Abstract
Purpose: To compare the efficacy and safety of 0.3% hydroxypropyl methylcellulose/dextran (HPMC/dextran) and 0.18% sodium hyaluronate (SH) in the treatment of ocular surface disease in patients using antiglaucoma drugs containing preservatives.
Methods: This was a double-blind, randomized, parallel-group study in 70 glaucoma patients with Ocular Surface Disease Index (OSDI) score greater than 20 points and/or presence of ocular signs. Patients were randomized to receive either preservative-free 0.3% HPMC/dextran (n=35) or preservative-free 0.18% SH (n=35). Treatment was 1 drop in each eye, 4 times a day. Data were collected at baseline, at day 7 and day 28.
Results: The groups were homogeneous at baseline. At day 28, both treatments showed significant improvements (P<0.05) in the mean OSDI score, lid skin and lid margin inflammation, conjunctival injection, and expressibility of meibomian glands, corneal staining score, fluorescein tear breakup time (FBUT), and Schirmer I test. However, the mean OSDI score, lid margin inflammation and conjunctival injection showed significant improvements (P<0.05) in the SH group at days 7 and 28, compared to the HPMC/dextran group. FBUT and the Schirmer I test also showed significant improvements (P<0.05) in the SH group compared to the HPMC/dextran group, at day 28. No adverse reactions were observed in either group.
Conclusions: Preservative-free artificial tear, 0.3% HPMC/dextran, and 0.18% SH, caused a significant relief of the ocular surface disease in glaucoma patients. However, 0.18% SH led to a greater improvement in ocular signs and symptoms than 0.3% HPMC/dextran.
Introduction
Glaucoma is the second leading cause of blindness in Thailand, and more generally worldwide.1,2 Nowadays, various medications are mandatory for the treatment of glaucoma to prevent blindness from hypertensive optic nerve damage. Eye drops usually containing preservatives, are the mainstay of treatment. However, the long-term duration of such treatments can also cause ocular surface disease, especially dry eye. Several previous studies3–9 have shown that the presence of preservatives in antiglaucoma medications is a main cause of ocular surface problems such as keratopathy, conjunctival inflammation, abnormal tear film production, tear film instability, and meibomian gland dysfunction. These adverse effects can lead to poor adherence to treatment.
Thus, given the need to continue glaucoma treatments and concern for the ocular surface damage they cause, it is important to find a medication that would decrease these ocular surface side effects. Previous reports have demonstrated the efficacy of nonpreserved artificial tears in increasing tear film production, tear film stability, and improving ocular surface in dry eye patients.10–14 Sodium hyaluronate (SH) was shown in vitro to reduce ocular toxicity due to benzalkonium chloride (BAK), a preservative often used in antiglaucoma drugs.15 Hydroxypropyl methylcellulose (HPMC) is a conventional therapy for tear film disturbances13,14 and is included in the essential drug list in Thailand.
The purpose of this study was to investigate the efficacy and safety of these 2 preservative-free artificial tears (0.3% HPMC and 0.18% SH) in reducing ocular surface toxicity induced by preserved antiglaucoma medication. To the best of our knowledge, this is the first study comparing the efficacy of these 2 artificial tears in the treatment of ocular surface disease in glaucoma patients.
Methods
Study design
This was a double-blind, randomized, parallel-group study carried out at a single center in Thailand. The treatment duration was 4 weeks and the study was divided into 3 visits for assessments (baseline, D7, and D28). After a washout period of 7 days for patients who were using artificial tears at the time of study inclusion, treatment was 1 drop in each eye, 4 times a day. No artificial tears were given during the washout period. Ethics committee approval was obtained from the Committee for the Protection of Human Participants in Research at the Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand [655/2553(EC4)]. The trial was registered with the following identification number: NCT 01284439 (www.clinicaltrials.gov). All subjects were recruited from an outpatient department in the Siriraj hospital during the period from October 2009 to December 2011. Informed consent was obtained from all patients before they were subjected to any study-specific examination. This study was conducted in accordance with the Helsinki Declaration and Good Clinical Practice.
Study population
Male and female patients were eligible for the study if they were over 18 years of age, diagnosed with glaucoma, that is, primary open angle glaucoma or primary angle closure glaucoma, and receiving topical antiglaucoma medications. All eligible patients must have an ocular surface disease index (OSDI; with the permission of Allergan, Inc., Irvine, CA) score equal to or greater than 20 and/or ocular surface diseases presenting any of the following: erythematous rash at eyelid skin/lid margin, signs of meibomian gland dysfunction, bulbar/tarsal conjunctival hyperemia, follicle at tarsal conjunctiva, fluorescein staining score using the National Eye Institute (NEI) system score equal to or greater than 3 points, Rose Bengal staining score (NEI system score) equal to or greater than 3 points, Schirmer's test equal to or less than 5 mm, and/or fluorescein tear breakup time (FBUT) equal to or less than 8 s.
Patients less than 18 years of age, those with secondary glaucoma from known causes, immunocompromised patients, those with an active or recent ocular infection, those with known allergies to any ingredient of study medications, pregnant or lactating women, contact lens wearers, and patients who were not able to follow the study instructions, were excluded from the study.
Study material
Vislube® (0.18% SH, molecular weight 1.2×106 Da; TRB Chemedica, Munich, Germany) and TearsNaturale® Free (0.3% HPMC/0.1% dextran; Alcon, FortWorth, TX) were used as study products. Vislube is a patented hypotonic (150 mOsm/kg) solution containing the electrolytes potassium, calcium, magnesium, sodium, and chloride. TearsNaturale Free is an isotonic (274 mOsm/kg) solution containing sodium, potassium, calcium, magnesium, zinc, chloride, and bicarbonate as electrolytes. Both products are preservative free. Both the patients and the evaluating investigator (PP) were blinded to the treatments received. The products were packed in their original monodose units, from which the label was removed. Therefore, the patients did not know which drug they were using. In addition, the evaluating investigator (PP) did not know which product was used.
Study proceedings
At the baseline visit (day 0), patients were assessed for the inclusion and exclusion criteria. Eligible patients were then randomized, by blocks of 4, into 2 groups using a computer-generated randomization list. The type of antiglaucoma products used was recorded.
Thereafter, all patients were classified as having either severe or nonsevere complications due to their antiglaucoma medications. If patients had any serious corneal complication, such as corneal epithelial defect, pseudodendritic corneal lesion, or conjunctival pseudomembrane, these patients were instructed to discontinue their current antiglaucoma medications and were switched to other systemic treatment modalities. However, no serious case occurred during the study.
All included patients underwent ocular examinations and completed 12 questions of the OSDI16 (Thai version) at baseline and at days 7 and 28. A detailed slit-lamp biomicroscopy was performed to evaluate and grade the severity of lid skin inflammation (Grade 0=no injection, Grade 1=lid skin injection/erythematous rash). Lid margin inflammation (hyperemia and telangiectasia) and bulbar conjunctival injection were graded as shown in our previous study17: (Grade 0=no injection, Grade 1=mild injection, Grade 2=moderate injection, and Grade 3=severe injection).17 The presence of follicles at the tarsal conjunctiva (Grade 0=none, Grade 1=presence of follicle) was assessed.
FBUT test was assessed with 2% fluorescein solution instilled into the lower fornix. FBUT recording was stopped when the first tear disruption spot was seen. This test was done 3 times and the mean time was used to calculate the FBUT.
Corneal and conjunctival staining with fluorescein and Rose Bengal was scored and recorded following the NEI system,18 in which the Rose Bengal staining score was recorded, after totally rinsing out the fluorescein solution with normal saline solution. The meibomian glands were evaluated in the next step to prevent pressure on the cornea from the observer's hand, which could interfere with the grading of corneal staining score. Grading of meibomian gland dysfunction was carried out according to the method reported in our previous study.17 In brief, the characteristics of meibomian gland secretions and meibomian gland expressibility were graded as in Table 1. Tear volume, measured by Schirmer I test, was performed in the last step. Additionally, the daily use of tear supplements was recorded by the patients.
Table 1.
Demographic Data and Baseline Characteristics
| Characteristics | HPMC/dextran (Group A) (n=35) | SH (Group B) (n=35) |
|---|---|---|
| Gender | ||
| Male, n (%) | 10 (28.6) | 13 (37.1) |
| Female, n (%) | 25 (71.4) | 22 (62.9) |
| Signs | ||
| Lid margin inflammation severity, n (%) | ||
| No injection | 4 (11.4) | 6 (17.1) |
| Mild inflammation | 28 (80.0) | 23 (65.8) |
| Moderate inflammation, telangiectasia | 3 (8.6) | 6 (17.1) |
| Severe inflammation, marked telangiectasia | 0 | 0 |
| Meibomian gland secretion, n (%) | ||
| Clear fluid | 8 (22.9) | 8 (22.9) |
| Cloudy fluid | 22 (62.9) | 25 (71.4) |
| Cloudy/particulate fluid | 4 (11.4) | 2 (5.7) |
| Inspissated/toothpaste-like | 1 (2.9) | 0 |
| Expressibility of meibomian gland n (%) | ||
| Well express | 8 (22.9) | 12 (34.3) |
| 2/3 expressibility | 20 (57.1) | 14 (40.0) |
| 1/3–2/3 expressibility | 7 (20.0) | 8 (22.9) |
| <1/3 expressibility | 0 | 1 (2.9) |
| Bulbar conjunctival injection n (%) | ||
| No injection | 5 (14.3) | 8 (22.9) |
| Mild injection | 26 (74.3) | 23 (65.7) |
| Moderate injection | 4 (11.4) | 4 (11.4) |
| Follicle n (%) | ||
| None | 15 (42.9) | 15 (42.9) |
| Presence | 20 (57.1) | 20 (57.1) |
| Corneal fluorescein score (mean±SD) | 5.86±3.33 | 6.37±4.27 |
| Corneal Rose Bengal score (mean±SD) | 0.37±0.69 | 0.37±0.84 |
| Fluorescein tear breakup time, s (mean±SD) | 3.83±1.54 | 4.65±1.85 |
| Schirmer's I test, mm (mean±SD) | 6.60±2.55 | 6.46±2.56 |
| Symptoms | ||
| OSDI [mean±SD] | 31.47±11.11 | 31.50±13.60 |
HPMC/Dextran, hydroxypropyl methylcellulose/dextran; OSDI, ocular surface disease index; SD, standard deviation; SH, sodium hyaluronate.
The main outcome measures for efficacy were OSDI score, lid margin inflammation, meibomian gland secretions and expressibility, conjunctival injection, corneal and interpalpebral staining, FBUT, and Schirmer I test. Safety and ocular side effects were recorded at each visit.
Statistical analyses
As this was a prospective study, no sample size determination was performed. It was hypothesized that a total of 70 eyes (ie, 70 patients) would provide sufficient power to show a difference between groups for the primary efficacy parameter, the OSDI score. The right eye was arbitrarily chosen for assessment.
Primary efficacy parameters were OSDI score, FBUT, corneal staining score, and Schirmer I tear test. Descriptive statistics were represented as mean values and standard errors of the mean. Intragroup comparisons for continuous variables were performed using a paired t-test or Wilcoxon signed-rank test (abnormal distribution).
Comparisons between groups for continuous variables were performed using an independent t-test or Mann–Whitney U test (abnormal distribution), and a Chi-square test was used for categorical variables. Statistical significance was considered as a P value<0.05 (two-sided, 95% confidence interval) and was performed using the Statistical Package for the Social Sciences software, version 14.0 (SPSS, Inc., Chicago, IL).
Results
A total of 70 patients were enrolled and randomly assigned to the 2 treatment groups, each containing 35 patients (eyes). No patients were withdrawn or lost to follow-up. Table 1 presents the demographic data and baseline characteristics. There were no statistically significant differences between groups for ocular signs and symptoms at baseline. Table 2 presents the antiglaucoma medications used by the patients before inclusion. Most patients used prostaglandin analogues and/or β-blockers.
Table 2.
Antiglaucoma Medications Before Inclusion
| Antiglaucoma medications | No. of eyes (%) |
|---|---|
| β-blockers | 48 (68.6) |
| Prostaglandin analogs | 43 (61.4) |
| α-agonists | 31 (44.3) |
| Topical CAIs | 8 (11.4) |
| Fixed-combinations | 9 (12.9) |
CAIs, carbonic anhydrase inhibitors.
The frequency of antiglaucoma medication use (drops per day) and the mean OSDI score at baseline are presented in Table 3.
Table 3.
Relationship Between Frequency of Antiglaucoma Eye Drops Administration and Mean OSDI Score at Baseline
| No. of drops/day | No. of patients (%) | Mean OSDI baseline |
|---|---|---|
| 1–2 | 23 (32.9) | 29.3 |
| 3–4 | 28 (40.0) | 31.4 |
| 5–7 | 19 (27.1) | 34.2 |
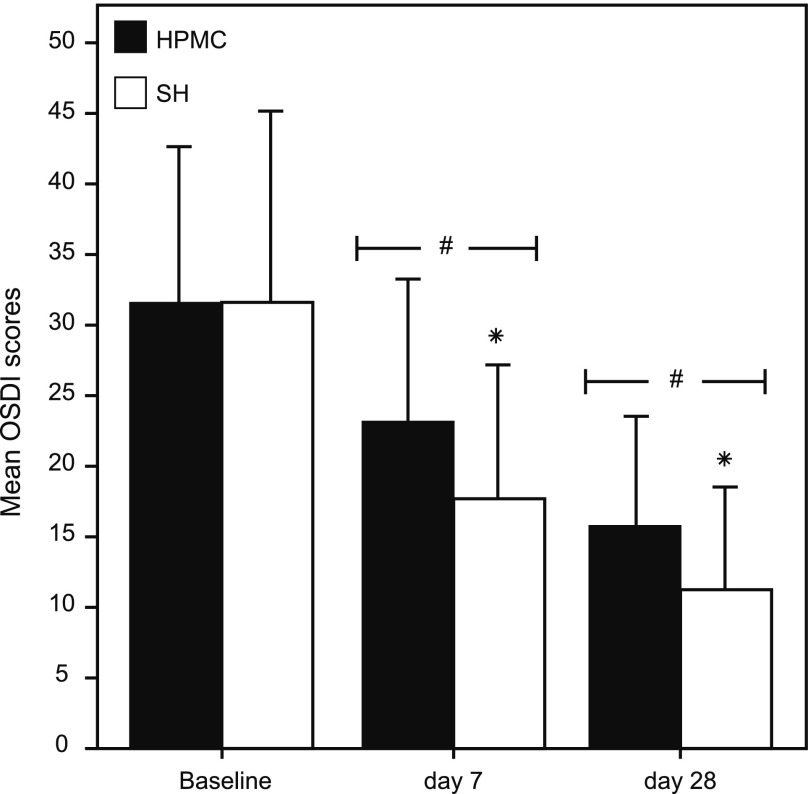
Results showed that the OSDI scores had significantly (P<0.0001) improved in both groups at D28, compared to baseline values (Fig. 1). The OSDI scores at baseline were 31.47±11.11 and 31.50±13.60 in the HPMC/dextran and SH groups, respectively. At D28, they were 15.76±7.75 and 11.14±7.31 in the HPMC/dextran and SH groups, respectively. The SH group showed a statistically significant (P=0.012) improvement in OSDI scores, compared to the HPMC/dextran group, at D28. A significant improvement (P=0.024) was already observed at D7 in the SH group, compared to the HPMC/dextran group.
FIG. 1.
Mean ocular surface disease index (OSDI) scores (±SD) at baseline, D7 and D28 in both groups. The symbols # and * represent a statistically significant (P<0.05) difference versus baseline and between groups, respectively. SD, standard deviation.
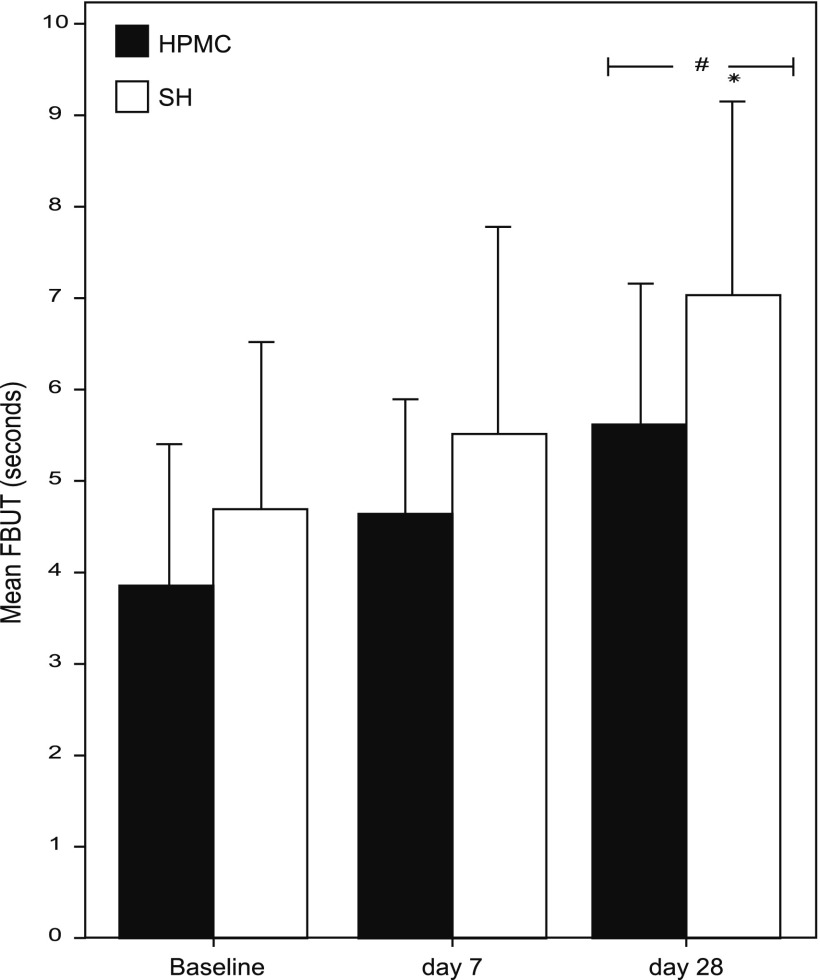
Regarding the signs, FBUT was statistically (P<0.0001) improved in both groups at D28 (5.57±1.59 and 6.99±2.17 in the HPMC/dextran and SH groups, respectively), compared to baseline (3.83±1.54 and 4.65±1.85 in the HPMC/dextran and SH groups, respectively). The Figure 2 evidences clearly that the SH induced a greater improvement in FBUT, than the HPMC/dextran, and that this difference is statistically significant (P=0.036).
FIG. 2.
Mean fluorescein tear breakup time (FBUT) (±SD) at baseline, D7 and D28 in both groups. The symbols # and * represent a statistically significant (P<0.05) difference versus baseline and between groups, respectively.
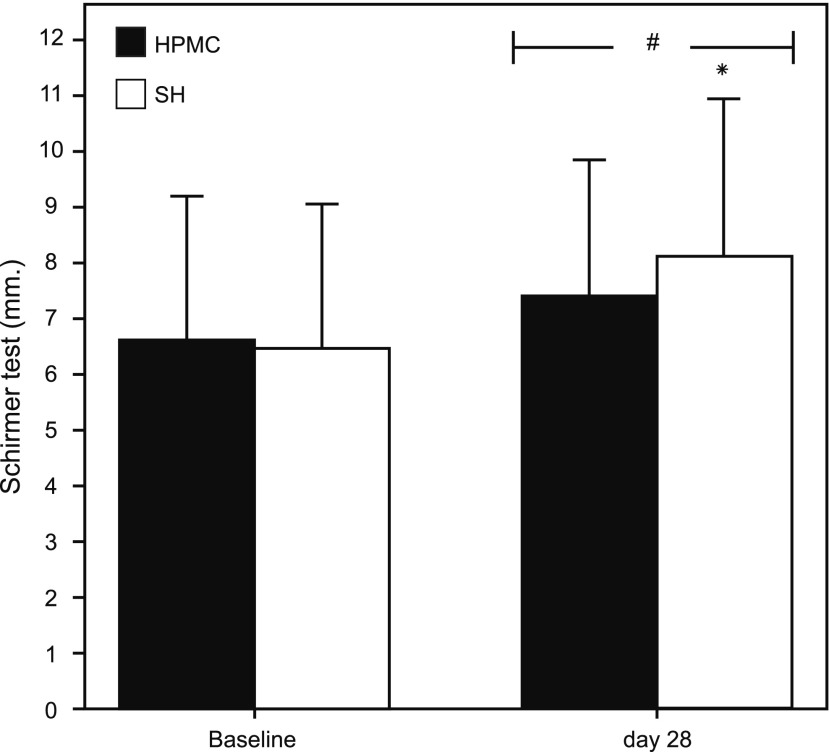
The SH group showed also a greater improvement in the tear amount than HPMC/dextran, at D28 (Fig. 3) with statistically significant (P<0.05), which at baseline Schirmer test was 6.60±2.55 and 6.46±2.56 for the HPMC/dextran and SH groups, respectively and at D28, 7.40±2.41 and 8.10±2.81 for the HPMC/dextran and SH groups, respectively.
FIG. 3.
Mean Schirmer's test value (±SD) at baseline and D28 in both groups. The symbols # and * represent a statistically significant (P<0.05) difference versus baseline and between groups, respectively.
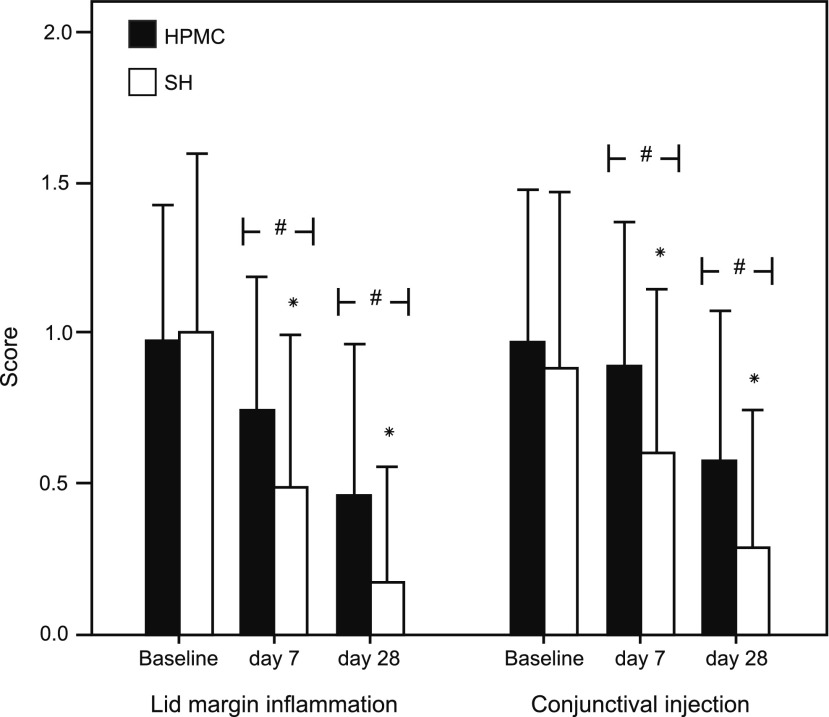
Lid skin and lid margin inflammation, as long as conjunctival injection showed significant improvements (P=0.011 and P=0.016, respectively) at D28, compared to baseline levels. For these parameters, the SH group showed an earlier and significant (P<0.05) improvement at D7, compared to baseline (Fig. 4).
FIG. 4.
Mean lid margin inflammation and conjunctival injection scores (±SD) at baseline, D7 and D28 in both groups. The symbols # and * represent a statistically significant (P<0.005) difference versus baseline and between groups, respectively.
There were no statistically significant differences between groups at D28, compared to baseline for meibomian gland secretion and expressibility, conjunctival follicle, and interpalpebral staining score. However, all of these parameters, except meibomian gland secretion, showed statistically significant improvements at D28 in each group compared to baseline values.
Subgroup analysis of the patients using monotherapy antiglaucoma medication (22 patients) revealed that the mean OSDI score (31.1±8.1) was significantly higher in beta-blocker group (13 patients) compared with prostaglandin group (23.9±7.1, P=0.045, paired t-test) while other parameters were no different. However, due to the small sample size, subgroup analysis to assess the relative effectiveness of different artificial tears in the treatment of toxic medicamentosa from different antiglaucoma medications could not be performed.
No serious adverse events occurred in any group during the study.
Discussion
The goal of glaucoma treatment is the prevention of blindness from glaucomatous optic nerve damage and thus antiglaucoma medications are mandatory treatments. However, these medications can cause adverse effects, such as ocular irritation and discomfort, lid and conjunctival redness and fibrosis, abnormal ocular surface dye staining, and punctate epitheliopathy or persistent epithelial defect,8,19,20 resulting in ocular surface diseases and poor compliance. Previous studies3,4,7,19,20 suggest that ocular surface toxicity as a result of glaucoma treatment is mainly due to preservatives contained in the antiglaucoma medications. Preservatives, especially BAK, decrease goblet cells,19,21 reduce tear secretion, and lower Schirmer test.9,19,22 Additionally, it interferes with tear film stability, increases tear evaporation, and shortens tear film breakup time with disturbances of the tear film lipid layer.9,11,19,22,23 These toxicities are from mechanisms of detergent effects leading to cellular apoptosis, oxidative stress,24 epithelial cell dysfunction, and inhibition of cell proliferation.24–26 Taken together, these effects can cause abnormal ocular surface inflammation and impaired corneal wound healing.6,27,28
In concordance with previous reports,8,19,20,29 the present study evidenced the relationship between the symptoms of a damaged ocular surface and the frequency of antiglaucoma eye drops administration (Table 3).
From this study, both preservative-free artificial tears (SH and HPMC/dextran) could improve the signs and symptoms of ocular surface disease in glaucoma patients within 1 month. The use of preservative-free artificial tears, concomitantly with antiglaucoma drugs, allowed counteracting their side effects onto the ocular surface. The study demonstrated that SH could improve ocular surface to a larger extent than HPMC/dextran, the following parameters: OSDI score, FBUT, conjunctival and lid inflammation as long as tear amount after 1 month of treatment with statistically significant difference (P<0.05). The viscoelasticity property of SH is probably its main asset to protect ocular surface damage.30 SH has also mucomimetic and mucoadhesive effects, which stabilized the precorneal tear film, and thus prolonged the residence time.31,32 Clinical studies comparing tear film stability in SH versus HPMC/dextran, supporting these results, demonstrated a significant improvement in SH than the HPMC group.10,33 Moreover, several studies reported that SH is able to promote epithelial migration,12 therefore, reducing punctate epitheliopathy.11,34 An in vitro study highlighted the positive effect of SH on oxidative stress, apoptosis, and necrosis of conjunctival and corneal epithelial cell line induced by BAK.15 Additionally, SH, in this study, is a hypotonic preservative-free artificial tear (150 mOsm/kg), which benefits the hyperosmolarity state of dry eye.35,36 This might explain the significantly improved result different from the previous study of Monaco et al.,37 which failed to demonstrate the advantage of using 0.2% isotonic (300 mOsm/kg) SH to treat ocular surface damage from glaucoma medications. However, in the Monaco study, carboxymethylcellulose with osmoprotection improved both OSDI and lissamine green staining score in patients having ocular surface disease (OSD) from glaucoma medications.
In serious cases such as severe lid edema, persistent epithelial defect, or severe keratitis, which were not included in this study, artificial tear supplement only may not be adequate. The treatment may require other modalities such as discontinue or change the medications, avoid toxic preservative such as BAK as demonstrated by Konstas,38 use nonpreservative drugs, add some more potent therapy such as nonpreservative steroid to reduce the inflammation, biological substance such as autologous serum which has growth factor39 to promote corneal integrity, or even switch to surgical treatment.
Different types of antiglaucoma medications may affect OSD differently. In this study, patients in beta-blocker monotherapy group had significantly more symptoms than prostaglandin group before the treatment. This finding was consistent with the Monaco study.37 However, OSDI score could be improved in both groups by artificial tears as previously mentioned.
The present randomized and controlled trial demonstrates the benefit of preservative-free artificial tears in a glaucoma patient, to reduce the ocular surface toxicity from glaucoma medication. However, the weakness of this study is the limitation in the number of patients. A larger or multicenter study may confirm the result.
In conclusion, ocular surface collateral damage or preservatives contained in antiglaucoma drugs are now well recognized. This study establishes that the concomitant use of preservative-free artificial tears, especially SH, may be helpful to relieve the patient's discomfort and improve the ocular surface health and could promote a reasonable compliance of the patient to antiglaucoma treatment.
Acknowledgments
The authors are grateful to Assistant Professor Chulaluk Komoltri, DrPH (Biostatistics) and Pimrapat Tengtrakulcharoen, MBH, from the Office for Research and Development, for their assistance with statistical analysis, and to Mathuwan Srikong from the Medical Education Technology Center, Faculty of Medicine Siriraj Hospital, Mahidol University, for preparing the figures.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.The First Visual Impairment Project in Thailand-TVIP, The Fourth National Survey of Blindness, Low Vision and Major Eye Disease in Thailand, 2006–2007 [Google Scholar]
- 2.Quigley H.A., and Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90:262–267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherwood M.B., Grierson I., Millar L., and Hitchings R.A. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon's capsule in glaucomatous patients. Ophthalmology 96:327–335, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Kuppens E.V., Stolwijk T.R., de Keizer R.J., and van Best J.A. Basal tear turnover and topical timolol in glaucoma patients and healthy controls by fluorophotometry. Invest. Ophthalmol. Vis. Sci. 33:3442–3448, 1992 [PubMed] [Google Scholar]
- 5.Herreras J.M., Pastor J.C., Calonge M., and Asensio V.M. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology 99:1082–1088, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Baudouin C. Side effects of antiglaucomatous drugs on the ocular surface. Curr. Opin. Ophthalmol. 7:80–86, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Feijoo J., and Sampaolesi J.R. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin. Ophthalmol. 6:441–446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung E.W., Medeiros F.A., and Weinreb R.N. Prevalence of ocular surface disease in glaucoma patients. J. Glaucoma 17:350–355, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Inoue K., Okugawa K., Kato S., Inoue Y., Tomita G., Oshika T., et al. . Ocular factors relevant to anti-glaucomatous eyedrop-related keratoepitheliopathy. J. Glaucoma 12:480–485, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Prabhasawat P., Tesavibul N., and Kasetsuwan N. Performance profile of sodium hyaluronate in patients with lipid tear deficiency: randomised, double-blind, controlled, exploratory study. Br. J. Ophthalmol. 91:47–50, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aragona P., Papa V., Micali A., Santocono M., and Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br. J. Ophthalmol. 86:181–184, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes J.A., Amankwah R., Powell-Richards A., and Dua H.S. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br. J. Ophthalmol. 88:821–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Versura P., Maltarello M.C., Stecher F., Caramazza R., and Laschi R. Dry eye before and after therapy with hydroxypropyl methylcellulose. Ultrastructural and cytochemical study in 20 patients. Ophthalmologica 198:152–162, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Toda I., Shinozaki N., and Tsubota K. Hydroxypropyl methylcellulose for the treatment of severe dry eye associated with Sjogren's syndrome. Cornea 15:120–128, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Pauloin T., Dutot M., Warnet J.M., and Rat P. In vitro modulation of preservative toxicity: high molecular weight hyaluronan decreases apoptosis and oxidative stress induced by benzalkonium chloride. Eur. J. Pharm. Sci. 34:263–273, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Schiffman R.M., Christianson M.D., Jacobsen G., Hirsch J.D., and Reis B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 118:615–621, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Prabhasawat P., Tesavibul N., and Mahawong W. A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea 31:1386–1393, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Lemp M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 21:221–232, 1995 [PubMed] [Google Scholar]
- 19.Baudouin C. The ocular surface in glucoma. Cornea 28:S14–S19, 2009 [Google Scholar]
- 20.Pisella P.J., Pouliquen P., and Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 86:418–423, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung S.H., Lee S.K., Cristol S.M., Lee E.S., Lee D.W., Seo K.Y., et al. . Impact of short-term exposure of commercial eyedrops preserved with benzalkonium chloride on precorneal mucin. Mol Vis 12:415–421, 2006 [PubMed] [Google Scholar]
- 22.Yalvac I.S., Gedikoglu G., Karagoz Y., Akgun U., Nurozler A., Koc F., et al. . Effects of antiglaucoma drugs on ocular surface. Acta Ophthalmol. Scand. 73:246–248, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Wilson W.S., Duncan A.J., and Jay J.L. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br. J. Ophthalmol. 59:667–669, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Saint Jean, M., Brignole F., Bringuier A.F., Bauchet A., Feldmann G., and Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 40:619–630, 1999 [PubMed] [Google Scholar]
- 25.Dogan A.S., Orhan M., Soylemezoglu F., Irkec M., and Bozkurt B. Effects of topical antiglaucoma drugs on apoptosis rates of conjunctival epithelial cells in glaucoma patients. Clin. Experiment. Ophthalmol. 32:62–66, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Cha S.H., Lee J.S., Oum B.S., and Kim C.D. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin. Experiment. Ophthalmol. 32:180–184, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Baudouin C., Pisella P.J., Fillacier K., Goldschild M., Becquet F., De Saint Jean, M., et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology 106:556–563, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Baudouin C., Hamard P., Liang H., Creuzot-Garcher C., Bensoussan L., and Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology 111:2186–2192, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Erb C., Gast U., and Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 246:1593–1601, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Abelson M.B., Nally L., and Ousler G.W. Dry eye, today and tomorrow. Rev. Ophthalmol. VII:132–134, 2000 [Google Scholar]
- 31.Nakamura M., Hikida M., Nakano T., Hamano T., Kinoshita S. Characterization of water retentive properties of hyaluronan. Cornea 12:433–436, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Saettone M.F., Chetoni P., Torracca M.T., Burgalassi S., Giannacini B. Evaluation of mucoadhesive properties and in vivo activity of opthalmic vehicles based on hyaluronic acid. Int. J. Pharm. 51:203–212, 1989 [Google Scholar]
- 33.McCann L.C., Tomlinson A., Pearce E.I., and Papa V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea 31:1–5, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Brignole F., Pisella P.J., Dupas B., Baeyens V., and Baudouin C. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch. Clin. Exp. Ophthalmol. 243:531–538, 2005 [DOI] [PubMed] [Google Scholar]
- 35.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 5:75–92, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 5:163–178, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Monaco G., Cacioppo V., Consonni D., and Troiano P. Effects of osmoprotection on symptoms, ocular surface damage, and tear film modifications caused by glaucoma therapy. Eur. J. Ophthalmol. 21:243–250, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Konstas A.G., Voudouragkaki I.C., Boboridis K.G., Haidich A.B., Paschalinou E., Giannopoulos T., et al. . 24-hour efficacy of travoprost/timolol BAK-free versus latanoprost/timolol fixed combinations in patients insufficiently controlled with latanoprost. Adv. Ther. 31:592–603, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Phasukkijwatana N., Lertrit P., Liammongkolkul S., and Prabhasawat P. Stability of epitheliotrophic factors in autologous serum eye drops from chronic Stevens-Johnson syndrome dry eye compared to non-autoimmune dry eye. Curr. Eye Res. 36:775–781, 2011 [DOI] [PubMed] [Google Scholar]