretzel larvae and mature stretched microfilariae (Breton et al., 1997).
2.4. Antibody production and immunohistochemical labeling
Anti-peptide antibody was produced and purified by LifeTein LLC (South Plainfield, NJ, USA). Briefly, target peptide of XP_001901226 (RDDNDDNQVTDEQR-Bm1_48815) was selected based on predicted chemical and immunogenic properties by the company. Target peptide was synthesized and coupled to a carrier protein (keyhole limpit hemocyanin, KLH). Rabbits were immunized with the peptide-KLH conjugates and boosted twice every two weeks after primary immunization. Rabbit polyclonal antibodies were affinity purified using the target peptide (without KLH).
Worms intended for histological examinations were fixed in 4% buffered formalin, embedded in paraffin and sectioned at 6um thickness according to standard histological procedure. Immunohistochemical labeling was as previously described (Fischer et al., 2011). Briefly, various dilutions of primary antibodies were tested in order to optimize signal to background. Antibody against Bm_48815 was used at 7.5ug/ml and Anti-KLH (negative control) was used at 1:1000 as recommended by the manufacturer. The alkaline phosphatase anti-alkaline phosphatase (APAAP, Dako) was used as developing agent for visualization as previously described (Fischer et al., 2011), and slides were developed with Fast Red (Sigma) and counterstained with Mayer's Hematoxylin Solution, and were viewed using an Olympus-BX40 microscope and photographed.
2.5. Assessment of expression of AChR subunit genes at the whole worm level by SYBR green I quantitative real-time PCR (qRT-PCR)
We performed qRT-PCR to assess expression of AChR subunit genes at the whole worm level in different life cycle stages. Primer design and optimization, RNA isolation, and reverse transcription were performed as previously described (Li et al., 2004), and the primer sequences are presented in a supplemental table. We used Bm1_09760 as a control gene for qRT-PCR, because prior studies showed that this gene is consistently expressed across the B. malayi life cycle (Li et al., 2012). We revalidated this gene as an internal control for the present study across three stages (male, female, and Mf), and consistent Ct values were observed (all within one cycle). Therefore we used this as an internal control for template quantity and PCR efficiency. Ten ng of RNA template isolated from each life cycle stage was used as starting material for qRT-PCR.
3. Results and discussion
3.1. Expression patterns of Bma-unc-29, Bma-unc-38, Bma-unc-63 and Bma-acr-12 in B. malayi adult worms
Preliminary studies were performed to assess expression level of AChR subunit genes at the whole worm level in different life cycle stages. Results in Table 2 show that expression of these genes was fairly consistent in microfilariae, adult male and adult female worms. qRT-PCR measures gene expression at the whole worm level, while in situ hybridization localizes gene expression in specific tissues.
Table 2.
Expression data (Ct values) for AChR subunit genes in Brugia malayi microfilariae, adult female and adult male worms as assessed by qRT-PCR.
| Brugia malayi gene | Microfilariae | Adult female | Adult male |
|---|---|---|---|
| Bm1_35890 (Bma-unc-29) | 25.3 | 24.8 | 25.2 |
| Bm1_48815 (Bma-acr-26) | 28.6 | 28.7 | 29.1 |
| Bm1_20330 (Bma-unc-38) | 25.6 | 26.9 | 28.4 |
| Bm1_38195 (Bma-unc-63) | 24.6 | 25 | 25.3 |
| Bm1_40515 (Bma-acr-12) | 31.6 | 27.9 | 30.7 |
| Bm1_09760a | 21.3 | 21.5 | 22.2 |
This gene was used as the internal control for qRT-PCR.
In situ expression patterns observed for these subunit genes in B. malayi worms are summarized in Table 3. Four of five gene candidates (except Bm1_48815, Bma-acr-26) were co-expressed in the body muscle in male and female worms (Figs. 1–5). These results are consistent with prior evidence that levamisole acts directly on body wall muscle (Richmond and Jorgensen, 1999), and they provide new molecular evidence for localization of binding sites for levamisole, which opens L-AChRs ion channels in nematode muscle leading to depolarization (Puttachary et al., 2010), spastic muscle contraction, and paralysis (Robertson et al., 2010). The majority of C. elegans genes that encode AChRs (23/30) are also expressed in body wall muscle and/or nervous tissue (Holden-Dye et al., 2013). A. suum orthologues of unc-29 and -38 are co-expressed in the muscle cell membrane and not confined to the neuromuscular junction (Williamson et al., 2009). Our expression data are consistent with results from whole-cell patch-clamp studies in B. malayi that found that AChRs are activated by cholinergic anthelmintics (levamisole, pyrantel, bephenium and tribendimidine) and that levamisole reversibly paralyzed adult worms (Robertson et al., 2011). Biophysical and pharmacological features of body muscle ACh receptors in B. malayi (Robertson et al., 2011, 2013) suggest that the B. malayi AChRs are different from those in A. suum [(Martin et al., 2004; Trailovic et al., 2008) and H. contortus (Boulin et al., 2011) and possibly more similar to those in C. elegans (Boulin et al., 2008). The B. malayi AChRs in body muscle are less sensitive to both acetylcholine and levamisole than that in C. elegans, A. suum or O. dentatum (Robertson et al., 2011). This may explain partially why levamisole is less effective in killing B. malayi adult worms than that in A. suum.
Table 3.
AChRs gene expression patterns in B. malayi adult worms by in situ hybridization.a
| Pub_Locus | Female reproductive system |
Lateral chord (F/M) | Male reproductive system |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uteral-epithelium | Oocytes | Morulae | Pretzel | Stretched MF | Spermotogonia | Spermotocytes | Spermatids | Spermatozoa | Vas deferens | Muscle F/M | ||
| Bm1_20330 | 3 | 3 | 2 | 1 | 1 | 1/1 | 3 | 2 | 0 | 0 | 3 | 2/2 |
| Bm1_38195 | 3 | 3 | 2 | 1 | 1 | 2/2 | 3 | 0 | 0 | 0 | 3 | 2/2 |
| Bm1_35890 | 3 | 3 | 2 | 1 | 1 | 2/2 | 3 | 0 | 0 | 0 | 3 | 2/2 |
| Bm1_48815 | 3 | 2 | 2 | 1 | 1 | 2/3 | 3 | 1 | 0 | 0 | 3 | 0/2 |
| Bm1_40515 | 3 | 3 | 3 | 0 | 0 | 2/2 | 3 | 2 | 1 | 0 | 2 | 2/2 |
Signal intensity was scored as follows: 1, weak; 2, moderate; 3, strong.
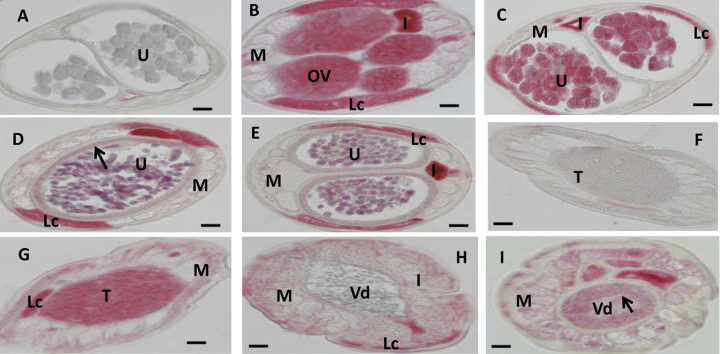
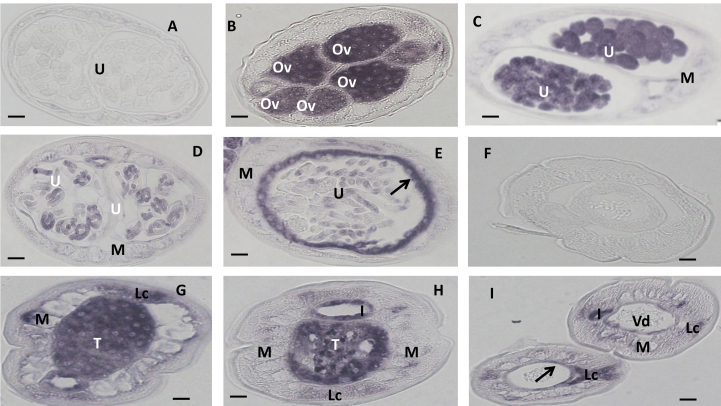
Fig. 1.
In situ hybridization patterns for Bm1_20330 in adult B. malayi adult worms. The sense RNA probe (negative control) did not label tissues in female (A) or male worms (F). In contrast, the antisense probe produced strong signals in female (B–E) and male worms (G–I). Oocytes in ovary (B), morulae stage embryos (C) and the uterine wall (arrow in E) adjacent to pretzel or stretched microfilariae (Mf) were intensely labeled and stretched Mf was partially labeled (E). The antisense probe also strongly labeled spermatogonia in the male testis (G, H) and the wall of the Vas deferens (arrow) (I), the lateral cord was weakly labeled (G, H). Weak to moderate labeling was also observed in both male and female body muscle. Abbreviations: Ov, ovary; I, intestine; U, uterus; M, muscle; Lc, lateral cord, Vd, Vas deferens; T, testis. Scale bar 10 μm in panel A–E and 5 μm in panel F–I.
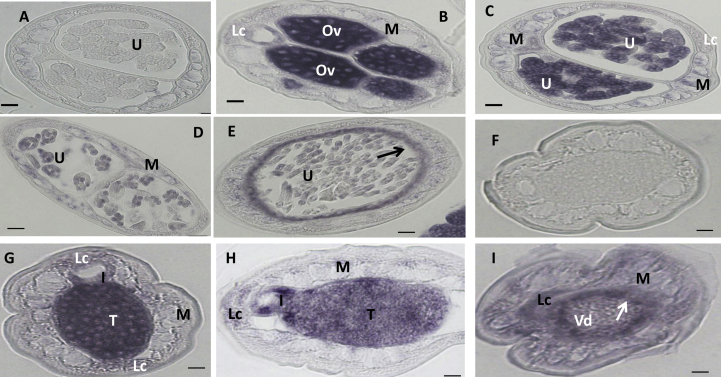
Fig. 2.
In situ hybridization patterns for Bm1_38195 in adult B. malayi adult worms. The sense RNA probe (negative control) did not label tissues in female (A) or male worms (F). In contrast, the antisense probe produced moderate to strong signals in female (B–E) and male worms (G–I). Oocytes in ovary (B), morulae stage embryos (C), and the uterine wall (E, arrow) adjacent to pretzel or stretched Mf were intensely labeled; and pretzel stage and stretched Mf were partially labeled (D, E). The antisense probe also strongly labeled spermatogonia in the male testis (G) and the wall of the Vas deferens (arrow) (I), the lateral cord was weakly labeled in female (B, C) and male (H, I), respectively. Weak to moderate labeling was also observed in both female and male body muscle. Abbreviations: Ov, ovary; I, intestine; U, uterus; M, muscle; Lc, lateral cord, Vd, Vas deferens; T, testis. Scale bar 10 μm in panel A–E and 5 μm in panel F–I.
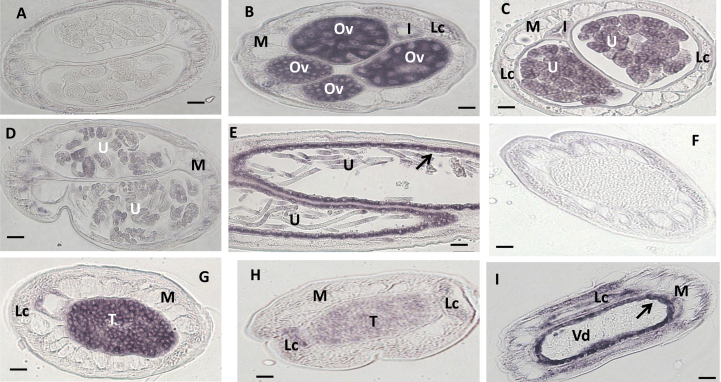
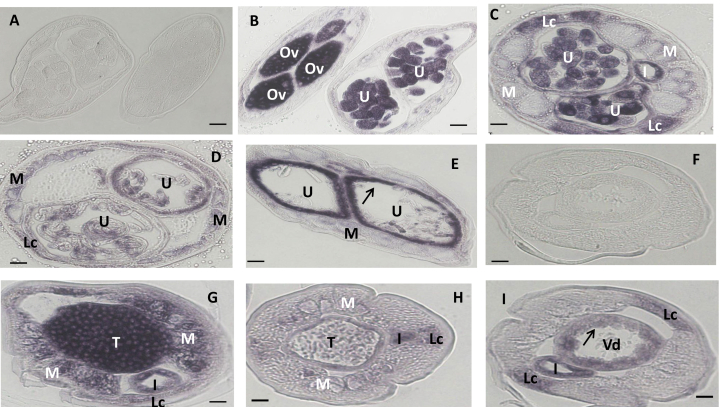
Fig. 3.
In situ hybridization patterns for Bm1_35890 in adult B. malayi adult worms. The sense RNA probe (negative control) did not label tissues in female (A) or male worms (F). In contrast, the antisense probe produced strong signals in female (B–E) and male worms (G–I). Oocytes in ovary (B), morulae stage embryos (B and C), pretzel stage (D), and the uterine wall (arrow) adjacent to pretzel or stretched Mf (E, arrow) were intensely labeled; stretched Mf was partially labeled (E). The antisense probe also strongly labeled spermatogonia in the male testis (G) and the wall of the Vas deferens (arrow) (H, I), and sperm was not labeled (I). The lateral cord was moderately labeled in male and female. Weak to moderate labeling was also observed in both male and female body muscle. Abbreviations: Ov, ovary; I, intestine; U, uterus; M, muscle; Lc, lateral cord, Vd, Vas deferens; T, testis. Scale bar 10 μm in panel A–E and 5 μm in panel F–I.
Fig. 4.
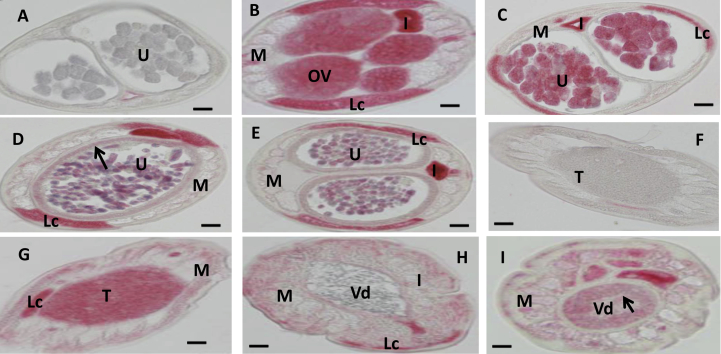
In situ hybridization patterns for Bm1_48815 in adult B. malayi adult worms. The sense RNA probe (negative control) did not label tissues in female (A) or male worms (F). In contrast, the antisense probe produced strong signals in female (B–E) and male worms (G–I). Oocytes in ovary (B), morulae stage embryos (C) and the uterine wall adjacent to pretzel or stretched Mf (E, arrow) were intensely labeled; pretzel stage (D) and stretched Mf (E) were partially labeled. The antisense probe also strongly labeled spermatogonia in the male testis (G, H) and the wall of the Vas deferens (arrow) (I); the lateral cord was strongly labeled in male (G, H). Weak to moderate labeling was also observed in male body muscle. Abbreviations: Ov, ovary; I, intestine; U, uterus; M, muscle; Lc, lateral cord, Vd, Vas deferens; T, testis. Scale bar 10 μm in panel A–E and 5 μm in panel F–I.
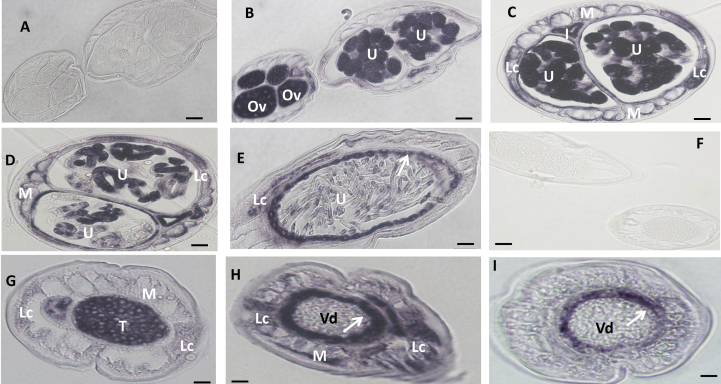
Fig. 5.
In situ hybridization patterns for Bm1_40515 in adult B. malayi adult worms. The sense RNA probe (negative control) did not label tissues in female (A) or male worms (F). In contrast, the antisense probe produced strong signals in female (B–E) and male worms (G–I). Oocytes in ovary (B), morulae stage embryos (C) and the uterine wall (arrow) adjacent to pretzel or stretched Mf (E) were intensely labeled and stretched Mf was partially labeled (D). The antisense probe also strongly labeled spermatogonia in the male testis (G) and the wall of the Vas deferens (arrow) (I); the lateral cord was strongly labeled (G, H). Weak to moderate labeling was also observed in both male and female body muscle. Abbreviations: Ov, ovary; I, intestine; U, uterus; M, muscle; Lc, lateral cord, Vd, Vas deferens; T, testis. Scale bar 10 μm in panel A–E and 5 μm in panel F–I.
Localization studies showed that Bm1_40515, an orthologue of the C. elegans AChR subunit gene acr-12, is also expressed in body muscle of B. malayi adult worms. In C. elegans, protein ACR-12 co-purified with levamisole receptor subunits by tandem affinity purification, and the authors suggest that ACR-12 may contribute to levamisole receptors in a subset of postsynaptic clusters (Gottschalk et al., 2005). The overlapping cellular localization of ACR-12 with genes known to be levamisole receptor subunits (UNC-63, UNC-38, and UNC-29) in B. malayi suggests possible association with these subunits in vivo. In C. elegans, ACR-12 is differentially expressed in both cholinergic and GABAergic motor neuron within the motor circuit and it is present in two distinct AChR subtypes. Other studies showed that these two receptor subtypes regulate the coordinated activity of excitatory (cholinergic) and inhibitory (GABAergic) motor neurons (Jospin et al., 2009; Barbagallo et al., 2010). Acr-12 is one of five components in the heteromeric acetylcholine-gated ion channel ACR-2R that is essential for regulating motor circuit activity (Petrash et al., 2013). Very recently, an orthologue of this gene has been identified in H. contortus as a new drug target (Laing et al., 2013; Beech and Neveu, 2015). It will be interesting to explore the biological function of ACR-12 in other parasitic nematodes along with its potential as a new anthelmintic target.
3.2. Bm1_48815 (Bma-acr-26) is expressed in male but not in female body muscle
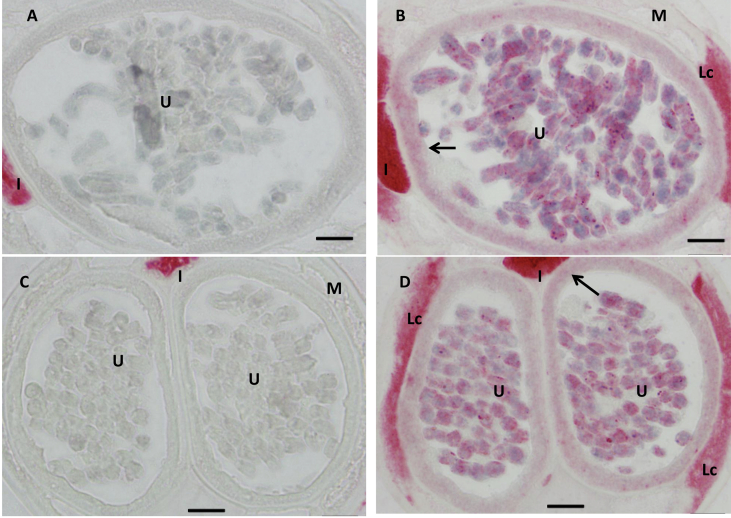
Bm1_48815, an orthologue of a novel receptor subunit acr-26, is expressed in most animal parasitic nematode species in clades III, IV and V, including filarial parasites and Strongyloides ratti, but it is not present in C. elegans or in plant parasitic nematodes (Bennett et al., 2012). It has been suggested as a potential target for the development of cholinergic anthelmintic specific for the parasitic nematodes (Bennett et al., 2012). Hybridization results showed that Bm1_48815 has an expression pattern similar to those of AChRs subunit genes mentioned above in male worms (Fig. 4G–I), but expression was not observed in female worm muscle (Fig. 4B–E). The labeling pattern produced by antibodies against the predicted peptide from XP_001901226 was consistent with in situ results (Fig. 6 and Fig. 7). Antibodies against the KLH carrier protein used for antibody production produced no tissue-specific labeling in female worms (Fig. 6A and Fig. 7A and C) or in males (Fig. 6F) other than non-specific background staining in the intestine that was most-likely due to internal alkaline phosphatase in that organ. In contrast, antibodies against the specific peptides strongly labeled the tissues corresponding to the early stages of sperm development (Fig. 6G), body muscle (Fig. 6G–I) and the wall of Vas deferens (arrow in Fig. 6I) in male worm. They also labeled early embryos (Fig. 4B–E), and the uterus wall at the level of stretched Mf (arrows in Fig. 6D and in Fig. 7B and C) but not body muscle in females. It is interesting that the A. suum orthologue of acr-26 subunit (Asu-acr-26) is expressed in muscle in the head but not in the body-wall of A. suum, and this is different from expression patterns of other AChR subunit genes such as unc-29 and unc-38 which are expressed in both muscle types (Williamson et al., 2009; Bennett et al., 2012). The homomeric channels formed by Asu-ACR-26 in Xenopus oocytes were extremely sensitive to acetylcholine and nicotine despite its sporadic expression (Bennett et al., 2012). The pharmacology of this receptor is also different from those previously reported for reconstituted levamisole receptors (Boulin et al., 2008; Williamson et al., 2009; Boulin et al., 2011). Williamson et al. suggested that expression limited to muscles in the head may be related to the more complex movements of the head in Ascaris. It is tempting to relate the function of Bma-acr-26 to the high motility of B. malayi male worms relative to females. Further study is needed to explore the potential function of this gene in B. malayi.
Fig. 6.
Localization of the predicted peptide from Bm1_48815 in adult B. malayi adult by immunohistochemical labeling. Negative control did not label any tissue except areas where the internal enzymes are high such as intestine in female (A) or male (F). Polyclonal antibodies against a predicted peptide from Bm_48815 labeled developing embryos, lateral cord, the wall of uteri with stretched Mf (arrow) in female (B–D); the antibody also labeled spermatogonia (G) and the body muscle and lateral cord (G–I), and the wall of the Vas deference with mature sperms (arrow, I). Abbreviations: Ov, ovary; I, intestine; U, uterus; M, muscle; Lc, lateral cord, Vd, Vas deferens; T, testis. Scale bar 10 μm in panel A–E and 5 μm in panel F–I.
Fig. 7.
Higher magnification of the Localization of the predicted peptide from Bm1_48815 in B. malayi female by immunohistochemical labeling (100×). Negative control did not label any tissue except areas where the internal enzymes are high such as intestine in female (A and C) while the uterine walls (arrows in B and D corresponding to Fig. 6D and E) were moderately labeled. Scale bar 10 um.
3.3. Localization of AChR subunit genes suggests that they may play a functional role in embryogenesis and spermatogenesis
Probes for these subunits produced very similar expression signals in embryos and spermatids in adult worms. Intense labeling for AChR transcripts was seen in oocytes in the ovaries (Figs. 1–5B), developing morulae (Figs. 1–5C), and pretzel stage larvae in females (Figs. 1–5D), and in spermatogonia and spermatocytes in males (Fig. 1–5G and H). The protein encoded by one of these channel genes (Bm1_48815) was easily detected in these stages by immunohistology (Fig. 6B, C, G), and this is consistent with in situ results. Thus, the in situ results are likely to reflect specific binding of the mRNA probe, and are unlikely to be caused by the probe binding to nonsense or nonspecific RNAs usually found in developing cells (Han et al., 2009; Vasale et al., 2010). However, stretched Mf in the uterus were only partially labeled (Figs. 1–5E) in areas where sections cut through the worms. This may be because the probes may not be able to penetrate the Mf sheath. Levamisole is a potent microfilaricidal drug in Mastomys coucha infected with Brugia pahangi, A. viteae, B malayi and L. carinii, and it rapidly eliminates Mf of these species from the peripheral circulation (Zahner and Schares, 1993). This rapid effect of levamisole on Mf requires the drug to directly interact with Mf in the circulation.
Strong expression of these genes in developing embryos and spermatozoa suggests that they may play a functional role in embryogenesis and spermatogenesis. This hypothesis is further supported by earlier observations on the embryotoxic activity of levamisole in L. carinii (Lammler et al., 1971), B. malayi (Joon-Wah et al., 1974) and O. volvulus (Rivas-Alcala et al., 1984). When given in combination with other anthelmintics such as mebendazole, levamisole increased the embryotoxic effect of the benzimidazole in O. volvulus (Awadzi et al., 1982) and hastened the clearance of Mf in patients infected with B. malayi (Mak and Chan, 1983). Levamisole was reported to have a delayed effect on Mf (at 60 and 91 days after treatment) in M. coucha infected with B. malayi (Tyagi et al., 1986). This might have been caused by an embryotoxic effect of levamisole on adult worms. Levamisole has also been reported to have better efficacy against developing filarial larvae than against adult worms in mammalian hosts (L. carinii, A. viteae and Dirofilaria immitis) (Lammler and Wolf, 1977; Hayasaki and Ohishi, 1984). It is interesting that AChR proteins and transcripts are present in early embryos and developmentally regulated in mammals (Zoli et al., 1995; Role and Berg, 1996; Conroy and Berg, 1998). These findings are consistent with the results from global gene expression analysis in C. elegans. In C. elegans, acetylcholine receptors and motor neurons are enriched in embryonic stage (Von Stetina et al., 2007; Chikina et al., 2009). Our expression data suggest that these genes are also developmentally regulated in parasitic nematodes. These results provide a deeper understanding of the embryotoxic effect of levamisole in nematodes.
3.4. Expression of AChR subunit genes s in the wall of the uterus and Vas deferens
We studied expression patterns of AChR genes in adult filarial worms to improve understanding of how cholinergic anthelmintics might affect Mf reproduction and release. Early clinical studies found that levamisole reduced Mf counts in patients with W. bancrofti and B. malayi (Zaman and Lal, 1973; O'Holohan and Zaman, 1974). Hybridization signals of AChRs were observed in sections of the uterine wall or Vas deferens that contained stretched Mf or sperms, respectively. These results suggest that AChRs may be involved in the release of Mf and sperm. This would be consistent with the rapid reduction of Mf release observed in animals treated with levamisole.
3.5. Conclusion
The recent finding that neuromuscular junction AChRs are the targets of the promising new anthelmintics derquantel and tribendimidine demonstrates that AChRs continue to be important targets for novel therapies for nematode infections. Our study used in situ hybridization to show that AChRs genes are highly expressed in body muscle in adult filarial worms, the target site of cholinergic anthelmintics. Importantly, our results show that these genes are also expressed in a developmentally controlled manner in developing embryos and sperm precursors in B. malayi. These results have shed new light on the action of cholinergic drugs in filarial parasites. Improved understanding of the localization of AChRs may contribute to development of new treatments for filarial infections.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2015.04.003.
Supplementary data
The following is the supplementary data related to this article:
References
- Awadzi K., Schulz-Key H., Howells R.E., Haddock D.R., Gilles H.M. The chemotherapy of onchocerciasis VIII Levamisole and its combination with the benzimidazoles. Ann. Trop. Med. Parasitol. 1982;76:459–473. doi: 10.1080/00034983.1982.11687567. [DOI] [PubMed] [Google Scholar]
- Barbagallo B., Prescott H.A., Boyle P., Climer J., Francis M.M. A dominant mutation in a neuronal acetylcholine receptor subunit leads to motor neuron degeneration in Caenorhabditis elegans. J. Neurosci. 2010;30:13932–13942. doi: 10.1523/JNEUROSCI.1515-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech R.N., Neveu C. The evolution of pentameric ligand-gated ion-channels and the changing family of anthelmintic drug targets. Parasitology. 2015;142:303–317. doi: 10.1017/S003118201400170X. [DOI] [PubMed] [Google Scholar]
- Beech R.N., Wolstenholme A.J., Neveu C., Dent J.A. Nematode parasite genes: what's in a name? Trends Parasitol. 2010;26:334–340. doi: 10.1016/j.pt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Bennett H.M., Williamson S.M., Walsh T.K., Woods D.J., Wolstenholme A.J. ACR-26: a novel nicotinic receptor subunit of parasitic nematodes. Mol. Biochem. Parasitol. 2012;183:151–157. doi: 10.1016/j.molbiopara.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Boulin T., Fauvin A., Charvet C.L., Cortet J., Cabaret J., Bessereau J.L., Neveu C. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br. J. Pharmacol. 2011;164:1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T., Gielen M., Richmond J.E., Williams D.C., Paoletti P., Bessereau J.L. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton B., Diagne M., Wanji S., Bougnoux M.E., Chandre F., Marechal P., Petit G., Vuong P.N., Bain O. Ivermectin and moxidectin in two filarial systems: resistance of Monanema martini; inhibition of Litomosoides sigmodontis insemination. Parasitologia. 1997;39:19–28. [PubMed] [Google Scholar]
- Brooker S., Hotez P.J., Bundy D.A. The global atlas of helminth infection: mapping the way forward in neglected tropical disease control. PLoS Negl. Trop. Dis. 2010;4:e779. doi: 10.1371/journal.pntd.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton S.K., Charvet C.L., Neveu C., Cabaret J., Cortet J., Peineau N., Abongwa M., Courtot E., Robertson A.P., Martin R.J. Investigation of acetylcholine receptor diversity in a nematode parasite leads to characterization of tribendimidine- and derquantel-sensitive AChRs. PLoS Pathog. 2014;10:e1003870. doi: 10.1371/journal.ppat.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikina M.D., Huttenhower C., Murphy C.T., Troyanskaya O.G. Global prediction of tissue-specific gene expression and context-dependent gene networks in Caenorhabditis elegans. PLoS Comput. Biol. 2009;5:e1000417. doi: 10.1371/journal.pcbi.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ D., Goebel M., Saz H.J. Actions of acetylcholine and GABA on spontaneous contractions of the filariid, Dipetalonema viteae. Br. J. Pharmacol. 1990;101:971–977. doi: 10.1111/j.1476-5381.1990.tb14190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ D., Oh J., Saz H.J. Contractions of the filariid Acanthocheilonema viteae induced by potassium chloride. Parasitol. Res. 1994;80:449–453. doi: 10.1007/BF00932689. [DOI] [PubMed] [Google Scholar]
- Conroy W.G., Berg D.K. Nicotinic receptor subtypes in the developing chick brain: appearance of a species containing the alpha4, beta2, and alpha5 gene products. Mol. Pharmacol. 1998;53:392–401. doi: 10.1124/mol.53.3.392. [DOI] [PubMed] [Google Scholar]
- Culetto E., Baylis H.A., Richmond J.E., Jones A.K., Fleming J.T., Squire M.D., Lewis J.A., Sattelle D.B. The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J. Biol. Chem. 2004;279:42476–42483. doi: 10.1074/jbc.M404370200. [DOI] [PubMed] [Google Scholar]
- Evans A.M., Martin R.J. Activation and cooperative multi-ion block of single nicotinic-acetylcholine channel currents of Ascaris muscle by the tetrahydropyrimidine anthelmintic, morantel. Br. J. Pharmacol. 1996;118:1127–1140. doi: 10.1111/j.1476-5381.1996.tb15515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvin A., Charvet C., Issouf M., Cortet J., Cabaret J., Neveu C. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Mol. Biochem. Parasitol. 2010;170:105–107. doi: 10.1016/j.molbiopara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Fischer K., Beatty W.L., Jiang D., Weil G.J., Fischer P.U. Tissue and stage-specific distribution of Wolbachia in Brugia malayi. PLoS Negl. Trop. Dis. 2011;5:e1174. doi: 10.1371/journal.pntd.0001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J.T., Squire M.D., Barnes T.M., Tornoe C., Matsuda K., Ahnn J., Fire A., Sulston J.E., Barnard E.A., Sattelle D.B., Lewis J.A. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J. Neurosci. 1997;17:5843–5857. doi: 10.1523/JNEUROSCI.17-15-05843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghedin E., Wang S., Spiro D., Caler E., Zhao Q., Crabtree J., Allen J.E., Delcher A.L., Guiliano D.B., Miranda-Saavedra D., Angiuoli S.V., Creasy T., Amedeo P., Haas B., El-Sayed N.M., Wortman J.R., Feldblyum T., Tallon L., Schatz M., Shumway M., Koo H., Salzberg S.L., Schobel S., Pertea M., Pop M., White O., Barton G.J., Carlow C.K., Crawford M.J., Daub J., Dimmic M.W., Estes C.F., Foster J.M., Ganatra M., Gregory W.F., Johnson N.M., Jin J., Komuniecki R., Korf I., Kumar S., Laney S., Li B.W., Li W., Lindblom T.H., Lustigman S., Ma D., Maina C.V., Martin D.M., McCarter J.P., McReynolds L., Mitreva M., Nutman T.B., Parkinson J., Peregrin-Alvarez J.M., Poole C., Ren Q., Saunders L., Sluder A.E., Smith K., Stanke M., Unnasch T.R., Ware J., Wei A.D., Weil G., Williams D.J., Zhang Y., Williams S.A., Fraser-Liggett C., Slatko B., Blaxter M.L., Scott A.L. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk A., Almedom R.B., Schedletzky T., Anderson S.D., Yates J.R., 3rd, Schafer W.R. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. Embo J. 2005;24:2566–2578. doi: 10.1038/sj.emboj.7600741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T., Manoharan A.P., Harkins T.T., Bouffard P., Fitzpatrick C., Chu D.S., Thierry-Mieg D., Thierry-Mieg J., Kim J.K. 26G endo-siRNAs regulate spermatogenic and zygotic gene expression in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18674–18679. doi: 10.1073/pnas.0906378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaki M.N.,K., Ohishi I. Larvicidal effects of the short-term medication of levamisole hydrochloride to developing stages of Dirofilaria immitis in infected dogs. Jpn. J. Parasitol. 1984;33:573–576. [Google Scholar]
- Holden-Dye L., Joyner M., O'Connor V., Walker R.J. Nicotinic acetylcholine receptors: a comparison of the AChRs of Caenorhabditis elegans and parasitic nematodes. Parasitol. Int. 2013;62:606–615. doi: 10.1016/j.parint.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Jiang D., Li B.W., Fischer P.U., Weil G.J. Localization of gender-regulated gene expression in the filarial nematode Brugia malayi. Int. J. Parasitol. 2008;38:503–512. doi: 10.1016/j.ijpara.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Jones A.K., Davis P., Hodgkin J., Sattelle D.B. The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: an update on nomenclature. Invert. Neurosci. 2007;7:129–131. doi: 10.1007/s10158-007-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.K., Sattelle D.B. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays. 2004;26:39–49. doi: 10.1002/bies.10377. [DOI] [PubMed] [Google Scholar]
- Joon-Wah M., Zaman V., Sivanandam S. Antifilarial activity of levamisole hydrochloride against subperiodic Brugia malayi infection of domestic cats. Am. J. Trop. Med. Hyg. 1974;23:369–374. doi: 10.4269/ajtmh.1974.23.369. [DOI] [PubMed] [Google Scholar]
- Jospin M., Qi Y.B., Stawicki T.M., Boulin T., Schuske K.R., Horvitz H.R., Bessereau J.L., Jorgensen E.M., Jin Y. A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biol. 2009;7:e1000265. doi: 10.1371/journal.pbio.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky R., Gauvry N., Schorderet Weber S., Skripsky T., Bouvier J., Wenger A., Schroeder F., Desaules Y., Hotz R., Goebel T., Hosking B.C., Pautrat F., Wieland-Berghausen S., Ducray P. Identification of the amino-acetonitrile derivative monepantel (AAD 1566) as a new anthelmintic drug development candidate. Parasitol. Res. 2008;103:931–939. doi: 10.1007/s00436-008-1080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Lammler G., Herzog H., Schutze H.R. Chemotherapeutic studies on Litomosoides carinii infection of Mastomys natalensis. 2. The activity of drugs against microfilariae. Bull. World Health Organ. 1971;44:757–763. [PMC free article] [PubMed] [Google Scholar]
- Lammler G., Wolf E. Chemoprophylactic activity of filaricidal compounds on litomosoides carinii infection of Mastomys natalensis (author's transl) Tropenmed Parasitol. 1977;28:205–225. [PubMed] [Google Scholar]
- Li B.W., Rush A.C., Tan J., Weil G.J. Quantitative analysis of gender-regulated transcripts in the filarial nematode Brugia malayi by real-time RT-PCR. Mol. Biochem. Parasitol. 2004;137:329–337. doi: 10.1016/j.molbiopara.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Li B.W., Wang Z.Y., Rush A.C., Mitreva M., Weil G.J. Transcription profiling reveals stage- and function-dependent expression patterns in the filarial nematode Brugia malayi. BMC Genomics. 2012;13 doi: 10.1186/1471-2164-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.W., Rush A.C., Weil G.J. High level expression of a glutamate-gated chloride channel gene in reproductive tissues of Brugia malayi may explain the sterilizing effect of ivermectin on filarial worms. Int. J. Parasitol. Drugs Drug Resist. 2014;4:71–76. doi: 10.1016/j.ijpddr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing R., Kikuchi T., Martinelli A., Tsai I.J., Beech R.N., Redman E., Holroyd N., Bartley D.J., Beasley H., Britton C. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome biol. 2013;14:R88. doi: 10.1186/gb-2013-14-8-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P.R., Hodge A., Maeder S.J., Wirtherle N.C., Nicholas D.R., Cox G.G., Conder G.A. Efficacy of a combined oral formulation of derquantel-abamectin against the adult and larval stages of nematodes in sheep, including anthelmintic-resistant strains. Vet. Parasitol. 2011;181:180–193. doi: 10.1016/j.vetpar.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Lok J.B., Harpaz T., Knight D.H. Abnormal patterns of embryogenesis in Dirofilaria immitis treated with ivermectin. J. Helminthol. 1988;62:175–180. doi: 10.1017/s0022149x00011482. [DOI] [PubMed] [Google Scholar]
- Mak J.W., Chan W.C. Treatment of Brugia malayi infection with mebendazole and levamisole. Southeast Asian J. Trop. Med. Public Health. 1983;14:510–514. [PubMed] [Google Scholar]
- Mak J.W., Zaman V. Drug trials with levamisole hydrochloride and diethylcarbamazine citrate in Bancroftian and Malayan filariasis. Trans. R. Soc. Trop. Med. Hyg. 1980;74:285–291. doi: 10.1016/0035-9203(80)90081-4. [DOI] [PubMed] [Google Scholar]
- Martin R.J. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br. J. Pharmacol. 1982;77:255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J. gamma-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br. J. Pharmacol. 1985;84:445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Buxton S.K., Neveu C., Charvet C.L., Robertson A.P. Emodepside and SL0-1 potassium channels: a review. Exp. Parasitol. 2012;132:40–46. doi: 10.1016/j.exppara.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Clark C.L., Trailovic S.M., Robertson A.P. Oxantel is an N-type (methyridine and nicotine) agonist not an L-type (levamisole and pyrantel) agonist: classification of cholinergic anthelmintics in Ascaris. Int. J. Parasitol. 2004;34:1083–1090. doi: 10.1016/j.ijpara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134:1093–1104. doi: 10.1017/S0031182007000029. [DOI] [PubMed] [Google Scholar]
- McKay J.P., Raizen D.M., Gottschalk A., Schafer W.R., Avery L. eat-2 and eat-18 are required for nicotinic neurotransmission in the Caenorhabditis elegans pharynx. Genetics. 2004;166:161–169. doi: 10.1534/genetics.166.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu C., Charvet C.L., Fauvin A., Cortet J., Beech R.N., Cabaret J. Genetic diversity of levamisole receptor subunits in parasitic nematode species and abbreviated transcripts associated with resistance. Pharmacogenet Genomics. 2010;20:414–425. doi: 10.1097/FPC.0b013e328338ac8c. [DOI] [PubMed] [Google Scholar]
- O'Holohan D.R., Zaman V. Treatment of Brugia malayi infection with levamisole. J. Trop. Med. Hyg. 1974;77:113–115. [PubMed] [Google Scholar]
- Osei-Atweneboana M.Y., Awadzi K., Attah S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011;5:e998. doi: 10.1371/journal.pntd.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrash H.A., Philbrook A., Haburcak M., Barbagallo B., Francis M.M. ACR-12 ionotropic acetylcholine receptor complexes regulate inhibitory motor neuron activity in Caenorhabditis elegans. J. Neurosci. 2013;33:5524–5532. doi: 10.1523/JNEUROSCI.4384-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttachary S., Robertson A.P., Clark C.L., Martin R.J. Levamisole and ryanodine receptors. II: an electrophysiological study in Ascaris suum. Mol. Biochem. Parasitol. 2010;171:8–16. doi: 10.1016/j.molbiopara.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Martin R.J., Robertson A.P. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. Faseb J. 2006;20:2606–2608. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- Richmond J.E., Jorgensen E.M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Alcala R., Mackenzie C.D., Gomez-Rojo E., Greene B.M., Taylor H.R. The effects of diethylcarbamazine, mebendazole and levamisole on Onchocerca volvulus in vivo and in vitro. Tropenmed Parasitol. 1984;35:71–77. [PubMed] [Google Scholar]
- Robertson A.P., Bjorn H.E., Martin R.J. Resistance to levamisole resolved at the single-channel level. Faseb J. 1999;13:749–760. doi: 10.1096/fasebj.13.6.749. [DOI] [PubMed] [Google Scholar]
- Robertson A.P., Buxton S.K., Martin R.J. Whole-cell patch-clamp recording of nicotinic acetylcholine receptors in adult Brugia malayi muscle. Parasitol. Int. 2013;62:616–618. doi: 10.1016/j.parint.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A.P., Clark C.L., Burns T.A., Thompson D.P., Geary T.G., Trailovic S.M., Martin R.J. Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J. Pharmacol. Exp. Ther. 2002;302:853–860. doi: 10.1124/jpet.102.034272. [DOI] [PubMed] [Google Scholar]
- Robertson A.P., Clark C.L., Martin R.J. Levamisole and ryanodine receptors. I: a contraction study in Ascaris suum. Mol. Biochem. Parasitol. 2010;171:1–7. doi: 10.1016/j.molbiopara.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A.P., Puttachary S., Martin R.J. Single-channel recording from adult Brugia malayi. Invert. Neurosci. 2011;11:53–57. doi: 10.1007/s10158-011-0118-1. [DOI] [PubMed] [Google Scholar]
- Robertson S.J., Martin R.J. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br. J. Pharmacol. 1993;108:170–178. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer W.H., Esch H., Saz H.J. Neuromuscular electrophysiology of the filarial helminth Dipetalonema viteae. Comp. Biochem. Physiol. C. 1988;91:517–523. doi: 10.1016/0742-8413(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Role L.W., Berg D.K. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Sattelle D.B., Buckingham S.D., Akamatsu M., Matsuda K., Pienaar I.S., Jones A.K., Sattelle B.M., Almond A., Blundell C.D. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human alpha7 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2009;78:836–843. doi: 10.1016/j.bcp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Touroutine D., Fox R.M., Von Stetina S.E., Burdina A., Miller D.M., 3rd, Richmond J.E. Acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J. Biol. Chem. 2005;280:27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- Trailovic S.M., Verma S., Clark C.L., Robertson A.P., Martin R.J. Effects of the muscarinic agonist, 5-methylfurmethiodide, on contraction and electrophysiology of Ascaris suum muscle. Int. J. Parasitol. 2008;38:945–957. doi: 10.1016/j.ijpara.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinin M., Gillo B., Liebman L., Chalfie M. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15492–15495. doi: 10.1073/pnas.95.26.15492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi K., Murthy P.K., Chowdhury T.K.R., Chatterjee R.K., Sen A.B. Chemotherapeutic response of Brugia malayi to antifilarials in Mastomys natalensis. Ind. J. Parasitol. 1986;10:195–207. [Google Scholar]
- Vasale J.J., Gu W., Thivierge C., Batista P.J., Claycomb J.M., Youngman E.M., Duchaine T.F., Mello C.C., Conte D., Jr. Sequential rounds of RNA-dependent RNA transcription drive endogenous small-RNA biogenesis in the ERGO-1/argonaute pathway. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3582–3587. doi: 10.1073/pnas.0911908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina S.E., Watson J.D., Fox R.M., Olszewski K.L., Spencer W.C., Roy P.J., Miller D.M., 3rd Cell-specific microarray profiling experiments reveal a comprehensive picture of gene expression in the C. elegans nervous system. Genome Biol. 2007;8:R135. doi: 10.1186/gb-2007-8-7-r135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S.M., Robertson A.P., Brown L., Williams T., Woods D.J., Martin R.J., Sattelle D.B., Wolstenholme A.J. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog. 2009;5:e1000517. doi: 10.1371/journal.ppat.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S.M., Walsh T.K., Wolstenholme A.J. The cys-loop ligand-gated ion channel gene family of Brugia malayi and Trichinella spiralis: a comparison with Caenorhabditis elegans. Invert. Neurosci. 2007;7:219–226. doi: 10.1007/s10158-007-0056-0. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J. Ion channels and receptor as targets for the control of parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2011;1:2–13. doi: 10.1016/j.ijpddr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Xiao S.H., Hui-Ming W., Tanner M., Utzinger J., Chong W. Tribendimidine: a promising, safe and broad-spectrum anthelmintic agent from China. Acta Trop. 2005;94:1–14. doi: 10.1016/j.actatropica.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Zahner H., Schares G. Experimental chemotherapy of filariasis: comparative evaluation of the efficacy of filaricidal compounds in Mastomys coucha infected with Litomosoides carinii, Acanthocheilonema viteae, Brugia malayi and B. pahangi. Acta Trop. 1993;52:221–266. doi: 10.1016/0001-706x(93)90010-9. [DOI] [PubMed] [Google Scholar]
- Zaman V., Lal M. Letter: treatment of Wuchereria bancrofti with levamisole. Trans. R. Soc. Trop. Med. Hyg. 1973;67:610. doi: 10.1016/0035-9203(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Zoli M., Le Novere N., Hill J.A., Jr., Changeux J.P. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J. Neurosci. 1995;15:1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.