Abstract
Background
Conventional treatments for eating disorders are associated with poor response rates and frequent relapse. Novel treatments are needed, in combination with markers to characterize and predict treatment response. Here, resting-state functional magnetic resonance imaging (rs-fMRI) was used to identify predictors and correlates of response to repetitive transcranial magnetic stimulation (rTMS) of the dorsomedial prefrontal cortex (dmPFC) at 10 Hz for eating disorders with refractory binge/purge symptomatology.
Methods
28 subjects with anorexia nervosa, binge−purge subtype or bulimia nervosa underwent 20–30 sessions of 10 Hz dmPFC rTMS. rs-fMRI data were collected before and after rTMS. Subjects were stratified into responder and nonresponder groups using a criterion of ≥50% reduction in weekly binge/purge frequency. Neural predictors and correlates of response were identified using seed-based functional connectivity (FC), using the dmPFC and adjacent dorsal anterior cingulate cortex (dACC) as regions of interest.
Results
16 of 28 subjects met response criteria. Treatment responders had lower baseline FC from dmPFC to lateral orbitofrontal cortex and right posterior insula, and from dACC to right posterior insula and hippocampus. Responders had low baseline FC from the dACC to the ventral striatum and anterior insula; this connectivity increased over treatment. However, in nonresponders, frontostriatal FC was high at baseline, and dmPFC-rTMS suppressed FC in association with symptomatic worsening.
Conclusions
Enhanced frontostriatal connectivity was associated with responders to dmPFC-rTMS for binge/purge behavior. rTMS caused paradoxical suppression of frontostriatal connectivity in nonresponders. rs-fMRI could prove critical for optimizing stimulation parameters in a future sham-controlled trial of rTMS in disordered eating.
Highlights
-
•
dmPFC-rTMS was performed on patients with treatment-refractory AN and BN.
-
•
Resting-state fMRI was collected to identify predictors and correlates of response.
-
•
dmPFC-rTMS achieves robust improvement on bingeing and purging in AN and BN.
-
•
Responders have lower baseline corticostriatal connectivity compared to nonresponders.
-
•
Increased corticostriatal connectivity is associated with treatment response.
1. Introduction
Recurrent episodes of binge eating and purging behavior occur in both anorexia nervosa, binge−purge subtype (AN−BP) and bulimia nervosa (BN). In women, the lifetime prevalence of AN and BN is 0.9% and 1.5% respectively (Hudson et al., 2007). Eating disorders, in particular AN, have among the highest mortality rate of all psychiatric disorders (Sullivan, 1995), and treatment options are limited for severe forms of AN and BN, particularly for AN−BP (Arcelus et al., 2011). Conventional treatments include psychotherapy, pharmacotherapy and inpatient treatments, but rates of treatment dropout, limited treatment response and relapse rates are substantial (Carter et al., 2012; Hay et al., 2012; Mitchell et al., 2007; Olmsted et al., 2005; Shapiro et al., 2007). New treatment options are urgently needed.
Therapeutic brain stimulation is a novel approach in treatment-refractory eating disorders (ED), as recently demonstrated in a pilot study using deep brain stimulation (DBS) in AN (Lipsman et al., 2013). Non-invasive techniques such as repetitive transcranial magnetic stimulation (rTMS) would offer greater accessibility and lower medical risk than DBS, if suitable stimulation targets could be identified. Neuroimaging research has identified a variety of neuroanatomical substrates of ED pathophysiology. For example, on structural imaging, ED patients show reductions in gray matter volume in regions involved in reward, impulse control, and emotion regulation: the caudate nucleus, ventral striatum (VS), anterior cingulate (ACC) and orbitofrontal cortex (OFC) (Friederich et al., 2012; Schäfer et al., 2010; Titova et al., 2013; Van den Eynde et al., 2012). Likewise, functional magnetic resonance imaging (fMRI) studies in ED reveal abnormal patterns of resting-state connectivity in the default-mode network (Cowdrey et al., 2014) and other intrinsic brain networks incorporating the ACC and insula (Amianto et al., 2013). ED patients also show abnormal VS activation in response to rewarding and aversive stimuli (Wagner et al., 2010, 2007). Patients with BN show hyperactivity in medial frontal lobe regions during inhibition of prepotent actions (Lock et al., 2011), and hypoactivity in response to food cues (Joos et al., 2011).
Among the various neural substrates implicated in ED pathology, a target of particular interest that is accessible to rTMS is the dorsomedial prefrontal cortex (dmPFC). The dmPFC plays an important role in forms of self-control, including self-inhibition of movements (Brass and Haggard, 2007), self-cessation of loss-chasing in pathological gamblers (Campbell-Meiklejohn et al., 2008), self-suppression of emotional responses (Kühn et al., 2011), and impulse control (Cho et al., 2013). Likewise, non-invasive stimulation of medial prefrontal areas, via rTMS, enhances inhibitory control over prepotent responses (Obeso et al., 2013), improves subjective choice for delayed rewards in a delayed discounting task, and interferes with striatal dopamine (Cho et al., 2015). In sum, stimulation of the medial prefrontal cortex using rTMS may alter the top-down executive control of the dmPFC to striatal regions associated with the urge to binge and purge, thereby improving symptom severity.
Considering this literature on the role for the DMPFC in self-regulation, one potential implication is that DMPFC-rTMS might be worth exploring as a therapeutic approach for addressing self-regulatory deficits in ED. To date, most previous rTMS studies in ED have focused on the conventional target, the left dorsolateral prefrontal cortex (dlPFC). Although dlPFC-rTMS reduces cue-induced food craving in BN (Uher et al., 2005; Van den Eynde et al., 2010), a double-blind trial of dlPFC-rTMS for binge−purge symptoms found no significant improvement over sham (Walpoth et al., 2008).
There are few reported studies of dmPFC-targeted rTMS to date. However, we recently investigated dmPFC-rTMS in major depression (MDD), finding efficacy rates comparable to dlPFC-rTMS, but with sharply dichotomous outcomes of improvement (Downar et al., 2014). Using resting-state functional MRI (rsfMRI), we found that low dmPFC–subcortical connectivity predicted successful outcome, and that symptomatic improvements were associated with increased dmPFC connectivity. We also reported the serendipitous finding of full remission from binge−purge symptoms during dmPFC-rTMS for comorbid depression, in a patient with treatment-refractory BN (Downar et al., 2012). These observations suggested that dmPFC-rTMS might treat a subset of BN patients, and that enhanced frontal−subcortical connectivity on rsfMRI might accompany symptomatic improvement.
Here we used rsfMRI to identify patterns of functional connectivity associated with response to dmPFC-rTMS, delivered as an intervention-probe to a series of 28 ED patients with treatment-refractory binge and purge behaviors. Based on our previous findings, we formulated two hypotheses: first, that lower baseline dmPFC connectivity on rsfMRI would predict successful treatment; second, that successful treatment would be associated with increases in connectivity through a fronto-subcortical circuit incorporating the dmPFC.
2. Methods
2.1. Subjects
28 subjects (26 female, age range = 20 − 56 years, mean = 31.0 ± 9.5 years) meeting DSM-5 criteria for AN−BP (n = 11), BN (n = 17) participated in this proof-of-concept, open-label study. Subjects included individuals with bingeing and purging behavior, both at a normal and lower than normal weight. BMI ranged from 14.5–28.8. AN−BP participants displayed both bingeing and purging behaviors. While there are certainly important differences in the clinical features of these disorders (e.g., failure to maintain normal body weight, or the presence of restricting symptoms), binge and purge behaviors were the specific target symptom for the purposes of this pilot study. Subjects engaged in ≥2 cumulative objective binge/purge episodes weekly at baseline to be included in the study. Diagnoses were established through interviews with 2 independent, Canadian Royal College-certified psychiatrists (authors JD, PG, PC, BW). In recognition of the high prevalence of psychiatric comorbidities in this population, and in order to characterize rather than minimizing sample heterogeneity, common comorbidities were not excluded This inclusion of comorbidities is in line with our previous work in MDD (Downar et al., 2014). Comorbid diagnoses included MDD (n = 16), obsessive–compulsive disorder (n = 6), post-traumatic stress disorder (n = 8), and bipolar disorder (n = 6). Patients with a history of a psychotic disorder, neurological disorder, active substance abuse, or contraindications to MRI or rTMS were excluded. All subjects had no improvements in bingeing/purging to or were unable to tolerate at least 1 previous medication trial (n = 27), and had also not responded to previous inpatient/outpatient treatment courses in terms of an improvement of binges and purges (n = 25). On average, patients had not respond to 2.61 ± 2.44 inpatient/outpatient treatments, and 3.00 ± 2.37 medication trials. Current medications included SSRIs (n = 11), antipsychotics (n = 11), benzodiazepines (n = 8), trazodone (n = 5), and SNRIs (n = 3). As routinely stipulated (Salomons et al., 2014), subjects were required to refrain from any medication changes during and for ≥4 weeks before rTMS.
2.2. Clinical outcomes
The clinical outcome of interest, weekly frequency of binge and purge episodes, was monitored via a structured clinical interview, the Eating Disorder Examination (EDE), administered 1 week before treatment, at each week during treatment, and at 4 weeks post-treatment. For the purposes of stratification, response was defined as ≥50% decrease in objective binge (>1000 calories per binge) and purge episode frequency from pre-rTMS to 4 weeks post-rTMS (at follow-up). Pre-treatment EDE assessments acquired binge/purge frequency for the 4 weeks prior to treatment, and the post-treatment EDE acquired frequency for the 4 weeks following treatment, and so the pre- and post-treatment scores were obtained from weeks outside of the rTMS treatment sessions. Data on bingeing and purging frequencies derived from the EDE was divided by 4 to generate a weekly frequency for bingeing and purging. To obtain weekly scores, EDE frequencies (which measure severity over 4 weeks) were divided over the 4 weeks. Although a more conservative criterion is sometimes used in clinical efficacy studies, our primary aim was to characterize and distinguish the neural activity of subpopulations of patients showing improvement versus non-improvement in this population. A set of clinical and psychometric data, including the 17-item Hamilton Rating Scale for Depression (HamD17), Beck Depression Index-II (BDI-II) and Beck Anxiety Index (BAI) were also collected as secondary metrics. To assess the distribution of outcomes across the study sample, we employed kernel density estimation by applying the Epanechnikov kernel (Epanechnikov, 1969) in Stata13 (StataCorp). Two-tailed t-tests (Bonferroni-adjusted) were performed to determine the significance of differences in clinical measures between groups and between timepoints. The nonparametric Mann–Whitney U test was performed for comparisons of binge and purge frequency do to the markedly non-normal distribution of these data.
2.3. Neuroimaging acquisition
The neuroimaging protocol followed parameters reported in detail in our previous studies of rsfMRI with dmPFC-rTMS (Downar et al., 2014; Salomons et al., 2014). All subjects underwent 3 T MRI the week prior to and the week after rTMS, with a T1-weighted (TE = 12 ms, TI = 300 ms, flip-angle = 20°, 0.94 × 0.94 × 1.5 mm voxels) and a 10-min eyes-open resting state functional MRI scan (TE = 30 ms, TR = 2000 ms, flip-angle = 85°, 3.4 × 3.4 × 5 mm voxels).
2.4. Neuronavigation and rTMS treatment
Neuronavigation and rTMS treatment followed our previously reported protocols for dmPFC-rTMS in MDD (Downar et al., 2014; Salomons et al., 2014). Neuronavigation employed the Visor 2.0 system (Advanced Neuro Technologies, Enschede, Netherlands) to target the stereotaxic coordinate (X = 0, Y = +30, Z = +30) in Talairach space (Talairach and Tournoux, 1988). rTMS employed the MagPro-R30 system (MagVenture, Farum, Denmark) and a Cool-DB80 coil. Motor thresholds were determined via contractions of the extensor hallucis longus (Hayward et al., 2007). Stimulation of the dmPFC was delivered at 120% resting motor threshold, at 10 Hz, 5 s on, 10 s off, 3000 pulses/hemisphere, with left then right lateralized coil orientation (Terao et al., 2001). Unilateral stimulation was achieved by orienting the coil vertex at the stereotactic target laterally, with current flow oriented towards the desired hemisphere (Harmer et al., 2001). If a patient missed an rTMS session for logistical reasons, an additional session was added at the end of the course of treatment (no patient required more than 4 such sessions). Patients underwent 20 sessions of dmPFC-rTMS on weekdays; responders with any residual binge/purge symptoms were extended to 30 sessions (mean = 21.2 ± 3.7 sessions, range = 18–30).
2.5. MRI preprocessing, seed selection and statistical analysis
Preprocessing of resting-state fMRI data from patients employed FSL (Jenkinson et al., 2012), following methods as reported in our previous dmPFC-rTMS studies (Salomons et al., 2014) for slice-timing correction, segmentation, motion correction, spatial smoothing (6 mm FWHM Gaussian kernel), correction for white matter and cerebrospinal fluid signal artifacts, bandpass filtering (0.009–0.09 Hz), and linear co-registration to the MNI-152 template.
Seed regions-of-interest (ROIs) were defined a priori from the parcellation atlas of Craddock et al. (2012) for the dmPFC and adjacent dorsal ACC (dACC) based on proximity to the stimulation target, following our analyses in a previous study of dmPFC-rTMS in MDD (Salomons et al., 2014). The seeds are described in detail in that study; their centroids were MNI = −4, 44, 42 for dmPFC and MNI = 0, 38, 24 for dACC (see Figs. 2 and 3 for images of the ROIs). For first-level analysis, each seed ROI was co-registered to each subject's brain via nonlinear transformation, then applied as masks to extract the mean ROI time series. These time series were used to generate whole-brain maps of positively and negatively correlated voxels with the seed ROI before and after treatment, via a linear regression analysis using a fixed effects model at the individual-subject level.
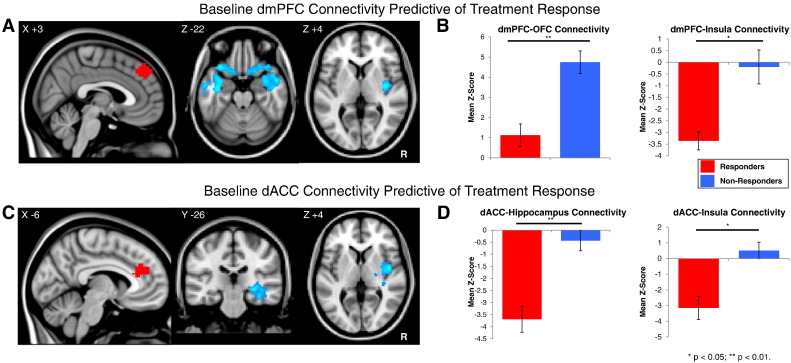
Fig. 2.
A: Regions with lower pre-treatment functional connectivity to the dmPFC seed (red) in responders versus nonresponders to dmPFC-rTMS. B: Parameter estimates for pre-treatment functional connectivity between the dmPFC seed and the bilateral orbitofrontal cortex (OFC) and right posterior insula in responders and non-responders prior to treatment. C: Regions with lower pre-treatment functional connectivity to the dACC seed (red) in responders versus nonresponders to dmPFC-rTMS. D: Parameter estimates for functional connectivity between the dmPFC seed and right hippocampus and posterior insula in responders and non-responders prior to treatment.
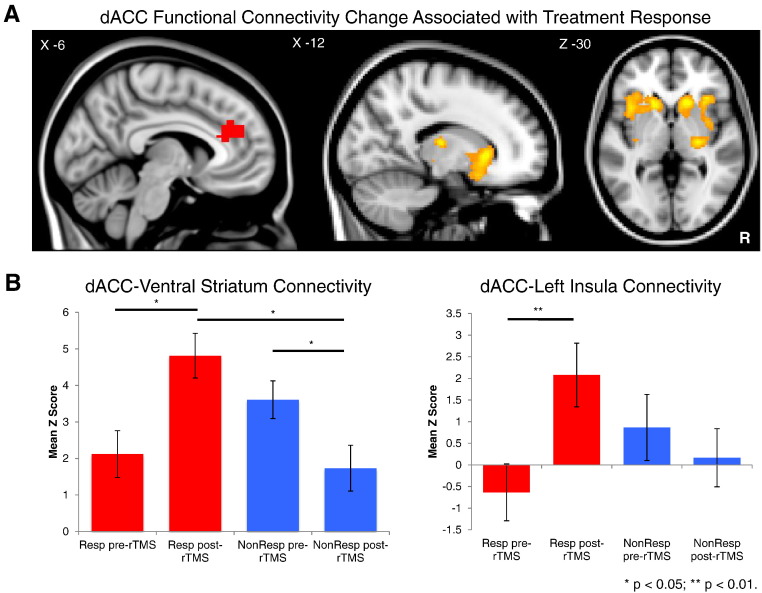
Fig. 3.
A: Regions showing significantly greater increases in functional connectivity to the dACC seed from pre- to post-treatment in responders versus nonresponders to dmPFC-rTMS. B: Parameter estimates for functional connectivity between the dACC seed and the ventral striatum (right) and insula (left) in responders and nonresponders before and after undergoing dmPFC-rTMS.
Group level analysis was performed using FSL's FLAME mixed effects model (Beckmann et al., 2003). We first identified regions where the degree of correlation at baseline to the ROIs differed significantly between responders and non-responders. To assess whether differing changes in resting-state connectivity over treatment would be associated with different outcomes, we then compared pre- and post-treatment scans to identify regions where the pre- to post-treatment change in correlation to the seed ROIs differed significantly between responders and non-responders. The results of these analyses were transformed into z-score maps, correcting for multiple comparisons using a Gaussian random field theory cluster-based correction (z-score > 1.96, cluster significance p > 0.05, corrected). Finally, the clusters previously obtained from group-level baseline predictor and change analyses were co-registered to patients' individual scans, and connectivity values were extracted for each subject.
3. Results
3.1. Primary clinical outcomes
No serious or treatment-limiting adverse effects occurred, and subjects reported only the localized scalp discomfort and transient headache routinely associated with rTMS treatment sessions.
Baseline binge and purge episode frequency per week was 11.1 ± SD 18.6 and 17.6 ± SD 31.7 (Table 1). Combining both subpopulations, there was no significant overall change in binge frequency (post-rTMS mean = 8.6 ± 2.9, mean percent improvement = 20.4 ± 77.0, Wilcoxon signed-rank W27 = 1.29, p = 0.20) but a significant decrease in purge frequency was found (post-rTMS mean = 20.33 ± 10.2, mean percent improvement = 35.7 ± 62.1, Wilcoxon signed-rank W27 = 2.20, p = 0.03). 16 of 28 subjects achieved ≥50% reduction in binge and purge frequency from baseline to follow-up. However, outcomes were widely divergent across individuals, ranging from full remission to marked worsening of symptoms (Fig. 1). The degree of improvement across individuals followed a non-normal distribution (Shapiro–Wilk W27 = 0.85, p = 0.001). For this reason, subsequent analyses were performed to characterize responder and non-responder subpopulations separately.
Table 1.
Descriptive statistics for all patients, rTMS responders and non-responders.
| All subjects (n = 28) | rTMS responders (n = 16) | rTMS non-responders (n = 12) | Responder vs. non-responder difference (p) | |
|---|---|---|---|---|
| Females (males) | 26 (2) | 15 (1) | 11 (1) | t0.04 (0.97) |
| Age | 31.04 (±9.48) | 30.75 (±8.23) | 31.42 (±11.32) | t0.04 (0.97) |
| Duration of ED (years) | 14.75 (±10.19) | 12.75 (±7.51) | 17.42 (±12.83) | t0.84 (0.41) |
| # Prior treatments | 2.61 (±2.44) | 2.63 (±2.06) | 2.58 (±2.97) | t0.04 (0.97) |
| # Prior medications | 3.00 (±2.37) | 2.38 (±2.00) | 3.83 (±2.66) | t0.99 (0.33) |
| # of hospitalizations | 3.57 (±4.20) | 5.00 (±3.14) | 6.42 (±5.35) | t0.65 (0.52) |
| Baseline BMI | 19.03 (±5.33) | 19.81 (±3.68) | 18.05 (±3.22) | t1.35 (0.19) |
| AN−BP (BN) | 12 (16) | 5 (11) | 7 (5) | t0.87 (0.39) |
| Weekly binge frequency (pre-rTMS) | 11.14 (±18.59) | 7.48 (±5.41) | 20.55 (±26.82) | U = 0.91 (0.36) |
| Weekly purge frequency (pre-rTMS) | 17.57 (±31.72) | 8.75 (±6.93) | 36.68 (±44.30) | U 1.21 (0.23) |
| Weekly binge frequency (post-rTMS) | 8.32 (±15.09) | 1.39 (±1.88) | 18.25 (±19.41) | U 4.12 (<0.0001)** |
| Weekly purge frequency (post-rTMS) | 19.63 (±53.96) | 1.34 (±2.01) | 54.53 (±76.93) | U 3.51 (0.0004)** |
Abbreviations: AN−BP = Anorexia nervosa binge/purge subtype; BMI = body mass index; BN = bulimia nervosa; ED = eating disorders; rTMS = repetitive transcranial magnetic stimulation. Values indicate means, with standard deviations reported in brackets.
U indicates nonparametric significance testing using the Mann–Whitney U.
** p < 0.05.
Fig. 1.
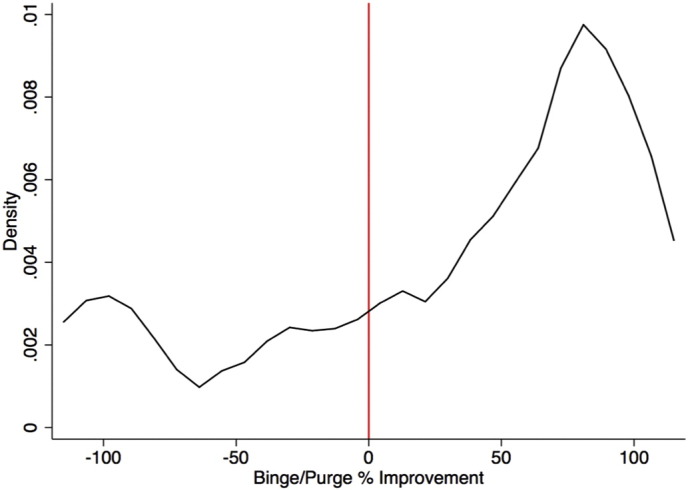
Probability distribution function of binge and purge percent improvement across all patients following dmPFC-rTMS.
Among responders, symptom frequency improved from 7.5 ± SD 5.4–1.4 ± SD 1.9 binge episodes/week (Wilcoxon signed-rank W15 = 3.39, p = 0.0007) and from 8.7 ± SD 6.9–1.3 ± SD 2.0 purge episodes/week (W15 = 3.49, p = 0.0005). Among non-responders, symptom frequency before and after treatment showed a nonsignificant worsening from 20.5 ± SD 26.8–18.3 ± SD 19.41 binge episodes/week (W11 = −1.30, p = 0.20) and 36.7 ± SD 44.3–54.5 ± SD 76.9 purge episodes/week (W11 = −0.59, p = 0.55). There was no significant difference between responders and non-responders at baseline for either binge (Mann–Whitney U26 = 0.91, p = 0.36) or purge (U26 = 1.21, p = 0.23) frequency. Further results pertaining to global and subscale changes on the EDE are reported in our supplementary document.
3.2. Psychometric outcomes
We also assessed the relationships between the following pre-treatment clinical measures and binge/purge percent improvement: baseline BMI, illness duration, baseline binge/purge severity, age, ED diagnosis (AN−BP or BN), presence of co-morbidity (MDD, obsessive–compulsive disorder, post-traumatic stress disorder, bipolar disorder), current medication (benzodiazepine, selective serotonin reuptake inhibitor, antipsychotic), and baseline severity and percent improvement of secondary measures (HamD17, BDI-II, BAI). No clinical measures, diagnoses, or medications predicted rTMS treatment response, or percent improvement in binge/purge frequency, either before or after Bonferroni correction for multiple comparisons (Spearman's rank correlation coefficient ρ26 < 0.30, p > 0.12 for each comparison above).
3.3. Functional connectivity predictors of response
Pre-treatment rsfMRI revealed significant differences between responders and non-responders from the dmPFC and dACC ROIs (Fig. 2). From the dmPFC seed, responders had significantly lower pre-treatment connectivity to bilateral temporal pole, OFC, and right posterior insula, and higher connectivity to bilateral lateral and medial occipital cortex (Table 2). Pre-treatment functional connectivity from dmPFC to left and right lateral OFC was slightly positive in responders (z = 1.12 ± 0.56), and positive in both non-responders (z = 4.75 ± 0.56). Parameter estimates also revealed that pre-treatment functional connectivity from dmPFC to right insula was negative in responders (z = −3.36 ± 0.39) and non-responders (z = −0.20 ± 0.73) (Fig. 2B). Both dmPFC–insula and dmPFC–OFC connectivity at baseline were significantly anti-correlated to binge/purge percent improvement (r = −0.42 p = 0.03 and r = −0.46 p = 0.01, respectively).
Table 2.
Brain regions where pre-treatment functional connectivity to dmPFC and dACC seeds differed significantly between rTMS responders and nonresponders.
| Seed | Brain region | Brodmann area | MNI coordinates |
z | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| dACC | Nonresponders > responders | ||||||
| R | Posterior insula | 13 | 44 | −6 | 4 | 3.70 | |
| R | Hippocampus | 32 | −28 | −8 | 3.61 | ||
| R | Posterior superior/middle temporal gyrus | 21/22 | 50 | −18 | −8 | 3.78 | |
| Responders > nonresponders | |||||||
| R | Precuneus | 7 | 10 | −58 | 58 | 3.64 | |
| L | Precuneus | 7 | −20 | −58 | 60 | 4.00 | |
| R | Intracalcarine cortex | 18 | 8 | −80 | 2 | 3.04 | |
| B | Cuneus | 18 | 8 | −90 | 22 | 3.11 | |
| dmPFC | Nonresponders > responders | ||||||
| R | Lateral OFC | 25/47 | 34 | 24 | −14 | 2.83 | |
| L | Lateral OFC | 47 | −34 | 22 | −24 | 3.87 | |
| R | Posterior insula | 13 | 38 | −10 | 4 | 3.79 | |
| L | Temporal pole | 28/38 | −38 | −10 | −20 | 3.84 | |
| R | Temporal pole | 28/38 | 52 | 4 | −24 | 3.35 | |
| Responders > nonresponders | |||||||
| B | Intracalcarine cortex, lingual gyrus | 18 | 4 | −80 | 2 | 4.06 | |
From the dACC seed, responders had significantly lower pre-treatment connectivity to the right posterior insula, putamen, hippocampus, and middle temporal gyrus, and higher connectivity to superior parietal and medial occipital cortices and precuneus (Table 2; Fig. 2C). Parameter estimates revealed that pre-treatment functional connectivity from dACC to right hippocampus was negative in responders (z = −3.69 ± 0.42), non-responders (z = −0.44 ± 0.46). Pre-treatment functional connectivity from dACC to right insula and thalamus was negative in responders (z = −3.15 ± 0.74) but positive in non-responders (z = 0.51 ± 0.53) (Fig. 2D). dACC–insula connectivity at baseline significantly anti-correlated to binge/purge percent improvement (r = −0.38 p = 0.01).
3.4. Functional connectivity changes associated with response
There were also significant differences between responders and non-responders in terms of the observed changes in functional connectivity to the dACC and dmPFC before and after treatment. Compared to non-responders, responders underwent significantly greater increases in functional connectivity between the dACC and bilateral caudate nucleus and VS, anterior insula, inferior frontal gyrus and adjacent OFC, and right putamen (Table 3, Fig. 3A). Responders also showed greater increases in functional connectivity between the dmPFC and right middle temporal gyrus, and greater decreases in functional connectivity between the dmPFC and bilateral thalamus (Table 3).
Table 3.
Brain regions where the change in functional connectivity to dACC and dmPFC seed from pre- to post-treatment differed significantly between rTMS responders and nonresponders.
| Seed | Brain region | Brodmann area | MNI coordinates |
z | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| dACC | Increased connectivity in responders > nonresponders | ||||||
| L | Caudate/ventral striatum | −14 | 18 | 2 | 3.89 | ||
| R | Caudate/ventral striatum | 16 | 20 | 0 | 4.02 | ||
| R | Insula, putamen | 13 | 32 | −18 | −2 | 4.03 | |
| R | Anterior insula/OFC | 13/47 | 36 | 18 | −8 | 3.49 | |
| L | Anterior insula/OFC | 13/47 | −38 | 14 | 2 | 3.16 | |
| L | Inferior frontal gyrus | 47 | −34 | 22 | −14 | 2.66 | |
| B | Thalamus | 14 | −18 | 14 | 3.13 | ||
| dmPFC | Increased connectivity in responders > nonresponders | ||||||
| R | Middle temporal gyrus | 21/22 | 50 | −26 | −10 | 4.27 | |
| Decreased connectivity in responders > nonresponders | |||||||
| B | Thalamus | −4 | −26 | 6 | 3.08 | ||
Inspection of parameter estimates revealed marked differences between responders and non-responders in how dACC–VS functional connectivity changed following treatment (Fig. 3B). The responder group showed lower functional connectivity between the dACC and VS prior to treatment, increasing significantly following successful treatment (t12 = 2.37, p = 0.035). Conversely, connectivity in non-responders decreased significantly (t8 = 2.316, p = 0.05), becoming significantly lower than treatment responders post-treatment (t20 = 2.409, p = 0.03). A similar pattern of significant increases in functional connectivity in responders (t12 = 3.314, p = 0.006), but decreases in functional connectivity in non-responders, trending to significance (t8 = 2.078, p = 0.07), was observed between the dACC and left anterior insula over the course of treatment (Fig. 3B). Binge/purge percent improvement was significantly correlated with the change in dACC–left anterior insula connectivity (r = 0.45, p = 0.04) and trended to significant correlation with the change in dACC–VS connectivity (r = 0.39, p = 0.07).
4. Discussion
To our knowledge, this is the first report of neuroimaging findings in ED patients undergoing non-invasive brain stimulation. As an intervention-probe, dmPFC-rTMS divided patients into treatment-responsive and nonresponsive groups, with widespread differences in resting-state connectivity apparent between these groups at baseline. rTMS-responsive patients showed baseline hypoconnectivity from the stimulation target to other cortical and subcortical regions, relative to non-responders. In responders, frontostriatal connectivity was enhanced following dmPFC-rTMS, in association with improvement in binge and purge frequency. Conversely, in patients with higher baseline connectivity, the same intervention produced the opposite effect, reducing frontostriatal connectivity, in association with non-improvement or worsening of symptoms.
Of particular interest were the divergent trajectories of outcome across individuals. While many subjects showed marked improvement, a large proportion of subjects did not improve, or even showed deterioration, when exposed to the same intervention. The observation of divergent responses to dmPFC-rTMS in ED is in keeping with our previous report of a sharply bimodal distribution of responses to dmPFC-rTMS in MDD (Downar et al., 2014). Of note, similarly dichotomous trajectories of improvement have been identified in clinical trials of duloxetine (Gueorguieva et al., 2011) and escitalopram (Thase et al., 2011).
The possibility of a neurobiological basis for the responder–non-responder dichotomy in the present study is supported by differences in resting-state functional connectivity observed between the two groups before treatment. In keeping with our first hypothesis, responders had significantly lower functional connectivity from the dmPFC and adjacent dACC to subcortical and cortical areas involved in emotion generation and regulation: hippocampus, lateral orbitofrontal cortex, and insula. These findings are consistent with several previous studies in MDD patients, linking baseline resting-state hypoconnectivity to better rTMS response (Fox et al., 2013, 2012; Liston et al., 2014; Salomons et al., 2014).
Another key finding in the present study is that the same 10 Hz intervention had widely divergent effects on functional connectivity across individuals: patients with high baseline cortico-cortical and fronto-insular connectivity saw significant reductions after 10 Hz rTMS, while patients with low baseline frontostriatal and fronto-insular connectivity saw significant increases after stimulation at the same frequency. This phenomenon is unlikely to represent a regression to the mean, as the groups showed divergence rather than convergence in both symptomatology and functional connectivity over treatment. The results are also consistent with our previously reported observations of markedly dichotomous effects on striatal, thalamic and cortical functional connectivity from 10 Hz dmPFC-rTMS in patients with MDD (Salomons et al., 2014). The results are also consistent with previous studies of dlPFC-rTMS in depression: while some improved with high-frequency rTMS, others worsened on this regimen, and instead responded better to low-frequency stimulation of the same target (Kimbrell et al., 1999; Speer et al., 2009, 2000). These findings are consistent with mounting evidence that the physiological effects of a given rTMS protocol can vary widely across individuals, not just in magnitude but also in direction (Cárdenas-Morales et al., 2014; Eldaief et al., 2011; Hallett, 2007; Maeda et al., 2000).
One limitation of this preliminary study involves its relatively small sample size. Though the sample was slightly larger than those used in several recent rTMS-fMRI studies in more prevalent disorders such as MDD (Baeken et al., 2014; Liston et al., 2014; Salomons et al., 2014), the present study does not allow a more detailed characterization of potentially important clinical markers that could help distinguish treatment subpopulations. Although the present investigation did not find statistically significant pre-treatment differences between responders and non-responders in terms of duration of illness, BMI, and binge/purge frequency, future studies with higher power will be necessary to better assess whether those with more severe illness in general are less likely to respond to treatment. Another caveat relates to the inclusion of multiple DSM-5 diagnostic categories in the current sample, which embraced ED patients with binge−purge behaviors alongside common comorbidities. On this point, we note that the sample composition was chosen to be representative of clinical populations presenting with refractory binge−purge symptoms, and that no DSM-5 diagnosis significantly correlated with rTMS response. More generally, it is unclear that current categorical nosologies map particularly well on to rTMS responsiveness in general; the distinction between unipolar and bipolar depression had no bearing on outcome in a recent meta-analysis of rTMS efficacy (Berlim et al., 2014). We aware that some of our subjects were underweight, and that improvements in bingeing and purging represent a limited area of improvement for such individuals. However, given the exploratory nature of this trial, and the notorious difficulty in treatment individuals with ANBN, we felt it was important to include such individuals in the trial. It is clear that the question of whether dmPFC-rTMS aids other forms of disordered eating (including restrictive-type behaviors) is still an important area for future investigation.
Another potential criticism relates to the use of an open-label design without sham stimulation. Since no previous studies have examined dmPFC-rTMS in ED, and since the primary aim of the study was to apply an intervention-probe and characterize neural predictors and correlates of response (as in our recent work in depression), we opted for the present approach in order to identify sources of heterogeneity that might confound a future sham-controlled trial. The results suggest that three potentially critical confounds will need to be accommodated in future trials: first, the presence of distinct neural endophenotypes, not readily apparent on standard diagnostic criteria, but with differential responses to the intervention at both the neural and the clinical level; second, the need to tailor rTMS parameters to the individual patient in order to avoid paradoxical effects; third, whether predictors of improvement relate to illness mechanism, or a capacity to change with rTMS treatment, or both. Our results suggest that rsfMRI techniques help address both of these issues in any future randomized controlled study of rTMS in ED.
In conclusion, dmPFC-rTMS shows promising therapeutic effects in a subset of ED patients with refractory binge and purge behaviors. As in MDD, therapeutic effects are associated with increases in initially low levels of fronto-striatal functional connectivity, detectable on pre-treatment rsfMRI. However, a significant proportion of patients have initially higher baseline fronto-striatal connectivity, and in these patients, the same intervention produces paradoxical decreases in frontostriatal connectivity after treatment, alongside non-response or worsening of clinical symptoms. A randomized controlled trial of dmPFC-rTMS in ED would be a reasonable next step. However, the results of this study join a growing body of evidence (Fox et al., 2013), suggesting that future trials of rTMS will need to take into account both the heterogeneity of individual patients' neural activity, and the heterogeneity of effects ensuing from the stimulation itself. Pre-treatment neuroimaging may eventually play a crucial role in optimizing stimulation parameters, to maximize the chances of success in each patient presenting for treatment.
Conflicts of interest
Dr. Downar has received research support from the Canadian Institutes of Health Research, the National Institutes of Health, the Klarman Family Foundation, the Buchan Family Foundation, and the Toronto General and Western Hospital Foundation, as well as a travel stipend from Lundbeck and in-kind equipment support for an investigator-initiated study from MagVenture. Dr. Giacobbe is a consultant for St. Jude Medical and has received personal fees from Eli Lilly Canada, Bristol-Myers Squibb, AstraZeneca, and Pfizer. He has also received research support from the Canadian Institutes of Health Research, Michael J. Fox Foundation for Parkinson's Research, the Brain and Behavior Research Foundation (formerly National Alliance for Research on Schizophrenia and Depression), and the National Institutes of Health. Dr. Woodside has received funding from the National Institute of Mental Health, The Price Foundation, and the Klarman Foundation, and Drs. Colton, Olmsted, Ms. Lam, and Ms. Dunlop report no financial relationships with commercial interests.
Acknowledgements
This study was supported by an award from the Klarman Family Foundation Grants Program in Eating Disorders Research. Authors KD and JD had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Sources of funding did not have a role in the design and conduct of the study; data collection, management, analysis and interpretation of the data; and the preparation, review, and approval of the manuscript.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.06.008.
Supplementary material
Supplementary material.
References
- Amianto F., D'Agata F., Lavagnino L., Caroppo P., Abbate-Daga G., Righi D., Scarone S., Bergui M., Mortara P., Fassino S. Intrinsic connectivity networks within cerebellum and beyond in eating disorders. Cerebellum. Lond. Engl. 2013;12(5):623–631. doi: 10.1007/s12311-013-0471-1. 23553468 [DOI] [PubMed] [Google Scholar]
- Arcelus J., Mitchell A.J., Wales J., Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry. 2011;68(7):724–731. doi: 10.1001/archgenpsychiatry.2011.74. 21727255 [DOI] [PubMed] [Google Scholar]
- Baeken C., Marinazzo D., Wu G.-R., Van Schuerbeek P., De Mey J., Marchetti I., Vanderhasselt M.-A., Remue J., Luypaert R., De Raedt R. Accelerated HF-rTMS in treatment-resistant unipolar depression: insights from subgenual anterior cingulate functional connectivity. World J. Biol. Psychiatry. 2014;15(4):286–297. doi: 10.3109/15622975.2013.872295. 24447053 [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. 14568475 [DOI] [PubMed] [Google Scholar]
- Berlim M.T., van den Eynde F., Tovar-Perdomo S., Daskalakis Z.J. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol. Med. 2014;44(2):225–239. doi: 10.1017/S0033291713000512. 23507264 [DOI] [PubMed] [Google Scholar]
- Brass M., Haggard P. To do or not to do: the neural signature of self-control. J. Neurosci. 2007;27(34):9141–9145. doi: 10.1523/JNEUROSCI.0924-07.2007. 17715350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn D.K., Woolrich M.W., Passingham R.E., Rogers R.D. Knowing when to stop: the brain mechanisms of chasing losses. Biol. Psychiatry. 2008;63(3):293–300. doi: 10.1016/j.biopsych.2007.05.014. 17662257 [DOI] [PubMed] [Google Scholar]
- Cárdenas-Morales L., Volz L.J., Michely J., Rehme A.K., Pool E.-M., Nettekoven C., Eickhoff S.B., Fink G.R., Grefkes C. Network connectivity and individual responses to brain stimulation in the human motor system. Cereb. Cortex. 2014;24(7):1697–1707. doi: 10.1093/cercor/bht023. 23395849 [DOI] [PubMed] [Google Scholar]
- Carter J.C., Mercer-Lynn K.B., Norwood S.J., Bewell-Weiss C.V., Crosby R.D., Woodside D.B., Olmsted M.P. A prospective study of predictors of relapse in anorexia nervosa: implications for relapse prevention. Psychiatry Res. 2012;200(2–3):518–523. doi: 10.1016/j.psychres.2012.04.037. 22657951 [DOI] [PubMed] [Google Scholar]
- Cho S.S., Koshimori Y., Aminian K., Obeso I., Rusjan P., Lang A.E., Daskalakis Z.J., Houle S., Strafella A.P. Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology. 2015;40(3):546–553. doi: 10.1038/npp.2014.211. 25168685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.S., Pellecchia G., Aminian K., Ray N., Segura B., Obeso I., Strafella A.P. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr. 2013;26(3):479–487. doi: 10.1007/s10548-012-0270-x. 23274773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdrey F.A., Filippini N., Park R.J., Smith S.M., McCabe C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum. Brain Mapp. 2014;35(2):483–491. doi: 10.1002/hbm.22202. 23033154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock R.C., James G.A., Holtzheimer P.E., Hu X.P., Mayberg H.S. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 2012;33(8):1914–1928. doi: 10.1002/hbm.21333. 21769991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J., Geraci J., Salomons T.V., Dunlop K., Wheeler S., McAndrews M.P., Bakker N., Blumberger D.M., Daskalakis Z.J., Kennedy S.H., Flint A.J., Giacobbe P. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol. Psychiatry. 2014;76(3):176–185. doi: 10.1016/j.biopsych.2013.10.026. 24388670 [DOI] [PubMed] [Google Scholar]
- Downar J., Sankar A., Giacobbe P., Woodside B., Colton P. Unanticipated rapid remission of refractory bulimia nervosa, during high-dose repetitive transcranial magnetic stimulation of the dorsomedial prefrontal cortex: a case report. Front. Psychiatry. 2012;3:30. doi: 10.3389/fpsyt.2012.00030. 22529822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief M.C., Halko M.A., Buckner R.L., Pascual-Leone A. Transcranial magnetic stimulation modulates the brain's intrinsic activity in a frequency-dependent manner. Proc. Natl. Acad. Sci. U. S. A. 2011;108(52):21229–21234. doi: 10.1073/pnas.1113103109. 22160708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epanechnikov V. Non-parametric estimation of a multivariate probability density. Theory Probab. Appl. 1969;14(1):153–158. [Google Scholar]
- Fox M.D., Buckner R.L., White M.P., Greicius M.D., Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry. 2012;72(7):595–603. doi: 10.1016/j.biopsych.2012.04.028. 22658708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Liu H., Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. 23142067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich H.-C., Walther S., Bendszus M., Biller A., Thomann P., Zeigermann S., Katus T., Brunner R., Zastrow A., Herzog W. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59(2):1106–1113. doi: 10.1016/j.neuroimage.2011.09.042. 21967727 [DOI] [PubMed] [Google Scholar]
- Gueorguieva R., Mallinckrodt C., Krystal J.H. Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses. Arch. Gen. Psychiatry. 2011;68(12):1227–1237. doi: 10.1001/archgenpsychiatry.2011.132. 22147842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. 17640522 [DOI] [PubMed] [Google Scholar]
- Harmer C.J., Thilo K.V., Rothwell J.C., Goodwin G.M. Transcranial magnetic stimulation of medial–frontal cortex impairs the processing of angry facial expressions. Nat. Neurosci. 2001;4(1):17–18. doi: 10.1038/82854. 11135640 [DOI] [PubMed] [Google Scholar]
- Hay P.J., Touyz S., Sud R. Treatment for severe and enduring anorexia nervosa: a review. Aust. N. Z. J. Psychiatry. 2012;46(12):1136–1144. doi: 10.1177/0004867412450469. 22696548 [DOI] [PubMed] [Google Scholar]
- Hayward G., Mehta M.A., Harmer C., Spinks T.J., Grasby P.M., Goodwin G.M. Exploring the physiological effects of double-cone coil TMS over the medial frontal cortex on the anterior cingulate cortex: an H2(15)O PET study. Eur. J. Neurosci. 2007;25(7):2224–2233. doi: 10.1111/j.1460-9568.2007.05430.x. 17439499 [DOI] [PubMed] [Google Scholar]
- Hudson J.I., Hiripi E., Pope H.G., Kessler R.C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. 16815322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. 21979382 [DOI] [PubMed] [Google Scholar]
- Joos A.A., Saum B., Zeeck A., Perlov E., Glauche V., Hartmann A., Freyer T., Sandholz A., Unterbrink T., van Elst L.T., Tüscher O. Frontocingular dysfunction in bulimia nervosa when confronted with disease-specific Stimuli. Eur. Eat. Disord. Rev. 2011;19(5):447–453. doi: 10.1002/erv.1150. 21809423 [DOI] [PubMed] [Google Scholar]
- Kimbrell T.A., Little J.T., Dunn R.T., Frye M.A., Greenberg B.D., Wassermann E.M., Repella J.D., Danielson A.L., Willis M.W., Benson B.E., Speer A.M., Osuch E., George M.S., Post R.M. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol. Psychiatry. 1999 doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gallinat J., Brass M. Vol. 6. 2011. pp. 1–4. (“Keep Calm and Carry On”: Structural Correlates of Expressive Suppression of Emotions. PLOS One). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N., Woodside D.B., Giacobbe P., Hamani C., Carter J.C., Norwood S.J., Sutandar K., Staab R., Elias G., Lyman C.H., Smith G.S., Lozano A.M. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. 2013;381(9875):1361–1370. doi: 10.1016/S0140-6736(12)62188-6. 23473846 [DOI] [PubMed] [Google Scholar]
- Liston C., Chen A.C., Zebley B.D., Drysdale A.T., Gordon R., Leuchter B., Voss H.U., Casey B.J., Etkin A., Dubin M.J. Default Mode Network Mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023. 24629537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J., Garrett A., Beenhakker J., Reiss A.L. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am. J. Psychiatry. 2011;168(1):55–64. doi: 10.1176/appi.ajp.2010.10010056. 21123315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F., Keenan J.P., Tormos J.M., Topka H., Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000;133(4):425–430. doi: 10.1007/s002210000432. 10985677 [DOI] [PubMed] [Google Scholar]
- Mitchell J.E., Agras S., Wonderlich S. Treatment of bulimia nervosa: where are we and where are we going? Int. J. Eat. Disord. 2007;40(2):95–101. doi: 10.1002/eat.20343. 17080448 [DOI] [PubMed] [Google Scholar]
- Obeso I., Cho S.S., Antonelli F., Houle S., Jahanshahi M., Ko J.H., Strafella A.P. Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul. 2013;6(5):769–776. doi: 10.1016/j.brs.2013.02.002. 23545472 [DOI] [PubMed] [Google Scholar]
- Olmsted M.P., Kaplan A.S., Rockert W. Defining remission and relapse in bulimia nervosa. Int. J. Eat. Disord. 2005;38(1):1–6. doi: 10.1002/eat.20144. 15971233 [DOI] [PubMed] [Google Scholar]
- Salomons T.V., Dunlop K., Kennedy S.H., Flint A., Geraci J., Giacobbe P., Downar J. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39(2):488–498. doi: 10.1038/npp.2013.222. 24150516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A., Vaitl D., Schienle A. Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. Neuroimage. 2010;50(2):639–643. doi: 10.1016/j.neuroimage.2009.12.063. 20035881 [DOI] [PubMed] [Google Scholar]
- Shapiro J.R., Berkman N.D., Brownley K.A., Sedway J.A., Lohr K.N., Bulik C.M. Bulimia nervosa treatment: a systematic review of randomized controlled trials. Int. J. Eat. Disord. 2007;40(4):321–336. doi: 10.1002/eat.20372. 17370288 [DOI] [PubMed] [Google Scholar]
- Speer A.M., Benson B.E., Kimbrell T.K., Wassermann E.M., Willis M.W., Herscovitch P., Post R.M. Opposite effects of high and low frequency rTMS on mood in depressed patients: relationship to baseline cerebral activity on PET. J. Affect. Disord. 2009;115(3):386–394. doi: 10.1016/j.jad.2008.10.006. 19027962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer A.M., Kimbrell T.A., Wassermann E.M., Repella J.D., Willis M.W., Herscovitch P., Post R.M. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry. 2000;48(12):1133–1141. doi: 10.1016/s0006-3223(00)01065-9. 11137053 [DOI] [PubMed] [Google Scholar]
- Sullivan P.F. Mortality in anorexia nervosa. Am. J. Psychiatry. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. 7793446 [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Neuropsychologia. 1988;39:145. [Google Scholar]
- Terao Y., Ugawa Y., Hanajima R., Machii K., Furubayashi T., Mochizuki H., Enomoto H., Shiio Y., Uesugi H., Iwata N.K., Kanazawa I. A single motor unit recording technique for studying the differential activation of corticospinal volleys by transcranial magnetic stimulation. Brain Res. Protoc. 2001;7(1):61–67. doi: 10.1016/s1385-299x(00)00063-5. [DOI] [PubMed] [Google Scholar]
- Thase M.E., Larsen K.G., Kennedy S.H. Assessing the “true” effect of active antidepressant therapy v. placebo in major depressive disorder: use of a mixture model. Br. J. Psychiatry. 2011;199(6):501–507. doi: 10.1192/bjp.bp.111.093336. 22130749 [DOI] [PubMed] [Google Scholar]
- Titova O.E., Hjorth O.C., Schiöth H.B., Brooks S.J. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies. B.M.C. Psychiatry. 2013;13:110. doi: 10.1186/1471-244X-13-110. 23570420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R., Yoganathan D., Mogg A., Eranti S.V., Treasure J., Campbell I.C., McLoughlin D.M., Schmidt U. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol. Psychiatry. 2005;58(10):840–842. doi: 10.1016/j.biopsych.2005.05.043. 16084855 [DOI] [PubMed] [Google Scholar]
- Van den Eynde F., Claudino A.M., Mogg A., Horrell L., Stahl D., Ribeiro W., Uher R., Campbell I., Schmidt U. Repetitive transcranial magnetic stimulation reduces cue-induced food craving in bulimic disorders. Biol. Psychiatry. 2010;67(8):793–795. doi: 10.1016/j.biopsych.2009.11.023. 20060105 [DOI] [PubMed] [Google Scholar]
- Van den Eynde F., Suda M., Broadbent H., Guillaume S., Van den Eynde M., Steiger H., Israel M., Berlim M., Giampietro V., Simmons A., Treasure J., Campbell I., Schmidt U. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur. Eat. Disord. Rev. 2012;20(2):94–105. doi: 10.1002/erv.1163. 22052722 [DOI] [PubMed] [Google Scholar]
- Wagner A., Aizenstein H., Venkatraman V.K., Bischoff-Grethe A., Fudge J., May J.C., Frank G.K., Bailer U.F., Fischer L., Putnam K., Kaye W.H. Altered striatal response to reward in bulimia nervosa after recovery. Int. J. Eat. Disord. 2010;43(4):289–294. doi: 10.1002/eat.20699. 19434606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A., Aizenstein H., Venkatraman V.K., Fudge J., May J.C., Mazurkewicz L., Frank G.K., Bailer U.F., Fischer L., Nguyen V., Carter C., Putnam K., Kaye W.H. Altered reward processing in women recovered from anorexia nervosa. Am. J. Psychiatry. 2007;164(12):1842–1849. doi: 10.1176/appi.ajp.2007.07040575. 18056239 [DOI] [PubMed] [Google Scholar]
- Walpoth M., Hoertnagl C., Mangweth-Matzek B., Kemmler G., Hinterhölzl J., Conca A., Hausmann A. Repetitive transcranial magnetic stimulation in bulimia nervosa: preliminary results of a single-centre, randomised, double-blind, sham-controlled trial in female outpatients. Psychother. Psychosom. 2008;77(1):57–60. doi: 10.1159/000110061. 18087209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.