Abstract
Smad-7 inhibited the transforming growth factor beta (TGF-β)-induced proteoglycan synthesis in chondrocytes and completely antagonized the effect of TGF-β on the proliferation of the cells. The aim of this study was to evaluate the contribution of Smad-7 to the pathophysiology of disc degeneration by determining the expression of Smad-7 in the degenerative intervertebral discs and its effect on the extracellular matrix metabolism of disc cells. Instability of the lumbar spine produced by imbalanced dynamic and static forces was used to induce intervertebral disc degeneration in rats. The expression of Smad-7 was assessed by the immunohistochemical method. Disc cell apoptosis was detected by in situ TUNEL staining. The effect of Smad-7 overexpression on the matrix metabolism of disc cells was analyzed in vitro by real-time polymerase chain reaction (PCR) and Western blotting. Finally, intradiscal injection of the Smad-7 overexpression lentivirus was performed to evaluate the in vivo effect of Smad-7 on disc degeneration. Radiographic and histomorphological examinations showed that lumbar disc degeneration became more and more severe in the rats with induced instability. Immunohistochemical observation demonstrated increasing protein expression of Smad-7 in the degenerative discs. A significantly positive correlation was found between Smad-7 expression and the degree of disc degeneration and between Smad-7 expression and disc cell apoptosis. Overexpression of Smad-7 in disc cells inhibited the expression of TGF-β1, collagen type-I, collagen type-II, and aggrecan and promoted the expression of MMP-13, but did not change the expression of ADAMTS-5. The in vivo findings illustrated that intradiscal injection of lentivirus vector with Smad-7 overexpression accelerated the progress of disc degeneration. In conclusion, Smad-7 was highly expressed in the degenerative discs. Overexpression of Smad-7 weakened the protective role of TGF-β and accelerated the progress of disc degeneration. Interference on Smad-7 might be a potential therapeutic method for the prevention and treatment of degenerative disc diseases.
Introduction
Transforming growth factor beta (TGF-β) is a pleiotropic cytokine that regulates diverse cellular and physiological processes, such as cell proliferation, differentiation, apoptosis, migration, adhesion, and intracellular matrix production. It could stimulate chondrocyte matrix production and has been considered as a potent agent to repair already damaged cartilage (Blaney Davidson and others 2007; Blom and others 2007). It was reported that TGF-β and its receptors were highly expressed in the disc tissues from patients with degenerative disc disease (DDD) (Peng and others 2006; Tolonen and others 2006), and the level of TGF-β expression was higher in patients with degenerated discs than in those with herniated discs (Lee and others 2009). TGF-β was also reported to be able to upregulate the synthesis of the key chondrocytic matrix molecules in disc cells in vitro (Murakami and others 2006; Risbud and others 2006; Tolonen and others 2006) and has been proposed as a therapeutic agent to prevent disc degeneration (Zhan and others 2004; Chen and others 2006).
TGF-β exerts its cellular effects by forming a complex with its type-II and type-I serine/threonine kinase receptors, and then the type-I receptor is phosphorylated and activated by the constitutively active type-II receptor. The activated TGF-β receptors phosphorylate Smad-2 and Smad-3, which form a heteromeric complex with Smad-4 and enter the nucleus, bind to DNA in a sequence-specific manner, and regulate gene transcription in cooperation with various transcriptional factors and coactivators and/or corepressors (Nakao and others 2002). At the same time, the inhibitory Smads (I-Smads, mainly Smad-7) can block the signal transduction. It either interferes with the binding of TGF-β to type-I receptors, preventing the activation of Smad-2/3 (Hayashi and others 1997), or recruits Smurf, an E3 ubiquitin ligase to receptors, targeting them for proteasome-mediated degradation (Hayashi and others 1997; Kavsak and others 2000). Therefore, Smad-7 performs a critical function in the process of TGF-β signal transduction.
It has been reported that altered expression of Smad-7 was associated with several human disease processes, such as cancers, inflammatory diseases, and tissue fibrosis (Briones-Orta and others 2011). Overexpression of Smad-7 was seen in renal carcinomas (Higgins and others 2003; Gumz and others 2007), squamous cell carcinomas of the head and neck region (Cromer and others 2004; Ginos and others 2004; Toruner and others 2004; Pyeon and others 2007), pancreatic (Kleeff and others 1999), and colorectal cancers (Stolfi and others 2014). Smad-7 expression was upregulated in inflammatory bowel disease mucosa and purified mucosal T cells (Monteleone and others 2001). Smad-7 overexpression in transgenic mice resulted in severe pathological alterations in multiple epithelial tissues (He and others 2002), whereas decreased Smad-7 expression contributed to cardiac fibrosis in the infarcted rat heart (Wang and others 2002), and deficient Smad-7 expression was causally linked to scleroderma (Dong and others 2002). In all of these diseases, the expression of TGF-β was abnormal.
Thus, appropriate expression of Smad-7 is critical for balanced TGF-β activity. It was also found that Smad-7 could mediate TGF-β-induced apoptosis of human prostate carcinoma cells, podocytes, Mv1Lu, MDCK, and COS7 cells (Landstrom and others 2000; Lallemand and others 2001; Mazars and others 2001; Schiffer and others 2001; Okado and others 2002; Sanchez-Capelo 2005). Overexpression of Smad-7 could inhibit important TGF-β-mediated biological responses such as cell proliferation and proteoglycan synthesis in chondrocytes (Scharstuhl and others 2003). However, there have never been investigative reports, at present, on the role of Smad-7 in disc cells and in the process of intervertebral disc degeneration (IVDD).
The present study was undertaken to evaluate the contribution of Smad-7 to IVDD and it has tried to elucidate its possible mechanisms by investigating the effect of Smad-7 on the catabolism and anabolism of disc cells. We believe that our study will deepen the understanding of disc degeneration and provide novel targets for pharmacological intervention of DDDs.
Materials and Methods
Animals and study protocols
Male Sprague–Dawley rats, aged 2 months, were used in this study. The rats were housed under controlled temperature and lighting conditions and received food and water ad libitum. The local Animal Care and Use Committee approved the experimental protocols and guidelines for laboratory procedures were followed at all times.
To testify our hypothesis that Smad-7 has an important role in the pathophysiology of IVDD, we designed our experiments to observe the following: (1) the expression of Smad-7 in the intervertebral discs and evaluate its association with the severity of degeneration and disc cell apoptosis, (2) the effect of Smad-7 on the metabolism of isolated disc cells, and (3) the in vivo effect of Smad-7 on disc degeneration.
Establishment for a rat model of lumbar IVDD
A modified surgical procedure was performed in 24 rats to induce imbalanced dynamic and static forces of the lumbar spine (Wang and others 2006). Other 24 rats just received a skin incision and suturation and severed as controls. At 6, 12, and 18 weeks, 8 operated and 8 control rats were euthanized by intraperitoneal administration of overdose of pentobarbital sodium separately. Lateral radiographic examination was performed for each rat. After that, lumbar spines, including L3 to L6 vertebral levels, were taken out en bloc; the paravertebral muscles and the posterior columns were removed thoroughly. Histomorphology evaluation was performed to verify the success of the rat model of lumbar IVDD, and TUNEL staining was used to detect the disc cell apoptosis as in our previous report (Zhao and others 2010).
Immunohistochemistry for Smad-7 expression
The obtained lumbar tissues were immediately washed with physiologic saline solution twice, followed by fixation in 4% paraformaldehyde for 24 h. After decalcification and embedding in paraffin, serial sections in 4 mm thickness were obtained in the sagittal plane and treated with xylene to remove paraffin and rehydrated in graded alcohol baths, followed by 3 rinses with phosphate-buffered saline (PBS). Slides were immunostained with the streptavidin-biotin peroxidase technique according to our previously described procedure (Zhao and others 2008). Antigen retrieval was performed in 10 mM citrate buffer (pH6.0) at 98°C for 10 min, allowing them to cool down to room temperature for 20 min. Then, sections were incubated in 1% H2O2 for 15 min for blocking the endogenous peroxidase activity. Sections were incubated overnight at 4°C with mouse Smad-7 monoclonal antibody (MAB2029; R&D Systems) after preincubation with 5% normal goat serum (S-1000; Vector) for 30 min at room temperature. Then, sections were incubated with the corresponding biotinylated goat anti-rabbit IgG (BA-1000; Vector), applied for 30 min at a dilution of 1:200, followed by a triple wash in PBS. Finally, at room temperature, the sections were incubated in ABC complex (Vectastain ABC kit, Cat#PK-6100; Vector) for 30 min. Staining was detected with DAB peroxides substrate solution for 5 min, followed by rinsing in distilled water briefly. The slides were dehydrated in graded ethanol, cleared in xylene, and mounted with Permount medium after counterstaining with Gill's hematoxylin solution for 3 min. Control experiments were incubated with the antibody preincubated with a blocking peptide. A set of sections, in which the primary antibodies were omitted, were used as negative controls. The Smad-7-positive disc cells were counted under 3 to 5 noncontinuous high-power fields (magnification, ×200) in each of 3 regions [cartilaginous endplate, annulus fibrosus (AF), and nucleus pulposus] from each of 3 discs per specimen and summed up. The percentage of Smad-7-positive disc cells compared with total disc cells was then calculated.
Isolation and culture of AF cells
AF cells were isolated from 2-month-old male Sprague–Dawley rats and cultured according to our previous method (Zhao and others 2010; Zhang and others 2011). First-passage cells maintained in a monolayer were used throughout the experiments.
Transfection of lentivirus vectors with Smad-7 overexpression into AF cells
The lentivirus vector with Smad-7 gene overexpression was constructed by Shanghai Genechem Co., Ltd. On the day of transfection, AF cells were replated at 5×104 cells/well in 24-well plates containing serum-free growth medium with polybrene (5 mg/mL). When they reached 50% confluence, cells were transfected with the Smad-7 overexpression lentivirus or negative control lentivirus at a multiplicity of infection of 100 and cells without transfection were used as control and cultured at 37°C and 5% CO2 for 24 h. Then, the supernatant was discarded and serum containing growth medium was added. Three days later, the transfection efficiency was measured by the frequency of green fluorescent protein (GFP)-positive cells. Real-time PCR and Western blotting were used to detect the expression level of Smad-7.
RNA isolation and quantitative real-time PCR for the transfected cells
At 3 days after the lentivirus infection, the AF cells were collected and lysed in Trizol reagent. Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's protocol. The quantity and purity of the isolated RNA were measured at OD260 and OD280 and analyzed on a 0.5×5 TBE (0.045 M Tris borate, 0.001 M EDTA) 1% agarose gel to check the integrity of the RNA. Then total RNA was reverse transcribed using the One-Step TaKaRa Primescript TM RT Reagent kit (TaKaRa). First-strand cDNA was synthesized from 1 μg total RNA. The gene expression of collagen type-I, collagen type-II, aggrecan, MMP-13, and ADAMTS-5 was measured by real-time PCR using SYBR Premix Ex Taq (Takara) and an ABI Prism 7500 sequence detection system (Applied Biosystems). The primers for actin, Smad-7, TGF-β1, collagen type-I, collagen type-II, aggrecan, MMP-13, and ADAMTS-5 were designed by TaKaRa Biotechnology. The target sites on these genes and PCR product length are shown in Table 1.
Table 1.
Sequences of Primers Used in the Real-Time Polymerase Chain Reaction
| Gene sequences of primers product size (bp) | ||
|---|---|---|
| β-actin | F: 5′-GGCACAGTCAAGGCTGAGAATG-3′ | 230 |
| R: 5′-ATGGTGGTGAAGACGCCAGTA-3′ | ||
| Smad-7 | F: 5′-GGGGGAACGAATTATCTGGC-3′ | 478 |
| R: 5′-CGCCATCCACTTCCCTTGT-3′ | ||
| TGF-β1 | F: 5′-GTGGCTGAACCAAGGAGACG-3′ | 461 |
| R: 5′-CAGGTGTTGAGCCCTTTCCAG-3′ | ||
| Col1α1 | F: 5′-GACATCCCTGAAGTCAGCTGC-3′ | 243 |
| R: 5′-TCCCTTGGGTCCCTCGAC-3′ | ||
| Col2α1 | F: 5′-GCCTTCCCATTGTTGACATTG-3′ | 131 |
| R: 5′-GTCCACACCAAATTCCTGATCA-3′ | ||
| Aggrecan | F: 5′-GCAACCTCCTGGGTGTAAGG-3′ | 197 |
| R: 5′-GCGTCGTAGCGGGATGAG-3′ | ||
| MMP-13 | F: 5′-TGACCTGGGATTTCCAAAAGA-3′ | 91 |
| R: 5′-TCCCCGTGTCCTCAAAGTG-3′ | ||
| ADATMS-5 | F: 5′-CGACAAGAGTCTGGAGGTGAG-3′ | 260 |
| R: 5′-CGTGAGCCACAGTGAAAGC-3′ | ||
The reaction mixture was amplified at 50°C for 2 min and 95°C for 30 s, and then 40 cycles at 95°C for 5 s, followed by 60°C for 34 s. The optimal concentrations of primers and templates used in each reaction were established according to the standard curve created before the reaction and corresponding to the nearly 100% efficiency of the reaction. Gene expression relative to the control was calculated using the 2−ΔΔCT formula method. The Ct value of each cDNA sample was defined as the cycle number at which the fluorescence intensity of each target gene was amplified within the linear range of the reaction.
Western blot analysis for the transfected cells
At 3 days after the lentivirus infection, the AF cells were collected and lysed in RIPA buffer (150 mM NaCl, 100 mM Tris-HCl, 1% Tween-20, 1% sodium deoxycholate, and 0.1% SDS) supplemented with 1 mM PMSF and protease inhibitor cocktail. The concentration of protein in the cell lysate was determined using a Bio-Rad assay according to the manufacturer's protocol (Bio-Rad). For each sample, whole cell extracts equivalent to 20 mg total protein were loaded into an 8% SDS–polyacrylamide gel, separated by electrophoresis, and the proteins were electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Nonspecific binding was blocked by incubating the PVDF membrane with 10 mM TBS with 1.0% Tween 20 and 10% dehydrated skimmed milk. Following the blocking procedure, the membranes were incubated overnight with mouse monoclonal antibody against Smad-7 (MAB2029; R&D Systems), mouse monoclonal antibodies against TGF-β1 (ab64715; abcam), rabbit monoclonal antibody against total Smad2\Smad3 (#8685; CST), rabbit polyclonal antibodies against P-Smad2 and rabbit monoclonal antibody against P-Smad3 (#3104 and #9520; CST), rabbit polyclonal antibodies against collagen type-I and type-II (ab34710 and ab34712; abcam), rabbit polyclonal antibodies against aggrecan (novus-NBP1-71370), rabbit polyclonal antibodies against MMP-13 (ab53047; abcam), and rabbit polyclonal antibodies against ADAMTS-5 (ab75606; abcam). The performance of immunoblotting on the same membranes using antibodies against GAPDH (Beyotime) was used as a loading control to assay the relative amounts of protein loaded into each gel. Following incubation with a primary antibody, the membranes were washed with TBST and incubated with alkaline phosphatase-linked secondary antibodies (Jackson Immunoresearch). The membranes were then washed thrice and the immunoreactive bands visualized using NBT/BCIP as a substrate. Densitometric analysis was done using the NIH ImageJ Software to quantify the protein present in the detected bands. GAPDH content was assayed as standardization of sample loading. Quantitative densitometric values of each protein of interest were normalized to GAPDH.
Intradiscal injection of the Smad-7 overexpression lentivirus
Another 48 male Sprague–Dawley rats, aged 2 months, were used for this part of the experiments. Under general anesthesia, the intervertebral discs were surgically exposed through a ventral approach (Rousseau and others 2004). A total of 2 μL solution (∼106 plaque-forming units) containing Smad-7 overexpression lentivirus was injected into the L4/L5 and L5/L6 intervertebral discs of half of the rats, with the depth of injection about 2 mm using a 28-gauge needle attached to a microsyringe (Nishida and others 2006; Zhang and others 2011). The negative control lentivirus was injected into the other half of the rats. After the surgery, antibiotics were supplied to the animals immediately.
Magnetic resonance imaging examination
At 8 and 12 weeks after injection, magnetic resonance imaging (MRI) examination for the lumbar spine was performed on a clinical 1.5-T scanner (Siemens Symphony) with a dedicated custom-made animal volume resonator at room temperature. T2-weighted sections in the sagittal plane were obtained. Then, a modified Thompson classification was used, by 2 observers who were blinded to the study groups, to evaluate the images based on changes in the degree and area of signal intensity from grade 1 to 4 (1, normal; 2, minimal decrease in signal intensity, but obvious narrowing of high signal area; 3, moderate decrease in signal intensity; and 4, severe decrease in signal intensity) (Masuda and others 2005).
Histomorphological evaluation
Just after MRI examination, lumbar spines, including L4–L6 vertebral levels, were harvested en bloc for histomorphological evaluation. They were fixed, decalcified, dehydrated, cleared with dimethylbenzene, and then embedded in paraffin. Serial sections in 4 μm thickness were obtained in the sagittal plane and stained with either hematoxylin–eosin and alcian blue-PAS, or safranin-O, or picrosirius red (Junqueira and others 1979; Borges and others 2007). The degenerative extent of IVD was graded according to the classification system for the histological features of age-related changes in the lumbar disc (Boos and others 2002; Furukawa and others 2009). A higher score represented a more severe extent of disc degeneration. Morphometric evaluation was carried out under an image autoanalysis system and a polar scope system (ZEISS Imager M1). The sections were evaluated by 2 observers, independently and blindly. The final results presented were the averages of the 2 observers.
RNA isolation and quantitative real-time PCR for the injected discs
The injected intervertebral discs from the L4–L6 vertebral levels harvested en bloc were dissected. Total RNA from the discs was isolated by grinding the frozen sample in liquid nitrogen using a mortar and pestle, followed by extraction using Trizol reagent (Invitrogen) according to the manufacturer's protocol. Primers for detection of actin, Smad-7, TGF-β1, collagen type-I, collagen type-II, aggrecan, MMP-13, and ADAMTS-5 were designed by TaKaRa Biotechnology. All the quantitative real-time PCR experiments were performed as previously described above.
Western blot analysis for the injected discs
Total protein was extracted from the injected IVD specimens using a Western and IP Cell Lysis kit (Beyotime). After centrifuge, the supernatant was collected and boiled with 2×SDS protein sample buffer. Then, the proteins were separated in SDS-PAGE and transferred to PVDF membranes, which were probed with different antibodies. The primary antibodies used in this study were the same as previously described above.
Statistical analyses
All data are expressed as mean±standard deviation. Statistical analyses were performed using the SPSS 11.5 statistical software program. All experiments in vitro were performed at least thrice in triplicate. Analysis of variance with subsequent Fisher's least significant difference test was performed to determine the significance of the difference in multiple comparisons. For the in vivo comparison of the differences between the Smad-7 overexpression lentivirus and negative control lentivirus injections, the Mann–Whitney U test was used. Pearson correlation test or Spearman rank correlation test was employed for correlation analysis. A P value<0.05 was considered to be the level of significance.
Results
Verification of the rat lumbar IVDD model
Successful establishment of the rat model of IVDD was confirmed by serial X-ray examinations and histomorphology evaluation. As in our previous report, imbalanced dynamic and static forces of the lumbar spine led to progressive degenerative changes, including lumbar spine malalignment, disc space narrowing, and endplate calcification on lateral radiographs. Meanwhile, histomorphological evaluation showed tears and clefts in the nucleus pulposus and AF and continuous new bone formation in the cartilaginous endplates. The total degenerative score gradually increased over time. TUNEL staining also revealed increased apoptosis of the disc cells with the increased severity of disc degeneration (Zhao and others 2010).
Expression of Smad-7 in the degenerative discs
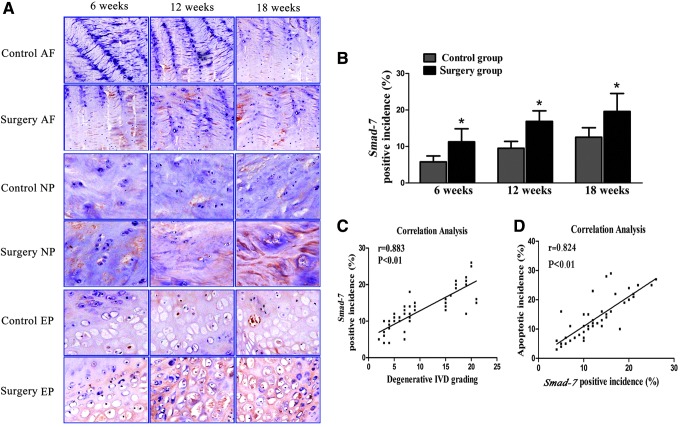
Expression of Smad-7 in the intervertebral discs was assessed by immunohistochemical analysis. Positive staining of Smad-7 was mainly localized in the nucleus and cytoplasm of disc cells. The number of Smad-7-positive cells progressively increased in the discs of the rats with induced instability at 6, 12, and 18 weeks, and there were significantly more Smad-7-positive cells in the discs of rats with induced instability than in those of the control rats at the 3 detection time points (P<0.05) (Fig. 1A, B). Correlation analysis revealed that the expression of Smad-7 was significantly correlated with the histomorphological grade of IVDD (r=0.883, P<0.01) and to the apoptosis incidence of the disc cells (r=0.824, P<0.01) (Fig. 1C, D). These results indicated that Smad-7 might have an important role in the pathophysiology of IVDD.
FIG. 1.
Expression of Smad-7 in the degenerative discs. (A) Immunohistochemical staining for Smad-7 (positively stained into brown) in the annulus fibrosus (AF), nucleus pulposus (NP), and endplate (EP) (×200). (B) Relative percentage of Smad-7-positive cells in the discs. *P<0.05. Correlation analysis between expression of Smad-7 and degenerative grading of the discs (C) and between expression of Smad-7 and disc cell apoptosis (D).
The effect of Smad-7 on the metabolism of isolated disc cells
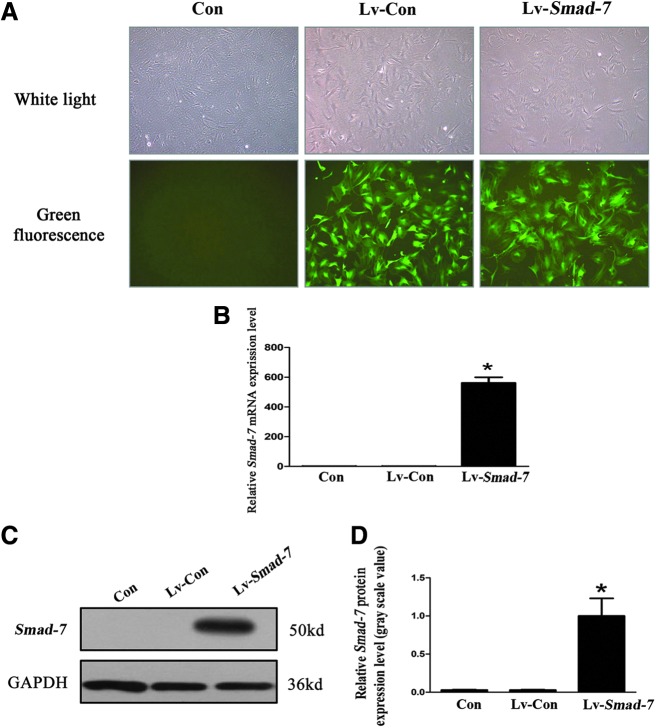
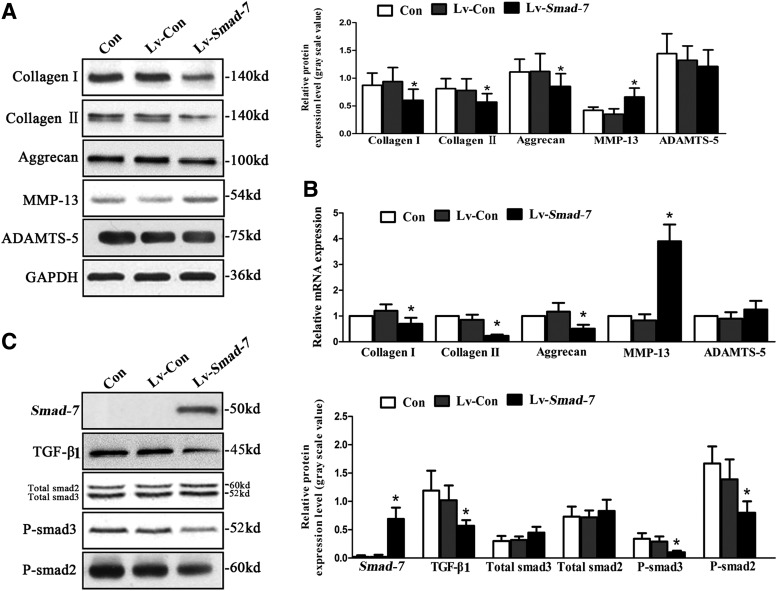
Successful transfection of the lentivirus vectors with Smad-7 overexpression into the isolated AF cells was verified (Fig. 2). To explore the role of Smad-7 in disc degeneration, we evaluated the effect of Smad-7 on the extracellular matrix metabolism of disc cells by real-time PCR and Western blotting. Three days after transfection of the lentivirus vectors with Smad-7 overexpression into the AF cells, the protein (Fig. 3A) and mRNA (Fig. 3B) expression of collagen type-I, collagen type-II, and aggrecan significantly decreased, while those of MMP-13 significantly increased. However, no significant change of the expression of ADAMTS-5 was noted. Meanwhile, Smad-7 overexpression significantly inhibited the expression of TGF-β1 and the phosphorylation of Smad-2 and Smad-3 without affecting the total amount of Smad-2 and Smad-3 (Fig. 3C). These results suggested that Smad-7 could inhibit the extracellular matrix synthesis and promote the degradation functions in the disc cells, and these effects of Smad-7 on the metabolism of disc cells were probably through its regulation on the biological behaviors of TGF-β.
FIG. 2.
Successful transfection of the isolated AF cells. (A) Fluorescence microscopy. The infection efficiency of the lentivirus vectors with Smad-7 overexpression (multiplicity of infection=100) in AF cells was greater than 80%. (B) Real-time polymerase chain reaction (PCR) for the mRNA expression of Smad-7 in the AF cells. (C) Western blot assay for the protein expression of Smad-7 in the AF cells. (D) Quantitative analysis of Smad-7 protein levels. *P<0.05. Con, controls without transfection; Lv-Con, negative control lentivirus vectors transfected; Lv-Smad-7, Smad-7 overexpression lentivirus vectors transfected.
FIG. 3.
The effect of Smad-7 on the metabolism of isolated AF cells. (A) Western blotting for the protein expression of collagen type-I, type-II, aggrecan, MMP-13, and ADAMTS-5. (B) Real-time PCR for the mRNA expression of collagen type-I, type-II, aggrecan, MMP-13, and ADAMTS-5. (C) Western blotting for the protein expression of Smad-7, TGF-β1, total smad3, total smad2, P-smad3, and P-smad2. *P<0.05. Con, controls without transfection; Lv-Con, negative control lentivirus vectors transfected; Lv-Smad-7, Smad-7 overexpression lentivirus vectors transfected; TGF-β1, transforming growth factor beta 1.
The in vivo effect of Smad-7 on disc degeneration
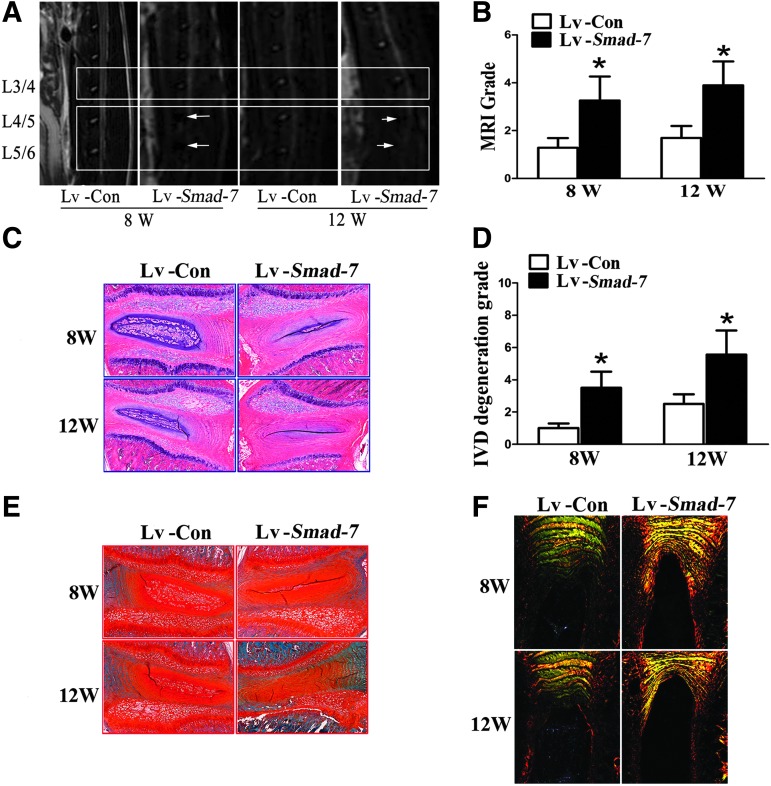
At 8 and 12 weeks after intradiscal injection, MRI examination of the lumbar spine was carried out in the rats. As shown in Fig. 4A, the signal intensities of the discs with negative control virus injections were much higher than those with Smad-7 overexpression virus injections. The degeneration score according to Thompson's classification was significantly higher in the discs with Smad-7 overexpression virus injections (Fig. 4B).
FIG. 4.
The in vivo effect of Smad-7 on disc degeneration. (A) MRI examinations at 8 and 12 weeks after intradiscal injections. White arrows indicated decreased signal intensities of the discs. (B) The MRI grading of disc degeneration. (C) HE staining of the injected discs. The number of notochordal cells significantly decreased in the nucleus pulposus and concentric tears could be identified in the AF of the discs with Smad-7 overexpression virus injections. (D) The histomorphological grade of disc degeneration. (E) Safranin-O staining of the injected discs. The red staining intensity for proteoglycan gradually decreased in the nucleus pulposus and AF of the discs with Smad-7 overexpression virus injections. (F) Picric acid–sirius red staining of the injected discs. The inner and outer AF in the negative control virus-injected discs were clearly divided and well arranged like concentric circles. However, they were vaguely divided in the Smad-7 overexpression virus-injected discs and were merged together and the boundary could not be clearly differentiated at 12 weeks. *P<0.05. Lv-Con, negative control lentivirus vectors injected; Lv-Smad-7, Smad-7 overexpression lentivirus vectors injected.
Histomorphologically, at 8 weeks after Smad-7 overexpression virus injections, the number of notochordal cells significantly decreased in the nucleus pulposus, and concentric tears could be identified in the AF of the injected discs. These characteristics of disc degeneration were more evident at 12 weeks (Fig. 4C). The grade of disc degeneration was also significantly higher in the rats with intradiscal Smad-7 overexpression virus injections (Fig. 4D). Meanwhile, safranin-O staining and picrosirius red staining were used to evaluate the content of proteoglycan and collagen fibrils as well as their changes. The red staining intensity for proteoglycan gradually decreased in the nucleus pulposus and AF in the rats with Smad-7 overexpression virus injections at 8 and 12 weeks (Fig. 4E). Under polarization microscopy, collagen type-I fibrils showed intense birefringence in yellow or red, while collagen type-II fibrils revealed mild birefringence in aquamarine blue or multicolor. In the discs with negative control virus injections, the inner AF was stained into aquamarine blue and the outer AF into bright yellow, indicating mainly collagen type-II fibrils in the inner AF and collagen type-I fibrils in the outer AF. These 2 parts were clearly divided and well arranged in concentric circles at 8 and 12 weeks. However, the inner and outer AF of the discs with Smad-7 overexpression virus injections were vaguely divided and appeared as yellow or thin green, suggesting their main component of collagen type-I fibrils (Fig. 4F).
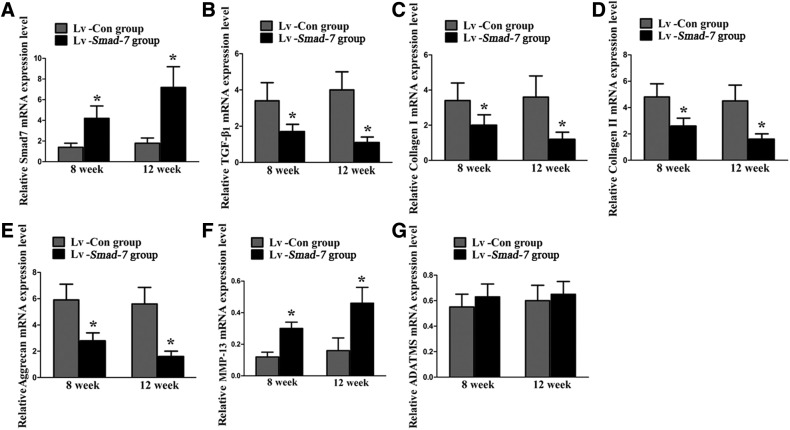
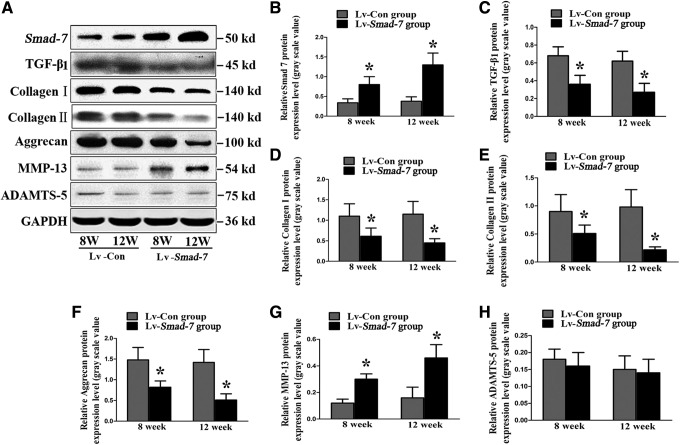
Finally, the in vivo effect of Smad-7 on the extracellular matrix metabolism of the disc cells was also investigated. Real-time PCR (Fig. 5) and Western blotting (Fig. 6) revealed that intradiscal injections with Smad-7 overexpression virus gradually and significantly increased the mRNA and protein expression of Smad-7 in the discs. The increased expression of Smad-7 inhibited the expression of TGF-β1, collagen type-I, collagen type-II, and aggrecan and promoted the expression of MMP-13. However, no significant change of ADAMTS-5 expression was noted, which was consistent with our in vitro results. These results suggested that Smad-7 did have an important role in the extracellular matrix metabolism of disc cells. Its high expression could lead to the metabolic disturbance of disc cells and thus the occurrence of disc degeneration.
FIG. 5.
Real-time PCR analysis for the extracellular matrix metabolism of the disc cells in vivo. (A) Smad-7 mRNA expression. (B) TGF-β1 mRNA expression. (C) Collagen type-I mRNA expression. (D) Collagen type-II mRNA expression. (E) Aggrecan mRNA expression. (F) MMP-13 mRNA expression. (G) ADAMTS-5 mRNA expression, *P<0.05. Lv-Con, negative control lentivirus vectors injected; Lv-Smad-7, Smad-7 overexpression lentivirus vectors injected.
FIG. 6.
Western blot analysis for the extracellular matrix metabolism of the disc cells in vivo. (A) Typical images of Western blot study. (B) Smad-7 protein expression. (C) TGF-β1 protein expression. (D) Collagen type-I protein expression. (E) Collagen type-II protein expression. (F) Aggrecan protein expression. (G) MMP-13 protein expression. (H) ADAMTS-5 protein expression. *P<0.05. Lv-Con, negative control lentivirus vectors injected; Lv-Smad-7, Smad-7 overexpression lentivirus vectors injected.
Discussion
Smad-7 has been characterized as an antagonist of TGF-β-initiated signaling (Hayashi and others 1997; Nakao and others 1997) and plays a key role in control of TGF-β signal transduction. TGF-β could stimulate the proteoglycan synthesis in the intervertebral disc (Thompson and others 1991; Nishida and others 1999) and was regarded as a crucial growth factor for the management of DDD (Zhan and others 2004; Chen and others 2006). However, the regulatory effect of Smad-7 on IVDD has never been investigated. In this study, we first employed imbalanced dynamic and static forces to induce lumbar IVDD in rats as in our previous report (Zhao and others 2010) and found that Smad-7 was highly expressed in the degenerative intervertebral discs. Significant positive correlations existed among the expression of Smad-7, the histomorphological severity of IVDD, and disc cell apoptosis. These results indicated that Smad-7 might have an important role in the pathophysiology of IVDD.
So far as we know, activation of Smad-2 and Smad-3 is mandatory for the proper function of TGF-β. However, Smad-7 interferes with the binding of TGF-β to its receptors, preventing the activation of Smad-2 and Smad-3 (Nakao and others 2002). In addition, this inhibitory Smad in the nucleus potently repressed the transcription of TGF-β (Nakao and others 1997). It has been reported that TGF-β was a protective factor against IVDD and it could increase proteoglycan and collagen synthesis rates in degenerated human disc cells (Lee and others 2001) and decrease the level of active MMPs in nucleus pulposus cells (Pattison and others 2001). Smad-7 inhibited the TGF-β-induced proteoglycan synthesis in chondrocytes and it completely antagonized the effect of TGF-β on the proliferation of the cells (Scharstuhl and others 2003). To elucidate the role of Smad-7 in the process of disc degeneration and explore the possible pathogenesis, we transfected the isolated disc cells with Smad-7 overexpression virus vectors and observed the effect of Smad-7 on the metabolic activities of the cells. Smad-7 upregulation could significantly inhibit the protein and mRNA expression of collagen type-I, collagen type-II, and aggrecan, while promoting the expression of MMP-13 in the AF cells. Meanwhile, the expression of TGF-β1 and the activation of Smad-2 and Smad-3 were obviously inhibited by Smad-7 overexpression in the cells. These in vitro results indicated that Smad-7 could inhibit the extracellular matrix synthesis and promote its degradation in the AF cells. Such effect of Smad-7 was probably through inhibiting the biological functions of TGF-β. Similar results were found in inflammatory bowel disease showing that highly expressed Smad-7 inhibited the TGF-β signal transduction, while activating the proinflammatory pathways. Meanwhile, knockdown of Smad-7 by antisense oligonucleotides could restore the TGF-β signaling and inhibit the production of inflammatory cytokines (Monteleone and others 2001, 2004). Thus, we speculate that inhibition of Smad-7 expression should improve the TGF-β signal transduction and promote the anabolism activities in disc cells and should be useful in the management of degenerative disc disorders.
Furthermore, we confirmed in our in vivo study that intradiscal injection of Smad-7 overexpression lentivirus could promote the catabolism and inhibit the anabolism in the discs. Similar to our in vitro results, the expression of collagen type-I, collagen type-II, and aggrecan significantly decreased, while the expression of MMP-13 significantly increased in the injected discs. The TGF-β1 expression in the injected discs was also significantly downregulated. Histomorphological analysis and MRI assessment also indicated progressive degeneration of the injected discs. These results further support that Smad-7 must have an important role in the pathophysiology of disc degeneration, and such effect of Smad-7 was probably through the TGF-β signaling pathway.
MMP-13 is known to be involved in IVD degeneration by hydrolyzing the collagens, and ADAMTS-5 is designated as an aggrecanase, which can specifically cleave cartilage proteoglycans. Previously, we found that ADAMTS-5 was involved in the process of IVD degeneration and its expression was closely related with aging (Zhao and others 2010). However, in this study, we found no significant change of ADAMTS-5 expression in the transfected disc cells or injected discs with Smad-7 overexpression virus. These seemingly unreasonable results were also observed in the studies showing that TGF-β had no effect on the expression and regulation of aggrecanase in human chondrocytes in vitro and in osteoarthritis (Yamanishi and others 2002; Moulharat and others 2004).
Our study also revealed that the degree of disc cell apoptosis was significantly correlated with the expression of smad-7 in the degenerative discs. Smad-7 was reported to induce apoptosis in podocytes and had a synergistic effect with TGF-β to promote podocyte apoptosis (Schiffer and others 2001). Likewise, Smad-7 could sensitize the tumor cells to TNF-induced apoptosis through the inhibition of expression of antiapoptotic NF-KB target genes (Hong and others 2007). However, in B-lymphocytes, Smad-7 was upregulated to inhibit TGF-β and activin-A-induced apoptosis (Ishisaki and others 1998; Patil and others 2000) and it could also inhibit the survival factor NF-kB and potentiate TGF-β-mediated apoptosis in epithelial cells (Lallemand and others 2001). Therefore, the functional role of Smad-7 in apoptosis seems to be cell type and tissue specific. How Smad-7 regulates apoptosis in disc cells needs to be further explored.
In conclusion, the current study demonstrated that Smad-7 was highly expressed in the degenerative discs. Disc degeneration might be modulated by the TGF-β/Smad-7 signaling pathway. Overexpression of Smad-7 probably weakened the protective role of TGF-β and accelerated the progress of disc degeneration. Interference on Smad-7 might be a potential therapeutic method for the prevention and treatment of DDDs.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (81171757, U1032001).
Author Disclosure Statement
No conflicts of interest to disclose.
References
- Blaney Davidson EN, van der Kraan PM, van den Berg WB. 2007. TGF-beta and osteoarthritis. Osteoarthritis Cartilage 15:597–604 [DOI] [PubMed] [Google Scholar]
- Blom AB, van der Kraan PM, van den Berg WB. 2007. Cytokine targeting in osteoarthritis. Curr Drug Targets 8:283–292 [DOI] [PubMed] [Google Scholar]
- Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. 2002. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 27:2631–2644 [DOI] [PubMed] [Google Scholar]
- Borges LF, Gutierrez PS, Marana HR, Taboga SR. 2007. Picrosirius-polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron (Oxford, England: 1993) 38:580–583 [DOI] [PubMed] [Google Scholar]
- Briones-Orta MA, Tecalco-Cruz AC, Sosa-Garrocho M, Caligaris C, Macias-Silva M. 2011. Inhibitory Smad7: emerging roles in health and disease. Curr Mol Pharmacol 4:141–153 [PubMed] [Google Scholar]
- Chen WH, et al. 2006. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol 209:744–754 [DOI] [PubMed] [Google Scholar]
- Cromer A, et al. 2004. Identification of genes associated with tumorigenesis and metastatic potential of hypopharyngeal cancer by microarray analysis. Oncogene 23:2484–2498 [DOI] [PubMed] [Google Scholar]
- Dong C, et al. 2002. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc Natl Acad Sci U S A 99:3908–3913 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Furukawa T, et al. 2009. Absence of biglycan accelerates the degenerative process in mouse intervertebral disc. Spine 34:E911–E917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginos MA, et al. 2004. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res 64:55–63 [DOI] [PubMed] [Google Scholar]
- Gumz ML, et al. 2007. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res 13:4740–4749 [DOI] [PubMed] [Google Scholar]
- Hayashi H, et al. 1997. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 89:1165–1173 [DOI] [PubMed] [Google Scholar]
- He W, et al. 2002. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J 21:2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, et al. 2003. Gene expression patterns in renal cell carcinoma assessed by complementary DNA microarray. Am J Pathol 162:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Lee C, Kim SJ. 2007. Smad7 sensitizes tumor necrosis factor induced apoptosis through the inhibition of antiapoptotic gene expression by suppressing activation of the nuclear factor-kappaB pathway. Cancer Res 67:9577–9583 [DOI] [PubMed] [Google Scholar]
- Ishisaki A, Yamato K, Nakao A, Nonaka K, Ohguchi M, ten Dijke P, Nishihara T. 1998. Smad7 is an activin-inducible inhibitor of activin-induced growth arrest and apoptosis in mouse B cells. J Biol Chem 273:24293–24296 [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. 1979. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447–455 [DOI] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell 6:1365–1375 [DOI] [PubMed] [Google Scholar]
- Kleeff J, et al. 1999. The TGF-beta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene 18:5363–5372 [DOI] [PubMed] [Google Scholar]
- Lallemand F, et al. 2001. Smad7 inhibits the survival nuclear factor kappaB and potentiates apoptosis in epithelial cells. Oncogene 20:879–884 [DOI] [PubMed] [Google Scholar]
- Landstrom M, Heldin NE, Bu S, Hermansson A, Itoh S, ten Dijke P, Heldin CH. 2000. Smad7 mediates apoptosis induced by transforming growth factor beta in prostatic carcinoma cells. Curr Biol 10:535–538 [DOI] [PubMed] [Google Scholar]
- Lee JY, et al. 2001. New use of a three-dimensional pellet culture system for human intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine 26:2316–2322 [DOI] [PubMed] [Google Scholar]
- Lee S, et al. 2009. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem 42:1504–1511 [DOI] [PubMed] [Google Scholar]
- Masuda K, et al. 2005. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14 [DOI] [PubMed] [Google Scholar]
- Mazars A, et al. 2001. Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J Biol Chem 276:36797–36803 [DOI] [PubMed] [Google Scholar]
- Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. 2001. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest 108:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, et al. 2004. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-kappaB activation in gut inflammation. J Biol Chem 279:3925–3932 [DOI] [PubMed] [Google Scholar]
- Moulharat N, et al. 2004. Effects of transforming growth factor-beta on aggrecanase production and proteoglycan degradation by human chondrocytes in vitro. Osteoarthritis Cartilage 12:296–305 [DOI] [PubMed] [Google Scholar]
- Murakami H, Yoon ST, Attallah-Wasif ES, Tsai KJ, Fei Q, Hutton WC. 2006. The expression of anabolic cytokines in intervertebral discs in age-related degeneration. Spine 31:1770–1774 [DOI] [PubMed] [Google Scholar]
- Nakao A, et al. 1997. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 389:631–635 [DOI] [PubMed] [Google Scholar]
- Nakao A, Okumura K, Ogawa H. 2002. Smad7: a new key player in TGF-beta-associated disease. Trends Mol Med 8:361–363 [DOI] [PubMed] [Google Scholar]
- Nishida K, et al. 1999. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine 24:2419–2425 [DOI] [PubMed] [Google Scholar]
- Nishida K, et al. 2006. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine 31:1415–1419 [DOI] [PubMed] [Google Scholar]
- Okado T, Terada Y, Tanaka H, Inoshita S, Nakao A, Sasaki S. 2002. Smad7 mediates transforming growth factor-beta-induced apoptosis in mesangial cells. Kidney Int 62:1178–1186 [DOI] [PubMed] [Google Scholar]
- Patil S, Wildey GM, Brown TL, Choy L, Derynck R, Howe PH. 2000. Smad7 is induced by CD40 and protects WEHI 231 B-lymphocytes from transforming growth factor-beta -induced growth inhibition and apoptosis. J Biol Chem 275:38363–38370 [DOI] [PubMed] [Google Scholar]
- Pattison ST, Melrose J, Ghosh P, Taylor TK. 2001. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by transforming growth factor-beta(1)and insulin like growth factor-I. Cell Biol Int 25:679–689 [DOI] [PubMed] [Google Scholar]
- Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y. 2006. Possible pathogenesis of painful intervertebral disc degeneration. Spine 31:560–566 [DOI] [PubMed] [Google Scholar]
- Pyeon D, et al. 2007. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res 67:4605–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, et al. 2006. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine 31:884–890 [DOI] [PubMed] [Google Scholar]
- Rousseau MA, Bass EC, Lotz JC. 2004. Ventral approach to the lumbar spine of the Sprague-Dawley rat. Lab Anim 33:43–45 [DOI] [PubMed] [Google Scholar]
- Sanchez-Capelo A. 2005. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev 16:15–34 [DOI] [PubMed] [Google Scholar]
- Scharstuhl A, Diepens R, Lensen J, Vitters E, van Beuningen H, van der Kraan P, van den Berg W. 2003. Adenoviral overexpression of Smad-7 and Smad-6 differentially regulates TGF-beta-mediated chondrocyte proliferation and proteoglycan synthesis. Osteoarthritis Cartilage 11:773–782 [DOI] [PubMed] [Google Scholar]
- Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Bottinger EP. 2001. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 108:807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi C, et al. 2014. A functional role for Smad7 in sustaining colon cancer cell growth and survival. Cell Death Dis 5:e1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Oegema TR, Jr., Bradford DS. 1991. Stimulation of mature canine intervertebral disc by growth factors. Spine 16:253–260 [DOI] [PubMed] [Google Scholar]
- Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, Karaharju EO. 2006. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J 15:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toruner GA, et al. 2004. Association between gene expression profile and tumor invasion in oral squamous cell carcinoma. Cancer Genet Cytogenet 154:27–35 [DOI] [PubMed] [Google Scholar]
- Wang B, Hao J, Jones SC, Yee MS, Roth JC, Dixon IM. 2002. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol 282:H1685–H1696 [DOI] [PubMed] [Google Scholar]
- Wang YJ, et al. 2006. Cervical intervertebral disc degeneration induced by unbalanced dynamic and static forces: a novel in vivo rat model. Spine 31:1532–1538 [DOI] [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, Firestein GS. 2002. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol (Baltimore, MD: 1950) 168:1405–1412 [DOI] [PubMed] [Google Scholar]
- Zhan Z, et al. 2004. Ad/CMV- hTGF-beta1 treats rabbit intervertebral discs degeneration in vivo. J Huazhong Univ Sci Technolog Med Sci 24:599–601, 624 [DOI] [PubMed] [Google Scholar]
- Zhang YH, Zhao CQ, Jiang LS, Dai LY. 2011. Lentiviral shRNA silencing of CHOP inhibits apoptosis induced by cyclic stretch in rat annular cells and attenuates disc degeneration in the rats. Apoptosis 16:594–605 [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Liu D, Li H, Jiang LS, Dai LY. 2008. Expression of leptin and its functional receptor on disc cells: contribution to cell proliferation. Spine 33:E858–E864 [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Zhang YH, Jiang SD, Jiang LS, Dai LY. 2010. Both endoplasmic reticulum and mitochondria are involved in disc cell apoptosis and intervertebral disc degeneration in rats. Age (Dordrecht, Netherlands) 32:161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]